Abstract
We previously reported that testosterone attenuated atherogenesis in LDLR−/− male mice, and that this effect of testosterone was most likely caused by its conversion to estradiol. Estradiol inhibits vascular cell adhesion molecule-1 (VCAM-1) expression, and expression of VCAM-1 is one of the early events in atherogenesis. We assessed the cellular mechanism(s) involved by which testosterone attenuates atherogenesis. We evaluated whether testosterone inhibited TNFα-induced VCAM-1 expression via its conversion to estradiol by the enzyme aromatase in human umbilical vein endothelial cells (HUVEC). Aromatase mRNA was dedected by reverse transcription–PCR in these cells. Testosterone (30 nM–1 μM) attenuated VCAM-1 mRNA expression in a concentration-dependent manner. The non aromatizable androgen, dihydrotestosterone, had no effect on VCAM-1 mRNA expression. Testosterone was less effective in attenuating VCAM-1 expression in the presence of anastrozole, an inhibitor of aromatase, indicating that testosterone inhibited VCAM-1 via conversion to estradiol. Estradiol also attenuated VCAM-1 mRNA expression, but this action was not abolished in the presence of anastrozole, indicating that anastrozole itself did not modulate VCAM-1 mRNA expression. The effect of testosterone on VCAM-1 mRNA expression was inhibited in the presence of the estrogen receptor antagonist, ICI-182780. Testosterone also attenuated TNFα-induced VCAM-1 protein expression, and this attenuation was abolished in the presence of anastrozole. In conclusion, testosterone inhibited VCAM-1 mRNA and protein expression in HUVEC by its conversion to estradiol via the enzyme aromatase present in the endothelial cells. Results from our study may help explain the mechanism by which testosterone may have beneficial effects on the cardiovascular system.
Keywords: atherosclerosisat‖sex steroids‖androgens
Men are twice as likely as women to die from coronary artery disease, probably because men lack the protection afforded by endogenous or exogenous estrogens (1, 2). Generally, it has been assumed that high levels of androgens in men are detrimental to the cardiovascular system (3). Testosterone administration has been associated with an increase in both total cholesterol and low density lipoprotein (LDL) levels, both of which correlated positively with coronary artery disease (4). However, the effects of testosterone administration on atherogenesis are controversial. Testosterone administration has been reported to increase the extent of atherosclerosis in subhuman primates (5) and in rabbits (6). On the other hand, its administration has also been reported to cause a decrease in atherosclerosis in castrated male rabbits (7) and in LDL-receptor knockout (LDLR−/−) mice (8). Similarly, androgens seem to have an antiatherogenic effect in men (9), and testosterone has been reported to be an effective antianginal agent (10). These differences may in part be because of the type of androgens administered and the route of administration.
We had previously speculated (11) that testosterone may attenuate early atherogenesis, at least in part by being converted to estradiol by the enzyme aromatase which is expressed in endothelial cells (12, 13). We subsequently demonstrated this possibility in LDLR−/− male mice and further demonstrated that this attenuating effect of testosterone on atherogenesis was abolished in the presence of an inhibitor of aromatase (8). The present work was undertaken to elucidate the cellular mechanism by which testosterone attenuates atherogenesis.
Materials and Methods
Materials.
The culture medium M199, penicillin-G sodium, streptomycin sulfate, amphotericin B, heparin, Hepes buffer, and collagenase-type 1 from porcine skin were obtained from Invitrogen. Gelatin, endothelial-derived growth factor, testosterone, 17β-estradiol, dihydrotestosterone, diethylpyrocarbonate, Salmon testes DNA, Denhardt's solution (0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA), SDS, and tumor necrosis factor-α (TNFα) were obtained from Sigma; charcoal-dextran-treated FBS was obtained from HyClone; FBS was obtained from Atlas (Fort Collins, CO), and ICI-182780 was obtained from Tocris Cookson (Ballwin, MO). Anastrozole was supplied as a gift from Zeneca Pharmaceuticals (Cheshire, U.K.).
The primer used for reverse transcription (RT)-PCR for aromatase was obtained from Custom Primers (GIBCO/BRL). Monoclonal antibodies to human vascular cell adhesion molecule-1 (VCAM-1) was obtained from American Qualex, San Clemente, CA)
Cell Culture.
Human umbilical vein endothelial cells (HUVEC) were isolated from freshly collected umbilical cords (female fetus), as described (14). The cells were cultured on 0.1% gelatin-coated 75-mm flasks in M199 medium supplemented with 20% FBS/5 μg/ml endothelial-derived growth factor/100 μg/ml heparin/100 units of penicillin G sodium/100 μg/ml of streptomycin sulfate/0.25 μg/ml of amphotercin-B/10 mM Hepes buffer, pH ≈7.5. Before the experiments, cells were shifted to phenol red-free M199 medium supplemented with 2% charcoal-dextran-treated FBS and the antibiotics mentioned above. Only second or third passage cells were used in all of the current studies.
RT-PCR and Southern Blot Analysis for Aromatase.
RNA extraction and purification were done by using RNeasy mini kit as described by the manufacturer's protocol (Qiagen, Chatsworth, CA). Total cellular RNA (3 μg) was used for RT-PCR analysis of human aromatase with 50 units of Moloney murine leukemia virus reverse transcriptase at 42°C for 25 min, as described (8). The resulting cDNA samples were PCR-amplified with the Gene Amp RNA PCR kit (Perkin–Elmer) according to the manufacturer's protocol. Oligonucleotide primers were designed for PCR amplification of specific DNA fragment contained in human aromatase P450 (15, 16) using the following set of primers: Forward primer sequence 5′-GAA TAT TGG AAG GAT GCA CAG ACT-3′, reverse primer sequence 5′-GGG TAA AGA TCA TTT CCA GCA TGT-3′. The PCR product (aromatase 293 bp) was size-fractionated on 1.5% agarose gel and transferred to nitrocellulose membranes. Membranes were hybridized with [α-32P]dCTP-labeled human aromatase cDNA probes (Research Genetics, Huntsville, AL; gene accession no. H66129).
Northern Blot Analysis for VCAM-1 mRNA.
Ten micrograms of total RNA was loaded on a 1% agarose-formaldehyde gel, electrophoresed, and transferred to Hybond membrane (Amersham Pharmacia) overnight by capillary action. The RNA was UV crosslinked by using GS Gene Linker (Bio-Rad). VCAM-1 (Research Genetics) cDNA was labeled with [α-32P]dCTP (ICN) by using the random priming method. Membranes were prehybridized at 42°C overnight followed by hybridization with respective labeled probes for another 24 h at 42°C. The membranes were washed twice at room temperature with 2× sodium chloride and sodium citrate (SSC) buffer and 0.5% SDS followed by washing at 65°C for 30 min twice with 0.5× SSC and 0.1% SDS; the respective bands were quantitated by using PhosphorImager (Molecular Dynamics). For visualization, Kodak X-Omat AR films were exposed at −70°C for 12 to 24 h. The VCAM-1 band was normalized with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band as an internal control.
VCAM-1 Detection by ELISA.
Endothelial cells were plated into 96-well plates and initially were incubated for 48 h at 37°C with or without testosterone. This procedure was followed by addition of either TNF-α (10 ng/ml) or vehicle for 4 h. The wells then were rinsed with PBS, which then was followed by fixation in 4% (wt/vol) paraformaldehyde for 20 min at room temperature. After adding the fixative, the cells were thoroughly rinsed with PBS containing 0.1% BSA (0.1% APBS). To block nonspecific binding, the cells were incubated in 3% APBS for 1 h at room temperature. Cells then were incubated overnight at 4°C with monoclonal antibodies to human VCAM-1 (1 μg/ml in 1% APBS) (American Qualex, La Mirada, CA). After the incubation with primary antibody, the wells were rinsed and blocked thoroughly before they were exposed to a horseradish peroxidase-conjugated secondary antibody. After the incubation, the cells were rinsed with PBS followed by double-distilled water. The peroxidase color reaction was developed in the dark by using orthophenylenediamine dihydrochloride (catalog no. P8787, Sigma). The plate was then read on a Molecular Devices kinetic microtiter plate reader with a Softmax 881 L-1 end-point program at an OD of 490 nm. The antibody concentration and incubation times were optimized to ensure testing in the linear range (17).
Data Analysis.
Values are expressed as means ± SE obtained from at least three separate experiments in each group. Differences between groups were assessed by one-way ANOVA and Newman-Keuls multiple comparison test where appropriate. P values <0.05 were considered as significant.
Results
Expression of Aromatase in HUVEC.
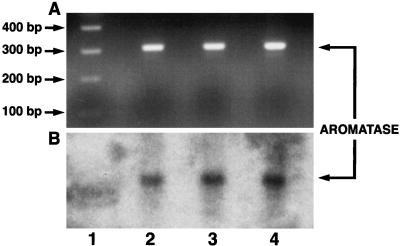
Aromatase mRNA levels were too low to be detected by Northern analysis in HUVEC. However, aromatase mRNA was detected by RT-PCR and confirmed by Southern blot analysis of RT-PCR products (Fig. 1).
Figure 1.
Southern blot analysis (B) of RT-PCR products of aromatase (A) in human umbilical vein endothelial cells from three separate experiments (lanes 2–4).
Effect of Various Concentrations of Testosterone and Dihydrotestosterone on TNFα-Stimulated VCAM-1 Expression.
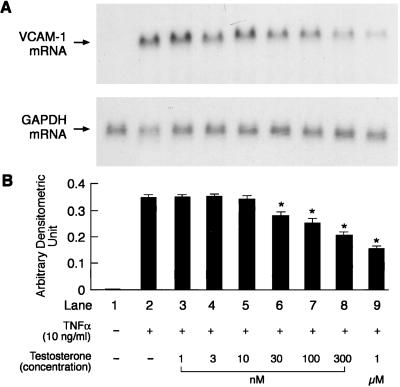
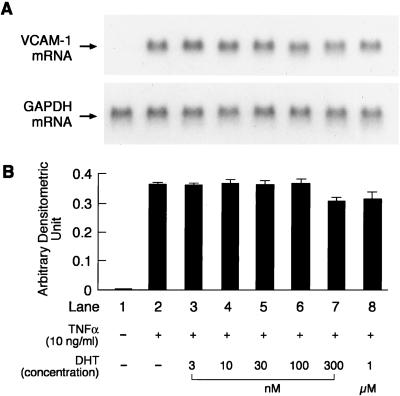
Second and third passage HUVEC, which were used for all our studies, expressed both estrogen reeptor (ER)α and ERβ mRNA (data not shown). No basal VCAM-1 mRNA expression was detected in the unstimulated cells or those treated with testosterone and dihydrotestosterone. Preliminary experiments indicated that HUVEC when exposed to 10 ng/ml TNFα for 4 h led to maximal VCAM-1 mRNA expression. This concentration and exposure time to TNFα was, therefore, used for all of the experiments. The effects of preincubation of HUVEC with different concentrations of testosterone (1 nM, 3 nM, 10 nM, 30 nM, 100 nM, 300 nM, and 1 μM) for 48 h on TNFα-induced VCAM-1 mRNA expression are shown in Fig. 2. Testosterone at concentrations ranging from 30 nM to 1 μM significantly attenuated the TNFα-induced VCAM-1 expression in a concentration-dependent manner, whereas lower concentrations did not have any significant effect when compared with that observed with TNFα alone. The effects of preincubation of HUVEC with different concentration of dihydrotestosterone (3 nM, 10 nM, 30 nM, 100 nM, 300 nM, and 1 μM) for 48 h on TNFα-induced VCAM-1 mRNA expression are shown in Fig. 3. Dihydrotestosterone did not have any effect on VCAM-1 mRNA expression at any of the concentrations tested.
Figure 2.
Representative picture of a Northern blot analysis showing the effects of different concentrations of testosterone on TNFα-induced VCAM-1 and GAPDH mRNA expression (A) and quantitation by PhosphorImager (B). Quantitation is expressed as means ± SEM of arbitrary densitometric units obtained from three separate experiments. GAPDH was used as internal control. *, Significant difference (P < 0.05) from TNFα-only treated cells.
Figure 3.
Representative picture of a Northern blot analysis showing the effects of different concentrations of dihydrotestosterone on TNFα-induced VCAM-1 and GAPDH mRNA expression (A) and quantitation by PhosphorImager (B). Quantitation is expressed as means ± SEM of arbitrary densitometric units obtained from three separate experiments. GAPDH was used as an internal control. No significant difference (P < 0.05) from TNFα-only treated cells was found.
Effect of Aromatase Inhibitor on Testosterone-Induced Reduction of VCAM-1 mRNA.
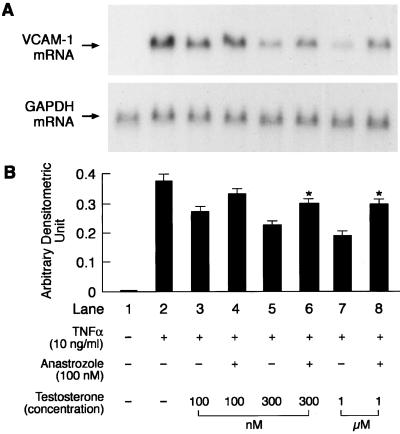
To elucidate whether testosterone itself or its conversion to estradiol was responsible for the attenuation of TNFα-induced VCAM-1 expression, we assessed the effects of testosterone (100 nM, 300 nM, and 1 μM) in the absence and presence of the aromatase inhibitor anastrozole (100 nM). These concentrations of testosterone were selected as they caused attenuation of VCAM-1 mRNA expression in a concentration-dependent manner (Fig. 2). The concentration of anastrozole selected was based on studies by other investigators who demonstrated significant aromatase inhibition in vitro at this concentration (18). Anastrozole was added 60 min before the addition of testosterone. Testosterone at concentrations of 300 nM and 1 μM was less effective in attenuating VCAM-1 mRNA expression in the presence of anastrozole when compared with values obtained in the absence of anastrozole (Fig. 4).
Figure 4.
Representative picture of a Northern blot analysis demonstrating the effects of different concentrations of testosterone (100 nM, 300 nM, and 1 μM) on TNFα-induced (10 ng/ml) VCAM-1 and GAPDH mRNA expression in the presence and absence of an aromatase inhibitor, anastrozole (100 nM) (A) and quantitation by PhosphorImager (B). Anastrozole was added to the culture medium containing HUVEC 60 min before the addition of testosterone. Quantitation is expressed as means ± SEM of arbitrary densitometric units obtained from three separate experiments. GAPDH was used as an internal control. *, Significant difference (P < 0.05) from values in preceding lane obtained in the absence of anastrozole.
Effect of the Aromatase Inhibitor Anastrozole on Estradiol-Induced Reduction of VCAM-1 mRNA.
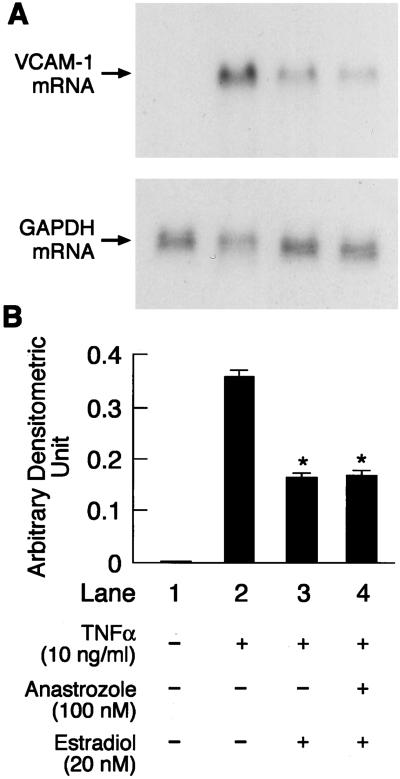
To confirm further that the effect of anastrozole in antagonizing testosterone-induced reduction in VCAM-1 mRNA expression was specific for this aromatizable androgen, we assessed the effect of estradiol, the byproduct of aromatization of testosterone, on TNFα-induced VCAM-1 expression in the absence and presence of anastrozole (100 nM) added 60 min before the addition of estradiol. HUVEC incubated with estradiol (20 nM) for 48 h significantly attenuated TNFα-induced VCAM-1 expression, and this attenuation was similar to that observed in the presence of anastrozole (Fig. 5).
Figure 5.
Representative picture of Northern blot analysis showing the effects of 20 nM estradiol on TNFα-induced (10 ng/ml) VCAM-1 and GAPDH mRNA expression in the absence and presence of an aromatase inhibitor (anastrozole, 100 nM) (A) and quantitation by PhosphorImager (B). Quantitation is expressed as means ± SEM of arbitrary densitometric units obtained from three separate experiments. GAPDH was used as internal control. *, Significant difference (P < 0.05) from TNFα-only treated cells. Anastrozole was added to the culture medium containing HUVEC 60 min before the addition of estradiol.
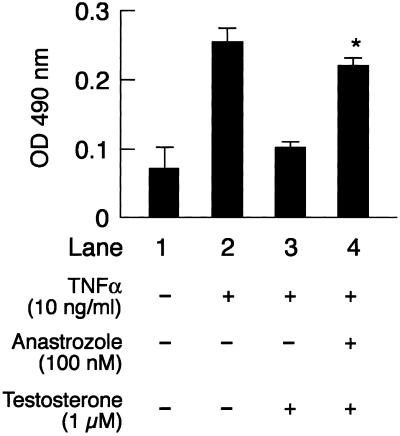
Effect of Testosterone on VCAM-1 Protein Expression in the Presence and Absence of the Aromatase Inhibitor.
Next, we assessed the effects of testosterone on VCAM-1 protein expression in the absence and presence of anastrozole (100 nM) added 60 mins before the addition of testosterone. We observed some basal VCAM-1 protein expression in unstimulated cells. Exposure of these cells to TNFα (10 ng/ml) for 4 h significantly increased VCAM-1 protein expression. Testosterone at a concentration of 1 μM significantly attenuated TNFα-induced VCAM-1 protein expression. In the presence of anastrozole, this attenuating effect was not observed, similar to that seen with VCAM-1 mRNA expression (Fig. 6).
Figure 6.
Effect of 1 μM testosterone in the absence and presence of an aromatase inhibitor, anastrozole, on TNFα-stimulated (10 ng/ml) VCAM-1 protein expression as analyzed by ELISA. Values represent absorbance (OD) at 490 nM. Quantitation is expressed as means ± SEM, obtained from three separate experiments. +, Significant difference (P < 0.05) from TNFα-only treated cells. *, Significant difference (P < 0.05) from the value in the preceding lane obtained in the absence of anastrozole (100 nM). Anastrozole was added to the culture medium containing HUVEC 60 min before the addition of testosterone.
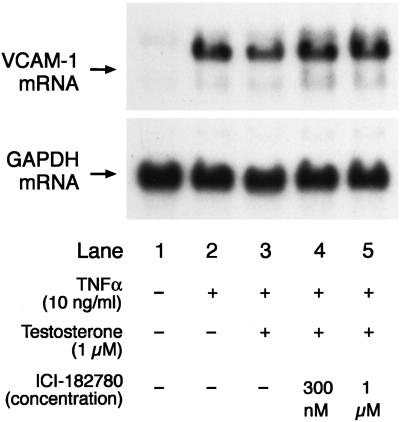
Effect of the ER Antagonist ICI-182780 on Testosterone-Induced Attenuation of VCAM-1 mRNA Expression.
Further, we wanted to confirm whether the attenuating effect of testosterone on TNFα-induced VCAM-1 mRNA expression was mediated by conversion of testosterone to estradiol, which in turn acted through the ER. Therefore, we assessed this effect of testosterone on VCAM-1 mRNA expression in the absence and presence of the ER antagonist ICI-182780 (300 nM and 1 μM) which was added 60 min before the addition of testosterone. The effect of testosterone (1 μM) in attenuating TNFα-induced VCAM-1 mRNA expression was significantly attenuated in the presence of ICI-182780 (Fig. 7). ICI-182780 did not affect either basal or TNFα-induced VCAM-1 mRNA expression (data not shown).
Figure 7.
Representative picture of a Northern blot analysis showing the effect of 1 μM testosterone on TNFα-induced (10 ng/ml) VCAM-1 and GAPDH mRNA expression in the absence and presence of two different concentrations (300 nM and 1 μM) of an ER antagonist (ICI-182780). The ER antagonist was added to the culture medium containing HUVEC 60 min before the addition of testosterone.
Discussion
The primary objective of this study was to elucidate the cellular mechanism(s) by which testosterone attenuates atherogenesis. Previously, we had demonstrated that the action of testosterone in attenuating atherogenesis in LDLR−/− male mice was not observed when the animals were treated simultaneously with an aromatase inhibitor, and, on this basis, we speculated that testosterone most likely attenuated atherogenesis by being converted to estradiol (8). Also, we had demonstrated previously that estradiol in vivo attenuates atherogenesis by inhibiting the adhesion of monocytes to endothelial cells, which is one of the early steps in the initiation of atherogenesis; this action of estradiol was associated with decreased expression of VCAM-1 (11).
Therefore, we decided to assess the effects of testosterone on VCAM-1 mRNA and protein expression in the presence and absence of an aromatase inhibitor. Testosterone is converted to estradiol by the enzyme aromatase and to dihydrotestosterone by the enzyme 5α reductase. Our results and those of others indicate that both aromatase (8, 12, 13) and 5α reductase (19) are present in HUVEC. We elected, therefore, to compare the effects of testosterone on VCAM-1 expression with that of dihydrotestosterone, which is a nonaromatizable androgen, to delineate further whether the effect of testosterone on VCAM-1 was caused by its conversion to estradiol or by an action mediated through the androgen receptor.
Our observation that testosterone caused a concentration-dependent decrease in the expression of TNFα-stimulated VCAM-1 mRNA is similar to that produced by estradiol (11, 14, 20). However, this effect was not observed with dihydrotestosterone, which indicated that the action of testosterone was most likely due to its conversion to estradiol by aromatase in the endothelial cells. Other investigators (21) have reported that dihydrotestosterone amplified the adhesion of monocytes to endothelial cells stimulated by IL-Iβ or TNFα in vitro, and that this was associated with increased expression of VCAM-1 protein when compared with cells treated with either IL-Iβ or TNFα alone. The lack of amplification of TNFα-stimulated VCAM-1 mRNA expression by dihydrotestosterone in our study may be due to maximal stimulation of VCAM-1 mRNA expression by the concentration of TNFα used in our study, as well as to slight differences in our experimental protocol. Pretreatment of the endothelial cells with the aromatase inhibitor anastrozole abolished the effect of testosterone in attenuating the TNFα-stimulated VCAM-1 mRNA and protein expression, indicating that this action of testosterone must be caused by its conversion to estradiol. Estradiol also decreased TNFα-induced VCAM-1 mRNA expression, which was unaffected in the presence of the aromatase inhibitor. This finding indicated that the aromatase inhibitor did not have any intrinsic activity of its own in modulating VCAM-1 mRNA expression. This action of testosterone in reducing VCAM-1 mRNA expression was abolished in the presence of the ER antagonist ICI-182780, which further confirmed that testosterone was converted to estradiol, which then acted to attenuate VCAM-1 mRNA and protein expression by an action mediated via the ER.
In this study, we did not assess the mechanism(s) by which estradiol attenuated VCAM-1 expression, although various mechanisms may be involved (20). Our observations taken together with those of others may help explain some of the contradictory reports with regard to the role of testosterone in atherogenesis. It seems that the extent of aromatization of testosterone or other androgens may determine whether testosterone is antiatherogenic by being converted to estradiol or proatherogenic by being converted to dihydrotestosterone. Our findings would explain, therefore, why low-dose transdermal testosterone therapy to men with chronic stable angina prolonged the time to myocardial ischemia (10), and why testosterone has vasodilatory effects on the coronary circulation (22, 23). Furthermore, local conversion of circulating testosterone to estradiol by the aromatase in endothelial cells would help explain why the vasodilatory effects on the coronary circulation after testosterone administration were not reflected in an increase in circulating concentration of estrogens (10). On the other hand, abuse of extremely high doses of anabolic steroids has been linked to cardiac death, leading many to believe that the physiologically high levels of androgens in men have a deleterious effect on the male cardiovascular system (3). Thus, excessive doses of aromatizable androgens like testosterone also may be diverted to the 5α reductase pathway, leading to the formation of dihydrotestosterone. This result would have adverse effects on atherogenesis, as has been suggested by other investigators (5, 6, 21). In addition, many nonaromatizable synthetic androgens may have only deleterious effects on the cardiovascular system rather than the beneficial effects seen with testosterone.
Testosterone administration has been shown to improve libido in postmenopausal women (24), a population that is more prone to clinically significant atherosclerosis. It would be interesting to assess whether testosterone administration either alone or in conjunction with low doses of estrogens is able to decrease cardiovascular mortality and morbidity in postmenopausal women. Postmenopausal women with intact ovaries continue to produce aromatizable androgens (25) for several years, unlike women after surgical menopause. It would, therefore, be interesting to assess in postmenopausal women who have never been on hormone replacement therapy whether the rate of progression of atherogenesis and the associated vascular dysfunction is greater in women who have undergone menopause after surgical removal of the ovaries compared to women who have undergone natural menopause and have their ovaries intact.
Acknowledgments
We thank Svetlana Arutyunova for her technical assistance. This work was supported in part by the National Institute of Aging Grant AG-15857. L.N. is a recipient of the Pfizer/Society for Women's Health Scholars Grant for faculty development in Women's Health. H.D. is a recipient of a graduate scholarship from The American Heart Association Southern California Chapter.
Abbreviations
- LDL
low density lipoprotein
- TNFα
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- HUVEC
human umbilical vein endothelial cells
- RT
reverse transcription
- ER
estrogen receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Nathan L, Chaudhuri G. Ann Rev Pharmacol. 1997;37:477–515. doi: 10.1146/annurev.pharmtox.37.1.477. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn M E, Karas R H. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan M L, Martinez C M, Gennis P. Prog Cardiovasc Dis. 1998;41:1–15. doi: 10.1016/s0033-0620(98)80019-4. [DOI] [PubMed] [Google Scholar]
- 4.Hayward C S, Kelly R P, Collins P. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 5.Adams M R, Williams J K, Kaplan J R. Arterioscler Thromb Basc Biol. 1995;15:562–570. doi: 10.1161/01.atv.15.5.562. [DOI] [PubMed] [Google Scholar]
- 6.Fischer G M, Bashey R I, Rosenbaum H, Lyttle C R. Exp Mol Pathol. 1985;43:288–296. doi: 10.1016/0014-4800(85)90066-8. [DOI] [PubMed] [Google Scholar]
- 7.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Circ Res. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Nathan L, Shi W, Dinh H, Mukherjee T K, Wang X, Lusis A J, Chaudhuri G. Proc Natl Acad Sci USA. 2000;98:3589–3593. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.English K M, Mandour O, Steeds R P, Diver M J, Jones T H, Channer K S. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 10.English K M, Steeds R P, Jones T H, Diver M J, Channer K S. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 11.Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- 12.Sasano H, Murakami H, Shizawa S, Satomi S, Nagura H, Harada N. Endocr J. 1999;46:233–242. doi: 10.1507/endocrj.46.233. [DOI] [PubMed] [Google Scholar]
- 13.Diano S, Horvath T L, Mor G, Register T, Adams M, Harada N, Naftolin F. Menopause. 1999;6:21–28. [PubMed] [Google Scholar]
- 14.Caulin-Glaser T, Watson C A, Pardi R, Bender J R. J Clin Invest. 1999;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Q, Nakamura J, Savinov A, Yue W, Weisz J, Dabbs D J, Wolz G, Brodie A. Endocrinology. 1996;137:3061–3068. doi: 10.1210/endo.137.7.8770932. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura J, Lu Q, Aberdeen E, Albrecht E, Brodie A. J Clin Endocrinol Metab. 1999;84:1432–1437. doi: 10.1210/jcem.84.4.5641. [DOI] [PubMed] [Google Scholar]
- 17.Shih P T, Elices M J, Fang Z T, Ugarova T P, Strahl D, Territo M C, Frank J S, Kovach N L, Cabanas C, Berliner J A, Vora D K. J Clin Invest. 1999;103:613–625. doi: 10.1172/JCI5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller W R. Endocr Relat Cancer. 1999;6:187–195. doi: 10.1677/erc.0.0060187. [DOI] [PubMed] [Google Scholar]
- 19.Milewich L, Kaimal V, Johnson A R. J Steroid Biochem. 1987;26:561–567. doi: 10.1016/0022-4731(87)90008-2. [DOI] [PubMed] [Google Scholar]
- 20.Simoncini T, Mafteri S, Basta G, Barsacchi G, Genazzani A R, Liao J K, De Caterina R. Circ Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- 21.McCrohon J A, Jessup W, Handelsman D J, Celermajer D S. Circulation. 1998;99:2317–2322. doi: 10.1161/01.cir.99.17.2317. [DOI] [PubMed] [Google Scholar]
- 22.Webb C M, McNeill J G, Hayward C S, de Zeigler D, Collins P. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 23.Chou T M, Krishnakutty S, Hutchinson S J, Ko E, Amidon T M, Collins P, Chatterjee K. Circulation. 1996;94:2614–2619. doi: 10.1161/01.cir.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 24.Shifren J L, Braunstein G D, Simon J A, Casson P R, Buster J E, Redmond G P, Burki R E, Ginsburg E S, Rosen R C, Leiblum S R, et al. N Engl J Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 25.Judd H L, Judd G E, Lucas W E, Yen S S. J Clin Endocrinol Metab. 1974;39:1020–1024. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]