Abstract
In the penis, nitric oxide (NO) can be formed by both neuronal NO synthase and endothelial NOS (eNOS). eNOS is activated by viscous drag/shear stress in blood vessels to produce NO continuously, a process mediated by the phosphatidylinositol 3-kinase (PI3kinase)/Akt pathway. Here we show that PI3-kinase/Akt physiologically mediates erection. Both electrical stimulation of the cavernous nerve and direct intracavernosal injection of the vasorelaxant drug papaverine cause rapid increases in phosphorylated (activated) Akt and eNOS. Phosphorylation is diminished by wortmannin and LY294002, inhibitors of PI3-kinase, the upstream activator of Akt. The two drugs also reduce erection. Penile erection elicited by papaverine is reduced profoundly in mice with targeted deletion of eNOS. Our findings support a model in which rapid, brief activation of neuronal NOS initiates the erectile process, whereas PI3-kinase/Akt-dependent phosphorylation and activation of eNOS leads to sustained NO production and maximal erection.
Nitric oxide (NO) serves many biological functions. As a neurotransmitter, it is produced by neuronal NOS (nNOS). Vascular tone is regulated by NO formed from endothelial NOS (eNOS). Inducible NOS accounts for diverse functions, especially responses to inflammatory stimuli (1–3). A substantial body of evidence implicates NO in normal erectile function: the nerves that regulate penile erection contain nNOS (4–7), NO donors and NOS inhibitors elicit and prevent erection, respectively (8–12), and mice lacking protein kinase G I (PKGI, a major target of NO/cGMP signaling in the penis) demonstrate a pronounced reduction in reproductive capacity (13).
Neurally derived NO is well established as a mediator of smooth muscle cell relaxation in the penis, engorgement of the cavernous sinusoids, and subsequent erection (14, 15). eNOS is abundant in the endothelial lining of the penile vessels and trabecular meshwork and is also a potential source of NO (16–18). Although erection elicited by electrical stimulation of cavernous nerves is abolished by NOS-inhibiting drugs (8, 19), it is preserved in mice with targeted deletion of nNOSα (nNOS−/−; ref. 16). However, these mice do not manifest total loss of nNOS in the brain, and in penile tissue from nNOS−/− mice NO production is not lost (ref. 20 and A.L.B., M.A.P., B.M., A. Sawa, J.K.C., K.J.H., and S.H.S., unpublished data). Penile erection is retained also in eNOS−/− mice (21).
nNOS and eNOS are activated by calcium entry into the cell, binding to calmodulin associated with the enzymes (22). Whereas physiologic penile erection lasts several minutes, the calcium-dependent activation of nNOS or eNOS is quite transient. Recently, several groups showed that the phosphatidylinositol 3-kinase (PI3-kinase) pathway that activates the serine/threonine protein kinase Akt (also known as PKB) causes direct phosphorylation of eNOS, reducing the enzyme's calcium requirement and causing increased production of NO (23–25). This pathway is responsible for both shear stress and growth-factor enhancement of blood flow that can last for hours (26–30). We have examined whether Akt regulation of eNOS occurs during penile erection and whether that regulation is important in producing and maintaining normal penile erection.
We now show that electrical stimulation as well as drug-induced relaxation of penile erectile tissue increases phosphorylation and activation of Akt as well as phosphorylation of eNOS. This response is reduced by PI3-kinase inhibitors. Moreover, penile erection monitored by changes in intracavernous pressure (ICP) elicited pharmacologically is diminished markedly in eNOS−/− animals. We propose a model integrating the neuronal and endothelial components of NO production in penile erection.
Materials and Methods
Reagents.
Wortmannin and LY294002 were from Calbiochem. Mouse monoclonal anti-eNOS antibody was from BD Transduction Laboratories (San Diego). Anti-phospho-Akt-S473, anti-Akt, and anti-phospho-eNOS-Ser-1177 antibodies were from Cell Signaling Technology (Beverly, MA).
Animal Models.
Age-matched adult male Sprague–Dawley rats (Charles River Breeding Laboratories) or C57BL6 (wild type; The Jackson Laboratory), eNOS−/− and nNOS−/− mice (31, 32) were anesthetized with 40 mg/kg pentobarbital (Abbot). In rats, systemic blood pressure (mean arterial pressure) was monitored continuously via the right carotid artery. To monitor ICP, the shaft of the penis was denuded of skin and fascia and the left corpus cavernosum perforated with a 23- (rat) or 25-gauge (mouse) needle connected via PE-50 tubing to a pressure transducer (DI-190; Dataq Instruments, Akron, OH) as described (33, 34). Response parameters were calculated by using MATLAB software (Mathworks, Natick, MA).
Physiologic Erection Studies.
For electrically stimulated penile erections, a bipolar electrode attached to a Grass Instruments S48 stimulator (Quincy, MA) was placed around the cavernous nerve as described (8). Stimulation parameters ranged between 4 and 6 volts at a frequency of 16 hertz with square-wave duration of 1–5 ms. To induce erection pharmacologically, the right corpus cavernosum was perforated with a 23- (rat) or 27-gauge (mouse) needle. Papaverine hydrochloride was injected intracavernosally (1.5 mg for rats and 0.4 mg for mice; refs. 16 and 35). PI3-kinase inhibitors wortmannin and LY294002 or inactive wortmannin were prepared in 30–50 μl of 0.15% DMSO and injected into the rat right corpus cavernosum 10 min before electrical stimulation or papaverine injection. The lowest dose of wortmannin within the penis was estimated at 2 nM and the highest at 2 μM, and the range for LY294002 was from 10 nM to 10 μM. In immunoblot studies, animals were stimulated once for 1 min or administered a single dose of papaverine after injecting the inhibitor. Penes were then excised and processed for phosphoprotein analysis. For ICP dose-response studies, the stimulus interval was 1 min with at least 20 min between stimulations at increasing doses of inhibitor or of vehicle alone.
Preparation of Protein Extracts and Western Immunoblot.
Minced tissue was homogenized in 8 volumes of ice-cold buffer containing 50 mM Tris, pH 7.5, 2 mM EDTA, 2 mM EGTA, 150 mM NaCl, 1% Triton X-100, 0.5% Nonidet P-40, 10% glycerol, and protease and phosphatase inhibitors (50 mM NaF/5 mM sodium pyrophosphate/1 mM benzamidine/1 μM Microcystin LR/20 mM β-glycerolphosphate/1 mM sodium vanadate/2 μg/ml aprotinin/10 μg/ml leupeptin/10 μg/ml trypsin inhibitor/1 mM Pefabloc). After centrifugation at 16,000 × g for 30 min, soluble protein was determined by using the BCA assay (Pierce). Protein (1–3 mg) was added to 40 μl of packed 2′,5′-ADP-Sepharose (22, 25). After 1.5 h, the beads were washed as follows: PBS/400 mM NaCl/1% Triton X-100; PBS/2% Triton X-100; and finally PBS alone. Bound protein was eluted in 30 μl of SDS loading buffer (62.5 mM Tris, pH 6.8/2% SDS/10% glycerol/2 mM EDTA) at 100°C for 3 min. Purified eNOS or 50 μg of crude homogenate (for Akt) was resolved on 7.5 or 12% Tris gels (Bio-Rad), transferred to nitrocellulose, probed at 4°C with rabbit polyclonal anti-phospho-eNOS-S1177 antibody (1:400) or rabbit polyclonal anti-phospho-Akt-S473 antibody (1:1,000), and then stripped and reprobed for total eNOS (1:1,000) or Akt (1:5,000). Results were quantified by densitometry, and the ratio of phospho to total protein was determined in arbitrary units and expressed relative to the ratio for sham-treated animals prepared and blotted at the same time.
Immunohistochemistry.
Whole penes removed from adult rats after electrical stimulation were embedded in Tissue-Tek (VWR Scientific). Transverse sections (6 μm) were cut and mounted on slides, fixed in 4% paraformaldehyde, permeabilized in 0.4% Triton X-100, and quenched in 3% H2O2 in PBS for 15 min. All slides were subsequently incubated in PBS containing 1% goat serum for 1 h and then at 4°C overnight in PBS with 0.2% BSA and the indicated commercial antibodies: eNOS, 1:250; phospho-eNOS-S1177, 1:200; Akt, 1:200; phopho-Akt-S473; 1:250; and Factor VIII, 1:200. Staining was visualized by using an Elite Vectastain kit (Vector Laboratories). Controls without primary antibody were run for each set of slides.
Statistical Evaluation.
Statistical analysis was performed by using one-way ANOVA followed by Newman–Keuls multiple comparison test or by t test when appropriate. The data are expressed as mean ± SE. A value of P < 0.05 was considered significant.
Results
Electrical Stimulation and Pharmacostimulation Elicit Phosphorylation of Akt and eNOS in the Penile Endothelium.
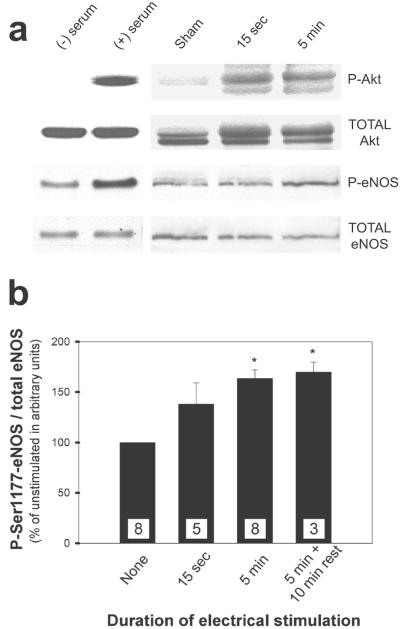
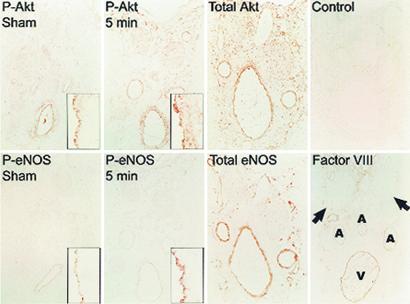
Akt is a serine/threonine kinase activated by phosphatidylinositol 3,4,5-trisphosphate (PIP3) and several phosphatidylinositol-dependent protein kinases (36). Phosphorylation of S473 on Akt is coincident with Akt activation in vivo and in vitro and has been used as a marker for Akt activity by using phospho-specific antibodies (37, 38). We elicited erection in rats by stimulating the cavernous nerve electrically (8, 33) for 15 sec or 5 min and then assessed changes in whole-organ Akt phosphorylation by Western blots. Electrical stimulation dramatically increased endogenous Akt-S473 phosphorylation as well as the phosphorylation of native eNOS at S1177 (eNOS-S1177), the site phosphorylated by Akt, as monitored with another phospho-specific antibody (Fig. 1). The ratio of phospho-eNOS to total eNOS increased by 30–40% after 15 sec and by 60–70% after 5 min. eNOS phosphorylation was maintained for at least 10 min after termination of the electrical stimulus. We localized phospho-Akt and phospho-eNOS immunohistochemically in serial sections of rat penis stimulated for 0 (sham) or 5 min (Fig. 2). Phospho-Akt and phospho-eNOS colocalized in the penile endothelium in these sections, and both increased after electrical stimulation.
Figure 1.
Electrical stimulation of the cavernous nerve enhances phosphorylation of Akt-S473 and eNOS-S1177 in the rat penis. (a) Representative Western immunoblots of phospho-Akt-S473 and total Akt (top two rows) or phospho-eNOS-S1177 and total eNOS (bottom two rows) after electrical stimulation of the cavernous nerve for 0 sec, 15 sec, or 5 min. Gel shift of phosphorylated form in the total Akt blot is consistent with phospho-Akt staining. HEK293 cells transfected with a plasmid expressing Akt or eNOS were starved of serum for 24 h [(−) serum] to abolish Akt activity or starved and then stimulated with 50 ng/ml insulin-like growth factor-1 for 5 min [(+) serum] as controls for each set of blots. (b) Changes in phospho-eNOS after electrical stimulation for 0 sec, 15 sec, 5 min, or 5 min followed by 10 min of rest. Bands were quantified in arbitrary units by densitometry. Each bar represents the mean ± SE of phospho-eNOS/eNOS expressed relative to sham control result. The number of measurements is indicated in each bar. *, P < 0.05 vs. control by t test.
Figure 2.
Immunohistochemical localization of Akt and eNOS in serial sections of rat penis shows increased staining of phosphorylated forms in the endothelium after electrical stimulation. Rats were sham-treated or electrically stimulated for 5 min before excising the penis and staining for the indicated proteins. Low-power views (×40) show a cross-section of the penis with major structures marked in the last micrograph. (Insets) Segments of penile vascular endothelium stained for phospho-Akt or phospho-eNOS (×400) are shown. Factor VIII staining is specific for the endothelium. A, artery; V, dorsal vein. The arrows indicate dorsal nerves.
Blood flow in the penis increases rapidly at the onset of erection (39), and shear stress associated with augmented blood flow activates Akt, leading to phosphorylation of eNOS-S1177 (23, 26, 40, 41). To determine whether the increase in eNOS phosphorylation elicited by cavernous nerve stimulation is secondary to augmented blood flow, we injected rats with papaverine intracavernosally, a procedure well known to elicit vasorelaxation and to increase ICP in rodents (42), and assessed the phosphorylation state of eNOS-S1177 and Akt-S473 (Table 1). Although papaverine responses are more variable than those after electrical stimulation, papaverine induced phosphorylation of Akt by 40% and of eNOS by 70%, indicating that augmented blood flow and endothelial shear stress may account for increased phosphorylation of penile eNOS.
Table 1.
Changes in the phosphorylation of Akt and eNOS during stimulated penile erection
| Electrical stimulation | % Phospho-Akt-S473 | % Phospho-eNOS-S1177 |
|---|---|---|
| None | 100 (4) | 100 (6) |
| 1 min | 176 ± 33* (3) | 164 ± 22* (6) |
| 1 min + wortmannin | 115 ± 7 (4) | 159 ± 14 (4) |
| 1 min + LY294002 | 106 ± 7 (4) | 123 ± 13 (4) |
| Papaverine | ||
| None | 100 (4) | 100 (9) |
| Papaverine alone | 135 ± 7 (4) | 171 ± 19* (9) |
| Papaverine + wortmannin | 86 ± 6 (4) | 127 ± 15 (4) |
| Papaverine + LY294002 | 80 ± 17 | 139 ± 24 (4) |
The numbers represent the mean change ± SE from unstimulated (sham) control for the ratio of phosphorylated to total protein as determined by densitometric analysis of Western blots using specific antibodies as described for Fig. 1. The numbers in parentheses indicate measurements per condition.
, P < 0.05 compared to unstimulated condition by t test.
PI3-Kinase Mediates Phosphorylation of Akt and eNOS and Augments ICP.
Akt phosphorylation and activation in most systems depend on PI3-kinase activation (36). To determine whether phosphorylation of Akt and eNOS during erection similarly depends on PI3-kinase, we injected the PI3-kinase inhibitors wortmannin and LY294002 directly into the penis before 1 min of electrical stimulation (Table 1). Wortmannin decreased the stimulated increase in phospho-Akt by 40% but had only a modest effect on stimulated phosphorylation of eNOS under these conditions. LY294002 decreased phosphorylation of both Akt and eNOS. Because we were unable to assay PI3-kinase activity in penile homogenates, we could not determine the exact relationship between the extent of inhibition of PI3-kinase by direct injection of the drugs and of Akt and eNOS phosphorylation.
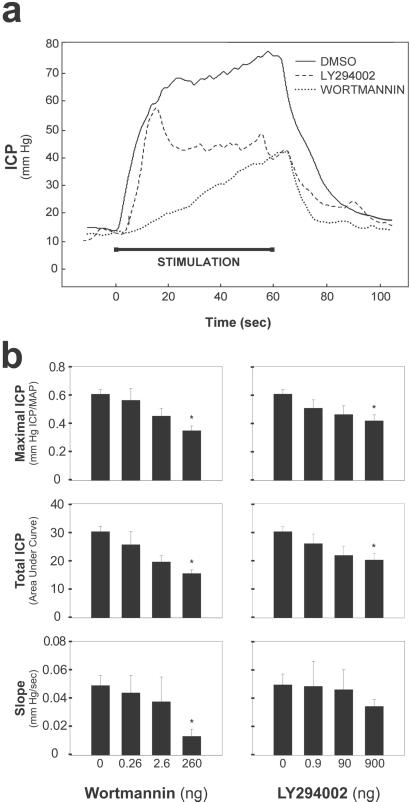
We assessed the physiologic consequence of eNOS phosphorylation/activation after electrical stimulation by monitoring changes in ICP (Fig. 3). Intracavernosal injection of wortmannin (260 ng) or LY294002 (900 ng) decreased ICP 50 and 30%, respectively. The rate of rise of ICP (slope) was diminished significantly by wortmannin. Repeated injection of vehicle alone (DMSO) or wortmannin that was inactivated by overnight incubation at 37°C failed to alter erection (data not shown).
Figure 3.
Wortmannin and LY294002 reduce ICP increases elicited by electrical stimulation of the cavernous nerve. (a) Representative ICP responses for individual rats stimulated 10 min after intracavernosal injection of wortmannin (260 ng), LY294002(900 ng), or DMSO alone (0.15%). The stimulus interval is indicated by a solid bar. (b) Mean maximal ICP, total ICP, and tumescence slope calculated for rats treated with the indicated doses of wortmannin (left side) or LY294002 (right side). The slope and area under the curve were calculated from ICP recordings normalized to mean arterial pressure (MAP). Each bar depicts the mean ± SE for n = 7 (wortmannin) or 8 (LY294002) animals. *, P < 0.05 vs. vehicle alone by ANOVA.
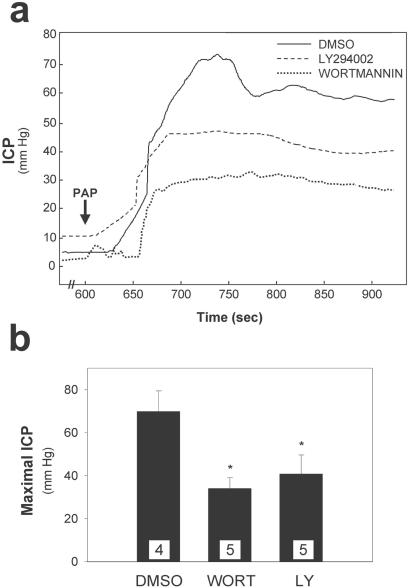
Finally, we tested whether the increases in ICP, phospho-Akt, and phospho-eNOS observed with papaverine injection were affected by treatment with PI3-kinase inhibitors. Stimulated phospho-Akt and phospho-eNOS are both decreased by wortmannin and LY294002 (Table 1). Moreover, both drugs affected maximal ICP attained with papaverine (Fig. 4). Wortmannin reduced maximal ICP by 60%, whereas LY294002 reduced it by 40%.
Figure 4.
Increased ICP after intracavernous papaverine injection (PAP) is reduced by PI3-kinase inhibitors. (a) Representative ICP tracings for erections elicited in rats by 1.5 mg of papaverine 10 min after intracavernous injection of wortmannin (WORT, 260 ng), LY294002 (LY, 900 ng), or DMSO alone (0.15%). (b) Mean ± SE for maximal ICP of the indicated number of animals in each treatment group. *, P < 0.05 vs. DMSO alone by ANOVA.
eNOS Deletion Impairs Penile Erection Elicited by Drug-Dependent Relaxation.
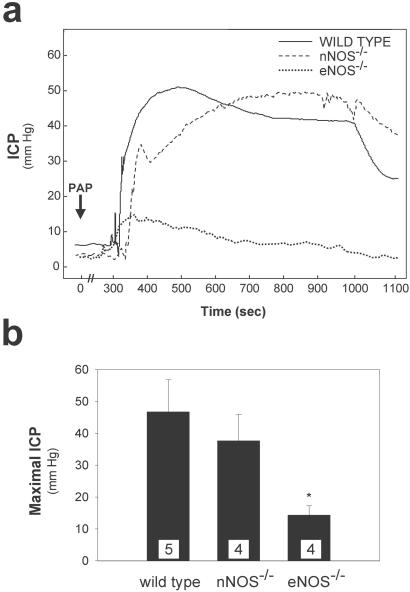
Our findings are consistent with a model in which penile erection is initiated by a neuronal pool of NO produced by nNOS, which is short-lived, followed by a prolonged shear stress-dependent activation of eNOS. This model predicts that deletion of eNOS should prevent maximal erections initiated by vasorelaxation. We monitored ICP after vasorelaxation elicited by papaverine in wild-type and eNOS−/− mice (Fig. 5). In wild-type mice intracavernosal injection of papaverine augmented ICP, although more gradually than electrical stimulation (33). ICP increased to maximal levels in 3–5 min in mice in contrast to the 10-sec time frame observed with electrical stimulation and was sustained for ≈10 min. The increase in maximal ICP for eNOS−/− mice was reduced by 75% after papaverine treatment. In marked contrast, the response of ICP to papaverine treatment in nNOS−/− animals was practically indistinguishable from wild-type mice.
Figure 5.
eNOS−/− mice show decreased erectile response to papaverine injection (PAP) compared with wild-type and nNOS−/− animals. (a) Representative ICP tracings showing 0.4 mg of papaverine's effect on ICP in individual mice. Injection of papaverine is indicated, with elevation of ICP occurring ≈5 min later. (b) Mean ± SE for maximal ICP response in wild-type, eNOS−/−, and nNOS−/− mice after intracavernous injection of papaverine. The number of animals tested is indicated in each bar. *, P < 0.01 vs. wild-type response by ANOVA.
Discussion
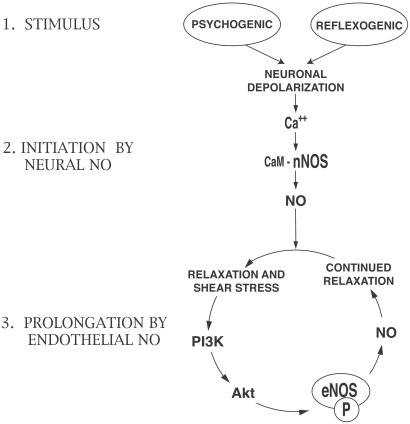
Our findings support a model in which nNOS and eNOS, respectively, mediate the initiation and sustained (tumescence) phases of maximal penile erection (Fig. 6). In the urologic literature, “sustained erection” often refers to long-term maintenance of maximal erection caused by venoocclusive and other factors (43). In this study, attainment of complete tumescence presumably involves amplification by eNOS of nNOS-initiated relaxation. Penile erection begins with psychogenic stimuli as well as tactile (reflexogenic) stimuli that excite pelvic plexus neurons and activate the postganglionic cavernous neurons that innervate the penis directly (44). Neural signals initiate penile erection by activating nNOS after depolarization-induced calcium entry and Ca2+/calmodulin stimulation of nNOS (22). The Ca2+/calmodulin activation of nNOS is reversible and brief, but the vasorelaxation from neurally derived NO causes increased blood flow and physical expansion of penile vasculature and sinusoidal spaces. The resulting shear force on the endothelium of these structures activates the PI3-kinase/Akt/eNOS phosphorylation cascade, causing more sustained NO release and relaxation.
Figure 6.
A model depicting initiation of erection by nNOS and its maintenance by eNOS. Initial stimuli from higher neural centers elicit a relatively short-lived burst of NO from nerve fibers throughout the penile tissue that relaxes smooth muscle, enhancing blood flow. PI3-kinase/Akt activation by the resulting shear stress phosphorylates eNOS (indicated with P) in endothelial cells and provides a sustained increase in NO leading to maximal relaxation that does not require continuous neuronal depolarization. CaM, calmodulin.
Consistent with this model, penile production of NO after electrical stimulation persists much longer than the duration of the stimulus (45). Direct evidence that vasorelaxant stimuli cause prolonged maximal erection comes from studies of intracavernosal injection of papaverine in humans (46) and animals (47). Papaverine is believed to be a nonselective phosphodiesterase inhibitor that promotes smooth muscle relaxation directly (48). Our finding that papaverine is less effective in eNOS−/− than in nNOS−/− mice indicates a crucial role for endothelial NO in papaverine's actions. A trabecular meshwork enriched in eNOS contains gap junctions that relay electrical activity among penile smooth muscle cells (49). Papaverine may induce an initial relaxation propagated by gap junctions that is accelerated by PI3-kinase/Akt stimulation of eNOS and sustained NO production. Our model is supported also by observations that prostaglandin E1, another clinically useful vasorelaxant with a pharmacologic mechanism distinct from that of papaverine, stimulates the formation of NO in the penis and enhances erectile responses to electrical stimulation (50).
Our findings clarify contradictory reports regarding the role of NO in penile erection. Both nNOS−/− and eNOS−/− animals breed normally, implying a capacity for functional erection. The nNOS−/− mice used by ourselves and many others were developed by the deletion of exon 2 (31). Bredt and coworkers (51) subsequently identified alternatively spliced forms of nNOS lacking exon 2, designated nNOSβ and nNOSγ, that are retained in nNOSα−/− animals. We demonstrated that the residual forms of NOS are physiologically active in generating NO in the nervous system (20). We also have observed continued generation of citrulline, a marker for nNOS activity, in the penis of nNOSα−/− animals (A.L.B., M.A.P., B.M., A. Sawa, J.K.C., K.J.H., and S.H.S., unpublished data). The ability of eNOS−/− mice to breed may be caused by the stimulus for erection provided by nNOS, which is retained wholly in the eNOS−/− animals. We have not observed differences in ICP for continuous electrically stimulated penile erection among wild-type, nNOS−/− (16), and eNOS−/− animals (52). Electrical stimulation presumably causes sustained NO production by nNOS, which can maintain an erectile response without the additional activation of eNOS. In combined eNOS−/−/nNOS−/− animals, ICP responses to electrical stimulation are disturbed markedly (M.A.P., D. J. Johns, J.K.C., P. L. Huang, and A.L.B., unpublished data). Before stimulation, the double-knockout mice manifest irregular phasic changes in ICP that are stabilized somewhat by electrical stimulation. When electrical stimulation is terminated, the phasic alterations in ICP resume or are more pronounced.
Our findings have therapeutic implications. Drugs that inhibit the dephosphorylation of eNOS might alleviate erectile dysfunction. eNOS abnormalities may play a role in diabetic erectile dysfunction. Hyperglycemia decreases NO production by eNOS via O-linked glycosylation of eNOS at the Akt target S1177 in hyperglycemic cell culture conditions and in animal models of diabetes (53). Erectile dysfunction in diabetes is associated with peripheral nerve damage but may involve diminished endothelial production of NO as well (54, 55).
Acknowledgments
We thank Bahman Agdhasi and Adam Resnick for helpful discussions and invaluable technical assistance. This work was supported by U.S. Public Health Service Grants DA-00266 (to S.H.S.), MH-18501 (to S.H.S.), DK02568 (to A.L.B.), and Research Scientist Award DA00074 (to S.H.S.).
Abbreviations
- nNOS
neuronal NO synthase
- eNOS
endothelial NO synthase
- PI3-kinase
phosphatidylinositol 3-kinase
- ICP
intracavernous pressure
References
- 1.Bredt D S. Free Radical Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Nat Immun. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 3.Huang P L. J Am Soc Nephrol. 2000;11, Suppl. 16:S120–S123. [PubMed] [Google Scholar]
- 4.Stanarius A, Uckert S, Machtens S A, Stief C G, Wolf G, Jonas U. Urol Res. 2001;29:168–172. doi: 10.1007/s002400100181. [DOI] [PubMed] [Google Scholar]
- 5.Alm P, Larsson B, Ekblad E, Sundler F, Andersson K E. Acta Physiol Scand. 1993;148:421–429. doi: 10.1111/j.1748-1716.1993.tb09578.x. [DOI] [PubMed] [Google Scholar]
- 6.Burnett A L, Tillman S L, Chang T S, Epstein J I, Lowenstein C J, Bredt D S, Snyder S H, Walsh P C. J Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- 7.Hedlund P, Larsson B, Alm P, Andersson K E. Histochem J. 1996;28:635–642. doi: 10.1007/BF02331384. [DOI] [PubMed] [Google Scholar]
- 8.Burnett A L, Lowenstein C J, Bredt D S, Chang T S, Snyder S H. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 9.Melis M R, Stancampiano R, Argiolas A. Neurosci Lett. 1994;179:9–12. doi: 10.1016/0304-3940(94)90922-9. [DOI] [PubMed] [Google Scholar]
- 10.Hellstrom W J, Monga M, Wang R, Domer F R, Kadowitz P J, Roberts J A. J Urol. 1994;151:1723–1727. doi: 10.1016/s0022-5347(17)35353-3. [DOI] [PubMed] [Google Scholar]
- 11.Trigo-Rocha F, Martinez-Pineiro L, Donatucci C F, Hsu G L, Lue T F, Tanagho E A. Int J Impot Res. 1995;7:49–56. [PubMed] [Google Scholar]
- 12.Trigo-Rocha F, Aronson W J, Hohenfellner M, Ignarro L J, Rajfer J, Lue T F. Am J Physiol. 1993;264:H419–H422. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- 13.Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, Fassler R, Andersson K E. Proc Natl Acad Sci USA. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett A L. J Urol. 1997;157:320–324. [PubMed] [Google Scholar]
- 15.Andersson K E. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- 16.Burnett A L, Nelson R J, Calvin D C, Liu J X, Demas G E, Klein S L, Kriegsfeld L J, Dawson V L, Dawson T M, Snyder S H. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- 17.Hedlund P, Ny L, Alm P, Andersson K E. J Urol. 2000;164:868–875. doi: 10.1097/00005392-200009010-00064. [DOI] [PubMed] [Google Scholar]
- 18.Dail W G, Barba V, Leyba L, Galindo R. Cell Tissue Res. 1995;282:109–116. doi: 10.1007/BF00319137. [DOI] [PubMed] [Google Scholar]
- 19.Holmquist F, Stief C G, Jonas U, Andersson K E. Acta Physiol Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- 20.Eliasson M J, Blackshaw S, Schell M J, Snyder S H. Proc Natl Acad Sci USA. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriegsfeld L J, Demas G E, Huang P L, Burnett A L, Nelson R J. Physiol Behav. 1999;67:561–566. doi: 10.1016/s0031-9384(99)00100-6. [DOI] [PubMed] [Google Scholar]
- 22.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A M. Nature (London) 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D, Gratton J P, McCabe T J, Fontana J, Fujio Y, Walsh K, Franke T F, Papapetropoulos A, Sessa W C. Nature (London) 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michell B J, Griffiths J E, Mitchelhill K I, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano P R, Kemp B E, Pearson R B. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 26.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- 27.Gallis B, Corthals G L, Goodlett D R, Ueba H, Kim F, Presnell S R, Figeys D, Harrison D G, Berk B C, Aebersold R, Corson M A. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 28.Fulton D, Fontana J, Sowa G, Gratton J P, Lin M, Li K X, Michell B, Kemp B E, Rodman D, Sessa W C. J. Biol. Chem. 2001. , 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- 29.Michell B J, Chen Z, Tiganis T, Stapleton D, Katsis F, Power D A, Sim A T, Kemp B E. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S, Dernbach E, Zeiher A M. FEBS Lett. 2000;477:258–262. doi: 10.1016/s0014-5793(00)01657-4. [DOI] [PubMed] [Google Scholar]
- 31.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 32.Huang P L, Huang Z, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 33.Sezen S F, Burnett A L. J Androl. 2000;21:311–315. [PubMed] [Google Scholar]
- 34.Mills T M, Wiedmeier V T, Stopper V S. Biol Reprod. 1992;46:342–348. doi: 10.1095/biolreprod46.3.342. [DOI] [PubMed] [Google Scholar]
- 35.Rehman J, Christ G, Melman A, Fleischmann J. Urology. 1998;51:640–644. doi: 10.1016/s0090-4295(97)00693-6. [DOI] [PubMed] [Google Scholar]
- 36.Chan T O, Rittenhouse S E, Tsichlis P N. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 37.Sun M, Wang G, Paciga J E, Feldman R I, Yuan Z Q, Ma X L, Shelley S A, Jove R, Tsichlis P N, Nicosia S V, Cheng J Q. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan Z Q, Sun M, Feldman R I, Wang G, Ma X, Jiang C, Coppola D, Nicosia S V, Cheng J Q. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 39.Kadioglu A, Erdogru T, Karsidag K, Dinccag N, Satman I, Yilmaz M T, Tellaloglu S. Eur Urol. 1995;27:311–314. doi: 10.1159/000475187. [DOI] [PubMed] [Google Scholar]
- 40.Go Y M, Park H, Maland M C, Darley-Usmar V M, Stoyanov B, Wetzker R, Jo H. Am J Physiol. 1998;275:H1898–H1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]
- 41.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher A M. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Pineiro L, Brock G, Trigo-Rocha F, Hsu G L, Lue T F, Tanagho E A. Eur Urol. 1994;25:62–70. doi: 10.1159/000475249. [DOI] [PubMed] [Google Scholar]
- 43.Lue T F, Takamura T, Schmidt R A, Palubinskas A J, Tanagho E A. J Urol. 1983;130:1237–1241. doi: 10.1016/s0022-5347(17)51768-1. [DOI] [PubMed] [Google Scholar]
- 44.Burnett A L. The Handbook of Sexual Dysfunction. San Francisco: Am. Soc. Androl.; 1998. pp. 12–17. [Google Scholar]
- 45.Escrig A, Gonzalez-Mora J L, Mas M. J Physiol. 1999;516:261–269. doi: 10.1111/j.1469-7793.1999.261aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lue T F, Hricak H, Marich K W, Tanagho E A. Semin Urol. 1985;3:43–48. [PubMed] [Google Scholar]
- 47.Chen K K, Chan J Y, Chang L S, Chen M T, Chan S H. J Urol. 1992;147:1124–1128. doi: 10.1016/s0022-5347(17)37500-6. [DOI] [PubMed] [Google Scholar]
- 48.Maggi M, Filippi S, Ledda F, Magini A, Forti G. Eur J Endocrinol. 2000;143:143–154. doi: 10.1530/eje.0.1430143. [DOI] [PubMed] [Google Scholar]
- 49.Campos de Carvalho A C, Roy C, Moreno A P, Melman A, Hertzberg E L, Christ G J, Spray D C. J Urol. 1993;149:1568–1575. doi: 10.1016/s0022-5347(17)36455-8. [DOI] [PubMed] [Google Scholar]
- 50.Escrig A, Marin R, Mas M. J Urol. 1999;162:2205–2210. doi: 10.1016/S0022-5347(05)68160-8. [DOI] [PubMed] [Google Scholar]
- 51.Brenman J E, Chao D S, Gee S H, McGee A W, Craven S E, Santillano D R, Wu Z, Huang F, Xia H, Peters M F, Froehner S C, Bredt D S. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 52.Burnett A L, Chang A G, Crone J K, Huang P L, Sezen S F. J Androl. 2002;23:92–97. doi: 10.1002/j.1939-4640.2002.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 53.Du X L, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Angelis L, Marfella M A, Siniscalchi M, Marino L, Nappo F, Giugliano F, De Lucia D, Giugliano D. Diabetologia. 2001;44:1155–1160. doi: 10.1007/s001250100616. [DOI] [PubMed] [Google Scholar]
- 55.Akingba A G, Burnett A L. Mol Urol. 2001;5:189–197. doi: 10.1089/10915360152745885. [DOI] [PubMed] [Google Scholar]