Abstract
Glial cells are traditionally regarded as elements for structural support and ionic homeostasis, but have recently attracted attention as putative integral elements of the machinery involved in synaptic transmission and plasticity. Here, we demonstrate that calcium-binding protein S100B, which is synthesized in considerable amounts in astrocytes (a major glial cell subtype), modulates long-term synaptic plasticity. Mutant mice devoid of S100B developed normally and had no detectable abnormalities in the cytoarchitecture of the brain. These mutant mice, however, had strengthened synaptic plasticity as identified by enhanced long-term potentiation (LTP) in the hippocampal CA1 region. Perfusion of hippocampal slices with recombinant S100B proteins reversed the levels of LTP in the mutant slices to those of the wild-type slices, indicating that S100B might act extracellularly. In addition to enhanced LTP, mutant mice had enhanced spatial memory in the Morris water maze test and enhanced fear memory in the contextual fear conditioning. The results indicate that S100B is a glial modulator of neuronal synaptic plasticity and strengthen the notion that glial–neuronal interaction is important for information processing in the brain.
Several lines of evidence suggest that glial cells play an active role in excitatory neurotransmission in the central nervous system (1, 2). For instance, astrocytes can release glutamate in response to physiological increases in their intracellular calcium concentration, and then evoke substantial glutamatergic currents in neighboring neurons (3). Oligodendrocyte precursor cells directly receive glutamatergic inputs from hippocampal pyramidal neurons (4). These findings, together with earlier studies showing that glial cells express many types of neurotransmitter receptors and respond to neurotransmitter by generating slowly propagating calcium waves (5), suggest that glial–neuronal reciprocal signaling may play a role in synaptic plasticity and eventually in information processing in the brain. Indeed, this notion has been supported by the findings that the mice devoid of glial fibrillary acidic protein (GFAP: an intermediate filament specific to astrocytes) showed enhanced long-term potentiation (LTP) in the hippocampal CA1 region and decreased long-term depression in the cerebellum associated with impaired eye-blink conditioning (6, 7). However, the molecular mechanism underlying glial–neuronal interactions is poorly understood.
One of the candidates that might be involved in glia-to-neuron signaling is the calcium-binding protein S100B. S100B is a member of the S100 family of proteins containing two EF-hand-type calcium-binding domains (8). The highest level of expression of the S100B protein is in the brain and is found primarily in the cytoplasm of astrocytes (9). Results of in vitro studies suggest a variety of intracellular functions of S100B, including cell growth, cell structure, energy metabolism, and calcium homeostasis (8). S100B is secreted from astrocytes, suggesting that it might also have extracellular functions. Exogenous S100B increases intracellular calcium concentrations in both cultured neurons and astrocytes (10). Elevated neuronal calcium might affect calcium-dependent processes involved in synaptic plasticity, and the role of S100B in neuronal synaptic plasticity is a subject of debate (1). Transgenic mice overexpressing human S100B exhibit impaired hippocampal LTP and spatial learning (11). Transgenic mice overexpressing S100B might not be appropriate for evaluating the physiological roles of S100B, however, because overexpression of S100B partly mimics pathological conditions in some neuronal diseases, such as Down's syndrome and Alzheimer's disease (12, 13). The constitutive overexpression of S100B might cause chronic neuronal damage (14, 15). Thus, there is no clear consensus regarding the significance of this glial protein in neuronal synaptic plasticity.
To clarify the precise role of S100B in neuronal synaptic plasticity, we generated mice devoid of S100B by using gene targeting methods. We here demonstrate that the deletion of S100B enhances hippocampal synaptic plasticity and hippocampus-dependent learning and memory.
Materials and Methods
Generation of S100B-Null Mice.
All experimental protocols were approved by the RIKEN Institutional Animal Care and Use Committee. A genomic clone of the murine S100b gene was isolated from 129/sv genomic DNA λFIXII library (Stratagene). A 3.5-kb SpeI–HindIII fragment including the entire S100b coding sequence was replaced by a neomycin selection marker, loxP-pgk-neo-loxP cassette. A 1.1-kb MC1-DT-A cassette, a negative selection marker, was added at the 3′ end of the targeting vector. The E14 embryonic stem cell line was used for gene targeting. Homologous recombinant clones were injected into mouse C57BL/6J blastocysts. Male chimeras were mated with C57BL/6J females to obtain the heterozygous mice. The heterozygotes were further crossbred with C57BL/6J mice five to seven times, and the resultant heterozygotes were interbred to obtain the wild-type and homozygous mice that were used in the present study. Genotypes were determined by Southern blot and/or PCR. PCR primers used were GTF1 (5′-GAGACGCTGGACGAAGATGG-3′), GTF2 (5′-CTTGACGAGTTCTTCTGAGG-3′), and SR1 (5′-CTGGGAAGGGTTGGGGTTTCA-3′). GTF1 and SR1 yield 280-bp fragments from the wild-type allele, and GTF2 and SR1 yield 160-bp fragments from the mutant allele.
Northern Blotting.
Total RNA (20 μg) from mouse brain was electrophoresed on formaldehyde agarose gels and transferred to Hybond N + filter (Amersham Pharmacia). The filter was hybridized with 32P- labeled 280 bp cDNA probe containing the entire S100b coding sequence.
Histological Analysis.
Mice were perfused intracardially with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) at 4°C. The brains were then removed and processed for paraffin embedding. Sagittal sections (4-μm-thick) were used for hematoxylin-eosin staining and S100B immunohistochemical staining (anti-S100B monoclonal antibody, clone SH-B1, diluted 1:2500; Sigma). Immunoreactivity was visualized using a Vectastain ABC kit (Vector Laboratories).
Electrophysiological Analysis.
Transverse hippocampal slices (400-μm-thick) from adult mice (3–6 months old) were perfused in an immersion-type recording chamber with artificial cerebrospinal fluid (ACSF, in mM; 118 NaCl/3 KCl/2 CaCl2/1 MgCl2/1 NaH2PO4/25 NaHCO3/10 glucose) at room temperature. Field excitatory postsynaptic potentials (fEPSPs) were recorded in the CA1 stratum radiatum by using glass pipettes filled with ACSF. Test stimuli of 0.1-ms duration were delivered to the Schaffer collaterals at 0.05 Hz with a monopolar platinum electrode and fEPSPs were amplified using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). The stimulus strength was adjusted to produce a response of 40–50% of the maximum fEPSP amplitude. fEPSP slope was calculated from the average of six responses evoked at 0.05 Hz and the magnitude of LTP was defined as the relative change in the fEPSP slope compared with the averaged baseline response monitored for 14 min before the tetanic stimulation. Stock solutions of drugs and recombinant S100B were dissolved in ACSF and stored in reservoirs. Drugs were applied by switching reservoirs. The electrophysiological experiments, except for D-APV [d(−)-2-amino-5-phosphonopentanoic acid] and picrotoxin treatments, were performed in a blind manner. Because we did not observed significant difference between genders, we pooled all data for analysis.
Calcium Imaging.
Hippocampal slices (400-μm-thick) were maintained in ACSF at room temperature for 1 h, and then incubated at 32°C for 1.5 h in ACSF supplemented by 10 μM calcium green-1 AM (Molecular Probes), 0.06% pluronic acid, 5 μM NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzoquinoxaline-7-sulfonamide, and 10 μM D-APV (final concentration of DMSO was 1.1%). Slices were then transferred to ASCF and incubated at room temperature for at least 1.5 h. Recording of fEPSP in the CA1 stratum radiatum and induction of LTP was performed as described above. Calcium green-1 was excited at 490 nm and fluorescence image (>515 nm) was obtained at the rate of 5 Hz, using a cooled CCD camera. Changes in dye fluorescence were expressed as ΔF/F0 values, with F0 being the baseline fluorescence intensity preceding the first tetanus.
Purification of S100B.
S100B was expressed from a murine S100b cDNA in Escherichia coli and purified using anion-exchange and hydrophobic chromatography, as described (10). The purified S100B was dialyzed against PBS, followed by oxidization with 5 mM sodium tetrathionate for 30 min (16). The mixture was dialyzed against PBS for 2 days at 4°C. After oxidation, approximately 80–90% of S100B consisted of disulfide-bonded dimers.
Behavioral Tests.
Group-housed adult male mice (3–6 months old) were used throughout behavioral tests. The genotype of the mice was always unknown to the experimenter.
Morris water maze test.
The water pool used in these experiments was 1 m in diameter and made of white polyvinyl chloride. The temperature of the water was held constant at 25 ± 1°C. In the hidden platform task, the acrylic transparent platform (diameter 10 cm) was submerged 0.7 cm below the surface of water made opaque by adding nontoxic white paint. The location of the platform was fixed over a series of trials for each mouse. If the mouse located the platform within 60 s, the mouse was allowed to remain on it for 30 s. Mice that failed to find the platform within 60 s were manually guided to the platform and allowed to remain on it for 30 s. Mice were given four trials per day for 5 consecutive days. A different starting point was used in each of the four trials. The time taken to reach the platform (escape latency) was recorded. A probe test was performed 3 h after the last hidden platform trial. In the probe test, the platform was removed and each mouse was allowed to swim for 60 s. The swimming path length, time spent in the quadrant that had contained the platform (trained quadrant), and number of times the mice crossed the area where the platform had been located were recorded. Three days after the probe test, mice were tested in a visible platform task for 3 consecutive days. In this task, the platform was made visible by attaching a black cubic landmark to the platform. In all of these experiments, movement of each mouse was monitored with a CCD camera and processed with NIH IMAGE WM 2.12 (O'hara & Co., Tokyo), a modified version of the software based on the public domain NIH IMAGE program.
Contextual fear conditioning test.
A fear conditioning shock chamber (10 × 10 × 10 cm high) was used. Mice were put in the chamber and conditioned by a single electrical foot shock (0.75 mA, 2 s). The mice were allowed to stay in the chamber for another 1 min for measurement of immediate freezing, and then placed back into their home cage. After 24 h, the mice were again put in the same chamber and monitored for 5 min without electrical shock. Movement of each mouse was monitored using a CCD camera and processed with NIH IMAGE FZ 2.17 (O'hara & Co.), a modified version of the software based on the public domain NIH IMAGE program.
Results
S100B-Null Mice Developed Normally.
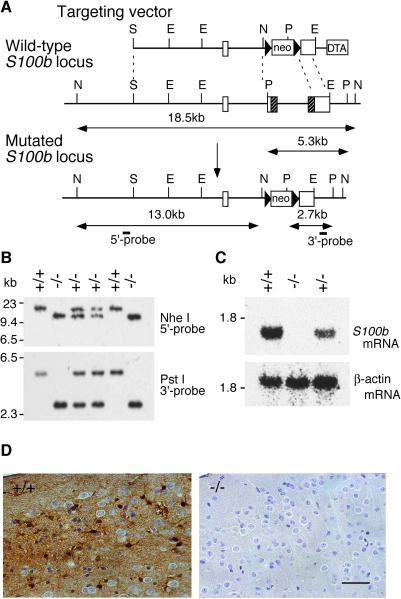
S100B-null mice were generated by homologous recombination in embryonic stem cells. Fig. 1A illustrates the targeting vector, the wild-type S100b locus, the mutated S100b locus, and the external probes used for genotyping in Southern blot analysis (Fig. 1B). Homozygous mutant mice completely lacked S100b mRNA and its product (Fig. 1 C and D). Mutant mice developed normally and were indistinguishable from the heterozygous and wild-type littermates. Mutant mice reached sexual maturity and both male and female mice were fertile. Despite several lines of evidences suggesting that S100B has trophic activity for glia and neurons (17–19), no anatomical abnormalities were observed in mutant brains (data not shown). We observed no abnormalities in their home cage behavior for up to 18 months of age.
Figure 1.
Targeted disruption of the S100b gene. (A) Schematic representation of the targeting vector, wild-type S100b locus, and mutated S100b locus. Boxes in the wild-type allele denote exons 1–3. Shaded parts of exons 2 and 3 correspond to protein coding regions. E, EcoRI; N, NheI; P, PstI; S, SalI. (B) Southern blot analysis of progeny from a cross of F1 heterozygotes by using the probes indicated in A. (C) Northern blot analysis of brain total RNA (20 μg) from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice by using a murine S100b protein coding sequence (Upper) and β-actin (Lower) as probes. Mutant mice completely lack S100b mRNA. (D) Immunohistochemistry of the cerebral cortex with an anti-S100B monoclonal antibody. The sections were lightly counterstained with hematoxylin to indicate cell nuclei. Soma and processes of cortical astrocytes were stained in wild-type mice (Left). There was no detectable immunoreactivity observed in mutant mice (Right). (Scale bar, 50 μm.)
Hippocampal LTP Was Enhanced in the S100B-Null Mice.
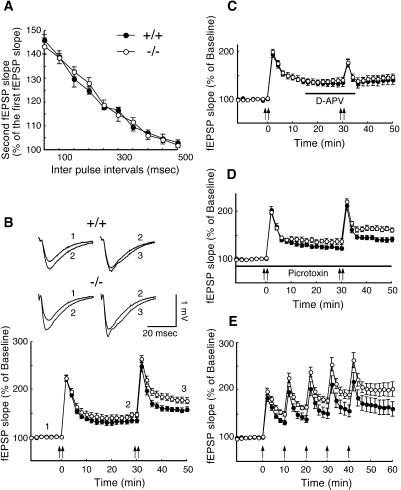
To examine the role of S100B in synaptic transmission, the Schaffer collateral-CA1 pyramidal cell synapses were analyzed in adult hippocampus. The input–output relationship, measured as the presynaptic fiber volley amplitude versus the slope of the fEPSP (data not shown), and paired pulse facilitation did not differ significantly between wild-type and mutant mouse slices, indicating that the basic synaptic transmission was normal in mutant mice (Fig. 2A). LTP was induced by tetanic stimulation (100 Hz, 1 s repeated twice with a 5-s interval), which was administered two times, 30 min apart (Fig. 2B). The first tetanic stimulation evoked reliable LTP (first LTP) in both wild-type and mutant mouse slices. The second tetanisation further enhanced potentiation (second LTP) in both groups. The mutant mouse slices exhibited greater potentiation than the wild-type mouse slices in both the first [wild-type = 134.9% ± 5.2%, mutant = 146.9% ± 3.7%; ANOVA, F (1,24) = 3.492, P = 0.074] and second LTP [wild-type = 157.7% ± 5.2%, mutant = 175.7% ± 6.2%; ANOVA, F (1,24) = 4.967, P < 0.05]. LTP at Schaffer collateral-CA1 pyramidal cell synapses is mediated by NMDA (N-methyl-D-aspartate) receptor-dependent mechanisms. To test whether NMDA receptor activation is required for the induction of a second LTP following a nonsaturated first LTP and whether the enhanced LTP is distinct from that in wild-type mice, we applied the NMDA receptor antagonist D-APV, before the second tetanus (Fig. 2C). D-APV completely blocked the second LTP in both wild-type and mutant mouse slices, indicating that the second LTP in both genotypes is NMDA receptor-dependent. The increased LTP observed in mutant mice could be due to a facilitation in the induction mechanism for LTP, such as an impairment in GABA (γ-aminobutyric acid)-mediated inhibitory systems (20). This is unlikely, however, because mutant mouse slices had an increased potentiation compared with wild-type mouse slices during both the first and second LTP in the presence of the GABAA receptor blocker picrotoxin [ANOVA, F (1,15) = 8.566, P < 0.05 for the second LTP; Fig. 2D]. To further investigate whether LTP induction is facilitated in mutant mice or whether naive synapses in mutant mice are more susceptible to LTP, we determined the level of saturated LTP. Tetanic stimulation was repeatedly applied at 10-min intervals (Fig. 2E). The magnitude of the LTP approached saturation by the fifth tetanus. The amplitude of saturated LTP was significantly greater in mutant mouse slices compared with wild-type mouse slices, at 20 min after the fifth tetanus (wild-type = 160.3% ± 15.6%, mutant = 202.2% ± 15.4%; Mann–Whitney U test, P < 0.05). These data suggested that the expression mechanisms of LTP were altered in mutant mice. If the enhanced LTP was solely a result of facilitated induction, such as an increased Ca2+ influx during potentiation, the level of saturated LTP would have been the same in both genotypes (21).
Figure 2.
Enhanced LTP in S100B-null mice. (A) Two consecutive stimulations were applied at various interpulse intervals. Paired pulse facilitation did not differ between wild-type (n = 6 slices/3 mice, closed circles) and mutant mouse slices (n = 6 slices/3 mice, open circles). (B) The averaged time course of LTP in wild-type (n = 13 slices/7 mice) and mutant (n = 13 slices/7 mice) mouse slices are shown. Tetanic stimulation was applied at time points 0 and 30 min, as indicated by the arrows. The representative traces, 1, 2, and 3, were taken from the time points 0, 30, and 50 min, respectively. (C) The NMDA receptor antagonist D-APV (50 μM) was applied during the time period indicated. D-APV completely prevented the second LTP in both wild-type (n = 9 slices/5 mice) and mutant (n = 9 slices/5 mice) mouse slices. (D) The mutant mouse slices (n = 9 slices/5 mice) had significantly greater potentiation than the wild-type mouse slices (n = 8 slices/5mice) in the presence of the GABAA receptor blocker picrotoxin (50 μM). (E) Saturated LTP was induced by five tetanic stimulations at 10-min intervals. Mutant mouse slices (n = 12 slices/5 mice) had significantly greater potentiation than wild-type mouse slices (n = 12 slices/5mice) at the saturated state. Data represent mean ± SE.
Calcium-Handling Capacity Was Normal in S100B-Null Astrocytes.
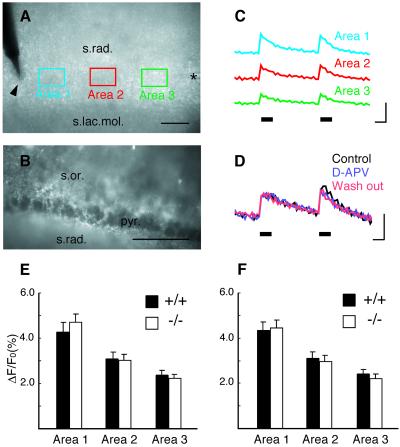
Neuronal synaptic activity can increase the cytosolic free Ca2+ concentration ([Ca2+]i) in neighboring astrocytes (22). An increase in [Ca2+]i in astrocytes at physiological levels triggers the release of glutamate from the astrocytes, and consequently stimulates neighboring neurons (3). Furthermore, alteration in stimulus-evoked calcium transient was reported in cultured cerebellar astrocytes derived from another line of S100B-null mice (23). These findings raise the possibility that tetanic stimulation required for the induction of LTP is accompanied by an enhanced [Ca2+]i increase and glutamate release in S100B-null astrocytes. To investigate this possibility, we first examined whether mature hippocampal astrocytes responded to tetanic stimulation with an elevation in [Ca2+]i. Slices were loaded with the fluorescent calcium indicator calcium green-1, which is preferentially sequestered in astrocytes (ref. 24; Fig. 3B). Tetanic stimulation produced a rapid rise in [Ca2+]i that peaked within 400 ms of the onset of the stimulation and decreased rapidly during the remaining stimulus period (Fig. 3C) in the CA1 stratum radiatum (Fig. 3A). The observed [Ca2+]i increase in mature hippocampal slices shared some common features with those reported in young astrocytes (22). The [Ca2+]i increase was completely blocked by application of tetrodotoxin (TTX, 0.5 μM; data not shown), and the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor antagonist NBQX (10 μM; data not shown) and the NMDA receptor antagonist D-APV (50 μM) did not block the [Ca2+]i increase (Fig. 3D). If the [Ca2+]i increase originated from the dendrites of CA1 pyramidal neurons, it would be sensitive to both NBQX and D-APV (25, 26). There were no differences observed in either duration or amplitude of the [Ca2+]i transients in wild-type or mutant mouse slices (Fig. 3 E and F). These results demonstrate that the [Ca2+]i handling capacity of the S100B-null astrocytes was normal within the range of [Ca2+]i changes evoked by tetanic stimulation, and that an alteration in astrocytic [Ca2+]i does not explain the difference observed in LTP in mutant mice.
Figure 3.
Normal calcium handling capacity in S100B-null astrocytes. Transillumination (A) and epifluorescence (B) images obtained from the CA1 region of a hippocampal slice loaded with a fluorescent calcium indicator. Arrowhead and asterisk in A indicate stimulation and recording electrodes, respectively. Rectangles (30 μm × 45 μm) separated by 40–50-μm intervals along the Schaffer collateral pathway indicate the areas measured. Note that pyramidal cell bodies in B are not labeled. (Scale bar, 50 μm.) (C) Relative fluorescent change evoked by tetanic stimulation (two 1-s, 100-Hz trains at 5-s intervals, indicated by solid bars). Traces were obtained from areas as indicated in A. [Scale bars, 1 s (horizontal) and 5% change in fluorescence (vertical).] (D) Same stimulation as in C before, during, and after superfusion of D-APV (50 μM). (E and F) Quantitative analysis of tetanus-induced calcium signals (mean ± SE). Amplitude of calcium transients evoked by the first and the second 100-Hz train are shown in E and F, respectively. There were no differences between wild-type (n = 12 slices/5 mice) and mutant (n = 12 slices/5 mice) mouse slices in all three areas. s.rad., stratum radiatum; s.lac.mol., stratum lacunosum molecular; s.or., stratum oriens; pyr., CA1 pyramidal cell layer.
Extracellular S100B Modulated Neuronal Synaptic Plasticity.
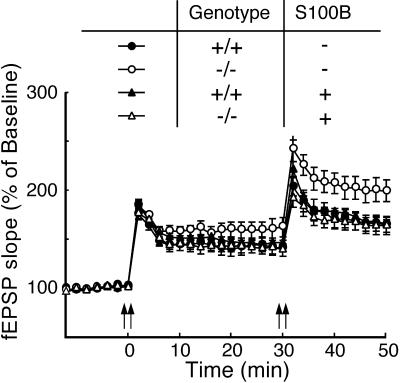
S100B protein is released by astrocytes into the extracellular space (27, 28) and could directly modulate synaptic mechanisms. To test this possibility, we perfused a disulfide-bonded dimer of S100B (10, 29) throughout the recording period at a concentration of 1 μg/ml, which is equivalent to the concentration in extracellular fluid of rat hippocampus (27). Exogenous S100B reversed the level of LTP in the mutant mouse slices to those of the wild-type mouse slices, whereas the same treatment did not affect the level of LTP in wild-type mouse slices (Fig. 4). This result indicates that S100B mediates effects via the extracellular space and, possibly, by direct action on the neurons. Further, the result suggests that the enhanced LTP in mutant mice is not a consequence of developmental deficits caused by the chronic loss of S100B.
Figure 4.
Extracellular S100B reversed the level of LTP in the mutant slices to that of the wild-type slices. A disulfide-bonded dimer of S100B (1 μg/ml) was applied throughout the experiment. The averaged time course of LTP with (wild-type, n = 10 slices/5 mice, closed triangles; mutant, n = 9 slices/5 mice, open triangles) or without (wild-type, n = 11 slices/5 mice, closed circles; mutant, n = 9 slices/5 mice, open circles) exogenous S100B is shown. The mutant mouse slices without S100B had significantly greater LTP than any other groups [ANOVA and post hoc Tukey–Kramer test, F (3, 35) = 4.062, P < 0.05].
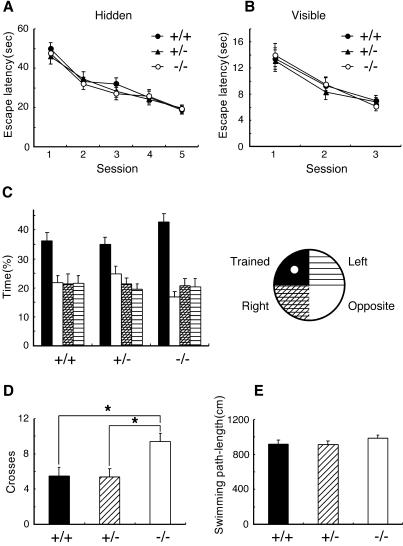
The S100B-Null Mice Exhibited Enhanced Spatial Memory.
The spatial learning ability of mutant mice was examined by using the hidden-platform Morris water maze test, which depends on hippocampal function (30). As shown in Fig. 5A, wild-type, heterozygous, and mutant mice improved their performance over 5 d of training. There was no significant difference in escape latency between genotypes on any day. Swimming path-length in the probe test and learning performance in the visible-platform task were not different between genotypes, indicating that mutant mice were normal in their vision, motor function, and the motivation required for the Morris water maze tasks (Fig. 5 B and E). In the probe test, however, mutant mice crossed the point where the platform had been located significantly more than the other genotypes [ANOVA and post hoc Tukey–Kramer test, F (2,53) = 5.550, P < 0.05; Fig. 5D]. Furthermore, mutant mice tended to spend a longer time in the trained quadrant than did the other genotypes [ANOVA, F (2,53) = 2.442, P = 0.097; Fig. 5C]. The probe test better reflects spatial learning than the measure of escape latencies during training (31). Therefore, these data indicated that mutant mice explored the platform site with higher spatial accuracy than did wild-type mice, suggesting superior spatial memory.
Figure 5.
Enhanced spatial memory in S100B-null mice. No difference was observed between genotypes in the escape latencies in water maze training (wild-type, n = 18; heterozygous, n = 19; mutant, n = 19) in the hidden-platform task (A) and the visible-platform task (B). (C) The probe test after the acquisition of the hidden-platform task. Times spent in each quadrant of the pool are shown. Mutant mice had a tendency to spend longer time in the trained quadrant than did the other genotypes (P = 0.097). (D) Number of crosses over the region where the platform had been located during the probe test. Mutant mice crossed the point significantly more than any other genotypes (P < 0.05). (E) Swimming path length during the probe test was not different between genotypes. Data represent mean ± SE. *, P < 0.05.
The S100B-Null Mice Exhibited Enhancement in Contextual Fear Memory.
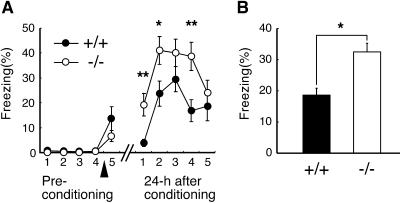
Finally, the associative emotional memory in mutant mice was examined by using the contextual fear conditioning test, which is also dependent on hippocampal function (32). Animals learn to fear a spatial context in which the animals were conditioned by the electrical foot shock. Before conditioning, both wild-type and mutant mice did not show freezing behavior at all. There was no significant difference between genotypes in freezing behavior immediately after conditioning (Fig. 6A). The freezing rate of mutant mice, however, was significantly higher than that of wild-type mice when they are tested at 24 h after conditioning [Fig. 6 A and B; repeated-measure ANOVA, F (1,21) = 6.563, P < 0.05]. There was no significant difference between genotypes either in nociceptive sensitivity to electrical foot shock or in locomotor activity assessed by the open field test (data not shown). The results indicated enhancement of contextual fear memory in mutant mice.
Figure 6.
Enhancement of contextual fear memory in S100B-null mice. (A) Movements of mice were monitored in a shock chamber for 5 min and percentage of freezing rate in each 1 min was plotted. Arrowhead indicates the time when the mice were conditioned by an electrical foot shock. Mutant mice (n = 11) had higher freezing rate than wild-type mice (n = 12) at 24 h after conditioning. (B) Each column represents averaged percentage of freezing rate during a 5-min recording period at 24 h after conditioning. Data represent mean ± SE. *, P < 0.05; **, P < 0.01.
Discussion
Several previous reports suggested that the S100B protein functions as a trophic factor (17–19). However, mice completely lacking S100B developed normally and there was no detectable abnormality in histological architecture of the mutant brain. There are two other S100 family proteins, S100A1 and S100A6 (33, 34), that are expressed in rodent glial cells albeit at much lower levels than S100B. We found no compensatory expression of S100A1 and S100A6 mRNA in the mutant brain (data not shown). This observation together with our finding that addition of exogenous S100B to the perfusing media reversed the level of LTP in the mutant slices to those of the wild-type slices suggest that the function of S100B is not substituted by other gene products in the mutant mice.
S100B-null mice exhibited enhanced performance in the hidden-platform task in the Morris water maze and the contextual fear conditioning together with an enhanced hippocampal LTP. Our results further strengthen the close correlation between hippocampal LTP and these behavioral paradigms.
Our data suggest that S100B modulates synaptic plasticity from the extracellular space. S100B can bind calcium (8). However, the low concentration of exogenously applied S100B unlikely affect extracellular free Ca2+ concentration. Popov et al. (35) reported alterations in S100 protein content in hippocampal slices during LTP. They showed that S100 protein content increased in the Triton-soluble membrane fractions and decreased in the water-soluble fractions at 1 h after tetanization. A fascinating hypothesis is that S100B secreted from astrocytes binds to a receptor on the neuronal membrane and drives subsequent signaling cascades involved in neuronal synaptic plasticity. RAGE (receptor for advanced glycation end products) is a receptor for a newly identified S100 family protein EN-RAGE (extracellular newly identified RAGE-binding protein) (36). RAGE is expressed at the highest level in the lung but is also expressed in neurons in adult human brain (37). Huttunen et al. (38) reported that S100B induces neurite outgrowth in RAGE-transfected cells. Although it is unknown whether RAGE is an endogenous receptor for S100B, RAGE or RAGE-related receptors might play a role underlying the synaptic plasticity modulated by S100B. The signaling cascades driven by extracellular S100B in neurons remain elusive. Identification of receptors for S100B should provide further insight into the complex regulatory processes involved in synaptic plasticity and biological significance of S100B.
Elevation of S100B expression is observed in various pathological conditions such as brain injury, ischemia, and in various neuronal diseases (12, 13, 39–42). These observations, together with our present results, tempt us to speculate that S100B might negatively regulate neuronal activity with the beneficial effect of preventing neuronal damage associated with hyper activities. Such a function as an “emergency valve” would extend the recognition of glial cells as passive supporters of homeostatic processes to active elements.
In conclusion, the present study provides strong evidence that a glial protein directly modulates neuronal synaptic plasticity, learning, and memory. Molecular mechanisms of glial–neuronal interactions are more elaborated than previously assumed and further studies on this line are required to understand brain plasticity under physiological and pathological conditions.
Acknowledgments
We thank Maria Elena Alojado, Toshio Ikeda, and Hiroshi Gomi for advice in experiments; Takuji Iwasato and Mika Tanaka for critical comments on the manuscript; and all members of Laboratories for Behavioral Genetics and Neuronal Circuit Dynamics for discussion and technical support. H.N. thanks Koreaki Ito for continuous encouragement. H.N. was supported by a Junior Research Associate program of RIKEN.
Abbreviations
- LTP
long-term potentiation
- fEPSP
field excitatory postsynaptic potentials
- D-APV
d(−)-2-amino-5-phosphonopentanoic acid
- NMDA
N-methyl-d-aspartate
References
- 1.Araque A, Carmignoto G, Haydon P. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 2.Laming P R, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C, Roots B, Ng K. Neurosci Biobehav Rev. 2000;24:295–340. doi: 10.1016/s0149-7634(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 3.Parpura V, Haydon P G. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergles D E, Roberts J D, Somogyi P, Jahr C E. Nature (London) 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 5.Kettenmann H, Ransom B R. Neuroglia. New York: Oxford Univ. Press; 1995. pp. 335–384. [Google Scholar]
- 6.McCall M A, Gregg R G, Behringer R R, Brenner M, Delaney C L, Galbreath E J, Zhang C L, Pearce R A, Chiu S Y, Messing A. Proc Natl Acad Sci USA. 1996;93:6361–6366. doi: 10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibuki K, Gomi H, Chen L, Bao S, Kim J J, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, et al. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer D B, Cornwall E H, Landar A, Song W. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 9.Van Eldik L J, Ehrenfried B, Jensen R A. Proc Natl Acad Sci USA. 1984;81:6034–6038. doi: 10.1073/pnas.81.19.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barger S W, Van Eldik L J. J Biol Chem. 1992;267:9689–9694. [PubMed] [Google Scholar]
- 11.Gerlai R, Wojtowicz J M, Marks A, Roder J. Learn Mem. 1995;2:26–39. doi: 10.1101/lm.2.1.26. [DOI] [PubMed] [Google Scholar]
- 12.Griffin W S, Stanley L C, Ling C, White L, MacLeod V, Perrot L J, White C L, III, Araoz C. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato K, Kurobe N, Suzuki F, Morishita R, Asano T, Sato T, Inagaki T. J Mol Neurosci. 1991;3:95–99. doi: 10.1007/BF02885530. [DOI] [PubMed] [Google Scholar]
- 14.Reeves R H, Yao J, Crowley M R, Buck S, Zhang X, Yarowsky P, Gearhart J D, Hilt D C. Proc Natl Acad Sci USA. 1994;91:5359–5363. doi: 10.1073/pnas.91.12.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker-Azmitia P M, Wingate M, Borella A, Gerlai R, Roder J, Azmitia E C. Brain Res. 1997;776:51–60. doi: 10.1016/s0006-8993(97)01002-0. [DOI] [PubMed] [Google Scholar]
- 16.Scotto C, Mely Y, Ohshima H, Garin J, Cochet C, Chambaz E, Baudier J. J Biol Chem. 1998;273:3901–3908. doi: 10.1074/jbc.273.7.3901. [DOI] [PubMed] [Google Scholar]
- 17.Kligman D, Marshak D R. Proc Natl Acad Sci USA. 1985;82:7136–7139. doi: 10.1073/pnas.82.20.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azmitia E C, Dolan K, Whitaker-Azmitia P M. Brain Res. 1990;516:354–356. doi: 10.1016/0006-8993(90)90942-5. [DOI] [PubMed] [Google Scholar]
- 19.Selinfreund R H, Barger S W, Pledger W J, Van Eldik L J. Proc Natl Acad Sci USA. 1991;88:3554–3558. doi: 10.1073/pnas.88.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzjohn S M, Morton R A, Kuenzi F, Davies C H, Seabrook G R, Collingridge G L. Neurosci Lett. 2000;288:9–12. doi: 10.1016/s0304-3940(00)01204-0. [DOI] [PubMed] [Google Scholar]
- 21.Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter J T, McCarthy K D. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong Z, O'Hanlon D, Becker L E, Roder J, MacDonald J F, Marks A. Exp Cell Res. 2000;257:281–289. doi: 10.1006/excr.2000.4902. [DOI] [PubMed] [Google Scholar]
- 24.Shelton M K, McCarthy K D. Glia. 1999;26:1–11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Regehr W G, Connor J A, Tank D W. Nature (London) 1989;341:533–536. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- 26.Regehr W G, Tank D W. Nature (London) 1990;345:807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- 27.Shashoua V E, Hesse G W, Moore B W. J Neurochem. 1984;42:1536–1541. doi: 10.1111/j.1471-4159.1984.tb12739.x. [DOI] [PubMed] [Google Scholar]
- 28.Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D'Alimonte I, D'Onofrio M, Nicoletti F, Caciagli F. Glia. 1999;27:275–281. [PubMed] [Google Scholar]
- 29.Winningham-Major F, Staecker J L, Barger S W, Coats S, Van Eldik L J. J Cell Biol. 1989;109:3063–3071. doi: 10.1083/jcb.109.6.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris R G, Anderson E, Lynch G S, Baudry M. Nature (London) 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 31.Brandeis R, Brandys Y, Yehuda S. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 33.Song W, Zimmer D B. Brain Res. 1996;721:204–216. doi: 10.1016/0006-8993(96)00172-2. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita N, Ilg E C, Schafer B W, Heizmann C W, Kosaka T. J Comp Neurol. 1999;404:235–257. [PubMed] [Google Scholar]
- 35.Popov N, Schulzeck S, Pankova T M, Ratushnyak A S, Starostina M V, Shtark M B, Matthies H. Biomed Biochim Acta. 1988;47:189–195. [PubMed] [Google Scholar]
- 36.Hofmann M A, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, Takeuchi M, Makita Z. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- 38.Huttunen H J, Kuja-Panula J, Sorci G, Agneletti A L, Donato R, Rauvala H. J Biol Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 39.Hardemark H G, Ericsson N, Kotwica Z, Rundstrom G, Mendel-Hartvig I, Olsson Y, Pahlman S, Persson L. J Neurosurg. 1989;71:727–731. doi: 10.3171/jns.1989.71.5.0727. [DOI] [PubMed] [Google Scholar]
- 40.Miyake T, Hattori T, Fukuda M, Kitamura T. Brain Res. 1989;489:31–40. doi: 10.1016/0006-8993(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 41.Hinkle D A, Baldwin S A, Scheff S W, Wise P M. J Neurotrauma. 1997;14:729–738. doi: 10.1089/neu.1997.14.729. [DOI] [PubMed] [Google Scholar]
- 42.Martinez G, Carnazza M L, Di Giacomo C, Sorrenti V, Avitabile M, Vanella A. Int J Dev Neurosci. 1998;16:519–526. doi: 10.1016/s0736-5748(98)00035-5. [DOI] [PubMed] [Google Scholar]