Abstract
Ion channels in roots allow the plant to gain access to nutrients. The composition of the individual ion channels and the functional contribution of different α-subunits is largely unknown. Focusing on K+-selective ion channels, we have characterized AtKC1, a new α-subunit from the Arabidopsis shaker-like ion channel family. Promoter-β-glucuronidase (GUS) studies identified AtKC1 expression predominantly in root hairs and root endodermis. Specific antibodies recognized AtKC1 at the plasma membrane. To analyze further the abundance and the functional contribution of the different K+ channels α-subunits in root cells, we performed real-time reverse transcription–PCR and patch-clamp experiments on isolated root hair protoplasts. Studying all shaker-like ion channel α-subunits, we only found the K+ inward rectifier AtKC1 and AKT1 and the K+ outward rectifier GORK to be expressed in this cell type. Akt1 knockout plants essentially lacked inward rectifying K+ currents. In contrast, inward rectifying K+ currents were present in AtKC1 knockout plants, but fundamentally altered with respect to gating and cation sensitivity. This indicates that the AtKC1 α-subunit represents an integral component of functional root hair K+ uptake channels.
Arabidopsis shaker-like potassium channel proteins can be divided into five groups: AKT1-, KAT1-, the stelar K+ outward rectifier (SKOR)-like α-subunits (Fig. 1A), and two separate groups, formed by AKT2/3 and AtKC1. Besides AKT5, AKT6, and AtKC1, all distinct α-subunits have been heterologously expressed and electrophysiologically characterized. SKOR and GORK form voltage-dependent outward-rectifying K+ channels (1, 2), whereas KAT1, AKT1, and KAT2 form voltage-dependent inward-rectifying K+ channels (3–5). AKT2/3 exhibits a weak voltage-dependence, conducting potassium at hyperpolarized and depolarized potentials (6, 7). The predominantly expressed channels in the root are AKT1 and SKOR, whereas AKT1 is expressed in the root cortex and epidermis, and SKOR is expressed exclusively in the stele (1, 8, 9). The relative small number of shaker-like channel proteins (10, 11) faces many different types of plant potassium currents (12, 13). Furthermore, there are remarkable differences between currents from native and heterologously expressed channels (14). How can the diversity of potassium currents be created by such a small number of proteins? Like their animal counterparts (15, 16), functional plant potassium channels are most likely formed by four α-subunits (17–19). Besides regulation of channel genes and proteins at the transcriptional (20) or at the posttranslational level (21), respectively, heteromeric assembly of different α-subunits might provide the molecular basis for the functional diversity observed in vivo (22–24). Although there is still controversy over them (25, 26), biochemical and electrophysiological data suggest that, like animal potassium channels (27), different α-subunits of one channel family form hetero-oligomeric channels (18, 25, 28–30).
Figure 1.
(A) Unrooted phylogenetic tree from all members of the shaker-like channel family of Arabidopsis thaliana. Multiple alignment and distance calculations were performed by the WISCONSIN PACKAGE V.10.0 (Genetics Computer Group, Madison, WI) with default parameters. Graphical presentation was done with TREEVIEW 1.6.5 (http://taxonomy.zoology.gla.ac.uk/rod/rod.html). AKT1 [Munich Information Center for Protein Sequences Arabidopsis thaliana database (MAtDB) protein entry code, At2g26650; AKT2/3 (At4g22200), AKT5 (At2g25600), AKT6 (At4g32500), AtKC1 (At4g32650, also named KAT3 or AKT4), KAT1 (At5g46240), KAT2 (At4g18290), SKOR (At3g02850), GORK (At5g37500, also assigned as SKOR2)]. (B) Position of the footprint mutation in plant line Atkc1-f. The diagram depicts the genomic organization of the AtKC1 gene (accession no. U73325) with exons (black boxes) and introns (lines). The sequence is given in detail below. The four are as follows: the derived amino acid and the nucleotide sequence (DNA) of the AtKC1 wild-type allele (wt) and the nucleotide and the derived amino acid sequence of the Atkc1-f mutant allele (footprint, fp). Gaps in the nucleotide sequence, which were introduced for optimal alignment, are indicated by a period (.). The splice site is indicated by an arrow. The primers for footprint sequencing (At-1, At-2) are indicated.
Here, we report on the molecular cloning and localization of AtKC1, a new member of the plant shaker-like channel family. After the isolation of AtKC1 knockout plants, we provide evidence that root hair K+ uptake is based on AtKC1/AKT1 heteromers. This particular type of interaction was not detected in previous studies using heterologous expression systems (17, 18, 25).
Methods
Plant Material, Growth Conditions, and Mutants.
A. thaliana (Col-0) were grown and mutants were isolated as described (31, 32). By PCR (forward primer At-1, 5′-GCC GTT GTT GAG AAG AGG AAG G-3′, position 36, and reverse primer At-2, 5′-CGC CGA ATA CCC AAC CAA TAT CAC C-3′, position 528), we identified a homozygous footprint mutant in which at nucleotide 258, 70 bp had been deleted, and 8 bp of unknown origin inserted (Fig. 1B). This plant was backcrossed against Col-0 wildtype. From F1, the segregating F2 population was generated by self-pollination. For subsequent experiments, plants were chosen that were either homozygous for the AtKC1 wild-type allele (wild-type control) or the Atkc1-f allele (knockout plant), both within a similar En-1 transposon background.
Antibody Production.
Polyclonal antibodies were raised in rabbits (33) and purified by using the PinPoint system (Qiagen, Hilden, Germany) or eight immobilized peptides derived from the AtKC1 C terminus (1, CSG-K528GLNDELKK; 2, CSG-E537IPFLRDLLDDADAQ; 3, CSG-T555VQSEETPQSNDEE; 4, CSG-T571VSRHENGQIEER; 5, CSG-I580EERRREGVPK; 6, C-Q597APPNQDNKNNGDSNGR; 7, CSG-E629KKLGKRGST; 8, CSG-Q649IDALRENDHLYI), according to the manufacturers instruction.
Protein Extraction and Western Blot Analysis.
Arabidopsis microsomes were isolated from roots grown in vitro in liquid culture and Arabidopsis membrane fractions by free-flow electrophoresis as described (34, 35). Proteins were separated on 10% polyacrylamide gels (36) and transferred to Immobilon-P membranes (Millipore). Membranes were blocked and incubated with affinity-purified anti-AtKC1-antibody followed by anti-rabbit antibody conjugated horseradish peroxidase. After washing, blots were developed by using SuperSignal Substrate (Pierce).
Plant Transformation.
For agrobacteria-mediated plant transformation, the binary vector pVKH-35S-pA1, in which the 35S promoter was replaced by the AtKC1 promoter, was used (32). This promoter was amplified by PCR by using the forward primer 5′-TAA TCA CAC AGC CCT TTT AGC C-3′ (position −1,867) and the reverse primer 5′-CAG TAG TCG TCG TAG AGA TTC-3′ (position 19; the mutation of ATG to ATC is underlined). The resulting vector, pVKH-PAtKC1-GUS, consists of a 1.9-kb promoter region, the AtKC1 translational start ATG mutated to ATC, the first six codons of AtKC1, and the full-length uidA gene. This vector was introduced into Agrobacterium tumefaciens GV3101 (37) and transformed into A. thaliana (Col-0) (38).
Quantitative Reverse Transcription (RT)-PCR.
mRNA of root hair protoplasts (see below) was purified 2-fold with the Dynabeads mRNA Direct kit (Dynal, Oslo, Norway) to minimize DNA contaminations. First strand cDNA was prepared by using Superscript RT (GIBCO/BRL) and diluted for RT-PCR 20-fold in water. PCR was performed in a LightCycler (Roche Molecular Biochemicals) with the LightCycler-FastStart DNA Master SYBR Green I Kit (Roche Molecular Biochemicals). Primers, gene accession numbers, and quantification were done according to ref. 29.
β-Glucuronidase (GUS) Assays.
GUS histochemical staining was performed on whole seedlings grown on agar plates. After vacuum infiltration with GUS staining solution (100 mM NaH2PO4, pH 7/10 mM EDTA/0.05% Triton X-100/0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-glucuronic acid) two times for 10 min each, seedlings were incubated overnight at 37°C. Tissue was cleared by treatment with ethanol and analyzed by light microscopy. For sectioning, stained roots were fixed (5% formaldehyde/1% glutaraldehyde/25 mM NaH2PO4, pH 7 for 3 h), embedded in methacrylate as described (39), and cut with the microtome RM2065 (Leica, Deerfield, IL).
Patch-Clamp Recordings.
Protoplasts were isolated according to Ivashikina et al. (40). Measurements were performed in whole-cell mode and data were analyzed as described (40).
Results and Discussion
Isolation and Structure of AtKC1.
The AtKC1 gene was isolated as a 3.8-kb fragment from an Arabidopsis genomic library by using a radioactive-labeled probe derived from the KAT1 gene (41). By using a restriction fragment from the genomic AtKC1 clone, a root-derived λYES cDNA library was screened, and a truncated AtKC1 cDNA, missing the two most N-terminal amino acid residues, was isolated. The full-length cDNA was reconstructed by PCR amplification of a genomic fragment and subsequent ligation as a PstI fragment into the cDNA (41). By sequence comparison of the genomic clone and the AtKC1 cDNA, 12 introns were identified (Fig. 1B). Several in-frame stop codons upstream of the coding sequence and putative polyadenylation signals located at cDNA nucleotides 2,020 (AATATA) and 2,054 (AATAAT) suggested that the cDNA contained the full-length coding region. AtKC1 encodes a deduced protein of 662 amino acids with a predicted molecular mass of 75.6 kDa. Based on protein comparison, AtKC1 shares up to 65% similarity and 45% identity with other known plant shaker-like K+ channels. Within this group, AtKC1 represents the only member of a separate branch (Fig. 1A). Its closest orthologue in plants is KDC1, a carrot root hair K+ channel (42). AtKC1 exhibits typical structural domains of the plant shaker like K+ channels: (i) six transmembrane regions (S1-S6, residues 91 to 329); (ii) eight basic residues (H, K, R) in the S4 voltage sensor (residue 198 to 219); (iii) a pore-region (P) located between S5 and S6 which contained the GYGD motif for K+-selective ion channels; (iv) a putative cyclic nucleotide-binding domain downstream of the S6 segment (residues 421 to 513); and (v) a hydrophobic and acidic C terminus (KHA), which has been suggested to be involved in oligomerization of plant K+ channel α-subunits (18).
AtKC1 Is Predominantly Expressed in Roots.
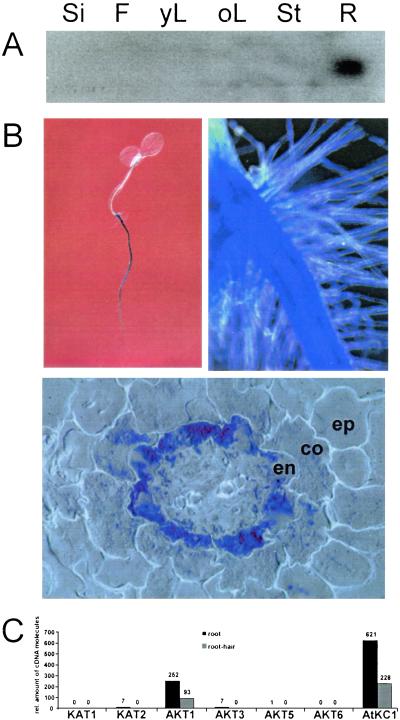
Northern blot analyses were performed to follow the expression pattern of AtKC1. When probing mRNA derived form various Arabidopsis tissues, AtKC1 transcription was detectable in roots only (Fig. 2A). To analyze the expression pattern of AtKC1 at the cellular level, we performed a promoter activity analysis. For this purpose, a 1.9-kb fragment of the AtKC1 promoter was fused to the uidA reporter gene and transformed into Arabidopsis. Tissue-specific expression patterns were analyzed over three generations by histochemical detection of GUS activity in different transgenic lines, which showed the same qualitative staining pattern. Microscopic analysis revealed promoter activity throughout the entire root with decreasing activity toward the root tip (Fig. 2B Upper Left). The highest level of promoter activity was observed in root hairs (Fig. 2B Upper Right) and around the central stele. Semithin sections through roots indicated a gradient of promoter activity ranging from the endodermis to the epidermis with decreasing intensity (Fig. 2B Lower). Because of the higher sensitivity of the promoter-GUS assay compared with Northern Blot analyses, AtKC1 expression also could be observed in leaf nodes, trichomes, and hydathodes (data not shown) of developing Arabidopsis seedlings. A similar expression was found for the AtKC1 orthologue KDC1 from carrot (42).
Figure 2.
AtKC1 expression. (A) Northern Blot analysis of AtKC1 transcripts in different Arabidopsis tissues: Si, silique; F, flower; yL, young leaf; oL, old leaf; St, Stem; R, root. Actin controls (data not shown) confirmed that the gels were identically loaded. (B and C) Analysis of AtKC1 promoter activity in transgenic A. thaliana plants. Upon staining with X-gluc, blue-colored tissue represented areas of AtKC1 promoter activity. Staining can be observed in seedlings throughout the whole root (B, Upper Left) and with high intensity in root hairs (B, Upper Right). On semithin cross sections through the root, staining intensity increased from the epidermis (ep) through cortex (co) to the endodermis (en) (B Lower). (C) AtKC1 and AKT1 represent the major K+-uptake channel transcripts in roots and root hairs. Quantitative RT-PCR was used on total RNA isolated from either root tissue (black bars) or root-hair protoplasts (gray bars) with specific primers for all inward rectifying members of the plant shaker-like potassium channels. The numbers above the bars represent the calculated numbers of cDNA molecules in the individual probes according to (29).
AtKC1 Is a Plasma Membrane-Located Protein.
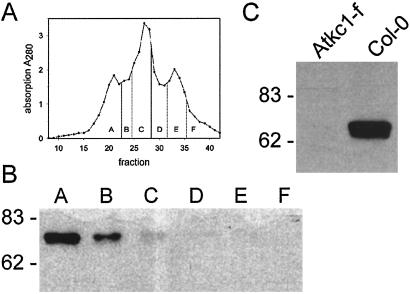
Protein sequence analysis with TargedP (43) indicated the possibility of AtKC1 being targeted to chloroplasts membranes. To reveal the subcellular localization of AtKC1, we used an immunological approach. By using an affinity-purified polyclonal antibody raised against the C-terminal region of the AtKC1 protein (residues 515 to 662), we were able to follow AtKC1 abundance in Arabidopsis microsomal fractions isolated by free-flow electrophoresis (ref. 35; Fig. 3A). According to marker enzyme activity in the membrane fractions (data not shown), subsequent Western blot analyses revealed that AtKC1 localization was confined to the plasma membrane (Fig. 3B, lanes A and B). The recognized protein band of about 76 kDa was well in agreement with the predicted size of the AtKC1 protein. No expression was detectable in the ER, mitochondria or tonoplast.
Figure 3.
(A) The diagram depicts the absorbance at a wavelength λ = 280 nm of fractions obtained by free-flow electrophoresis. According to marker enzyme analysis (not shown), single fractions were pooled into A, plasma membrane; C, endoplasmic reticulum and mitochondria; and E, tonoplast as well as three additional intermediate pools (B, D, and F). (B) Western blot probed with an anti-AtKC1 antibody (1:5,000 dilution) with the main signal in the plasma membrane fraction and a weaker signal in the intermediate fraction. (C) Western blot analysis of root microsomes isolated from wildtype (wt) and Atkc1-f Arabidopsis plants. The blot was probed as in B.
Isolation and Characterization of Atkc1-f.
For functional analysis of the AtKC1 gene, we isolated a knockout mutant (32). We were able to identify Atkc1-f, a stable footprint mutant (Fig. 1B). This mutant exhibited a 70-bp deletion upstream of the En-1 insertion site as well as an additional 8-bp insertion of unknown origin, resulting in the appearance of a PstI site and an in-frame stop codon following Ala-62 of the deduced AtKC1 protein sequence. To demonstrate that the mRNA carrying the footprint mutation did not encode the entire AtKC1 protein, microsomal membranes from Arabidopsis root cultures were subjected to Western blot analysis (Fig. 3C). In microsomes of Arabidopsis wild-type plants, a 76-kDa protein corresponding in size to the deduced AtKC1 protein was detected. In contrast, no immunological signal was observed in Atkc1-f.
Major K+ Channel Transcripts in Root Hairs.
Previous studies (25) have shown that AtKC1 seems not to form functional K+ channels when solely expressed in Xenopus oocytes, human embryo kidney 293 cells, or Sf9 insect cells (unpublished observations). Coexpression of AtKC1 or its orthologue KDC1 with members of other subfamilies strongly affected gating, kinetics, selectivity, and heavy metal resistance (25, 30). Therefore, we studied the potassium conductance of AtKC1 expressing root hair protoplasts isolated from 7-day-old seedlings grown on filter paper. In contact with cell wall degrading enzymes, these cells are the first to release protoplasts (40, cf. 44). After the isolation of mRNA from this protoplast preparation, we used quantitative RT-PCR to determine the expression levels of Arabidopsis shaker-like potassium channels in this cell type. In line with the GUS staining (Fig. 2B), all root hair protoplast preparations tested contained AtKC1 transcripts (Fig. 2C). In contrast to mRNA isolated from young seedlings (data not shown) or whole roots, the root hair protoplast preparation did not contain KAT1 or KAT2, two K+ channels expressed in guard cells, or SKOR, the stelar K+ outward rectifier expressed in root pericycle and xylem parenchyma cells, respectively (Fig. 2C; cf. refs. 1, 5, and 45). Furthermore, transcripts encoding AKT3, a phloem K+ channel (6, 7, 46) were not detected. We found GORK, a SKOR-like outward rectifier initially identified in guard cells (2, 40, and data not shown). The AKT1 and AtKC1 expression in root hairs is in agreement with expression sites of their orthologues LKT1, SKT1, or KDC1 in tomato, potato, or carrot, respectively (42, 47, 48). Based on these expression patterns, the outward rectifier GORK and the two inward rectifiers AKT1 and AtKC1 were supposed to dominate the electrical properties of the root hair plasma membrane.
K+ Currents in Root Hair Protoplasts.
To determine the biophysical fingerprint of the K+ channels functionally expressed in root hairs, we used the patch-clamp technique to study protoplasts isolated from wildtype, Atkc1-f, and Akt1–1 loss-of-function plants. When wild-type root hair protoplasts in the whole-cell configuration were challenged with hyperpolarizing and depolarizing voltage pulses, two types of K+ currents were elicited (Fig. 4A). In the presence of 150 mM K+ in the pipette and 30 mM K+ in the bath, inward rectifying potassium currents were elicited by voltage pulses negative to −80 mV, while outward rectifying K+ currents appeared in response to pulses positive to −30 mV. Inward and outward currents were blocked by extracellular Cs+ or Ba2+, respectively (data not shown). The basic biophysical properties of the inward and outward currents were similar to AKT1- and GORK-mediated currents in heterologous expression systems (2, 49).
Figure 4.
Electrophysiological analyses of whole-cell K+ currents in Arabidopsis root hair protoplasts. In the whole-cell configuration, voltage pulses (A and B) were applied from a holding potential of −48 mV in 20-mV decrements in the range from +52 to −188 mV, with subsequent voltage steps to −88 mV. (A) Voltage- and time-dependent of inward and outward K+ currents in root hair protoplasts from Arabidopsis wild type. (B) Representative outward K+ currents in root hair protoplasts from the Arabidopsis akt1–1 knockout mutant. Voltage- and time-dependent inward K+ currents in root hair protoplasts from Arabidopsis wildtype (C, E, G, I, and K) and Atkc1-f knockout mutant (D, F, H, J, and L). The voltage-current characteristics of inward K+ currents in wild-type (C and E) and Atkc1-f knockout protoplasts (D and F) in the presence of 20 mM (●) or 1 mM (○) CaCl2. Current amplitudes were sampled at the end of 1-s pulses to different voltages in the range from +12 to −188 mV, applied from a holding potential of −48 mV. The data points represent mean values ± SD for n = 10 protoplasts. Representative whole-cell K+ currents in wild-type (C) and Atkc1 knockout (D) protoplasts were measured in an external solution containing 30 mM K-gluconate, 10 mM Mes/Tris (pH 5.6), and 20 mM CaCl2. Voltage pulses were applied from a holding potential of −48 mV in 20-mV decrements in the range from +12 to −188 mV. Steady-state currents were normalized to currents at −168 mV. (G and H) Effect of pH on inward K+ currents in root hair protoplasts from Arabidopsis wildtype and the Atkc1-f mutant. Whole-cell K+ currents in wild-type (G) and knockout protoplasts (H) were elicited by voltage pulse to −188 mV from a holding potential −48 mV. External solutions contained 1 mM CaCl2, 30 mM K-gluconate, and 10 mM Mes/Tris (pH 5.6) or Hepes/Tris (pH 7.0). Current traces represent typical recordings obtained in nine independent experiments. Under these conditions, changes in steady-state current amplitudes in response to alkalization of the bathing medium where −26 ± 5.25% for wildtype and +15 ± 4.24% for Atkc1-f, respectively (values are given in means ± SE). Note the different scales of the current axis. (I–L) Block of inward K+ currents by Rb+ in root hair protoplasts from Arabidopsis wild-type and the Atkc1-f mutant. Whole-cell K+ currents in wild-type (I and K) and Atkc1-f protoplasts (J and L) were elicited by voltage pulses from a holding potential of −48 mV in 20-mV decrements in the range from +12 to −188 mV. (I and J) External solution contained 1 mM CaCl2, 30 mM K-gluconate, and 10 mM Mes/Tris (pH 5.6). (K and L) RbCl (1 mM) was added to external solution.
To separate the individual components of the hyperpolarization-activated whole-cell K+ currents, we analyzed the corresponding currents from the Akt1–1 and Atkc1-f mutant plants. Root hair protoplasts from Akt1–1 plants completely lacked inward rectifying K+ currents (Fig. 4B). In this respect, these root hair protoplasts resembled the properties of protoplasts from nonepidermal root cells isolated from Akt1–1 seedlings (9). Under the same experimental conditions, the amplitude and properties of the outward K+ currents remained essentially like wild type. This feature is well in agreement with the finding that the transcriptional regulation of GORK in guard cells is not affected by the loss of KAT1 function (29).
Protoplasts isolated from Atkc1-f plants, in contrast to the Akt1–1 mutant, exhibited a pronounced inward rectifying K+ current, which was characterized by a positively shifted I/V-curve (about −50 mV; Fig. 4 E and F, open symbols) and faster activation kinetics when compared with the wild type (Fig. 4 I and J). With respect to block of ionic currents by Cs+ or Ba2+, Atkc1-f behaved similarly to wild type (data not shown). Pronounced differences of the Atkc1-f mutant became obvious when protoplasts were challenged with 20 mM Ca2+ in the presence of 30 mM K+ (Fig. 4 C and D). Upon the application of progressively more hyperpolarizing voltage steps, a strong time- and voltage-dependent calcium block appeared in the Atkc1-f plant only (Fig. 4 D and F, closed symbols). A 10-fold reduction in both the K+ and Ca2+ concentrations—closer to the physiological conditions in the rhizosphere—did not alter the basic features of the interaction between the K+ channel and calcium ions (not shown). We found, however, that the susceptibility of inward K+ currents toward protons in Atkc1-f plants were dramatically altered when compared with wild type. In contrast to wild-type root hair protoplasts, extracellular acidification caused a reduction in inward K+ currents (Fig. 4 G and H).
Taken together, the absence of inward K+ currents in root hair protoplasts isolated from Akt1–1 loss-of-function plants, the pronounced changes of K+ inward currents in the Atck1-f mutant, and the inability of AtKC1 to generate a functional channel by its own, strongly suggest that the AtKC1 subunit represents a component of the root hair inward rectifier K+ channel. To prove this, we measured the selectivity for alkali ions by replacing extracellular potassium with sodium or rubidium. In line with a K+-selective channel complex, the exchange of potassium by sodium completely suppressed time-dependent inward currents in both wild-type and Atkc1-f protoplasts (data not shown). Upon replacement of 30 mM K+ by 30 mM Rb+, currents carried by the wild-type root hair inward rectifier dropped by about 70–80%, whereas no inward currents were observed in Atkc1-f protoplasts (data not shown). When 1 mM Na+ was applied in the presence of 30 mM K+ in the bath solution, inward K+ currents remained largely unaffected in both wild-type and Atkc1-f protoplasts (data not shown). However, 1 mM Rb+ in the presence of 30 mM K+ reduced the wild-type K+ currents by 20–30% (Fig. 4K), whereas the inward rectifier in Atkc1-f protoplasts was blocked completely (Fig. 4L). This mutant behavior points to a dramatically changed pore structure of the root-hair inward rectifier channel. AKT1 represents a likely candidate mediating inward potassium currents in Atkc1-f. This hypothesis is further strengthened by the finding that AKT1, when heterologously expressed in insect cells, has a marked susceptibility toward Rb+ and Cs+ ions, although there are some differences with respect to blocking by Ba2+ ions and pH dependencies (49, 50).
Because the biophysical properties of the K+-uptake channel, such as gating, kinetics, and selectivity, are altered in AtKC1-f, AtKC1 seems to represent an integral, pore-lining ion channel subunit rather than a peripheral auxiliary subunit, as known for plant and animal β-subunits (51, 52). In fact, the phenomenon of a “silent” α-subunit with modulating effects is reminiscent of silent α-like subunits of the family of voltage-dependent channels (53–55) as well as cyclic-nucleotide gated channels (56) in the animal kingdom, which alter gating, kinetic, and selectivity because of formation of heteromeric channels. AtKC1 and ATK1 did not form functional K+ channels in heterologous expression systems (25). By using in vivo studies, we provide evidence that, in Arabidopsis root hairs, AtKC1 and AKT1 are part of a functional K+-influx channel.
Root hairs and the endodermis with the casparian strip are exposed places for potassium uptake (57). Knowing that AtKC1 influences the apparent K+ conductance of whole-cell inward currents and has a maximum expression in these two cell types, AtKC1 is likely to be a K+-uptake modulatory subunit needed to adjust the characteristics of plant potassium uptake channels such as AKT1.
Acknowledgments
We thank the service group Automated DNA Isolation and Sequencing (MPJ2), Susanne Michel (Central Analytics of Sonderforschungsbereich 567) for DNA sequencing, and the Center for Functional Genomics in Arabidopsis for En lines and support. We also thank Hervé Canut (Centre National de la Recherche Scientifique, Toulouse, France) for providing FFE fractions. We thank Drs. Petra Dietrich, Benoit Lacombe, and Patricia Wolff for their critical reading and helpful comments on the manuscript. We also thank A. Küch for help in the early steps of analysis and Professor U. J. Flügge and members of his group for support. This work was funded by the Deutsche Forschungsgemeinschaft (SFB 567, SPP Apoplast, Phytohormone und Membrantransport), European Community's BIOTECH program, the International Cooperation Copernicus program, and European Communities V Framework program Grant QLRT-1999–01209 (to R.H and K.P.).
Abbreviations
- GUS
β-glucuronidase
- SKOR
stelar K+ outward rectifier
References
- 1.Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud J B, Sentenac H. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- 2.Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema M R, Hedrich R. FEBS Lett. 2000;486:93–98. doi: 10.1016/s0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- 3.Schachtman D P, Schroeder J I, Lucas W J, Anderson J A, Gaber R F. Science. 1994;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 4.Bertl A, Anderson J A, Slayman C L, Sentenac H, Gaber R F. Folia Microbiol (Praha) 1994;39:507–509. doi: 10.1007/BF02814074. [DOI] [PubMed] [Google Scholar]
- 5.Pilot G, Lacombe B, Gaymard F, Cherel I, Boucherez J, Thibaud J B, Sentenac H. J Biol Chem. 2001;276:3215–3221. doi: 10.1074/jbc.M007303200. [DOI] [PubMed] [Google Scholar]
- 6.Marten I, Hoth S, Deeken R, Ache P, Ketchum K A, Hoshi T, Hedrich R. Proc Natl Acad Sci USA. 1999;96:7581–7586. doi: 10.1073/pnas.96.13.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud J B. Plant Cell. 2000;12:837–851. doi: 10.1105/tpc.12.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C. Plant J. 1996;9:195–203. doi: 10.1046/j.1365-313x.1996.09020195.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch R E, Lewis B D, Spalding E P, Sussman M R. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 10.Czempinski K, Gaedecke N, Zimmermann S, Mueller-Roeber B. J Exp Bot. 1999;50:955–966. [Google Scholar]
- 11.Roelfsema M R G, Hedrich R. Encyclopedia of Life Sciences. 1999. http://www.els.net , http://www.els.net, (Nature Publishing Group, London). , (Nature Publishing Group, London). [Google Scholar]
- 12.Tester M. New Phytol. 1990;114:305–340. doi: 10.1111/j.1469-8137.1990.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 13.Krol E, Trebacz K. Ann Bot. 2000;86:449–469. [Google Scholar]
- 14.Brüggemann L, Dietrich P, Dreyer I, Hedrich R. Planta. 1999;207:370–376. doi: 10.1007/s004250050494. [DOI] [PubMed] [Google Scholar]
- 15.MacKinnon R. Nature (London) 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 16.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 17.Daram P, Urbach S, Gaymard F, Sentenac H, Cherel I. EMBO J. 1997;16:3455–3463. doi: 10.1093/emboj/16.12.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrhardt T, Zimmermann S, Muller-Rober B. FEBS Lett. 1997;409:166–170. doi: 10.1016/s0014-5793(97)00502-4. [DOI] [PubMed] [Google Scholar]
- 19.Uozumi N, Nakamura T, Schroeder J I, Muto S. Proc Natl Acad Sci USA. 1998;95:9773–9778. doi: 10.1073/pnas.95.17.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philippar K, Fuchs I, Luthen H, Hoth S, Bauer C S, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M, et al. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori I C, Uozumi N, Muto S. Plant Cell Physiol. 2000;41:850–856. doi: 10.1093/pcp/pcd003. [DOI] [PubMed] [Google Scholar]
- 22.Wang X Q, Ullah H, Jones A M, Assmann S M. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich P, Dreyer I, Wiesner P, Hedrich R. Planta. 1998;205:277–287. [Google Scholar]
- 24.Hoth S, Dreyer I, Dietrich P, Becker D, Mueller-Roeber B, Hedrich R. Proc Natl Acad Sci USA. 1997;94:4806–4810. doi: 10.1073/pnas.94.9.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyer I, Antunes S, Hoshi T, Mueller-Roeber B, Palme K, Pongs O, Reintanz B, Hedrich R. Biophys J. 1997;72:2143–2150. doi: 10.1016/S0006-3495(97)78857-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbach S, Cherel I, Sentenac H, Gaymard F. Plant J. 2000;23:527–538. doi: 10.1046/j.1365-313x.2000.00828.x. [DOI] [PubMed] [Google Scholar]
- 27.Coetzee W A, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal M S, Ozaita A, Pountney D, et al. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 28.Baizabal-Aguirre V M, Clemens S, Uozumi N, Schroeder J I. J Membr Biol. 1999;167:119–125. doi: 10.1007/s002329900476. [DOI] [PubMed] [Google Scholar]
- 29.Szyroki A, Ivashikina N, Dietrich P, Roelfsema M R, Ache P, Reintanz B, Deeken R, Godde M, Felle H, Steinmeyer R, et al. Proc Natl Acad Sci USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paganetto A, Bregante M, Downey P, Lo S F, Hoth S, Hedrich R, Gambale F. J Bioenerg Biomembr. 2001;33:63–71. doi: 10.1023/a:1005676724618. [DOI] [PubMed] [Google Scholar]
- 31.Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, Saedler H, Weisshaar B. Proc Natl Acad Sci USA. 1998;95:12432–12437. doi: 10.1073/pnas.95.21.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumann E, Lewald J, Saedler H, Schulz B, Wisman E. Theor Appl Genet. 1998;97:729–734. [Google Scholar]
- 33.Reintanz B. PhD thesis. Cologne, Germany: Univ. of Cologne; 1997. [Google Scholar]
- 34.Possee R D, Thomas C J, King L A. Biochem Soc Trans. 1999;27:928–932. doi: 10.1042/bst0270928. [DOI] [PubMed] [Google Scholar]
- 35.Bardy N, Carrasco A, Galaud J P, Pont-Lezica R, Canut H. Electrophoresis. 1998;19:1145–1153. doi: 10.1002/elps.1150190715. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Koncz C, Schell J. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- 38.Bechthold N, Ellis J, Pelletier G. C R Acad Sci Paris (Life Sci) 1993;316:1194–1199. [Google Scholar]
- 39.Kronenberger J, Desprez T, Höfte H, Caboche M, Traas J. Cell Biol Int Rep. 1993;17:1013–1021. [Google Scholar]
- 40.Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle H H, Hedrich R. FEBS Lett. 2001;201:463–469. doi: 10.1016/s0014-5793(01)03114-3. [DOI] [PubMed] [Google Scholar]
- 41.Küch A. PhD thesis. Cologne, Germany: Univ. of Cologne; 1994. [Google Scholar]
- 42.Downey P, Szabo I, Ivashikina N, Negro A, Guzzo F, Ache P, Hedrich R, Terzi M, Schiavo F L. J Biol Chem. 2000;275:39420–39426. doi: 10.1074/jbc.M002962200. [DOI] [PubMed] [Google Scholar]
- 43.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 44.Gassmann W, Schroeder J I. Plant Physiol. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura R L, McKendree W L, Jr, Hirsch R E, Sedbrook J C, Gaber R F, Sussman M R. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deeken R, Sanders C, Ache P, Hedrich R. Plant J. 2000;23:285–290. doi: 10.1046/j.1365-313x.2000.00791.x. [DOI] [PubMed] [Google Scholar]
- 47.Hartje S, Zimmermann S, Klonus D, Mueller-Roeber B. Planta. 2000;210:723–731. doi: 10.1007/s004250050673. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann S, Talke I, Ehrhardt T, Nast G, Muller-Rober B. Plant Physiol. 1998;116:879–890. doi: 10.1104/pp.116.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertl A, Reid J D, Slayman C L. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- 50.Horeau C, Thibaud J B. Experimental Biology Online 3. Cambridge, U.K.: Suppl. 11th International Workshop on Plant Membrane Biology; 1998. [Google Scholar]
- 51.Zhang X, Ma J, Berkowitz G A. Plant Physiol. 1999;121:995–1002. doi: 10.1104/pp.121.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese K P, Silva A J, Storm J F. Ann NY Acad Sci. 1999;868:344–355. doi: 10.1111/j.1749-6632.1999.tb11296.x. [DOI] [PubMed] [Google Scholar]
- 53.Kramer J W, Post M A, Brown A M, Kirsch G E. Am J Physiol. 1998;274:C1501–C1510. doi: 10.1152/ajpcell.1998.274.6.C1501. [DOI] [PubMed] [Google Scholar]
- 54.Salinas M, Duprat F, Heurteaux C, Hugnot J P, Lazdunski M. J Biol Chem. 1997;272:24371–24379. doi: 10.1074/jbc.272.39.24371. [DOI] [PubMed] [Google Scholar]
- 55.Stocker M, Hellwig M, Kerschensteiner D. J Neurochem. 1999;72:1725–1734. doi: 10.1046/j.1471-4159.1999.721725.x. [DOI] [PubMed] [Google Scholar]
- 56.Körschen H G, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Müller F, Dose A, Godde M, et al. Neuron. 1995;15:627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 57.Tester M, Leigh R A. J Exp Bot. 2001;52:445–457. doi: 10.1093/jexbot/52.suppl_1.445. [DOI] [PubMed] [Google Scholar]