Abstract
Previous studies have established that deactivation of the superior colliculus severely retards the normally rapid learning of pattern discriminations in the mature cat. The purpose of this study was to test the hypothesis that the midbrain plays an important role in the learning of simple pattern discriminations and that the contribution of this pathway is to the perception of global, rather than local, features of a figure. To answer this question, pattern discrimination learning was studied in three intact cats and in three experimental cats during bilateral reversible deactivation of the superficial layers of the superior colliculus (SC). The animals concurrently learned to discriminate three pairs of compound visual patterns composed of small (local element) Ts or 7s. Congruent and incongruent stimulus pairs were large (global) Ts vs. 7s comprising the same or different local elements, respectively. The random stimulus pair consisted of randomly scattered local elements (Ts vs. 7s). The animals were trained to respond to the large Ts in the congruent and incongruent pairs and the small Ts in the random pair. In the normal cats, learning of the random pair was much slower than the learning of the congruent and incongruent pairs. This finding demonstrated the theory of global precedence, because the animals learned the global features of the congruent and incongruent pairs much more quickly than the local features of the random pair. In contrast, during bilateral deactivation of the superficial layers of the SC, the learning of the incongruent pair was significantly retarded and took longer to learn than the random pair. Congruent and random pair learning rates were unchanged. The specific deficit in learning the incongruent pair indicates that the learning of global, but not local, elements of the visual pattern is impaired during deactivation of the SC. The unimpaired use of local features permitted the animals to learn the congruent and random pairs at normal rates. Therefore, deactivation of the superficial layers of the SC during pattern discrimination learning reverses the precedence for global visual features that is typical of normal learning.
Keywords: concurrent learning‖global precedence‖feature detection‖form discrimination‖cat
In the study of visual perception, the theory of global precedence states that global features of a figure are processed before its local features (1, 2). It has been suggested that this precedence is not one of prior processing, but of attention to one feature or another (3). More recent studies have proposed that local and global feature detection may be separate processes, mediated by different visual pathways, and thereby be dissociable (4). The two principal pathways, or information streams, for visual signals are the geniculate pathway, mediated by the lateral geniculate nucleus, and the midbrain pathway, mediated by the superior colliculus (SC). Early proposals concerning the functions of these two pathways suggested that the geniculate pathway subserves visual discrimination, whereas the midbrain pathway is responsible for visual localization (5). This hypothesis was later modified when it was discovered that deactivations of the cat SC also result in pattern discrimination learning deficits (6–8). The revised hypothesis suggests that the midbrain pathway provides the first stage of simple, coarse pattern perception (9).
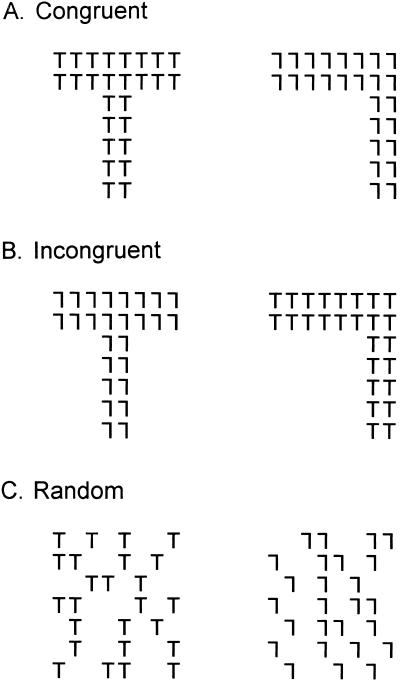
The purpose of this study was to test the hypothesis that the midbrain plays an important role in the learning of simple pattern discriminations and that the contribution of this pathway is to the perception of global, rather than local, features of a figure. To accomplish this test, we examined the ability of mature cats to learn three pattern discriminations concurrently (Fig. 1) while the superficial layers of the SC were deactivated by cooling. We used small Ts and 7s, the local elements, to assemble global Ts and 7s. The global Ts and 7s were either made of the same or different local elements to create congruent or incongruent discrimination pairs, respectively. The cats learned to classify the patterns as follows. For the congruent pair, the animal was rewarded for responding to the T, large or small. For the incongruent pair, the animal was rewarded for responding to the large T. Responses to the small Ts, which formed the large 7, were incorrect, and were used to detect the potential failure to perceive the global figure while sparing perception of the local element. The third pair consisted of randomly scattered small Ts in one figure and small 7s in the other, which lacked any global form. The animals were rewarded for responding to the small Ts. This discrimination was used to test the animal's ability to respond to the small Ts, when they could not discern a global pattern.
Figure 1.
The three discriminations that were concurrently learned. (A) Congruent pair in which both the local and global cues matched. (B) Incongruent pair in which the local and global cues did not match. (C) Random stimulus that consists of only the local cues with no global pattern. Note that these random discriminanda are only an example as the distribution of the local elements changed on each trial. For each stimulus pair, responses to the stimuli in the left column were rewarded. Stimuli were never paired with stimuli of another class. This task was developed originally by Horel (4).
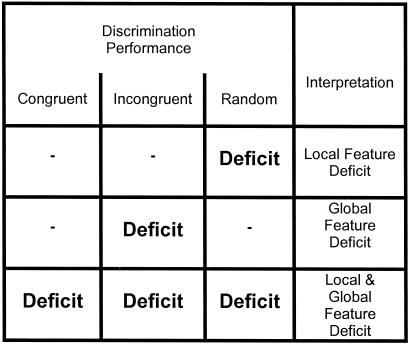
Examination of the learning rates of these three stimulus pairs permits three different perceptual consequences to be determined. (i) If deactivation of the SC disrupts the perception of the global, but not the local features, the animals should respond to the small-7 elements that form the large T of the incongruent pair and make more errors in learning this discrimination (Fig. 2). (ii) On the other hand, if deactivation of the SC disrupts perception of local, but not global features, then learning of the randomly distributed Ts and 7s should be deleteriously affected, whereas learning rates for the congruent and incongruent discrimination pairs would not change (Fig. 2). (iii) Finally, if deactivation of the SC affects the perception of all figures, regardless of size (local and global), learning of all discrimination pairs would be affected (Fig. 2).
Figure 2.
Chart to summarize how specific deficits on the three discrimination pairs (congruent, incongruent, and random) are interpreted in terms of global and local feature processing.
Methods
Subjects.
Six adult domestic cats were tested in this study. The animals were obtained from a U.S. Department of Agriculture-licensed commercial laboratory animal breeding facility (Liberty Laboratories, Waverly, NY). Three of the cats served as intact controls, and the other three cats had bilateral cooling loops placed over the dorsal surface of the superior colliculi. The cats were housed in an enriched-environment colony room with free access to water. Caloric intake was regulated to the testing sessions and to 1 h at the end of each day, when the animals had dry cat food ad libitum. All treatments met or exceeded the guidelines published in the National Research Council's Guide for the Care and Use of Laboratory Animals (1986) and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Dallas.
Experimental Apparatus.
A two-choice Pennsylvania General Testing Apparatus was used, which consisted of a gray start box (25 × 45 cm) that opened into a gray decision area that was 25 cm deep. The start box was separated from the decision area by two doors; one was gray and opaque, and one was transparent Plexiglas. The decision area led to two white goal compartments (30 × 40 cm) that were separated by a white opaque wall. Discriminanda were displayed on 17-cm-diagonal black-and-white video monitors. For more details and an illustration, see figure 2 of Lomber et al. (10). Before the initiation of these experiments all six cats were conditioned to perform discriminations in the Pennsylvania General Testing Apparatus as described in Cornwell et al. (11, 12), had learned and recalled more than 100 pattern or object discriminations, and performed within the normal range.
Cryoloop Implantation.
During the 24 h before cryoloop implantation, the cats were fasted and received dexamethasone (1.0 mg/kg, i.m.). Eighteen hours before surgery, each cat was sedated with ketamine (20 mg/kg, i.m.), and an indwelling feline catheter was placed in the cephalic vein. On the day of surgery, all animals received additional dexamethasone and atropine (0.03 mg/kg, s.c.). Sodium pentobarbital (≈25 mg/kg to effect) was infused (i.v.) to induce general anesthesia. The cat was then installed in a stereotaxic apparatus and prepared for surgery by using antiseptic procedures. Heart and respiration rates and body temperature were monitored continuously.
Cooling loops fashioned from 23-gauge stainless steel hypodermic tubing (13) were shaped to conform to the dorsal surface of the SC. The SC cryoloops were circular with an inside-loop diameter of 2.5 mm. Attached to the union of each loop was a microthermocouple. Collicular cryoloops were implanted with standard procedures (13–15). The insulated shafts of the cryoloops were in contact intracranially with the bony tentorium and exited the cranial vault posteriorly. The cryoloops were fixed to the skull with bone screws and dental acrylic.
After cryoloop implantation, the dura mater was replaced and bone defects were repaired with bone and Gelfoam (Pharmacia, Peapack, NJ). Dermal incisions were repaired with silk sutures that were removed approximately 10 days later. During the initial period after awaking, the cats were also given buprenorphine analgesic (0.01 mg/kg, s.c.). Ambi-pen systemic antibiotic (300,000 units, i.m.; The Butler Company, Columbus, OH) was administered every 2 or 3 days for 1 week. In all cases, postsurgical recovery was uneventful.
Stimuli.
The stimuli were displayed on black-and-white video monitors and are shown in Fig. 1. The stimuli were black on a white background. Each of the small (local) letters was composed of a horizontal bar at the top with a vertical bar centered on the horizontal line (T) or displaced to the right (7). The bars were 0.2° wide when viewed from the decision line of the Pennsylvania General Testing Apparatus. The height of the vertical bars was 1.2°, and the width of the horizontal bar was 1.0°. The global figures (Fig. 1 A and B) were composed of small Ts and 7s as shown in Fig. 1. The thickness of the horizontal bars was 2.2° and that of the vertical bars was 3.0°. The width of the global horizontal bars was 9.5°, and the height of the vertical bars was 11.5°. The random figures (Fig. 1C) were created with the same local figures (Ts and 7s), but they were scattered randomly across a rectangular box that could enclose the global figures. The same number of local elements was used in both the global (Fig. 1 A and B) and random discrimination (Fig. 1C) pairs.
Behavioral Task.
The three control animals were trained concurrently to discriminate the three stimulus pairs (Fig. 1), which appeared in random order. The congruent pair consisted of a global T formed by small Ts and a global 7 formed by small 7s. The animals were rewarded for responding to the T, whether it was local or global. The incongruent pair consisted of a global T formed by small 7s and a global 7 formed by small Ts. The animals were rewarded for responding to the global T, but not for responding to the small Ts forming the global 7. In the random pair, the animals were rewarded for responding to the small Ts, but not the small 7s. In summary, if the animals could identify a global figure, they were rewarded for responding to a global T, regardless of the elements that made up the figure. If they could not identify a global figure, they were rewarded for responding to a small T.
The animals learned all three discriminations concurrently with each of three pairs appearing in random order, one pair at a time. The cats were rewarded with soft commercial cat food that was placed behind a raised lip above the video monitor that presented the rewarded stimulus. The animals were not allowed to correct an error and incorrect responses went unrewarded. During daily learning of the three discrimination pairs, the animals performed four blocks of 30 trials (120 trials per day). Each block consisted of a balanced, irregular sequence whereby each pair appeared 10 times and alternated between the left and right sides of the apparatus according to a Gellerman series (16). The animals were tested daily, 7 days a week. Concurrent learning of all three discrimination pairs continued until a criterion of 90% correct or better, on 2 consecutive days, was reached for each of the three pairs.
For the three experimental animals, the same training paradigm as described above was used, with one exception. Ten minutes before the initiation of each daily training session, the bilateral SC cryoloops were deactivated to a loop temperature of 10°C. Previous studies (17) have identified that cooling an SC cryoloop to 10°C deactivates the superficial layers of the SC [layers stratum zonale, stratum griseum superficiale, and stratum opticum of Kanaseki and Sprague (18)]. All discriminations were performed during bilateral deactivation of the superficial layers of the SC. After completion of daily training, the deactivation was terminated and the animal was returned to the colony.
Terminal Procedures.
At the conclusion of all behavioral work, each experimental animal received a systemic i.v. injection of [2-14C]deoxyglucose (2DG; 100 μCi/kg, i.v.) while both collicular cryoloops were cooled to 10°C. During administration of the 2DG, the animal was alert and viewed the regular activity of the laboratory while being maintained in a veterinary cat sack. Thirty minutes later, the cats were anesthetized with an overdose of sodium pentobarbital (50 mg/kg, i.v.) and perfused with fixatives in accord with the recommendations of the American Veterinary Medical Association Panel on Euthanasia (19). The fixed brain was exposed, photographed, frozen at −30°C, stored at −80°C, and cut into 23- to 26-μm sections in the coronal plane in a cryostat. Dried sections were apposed to x-ray film and processed by using routine procedures (20). The extent of the deactivated area was then determined by delineating the region of decreased 2DG uptake. Adjacent coronal sections were stained for Nissl substance or cytochrome oxidase to verify both the location of the cryoloop and absence of surgical or cooling-induced damage.
Results
Deactivation Extent.
Exposure and dissection of the fixed brain revealed that the cryoloops were in correct position and in contact with the dorsal surface of the SC. On histological examination, the contact points were marked by small depressions that indicated the locus of contact with the collicular surface. The same sections revealed that neither the surgical procedures to implant the cooling loops nor the cooling itself disturbed collicular structure.
In agreement with previous studies (17), examination of the autoradiograms from all three experimental cats confirmed low 2DG-uptake levels, and by extension low neural activity, in the stratum zonale, stratum griseum superficiale, and a major portion of stratum opticum. Little or no impact on 2DG uptake occurred in stratum griseum intermediale or profundum. Finally, the functional impact of the cooling was confined to the SC, with no evidence of any cooling spread to the inferior colliculus or cingulate cortex.
Controls.
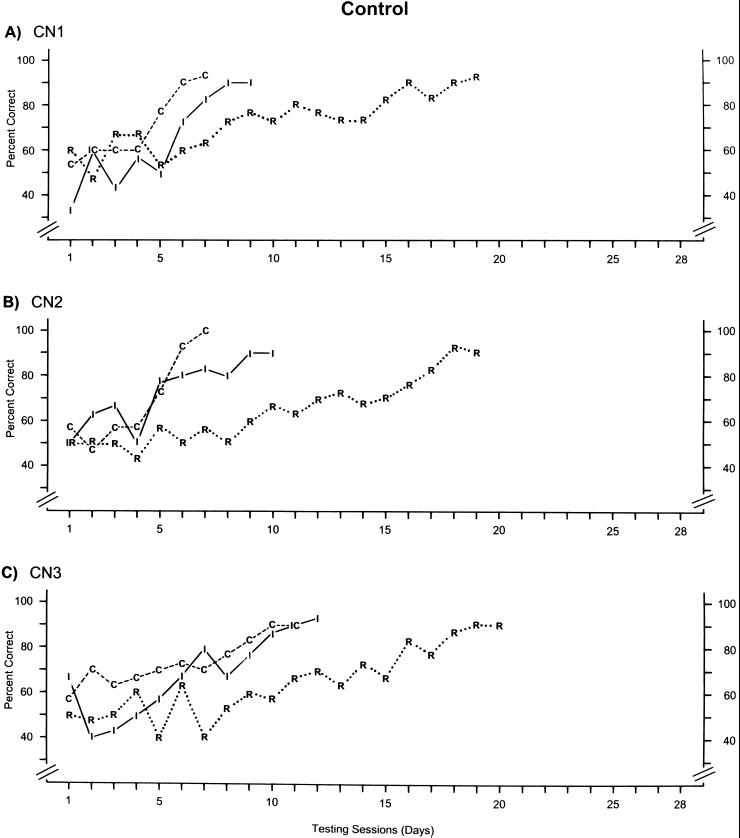
For the three control animals, the learning rates for each of the three concurrently learned discriminations are provided in Fig. 3. Overall, the learning curves for each of the three pairs are quite similar among the three animals. For the most part, performance levels for learning the congruent (C) and incongruent (I) pairs commingle until criterion was ultimately reached for the congruent pair just prior to the incongruent pair. For cats CN1 and CN2, criterion on the congruent pair was reached in seven testing sessions, and criterion was reached on the incongruent pair in 9 and 10 sessions, respectively. Cat CN3 took slightly longer, requiring 11 and 12 sessions to reach criterion on the congruent and incongruent pairs, respectively. However, every cat required nearly double the number of sessions to reach criterion on the random pair (R) compared with the congruent or incongruent pairs. Each cat needed 19 or 20 sessions to reach criterion. Therefore, it took the cats only half as long to learn the two discriminations that consisted of global patterns made up of local elements compared with the pairs consisting of randomly scattered local elements, thus demonstrating global precedence in the learning of visual forms in cats.
Figure 3.
Learning rates for each of the three discrimination pairs in each of the three control (CN) animals. Each data point shows the percent correct on that particular discrimination during each testing session (day). Performance on the congruent discrimination is indicated by the letter “C” and dashed lines. The letter “I” and solid lines indicate performance on the incongruent discrimination, and the letter “R” and dotted lines indicate performance on the random discrimination. For the sake of clarity, data from a specific discrimination pair are not shown after criterion (two consecutive days of ≥90% correct) was reached for that pair.
Superior Colliculus Deactivation.
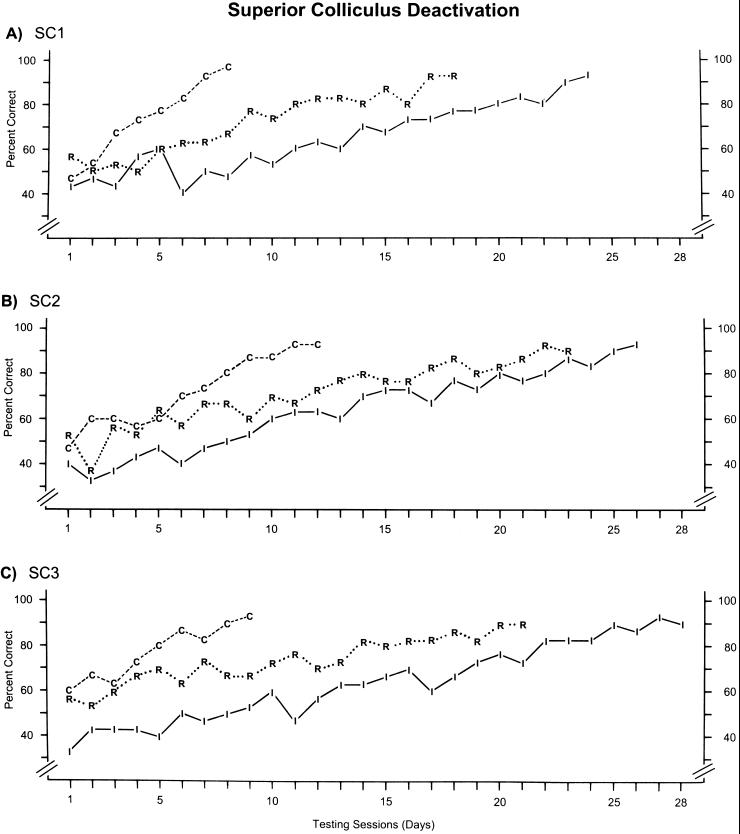
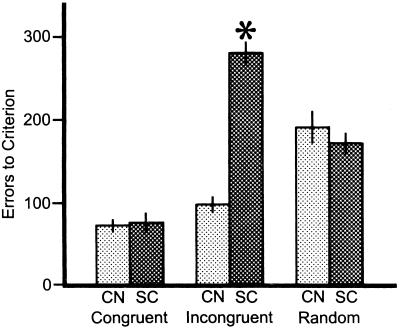
The learning curves for the three animals that concurrently learned the three discriminations while the SC was bilaterally deactivated with cooling are shown in Fig. 4. The most striking difference compared with the control animals was the greatly retarded learning of the incongruent pair. Learning of the incongruent pair took from 25 to 28 sessions during SC deactivation, compared with 9–12 sessions for the control cats. However, learning curves for the congruent and random pairs during SC deactivation (Fig. 4) were very similar to those of the control animals (Fig. 3). Statistically, these differences can be easily identified when errors to criterion are examined (Fig. 5). For the congruent pairs, no difference occurred in performance for the control and SC deactivation cats, with mean errors to criterion of 71.3 ± 7.9 and 74.7 ± 12.7 (x̄ ± SE), respectively. Similarly, the errors to criterion for the random pairs of the control (191.0 ± 18.2) and SC deactivation (171.0 ± 13.1) cats did not differ. However, the number of errors made by the cats during SC deactivation on the incongruent pair was increased by nearly 200% (280.3 ± 13.6), compared with the control cats (97.3 ± 9.8). This result was significant (P < 0.001).
Figure 4.
Learning rates for each of the three discrimination pairs (congruent, incongruent, and random) for each of the three experimental animals (SC) during bilateral deactivation of the superior colliculi. For conventions, see Fig. 3.
Figure 5.
The mean number of errors to criterion for each of the three discrimination classes (congruent, incongruent, and random) for the three control (CN) cats and the three animals that that the superior colliculus (SC) deactivated during concurrent learning of the three discriminations. Student's t tests were used to compare the control group with the SC deactivation group. Error bars indicate one SEM.
The specific deficit in learning the incongruent pair indicates that the learning of global, but not local, elements of the visual pattern is impaired during deactivation of the SC. The unimpaired use of local features permitted the animals to learn the congruent and random pairs at normal rates. Therefore, deactivation of the superficial layers of the SC during pattern discrimination learning reverses the precedence for global visual features that was identified in the control cats.
Discussion
Global Precedence.
The theory of global precedence states that when visual patterns present information on both a local and global spatial scale, subjects respond more quickly to the global cues than the local cues. As seen in the control cats, this study supports global precedence theory. Specifically, learning the global features of the congruent and incongruent discrimination pairs was much more rapid than the learning of the local features of the random discrimination pair. In accord with earlier studies on the cat (21), these results parallel those in humans (1, 2, 22, 23) and monkeys (4, 24) and are in agreement with the theory that global pattern cues dominate local cues. In contrast, during bilateral deactivation of the superficial layers of the SC, the learning of the incongruent pair was significantly slowed relative to the learning rates of control cats. The specific deficit in learning the incongruent pair indicates that the learning of global, but not local, elements of the visual pattern is impaired during deactivation of the SC. This result is buttressed by the fact that all three experimental cats started well below chance on the incongruent pair (Fig. 4), indicating that the new dominance of the local cues interfered with the global cues. Furthermore, the control cats demonstrated much less interference from the local cues on the incongruent pair (Fig. 3), thus supporting the conclusion that cats respond preferentially to the global cues. Deactivation of the superficial layers of the SC during pattern discrimination learning therefore reverses the precedence for global visual features that is typical of normal learning.
Attention or Perception?
Although the global precedence deficit during SC deactivation is quite robust, several possible interpretations exist. One interpretation is that the deficit could be based on altered attention to the stimuli. Investigators of local and global visual perception have been at loggerheads as to whether global precedence is an attentional or perceptual effect (see refs. 1, 2, and 23 as examples). Our own work has identified that, in the cat, an impairment exists in visual attention to the most peripheral positions during the bilateral deactivation of the SC, as assessed with visual-orienting responses to moving stimuli (15, 17). However, attention to positions within the central 120° of the visual field is not impaired and responses are quantitatively and qualitatively no different from normal. Considering that the testing apparatus is a relatively confined space, it is doubtful that the animal uses regions outside its central 120° to make the discriminations. We expect that this lack of attention to visual stimuli in the periphery during bilateral SC deactivation does not produce the global-processing impairment. Therefore, the reversal of global precedence during bilateral SC deactivation is unlikely to be an attentional deficit.
The second interpretation of the reversal of global precedence during bilateral cooling of the SC is perceptual. This view is supported by the types of visual signals that are transmitted through the midbrain, en route to the extrageniculate thalamus and extrastriate cortex. Alpha and gamma-type retinal ganglion cells provide the dominant, if not the only, retinal input to the superficial layers of the SC (25–29). Neither of these types of ganglion cells is responsible for high-acuity vision, which would seem to be essential for the discrimination of local visual features (30). Higher-acuity vision is associated with beta ganglion cells (31, 32), which project exclusively to the lateral geniculate nucleus (27, 28). Therefore, the anatomical and physiological characteristics of the midbrain pathway support the finding that global, but not local, feature processing is disrupted by bilateral deactivation of the SC.
Another interpretation of this deficit, which also concerns perceptual processing, views the early stages of visual processing as comprising a set of filtering operations, whereby the image passes through a set of channels or filters that are sensitive to different spatial frequency bands (33–35). The results presented here suggest that the global features would be detectable by low-frequency channels while the local features would be detectable by only medium- or high-frequency channels. Although the anatomical locations of these channels is rarely addressed, it is generally assumed that the full spectrum of channels are present in the visual cortex, rather than being spread out over a number of separate pathways or anatomical regions. The fact that deactivation of the superior colliculus seems to only affect low spatial frequency channels would explain the deficits in global processing and suggests that at least some of these channels are located within the superior colliculus.
Acknowledgments
I thank Bertram Payne for surgical assistance and James Horel, Alice O'Toole, and two anonymous reviewers for very helpful comments on this manuscript. This work was supported by the National Science Foundation.
Abbreviations
- SC
superior colliculus
- 2DG
[2-14C]deoxyglucose
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Navon D. Cognit Psychol. 1977;9:153–183. [Google Scholar]
- 2.Hughes H C, Layton W M, Baird J C, Lester L S. Percept Psychophys. 1984;35:361–371. doi: 10.3758/bf03206340. [DOI] [PubMed] [Google Scholar]
- 3.Kinchla R, Solis-Marcias V, Hoffman J. Percept Psychophys. 1983;33:1–10. doi: 10.3758/bf03205860. [DOI] [PubMed] [Google Scholar]
- 4.Horel J A. Behav Brain Res. 1994;65:157–164. doi: 10.1016/0166-4328(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 5.Schneider G E. Science. 1969;163:895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- 6.Sprague J M, Berlucchi G, DiBerardino A. Brain Behav Evol. 1970;3:285–294. doi: 10.1159/000125478. [DOI] [PubMed] [Google Scholar]
- 7.Berlucchi G, Sprague J M, Levy J, DiBerardino A C. J Comp Physiol Psychol. 1972;78:123–172. doi: 10.1037/h0032172. [DOI] [PubMed] [Google Scholar]
- 8.Tunkl J E, Berkley M A. J Comp Neurol. 1977;176:575–588. doi: 10.1002/cne.901760408. [DOI] [PubMed] [Google Scholar]
- 9.Sprague J M, Levy J, DiBerardino A, Berlucchi G. J Comp Neurol. 1977;172:441–488. doi: 10.1002/cne.901720305. [DOI] [PubMed] [Google Scholar]
- 10.Lomber S G, Payne B R, Cornwell P, Long K D. Cereb Cortex. 1996;6:673–695. doi: 10.1093/cercor/6.5.673. [DOI] [PubMed] [Google Scholar]
- 11.Cornwell P, Herbein S, Corso C, Eskew R, Warren J M, Payne B R. Behav Neurosci. 1998;103:1176–1190. doi: 10.1037//0735-7044.103.6.1176. [DOI] [PubMed] [Google Scholar]
- 12.Cornwell P, Nudo R J, Straussfogel D, Lomber S G, Payne B R. Behav Neurosci. 1998;112:800–811. doi: 10.1037//0735-7044.112.4.800. [DOI] [PubMed] [Google Scholar]
- 13.Lomber S G, Payne B R, Horel J A. J Neurosci Methods. 1999;86:179–194. doi: 10.1016/s0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- 14.Keating E G, Gooley S G. Brain Res. 1988;438:247–255. doi: 10.1016/0006-8993(88)91343-1. [DOI] [PubMed] [Google Scholar]
- 15.Lomber S G, Payne B R. Visual Neurosci. 1996;13:1143–1156. doi: 10.1017/s0952523800007781. [DOI] [PubMed] [Google Scholar]
- 16.Fellows B J. Psychol Bull. 1967;57:87–92. doi: 10.1037/h0024098. [DOI] [PubMed] [Google Scholar]
- 17.Lomber S G, Payne B R, Cornwell P. J Comp Neurol. 2001;441:44–57. doi: 10.1002/cne.1396. [DOI] [PubMed] [Google Scholar]
- 18.Kanaseki T, Sprague J M. J Comp Neurol. 1974;158:319–338. doi: 10.1002/cne.901580307. [DOI] [PubMed] [Google Scholar]
- 19.Beaver B V, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett B T, Pascoe P, Shull E, Cork L C, et al. J Am Vet Med Assoc. 2001;218:669–696. [Google Scholar]
- 20.Payne B R, Lomber S G. J Neurosci Methods. 1999;86:195–208. doi: 10.1016/s0165-0270(98)00166-6. [DOI] [PubMed] [Google Scholar]
- 21.Hughes H C, Sprague J M. Exp Brain Res. 1986;61:332–354. doi: 10.1007/BF00239523. [DOI] [PubMed] [Google Scholar]
- 22.Navon D. Perception. 1983;12:239–254. doi: 10.1068/p120239. [DOI] [PubMed] [Google Scholar]
- 23.Miller J. J Exp Psychol Human Percept Perform. 1981;9:353–383. [Google Scholar]
- 24.Tanaka H, Fujita I. NeuroReport. 2000;11:2881–2884. doi: 10.1097/00001756-200009110-00010. [DOI] [PubMed] [Google Scholar]
- 25.Graybiel A M. Brain Res. 1975;96:1–23. doi: 10.1016/0006-8993(75)90566-1. [DOI] [PubMed] [Google Scholar]
- 26.Harting J K, Guillery R W. J Comp Neurol. 1976;166:133–144. doi: 10.1002/cne.901660202. [DOI] [PubMed] [Google Scholar]
- 27.Illing R B, Wässle H. J Comp Neurol. 1981;202:265–285. doi: 10.1002/cne.902020211. [DOI] [PubMed] [Google Scholar]
- 28.Leventhal A G, Rodieck R W, Dreher B. J Comp Neurol. 1985;237:216–226. doi: 10.1002/cne.902370206. [DOI] [PubMed] [Google Scholar]
- 29.Berson D M. Prog Brain Res. 1988;75:17–26. doi: 10.1016/s0079-6123(08)60462-8. [DOI] [PubMed] [Google Scholar]
- 30.Sherman S M. In: Progress in Psychobiology and Physiological Psychology. Sprague J M, Epstein A N, editors. Vol. 11. New York: Academic; 1985. pp. 233–314. [Google Scholar]
- 31.Berkley M A, Sprague J M. J Comp Neurol. 1979;187:679–702. doi: 10.1002/cne.901870404. [DOI] [PubMed] [Google Scholar]
- 32.Orban G A. Neuronal Operations in Visual Cortex. New York: Springer; 1984. [Google Scholar]
- 33.Wilson H R. In: Spatial Vision. Regan D, editor. London: MacMillan; 1991. pp. 64–86. [Google Scholar]
- 34.Wilson H R, Wilkinson F. Vision Res. 1998;38:2933–2947. doi: 10.1016/s0042-6989(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 35.Hess R F, Wang Y Z, Dakin S C. Vision Res. 1999;39:4354–4360. doi: 10.1016/s0042-6989(99)00153-4. [DOI] [PubMed] [Google Scholar]