Abstract
Conventional memory assessment may fail to identify memory dysfunction characterized by intact recall for a relatively brief period but rapid forgetting thereafter. This study assessed learning and retention after 30-min and 24-hr delays on auditory and visual selective reminding tests (SRTs) in right (n = 20) and left (n = 22) temporal lobe epilepsy (TLE) patients and controls (n = 49). The left TLE group performed significantly worse than controls on all 3 trials of both tests. The right TLE group differed from the controls on all 3 visual SRT trials and on learning for the auditory SRT. There were no between-groups differences in rate of information lost at the 30-min versus the 24-hr delay. At the individual level, there was no difference in the percentage of patients versus controls who demonstrated isolated memory impairment at the 24-hr delay. Accelerated forgetting over 24 hr is uncommon in TLE patients.
Learning and memory impairment are common in patients with temporal lobe epilepsy (TLE; Chelune, Naugle, Luders, & Awad, 1991; Wilde et al., 2001). Traditionally, this impairment has been identified through an assessment of immediate memory or learning and then delayed memory or retention for the same information following a 20- to 30-min interval. Although a reliable association between visual memory test performance and the right hippocampus has not been found, conventional auditory learning and retention scores in groups of TLE patients have correlated with left hippocampal volume or transverse (T2) relaxation time on magnetic resonance imaging (MRI; Kalviainen et al., 1997; Kilpatrick et al., 1997; Lencz et al., 1992; Loring et al., 1993; Trenerry, Jack, Cascino, Sharbrough, & Ivnik, 1995; Wood, Saling, O’Shea, Berkovic, & Jackson, 2000); metabolic function, as measured by proton magnetic resonance spectroscopy (Kikuchi, Kubota, Hattori, Oya, & Mikuni, 2001; Martin et al., 1999; Sawrie et al., 2000, 2001); and hippocampal pathology ratings performed after anterior temporal lobectomy (Baxendale et al., 1998; Rausch & Babb, 1993; Sass et al., 1990).
A normal retention or savings score after the standard 30-min delay has been considered to reflect an intact initial consolidation process, with the information retained considered part of long-term episodic memory. However, there is evidence that retention of information over this relatively short delay is not always a good predictor of the permanence of that information in memory, and this evidence suggests that consolidation of information into long-term storage may be a multistage process (De Renzi & Lucchelli, 1993; Kapur et al., 1997). For example, three recent case studies of TLE patients revealed a syndrome of amnesia in which retention was normal when assessed after relatively brief delays but impaired as a result of accelerated forgetting when assessed after days or weeks (Holdstock, Mayes, Isaac, Gong, & Roberts, 2002; Kapur et al., 1997; O’Connor, Sieggreen, Ahern, Schomer, & Mesulam, 1997). Accelerated forgetting over time had a clear association with seizure activity in one patient who was tested at many sessions across several years (O’Connor et al., 1997). It was not possible to determine whether there was a relation between seizure frequency and memory loss in the other two cases, because they were not tested repeatedly, and at least one had a stable seizure pattern (Holdstock et al., 2002, p. 763). It is notable that none of these three cases had typical mesial TLE (Engel, Williamson, & Wieser, 1997). The patient described by O’Connor et al. developed late-onset bilateral TLE that was thought to be associated with neoplastic limbic encephalitis and caused up to 30 complex partial seizures per day. Holdstock et al. (2002) described a 40-year-old patient (J. L.) who had developed TLE at age 17 after a traumatic brain injury associated with an 11-day coma. Imaging revealed normal hippocampi, but extensive atrophy was present in other temporal lobe regions, including the superior, middle, and inferior gyri bilaterally. At the time her memory test data were acquired, J. L. was experiencing approximately 35 seizures per month. Bilateral temporal lobe electroencephalogram (EEG) abnormalities were identified in the third patient after she presented with amnesia for recent events at about age 60, but this patient had no history of typical complex partial temporal lobe seizures (Kapur et al., 1997).
There have been two reports that accelerated forgetting occurred at the group level among TLE patients following adequate auditory immediate memory and 30-min retention performance (Blake, Wroe, Breen, & McCarthy, 2000; Martin et al., 1991). Blake et al. reported that, compared with controls, a small group of left TLE patients (n = 9), but not a right TLE group (n = 5), showed accelerated memory loss for a story between 30-min and 8-week delay recall trials. Participants were presented the story until they met the learning criterion or a maximum of 10 times. In the Martin et al. study, which included some anterior temporal lobectomy patients, both left (n = 13) and right (n = 8) TLE groups showed accelerated forgetting between the 30-min and 24-hr recall trials on an auditory selective reminding test (SRT). Participants were presented the word list until they met the learning criterion or a maximum of 12 times.
In the present study of groups of right and left TLE patients and controls, learning and free recall after 30-min and 24-hr delays were assessed on an auditory SRT and a visual SRT. A maximum of six learning trials were presented for each SRT. We tested the prediction that there would be a disproportionate loss of memory between the 30-min and 24-hr delayed recall trials in TLE patients compared with controls at the group level. Memory test performance at a 30-min versus a longer delay has not yet been analyzed at the individual level among a group of TLE patients. Determining the percentage of patients who had unimpaired scores at both the initial learning and the 30-min retention trials but impaired retention after the longer delay would provide information about the cost–benefit ratio of performing an extra memory assessment session. Therefore, the data also were analyzed at the individual level, with impairment on each variable defined as a score falling one standard deviation or more below the control mean. Finally, because previous findings have been mixed, we made no specific prediction about the relation between memory performance after the 24-hr delay and seizure activity during those 24 hr.
Method
Participants
Between 1998 and 2002, 85 TLE patients and 75 controls were administered a neuropsychological battery for a research study of the neurobehavioral consequences of TLE at the University of Wisconsin Hospital in Madison. The individuals with TLE were recruited after their medical records were reviewed by a board-certified neurologist with expertise in epilepsy. This review included information pertaining to seizure semiology, previous EEGs and neuroimaging, and clinical history and course. Each patient then was classified as having seizures of definite, probable, or possible temporal lobe origin. Definite TLE was defined by continuous video and EEG monitoring demonstrating temporal lobe seizure onset. Probable TLE was defined by clinical semiology reported to reliably identify complex partial seizures of temporal lobe origin, in conjunction with interictal EEGs, neuroimaging findings, and developmental and clinical history. Only those patients meeting criteria for definite or probable TLE were recruited for study participation. The majority of the controls were friends, relatives, or spouses of the individuals with TLE. They met Wechsler Memory Scale—Third Edition (WMS–III) criteria for healthy controls (Psychological Corporation, 2002). All participants were between the ages of 18 and 59, had a Wechsler Adult Intelligence Scale—Third Edition (WAIS–III) seven-subtest short form (Pilgrim, Meyers, Bayless, & Whetstone, 2000; Psychological Corporation, 2002) full scale IQ greater than or equal to 70, had no MRI abnormalities other than atrophy, and had not undergone epilepsy surgery.
Ninety-one of the 160 research participants were included in this study. These 91 participants (controls = 49, right TLE = 20, left TLE = 22) were included because they had been administered all three trials of both the auditory and the visual SRTs and, in the case of the participants with TLE, had lateralized seizure onset identified by either interictal or ictal EEG.
Twenty patients were excluded from the study because their laterality of seizure onset was bilateral or indeterminate. Forty-nine additional participants (26 controls and 23 patients) from the total of 160 were excluded from the study because they were not administered the auditory and/or visual SRT 24-hr delay trial. The 24-hr delay was not administered to these cases because it had been acknowledged in advance that they would not be available for a 2nd day of testing or because they completed all other phases of the research study in 1 day.
For the patient (n = 42 [no 24 hr trial], n = 23, respectively) and control (n = 49 [no 24 hr trial], n = 26, respectively) groups considered separately, t tests indicate that the mean auditory SRT total learning score of participants who were administered the 24-hr delay did not differ significantly from the mean score of participants who were not administered the 24-hr delay trial: patients, t(63) = 1.37, p = .18; controls, t(73) = 0.88, p = .38. The 30-min delay score also did not differ significantly between the patients who were and were not administered the 24-hr delay and the controls who did and did not have this trial administered: patients, t(63) = −0.20, p = .85; controls, t(73) = 0.71, p = .48.
In addition, within each group, the participants who were administered the 24-hr delay trial did not differ significantly from the participants who were not administered the 24-hr delay on the visual SRT total learning score—patients, t(63) = 0.58, p = 56; controls, t(73) = 1.41, p = .16—or the 30-min delay score—patients, t(63) = 0.52, p = .61; controls, t(73) = 0.21, p = .84. For the visual SRT total learning score, the controls who were not administered the 24-hr delay recalled only 2.21 more words than the controls who were administered this trial (M = 63.19, SD = 7.13, vs. M = 60.98, SD = 5.02), which results in an effect size (Cohen’s d) of .36. With power = .80 and p = .05, it is necessary to have 129 participants in each subgroup to find a significant difference. This finding further illustrates that the mean memory score of the group that was not administered the 24-hr delay trial did not differ substantially from the group that was administered this trial.
Information about each patient’s medical and demographic history was acquired from medical records and an interview with each patient and/or spouse or a relative (see Table 1). On the basis of ictal or interictal EEG data, 20 of the patients in this study had a right temporal EEG focus, and 22 had a left temporal lobe focus. All patients were taking at least one antiepilepsy drug at the time of their participation in the study. Fourteen percent of the patients reported having been seizure free during the year preceding the study. Forty-five percent of the right TLE group and 41% of the left TLE group had had at least one secondarily generalized seizure during their lifetime. Seizure frequency data were based on self-report and the report of family members. It should be noted that all available TLE patients were recruited, even those who were not necessarily candidates for anterior temporal lobectomy (e.g., because of late age of seizure onset or relatively infrequent seizures).
Table 1.
Demographic and Seizure History Data
| Group
|
|||
|---|---|---|---|
| Variable | Control | Right TLE | Left TLE |
| n | 49 | 20 | 22 |
| Age | |||
| M | 37.0 | 40.0 | 34.0 |
| SD | 11.8 | 9.8 | 13.0 |
| Education | |||
| M | 13.7 | 13.1 | 12.6 |
| SD | 2.1 | 1.8 | 2.5 |
| WAIS–III FSIQ | |||
| M | 104.0a | 95.0 | 92.0 |
| SD | 12.7 | 13.8 | 14.6 |
| % Female | 55 | 55 | 77 |
| Right hippocampal volume z | |||
| M | −0.03b | −1.75 | −0.45 |
| SD | 1.00 | 1.40 | 1.20 |
| Left hippocampal volume z | |||
| M | 0.04c | −0.55 | −1.42 |
| SD | 1.00 | 1.40 | 1.10 |
| Age of onset (years) | |||
| M | 13.1 | 13.9 | |
| SD | 9.4 | 13.0 | |
| Epilepsy duration (years) | |||
| M | 26.8 | 20.1 | |
| SD | 14.0 | 11.8 | |
| No. AEDs | |||
| M | 1.9 | 1.7 | |
| SD | 0.75 | 0.70 | |
| No. participants with seizure during 24-hr delay | 2 | 2 | |
| Seizure frequency in past year (Mdn)d | monthly | monthly | |
Note. Hippocampal data were available for 39 controls, 16 individuals with right temporal lobe epilepsy (TLE), and 15 individuals with left TLE. WAIS–III FSIQ = Wechsler Adult Intelligence Scale—Third Edition full scale IQ; AED = anti-epilepsy drug.
Significantly different from right and left TLE groups.
Significantly different from right TLE group.
Significantly different from left TLE group.
Participants estimated whether seizures occurred on a daily, weekly, monthly, or yearly basis or did not occur within the year prior to the memory assessment. This estimate includes all seizure types.
Only 4 patients (2 with left TLE, 2 with right TLE) experienced a seizure, either witnessed or by self-report, during the 24-hr delay interval. The type of seizure that occurred during this interval was not recorded. None of the patients underwent EEG monitoring during the 24-hr delay.
Analyses of variance showed there were no significant differences among the three groups in age, F(2, 88) = 1.3, p = .28, education, F(2, 88) = 1.9, p = .15, or male to female ratio, χ2(2, N = 91) = 3.44, p = .18. The groups differed significantly on full scale IQ, F(2, 88) = 7.9, p < .01, with the control group’s IQ being higher than the left (mean difference = 12.10, SE = 3.40, p < .01) and right (mean difference = 9.80, SE = 3.60, p < .05) TLE groups. Left hippocampal volume was smaller in the left TLE patients compared with controls (mean difference = −1.50, SE = 0.35, p < .01) and the right TLE group (mean difference = −0.87, SE = 0.41, p < .05). Right hippocampal volume was smaller in the right TLE patients compared with controls (mean difference = −1.70, SE = 0.35, p < .01) and the left TLE group (mean difference = −1.30, SE = 0.42, p < .01). These IQ and hippocampal volume group differences are consistent with the TLE literature.
Assessment
Auditory and visual memory assessment involved the presentation of a maximum of six trials of a 12-item list via the SRT procedure, in which the examiner presents only those items that were not recalled on the immediately preceding trial (Buschke, 1973). The auditory SRT was always administered immediately before the visual SRT. The former consisted of 12 one- or two-syllable words that were read aloud to the participants. On each trial, the participants were asked to say all the words from the list that they could remember. The visual SRT consisted of 12 geometric figures that were presented one at a time on 3-in. (7.62-cm) × 5-in. (12.70-cm) index cards for 10 s each. On each trial, the participants were asked to draw all the figures from the list that they could remember. The drawings were scored as correct or incorrect on the basis of explicit scoring criteria (M. Westerveld, personal communication, 1998).
Presentation of the list was discontinued before the sixth trial if all 12 items were recalled on two consecutive trials. For the auditory SRT, only 2 (4%) of the controls and none of the patients were administered fewer than six learning trials because they met this criterion. Twenty-four percent of the controls versus 7% of the patients recalled all 12 words on the auditory SRT list by the sixth trial, χ2(1, N = 91) = 4.94, p < .05. For the visual SRT, 33% of the controls and 14% of the patients were administered fewer than six learning trials because they met the learning criterion before the sixth trial. Sixty-five percent of the controls versus 26% of the patients recalled all 12 geometric figures on the visual SRT list by the final trial, χ2(1, N = 91) = 13.88, p < .01.
The following two sets of SRT variables were examined in the analyses: (a) number of words recalled on Trial 6, after the 30-min delay, and after the 24-hr delay, and (b) the rate of information lost between Trial 6 and the 30-min delay and between the 30-min delay and the 24-hr delay. We corrected for the large difference between the two delay periods (0.5 hr vs. 23.5 hr) by dividing the difference between the memory scores at the 30-min trial and Trial 6 trials by 0.5 ([30-min score − Trial 6 score]/0.5) and by dividing the difference between the memory scores at the 24-hr trial and 30-min trial by 23.5 ([24-hr score − 30-min score]/23.5).
The participants were not informed in advance that the delay trials would be administered. All SRT trials were administered in person at the neuropsychology laboratory. Recognition memory was not assessed for the SRT measures. Non-SRT memory measures also were administered, but not after 24 hr.
To determine the number of participants in each group with an impaired score on each variable, we defined memory impairment as a score less than or equal to − 1.0 standard deviation below the mean of the control group. The cut-off of one standard deviation has been used to define scores as abnormal on standardized tests of memory and cognition (Heaton, Grant, & Matthews, 1991; Heaton, Taylor, & Manly, 2003). For the auditory SRT, a score less than or equal to − 1.0 standard deviation corresponded to raw scores less than or equal to 8, 5, and 3 for Trial 6 and the 30-min and 24-hr delays, respectively. For the visual SRT, raw scores less than or equal to − 1.0 standard deviation from the control mean fell at or below 10, 10, and 8 for Trial 6 and the 30-min and 24-hr delays, respectively. The finding of better retention on the visual compared with the auditory SRT is consistent with the pattern of visual and auditory memory savings scores for the standardization sample on the core subtests of the WMS–III (Psychological Corporation, 2002). However, the 10-s exposure to each visual SRT target probably explains, in part, the higher learning and retention scores for the visual SRT in both groups.
Brain Imaging
Images were obtained on a 1.5-T GE Signa (Milwaukee, WI) magnetic resonance (MR) scanner. MR images were acquired at the University of Wisconsin—Madison and transferred to the University of Iowa, where they were processed using a semiautomated software package known as Brain Research: Analysis of Images, Networks, and Systems (Andreasen et al., 1992). The hippocampi were manually traced there also to obtain the hippocampal volumes (Pantel et al., 2000). Hippocampal volumes were adjusted for intracranial volume (ICV) via multiple regression analyses. These analyses were based on all our healthy controls for whom MRI data were available (n = 66), which is why the hippocampal z scores for the controls in Table 1 are not equal to zero precisely. The regression equations were then applied to TLE patient volumes, and the predicted variance was removed from the observed hippocampal volume values of the TLE patients. The result is a residual score that removes variance due to ICV. The z score transformations of the adjusted volumes were computed on the basis of the control group’s mean adjusted volumes.
Because some participants in the study had claustrophobia, were too large for the MRI apparatus, or moved excessively during MRI, hippocampal data were available for 80% of the controls, 80% the right TLE patients, and 68% of the left TLE patients. MRI usually took place on the same day as the neuropsychological assessment.
Results
Auditory SRT
Group comparisons.
A multivariate analysis of variance (MANOVA), with the three auditory SRT trials as the dependent variables, showed that there was a significant overall group difference, Wilks’s λ(6, 172) = 0.80, p < .01, partial η2 = .11. Pairwise comparison tests showed that the control group differed significantly from the left TLE patients on each of the three auditory SRT variables (Trial 6: mean difference = 1.72, SE = 0.51, p < .01; 30-min delay: mean difference = 2.76, SE = 0.77, p < .01; 24-hr delay: mean difference = 2.93, SE = 0.73, p < .01). The control group differed from the right TLE group only on Trial 6 (mean difference = 1.44, SE = 0.53, p < .05). Scores for the left and right TLE groups did not differ on any of the three trials (see Table 2 and Figure 1).
Table 2.
Auditory and Visual Selective Reminding Test (SRT) Data
| Group
|
||||||
|---|---|---|---|---|---|---|
| Control (n = 49)
|
Right TLE (n = 20)
|
Left TLE (n = 22)
|
||||
| Variable | M | SD | M | SD | M | SD |
| Auditory SRT | ||||||
| Total learning | 51.3 | 7.5 | 45.1 | 10.2 | 44.6 | 11.4 |
| Trial 6 | 10.0 | 1.6 | 8.6 | 2.4 | 8.3 | 2.3 |
| 30-min raw | 7.7 | 2.8 | 5.8 | 3.4 | 5.0 | 3.1 |
| 24-hr raw | 6.0 | 3.0 | 4.7 | 2.8 | 3.1 | 2.7 |
| Visual SRT | ||||||
| Total learning | 61.0 | 7.1 | 49.7 | 15.2 | 45.5 | 17.1 |
| Trial 6 | 11.4 | 0.94 | 9.4 | 2.9 | 8.6 | 3.3 |
| 30-min raw | 11.1 | 1.0 | 9.7 | 2.3 | 8.2 | 3.2 |
| 24-hr raw | 10.2 | 1.7 | 8.3 | 2.8 | 7.5 | 3.4 |
Note. TLE = temporal lobe epilepsy.
Figure 1.
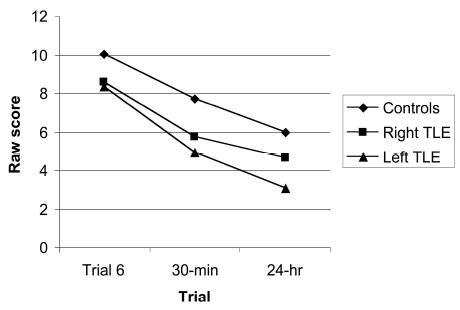
Auditory selective reminding test scores by trial and group. TLE = temporal lobe epilepsy.
A second MANOVA indicated that there was no significant difference among the groups in rate of information loss across the two delays, Wilks’s λ(4, 174) = 0.93, p = .15, partial η2 = .04 (see Table 3). However, because there was a slight trend toward significance, we investigated the rate of information loss further. Pairwise comparison tests showed that there were no differences between controls and the left TLE group in rate of information loss at the 30-min delay (mean difference = 2.07, SE = 1.03, p = .14) or at the 24-hr delay (mean difference = 0.01, SE = 0.02, p = 1.0). In addition, there were no differences between controls and the right TLE group in rate of information loss at the 30-min delay (mean difference = 1.05, SE = 1.07, p = .99) or at the 24-hr delay (mean difference = −0.03, SE = 0.02, p = .60). Finally, the two TLE groups did not differ in rate of information loss between Trial 6 and the 30-min delay (mean difference = 1.03, SE = 1.24, p = 1.0) or between the 30-min and 24-hr delays (mean difference = 0.03, SE = 0.02, p = .49).
Table 3.
Rate of Information Loss per Hour on the Auditory and Visual SRTs by Group
| Group
|
||||||
|---|---|---|---|---|---|---|
| Control
|
Right TLE
|
Left TLE
|
||||
| Variable | M | SD | M | SD | M | SD |
| Auditory SRT | ||||||
| 30-min delay | −4.70 | 3.80 | −5.50 | 4.10 | −6.70 | 4.50 |
| 24-hr delay | −0.07 | 0.08 | −0.05 | 0.07 | −0.08 | 0.08 |
| Visual SRT | ||||||
| 30-min delay | −0.61 | 2.10 | 0.63 | 2.70 | −0.81 | 3.10 |
| 24-hr delay | −0.04 | 0.06 | −0.06 | 0.07 | −0.03 | 0.08 |
Note. TLE = temporal lobe epilepsy; SRT = selective reminding test.
Finally, we examined the within-group rate of information loss across the two delay trials. Paired samples t tests indicated that all three groups lost information at a faster rate per hour over the 30-min delay compared with the 24-hr delay: controls, t(48) = − 8.46, p < .01; left TLE group, t(21) = −6.87, p < .01; and right TLE group, t(18) = −5.80, p < .01.
Analysis of the auditory SRT at the individual level.
Table 4 shows the percentage of participants who had an impaired score (less than or equal to − 1.0 standard deviation from control mean) on at least one of the three auditory SRT variables. As is evident from the table, 33% of the controls, 60% of the right TLE group, and 77% of the left TLE group were impaired on at least one of the auditory SRT variables. Table 4 also shows that 2 controls (4%) and 2 left TLE patients (9%) but no individuals with right TLE showed isolated impairment at 24 hr. There was no significant difference in percentage of cases with isolated 24-hr memory impairment (Bruning & Kintz, 1977) between the control and left TLE groups (z = −0.85, p = .36), the control and right TLE groups (z = 1.00, p = .30), or the left and right TLE groups (z = 1.38, p = .15).
Table 4.
Number of Patients and Controls With Impairment on at Least One of the Three SRT Scores and Number With Impairment on the 24-hr Delay Trial Only
| Group
|
||||||
|---|---|---|---|---|---|---|
| Control (n = 49)
|
Right TLE (n = 20)
|
Left TLE (n = 22)
|
||||
| Test | n | % | n | % | n | % |
| Auditory SRT | ||||||
| Total impaired on at least one variable | 16 | 33 | 12 | 60 | 17 | 77 |
| Impaired at 24-hr savings only | 2 | 4 | 0 | 0 | 2 | 9 |
| Visual SRT | ||||||
| Total impaired on at least one variable | 19 | 39 | 14 | 70 | 13 | 59 |
| Impaired at 24-hr savings only | 2 | 4 | 0 | 0 | 0 | 0 |
Note. TLE = temporal lobe epilepsy; SRT = selective reminding-test.
Correlation between auditory SRT scores and seizures.
A total of 4 patients, 2 with right TLE and 2 with left TLE, experienced a seizure during the 24-hr delay. One of these left TLE patients showed an isolated auditory SRT deficit at the 24-hr delay. There was no significant correlation between presence or absence of a seizure and 24-hr delayed memory on the auditory SRT for either group
Visual SRT
Group comparisons.
A MANOVA, with the three visual SRT trials as the dependent variables, showed that there was a significant overall group difference, Wilks’s λ(6, 172) = 0.69, p < .01, partial η2 = .17. Pairwise comparison tests showed that the control group differed significantly from the left TLE patients on each of the three visual SRT variables (Trial 6: mean difference = 2.80, SE = 0.56, p < .01; 30-min delay: mean difference = 2.90, SE = 0.52, p < .01; 24-hr delay: mean difference = 2.73, SE = 0.64, p < .01). The control group also differed from the right TLE group on each of the three visual SRT variables (Trial 6: mean difference = 2.03, SE = 0.58, p < .01; 30-min delay: mean difference = 1.47, SE = 0.54, p < .05; 24-hr delay: mean difference = 1.93, SE = 0.66, p < .01). Scores for the left and right TLE groups did not differ significantly on any of the three trials, although there was a trend for the left TLE group to perform worse at the 30-min trial compared with the right TLE group (30-min delay: mean difference = 1.42, SE = 0.63, p = .08; see Table 2 and Figure 2).
Figure 2.
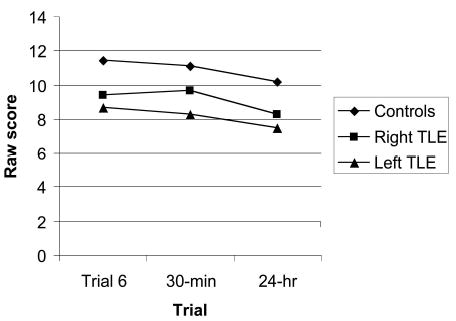
Visual selective reminding test scores by trial and group. TLE = temporal lobe epilepsy.
A second MANOVA tested whether the groups differed in rate of information loss across the two visual SRT delay trials (see Table 3). This MANOVA indicated that there was no significant difference between the two groups in rate of information loss across the two delays, Wilks’s λ(4, 174) = 0.96, p = .40, partial η2 = .02.
Finally, we examined the within-group rate of information loss across the two delay trials. Paired samples t tests indicated that none of the three groups showed a significant difference in rate of information lost per hour over the 30-min delay compared with the later delay, although the controls showed a trend to lose information faster from Trial 6 to the 30-min delay, as compared with the loss between the 30-min and the 24-hr delay: controls, t(48) = − 1.87, p = .07; left TLE group, t(21) = − 1.19, p = .25; and right TLE group, t(18) = 1.13, p = .28.
Analysis of the visual SRT at the individual level.
Table 4 shows the percentage of patients identified as memory impaired (less than or equal to − 1.00 standard deviation from control mean) on the visual SRT variables. As is evident from the table, 39% of the controls, 70% of the right TLE group, and 59% of the left TLE group were impaired on at least one of the visual SRT variables. Table 4 also shows that 2 controls (4%) but no patients in either group had normal Trial 6 and 30-min delay scores but impaired 24-hr recall on the visual SRT. For tests of the significance of the difference between group proportions, all ps ≥ .30.
Correlation between visual SRT scores and seizures.
There was no significant correlation between presence or absence of a seizure and 24-hr delayed memory on the visual SRT for either TLE group.
Discussion
In this study, groups of left and right TLE patients and controls were assessed on auditory and visual SRTs. This memory evaluation included the traditional immediate and 30-min delay trials as well as a 24-hr delay trial. The left TLE group performed worse than controls on all three test variables from both the auditory and the visual SRTs. The right TLE group performed worse than the controls on Trial 6 from the auditory SRT and on all three variables from the visual SRT. The two TLE groups did not differ from each other on any of the SRT variables. There were no between-groups differences in rate of information loss at the 30-min or 24-hr delays. Within each group, the rate of information loss between Trial 6 and the 30-min delay did not differ from the rate of loss between the 30-min and 24-hr delays for any of the groups.
To determine whether a sizable subset of the TLE groups might have demonstrated isolated memory impairment at the 24-hr delay, we conducted analyses at the individual level. These demonstrated that isolated memory impairment at the 24-hr delay was quite rare in all three groups on both the auditory and the visual SRT.
We do not consider lack of statistical power to be a major issue in this study. For example, considering the left TLE versus control group comparison for the auditory SRT at the 30-min delay, there was a difference of 2.00 in the mean rate of information lost per hour, with a standard deviation of approximately 4.00 for each group. At power = .80 and p = .05, 63 controls and 63 left TLE patients would be required to reach significance. Sample sizes this large are rarely reported in the TLE literature and would be difficult to acquire given that all participants have to be contacted 24 hr after the initial memory assessment. In addition, it could be argued that an effect that requires sample sizes of that magnitude might not have important clinical significance. Moreover, when one group has 5% of cases with impairment and the other has 10%, which is similar to the actual difference between the control and left TLE groups in this study with regard to isolated memory impairment at the 24-hr delay on the auditory SRT, 435 controls and 435 patients are required to detect a statistically significant group difference. This calculation supports the observation that intact memory up to the point of a 30-min delay but impaired memory after a 24-hr delay is rare in patients with TLE.
Our findings differ from those of Martin et al. (1991) and Blake et al. (2000), who reported accelerated forgetting in TLE groups compared with controls. This inconsistency in findings might be explained in part by the different testing procedures used and the patients assessed. For example, Martin et al. included some patients who had undergone anterior temporal lobectomy.
Blake et al. (2000) reported finding no relation between seizure frequency, as estimated by their patients, and forgetting over the course of 8 weeks. This is consistent with another study that found no association between the occurrence of partial seizures and forgetting over 48 hr (Bergin, Thompson, Fish, & Shorvon, 1995). However, Jokeit, Daamen, Zang, Janszky, and Ebner (2001) reported that auditory recall after 24 hr was related significantly to the occurrence of seizures during that interval in left TLE patients.
In the present study, there was no significant correlation at the group level between presence of a seizure during the 24-hr delay and memory performance after that delay for the auditory or visual SRT, but only 4 patients experienced a seizure during that delay. At the individual level, 1 of the 4 TLE patients who experienced a seizure during the 24-hr delay showed an isolated memory deficit at 24 hr on the auditory SRT. Because of the rarity of seizures during the 24-hr delay in this study, our results cannot provide definitive data about the relation between seizure activity and long-term consolidation of information. However, the data do suggest that, in the absence of overt seizure activity, accelerated memory loss is not common among individuals with TLE within a 24-hr period.
Studies of retrograde amnesia have suggested that the consolidation of memory as mediated by the mesial temporal lobe is a gradual process that may continue for years in humans (Kopelman, 2002; Squire & Alvarez, 1995). This period of consolidation appears to involve multiple regions, mechanisms, and time courses (De Renzi & Lucchelli, 1993; McGaugh, 2000). Additional examination of forgetting on clinical memory tests over relatively lengthy delays could advance understanding of the way TLE might sometimes interfere with the consolidation process. Prospective analysis of memory for everyday events (e.g., Schmolck, Buffalo, & Squire, 2000) also could prove fruitful if applied to this group. However, the primary deficit of amnesic patients has been described as a problem in the initial acquisition of information, with retention being a more subtle deficit (Kopelman, 2002), and this hypothesis might also apply to the memory dysfunction of TLE patients (Wilde et al., 2003).
It is possible that assessment of retention after a longer interval might have revealed substantial forgetting among the patients in this study who were not impaired relative to controls on any of the memory trials administered. This possibility deserves exploration in future investigations. In a study by Helmstaeder, Hauff, and Elger (1998), TLE patients and controls underwent assessment of memory for a word list after 30 min and after 1 week. It is notable that the patients and controls differed significantly in memory performance at all three evaluation points. The authors did not analyze whether there was an interaction effect between time and group. At the 1-week assessment, recall for aspects of the test-taking situation, namely the materials, procedures, and purposes of the tests, also was assessed. A multiple regression analysis showed that, when the 1-week delay word list scores were excluded from the regression, 30-min delay scores were a significant predictor of memory for the testing environment after the 1-week delay. This finding suggests that results from the conventional 30-min delay had ecological validity.
In conclusion, the data suggest that accelerated forgetting during the 24 hr after initial memory testing is relatively rare among TLE patients, at least in the context of the methods and procedures used in this study. It appears that memory impairment in this group can be identified by conventional memory tests. Assessment over a longer interval is necessary to confirm this finding. When a patient emphatically complains of impaired memory in everyday life but performs normally on standard memory measures, assessment after a longer delay might prove informative, but control data should be acquired for comparison, and mood disturbance should be considered as a possible basis for such complaints (Elixhauser, Kline Leidy, Meador, Means, & Willian, 1999; Vermuelen, Aldenkamp, & Alpherts, 1993).
Acknowledgments
This study was supported by National Institutes of Health Grants NS 37738, NS 42251, and MO1 RR03186 (General Clinical Research Center). We thank Jana Jones, Paul Rutecki, Raj Sheth, and Lauren Koss Hensley, a University of Wisconsin—Madison undergraduate research scholar, for their invaluable assistance on this project.
References
- Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW. Image processing for the study of brain structure and function: Problems and programs. Journal of Neuropsychiatry and Clinical Neuroscience. 1992;4:125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, Van Paesschen W, Thompson PJ, Duncan JS, Harkness WF, Shorvon SD. Hippocampal cell loss and gliosis: Relationship to preoperative and postoperative memory function. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1998;11:12–21. [PubMed] [Google Scholar]
- Bergin PS, Thompson PJ, Fish DR, Shorvon SD. The effect of seizures on memory for recently learned material. Neurology. 1995;45:236–240. doi: 10.1212/wnl.45.2.236. [DOI] [PubMed] [Google Scholar]
- Blake R, Wroe S, Breen E, McCarthy R. Accelerated forgetting in patients with epilepsy: Evidence for an impairment in memory consolidation. Brain. 2000;123:472–483. doi: 10.1093/brain/123.3.472. [DOI] [PubMed] [Google Scholar]
- Bruning, J. L., & Kintz, B. L. (1977). Computational handbook of statistics (2nd ed.). Glenview, IL: Scott Foresman.
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Auditory Learning and Auditory Behavior. 1973;12:543–550. [Google Scholar]
- Chelune GJ, Naugle R, Luders H, Awad I. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Lucchelli F. Dense retrograde amnesia, intact learning capability and abnormal forgetting rate: A consolidation deficit? Cortex. 1993;29:449–466. doi: 10.1016/s0010-9452(13)80253-5. [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Kline Leidy N, Meador K, Means E, Willian MK. The relationship between memory performance, perceived cognitive function, and mood in patients with epilepsy. Epilepsy Research. 1999;37:13–24. doi: 10.1016/s0920-1211(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Engel, J., Jr., Williamson, P. D., & Wieser, H.-G. (1997). Mesial temporal lobe epilepsy. In J. Engel, Jr., & T. A. Pedley (Eds.), Epilepsy: A comprehensive textbook (pp. 2417–2425). Philadelphia: Lippincott-Raven.
- Heaton, R. K., Grant, I., & Matthews, C. G. (1991). Comprehensive norms for an expanded Halstead-Reitan battery. Odessa, FL: Psychological Assessment Resources.
- Heaton, R. K., Taylor, M. J., & Manly, J. (2003). Demographic effects and use of demographically corrected norms with the WAIS–III and WMS–III. In D. S. Tulsky, D. H. Saklofske, G. J. Chelune, R. K. Heaton, R. J. Ivnik, R. Bornstein, et al. (Eds.), Clinical interpretation of the WAIS–III and WMS–III (pp. 181–210). New York: Academic Press.
- Helmstaedter C, Hauff M, Elger CE. Ecological validity of list-learning tests and self-reported memory in healthy individuals and those with temporal lobe epilepsy. Journal of Clinical and Experimental Neuropsychology. 1998;20:365–375. doi: 10.1076/jcen.20.3.365.824. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Isaac CL, Gong Q, Roberts N. Differential involvement of the hippocampus and temporal lobe cortices in rapid and slow learning of new semantic information. Neuropsychologia. 2002;40:748–768. doi: 10.1016/s0028-3932(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Daamen M, Zang H, Janszky J, Ebner A. Seizures accelerate forgetting in patients with left-sided temporal lobe epilepsy. Neurology. 2001;57:125–126. doi: 10.1212/wnl.57.1.125. [DOI] [PubMed] [Google Scholar]
- Kalviainen R, Partanen K, Aikia M, Mervaala E, Vainio P, Riekkinen PJ, Pitkanen A. MRI-based hippocampal volumetry and T2 relaxometry: Correlation to auditory memory performance in newly diagnosed epilepsy patients with left-sided temporal lobe focus. Neurology. 1997;48:286–287. doi: 10.1212/wnl.48.1.286. [DOI] [PubMed] [Google Scholar]
- Kapur N, Millar J, Colbourn C, Abbott P, Kennedy P, Docherty T. Very long-term amnesia in association with temporal lobe epilepsy: Evidence for multiple-stage consolidation processes. Brain and Cognition. 1997;35:58–70. doi: 10.1006/brcg.1997.0927. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Kubota F, Hattori S, Oya N, Mikuni M. A study of the relationship between metabolism using H-MRS and function using several neuropsychological tests in temporal lobe epilepsy. Seizure. 2001;10:188–193. doi: 10.1053/seiz.2000.0498. [DOI] [PubMed] [Google Scholar]
- Kilpatrick C, Murrie V, Cook M, Andrewes D, Desmond P, Hopper J. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure. 1997;6:213–218. doi: 10.1016/s1059-1311(97)80008-8. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Lencz T, McCarthy G, Bronen R, Scott T, Inserni J, Sass K, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: Relationship to neuropathology and neuropsychological function. Annals of Neurology. 1992;31:629–637. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- Loring DW, Murro AM, Meador KJ, Lee GP, Gratton CA, Nichols ME, et al. Wada memory testing and hippocampal volume measurements in the evaluation for temporal lobectomy. Neurology. 1993;43:1789–1793. doi: 10.1212/wnl.43.9.1789. [DOI] [PubMed] [Google Scholar]
- Martin RC, Loring D, Meador K, Lee G, Thrash N, Arena J. Impaired long-term retention despite normal auditory learning in patients with temporal lobe dysfunction. Neuropsychology. 1991;5:3–12. [Google Scholar]
- Martin RC, Sawrie S, Hugg J, Gilliam F, Faught E, Kuzniecky R. Cognitive correlates of 1H MRSI-detected hippocampal abnormalities in temporal lobe epilepsy. Neurology. 1999;53:2052–2058. doi: 10.1212/wnl.53.9.2052. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. January 14). Memory—a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- O’Connor M, Sieggreen MA, Ahern G, Schomer D, Mesulam M. Accelerated forgetting in association with temporal lobe epilepsy and paraneoplastic encephalitis. Brain and Cognition. 1997;35:71–84. doi: 10.1006/brcg.1997.0928. [DOI] [PubMed] [Google Scholar]
- Pantel J, O’Leary D, Cretsinger K, Bockholt H, Keefe H, Magnotta V, Andreasen N. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus. 2000;10:752–758. doi: 10.1002/1098-1063(2000)10:6<752::AID-HIPO1012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pilgrim B, Meyers J, Bayless J, Whetstone M. Validity of the Ward seven-subtest WAIS–III short form in a neuropsychological population. Applied Neuropsychology. 2000;6:243–246. doi: 10.1207/s15324826an0604_7. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. (2002). WAIS–III/WMS–III technical manual (updated). San Antonio, TX: Author.
- Rausch R, Babb T. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Archives of Neurology. 1993;50:812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- Sass K, Spencer M, Kim J, Westerveld M, Novelly R, Lencz B. Auditory memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40:1694–1697. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- Sawrie S, Martin R, Gilliam F, Faught RE, Maton B, Hug J, et al. Visual confrontation naming and hippocampal function: A neural network study using quantitative 1H magnetic resonance spectroscopy. Brain. 2000;123:770–780. doi: 10.1093/brain/123.4.770. [DOI] [PubMed] [Google Scholar]
- Sawrie SM, Martin RC, Knowlton R, Faught E, Gilliam F, Kuzniecky R. Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy, and auditory memory in temporal lobe epilepsy. Epilepsia. 2001;42:1403–1407. doi: 10.1046/j.1528-1157.2001.018301.x. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Buffalo EA, Squire LR. Memory distortions develop over time: Recollections of the O. J. Simpson trial verdict after 15 and 32 months. Psychological Science. 2000;11:39–45. doi: 10.1111/1467-9280.00212. [DOI] [PubMed] [Google Scholar]
- Squire L, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Cascino GD, Sharbrough FW, Ivnik RJ. Gender differences in post-temporal lobectomy auditory memory and relationships between hippocampal volumes and preoperative auditory memory. Epilepsy Research. 1995;20:69–76. doi: 10.1016/0920-1211(94)00060-a. [DOI] [PubMed] [Google Scholar]
- Vermuelen J, Aldenkamp AP, Alpherts WC. Memory complaints in epilepsy: Correlations with cognitive performance and neuroticism. Epilepsy Research. 1993;15:157–170. doi: 10.1016/0920-1211(93)90096-p. [DOI] [PubMed] [Google Scholar]
- Wilde NJ, Strauss E, Chelune GJ, Hermann BP, Hunter M, Loring DW, et al. Confirmatory factor analysis of the WMS–III in patients with temporal lobe epilepsy. Psychological Assessment. 2003;15:56–63. doi: 10.1037/1040-3590.15.1.56. [DOI] [PubMed] [Google Scholar]
- Wilde N, Strauss E, Chelune G, Loring DW, Martin RC, Hermann B, et al. WMS–III performance in patients with temporal lobe epilepsy: Group differences and individual classification. Journal of the International Neuropsychological Society. 2001;7:881–891. [PubMed] [Google Scholar]
- Wood AG, Saling MM, O’Shea MF, Berkovic SF, Jackson GD. Components of auditory learning and hippocampal damage assessed by T2 relaxometry. Journal of the International Neuropsychological Society. 2000;6:529–538. doi: 10.1017/s1355617700655029. [DOI] [PubMed] [Google Scholar]


