Abstract
Neurons in the lateral intraparietal area, frontal eye field, and superior colliculus exhibit a pattern of activity known as remapping. When a salient visual stimulus is presented shortly before a saccade, the representation of that stimulus is updated, or remapped, at the time of the eye movement. This updating is presumably based on a corollary discharge of the eye movement command. To investigate whether visual areas also exhibit remapping, we recorded from single neurons in extrastriate and striate cortex while monkeys performed a saccade task. Around the time of the saccade, a visual stimulus was flashed either at the location occupied by the neuron's receptive field (RF) before the saccade (old RF) or at the location occupied by it after the saccade (new RF). More than half (52%) of V3A neurons responded to a stimulus flashed in the new RF even though the stimulus had already disappeared before the saccade. These neurons responded to a trace of the flashed stimulus brought into the RF by the saccade. In 16% of V3A neurons, remapped activity began even before saccade onset. Remapping also was observed at earlier stages of the visual hierarchy, including in areas V3 and V2. At these earlier stages, the proportion of neurons that exhibited remapping decreased, and the latency of remapped activity increased relative to saccade onset. Remapping was very rare in striate cortex. These results indicate that extrastriate visual areas are involved in the process of remapping.
The eyes are constantly moving yet we perceive a stable visual world. Psychophysical (1) and neurophysiological (2) experiments indicate that the brain can keep track of the location of visual objects despite eye movements. One neuronal mechanism that may contribute to spatial constancy is updating. Visual neurons in the lateral intraparietal area (LIP), the frontal eye field (FEF), and the intermediate layers of the superior colliculus update, or remap, the representation of a salient stimulus location when the eyes move. In LIP, most neurons (96%) neurons respond when a saccade brings the location of a previously flashed stimulus into the receptive field (RF) (3). Moreover, when the monkey makes a saccade that brings a stationary stimulus into the RF, 44% of LIP neurons, 30% of neurons in intermediate layers of superior colliculus, and 31% of FEF neurons respond at a latency that indicates that remapping is predictive (3–5). This phenomenon may contribute to the brain's ability to maintain a stable, spatially accurate representation despite eye movements. It enables neurons to represent recently stimulated locations immediately at the end of a saccade without the delay associated with retinal reafference. The brain regions in which remapping has been demonstrated to date all are strongly involved in spatial attention and the control of eye movements. We have now asked whether remapping also occurs in extrastriate and striate cortex, which are thought to serve more purely visual functions.
We hypothesized that remapping would occur in extrastriate area V3A based on previous anatomical and physiological studies. Area V3A provides a major input to LIP and receives reciprocal projections from it (6–10). Area LIP is also reciprocally connected with the FEF (11, 12). Moreover, physiological studies indicate the existence of top-down effects in V3A. Visual responses in V3A are modulated by eye position (13), and V3A neurons discriminate between real motion of a stimulus and retinally equivalent motion produced by eye movements (14). We have previously shown that the activity of V3A neurons is modulated by cognitive signals, such as anticipation and memory, and by saccadic eye movements (15). Thus, we expected that signals reflecting intended saccades might induce remapping in V3A. For comparison to V3A, we also recorded from neurons in V3, V2, and V1, using identical procedures. The results indicate that remapping occurs in a substantial proportion of neurons in V3A and even occurs at earlier stages in the hierarchical chain of visual areas.
Methods
Four hemispheres of two rhesus monkeys (Macaca mulatta) were studied by using the methods described (15).
During recording sessions the monkey sat with its head fixed in a primate chair in a darkened room facing a tangent screen, subtending 100 degrees (deg) horizontally and 76 deg vertically. The luminance of the background was 6 cd/m2. Visual stimuli (186 cd/m2) were back-projected onto the screen. For each neuron we mapped the RF with moving oriented bars. To determine whether a given neuron was recorded from V1, V2, V3, or V3A, we used three standard measures. First, we used a combination of grid position and recording depth to reconstruct the locations of recording sites on drawings from MR images. Second, the progression of RF locations across the cortex was charted. This procedure is comparable to that used by Gattass et al. (16) and was essential for determining the areal location of each neuron. Third, we took into account the size of the RF of each neuron. These procedures are described in detail elsewhere (15) and shown in Fig. 9, which is published as supporting information on the PNAS web site, www.pnas.org.
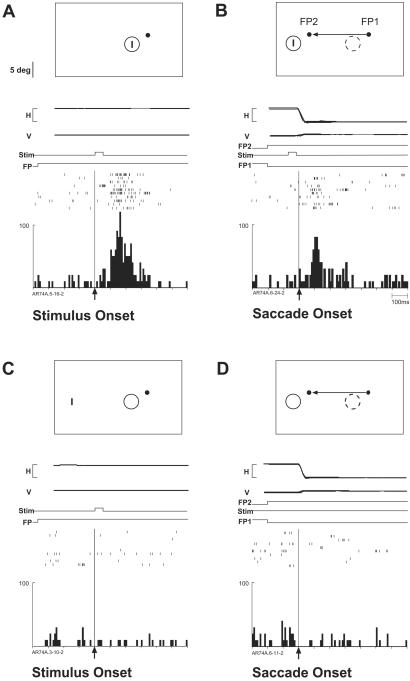
The monkey performed fixation and visually guided saccade tasks. In the fixation task, the monkey maintained fixation on a central fixation point (FP, 0.5 deg in diameter) for a variable period (400–600 ms), and then a stimulus was flashed for 50 ms in the neuron's RF. The animal was rewarded for continuing to hold eye position within a 1.5-deg window for another 1,000 ms. In the single-step task (Fig. 1), the monkey maintained fixation for 400–600 ms on an initial FP (FP1). Simultaneously, FP1 was extinguished and a new FP appeared at a different location (FP2). The monkey had to make a saccade from FP1 to FP2 and maintain fixation on FP2 for 500–1,000 ms. The second period of fixation was used to prevent the animal from looking at the task-irrelevant stimulus after the saccade. We analyzed the trials in which the end point of the saccade was within a 1.5-deg window around FP2. We refer to the location of the RF before the saccade as the old RF and to the location of the RF after the saccade as the new RF. The stimulus was presented for 50 ms in either the old or new RF (Fig. 1A).
Figure 1.
Location and timing of stimulus presentation in the single-step task. The monkey makes a visually guided saccade from FP1 to FP2. (A) The RF moves from the old location associated with FP1 (old RF) to a new location associated with FP2 (new RF). The visual stimulus is flashed in either old RF or new RF. (B) The visual stimulus (Stim) is flashed either before or after the saccade.
To contrast the effects of stimuli presented at different intervals relative to the saccade, we used four stimulus onset times: 33 ms (time 1) or 133 ms after the onset of the new FP (time 2), and 17 ms (time 3) or 217 ms after the end of the saccade (time 4) (Fig. 1B). In the first two cases, the stimulus appeared and was extinguished before saccade onset, while the eyes were still at FP1. In the latter two cases, the stimulus was flashed while the eyes were at FP2. This set of stimulus times allowed us to contrast the responses elicited when the stimulus was presented as close as possible to the time of the saccade (times 2 and 3), with the responses generated when the stimulus was presented long before (time 1) or long after the saccade (time 4). The condition that corresponds most closely to that used in previous studies (3) is the condition in which the stimulus was presented at time 1 in the new RF.
This set of two stimulus locations and four times made for eight conditions that were interleaved within a block until the monkey had performed 10–16 correct trials for each. The visual stimulus for a given neuron was either a spot or a bar of optimal length and orientation, depending on which was most effective. The positions of FP1 and FP2 were selected from five possible locations ranging in 10-deg intervals from 20 deg left to 20 deg right at 0 deg of elevation. Typically, we placed FP1 and FP2 20 deg apart, such that the new and old RF were in different visual hemifields. In separate blocks we ran two control tasks before the saccade tasks described above. In the stimulus-only control, the monkey gazed at FP1 while the visual stimulus was presented in the new RF location. In the saccade-only control, the monkey made a saccade without visual stimulus presentation. Neurons that were activated in either control task were excluded from further analysis.
Our aim was to determine whether neurons responded when the RF was brought by a saccade to the location where a stimulus had previously been visible but was not currently visible. Accordingly, we rejected from consideration any trials in which the monkey initiated a saccade while the stimulus was still on. Even when the stimulus had been turned off, however, it did not vanish instantaneously but rather decayed with a time constant of 13 ms, as determined by measurement with a phototransistor. The contrast of the stimulus, as a function of time after stimulus offset was: [L(t) − 6 cd/m2]/[L(t) + 6 cd/m2], where L(t) = 6 cd/m2 + {(186 cd/m2) × exp[−t/(13 msec)]} and t = time in ms. According to this calculation, the contrast of the stimulus was reduced to 7% at 70 ms after stimulus offset, 3% after 80 ms, and 1.5% after 90 ms. The contrast sensitivity of V3A neurons has not been determined in our paradigm. In previous studies, it has been shown that most neurons in the lateral geniculate nucleus, area V1, and area MT are unresponsive at 7% contrast, although a small population of the most sensitive MT neurons are still active (17). Although we cannot entirely exclude the possibility that the neuron may respond to the decaying stimulus in the time 2 condition, the range of saccade offset relative to the stimulus offset was 67–197 ms (mean 115 ms ± 31 ms SD), which indicates that most saccades occurred late enough that the neurons are not responding to a detectable stimulus.
For each neuron in each task, responses were measured off-line for 10–16 correct trials. Response latency was measured as the time when the activity during the detection window (duration, 20 ms) was significantly different from the baseline (100 ms before stimulus onset, P < 0.05, t test) (15). If this criterion was not met by any bins up to 200 ms after stimulus onset, we concluded that there was no response associated with visual stimulus presentation. The amplitude of a response was measured as the average firing rate during the 80 ms after the response latency for that neuron.
Results
We found that neurons in three extrastriate areas update visual signals. We classified a neuron as exhibiting remapping if it had a significant response in the single-step task (time 1 or 2, new RF) and was not activated in either of the two control conditions. More than half of V3A neurons (52%) met this criterion. An example is shown in Fig. 2. The RF was well defined and the neuron fired vigorously in response to the onset of a visual stimulus during fixation (Fig. 2A). In the two control conditions, the neuron did not fire when a stimulus was presented outside of the RF (Fig. 2C), nor did it fire in conjunction with a saccade from the initial FP (FP1) to a new FP (FP2) (Fig. 2D). Nevertheless, in the single-step task, the neuron fired after the same saccade brought the location of the stimulus into the RF (Fig. 2B). In this task, the visual stimulus was flashed in the new RF for 50 ms, beginning 133 ms after FP2 onset (simultaneous with FP1 offset). We analyzed only the trials in which the saccade started after stimulus offset, therefore, the stimulus was never in the RF. For this set of trials, the average latency from FP2 onset to saccade onset was 202 ms, thus, the stimulus was extinguished on average 19 ms before saccade onset. The neuron responded to the “trace” of the stimulus, even though the physical stimulus was no longer present.
Figure 2.
A V3A neuron (RF size, 4 deg; eccentricity, 5.2 deg) that responds to the stimulus (Stim) trace after a saccade. The cartoons show the location of stimuli on the screen. The horizontal (H) and vertical (V) eye position (calibration bar, 20 deg) and the timing of stimulus events also are shown. Response rasters from 10 successful trials are aligned on the specified events and summed to generate a histogram. (A) Fixation task: the stimulus, a 3-deg bar, is flashed in the RF while the monkey fixates the FP. (B) Single-step task: while the monkey fixates FP1 the stimulus is flashed in the new RF for 50 ms and is extinguished before the saccade to FP2. After the saccade, the neuron fires, even though the stimulus is already gone. (C) Stimulus-only control: presentation of a stimulus outside the RF does not drive the neuron in the absence of a saccade. (D) Saccade-only control: the saccade alone does not drive the neuron in the absence of a stimulus. Target distance, size, and location of RF are drawn to scale.
The response to the remapped visual signal occurred slightly earlier than might be expected on the basis of the visual response. This neuron's visual latency in the fixation task averaged 104 ms (Fig. 2A). Thus, if the neuron responded to the trace of the visual stimulus in its classic RF, it should start responding only 104 ms after the stimulated location entered the RF. However, as shown in Fig. 2B, activity began less than 50 ms after saccade onset. Thus, the response to the stimulus trace, even though it occurred after the saccade onset, was predictive.
To examine in more detail the time course of excitability changes, we presented the stimulus in either the old or the new RF at four different times. We found that excitability decreased at the old RF location as it increased at the new RF location. In Fig. 3, we show the responses of a single neuron in all eight conditions (same neuron as Fig. 2). In Fig. 3 Middle, the neuron responded strongly to a stimulus flashed in the old RF at time 1, well before the saccade. In contrast, the response to the same stimulus in the same location was significantly reduced at time 2, when that stimulus was presented just before the saccade to FP2. It is as though the RF had already begun to shift to the spatial location that it would occupy after the end of the saccade. When the stimulus was presented in the old RF location after the saccade had been initiated, there was, of course, no response (times 3 and 4).
Figure 3.
Response to the stimulus (Stim) presented in the old (Middle) or new (Bottom) RF at four different timings (same neuron as Fig. 2). Data are aligned on stimulus onset. Inverted triangles indicate the mean time of saccade onset. The excitability at the new RF increases while excitability at the old RF decreases even before saccade onset (time 2). H, eye position in horizontal axis.
These responses to stimuli in the old RF stand in contrast to responses to stimuli presented at the new RF location, shown in Fig. 3 Bottom. There was no response to a stimulus presented at the new RF location at time 1, well before the saccade occurs. The neuron did respond, however, to a stimulus presented in the new RF immediately before the saccade (time 2). The stimulus was presented for only 50 ms, therefore the neuron was responding to a stimulus that was no longer present. Note that these data for time 2 are the same data shown in Fig. 2B but are here aligned on stimulus onset rather than saccade onset. Again, it appears that the RF has shifted to the location that it will occupy after the saccade, and the neuron has become responsive to stimuli that would be in the RF after the saccade. At times 3 and 4, the amplitude of response increased as the neuron responded to an actual stimulus in the new RF.
We found that neurons that exhibited remapping of stimulus traces fell along a continuum. Some, like the neuron illustrated in Figs. 2 and 3, appear to shift the location of the RF around the time of an intended saccade and become less responsive at the old location while simultaneously becoming much more responsive at the future location of the RF. Others, like the neuron illustrated in Figs. 4 and 5, appear to undergo a momentary expansion of the RF immediately before a saccade. The V3A neuron illustrated in Fig. 4 responded at a latency of 94 ms in the fixation task (Fig. 4A). The same neuron responded to a visual stimulus presented in the new RF at time 2 and did so even before the saccade had been initiated (Fig. 4B). The visual stimulus was extinguished well before saccade onset and never appeared in the classical RF. Control experiments demonstrated that the neuron did not respond to a stimulus in the new RF in the absence of eye movement (Fig. 4C), nor did it respond when a saccade was made in the absence of the stimulus (Fig. 4D).
Figure 4.
(A) A V3A neuron (RF size, 12 deg; eccentricity, 21 deg) that responds to the stimulus (Stim) flashed in the new RF even before a saccade. Same format as Fig. 2. In the single-step task (B), a visual stimulus is flashed in the new RF 33 ms after FP2 onset (time 1). H, horizontal; V, vertical.
Figure 5.
Responses to stimuli (Stim) presented in the old or new RF (same neuron as in Fig. 4, same format as Fig. 3). This neuron responds to the stimulus presented in the new RF long before saccade onset (time 1). H, horizontal.
The time course of excitability at the old and new RF locations is shown in Fig. 5. The neuron responded to a stimulus in the old RF before, but not after the saccade (Fig. 5 Middle). In contrast, the neuron responded to a stimulus flashed in the new RF even long before saccade onset (Fig. 5 Bottom, time 1). Note that the same neuron responded to a stimulus presented in the old RF at the same timing (Fig. 5 Middle, time 1). This dual responsiveness to the stimulus in both old and new RF continued until saccade onset, indicating an expansion of the effective RF.
At the population level, 40 of 77 neurons (52%) in V3A, responded in the single-step task in which the stimulus was presented in the new RF at time 2.
The amplitude of response to the stimulus trace for a given neuron was usually smaller than its visual response (Fig. 6A). We compared the amplitude of response in the standard single-step task (time 2, new RF) to the amplitude of response in a condition equivalent to a simple fixation task, in which the stimulus was presented in the RF long after the monkey has acquired FP2 (time 4, new RF). The amplitude of response to the stimulus trace was on average 75.3% of the visual response.
Figure 6.
(A) Comparison of amplitude of response to the stimulus trace in the single-step task (new RF, time 2) to the response to the stimulus presented in the new RF at time 4 (217 ms after saccade offset). Filled bars indicate that the difference was significant (t test, P < 0, 05). (B) Response latency when the stimulus was presented 133 ms after FP2 onset (time 2), plotted against the latency of visual response in the fixation task. Each dot represents one neuron. Distribution histograms are shown at the side and bottom.
Most V3A neurons that respond to remapped visual signals do so predictively: they respond to a stimulus trace at a latency that is shorter than the neuron's visual latency. We compared the latency of the visual response in the standard fixation task with the latency of response to the stimulus trace (Fig. 6B). For the population of V3A neurons that exhibited remapping, the mean visual latency in the fixation task was 61 ms (±17 ms SD). In the single-step task, we measured the latency of response to the stimulus trace relative to saccade onset. In Fig. 6B, we show data from the condition in which the stimulus was presented in the new RF just before the saccade (time 2). For this condition, the latency of response averaged 25 ms (±53 ms SD), considerably shorter than the visual response. In other words, these V3A neurons began to respond in anticipation of the RF landing on a stimulated screen location. The majority (28/40 neurons) responded at a latency shorter than their ordinary visual latency. Among the 40 neurons that exhibited remapping in the single-step task (new RF at time 2) 12 responded at or even before saccade onset (Fig. 6B). Ten V3A neurons also responded when the stimulus was presented in the new RF at time 1, long before the saccade. Among these, nine began to respond before saccade onset.
All 28 neurons that responded predictively to a stimulus presented in the new RF also maintained their responsiveness to stimuli in the old RF. This finding suggests that the effective size of the RF expands around the time of the saccade. In contrast, the 12 neurons that did not respond predictively showed a reduced responsiveness at the old RF when responsiveness increased at the new RF. This finding suggests that the RF for these neurons shifted to the new location but did not expand.
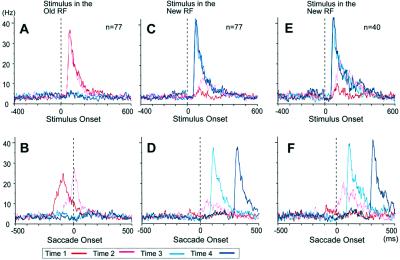
For the population as a whole, remapping was evident for stimuli presented in the new RF immediately before the saccade and even for stimuli presented long before the saccade. In Fig. 7, we show population data for all 77 V3A neurons studied (A–D) and for the subset of 40 V3A neurons that exhibited remapping (E and F). V3A neurons had robust visual responses to stimuli presented in the old RF at times 1 and 2 (Fig. 7A, red and pink lines). When the stimulus was presented only after the end of the saccade, at times 3 or 4, there was, of course, no visual response (Fig. 7B, light blue and dark blue lines). The data from Fig. 7A are replotted in Fig. 7B aligned on saccade onset. Here the visual response for time 2 (stimulus in old RF just before saccade) can be seen to decrease more sharply than that for time 1. This finding suggests that there is an active truncation of the visual response when the stimulus is presented just before an eye movement that will shift the RF away from the stimulated location. In Fig. 7 C and D, population responses are shown for stimuli presented in the new RF. Again, V3A neurons naturally had strong visual responses to the onset of a visual stimulus in the new RF when that stimulus was presented after the saccade (times 3 and 4). The population trace also shows responsiveness in the remapping conditions, both when the stimulus was presented immediately before the saccade (time 2) and even when it was presented long before the saccade (time 1). This responsiveness to remapped visual signals begins before saccade onset (Fig. 7D, times 1 and 2), indicating that remapping is predictive. These effects are even more pronounced when we restrict analysis to the subset of V3A neurons that showed significant remapping (Fig. 7 E and F).
Figure 7.
(A) Population activity of all V3A neurons studied. Mean activity for conditions in which the stimulus was presented in the old RF at four different times, aligned on stimulus onset. (B) Same data aligned on saccade onset. (C) Population response for the stimulus presented in the new RF. (D) Same data aligned on saccade onset. (E) Population activity for V3A neurons that exhibit remapping, for the stimulus presented in the new RF. (F) Same data aligned on saccade onset.
We considered whether responsiveness to remapped stimulus traces might be related to covert attention to the saccade target (FP2). All eight conditions were run in an interleaved fashion, and the monkey repeatedly made saccades from FP1 to FP2, which might have induced an anticipatory shift of attention toward FP2. To investigate this possibility, we added an experiment in which the stimulus-only control was interleaved with the single-step task. For all 33 V3A neurons tested, there was no response in the stimulus-only control when it was run as a separate block, and for 28 of these neurons there was still no response when the stimulus-only control was interleaved with single-step tasks. Five neurons, however, did respond in the interleaved stimulus-only control trials. This observation indicates that at least for some neurons, there may be an effect of shifting attention to the new FP or a longer-term memory mechanism (18).
We also considered whether remapping depends on the monkey's anticipation or preparation for a particular size and direction of saccades. We therefore tested, in 10 neurons that showed remapping in the original experiment, the effect of having two possible targets for FP2, one to the left, another to the right of FP1. We found that all of the neurons tested still showed remapping.
Does remapping occur in even earlier visual areas? To answer this question, we examined whether neurons in V1, V2, and V3 respond to the trace of the visual stimulus. We found that neurons in earlier visual areas also exhibit remapping (Fig. 8A). Interestingly, the fraction of neurons that responded to the stimulus trace became smaller at lower levels in the visual hierarchy; 35% of neurons (8/23) in V3, 11% (5/46) in V2, and 2% (1/64) in V1 responded to the stimulus trace (time 2). These proportions are lower than in V3A or LIP. Very few neurons in these earlier visual areas responded before saccade onset: 9% (2/23) in V3, 2% (1/46) in V2, and none in V1, as compared with 16% in V3A and 35% in LIP (3). Further, the mean latency of response to the stimulus trace relative to saccade onset was longer in visual areas lower in hierarchy: 46 ms (±56 SD) for V3, 78 ms (±101 SD) for V2, and 103 ms for V1 in contrast to V3A (25 ms ± 53 SD).
Figure 8.
(A) Percentage of neurons that responded to the stimulus flashed in the new RF at time 2 in each visual area. Filled bars, neurons started responding after saccade onset. Shaded bars, neurons that started responding before saccade onset. (B) Perisaccadic change in responsiveness to a stimulus presented in the new RF. Normalized firing rate is plotted against the time of stimulus presentation relative to saccade onset. Negative values on the horizontal axis indicate that the stimulus is flashed before saccade onset. For each neuron there are four data points, for the four times at which the stimulus appeared: blue, time 1; black, time 2, red, time 3, and green, time 4. Firing rate is normalized as (response to the stimulus in new RF at time X)/(response to the stimulus at time 4). Neurons are judged to have no response if the activity does not change significantly relative to the baseline (100 ms before stimulus onset) and are plotted as zero.
For neurons in each area, we examined excitability at the new RF location around the time of the saccade (Fig. 8B). We compared the amplitude of the response to a stimulus presented in the new RF in each of the four time conditions to the response in the fixation-like condition (new RF, time 4). We plotted the ratio of response against an average time of saccade onset for the each neuron. The activity of each neuron recorded in each area is represented by four data points. The visual responsiveness at the new RF for V1 neurons started to rise only after the saccade. In contrast, for V3A neurons, the responsiveness at the new RF began to rise about 200 ms before saccade onset. Some neurons in V2 and V3 also began to respond to the stimulus presented in the new RF before saccade onset.
Finally, we examined the possibility that responses to the stimulus in the new RF might vary systematically with the size of the RF. We computed the fraction of V3A neurons that responded to the stimulus trace for each different RF size (1-deg bins). There was no significant tendency for neurons with larger RFs to respond to the stimulus trace more often (Spearman Rank Correlation, ρ = −2.73, n = 60, p = 0.387). We also examined the dependence of remapping on saccade amplitude. For some neurons in which no remapping occurred with our standard 20-deg saccades (six neurons in V3A, five in V3, 16 in V2, and 21 in V1), we retested with 10-deg saccades. We observed remapping in conjunction with a 10-deg saccade in only one V3 neuron that had not shown remapping with a 20-deg saccade.
Discussion
We have found that visual signals are updated in extrastriate visual cortex. These results demonstrate that remapping is not restricted only to brain regions with attentional and oculomotor functions but also occurs in areas thought to have more purely visual functions. Our results are congruent with previous findings on the essentially visual nature of remapping. In LIP, purely visual neurons, which have no saccade-related activity, exhibit remapping (3). Likewise, in FEF, visual and visuomovement cells update stimulus traces whereas movement cells do not (4). Our finding that neurons in extrastriate areas V3A, V3, and V2 can remap visual signals provides further evidence to support the argument that updating reflects a coordinate transformation of a sensory signal.
We examined the possibility that the modulation of visual responsiveness in the single-step task might be explained as merely the result of an attentional shift. We have shown previously that responses of V3A neurons are modulated by attention (15). In the current experiment, the visual stimulus in the RF was behaviorally irrelevant. Nonetheless, the sudden onset of the stimulus would be expected to draw attention to the stimulated location. In addition, because the stimulus is repeatedly presented at a single location (new RF), the animal could anticipate that a stimulus would appear, and potentially attend to that location covertly. We found, however, that extrastriate neurons did not typically respond in the stimulus-only control, even when it was interleaved with single-step trials. This result indicates that attention to the stimulus location cannot in itself explain the modulation of responsiveness we observed. A subset of V3A neurons did respond in these interleaved stimulus-only trials. These responses may reflect effects of attention or memory, as has been described in FEF (18).
Receptive fields in extrastriate cortex are dynamic. Neurons fell along a continuum with respect to the remapping we observed. For some neurons, the responsive location shifts to the future RF. For other neurons, there appears to be an expansion of the RF around the time of the saccade, such that the neuron becomes responsive to stimuli in both the new and old RFs. Similar effects have been observed in LIP (19), where responsiveness at the new RF begins to increase long before saccade onset. The spatial nature of RFs around the time of a saccade has been studied by systematic presentation of the stimulus at several locations around the new and old RFs. For example, in extrastriate area V4, the RF becomes smaller and shifts in location toward the saccade goal before saccade onset (20–22). The neurophysiological results correspond well with the psychophysically demonstrated compression of visual space that occurs just before saccades (23).
Neurons at quite early stages of the visual hierarchy update visual signals. The gradient in the frequency of remapping across V3A, V3, and V2 cannot be explained by differences in the size or eccentricity of the RF locations. Instead, we suggest that this gradient reflects the anatomical and functional distance between each of these areas and the source of the central signal that drives remapping. The FEF projects extensively to V3A, V3, and V2 (11, 12). The density of projections decreases at earlier stages of the hierarchy, which parallels our observation of a reduced prevalence of remapping at earlier stages. Alternatively, remapping of visual signals may take place initially in parietal cortex and the remapping we observe in extrastriate cortex may reflect the influence of back projections from LIP (7–9).
The properties of visual neurons in striate and extrastriate cortex have been studied primarily during fixation or under anesthesia. Perception, however, normally takes place in the context of frequent eye movements. Understanding the dynamic nature of RFs around saccades provides important information about how we perceive the visual world in the natural environment. Specifically, predictive remapping allows spatial processing to proceed in advance of a saccade as although the saccade had already taken place. Further, it permits the maintenance of spatially accurate representations across saccades. This mechanism may be useful in constructing a stable image of the visual world despite eye movements.
Supplementary Material
Acknowledgments
We thank Dr. Donald Williams for performing MR scan, Karen Medler for animal care, J. Nadler for data analysis, and R. Berman, L. Heiser, E. Merriam, and Dr. C. Olson for helpful comments. This work was supported by the Uehara Foundation, the Human Frontier Science Program, the EJLB Foundation, the Whitehall Foundation, the McDonnel Pew Foundation, and National Institutes of Health Grants EY12032 and EY08098.
Abbreviations
- RF
receptive field
- FP
fixation point
- LIP
lateral intraparietal area
- FEF
frontal eye field
- deg
degrees
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hallett P E, Lightstone A D. Vision Res. 1976;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- 2.Mays L E, Sparks D L. J Neurophysiol. 1980;43:207–232. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- 3.Duhamel J R, Colby C L, Goldberg M E. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 4.Umeno M M, Goldberg M E. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- 5.Walker M F, Fitzgibbon E J, Goldberg M E. J Neurophysiol. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- 6.Andersen R A, Asanuma C, Essick G, Siegel R M. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- 7.Baizer J S, Ungerleider L G, Desimone R. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blatt G J, Andersen R A, Stoner G R. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- 9.Cavada C, Goldman-Rakic P S. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 10.Morel A, Bullier J. Visual Neurosci. 1990;4:555–578. doi: 10.1017/s0952523800005769. [DOI] [PubMed] [Google Scholar]
- 11.Schall J D, Morel A, King D J, Bullier J. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanton G B, Bruce C J, Goldberg M E. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- 13.Galletti C, Battaglini P P. J Neurosci. 1989;9:1112–1125. doi: 10.1523/JNEUROSCI.09-04-01112.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galletti C, Battaglini P P, Fattori P. Exp Brain Res. 1990;82:67–76. doi: 10.1007/BF00230838. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Colby C L. J Neurophysiol. 2000;84:677–692. doi: 10.1152/jn.2000.84.2.677. [DOI] [PubMed] [Google Scholar]
- 16.Gattass R, Sousa A P, Gross C G. J Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclar G, Maunsell J H, Lennie P. Vision Res. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- 18.Umeno M M, Goldberg M E. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- 19.Kusunoki M, Colby C L, Duhamel J-R, Goldberg M E. In: The Association Cortex: Structure and Function. Sakata H, Fuster J, Mikami A, editors. London: Harwood; 1997. pp. 89–97. [Google Scholar]
- 20.Moore T, Tolias A S, Schiller P H. Proc Natl Acad Sci USA. 1998;95:8981–8984. doi: 10.1073/pnas.95.15.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore T. Science. 1999;285:1914–1917. doi: 10.1126/science.285.5435.1914. [DOI] [PubMed] [Google Scholar]
- 22.Tolias A S, Moore T, Smirnakis S M, Tehovnik E J, Siapas A G, Schiller P H. Neuron. 2001;29:757–767. doi: 10.1016/s0896-6273(01)00250-1. [DOI] [PubMed] [Google Scholar]
- 23.Ross J, Morrone M C, Burr D C. Nature (London) 1997;386:598–601. doi: 10.1038/386598a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.