Abstract
Background
Spinal dural arteriovenous fistulas (SDAVFs) are the most common type of spinal arteriovenous malformation. Typically, these malformations present with a wide range of nonspecific symptoms indicative of thoracolumbar myelopathy. However, patients with spinal dural arteriovenous fistulas may rarely present with subarachnoid hemorrhage.
Methods
A systematic review of MEDLINE and Embase databases was performed querying for cases of spinal dural arteriovenous fistulas with subarachnoid hemorrhage. Patient characteristics and outcomes investigated included spinal level of the fistula, delay of diagnosis, Hunt and Hess grade, interventions, recurrence of the fistula, and postoperative disability. Additionally, we present a unique case in which subarachnoid hemorrhage resulted from a spinal dural arteriovenous fistula that was refractory to multiple endovascular and open surgical interventions.
Results
Of 116 records identified, 45 studies were included comprising 80 patients with spinal dural arteriovenous fistula and subarachnoid hemorrhage. The most common locations of the spinal dural arteriovenous fistula were in the cervical spine (57.5%) and at the craniocervical junction (35%). Patients were treated with open surgical ligation (60.0%), endovascular embolization (22.5%), or an open surgical procedure following persistent symptoms after endovascular treatment (10.0%). Overall, the prognoses among the treated patients were favorable with only two reported (2.5%) mortalities. Rates of neurologic recovery were similar when comparing endovascular and open surgical treatment. Endovascular treatment with coil embolization of a C1–C2 spinal dural arteriovenous fistula presenting as subarachnoid hemorrhage is also described.
Conclusion
Spinal dural arteriovenous fistulas, particularly in the cervical spine, could be considered as a potential etiology for subarachnoid hemorrhage patients with no obvious intracranial cause. Treatment with either open surgery or embolization appears to offer a positive prognosis for both functional and angiographic outcomes.
Keywords: Spinal dural arteriovenous fistula, subarachnoid hemorrhage, embolization, endovascular, n-butyl cyanoacrylate, Onyx™
Background
Spinal dural arteriovenous fistulas (SDAVFs) are acquired central nervous system vascular lesions caused by aberrant connections between radicular arteries and veins of the spinal venous plexus along the spinal dural surface. 1 These lesions account for approximately 70% of all reported spinal vascular malformations and are often difficult to diagnose due to their presentation via a myriad of nonspecific symptoms. 2 Classically, SDAVFs occur in men between the ages of 50 and 65 and present insidiously as progressive thoracolumbar myelopathy, demonstrated by symptoms such as lower extremity weakness, gait disturbances, sensory symptoms, and bowel and/or bladder incontinence. 3 In rare instances, occurring in less than 1% of cases, SDAVF can present as subarachnoid hemorrhage (SAH). 4
While up to 80% of SDAVFs occur between T6 and L2, SAH is an exceptionally more common presentation in cervical SDAVFs (cSDAVF), with up to 45% of cSDAVFs presenting as SAH.3,5 Additionally, there have been case reports of both thoracic and lumbar lesions causing SAH.6–10 This review aims to systematically describe the presentation, clinical management, and outcomes of cases of SDAVFs presenting as intracranial SAH. Furthermore, we present a case of a cSDAVF presenting as SAH that was refractory to multiple interventions. Although SDAVF-induced SAH is a rare entity, a comprehensive description of this disease process is useful to aid in the differential diagnosis of spontaneous, nontraumatic SAH without radiographic evidence of intracranial pathology. Finally, the composite results of surgical interventions and outcomes can guide clinical decision-making for patients presenting with SDAVFs as a cause of intracranial SAH.
Methods
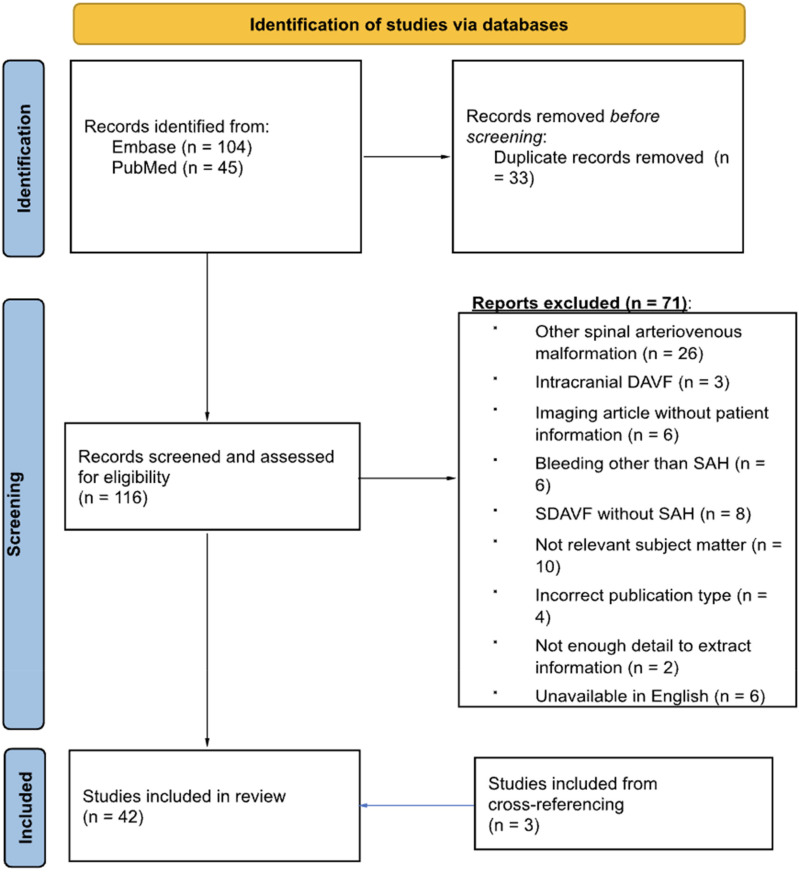
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were used to guide the literature review. 11 This systematic review was not registered in the state of New York and a protocol was not prepared. Both PubMed and Embase databases were interrogated (Figure 1) for clinical cases describing concurrent SDAVF and SAH.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram.
Eligibility criteria
The following inclusion criteria were used to screen for relevant articles: (1) clinical studies describing SDAVFs, (2) concurrent presentation with SAH, and (3) description of either open surgical or endovascular treatment modality. Exclusion criteria were the following: (1) description of other spinal arteriovenous malformations such as perimedullary spinal arteriovenous (AV) fistula, intracranial dural AV fistulas, (2) bleeding patterns other than SAH such as spinal subdurals, (3) studies describing SDAVF without SAH, (4) abstracts, unpublished studies, and reviews, and (5) non-English articles.
Information sources and search strategy
PubMed/MEDLINE and Embase databases were manually interrogated on December 23, 2022 using the following keywords: “Central Nervous System Vascular Malformations,” “Spinal Dural Arteriovenous Fistula,” “Dural Arteriovenous Fistula,” “Spinal Arteriovenous Malformations,” and “Subarachnoid Hemorrhage.” Full search terms, operators, and strings are available in Supplementary Table 1. Queried articles had no restrictions on years of publications, and all accessed articles were peer-reviewed, published, and publicly available without requiring contact with corresponding authors.
Selection process
Our search queries of PubMed/MEDLINE and Embase databases retrieved 150 records, which were extracted for deduplication. The retrieved records were deduplicated to identify 116 unique records and 34 duplicates. Articles were then copied into two separate spreadsheets for independent review based on our previously stated inclusion and exclusion criteria. The bibliographies of included articles were also cross-referenced for relevant articles to be screened using our inclusion and exclusion criteria. Ultimately, 45 articles were included, including three articles that were discovered via cross-referencing from the original search.
Data collection process and data items
Following screening, authors (BN and CR) independently extracted the following data into separate Google Spreadsheets from the included articles: age, gender, spinal level of fistula, presenting symptoms, Hunt and Hess (HH) grade, delay to intervention, intervention performed (open clipping vs. endovascular treatment), postoperative course and complications, clinical presentation at latest follow up, and angiographic findings at latest follow up. For patients that did not have HH grade explicitly stated, it was extrapolated from clinical vignettes that provided enough information to do so. Our primary outcomes investigated in this study were angiographic resolution of the fistula at latest follow up and neurologic improvement of the patient at latest follow up. Secondary outcomes included surgical complications and revision procedures.
Study risk of bias assessment
Included articles were independently assessed for bias using the Joanna Briggs Institute critical appraisal checklists for case reports and case series. 12 The checklists entailed evaluating each included article using a series of 8 or 10 questions for case reports and case series respectively to assess bias level. Studies with a score ≥70% of questions answered “yes” were attributed with a low risk of bias, studies with a score between 50% and 69% were attributed with a moderate risk of bias, and studies with a score of <50% of questions answered “yes” were attributed with a high risk of bias.
Statistical analysis
Statistical analysis of extracted data was performed using GraphPad Prism Version 10.1.1, with a significance level of p ≤ .05 as the threshold for determining statistical significance using comparison of categorical variables via Fisher's exact testing. Angiographic resolution at latest follow up, functional recovery at latest follow up, complications, and revision procedures were compared across baseline extracted data including spinal level of the SDAVF, HH grade, delay to diagnosis, and index intervention. All data is presented as mean and standard deviation or n (%). Comparisons between groups are presented as odds ratios (OR) with 95% confidence intervals (CIs).
Results
Baseline characteristics
Our comprehensive review of the literature yielded 45 publications from 1977 to 2021 with 80 distinct patients who presented with SAH as a result of SDAVF. All included articles were level IV evidence (case reports and case series) and therefore subject to selective reporting, incomplete outcome data, and reporting bias. Full assessment of bias for included articles can be found in Supplementary Tables 2 and 3. All included articles were assigned a low risk of bias except for two case series which were assigned a moderate risk of bias. A detailed summary describing the associated publication, spinal level of SDAVF, patient age, patient gender, presenting symptom, HH grade, delay to recognition, type of intervention, surgical approach, embolic agent used (if applicable), and repeat intervention for each patient can be found in Supplementary Table 4. A summary of demographic and baseline clinical characteristics is detailed in Table 1.
Table 1.
Baseline characteristics of patients with SDAVF and SAH (n = 80).
| Average age | 54.0 ± 14.2 |
| Female patients | 19 (23.8%) |
| Patients with a delayed diagnosis | 29 (36.3%) |
| Delay to diagnosis (days) | 314 ± 896 |
| Patients with Hunt and Hess score (n = 44) | |
| 1 | 14 (31.8%) |
| 2 | 25 (58.8%) |
| 3 | 3 (6.8%) |
| 4 | 2 (4.5%) |
| 5 | 0 (0.0%) |
| Level of fistula | |
| CCJ | 28 (35.0%) |
| Cervical | 46 (57.5%) |
| C1–C2 | 37 |
| C3–C7 | 9 |
| Thoracic | 4 (5.0%) |
| Lumbar | 2 (2.5%) |
| Treatment | |
| Surgery | 48 (60.0%) |
| Embolization | 18 (22.5%) |
| Both embolization and surgery | 8 (10.0%) |
| None | 6 (7.5%) |
| Embolic agent | |
| n-BCA | 16 (61.5%) |
| Onyx™ | 5 (19.2%) |
| Other | 5 (19.2%) |
SDAVF: spinal dural arteriovenous fistula; SAH: subarachnoid hemorrhage; CCJ: craniocervical junction; n-BCA: n-butyl cyanoacrylate.
Of the 80 included patients, 19 (23.8%) were female. The average age of the patients was 54.0 ± 14.2 years with a range of 3–80. Of note, a 3-year-old patient, who presented with a T9–T11 SDAVF and SAH, represented an outlier in the age category. If this outlier was excluded from our analysis, the average age becomes 54.6 ± 13 years. Delayed diagnosis of SDAVF from initial manifestation of symptoms occurred in 29 (36.3%) of patients with an average delay in diagnosis of 314 ± 896 days. However, there were four patients who were outliers in the timeline of their diagnoses, ranging from 7 months to 10 years, which contributed to the larger standard deviation in time to diagnosis in our patient sample.6,13–15 The majority of the SDAVFs occurred at either the craniocervical junction (CCJ) or cervical spine, accounting for 35% (28/80) and 57.5% (46/80) of all SDAVF cases, respectively. Of the lesions located within the cervical spine, 37/46 (80%) occurred at C1 or C2. HH grades were available or extrapolated from 44 patients (55.0%). They were predominantly mild, with 39 of 44 (88.6%) presenting with either HH 1 or 2. The most common HH score observed was grade 2 in 25/80 (58.8%) patients. No HH scores of 5 were observed in our patient sample.
Interventions
The 80 patient cases of SDAVF with SAH described in our review were managed with embolization, open surgery, or a combination of these two techniques. Table 1 details incidence of types of treatment and embolic agent if applicable. Of the 80 cases of SDAVF with SAH we reviewed, open surgery was the most common approach, composing 48 (60.0%) of our extracted cases. Surgical techniques used to resolve the SAH and SDAVF included ligation of the fistula following a laminectomy and/or craniectomy, direct surgical resection of the fistula, posterior fossa approaches to clip the draining vein, 16 and resection of the fistula using intraoperative indocyanine green angiogram guidance. 17
Eighteen (22.5%) cases were treated with embolization only, while 8 (10.0%) cases were treated with embolization and an open surgical procedure. All patients that had an open surgical procedure following embolization did so as a repeat intervention due to symptoms persisting following embolization, with one patient requiring stereotactic radiosurgery to occlude the fistula following initial open surgery and subsequent embolization treatment. 18 Revision procedures were significantly more common in patients initially treated with embolization than patients treated with open surgery (p = .002, OR: 18.67, 95% CI: 2.819–213.5). Further, HH 1 or HH 2 grades at presentation were associated with significantly decreased likelihood of requiring repeat interventions compared to HH 3 or HH 4 at presentation (p = 0.01, OR: 0.000, 95% CI: 0.000–0.2767).
Among cases that were managed with embolization, n-butyl cyanoacrylate (n-BCA) was used as the embolic agent in 16 (61.5%) cases and Onyx™ was used in 5 (19.2%) cases. Two studies did not specify the embolic agent used.19,20 Besides liquid embolic agents, other endovascular occlusive agents included a detachable balloon21,22 and liquid coils with an ethylene-vinyl copolymer. 13 However, these agents were only used in three of the extracted cases, none of which were published after 2000.
Six cases of SDAVF with SAH found in our review received no intervention. Among these patients, one did not survive until his planned surgery, 5 one reported continued moderate disability, 23 and four did not follow up. Besides the patient who passed away prior to his treatment (due to complications from a shunt), financial limitations were stated as the reason that no intervention was pursued in all cases.
Regarding overall trends in interventions to treat SDAVF with SAH, open surgery was the most commonly observed approach to manage these cases. Even in recent cases after 2010, open surgery remained more common compared to embolization. However, embolization was seen more frequently in recent cases with the majority of cases treated endovascular embolization being published after 2003. Patients who presented with a thoracolumbar DAVF were statistically more likely to receive endovascular embolization treatment than patients who presented with cervical DAVF (p = .04, OR: 5.667, 95% CI: 1.142–31.46). Notably, all but one of the cases that were treated with open surgery following embolization were published after 2008.
Outcomes
Comparisons of functional and radiographic outcomes of patients receiving endovascular and/or open surgical treatment are depicted in Table 2. Overall, patient outcomes were relatively favorable regardless of intervention, and only two (2.5%) cases of patient death were found. One patient succumbed to shunt-related complications before they could receive surgical intervention, 5 and one patient died as a result of recurrent SAH. This patient had received endovascular treatment 3 months prior, which included embolization of the feeder branches of the CCJ SDAVF and placement of a detachable balloon in the vertebral artery (VA). 22
Table 2.
Predictors of repeat intervention, fistula obliteration, and functional recovery.
| Spinal level of SDAVF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence of repeat intervention | Incidence of fistula obliteration | Incidence of functional recovery | |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| CCJ vs. cervical | 2.235 | [0.5860–8.309] | .42 | CCJ vs. cervical | 0.1351 | [0.01081–0.9471] | .048 | CCJ vs. cervical | 0.9829 | [0.2231–3.986] | >.99 |
| CCJ vs. thoracolumbar | 1.176 | [0.1137–16.99] | >.99 | CCJ vs. thoracolumbar | 0.000 | [0.000–5.609] | >.99 | CCJ vs. thoracolumbar | 5.111 | [0.6438–32.60] | .17 |
| Cervical vs. thoracolumbar | 0.5263 | [0.05467–7.617] | .50 | Cervical vs. thoracolumbar | 0.000 | [0.000–85.50] | >.99 | Cervical vs. thoracolumbar | 5.200 | [0.7405–1.350] | .14 |
| Hunt–Hess grade | |||||||||||
| Incidence of repeat intervention | Incidence of fistula obliteration | Incidence of functional recovery | |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| HH 1/2 vs. HH 3/4 | 0.000 | [0.000–0.2767] | .01 | HH 1/2 vs. HH 3/4 | N/A a | N/A a | >.99 | HH 1/2 vs. HH 3/4 | 5.500 | [0.7471–32.38] | .14 |
| Management | |||||||||||
| Incidence of fistula obliteration | Incidence of functional recovery | ||||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||||||
| Surgery vs. embolization | +Infinity | [1.515 to infinity] | .01 | Surgery vs. embolization | 3.385 | [0.7059–15.52] | .17 | ||||
| Surgery vs. both | +Infinity | [5.808 to infinity] | .003 | Surgery vs. both | 2.095 | [0.1426–15.64] | .48 | ||||
| Surgery vs. none | N/A | N/A | N/A | Surgery vs. none | 44.00 | [4.440–573.1] | .004 | ||||
| Embolization vs. both | 3.600 | [0.5529–23.61] | .31 | Embolization vs. both | 0.6190 | [0.04228–4.962] | >.99 | ||||
| Embolization vs. none | N/A | N/A | N/A | Embolization vs. none | 13.00 | [1.278–177.8] | .03 | ||||
| Both vs. none | N/A | N/A | N/A | Both vs. none | 21.00 | [1.254–298.7] | .03 | ||||
| Embolic agent | |||||||||||
| Incidence of fistula obliteration | Incidence of functional recovery | ||||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||||||
| n-BCA vs. all other agents | 2.750 | [0.4360–17.99] | .61 | n-BCA vs. all other agents | 0.5000 | [0.03467–4.000] | >.99 | ||||
| n-BCA vs. Onyx | 3.667 | [0.4006–29.00] | .53 | n-BCA vs. Onyx | 0.000 | [0.000–3.570] | .54 | ||||
CCJ: craniocervical junction; n-BCA: n-butyl cyanoacrylate; SDAVF: spinal dural arteriovenous fistula; OR; odds ratio; 95% CI: 95% confidence interval; CCJ: craniocervical junction; HH: Hunt and Hess grade.
Analysis could not be performed due to 100% fistula obliteration in each group.
Among the 18 patients treated with embolization alone, 12 (66.7%) patients achieved complete obliteration of the fistula, and 13 (72.2%) patients achieved good recovery from the procedure. Patients with cervical DAVFs were more likely to have evidence of radiographic obliteration of their fistula at their latest follow up than patients with DAVFs at the CCJ (p = .048, OR: 7.400, 95% CI: 1.059–92.50). One patient treated with only embolization had an incomplete obliteration of the fistula, but this individual also had a good recovery postoperatively. Moderate disability was observed in two (11.1%) patients following embolization, one as a result of surgical complications 24 and the other with a lumbar SDAVF treated with two n-BCA embolization procedures 3 months apart. 10 Four (22.2%) patients treated with embolization alone did not undergo follow-up angiograms, and two patients did not report functional outcomes following embolization.
Among the 48 patients treated with surgery alone, 44 (91.7%) patients had their SDAVFs completely resolved with surgery, and 44 (91.7%) patients had good recovery postoperatively. Four (8.3) patients treated with only open surgical techniques did not report or perform angiograms outcomes postoperatively. Two patients (4.2%) reported moderate disability postoperatively, one following a craniectomy and C1–C2 laminectomy 25 and another treated with a posterior transcondylar fossa approach with pre-existing damage to the medulla oblongata. 16 Additionally, a patient with a T10–T12 SDAVF and a history of recurrent encephalopathy and brain superficial siderosis reported severe disability postoperatively following a T10–T12 laminectomy and ligation of the fistula. 6 Three (6.3%) patients treated with open surgery did not report postoperative functional outcomes. Patients managed with embolization and patients with open surgery both had statistically higher rates of functional recovery than patients who declined treatment (p = .03, OR: 13.00, 95% CI: 1.278–177.8 and p = 0.004, OR: 44.00, 95% CI: 4.440–573.1, respectively).
When comparing embolization and open surgical treatment strategies, only embolization techniques demonstrated incomplete obliteration or recurrence of the SDAVF (n = 2 vs. n = 0), and surgical management alone demonstrated significantly decreased incidence of persistent SDAVF following management compared to embolization alone (p = .01, OR: 0.000, 95% CI: 0.000–0.6602). However, no difference in functional recovery was observed when comparing surgical management to endovascular management (p = .17, OR: 3.385, 95% CI: 0.7059–15.52). Therefore, to determine the effectiveness of either treatment more accurately in occluding the fistula and reducing morbidity, a larger patient cohort is necessary. Of the eight patients who received a combined treatment of open surgery following embolization, five (62.5%) patients showed complete angiographic of their SDAVFs postoperatively and seven patients (87.5%) had good functional outcomes. However, three patients (37.5%) had an incomplete obliteration of their fistula, and one patient exhibited moderate disability postoperatively. 15 Interestingly, patients receiving surgical management alone also had statistically lower rates of fistula recurrence than patients who received management with embolization and surgery (p = .003, OR: 0.000, 95% CI: 0.000–0.1722).
Case presentation
We describe a 42-year-old male patient with a remote history of childhood head trauma and no pertinent past medical history who was transferred to our institution for evaluation of a HH 2, modified Fisher 2 SAH. The patient woke up the morning prior to his transfer with a severe, persistent 10/10 occipital headache radiating down the back of his neck with associated nausea and vomiting. He presented to an outside hospital where his systolic blood pressure was noted to be in the 190s and a computed tomography (CT) demonstrated acute SAH primarily within the ventral posterior fossa. Following his transfer to our institution, a CT angiogram was performed and did not identify any intracranial aneurysm, so the patient was brought for a diagnostic cerebral angiogram. Injections of the right common carotid, internal carotid, external carotid, and VAs showed no arteriovenous malformations or other vascular anomalies. Injections into the left common, internal, and external carotid arteries also displayed no vascular anomalies, but the left VA run demonstrated contrast extravasation into the subdural and subarachnoid space at the level of C1–C2 from an indistinct feeder originating from the V2 segment of the left VA (Figure 2A).
Figure 2.
Pre- and postoperative angiography. (A) Left VA injection, cervical, lateral: initial digital subtraction angiography (DSA) illustrating contrast extravasation of V2 at the junction of the C1–C2 vertebrae (arrow). (B) Left VA injection, cervical, lateral: day 9 DSA of the C1–C2 SDAVF displays continued delayed arterial filling of the DAVF at C2 via a muscular feeding branch from the V2/V3 region (arrow). This branch is distinct from the previously embolized branch. A coil mass is seen in the previously embolized arterial feeder with no evidence of residual contribution. (C) Left VA injection, cervical, lateral: intraoperative DSA reveals a 50% reduction in the size of the DAVF with delayed venous phase filling, predominantly from a muscular feeder from the left VA. (D) Left VA injection, cervical, lateral. Angiography performed eight months after open surgical intervention shows residual SDAVF with supply from muscular branches of the V3 segment of the left VA at C2 level, supplying a fistulous pouch. VA: vertebral artery; SDAVF: spinal dural arteriovenous fistula.
To better characterize this lesion, magnetic resonance imaging of the cervical spine was performed, revealing mass effect at C1–C2 level due to extra-axial hematoma. However, further characterization of the lesion was not possible, so a second angiogram was performed. After multiple superselective injections of muscular branches of the left V2 segment of the VA, the arterial feeder was identified and demonstrated an early nonarterial opacification of a poorly defined structure most consistent with an SDAVF. Embolization of this muscular feeder was carried out using a Nano 1 mm × 1 cm coil. Follow-up angiography showed reduced flow to the vascular malformation from this branch with delayed filling from another muscular branch located more superiorly.
On day 9 of hospitalization, follow-up angiography demonstrated continued delayed arterial filling of the SDAVF at C2 through a muscular feeding branch distinct from the previously embolized branch (Figure 2B). Subsequently, an open surgical procedure was performed on the 18th day of hospitalization, utilizing a far-lateral approach for extradural coagulation of the fistula branches. Intraoperative angiogram revealed a 50% reduction in the SDAVF with delayed venous phase predominantly from a muscular feeder from the left VA (Figure 2C). He was discharged 23 days following admission with no neurologic deficits and resolution of his headache.
Follow-up angiography at 8 months postop displayed evidence of residual SDAVF with arterial feeders from two muscular branches of the V3 of the left VA at the level of C2 (Figure 2D). Despite the patient being asymptomatic at this time, it was decided to treat the patient with a flow-diverting stent given the patient's history of SAH and a substantial risk for rupture. He was started on aspirin 325 mg and clopidogrel 75 mg in anticipation of the flow diversion procedure. His plavix assay was therapeutic and aspirin was slightly hyporesponsive, so he was given a 650 mg dose of aspirin per orogastic tube during the procedure prior to the stent being placed. Following a balloon occlusion test of the left VA to confirm codominance of the vessels, a single 4.5 × 17 mm Surpass Evolve flow stent was placed proximal to the left posterior inferior cerebellar artery into the proximal V3 segment of the VA. Angiography at the end of the procedure demonstrated a well-apposed stent with stasis within the pouch of the fistula. The patient awoke from the procedure neurologically intact at his baseline and was discharged the next day. Unfortunately, the patient was lost to follow-up after this procedure, so no repeat angiography was performed.
Discussion
This systematic review of the literature details 80 distinct patients with intracranial SAH as a result of SDAVF. To the authors’ knowledge, this is the first systematic review describing the concurrent presentation of SDAVF and SAH. Although SDAVF is the most common spinal vascular anomaly, SAH remains an exceedingly rare manifestation of this pathology, accounting for less than 1% of reported cases. 17 Additionally, the etiology of SDAVF as a cause of SAH is poorly understood. It has been postulated that venous hypertension as a result of arterialization of the radial veins decreases the arterial-venous pressure gradient, thereby decreasing drainage in normal spinal veins.1,5,14,26,27 This venous congestion in combination with spontaneous thrombosis of these vessels is thought to lead to rapid increases in venous pressure, which can translate to anastomotic intracranial veins, as the radial and intramedullary veins share a common outflow.1,5 Resultant venous hypertension in the intracranial veins can lead to SAH. Congruent with this hypothesis, it has been shown that SDAVF patients with increased cranially directed venous outflow are at higher risk for SAH.
Despite our patient population presenting with an unusual manifestation of SDAVF, the demographics of this sample mirrored previous descriptions of the overall SDAVF population. Classically, SDAVF is a disease of middle-aged males, a trend reflected in our average patient age of 54 years and male-to-female ratio of 3:1.2,28,29 Current literature suggests that SDAVFs have an acquired component to their development that are consequent to spinal trauma or ischemia. 30 We see a similar correlation in our patient who had a documented history of childhood head trauma. Interestingly, the SAH and SDAVF patient population suffered from similar delays in diagnosis as did their counterparts who presented solely with myelopathic symptoms. Our patient cohort demonstrated an approximately 10-month delay in diagnosis, a timeframe comparable to Jellema et al. who reported an average of 15 months from symptom onset to diagnosis in a retrospective cohort of 80 patients. 28
Although the average age and gender of the SDAVF and SAH population reflected the overall SDAVF population, the location of the lesions in the SAH population differed significantly from general trends. While up to 80% of all SDAVFs occur between T6 and L2, 92.5% of patients in this report had lesions in the craniocervical region. This finding agrees with prior descriptions of SAH in SDAVF, which demonstrated that those with SDAVF in the craniocervical region are significantly more likely to present with SAH, with some estimates predicting that up to 45% of patients with cSDAVF present with SAH.1,5,26 As opposed to lower SDAVFs, cervical and CCJ SDAVF have been shown to have a larger distribution of venous drainage patterns than thoracic and lumbar DAVFs. 5 Superior or intracranial patterns of venous drainage of the fistulae have been demonstrated to be associated with SAH in multiple studies in the literature with the elevated venous pressure of the ascending venous drainage leading to intracranial venous hypertension and potential SAH.23,26,31 Further, venous drainage of higher SDAVF is often complicated by impaired inferior venous drainage due to varices. 5
Also aligned with prior reports of SAH in SDAVF, a vast majority of our described patients presented with a mild SAH, with 90.6% displaying a HH grade of 1 or 2. By contrast, between 20% and 30% of all aneurysmal SAH are high grade with HH grade of 4 or 5. Because many of our included cases described a negative cerebral angiography in their patients, we believe that the benign course of SAH due to SDAVF is likely due to the absence of intracranial arterial bleeding seen in aneurysmal SAH. Prior studies of angiography-negative SAH have similarly hypothesized that the benign course of this pathology is due to a low-venous bleed,32–34 and the angiographic architecture of SDAVFs with SAH is consistent with intracranial venous hypertension and subsequent hemorrhage. 26 However, there are two cases that are noteworthy exceptions to this generalization. First, Pierot et al. described a 56 male with a CCJ SDAVF treated with balloon embolization who died because of recurrence of SAH 3 months after initial treatment. 22 Second, Baharvahdat et al. described a patient who presented with altered mental status and extensive SAH with intraventricular hemorrhage. 10 Interestingly, imaging revealed a conus medullaris SDAVF, which is one of the only two described lumbar lesions causing SAH, that was ultimately treated with two sessions of embolization. These cases highlight the need to effectively treat these vascular lesions to prevent recurrence, and that high-grade SAH is not necessarily an exclusion for diagnosis of SDAVF. 35
Despite current trends for primary treatment of many cerebrovascular pathologies leaning toward endovascular intervention, our data showed that open surgery remained the most common treatment modality for SDAVF, with both modalities showing promise for treatment of this disease. This trend held steady with time, with 21.8% (7/32) of cases prior to 2008 involving embolization compared to 22.9% (11/48) of cases after 2008. This may be because prior data has shown that not only is open surgical approach more likely to cause complete obliteration, but recurrence is also less likely.
Unsurprisingly, patients that declined treatment had poorer outcomes than treated patients. Approximately 50% of patients with SDAVF become severely disabled following three years of onset of symptoms, 36 and in this cohort complicated by concurrent SAH, prompt treatment with either modality is potentially lifesaving. Bakker et al. demonstrated that endovascular treatment for SDAVF only has a complete occlusion rate of 72.2% occlusion rate compared to 96.6% for open surgery. 37 While Bretonnier et al. showed that recurrence is more likely with endovascular intervention, 21.4% versus 9.6% for open surgical ligation. Interestingly, despite these poorer radiographic outcomes in the endovascular group, neurologic outcomes were similar compared to the open surgical group. 38 Similarly, our study observed that complete obliteration of the fistula did not necessarily correlate with superior neurologic outcomes. Despite our surgical cohort demonstrating higher fistula occlusion rates, neurologic outcomes were similar to endovascular treatment. This is consistent with what is observed in the literature concerning occlusion of SDAVF. The primary purpose of fistula occlusion is to prevent rebleeding as persistence of an SDAVF is associated with a similar rebleeding risk prior to the intervention. 2 In the described case, the patient's fistula was treated with endovascular coiling, open surgical clipping, and a flow-diverting stent to minimize risk of fistula persistence and rebleeding.
Further, patients receiving both embolization and surgical treatment had a peculiar disparity in recovery rate with patients who received surgery alone (87.5% vs. 91.7%, respectively). In fact, retrospective studies of SDAVF have shown that either modality has good outcomes if only the index procedure is performed, but in cases where a second treatment modality is required, outcomes can be significantly poorer. 39 However, our cohort differs from prior literature in that the presenting symptoms of many of our included patients concerned their SAH rather than any spinal symptoms due to the SDAVF. Therefore, we cannot conclude whether the statistically higher fistula occlusion rate observed by surgical management in this study is relevant to long-term resolution of the SAH in these patients. A larger cohort of patients with long term follow-up of persistent SDAVFs following SAH would be needed to correlate persistence of the SDAVF with recurrence of SAH.
Additionally, we presented the case of a patient in their 40s who presented with HH 2, mF 2 SAH in the setting of a cSDAVF. He was treated with coil embolization and then open ligation with recurrence of the fistula at 8 months. To our knowledge, this is the first reported case of using flow diversion to treat an SDAVF. Unfortunately, this patient was lost to follow-up after this treatment so we cannot speak to the efficacy in preventing recurrence. However, the patient recovered well neurologically following his procedure and had no complaints at discharge.
Limitations
As this is a systematic review of case reports and case series for a rare pathology, we are limited to a small pool of heterogenous, retrospective data that is subject to selection bias and incomplete outcome data. As a result, multivariate analysis of our stated outcomes could not be performed due to limited power. Therefore, HH grades were extracted in an effort to give an estimate of baseline patient risk and frailty in this cohort. While our data represented overall positive outcomes, it is possible that poorer outcomes were not reported and we were limited to only assessing articles available in English. Additionally, techniques for both open surgical and endovascular embolization varied greatly between reports, and there was great variability in patient follow-up and reported outcomes, both functional and angiographic. Despite this, as this is the largest scale description of SDAVF causing SAH to date, we believe it provides valuable information for clinical decision-making by describing outcomes, interventions, and surgical trends.
Future directions
The novel presentation of SAH as a potential consequence of SDAVF necessitates future investigation given its potential harm to an affected patient. The current literature demonstrates a variety of open surgical and endovascular methodologies to treat this pathology, and there is current debate as to proper first-line treatment for SDAVFs in general. 30 Further, we present a unique case report that describes the novel application of a flow-diverting stent in the context of SAH and SDAVF. Current endovascular techniques have yet to have been applied to SDAVF, and as a result, open surgery remains the persistent mainstay of treatment. However, recent studies have shown decreasing endovascular failure rates with more sophisticated modern endovascular techniques for treatment. 40
Conclusion
This is a systematic review of patients presenting with intracranial SAH and SDAVF. Patients had an average age of 54, were predominantly male, presented largely with low grade SAH (HH 2), and had generally favorable outcomes, regardless of endovascular or open surgical intervention. Although a rare entity, a comprehensive description of this pathology can help provide valuable insights for diagnosis, prognosis, and intervention.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-2-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-3-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-4-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Footnotes
Consent for publication: Informed consent for publication of this study was obtained.
Data availability: All data are publicly available or available from the author with reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethics approval and informed consent were not required for this study.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Christian Rajkovic https://orcid.org/0000-0003-3559-2766
Ankita Jain https://orcid.org/0009-0002-4761-7828
Ariel Sacknovitz https://orcid.org/0009-0008-6236-1190
Fawaz Al-Mufti https://orcid.org/0000-0003-4461-7005
Supplemental material: Supplemental material for this article is available online.
References
- 1.Fassett DR, Rammos SK, Patel P, et al. Intracranial subarachnoid hemorrhage resulting from cervical spine dural arteriovenous fistulas: literature review and case presentation. Neurosurg Focus 2009; 26: E4. [DOI] [PubMed] [Google Scholar]
- 2.Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 2009; 30: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus J, Schwarz J, Singh IP, et al. Spinal dural arteriovenous fistulas: a review. Curr Atheroscler Rep 2013; 15: 335. [DOI] [PubMed] [Google Scholar]
- 4.Yue H, Ling W, Ou Y, et al. Intracranial subarachnoid hemorrhage resulting from non-cervical spinal arteriovenous lesions: analysis of possible cause of bleeding and literature review. Clin Neurol Neurosurg 2019; 184: 105371. [DOI] [PubMed] [Google Scholar]
- 5.Aviv RI, Shad A, Tomlinson G, et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: report of two cases and literature review. AJNR Am J Neuroradiol 2004; 25: 854–858. [PMC free article] [PubMed] [Google Scholar]
- 6.Gonella MC, Fischbein NJ, Lane B, et al. Episodic encephalopathy due to an occult spinal vascular malformation complicated by superficial siderosis. Clin Neurol Neurosurg 2010; 112: 82–84. [DOI] [PubMed] [Google Scholar]
- 7.Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg 2004; 100: 385–391. [DOI] [PubMed] [Google Scholar]
- 8.Tan AK, Dinesh SK, Lim WE, et al. Sudden severe chest pain: thoracic dural arteriovenous fistula aneurysm rupture with intracranial subarachnoid haemorrhage. Singapore Med J 2010; 51: e114–e117. [PubMed] [Google Scholar]
- 9.Hayward DM, Johans SJ, Rosenblum JD, et al. Balloon-occlusion catheter Onyx embolization of a spinal dural arteriovenous fistula presenting with subarachnoid hemorrhage in a pediatric patient. J Stroke Cerebrovasc Dis 2016; 25: e46–e49. [DOI] [PubMed] [Google Scholar]
- 10.Baharvahdat H, Ganjeifar B, Baradaran A. Diffuse subarachnoid and intraventricular hemorrhage as the presenting sign of a conus medullaris arteriovenous malformation: case report. Neurol Neurochir Pol 2016; 50: 487–490. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 2020; 18: 2127–2133. [DOI] [PubMed] [Google Scholar]
- 13.Kai Y, Hamada J, Morioka M, et al. Foramen magnum dural arteriovenous fistulae with repeated subarachnoid haemorrhage. Interv Neuroradiol 1998; 4: 171–176. [DOI] [PubMed] [Google Scholar]
- 14.Kinouchi H, Mizoi K, Takahashi A, et al. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg 1998; 89: 755–761. [DOI] [PubMed] [Google Scholar]
- 15.Willinsky R, TerBrugge K, Lasjaunias P, et al. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 1990; 34: 118–123. [DOI] [PubMed] [Google Scholar]
- 16.Kai Y, Hamada J-I, Morioka M, et al. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol 2005; 26: 1949–1954. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Esemen Y, Rane N, et al. Intracranial subarachnoid haemorrhage caused by cervical spinal dural arteriovenous fistulas: case report. Front Neurol 2021; 12: 685332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drazin D, Jeswani S, Shirzadi A, et al. Anterior spinal artery syndrome in a patient with vasospasm secondary to a ruptured cervical dural arteriovenous fistula. J Neuroimaging 2014; 24: 88–91. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Cao D, Wang H, et al. Spinal dural arteriovenous fistula presenting with subarachnoid hemorrhage: a case report. Medicine (Baltimore) 2018; 97: e0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimoto S, Takai K, Nakatomi H, et al. Three-dimensional angioarchitecture and microsurgical treatment of arteriovenous fistulas at the craniocervical junction. J Clin Neurosci 2018; 53: 140–146. [DOI] [PubMed] [Google Scholar]
- 21.Cahan LD, Higashida RT, Halbach VV, et al. Variants of radiculomeningeal vascular malformations of the spine. J Neurosurg 1987; 66: 333–337. [DOI] [PubMed] [Google Scholar]
- 22.Pierot L, Chiras J, Meder JF, et al. Dural arteriovenous fistulas of the posterior fossa draining into subarachnoid veins. AJNR Am J Neuroradiol 1992; 13: 315–323. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, Wang Q, Tian Y, et al. Dural arteriovenous fistulae at the craniocervical junction: the relation between clinical symptom and pattern of venous drainage. Acta Neurochir Suppl 2011; 110: 99–104. [DOI] [PubMed] [Google Scholar]
- 24.Byun JS, Hwang SN, Park SW, et al. Dural arteriovenous fistula of jugular foramen with subarachnoid hemorrhage: selective transarterial embolization. J Korean Neurosurg Soc 2009; 45: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwa J, Matsumura S, Maeda Y, et al. Transcondylar approach for dural arteriovenous fistulas of the cervicomedullary junction. Surg Neurol 1997; 48: 627–631. [DOI] [PubMed] [Google Scholar]
- 26.Wang JY, Molenda J, Bydon A, et al. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci 2015; 22: 1701–1707. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, Hayashi S, Ootani T, et al. Dural arteriovenous fistula manifesting as subarachnoid hemorrhage at the craniocervical junction. A case report. No Shinkei Geka 2008; 36: 901–906. [PubMed] [Google Scholar]
- 28.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain 2006; 129: 3150–3164. [DOI] [PubMed] [Google Scholar]
- 29.Donghai W, Ning Y, Peng Z, et al. The diagnosis of spinal dural arteriovenous fistulas. Spine 2013; 38: E546–E553. [DOI] [PubMed] [Google Scholar]
- 30.Alkhaibary A, Alharbi A, Alnefaie N, et al. Spinal dural arteriovenous fistula: a comprehensive review of the history, classification systems, management, and prognosis. Chin Neurosurg J 2024; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikkawa Y, Nakamizo A, Yamashita K, et al. Preoperative evaluation and surgical strategies for craniocervical junction dural arteriovenous fistula with multiple feeders: case report and review of the literature. Fukuoka Igaku Zasshi 2013; 104: 299–308. [PubMed] [Google Scholar]
- 32.Schievink WI, Wijdicks EF, Piepgras DG, et al. Perimesencephalic subarachnoid hemorrhage. Additional perspectives from four cases. Stroke 1994; 25: 1507–1511. [DOI] [PubMed] [Google Scholar]
- 33.Achrén A, Raj R, Siironen J, et al. Spontaneous angiogram-negative subarachnoid hemorrhage: a retrospective single center cohort study. Acta Neurochir (Wien) 2022; 164: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu G-D, Wang C, Zhao L-B, et al. Clinical outcomes of diffuse angiogram-negative subarachnoid hemorrhage versus aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. J Am Heart Assoc 2024; 13: e031066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C-L, Su Y-C, Chen C-C, et al. Ruptured cervical arteriovenous fistulas presenting with subarachnoid hemorrhage and quadriplegia: an uncommon case. Am J Emerg Med 2008; 26: 249.e1–2. [DOI] [PubMed] [Google Scholar]
- 36.Cenzato M, Debernardi A, Stefini R, et al. Spinal dural arteriovenous fistulas: outcome and prognostic factors. Neurosurg Focus 2012; 32: E11. [DOI] [PubMed] [Google Scholar]
- 37.Bakker NA, Uyttenboogaart M, Luijckx GJ, et al. Recurrence rates after surgical or endovascular treatment of spinal dural arteriovenous fistulas: a meta-analysis. Neurosurgery 2015; 77: 137–144. discussion 144. [DOI] [PubMed] [Google Scholar]
- 38.Bretonnier M, Hénaux P-L, Gaberel T, et al. Spinal dural arteriovenous fistulas: clinical outcome after surgery versus embolization: a retrospective study. World Neurosurg 2019; 127: e943–e949. [DOI] [PubMed] [Google Scholar]
- 39.Andres RH, Barth A, Guzman R, et al. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology 2008; 50: 869–876. [DOI] [PubMed] [Google Scholar]
- 40.Kirsch M, Berg-Dammer E, Musahl C, et al. Endovascular management of spinal dural arteriovenous fistulas in 78 patients. Neuroradiology 2013; 55: 337–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-2-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-3-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology
Supplemental material, sj-docx-4-ine-10.1177_15910199251328721 for Spinal dural arteriovenous fistulas presenting as intracranial subarachnoid hemorrhage: A systematic review by Bridget Nolan, Christian Rajkovic, Galadu Subah, Alis J Dicpinigaitis, Eric Feldstein, Ankita Jain, Eris Spirollari, Ariel Sacknovitz, Ilya Frid, Merritt Kinon, John Wainwright, Chirag D Gandhi, Gurmeen Kaur and Fawaz Al-Mufti in Interventional Neuroradiology