Abstract
Objective
To test the hypothesis that a voluntary pelvic muscle contraction initiated in preparation for a cough, a maneuver we call the Knack, significantly reduces vesical neck displacement.
Methods
A convenience sample of 22 women consisted of 11 young, continent nulliparas (mean age [± standard deviation] 24.8 ± 7.0 years) and 11 older, incontinent paras (mean age [± SD] 66.9 ± 3.9 years). With the use of perineal ultrasound, we quantified vesical neck displacement at rest and during coughs using caliper tracing and a coordinate system. The subjects coughed with and without voluntary pelvic floor muscle contraction.
Results
Vesical neck mobility during coughs was significantly decreased when voluntary contraction was used: from a median (range) of 5.4 (20.0) mm without volitional contraction to 2.9 (18.3) mm with volitional contraction (P < .001). The younger women demonstrated a median (range) decrease in excursion from 4.6 (19.5) to 0.0 (17.0) mm (P = .007), and the older incontinent women demonstrated a median (range) decrease from 6.2 (10.0) to 3.5 (15.4) mm (P = .003). At rest, the median vesical neck position in the group of older incontinent women was significantly further dorsocaudal (P = .001) than in the younger women.
Conclusion
A pelvic floor muscle contraction in preparation for, and throughout, a cough can augment proximal urethra support during stress, thereby reducing the amount of dorsocaudal displacement.
Excessive vesical neck movement during activities such as coughing has been associated with development of stress urinary incontinence.1–3 Let us suppose an individual coughs twice: once normally and once with the levator ani muscles purposely contracted.4 A reduction in the magnitude of the vesical neck movement when the muscles are contracted during a cough would indicate a temporary increase in resistance to stretch (stiffness) under conditions of stress. This argument is predicated on the knowledge that contraction of a striated muscle can increase its stiffness by up to five times compared with the resting state,5,6 primarily because of an increase in the number of strongly bound actin-myosin cross-bridges.
Improved vesical neck stabilization is of interest because we recently showed that a volitional contraction of the pelvic floor muscles just before and throughout a cough, a preemptive maneuver we call the Knack because skill is involved, can be used to reduce stress-related urine leakage significantly.7 However, the underlying mechanism by which the Knack reduces urine leakage has not been elucidated.
The aim of this study was to test the hypothesis that vesical neck displacement during a cough performed with the Knack is less than that measured when a cough is performed without the Knack. The hypothesis was tested in a sample composed of both young, healthy (continent) nulliparous women and older, incontinent parous women.
Materials and Methods
The institutional review board approved all experimental procedures. A convenience sample was recruited primarily through advertisements in local newspapers. Twenty-two women gave informed consent to participate; half were young, continent, and nulliparous, and half were older, incontinent, and parous. Continence status was confirmed by a standing full-bladder stress test using deep coughs and the protocol for bladder filling described later in this article.8 Baseline frequency of incontinence was estimated using a 7-day self-report diary.9 Descriptive statistics including leakage reports and voided volume obtained immediately after examination are shown in Table 1. Exclusion criteria included the following: prior bladder surgery, age less than 18 years, and inability to refrain from performing Valsalva’s maneuver when asked to contract the pelvic floor muscles volitionally.
Table 1.
Group-Specific Descriptions
| Group | n | Age (y) | Urine leakage (times/d) | Voided volume (ml) |
|---|---|---|---|---|
| Young, nulliparious women | 11 | 24.8 (7.0) | 0 (0) | 200 (106) |
| Older, parous women | 11 | 66.9 (3.9) | 0.5 (0.5) | 321 (179) |
Data are expressed as mean (standard deviation).
Women in the younger, continent group were trained briefly in the Knack immediately before initial data collection. Measures for these women were repeated during a return visit 1 week later, and measurements from the two visits were averaged for analysis. Women in the older, incontinent group already had completed a 6-month intervention aimed at evaluating the efficacy of the Knack as an incontinence management strategy. In this group, the ultrasound examination was performed during the patient’s last study visit.
We compared vesical neck displacement during coughing with and without use of the Knack. Within-visit cough evaluations were done within 2 minutes of one another so that bladder volume did not differ. To ensure adequate volume for ultrasound imaging,10 we instructed each woman to refrain from emptying her bladder for 2 hours before her visit and to drink 16 ounces of water 1 hour before her visit. Bladder volume during the procedure was estimated as voided volume measured immediately after the ultrasound examination.
The kinematic measures of vesical neck displacements were obtained using an ultrasound scanner (Siemens Sonoline SI-400; Siemens Medical Systems, Issaquah, WA) with a curved array probe (5 MHz). Women were asked to stand with feet slightly apart so that the probe could be maintained on the vulva in the midsagittal plane. Vesical neck position was measured at rest and again during maximal voluntary pelvic floor contraction, hard coughing without using the Knack, and finally hard coughing using the Knack.
Measures of resting vesical neck position (in millimeters) were done in the midsagittal plane using a rectangular coordinate system and the measurement strategy described by Schaer et al,10 who also demonstrated reliability of the method. A minor modification involved designating the positive X axis as being directed cranioventrally along the major diameter of the symphysis and the positive Y axis as being directed craniodorsally from the reference system origin at the dorsocaudal end of the symphysis. This orientation maintains compliance with the standard international convention for using a “right-handed” rectangular coordinate system (Figure 1). The point of peak cough pressure was identified, using frame-by-frame replay of the videotaped images, and then frozen for coordinate measurement purposes.
Figure 1.
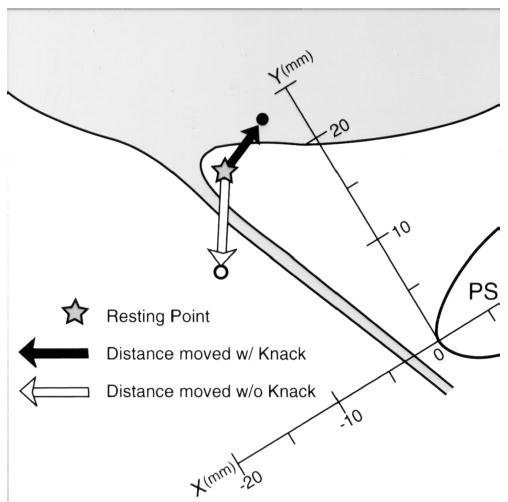
“Knack” schematic showing the measurement strategy and an example of potential bladder neck location above resting during coughing with the pelvic floor muscles contracted.
In addition, measurements of maximal vesical neck excursion during each cough were done using on-screen calipers by tracing the displacement of the vesical neck from its resting location to its location at peak cough pressure during slow-motion replay. For clarity, examples are shown in Figures 1 and 2. The black arrows demonstrate that during a cough performed using the Knack, the bladder neck position at peak cough pressure can be located either above (Figure 1) or below (Figure 2) the resting position.
Figure 2.
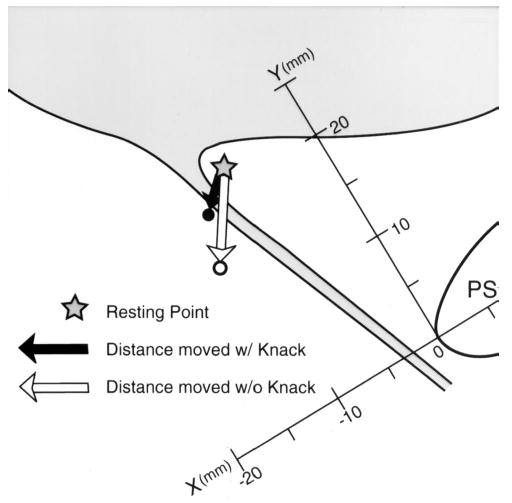
“Knack” schematic showing second example of potential bladder neck location below resting during coughing with the pelvic floor muscles contracted.
A single evaluator (DP) with extensive experience in perineal ultrasound obtained all measures. A second evaluator (LTC) confirmed that the original measurements were free of measurement error such as sign reversal by repeating the measurements.
Simultaneous measures of intra-abdominal cough pressure were done during the ultrasound examination, using a standard 8-Fr catheter with a dual-tip micro-transducer (Gaeltek; Medical Measurement Inc., Hackensack, NJ) placed in the upper fornix of the vagina. The transducer had a frequency response of 0–1 kHz and a dynamic range of 0–300 cm H2O. Pressure signals were amplified to volt levels before being digitized synchronously at 1 kHz using a 12-bit analog–to–digital converter board and microprocessor. Pressures also were displayed in digital form on the recorded videotape for the purpose of locating the video frame that specifically included peak cough pressure.
Because of the skewed distributions of the ultrasound measures observed on histograms, we analyzed these data by means of nonparametric statistical tests and reported central tendency as median and range. We used the Wilcoxon signed-rank test for within-group analysis and the Mann-Whitney U test for between-group statistical analysis. For the pressure measurements, which were more normally distributed, we used paired t tests and the Student t test for within-group and between-group analyses, respectively. P < .05 was considered statistically significant throughout. The sample size selected was appropriate for detecting a difference in displacement of 4 mm between coughs with and without Knack with a power of 90% at the 5% level of significance, given a mean (standard deviation [SD]) vesical neck displacement during coughs without use of the Knack of approximately 8 (9) mm estimated from similar measures by Schaer et al.10
Reproducibility of cough pressures was assessed using methods outlined by Bland and Altman.11–13 Limits of agreement were calculated between measures obtained with and without use of the Knack. The limits of agreement are equal to the mean difference ± twice the SD of the difference.11 A priori, we judged that cough pressure measures of 20 cm H2O or less would warrant clinically acceptable agreement. Similarly, reproducibility of vesical neck displacement measures was determined by calculating repeatability limits as twice the SD of the difference11 using the repeated measures data obtained in the healthy continent women’s two visits.
Results
Listed in Table 2 are overall descriptive statistics of the median (range) vesical neck position on the X and Y coordinates at rest, during coughing without use of the Knack, and during coughing with use of the Knack. With use of the Knack, the extent of displacement occurring during coughing was reduced significantly. Median (range) vesical neck displacement during a cough performed without the Knack was 5.4 (20.0) mm, and during a cough performed with the Knack median (range) vesical neck displacement was 2.9 (18.3) mm (P < .001).
Table 2.
Vesical Neck Position
| Vesical neck | X coordinate (mm) | Y coordinate (mm) |
|---|---|---|
| Without stress | ||
| Rest | −3.0 (42.0) | 19.8 (22.7) |
| With stress | ||
| Cough without Knack | −4.5 (35.8) | 15.3 (23.1) |
| Cough with Knack | −1.9 (33.0) | 17.5 (22.1) |
Data are expressed as median (range).
The range of vesical neck displacement for coughs performed without use of the Knack was 1–21 mm, with displacement consistently in the dorsocaudal direction. By contrast, when performing coughs using the Knack, five women were able to lift and maintain the vesical neck in a cranioventral position relative to the resting state. The maximal cranioventral displacement at peak Knack cough pressure was 7.3 mm, and the maximal dorsocaudal displacement in this sample of women was 11.0 mm. Displacement scores during coughing are shown graphically for each group in Figures 3 and 4. Overall, when coughs without use of the Knack were compared with those in which the Knack was performed, the difference in displacement ranged from 17.3 mm more cranioventral to 0.5 mm more dorsocaudal.
Figure 3.
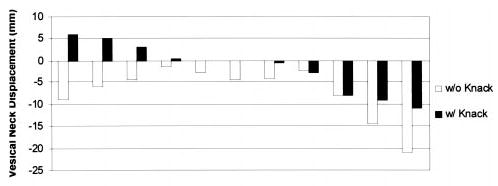
Vesical neck displacement scores of younger women, obtained during coughing without and then with (Knack) the pelvic floor muscles contracted.
Figure 4.
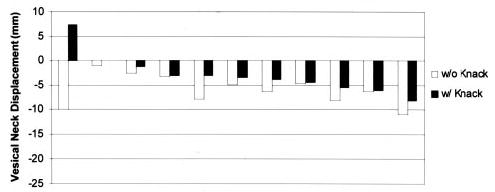
Vesical neck displacement scores of older women, obtained during coughing without and then with (Knack) the pelvic floor muscles contracted.
When analyzed by group, resting vesical neck position on the X and Y coordinates differed significantly between the young, continent women and the older, incontinent women (P = .001 and P < .001, respectively). Median (range) X and Y coordinate measures for the group of younger, continent women were 7.3 (20.8) and 22.0 (9.0) mm, respectively. For the group of older, incontinent women, median (range) X and Y coordinates were −9.0 (27.4) and 16.0 (20.7) mm, respectively. Individual X and Y scores for locating the resting vesical neck position on the coordinate system are shown graphically in Figure 5.
Figure 5.
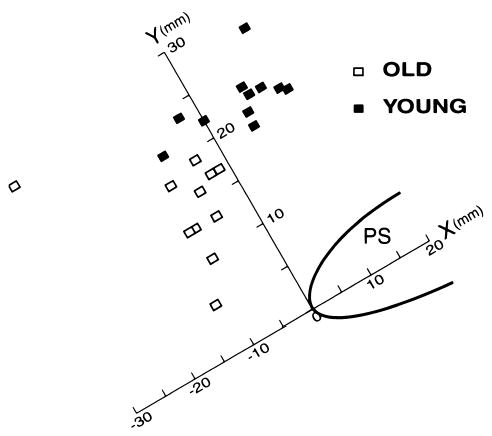
Vesical neck resting position by individual subject and group.
By using the Knack, the younger, continent women were able to reduce vesical neck displacement during coughs. Without use of the Knack, median (range) displacement was 4.6 (19.5) mm, with use of the Knack, median (range) displacement was 0.0 (17.0) mm (P = .007). Similarly, the older, incontinent women were able to reduce vesical neck displacement from a median (range) of 6.2 (10.0) mm (without use of the Knack) to 3.5 (15.4) mm (with use of the Knack) (P = .003). Individual scores are shown graphically for each group in Figures 3 and 4.
Within-visit repeated maximal cough pressures, measured with the pressure transducer placed at the upper fornix of the vagina, were acceptably consistent. The group mean (SD) scores of the two cough pressures measured about 1 minute apart (with and then without use of the Knack) were calculated as 129 (12.3) and 124 (11.6) cm H2O. The mean (SD) difference between these pressures (defined as cough pressure without use of the Knack minus cough pressure with use of the Knack) was 5.2 (6.9) cm H2O, indicating slightly higher mean pressure during the Knack coughs (P = .032). Lower and upper limits of agreement in the pressure measures for cough sets then were calculated as the mean difference ± twice the SD. The results (19.1 and −8.7 cm H2O, respectively) indicated that 95% of the sample women had consistent cough pressures within this range despite the manipulating conditions of Knack and no Knack.
In the next analysis, we considered repeatability in measures of displacement done for coughs 1 week apart in the minimally trained younger, continent women. The analysis was conducted for coughs performed under the same condition, either with the use of Knack or without the use of Knack. Hence, repeatability was calculated from the ideal difference of 0 mm. For the coughs performed without use of the Knack, repeated performance resulted in a mean (SD) group difference in displacement of 0.51 (3.9) mm. Repeatability was calculated as 0 ± twice the SD of the difference. The result was ± 7.9 mm; about 95% of the sample could repeat the without Knack coughs within 0–8 mm despite 1 week intervening. Similarly, for coughs performed using the Knack, repeated performance resulted in a mean (SD) group difference in displacement of 1.5 (6.9) mm, with repeatability calculated as ± 12.9 mm.
After instruction in basic pelvic floor muscle contraction technique, none of the women strained when asked to contract their pelvic floor muscles without coughing. Nineteen of the 22 women demonstrated a visible elevation of the vesical neck on the ultrasound image when asked to contract their pelvic floor muscles without coughing. The remaining three women (two young women and one older woman) demonstrated neither visible cranioventral vesical neck elevation nor the dorsocaudal displacement indicative of Valsalva’s maneuver and thus were allowed to remain in the study. The younger, continent women had a median (range) maximal cranioventral displacement during contraction of 4.9 (13.5) mm; similarly, the older, incontinent women had a median (range) maximal displacement of 5.6 (11.0) mm.
Discussion
The finding that a preemptive levator ani muscle contraction can stiffen vesical neck support during stress is consistent with findings of anatomic studies indicating that functional mobility of urethral anatomic support is governed in part by activation of these muscles.
In this investigation, we demonstrated women’s ability voluntarily to augment a normal levator ani muscle contraction or to accomplish a conscious contraction if the normal mechanisms generating it during a cough have been lost. Our study shows that the Knack improves vesical neck stability during stress in continent nulliparas, and the study provides indirect evidence that in most normal women, full activation of the muscle does not occur by reflex during a cough. Use of the Knack also resulted in improved stabilization of the vesical neck in certain women with stress urinary incontinence. However, the overall poorer improvement in this group of parous women is in keeping with evidence that the degree to which volitional muscle action can affect vesical neck position could be influenced by damage to the fascial attachments and/or damage to one or both of the levator ani muscles or their innervation.14–16
High intra- and intersubject variability in precision of control of the hidden pelvic floor muscles was well documented in other studies, and our findings concur.17 For example, the lesser repeatability of measures in the case of coughs involving use of the Knack may be explained by the fact that these measures were obtained in women who were not practiced in the maneuver. The poor repeatability warrants caution in interpretation of the findings. However, we are reassured by the consistency of the overall finding of reduced vesical neck displacement across nearly all subjects (20 of 22). Limitations of the study include lack of repeated measures for the older women and the fact that we could not conduct a blind review of the tapes (because the caliper tracings of the measures obtained during the actual data collection remained on the recordings, as did labels identifying the three activities performed). Overall, our measures of resting bladder neck position and bladder neck displacement during a cough are consistent with those of others18,19 when age and postural differences in sample selection and protocol are considered. This study provides scientific evidence that may help clarify the role that the levator ani muscles play in vesical neck support.
Footnotes
Financial support provided by Public Health Service grants R01 DK47516, R01 DK51405, P30 AG 08808, and T32 AG00114.
References
- 1.Jeffcoate TN, Roberts H. Observations on stress incontinence of urine. Acta Obstet Gynecol Scand. 1952;28:183–8. doi: 10.1016/s0002-9378(16)38792-0. [DOI] [PubMed] [Google Scholar]
- 2.Hodgkinson CP. Relationships of the female urethra in urinary incontinence. Am J Obstet Gynecol. 1953;65:560–73. doi: 10.1016/0002-9378(83)90612-9. [DOI] [PubMed] [Google Scholar]
- 3.Versi E, Lyell D, Griffiths D. Videourodynamic diagnosis of occult genuine stress incontinence in patients with anterior vaginal wall relaxation. J Soc Gynecol Investig. 1998;5:327–30. doi: 10.1016/s1071-5576(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JOL. Functional anatomy of the female lower urinary tract and pelvic floor. In: Bock G, Whelan J, eds. Neurobiology of incontinence (Ciba Foundation Symposium 151). Chichester, England: Wiley, 1990:57–76. [DOI] [PubMed]
- 5.Sinkjaer T, Toft E, Andreassen S, Hornemann BC. Muscle stiffness in human ankle dorsiflexors: Intrinsic and reflex components. J Neurophysiol. 1988;60:1110–21. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- 6.Blanpied P, Smidt GL. The difference in stiffness of the active plantarflexors between young and elderly human females. J Gerontol. 1993;48:M58–63. doi: 10.1093/geronj/48.2.m58. [DOI] [PubMed] [Google Scholar]
- 7.Miller JM, Ashton-Miller JA, DeLancey JOL. A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI. J Am Geriatr Soc. 1998;46:870–4. doi: 10.1111/j.1532-5415.1998.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller JM, Ashton-Miller JA, DeLancey JOL. Quantification of cough-related urine loss using the paper towel test. Obstet Gynecol. 1998;91:705–9. doi: 10.1016/s0029-7844(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 9.Wyman JF, Choi SC, Harkins SW, Wilson MS, Fantl JA. The urinary diary in evaluation of incontinent women: A test-retest analysis. Obstet Gynecol. 1988;71:812–7. [PubMed] [Google Scholar]
- 10.Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound: Determination of reliable examination procedures. Ultrasound Obstet Gynecol. 1996;7:347–52. doi: 10.1046/j.1469-0705.1996.07050347.x. [DOI] [PubMed] [Google Scholar]
- 11.Altman DG, Bland JM. Measurement in medicine: The analysis of method comparison studies. Statistician. 1983;32:307–17. [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Comparing two methods of clinical measurement: A personal history. Int J Epidemiol. 1995;24 (suppl 1):S7–14. doi: 10.1093/ije/24.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- 14.Taverner D. An electromyographic study of the normal function of the external anal sphincter and pelvic diaphragm. Dis Colon Rectum. 1959;2:153–60. doi: 10.1007/BF02616708. [DOI] [PubMed] [Google Scholar]
- 15.Dimpfl T, Jaegar C, Mueller-Felber W, Anthuber C, Hirsch A, Brandmaier R, et al. Myogenic changes of the levator ani muscle in premenopausal women: The impact of vaginal delivery and age. Neurourol Urodyn. 1998;17:197–205. doi: 10.1002/(sici)1520-6777(1998)17:3<197::aid-nau4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Hanzal E, Berger E, Koelbl H. Levator ani muscle morphology and recurrent genuine stress incontinence. Obstet Gynecol. 1993;81:426–9. [PubMed] [Google Scholar]
- 17.Bö K, Kvarstein B, Hagen R, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence. I. Reliability of vaginal pressure measurements of pelvic floor muscle strength. Neurourol Urodyn. 1990;9:471–7. [Google Scholar]
- 18.Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol. 1995;85:220–4. doi: 10.1016/0029-7844(94)00369-O. [DOI] [PubMed] [Google Scholar]
- 19.Wijma J, Tinga D, Visser G. Perineal ultrasonography in women with stress incontinence and controls. Gynecol Obstet Invest. 1991;32:176–9. doi: 10.1159/000293024. [DOI] [PubMed] [Google Scholar]


