Abstract
Background
Crinum macowanii is a therapeutic plant acknowledged for its rich phytochemical profile and traditional medicinal uses. Research on endophytic fungi has surged due to their ability to produce bioactive secondary metabolites, many of which have antimicrobial and antiproliferative or cytotoxic properties. This study evaluated the diversity, antimicrobial and antiproliferative potential, and metabolomic profiles of fungal endophytes isolated from C. macowanii.
Results
Fungal endophytes were isolated from fresh leaves and bulbs of C. macowanii collected from a botanical garden. The endophytes were identified using cultural and molecular techniques, and sequences were deposited in GenBank. Crude secondary metabolites were extracted from the fungal endophytes by fermentation, and the antibacterial activity and minimum inhibitory concentration (MIC) of the extracts were evaluated using a 96-well plate Resazurin Microtiter assay. Cytotoxicity assays were carried out using U87MG Glioblastoma cells and A549 Lung carcinoma cell lines to assess the antiproliferative effect of the secondary metabolites. An untargeted analysis of the bioactive components from two endophytes with the most active antibacterial metabolites was conducted using LC-Q-TOF-MS. The results revealed that six fungal endophytes (Filobasidium magnum, Filobasidium sp., Alternaria alternata, A. tenuissima, Penicillium sp., and P. chrysogenum) were identified from the leaves and bulb of C. macowanii. There was significant antibacterial activity, specifically from Penicillium sp., against several pathogens of public health importance. Cytotoxicity assessments on A549 lung carcinoma and UMG87 glioblastoma cell lines showed mild cytotoxicity compared to the auranofin control, with 87.13% cell viability at 100 µg/mL on A549 cells. LC-Q-TOF-MS analysis identified eight secondary metabolites. Four were shared between A. tenuissima and Penicillium sp., while each species produced two unique compounds. The high efficacy of some of the extracts from these endophytes against clinically relevant pathogens underscores their relevance to public health, especially in combating infectious diseases where traditional antibiotics could have been ineffective.
Conclusion
This study emphasizes the importance of endophytes in drug discovery, particularly in contexts where resistance to conventional treatments is rising. With AMR at critical levels, endophytes like Penicillium species offer leads for next-generation antibiotics to overcome pathogen resistance. The selective antiproliferative potential of the crude extract warrants further investigation, as it could pave the way for developing novel, targeted anticancer therapies with potentially fewer side effects.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-05011-9.
Keywords: Cytotoxicity, Antimicrobial resistance, Antiproliferation, Secondary metabolites, Chromatography
Background
The search for novel bioactive compounds has been intensified by the global surge in antimicrobial resistance (AMR) and the persistent challenge of developing effective and targeted therapeutics from natural sources [1–3]. Among these, endophytic fungi (fungi capable of stable occurrence within tissues of plants without causing apparent harm) have emerged as promising reservoirs of diverse secondary metabolites with probable medical and pharmaceutical applications [4–6]. These endophytes exist in a symbiotic relationship with their host plants and often produce unique bioactive compounds that usually contribute to the survival strategies and the host’s defence mechanisms [7, 8].
Crinum macowanii, a therapeutic plant acknowledged for its rich phytochemical profile and traditional medicinal uses [9, 10], presents an ideal model for investigating endophyte-host interactions and prospective biotechnological applications. C. macowanii belonged to the Amaryllidaceae family and has been historically utilized as herbs in various traditional medicine systems, suggesting the presence of bioactive compounds that may originate from or be influenced by its endophytic partners [9, 10]. The synergistic association between medicinal plants and their endophytic microbes often produces unique metabolites, making these associations particularly valuable for natural product discovery [6, 7, 11, 12]. Research on endophytic fungi, especially those from medicinal plants, has surged due to their unique ability to produce bioactive secondary metabolites, many of which have antimicrobial and cytotoxic or antiproliferative properties. Studies by Ogofure et al. [13] and Kumar et al. [14] have shown that these endophytes often produce metabolites such as tubulysin B, fecosterol and paclitaxel that mimic or complement the bioactivity of their host plants. Further, the symbiotic relationship between endophytes and their host plants is proposed to facilitate unique metabolic pathways distinct from free-living fungi, making them a reservoir of novel compounds with potentially novel mechanisms against pathogens [7, 12, 13, 15].
Current developments in analytical techniques, particularly Liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (LC-Q-TOF-MS), have transformed our ability to identify and characterize complex metabolites produced by fungal endophytes [16, 17]. This technological progress and molecular and phylogenetic analysis advancements enable a more comprehensive understanding of plant-associated microorganisms’ diversity and biosynthetic abilities. The tissue-specific colonization patterns by endophytes within different plant tissues add a layer of complexity to these interactions, potentially influencing the types and distributions of bioactive compounds produced [18, 19]. Thus, in the context of drug discovery, endophytic fungi offer numerous advantages as they can produce bioactive compounds independent of their host plant, once isolated and cultured, and present a sustainable alternative to plant extraction. Furthermore, the diversity of secondary metabolites produced by these endophytes frequently includes unique compounds with biological activities [20, 21]. The potential for antimicrobial and antiproliferative properties in endophyte-derived compounds is particularly relevant in the current pharmaceutical landscape due to the growing threat of AMR [22] and humanity’s bleak future if the war against multidrug-resistant pathogens is not won.
Therefore, exploring the vast and rich plant-microbe interactions to obtain antimicrobial and antiproliferative or cytotoxic compounds that benefit humanity is of renewed importance. As traditional antibiotics become increasingly ineffective, fungal endophytes, with their unique ecological niche and evolutionary history, may provide novel compounds with a unique action mechanism that can circumvent existing resistance mechanisms [3, 23]. Similarly, searching for anticancer compounds with selective cytotoxicity remains a critical area of research. Targeting cancer cells while minimizing damage to healthy tissues represents a significant challenge in cancer therapy. Endophyte-derived compounds, having evolved within the complex chemical environment of their host plants, may offer unique solutions to this challenge [24, 25]. Despite the potential of fungal endophytes as sources of bioactive compounds, several aspects of their diversity, metabolic capabilities, and host-specific adaptation still need attention [26]. A comprehensive analysis exploring metabolomic profiling, phylogeny and biological activity assessments is essential to appreciate and exploit the potential of these endophytes in their entirety. More so, a good understanding of the patterns of secondary metabolite distribution between different endophytic species and their relationship to observed biological activities can provide valuable insights into their potential applications and ecological roles.
In light of the foregoing, our study specifically sought to investigate the diversity and biosynthetic capabilities of fungal endophytes associated with the leaves and bulbs of Crinum macowanii, emphasizing their antimicrobial and antiproliferative potential. The core research question driving this work is: Do crude extracts from fungal endophytes isolated from different tissues of C. macowanii possess bioactive properties that justify further investigation for drug discovery? Our intention in this study was to establish a foundational understanding of the biosynthetic potential of the isolated endophytes (associated with C. macowanii) and to evaluate whether the crude extracts possess any biologically relevant activity worth pursuing further. While using crude extracts has limitations, it remains a widely accepted initial screening approach in natural product discovery [27–29], especially when dealing with organisms that produce structurally diverse secondary metabolites. Therefore, we aimed to thoroughly investigate the leaves and bulbs of C. macowanii for fungal endophytes with a special focus on metabolomic profiling and the bioactivity of its crude extract using advanced analytical methods. Ultimately, our objective was to assess the pharmacological promise of these fungal endophytes by integrating phylogenetic analysis, secondary metabolite profiling, and biological screening in a unified approach. Consequently, by employing a multi-faceted approach to assessing the biological activity of crude secondary metabolites, we seek to contribute to the growing body of knowledge on endophyte-derived bioactive compounds and their potential therapeutic applications.
Methodology
Sample collection and preparation
The bulbs and leaves of C. macowanii samples used in the study were collected once from the Walter Sisulu National Botanical Garden (Gauteng, South Africa (SA) 26°05’10.4"S 27°50’41.5” E) in February 2019. The samples were formally identified by a Botanist, Professor Annah Moteetee, and a voucher specimen (BTNST02) was assigned. All samples looked fresh, healthy and devoid of disease symptoms, such as spots, blotch, wilt rust, etc. Following harvesting, the samples were stored at 4 °C in sterile polyethene bags and transported to the Molecular Pathogenic and Molecular Epidemiology Research Group (MPMERG) Laboratory, University of Johannesburg, Doornfontein Campus, South Africa. Upon arrival, some samples were deposited in the University of Johannesburg herbarium with the assigned voucher number, while the other portions were taken to the laboratory. At the laboratory, samples were thoroughly washed with sterile distilled water and analyzed within 2 h.
Isolation of endophytes
Plant tissues were surface-sterilized using a sequential washing protocol consisting of 70% ethanol (1 min), 2% sodium hypochlorite (3 min), and a final rinse with sterile distilled water (2×). This protocol was adapted from Jasim et al. [27]. Fungal endophytes were identified using ITS region sequencing, following standard procedures for molecular classification as described by Kuklinsky-Sobral et al. [28]. Plant samples underwent rinsing multiple times (about ten times) with sterile distilled water before cold sterilization using 70% ethanol for 1 min. Ethanol traces were removed through five additional rinses with sterile distilled water. Furthermore, sodium hypochlorite (1%) was used to treat the samples for 10 min. Before another round of 5 times rinsing with sterilized distilled water. The final rinse water was employed as the control for the isolation of endophytic fungi from the samples. Culturing of endophytes was done after the plant material was in sterilized phosphate-buffered saline (PBS), and the resulting suspension was serially diluted up to a 10⁻³ dilution. The Spread plate method was used for the culture of the samples and the control, and 100 µL was plated on PDA (Potato Dextrose Agar) (HiMedia, USA) in five independent replicates. The PDA plates were incubated at 30º C in an incubator (IncoTherm, Labotec, Johannesburg, SA). The plates were carefully observed for microbial growth over 7 days. The sterilization effectiveness was confirmed by checking the control (wash/last rinse) plates to see whether there was any growth (as the presence of growth would indicate poor or inadequate sterilization). In cases where growth occurs in the wash plates, the corresponding sample plates are discarded, and the sterilization process for the sample is repeated. For samples whose control passed the sterility test, distinct colonies were selected and subcultured on PDA plates to obtain a pure culture. Sterile filter papers were used to preserve the pure fungal isolates after they were cultivated and preserved at 4 0C. The viability and purity of the respective fungus were evaluated bimonthly [29].
Cultural, microscopic and molecular identification
Following the methods employed by Navi et al. [30] and Pitt and Hocking [31], the pure fungal isolates were identified based on their macroscopic and microscopic characteristics. Each isolate was cultured on PDA at 25ºC for 5 d. After incubation, phenotypic or macroscopic features such as the colour of colonies (front and reverse plate culture), size of colonies, conidial structure and mycelial growth were examined. The microscopic identification was evaluated using the wet mount technique for each fungal isolate via the lactophenol cotton blue stain. The microscopic features assessed included phialides, metulae, vesicles, conidia, and stipes. Briefly, a drop of the lactophenol cotton blue stain was placed on a clean slide, and a small sample of the fungal mycelium was added to form a suspension before it was covered with a slip. The resulting slide was then viewed under a light microscope (BX51, Ultra 20 soft imaging system, Olympus, Japan) for a detailed analysis of the morphological characteristics.
The pure colonies of fungal isolates were cultured overnight in potato dextrose broth (PDB) at 30°C and then centrifuged at 13,000 g for 5 minutes. The supernatant was discarded immediately after centrifugation, and fungal DNA was extracted using Fungal Kit™ (Zymo Research, catalogue No. R2014), strictly following the directions stipulated by the manufacturers. Furthermore, the polymerase chain reaction (PCR) technique was used for the amplification of the ITS (Internal Transcribed Spacers) regions of the fungal endophytes. The primers ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’), with DreamTaq™ DNA polymerase (Thermo Scientific™), were used for the amplification of the ITS regions. Products of the PCR were extracted from the gel using the Zymoclean™ Gel DNA Recovery Kit (Zymo Research) before sequencing was carried out in both forward and reverse directions on the Genetic Analyser (ABI PRISM™ 3500xl). Cleaning of the PCR products was done using ExoSAP-it™, and for this process, the manufacturer’s recommendation was strictly adhered to. The sequencing products were further cleaned using the ZR-96 DNA Sequencing Clean-up Kit™ (Zymo Research). The sequences were analyzed using CLC Main Workbench 7, and identification was done through a BLAST search against the NCBI database [28]. The obtained sequences were screened for chimaeras and subjected to BLAST analysis on the NCBI database, and the selected species were those that showed 98–100% similarity. The ITS regions of the identified isolates were deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) to obtain accession numbers.
Extraction of crude extracts (secondary metabolites) by fermentation
The extraction of crude extracts from endophytes by fermentation was done following the adopted protocol by Prabavathy and Nachiyar [32] with minimal modification. Purified colonies of the fungal endophytes were cultured in a cool, adequately autoclaved 2 L PDB (HiMedia, Mumbai) within a 4 L Erlenmeyer flask, allowing space for aeration during fermentation. After inoculating the respective endophytes in the cooled PDB, fermentation began following incubation at 25 0C on a rotatory shaker (Amerex Gyromax, USA) at 100 rpm for 14 days. This rationale is supported by existing literature. For instance, Rumidatul et al. [33] emphasized that the production of secondary metabolites by endophytic and filamentous fungi often reaches a maximum between 10 and 15 days under standard culture conditions, with optimal bioactive compound production from endophytic fungi within this time frame in PDB. On completion of the fermentation period, the potato dextrose broth (PDB) was filtered through a three-layered muslin cloth to separate fungal mycelia. The broth was further filtered using Whatman filter paper No. 1 (Sigma-Aldrich, SA), and a 1:1 (v/v) relative to volume ratio of ethyl acetate was added to the filtrate. The mixture was thoroughly shaken for 10 min before being poured into a separating funnel to enable phase/layer separation. Each layer/phase was separately collected in a conical flask aseptically. Evaporation of the ethyl acetate fraction was carried out using a rotary evaporator. The obtained crude extract was stored in an amber bottle at a cool, dry location until further analysis, such as antibacterial, antiproliferative and metabolite profiling, was conducted.
Antibacterial assay
A 64 mg/mL stock solution of the crude endophytic fungal extract was prepared in 0.1% dimethyl sulfoxide (DMSO) and used for the antibacterial assays. Serial dilutions were made to obtain working concentrations ranging from 32 mg/mL to 0.25 mg/mL. The crude secondary metabolites’ minimum inhibitory concentration (MIC) was determined against a panel of reference bacterial strains of public health importance. These included Pseudomonas aeruginosa (ATCC10145), Proteus vulgaris (ATCC33420), Klebsiella pneumoniae (ATCC10031), Escherichia coli (ATCC10536), Enterobacter aerogenes (ATCC13048), Mycobacterium marinum (ATCC927), Mycobacterium smegmatis (ATCC21293), Staphylococcus aureus (ATCC25923), Streptococcus epidermidis (ATCC14990), Bacillus subtilis (ATCC19659), and Bacillus cereus (ATCC10876). The antibacterial activity was assessed using a broth microdilution method in 96-well microtiter plates, as described by Andrews [34] and Sebola et al. [35], with minor modifications.
A stock solution of the crude extract (64 mg/mL) was prepared in 0.1% dimethyl sulfoxide (DMSO), and serial two-fold dilutions were made in Mueller-Hinton (MH) broth to achieve final concentrations ranging from 32 mg/mL to 0.25 mg/mL. For the mycobacterial strains (M. marinum and M. smegmatis), Middlebrook 7H9 broth supplemented with OADC enrichment was used instead of MH broth. Each well received 100 µL of the bacterial suspension (adjusted to 0.5 McFarland standard) and 100 µL of the respective extract dilution. The outer wells were filled with sterile distilled water to minimize edge effects. Plates were incubated at 37 °C for 24 h, except for M. marinum, which was incubated at 30 °C for 14 days, and M. smegmatis, which was incubated at 37 °C for 24 h. Following incubation, 10 µL of 0.02% resazurin sodium salt solution was added to each well, and the plates were further incubated for 2 h. Colour change from blue to pink or colourless indicated bacterial viability (metabolic activity). The MIC was recorded as the lowest concentration of the extract that prevented colour change, indicating complete inhibition of bacterial growth. Streptomycin (0.064 mg/mL) served as the positive control, while 0.1% DMSO was used as the negative control [36–38].
Cytotoxic assay
The antiproliferative potential of the crude extracts obtained from the fungal endophytes was evaluated on two can [38]cer cell lines (UMG87 glioblastoma cells and A549 lung carcinoma cells) using an MTS (an MTS (3 (4,5-dimethylthiazol-2-yl)-5-(3-carboxy methoxy-phenyl)-2-(4-sulfophenyl)-2 H-tetrazolium) assay as suggested by McCauley et al. [39] and Handayani et al. [40]. These two cell lines are frequently employed in preliminary cytotoxicity screenings, where the aim is to identify broad-spectrum antiproliferative potential before advancing to more specialized or resistant cell lines.
Stock solutions of each extract (200 µg/mL) were prepared in 0.1% DMSO and sonicated for a few minutes until fully dissolved. Serial dilutions using Dulbecco’s Modified Eagle Medium (DMEM) (Merck, Johannesburg, SA) as the diluent were used to dilute the 200 µg/mL stock in a two-fold manner until a final concentration of 3.13 µg/mL was attained. The cells were maintained in DMEM supplemented with 10–15% fetal bovine serum (FBS) (Merck, Johannesburg, SA) under standard conditions (37 °C) in a humidified atmosphere containing 5% CO2 to mimic physiological conditions.
The antiproliferative assay was conducted to evaluate changes in cell viability via a colour change, which is visible at 490 nm under UV-VIS spectroscopy (Eppendorf AG 2231 Biospectrometer).
Consequently, cell viability is directly correlated with the measured absorbance at 490 nm. All samples were analyzed in duplicate across three plates (n = 6), and the average or mean values were reported. The ATCC-referenced cancer cell lines, U87MG (glioblastoma) and A549 (lung carcinoma) cells, were cultured using standard tissue culture techniques with Dulbecco’s Modified Eagle Medium (DMEM, Merck, SA). The medium was supplemented with 15% fetal bovine serum (FBS, Merck, Johannesburg, SA). The referenced cell lines were standardized (1 × 105 cells/mL) and incubated at 37 °C overnight in 96-well plates. Crude endophytic fungal extracts were then added at concentrations ranging from 0 to 100 µg/mL. After a 4-day incubation period, 5 µL of MTS reagent (Promega, USA) was added to each well. Absorbance values were measured at 490 nm after incubation for 1, 2, and 4 h, respectively. Auranofin was used as a positive control, and all assays were performed in triplicates [38, 40]. The cell viability was calculated using the following formula below:
 |
Where Ea stands for the absorbance of the extract, Ba is the absorbance of the blank, and Ca is the absorbance of the control.
LC-QTOF-MS/MS chemotaxonomic metabolomic profiling
The metabolomic profiling was performed using liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (LC-QTOF-MS/MS), following previously described protocols [38, 41, 42]. The system consisted of a Thermo Scientific (Darmstadt) Dionex UltiMate 3000 ultra-high-performance liquid chromatography (UHPLC) coupled to a Bruker Daltonics Compact™ QTOF mass spectrometer (Bremen) equipped with an ESI (electrospray ionization) interface. The crude extracts of the selected fungal endophytes A. tenuissima and Penicillium sp. were prepared for analysis by dissolving 1 mg/mL (w/v) crude extract in HPLC grade methanol (Merck, SA) and sonicated until full dissolution was achieved. The sample was filtered through 0.22 μm PVDF (polyvinylidene fluoride) membrane syringe filters into 1 mL LC auto-sampler vials. Instrument operation, control, and data acquisition were managed using HyStar software version 2.10 (Thermo Scientific, Darmstadt, Germany).
The LC-QTOF-MS/MS system was optimized for high-resolution profiling of fungal extracts. The separation was carried out using a C18 reverse-phase column (50 mm × 2.1 mm, 1.7 μm particle size), with a binary solvent system comprising water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B). The gradient elution started at 5% B, increased to 95% B over 25 min, and held for 5 min before re-equilibration. The flow rate was maintained at 0.3 mL/min, and the column temperature was set at 40 0C. The electrospray ionization (ESI) source operated in positive mode (ESI+) for mass spectrometry detection. The key parameters included a capillary voltage of 3.5 kV, a desolvation gas temperature of 350 °C, and a desolvation gas flow rate of 800 L/h. The collision energy for MS/MS fragmentation was set between 20 and 40 eV, and the scan range was set from 50 to 1200 m/z. Calibration was performed using sodium formate, and leucine-enkephalin was employed as a lock mass to ensure mass accuracy throughout the run. Maintaining the above specific conditions ensured high sensitivity and reproducibility for identifying bioactive compounds [43]. The blank used for properly calibrating the system was HPLC-grade methanol devoid of the analyte. These blanks were run under the same conditions as the sample extracts to account for any contributions from the filter material, and the results were considered during data analysis.
Data analysis and visualization
The data for antibacterial and cytotoxic activities and the comparison of metabolites from the selected endophytes were visualized in R Studio software (version 4.3.3) using specialized R packages [44]. The Complexheatmap and Pheatmap packages [45] facilitated the generation of heatmaps to display antibacterial and cytotoxicity (antiproliferative) results, incorporating hierarchical clustering and custom colour scales. Enhanced visualizations, including heatmaps with numerical data, were also employed using the ggplot2 package [46]. These visualization enhancements mimicked the patterns used by Ogofure and Green [47] and Ogofure et al. [13] for visualizing microbiological data. To compare the secondary metabolites among plant parts, a Venn diagram was constructed using the VennDiagram package [48], featuring custom label positions and colour schemes for clarity. The spectral data processing was performed using Bruker Compass Data Analysis software version 4.3 (Bruker Daltonics, Bremen, Germany). The resulting fragment spectra were characterized using the MetFrag web tool version 2.1 (Git Hub, California, USA), which links to three compound databases: PubChem, ChemSpider, and KEGG [43]. A blank sample containing PDB was analyzed under the same conditions to filter out impurities from the growth medium. Cytotoxic effects across different concentrations (3.13–100 µg/mL) were quantified and represented using a colour gradient scheme. The statistical significance was assessed through multiple approaches. One-way analysis of variance (ANOVA) was performed to evaluate overall differences in cytotoxic effects across all tested concentrations. Pairwise comparisons between individual concentrations were conducted using Student’s t-tests with Bonferroni correction for multiple comparisons. Additionally, paired t-tests were employed to compare each concentration against the highest test concentration (100 µg/mL) as a reference, with p < 005 regarded as the significance level. A two-tailed unpaired Student’s t-test was applied to compare the mean MIC values of fungal endophyte extracts against a control group. The test was performed independently for each treatment group (column-wise), and p-values were calculated using the base t.test() function in R. A p-value < 0.05 was considered statistically significant. Heatmaps were generated to visualize the MIC values and highlight statistically significant differences [38].
Results and discussion
Figure 1. illustrates the identification and phylogenetic analysis of fungal endophytes isolated from both the leaves and bulbs of C. macowanii. The fungal strains identified were deposited in the gene bank under accession numbers MF925700, MF925701, MF925702, MF925703, MF925704, and MF925706. Although ITS sequencing has limitations for species-level resolution, especially among closely related fungal taxa, we employed BLASTn against the NCBI database to obtain provisional taxonomic identities. A phylogenetic tree was constructed using the Interactive Tree of Life (iTOL) to illustrate the evolutionary relationships among the endophytic fungi, visually demonstrating clade differentiation and potential evolutionary convergence. The associated BLAST details (percent similarity, query coverage, and accession numbers) are also available in Supplementary Table S1 for clarity and reference. The constructed phylogenetic tree revealed that the isolated fungal endophytes primarily belong to two distinct phyla: Basidiomycota, represented by blue-coloured nodes, and Ascomycota, represented by red-coloured nodes. The classification underscores the phylogenetic diversity of the fungal endophytes associated with C. macowanii. Furthermore, the endophytes are clustered into two major clades based on the different plant parts wherewith they were isolated. The fungi in the green clades were isolated from the leaves of C. macowanii, whereas those in the blue clades were derived from the bulbs. Remarkably, the blue clades consist of fungal strains such as Filobasidium sp., Filobasidium magnum, Alternaria alternata and Penicillium sp., which are bulb-associated. In contrast, the green clades contain species such as Filobasidium sp. and Alternaria tenuissima from the leaves. Our findings clearly distinguished between the fungal species inhabiting the leaves and those residing in the bulbs, emphasizing the organ-specific colonization patterns of fungal endophytes in C. macowanii. These findings contribute valuable insights into the symbiotic relationships between plants and fungal endophytes, offering potential leads for further studies on plant-microbe interactions.
Fig. 1.

Molecular identification and phylogenetic analysis of fungal endophytes from leaves and bulbs of C. macowanii. The fungal endophytes were deposited in the gene bank with the following accession numbers: MF925700, MF925701, MF925702, MF925703, MF925704 and MF925706
Our study represents the first comprehensive investigation of fungal endophytes in C. macowanii, expanding beyond the previous study by Sebola et al. [49], which focused solely on bacterial endophytes from the same plant. Thus, findings from our study significantly advance our understanding of the microbial ecology of C. macowanii, offering the first comprehensive exploration of its rich and diverse fungal endophytic community/microbiome. Endophytes are microorganisms (bacteria or fungi) that live inside the host plant and participate in several biological processes without causing disease or other adverse effects [13, 50]. The phylogenetic analysis of the isolated fungal endophytes revealed a taxonomic distribution spanning Basidiomycota and Ascomycota (two major fungal phyla). This finding aligns with the broader understanding of the diversity of fungal endophytes across plant species, as noted by Faeth and Fagan [51]. As microbial symbionts, endophytes occupy internal tissues of plants such as Crinum macowanii [52] and play a crucial role in agricultural plant protection against non-insect pests, offering increased fitness and potential for integrated plant health management strategies [53]. More so, our findings support the growing understanding that plant organs often host-specific fungal communities, reflecting functional diversity, and this organ-specific colonization pattern is consistent with other studies on fungal endophytes [54], which demonstrated similar localization preferences in plant tissues. Moreover, the diversity of the isolated strains further emphasizes the ecological and biological significance of C. macowanii as a host for fungal endophytes, potentially linked to its medicinal properties.
The antibacterial activity of crude extracts from fungal endophytes isolated from the leaves and bulbs of C macowanii, tested against a spectrum of referenced pathogenic bacterial isolates, is shown in Fig. 2. The heatmap presents the MICs of the crude extracts of the fungal endophytes, with a colour gradient to show the varying levels of antibacterial efficacy. The MIC values are expressed in mg/mL for the fungal extracts, while the positive control (streptomycin) is expressed in µg/mL. The colour scheme of the heatmap is critical to interpreting the results, where green represents moderate to high antibacterial activity (reflecting MIC values ranging from 30 µg/mL to 8000 µg/mL for treatment aside from the control). The “white portions/cells” indicate mild antibacterial activity, corresponding to MICs between 8000 and 16,000 µg/mL, and the “Yellow” parts indicate low activity, with MIC values greater than 16,000 µg/mL. The heatmap was column clustered so that the antibacterial efficacy of the crude extracts of fungal endophytes could be visualized relative to the control agent used in the study. From the presented data, it was evident that crude extracts from the fungal endophytes, particularly those of Penicillium sp. and A. tenuissima, displayed better antibacterial activity against multiple pathogens, including Bacillus cereus, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae. Remarkably, extracts from Penicillium sp. were also effective against pathogenic strains of Mycobacterium. Filobasidium magnum exhibited lower activity, as highlighted by the yellow colour scheme and higher MIC values, indicating its crude extract’s low sensitivity. Significant differences (p < 0.05) in MIC values were observed between the extract and the control. Extracts showing significant activity were annotated with their respective p-values and marked with an asterisk (*). Notably, extracts from Penicillium chrysogenum showed the lowest MIC values and were significantly more active than the other extracts in our study. The heatmap clearly reveals variations in antibacterial potency among the extracts tested. The broad-spectrum antibacterial activity observed in a few of the extracts from the fungal endophyte has significant implications for public health and drug discovery. With the growing global concern over antimicrobial resistance (AMR), there is an urgent need for novel antibacterial agents. The endophytes from C. macowanii demonstrated considerable potential as sources of new antimicrobial compounds, which could serve as templates for the development of next-generation antibiotics. The ability of some of the extracts to inhibit both Gram-negative and Gram-positive bacteria suggests that their mode of action could be versatile, and this can be further exploited to elucidate their mechanisms of action for therapeutic applications. Our findings on the antibacterial efficacy of Penicillium sp. and A. tenuissima against both Gram-positive and Gram-negative bacteria align with those of Efendi et al. [55], who reported broad-spectrum activity in Penicillium isolates. Specifically, our results suggest that the metabolites from Penicillium sp. may interfere with bacterial cell walls, an effect noted in previous studies on the cell wall-targeting properties of endophyte-derived metabolites [55]. This is particularly relevant given the rise of AMR, where novel antibiotics are in urgent demand [1]. Furthermore, studies by Sebola et al. [37] on C. macowanii bacterial endophytes suggest that the antimicrobial properties of the host plant may, in part, be attributed to its microbial associates, supporting our hypothesis of a symbiotic relationship enhancing C. macowanii’s therapeutic effects.
Fig. 2.
Heatmap of the antibacterial activity (MICs) of crude extracts of fungal endophytes isolated from leaves and bulbs of C. macowanii. Each cell’s colours indicate the antibacterial activity level measured by their respective MICs (in mg/ml except for control (streptomycin) in µg/ml). The colour scale “green” is indicative of moderate to high activity, while “white” designates mild antibacterial activity, and the “yellow” colour designates low antibacterial activity
Furthermore, the high efficacy of these endophyte extracts against clinically relevant pathogens highlights their potential in combating antibiotic-resistant infections. The findings from our study highlight the untapped potential of fungal endophytes as a reservoir of novel bioactive compounds with significant antibacterial properties. Research focusing on isolating and characterizing the specific compounds responsible for these activities should be encouraged.
Although the MIC values recorded for the fungal endophyte crude extracts were comparatively higher than those of the positive control, their antimicrobial potential remains noteworthy. According to the Clinical and Laboratory Standards Institute (CLSI, 2020), as interpreted by Ogofure et al. [13], crude extracts with MIC values below 0.07 mg/mL (70 µg/mL) are considered promising and worthy of further investigation. It is essential to underscore that the extracts tested in this study were in their crude form, typically containing a complex mixture of compounds that may not exhibit optimal activity until further purification and fractionation. Previous studies have shown that bioactivity often improves significantly after isolation and structural refinement of active constituents. Therefore, the observed antimicrobial activity, despite being moderate in comparison to standard antibiotics, highlights the potential of these fungal endophytes as reservoirs of novel antimicrobial agents. These findings provide a strong basis for future studies on compound purification, structural elucidation, and mechanism-of-action analyses.
The antibacterial properties exhibited by the fungal endophyte extracts, particularly those from Penicillium sp. and A. tenuissima, demonstrate the potential of these microorganisms as sources of novel antimicrobial compounds. The broad-spectrum activity observed against both Gram-positive and Gram-negative bacteria is especially significant, corroborating the findings of Efendi et al. [55], who reported that Penicillium sp. isolated from Gambir leaves exhibited broad-spectrum activity against a panel of bacterial and fungal pathogens. The findings from our study were also consistent with previous studies on endophytes from Amaryllidaceae plants [56, 57]. Nonetheless, our findings extend beyond the earlier report on bacterial endophytes by Sebola et al. [49] from C. macowanii, proving that fungal endophytes contribute significantly to the plant’s antimicrobial properties and give perspective to its medicinal plant usage. Sebola et al. [37] evaluated the antibacterial activity against Gram-positive and Gram-negative bacteria. They observed that C. macowanii bulbs and leaves exerted a broad-spectrum activity, suggesting that the plant’s antimicrobial properties may be partially attributed to its fungal and bacterial endophytes. The findings from our study align with broader observations that fungal endophytes are a potential source of antibacterial compounds, with many endophytes showing varying metabolic capacities for synthesizing secondary metabolites [3]. The acceptance of Crinum species for several herbal and traditional uses is due to their potential as a source of pharmaceutical products for treating a plethora of diseases of man and animals. Still, the population (especially of C. macowanii) is declining due to over-harvesting of the plants, especially in southern Africa [9, 57]. As stated above, the fungal or bacterial endophytes might play a role in the medicinal property of the plant. More so, Fennell and van-Staden [10] stated that several biologically active compounds have been isolated from Crinum species on chemical investigation of the plant. Furthermore, our findings revealed the efficacy of Penicillium sp. extracts against Mycobacterium strains. This observation highlights the untapped potential of fungal endophytes in combating challenging pathogens. It emphasizes the importance of exploring diverse ecological niches for novel antimicrobial agents, as emphasized by Abdalla et al. [50].
The antiproliferative effects of secondary metabolites from crude extracts of fungal endophytes isolated from the leaves and bulbs of C. macowanii on A549 lung carcinoma cells are shown in Fig. 3 below. The effect on the extracts was assessed at various concentrations, ranging from 100 µg/mL to 3.13 µg/mL, with cell viability (in percentages) used as the indicator of cytotoxicity (because of their inverse relationship). The colour scheme in the heatmap ranges from “pink” (indicating low cell viability or high cytotoxicity) through “yellow-green” to “blue” (indicating high cell viability or low cytotoxicity). The positive control (auranofin), which was expected to exhibit potent cytotoxic activity, showed significantly low cell viability values across 100–25 µg/mL concentrations, confirming its strong cytotoxic effect on A549 cell lines (as demonstrated by the row clustering in the heatmap, having been separated from the treatments). The observed colour changes in the MTS assay indicate cell viability, where a transition from yellow to dark purple signifies the metabolic reduction of MTS by viable cells. In contrast, pink hues suggest cellular death and a lack of MTS metabolism.
Fig. 3.
Heatmap of the antiproliferative effects (cell viability %) of secondary metabolites of fungal endophytes from leaves and bulbs of C. macowanii on A549 Lung carcinoma cells. Auranofin was used as the positive control in this study
Interestingly, some cell viability values recorded in this study exceeded 100% relative to the untreated control. This outcome likely reflects enhanced metabolic activity in response to certain crude fungal metabolite extracts, suggesting a possible proliferative or stimulatory effect on UMG87 glioblastoma cells. Such responses are common in viability assays like MTT or resazurin, where increased absorbance may indicate elevated cellular activity rather than cytotoxicity. We reported the raw values without normalization to maintain transparency and accurately represent the biological responses observed. This approach enables a more nuanced interpretation of the metabolites’ effects, particularly in cases where stimulation rather than inhibition may be relevant for future therapeutic applications.
However, the metabolites from the fungal endophytes demonstrated low antiproliferative effects, as indicated by the high percentage of cell viability across all tested concentrations. Interestingly, a slight decrease in viability was observed with decreasing concentrations, suggesting a possible reverse dose-dependent trend in some cases. For instance, Alternaria tenuissima exhibited weak antiproliferative activity, with 87.13% cell viability at 100 µg/mL and slightly increased viability (87.51%) at the lowest tested concentration (3.13 µg/mL), indicating minimal inhibitory effects on UMG87 glioblastoma cells. These findings suggest that the crude metabolites are largely non-toxic in their current form and may require further purification or structural optimization to enhance their antiproliferative potential. The antiproliferative profile of the secondary metabolites from fungal endophytes is of considerable interest in the context of anticancer drug discovery. The high cell viability observed across all tested concentrations suggests that these crude metabolites exhibit low nonspecific toxicity, an important characteristic for compounds that selectively target cancer cells while sparing healthy ones. This low antiproliferative effect highlights their potential for further studies to identify selective cytotoxicity against specific cancer cell lines. Moreover, such properties may also support applications in tissue regeneration and therapeutic development, pending purification and mechanistic evaluation of the active constituents. Studies on the cytotoxic effects of endophyte-derived compounds have revealed varied activity across cancer cell lines, often dependent on the endophyte type and the host plant. For example, Varli et al. [58] reported low cytotoxicity of Nemania sp. extracts on lung cancer cells, similar to our findings with Penicillium and Alternaria isolates, which exhibited mild effects on A549 and U87MG cells. This low cytotoxicity pattern encourages selective anticancer therapy, suggesting these endophyte-derived compounds may target cancer cells with minimal harm to healthy cells. In contrast, Liu et al. [59] documented higher cytotoxicity in Aspergillus endophytes from marine environments, illustrating how environmental factors might influence endophyte bioactivity profiles. A One-Way analysis of variance for the data presented below revealed no statistically significant difference in the overall mean cytotoxicity values across the different concentrations when considered together (p > 0.05). The pairwise t-tests using Bonferroni correction show all p-values to be greater than 0.05 (confirming that no significant differences exist between any pair of concentrations, which is the result obtained for ANOVA analysis). The antiproliferative data across different concentrations show that cell viability generally remains high across all concentrations without a clear dose-dependent response. The crude extract, therefore, appears to have limited cytotoxicity against A549 lung carcinoma cells, with no statistically significant effect across the concentration range tested.
The cytotoxic effects of secondary metabolites from endophytes isolated from bulbs and leaves of C. macowanii on the UMG87 glioblastoma cell line are shown in Fig. 4. The heatmap demonstrates a spectrum of cytotoxicity, with the colour gradient ranging from “green” (high cytotoxicity/low cell viability) to “yellow” (low cytotoxicity/high cell viability). The positive control (auranofin), as expected, showed a powerful cytotoxic effect, as indicated by its low cell viability percentages at higher concentrations (1.92% at 100 µg/mL) and increased cell viability (low cytotoxicity) at lower concentrations (89.99% at 3.13 µg/mL), reflecting the expected dose-dependent cytotoxic effect. The row clustering in the heatmap highlighted the cytotoxicity of the crude extracts from the fungal endophytes relative to the control. The results showed that Penicillium chrysogenum, Alternaria tenuissima and Filobasidum magnum demonstrated a mild cytotoxic effect at the highest concentrations (as cell viability was less than 100% at the highest concentration for metabolites of both endophytes) on properties against UMG87 glioblastoma cells, with a remarkable dose-dependent effect. The secondary metabolites produced by these endophytes warrant further investigation, especially when targeting selective cell regeneration. Their mild to low cytotoxicity suggests that these endophytes could be explored as potential sources of novel compounds for selective cytotoxicity or safety in non-cancerous cells. Following a One-Way analysis of variance, there’s no statistically significant difference in the overall mean cytotoxicity values across the different concentrations when considered together (p = 0.893, F-value = 0.327). The pairwise t-tests using Bonferroni correction show all p-values as greater than 0.05 (p > 0.05), confirming no significant differences exist between any pair of concentrations. There appears to be a non-linear dose-response relationship, with the highest cytotoxicity at the low concentrations. In addition, the 12.5 µg/mL concentration shows moderately significant lower cytotoxicity (p = 0.03), and the lowest two concentrations (6.25 µg/mL and 3.13 µg/mL) do not differ significantly from the highest concentration. There appears to be a non-linear dose-response relationship, with the highest cytotoxicity at the lowest concentration (3.13 µg/mL = 85.22%), followed by the highest concentration (100 µg/mL = 82.44%), with lower cytotoxicity at the middle concentrations.
Fig. 4.
Heatmap of the antiproliferative effects (cell viability %) of secondary metabolites of fungal endophytes from leaves and bulbs of C. macowanii on UMG87 glioblastoma cells tested at different concentrations. Auranofin was used as the positive control
The antiproliferative effects of the crude fungal endophyte extracts present an interesting contrast to some existing literature. While our study found relatively low or little effects on A549 lung carcinoma cells and UMG87 Glioblastoma cells, it was at variance with the reports of Liu et al. [59], who showed that ten endophytic fungal strains were found to have very high effects against different cancer cell lines. In addition, the report of Taritla et al. [60] showed that the crude ethyl acetate extract of fungal endophytes showed maximum cytotoxicity on multiple cancer cell lines. However, the fungal endophyte evaluated in the aforementioned study was Aspergillus sp., not one of the fungal endophytes isolated in our study. The crude extract of Aspergillus sp. (an endophytic fungus of marine algae) was found to show better cytotoxic potential against different cervical, lung, skin, and breast cancer cell lines [61]. Weng et al. [62] reported that crude secondary metabolites from Penicillium sp. had some potential cytotoxic activity against various cancer cell lines, which was at variance with the findings of our study. We agree that solvent selection can influence the metabolite profile, and therefore, a comparative multi-solvent extraction approach (e.g., using methanol, chloroform, or hexane) for future work will broaden the chemical space captured and refine bioactivity outcomes (a limitation of our study).
Furthermore, a detailed investigation into the optimal physiological and nutritional parameters might enhance metabolite yield, diversity, and possible bioactivity. Therefore, optimization experiments to determine the ideal pH, temperature, and media composition tailored to each fungal endophyte would be suitable for improved metabolite production. More so, we acknowledge the limitations of using only two cell lines, as this current study was designed as a first-line investigation into the cytotoxic potential of crude extracts as additional cancer cell lines (e.g., breast, colorectal, and melanoma) and normal human cells would be appropriate to evaluate selectivity and therapeutic potential of the crude extract more comprehensively.
Nonetheless, our findings were consistent with the report of Varli et al. [58] regarding the low cytotoxicity of the endophytic fungal (Nemania sp.) extracts against lung cancer cell lines. The variability in the cytotoxic effects of crude extracts of the fungal endophytes across different studies and perhaps plant species underscores the complex nature of secondary metabolites produced by fungal endophytes and their potential influence on host plants’ bioactivity profiles. The mild cytotoxic effect observed on UMG87 glioblastoma by Tapfuma et al. [63]. cells corroborate the findings from our study, thus suggesting a consistent pattern in the interaction between fungal endophyte metabolites and certain cancer cell lines. This selective cytotoxicity warrants further investigation, as it could pave the way for developing novel, targeted anticancer therapies with potentially fewer side effects. Similarly, crude extracts of bacterial endophytes from the leaves of C. macowanii leaves were reported to have mild cytotoxicity against glioblastoma and lung carcinoma cell lines [49].
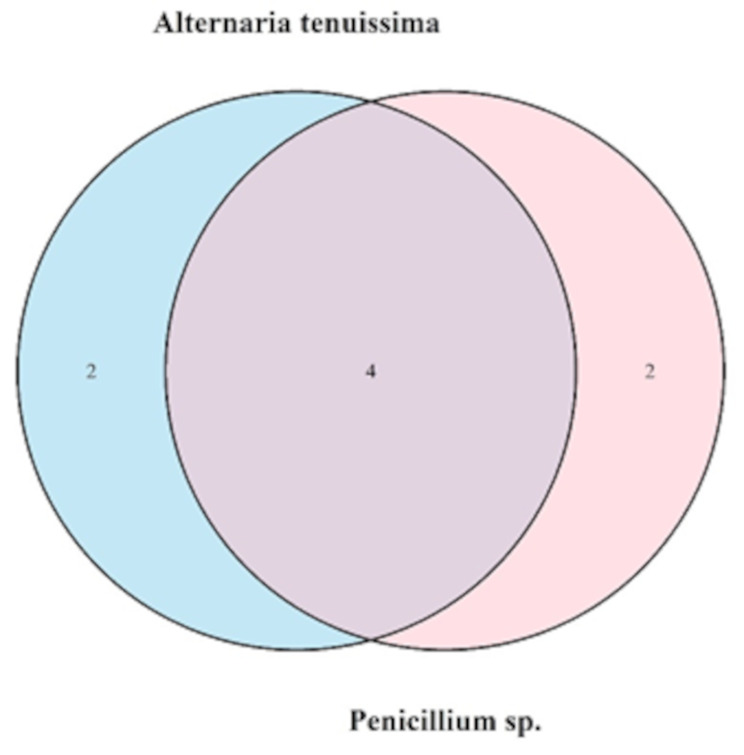
A Venn diagram representing the distribution of secondary metabolites produced by Penicillium sp. and A. tenuissima (the two fungal endophytes with more potent/better antibacterial activity) isolated from the leaves and bulbs of C. macowanii plant is shown in Fig. 5. The shared and unique metabolites between the selected endophytes are highlighted in the figure below. A. tenuissima produced 2 unique secondary metabolites not found in Penicillium sp.; similarly, Penicillium sp. produced 2 unique metabolites absent in A. tenuissima. Both fungal endophytes have 4 shared secondary metabolites in common. The overlap of 4 metabolites between these endophytes suggests a potential structural similarity or common biosynthetic pathways shared by these endophytes. These shared biosynthetic pathways or structural similarities can have significant implications for drug discovery, as they may contribute to the biological activities observed in earlier assays. The unique metabolites identified in each species of endophytes also highlight the biochemical diversity of the fungal endophytes and underscore the potential of these organisms as sources of novel bioactive compounds.
Fig. 5.
Ven-Diagram of distribution of secondary metabolites across selected fungal endophytes from leaves and bulbs of C. macowanii. Light blue (A. tenuissima), two metabolites and Pink (Penicillium sp.), two metabolites with four common shared metabolites for both endophytes
The LC-Q-TOF-MS analysis of crude extracts from the selected fungal endophytes of C. macowanii is shown in Table 1. A plethora of secondary metabolites produced by A. tenuissima and Penicillium sp. is shown below. Eight distinct metabolites with varying molecular formulas and retention times were identified. Four of the metabolites (crinamine, lunacrine, crinine, and justicidin B) were produced by both fungal endophytes, demonstrating shared biosynthetic capabilities. Two metabolites showed species-specific production by each of the endophytes. Oxamniquine and pedilstatin were exclusively produced by A. tenuissima, while melosatin A and isoquinoline was unique to Penicillium sp. The [M + H]+ (m/z) values, which correlate to the molecular weight/masses of the identified compounds, ranged from 130.0651 (isoquinoline) to 513.2808 (pedilstatin), indicating a broad spectrum of molecular complexities. The retention times also differ considerably, from 1.53 min. (justicidin B from extracts of A. tenuissima) to 28.49 min. (melosatin identified in Penicillium sp. extracts), and it reflects the diversity in the physicochemical properties of the metabolites produced by the endophytes. Our findings highlight the diverse and rich profile of secondary metabolites produced by the endophytic fungi associated with C. macowanii, thereby suggesting their potential as a rich source of bioactive compounds. Shared and species-specific metabolites indicate their distinct yet overlapping biosynthetic capabilities between A. tenuissima and Penicillium sp., contributing to our understanding of fungal chemical ecology and potential biotechnological applications. A comparison of retention times revealed that some compounds, such as crinamine and justicidin B, appeared at slightly different times in the two fungal extracts. This variation is most likely due to differences in the chemical background of the crude extracts, which can affect chromatographic separation. All identifications were made using accurate mass data and should be considered provisional, pending further confirmation by tandem MS analysis.
Table 1.
Analysis of crude extracts of fungal endophytes from leaves and bulbs of C. macowanii
| S/N | Metabolite name | Molecular formula | [M + H]+ (m/z) | Alternaria tenuissima | Rt (min) | Penicillium sp. | Rt (min) |
|---|---|---|---|---|---|---|---|
| 1 | Crinamine | C17H19N1O4 | 302.1401 | + | 18.50 | + | 25.58 |
| 2 | Lunacrine | C16H19N1O3 | 274.1442 | + | 15.51 | + | 15.52 |
| 3 | Crinine | C16H17N1O3 | 272.1278 | + | 16.32 | + | 8.94 |
| 4 | Justicidin B | C21H16O6 | 365.1033 | + | 1.53 | + | 13.57 |
| 5 | Oxamniquine | C14H21N3O3 | 280.1618 | + | 12.68 | - | 12.68 |
| 6 | Melosatin A | C21H23N1O4 | 354.1686 | - | 28.49 | + | 28.49 |
| 7 | Pedilstatin | C30H40O7 | 513.2808 | + | 5.48 | - | 5.48 |
| 8 | Isoquinoline | C9H7N1 | 130.0651 | - | 2.10 | + | 2.1 |
Our analysis revealed various secondary metabolites produced by Alternaria tenuissima and Penicillium sp. (fungal endophytes) isolated from C. macowanii. The variability in retention times observed for the same compounds between the A. tenuissima and Penicillium sp. extracts can be attributed to a variety of factors, such as the complexity of the crude matrices, potential interactions between co-extracted substances, and overlapping peaks, among other reasons [64–67]. These interactions can affect how metabolites behave during LC-MS analysis. Additionally, because identifications were based on accurate mass-to-charge ratio data and database matches alone, they should be considered provisional, pending further confirmation by tandem MS analysis or supporting MS/MS spectra. Future studies should aim to evaluate these findings using standard compounds or fragmentation-based approaches. Among the putatively identified metabolites were several alkaloids with notable pharmacological and bioactive properties, which aligns with existing literature on the role of fungal endophytes in enhancing or increasing the production of bioactive compounds (particularly alkaloids) in plants [68]. This, therefore, suggests a significant biotechnological potential of these endophytes, especially in the context of antibacterial and antifungal activities. The secondary metabolites reported in our findings include crinamine, a prominent alkaloid known for its broad pharmacological activity. It has been previously isolated from Crinum angustum and other members of the Amaryllidaceae family, such as Boophone disticha and Crinum asiaticum [57, 69–71]. Crinamine exhibits potent antibacterial and antifungal effects [72], and recent studies suggest it may also induce apoptosis and inhibit proliferation in cervical cancer cell lines [71], showcasing its anticancer potential. Lunacrine, another alkaloid identified in our study, is recognized for its cytotoxic, antibacterial, and antifungal properties [73, 74]. The compound has been isolated from Lunasia amara [75] and soybean (Glycine max), demonstrating its significance as a biologically active substance with potential therapeutic applications [76]. Similarly, crinine, identified in this study, is a bioactive compound of immense value. This alkaloid has been previously isolated from various plants, particularly within the Amaryllidaceae family [77–79]. Crinine is known for its broad-spectrum antibacterial and antifungal activities [77, 80], further supporting the bioactive potential of the fungal endophytes evaluated. Justicidin B, a lignan which has been isolated from the Justicia species, is another significant compound identified in this study. It has been reported to possess antibacterial and antifungal properties [81, 82]. Its presence in Penicillium sp. suggests that endophytic fungi may share biosynthetic pathways with their host plants, enhancing their pharmacological importance. The melosatin A compound, unique to Penicillium sp., is an indole derivative previously isolated from Melochia tomentosa [83, 84]. Melosatin A has been reported to exhibit both antibacterial and antitubercular activities [85], highlighting its relevance to clinical medicine and public health as a promising therapeutic agent [86]. Additionally, isoquinoline, an alkaloid with diverse bioactivities, was also identified in Penicillium sp. Isoquinoline derivatives are well-documented for their antibacterial, antifungal, antiviral, and antiplasmodial properties [78, 87, 88]. This compound has been isolated from both plant species and fungal endophytes, including Paraphaeosphaeria sporulosa, Aspergillus puniceus, and Penicillium spathulatum [89–91]. Oxamniquine, produced by Alternaria tenuissima, is a well-established antiparasitic agent primarily used against Schistosoma species [92, 93]. While its antibacterial potential has been explored in derivative forms [94, 95], its primary significance or usage has largely been its antiparasitic efficacy, especially against Schistosoma species. Lastly, pedilstatin, another important compound reported to be produced by Alternaria tenuissima, belongs to the statin family, known for its cholesterol-lowering or lipid-lowering properties [96]. Pedilstatin has been reported in plant leaves and endophytes [97, 98], and its identification in Alternaria highlights its potential relevance in medical applications, particularly in cardiovascular health and antimicrobial activity. Tentatively identifying these metabolites underscores the biosynthetic capabilities of fungal endophytes associated with C. macowanii and suggests their potential use in developing novel bioactive compounds for pharmaceutical, agricultural purposes, and clinical purposes. Our study contributes to the growing body of research on fungal chemical ecology and the potential for endophyte-derived bioactive compounds to address pressing medical and environmental challenges.
Conclusion
The comprehensive analysis of fungal endophytes isolated from C. macowanii has revealed significant insights into their diversity, bioactive potential, and metabolomic profiles, with important implications for basic research and therapeutic applications. The clear organ-specific colonization patterns observed between leaves and bulbs and the phylogenetic distribution across Ascomycota and Basidiomycota phyla enhance our understanding of plant-endophyte relationships and their evolutionary significance.
Identifying eight distinct secondary metabolites, with shared and unique distributions between Alternaria tenuissima and Penicillium sp., highlights these endophytes’ complex and diverse biosynthetic capabilities. This metabolic diversity contributes to our knowledge of fungal chemical ecology and presents opportunities for novel compound discovery. The overlapping metabolic profiles suggest common evolutionary pathways, while species-specific compounds indicate unique adaptations that merit further investigation.
The substantial antibacterial activity demonstrated by these endophytes, particularly against clinically relevant pathogens, is especially significant in the context of global antimicrobial resistance. Of particular novelty is identifying crude fungal metabolites exhibiting broad-spectrum antibacterial activity and low nonspecific cytotoxicity—an uncommon but desirable combination in early-stage therapeutic discovery. The broad-spectrum activity observed, especially in Penicillium sp. extracts, suggests potential new avenues for antibiotic development. Moreover, the reverse dose-dependent antiproliferative effects and enhanced cell viability in glioblastoma cells point to unexpected biological interactions that open new directions for anticancer compound development, tissue regeneration, or immune modulation. These findings collectively establish C. macowanii endophytes as promising sources of bioactive compounds with potential pharmaceutical applications. Rather than restating current public health concerns, our study underscores the novelty of uncovering underexplored fungal taxa from indigenous flora with unique bioactivities that could contribute to the next generation of therapeutics. The combination of antimicrobial efficacy and selective cytotoxicity presents opportunities for developing targeted therapeutic agents with minimal side effects. The study’s insights into the bioactive metabolites of fungal endophytes from C. macowanii add to the growing body of evidence supporting endophytes as viable sources for novel antimicrobial and anticancer compounds. By providing one of the first detailed metabolomic and functional evaluations of endophytes from this plant species, our study contributes unique findings to natural product drug discovery.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Acknowledgement: The authors are grateful to the University of Johannesburg, Faculty of Science, Department of Biotechnology and Food Technology, for providing an enabling environment and state-of-the-art facilities that supported the conceptualization, execution, and visualization of the data presented in this manuscript. We are also grateful to all members of the Molecular Pathogenic and Molecular Epidemiology Research Group (MPMERG) Laboratory, University of Johannesburg, for their team spirit and encouragement.
Author contributions
Author ContributionsAGO: Drafted the manuscript, visualized the data, and validated the findings. TS: Conducted the investigation and developed the methodology. EG: Conceptualized, designed, and supervised the project, reviewed the manuscript, and provided critical insights for clarity. All authors have read and approved the final version of the manuscript.
Funding
This research was funded by the University of Johannesburg University Research Committee, Project No. 075432.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study does not contain any research requiring ethical consent or approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abraham Goodness Ogofure, Email: aogofure@uj.ac.za.
Ezekiel Green, Email: egreen@uj.ac.za.
References
- 1.Elmaidomy AH, Shady NH, Abdeljawad KM, Elzamkan MB, Helmy HH, Tarshan EA, Adly AN, Hussien YH, Sayed NG, Zayed A, Abdelmohsen UR. Antimicrobial potentials of natural products against multidrug resistance pathogens: a comprehensive review. RSC Adv. 2022;12(45):29078–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nainu F, Permana AD, Djide NJN, Anjani QK, Utami RN, Rumata NR, Zhang J, Emran TB, Simal-Gandara J. Pharmaceutical approaches on antimicrobial resistance: prospects and challenges. Antibiot (Basel). 2021;10(8). [DOI] [PMC free article] [PubMed]
- 3.Deshmukh SK, Dufosse L, Chhipa H, Saxena S, Mahajan GB, Gupta MK. Fungal endophytes: A potential source of antibacterial compounds. J Fungi (Basel). 2022;8(2). [DOI] [PMC free article] [PubMed]
- 4.Gupta S, Chaturvedi P, Kulkarni MG, Van-Staden J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol Adv. 2020;39:107462. [DOI] [PubMed] [Google Scholar]
- 5.Torres-Mendoza D, Ortega HE, Cubilla-Rios L. Patents on endophytic Fungi related to secondary metabolites and biotransformation applications. J Fungi (Basel). 2020;6(2). [DOI] [PMC free article] [PubMed]
- 6.Mishra S, Sahu PK, Agarwal V, Singh N. Exploiting endophytic microbes as micro-factories for plant secondary metabolite production. Appl Microbiol Biotechnol. 2021;105(18):6579–96. [DOI] [PubMed] [Google Scholar]
- 7.Ek-Ramos MJ, Gomez-Flores R, Orozco-Flores AA, Rodriguez-Padilla C, Gonzalez-Ochoa G, Tamez-Guerra P. Bioactive products from Plant-Endophytic Gram-Positive Bacteria. Front Microbiol. 2019;10:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusari S. Editorial special issue endophytes. Planta Med. 2020;86(13–14):889. [DOI] [PubMed] [Google Scholar]
- 9.Maroyi A. A review of ethnoboatany, therapeutic value, phytochemistry and Pharmacology of Crinum macowanii baker: A highly traded bulbous plant in Southern Africa. J Ethnopharmacol. 2016;194:595–608. [DOI] [PubMed] [Google Scholar]
- 10.Fennell CW, van-Staden J. Crinum species in traditional and modern medicine. J Ethnopharmacol. 2001;78(1):15–26. [DOI] [PubMed] [Google Scholar]
- 11.Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP. A friendly relationship between endophytic Fungi and medicinal plants: A systematic review. Front Microbiol. 2016;7:906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez OC, Luiz JHH. Endophytic fungi isolated from medicinal plants: future prospects of bioactive natural products from tabebuia/handroanthus endophytes. Appl Microbiol Biotechnol. 2018;102(21):9105–19. [DOI] [PubMed] [Google Scholar]
- 13.Ogofure AG, Pelo SP, Green E. Identification and assessment of secondary metabolites from three fungal endophytes of Solanum mauritianum against public health pathogens. Molecules. 2024;29(20). [DOI] [PMC free article] [PubMed]
- 14.Kumar V, Soni R, Jain L, Dash B, Goel R. Endophytic Fungi: Recent Advances in Identification and Explorations. Advances in Endophytic Fungal Research. Fungal Biology 2019;267– 81.
- 15.Ogofure AG, Green E. Bioactivity and metabolic profiling of crude extracts from endophytic bacteria linked to Solanum mauritianum scope: discovery of antibacterial and anticancer properties. Heliyon. 2025;11(2). [DOI] [PMC free article] [PubMed]
- 16.Goh EML, Ng XQ, Yong CY, Hamzah A, Moy HY. Qualitative confirmation of 94 new psychoactive substances and metabolites in urine using liquid chromatography quadrupole Time-of-Flight mass spectrometry. J Anal Toxicol. 2023;47(4):366–78. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Edgar AS, Wong CH, Yang D. Liquid chromatography quadrupole Time-of-Flight mass spectrometry: A strategy for optimization, characterization, and quantification of antioxidant nitro derivatives. ACS Omega. 2022;7(36):32701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijesekara T, Xu B. Health-Promoting effects of bioactive compounds from plant endophytic Fungi. J Fungi (Basel). 2023;9(10). [DOI] [PMC free article] [PubMed]
- 19.Adeleke BS, Babalola OO. The plant endosphere-hidden treasures: a review of fungal endophytes. Biotechnol Genet Eng Rev. 2021;37(2):154–77. [DOI] [PubMed] [Google Scholar]
- 20.Rustamova N, Bozorov K, Efferth T, Yili A. Novel secondary metabolites from endophytic fungi: synthesis and biological properties. Phytochem Rev. 2020;19(2):425–48. [Google Scholar]
- 21.Gao H, Li G, Lou HX. Structural diversity and biological activities of novel secondary metabolites from endophytes. Molecules. 2018;23(3). [DOI] [PMC free article] [PubMed]
- 22.Hridoy M, Gorapi MZH, Noor S, Chowdhury NS, Rahman MM, Muscari I, Masia F, Adorisio S, Delfino DV, Mazid MA. Putative anticancer compounds from Plant-Derived endophytic fungi: A review. Molecules. 2022;27(1). [DOI] [PMC free article] [PubMed]
- 23.Mbekou MIK, Dize D, Yimgang VL, Djague F, Toghueo RMK, Sewald N, Lenta BN, Boyom FF. Antibacterial and mode of action of extracts from endophytic Fungi derived from Terminalia mantaly, Terminalia catappa, and Cananga odorata. Biomed Res Int. 2021;2021:6697973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez RM, Gonzalez AM, Ramirez AM. Compounds derived from endophytes: a review of phytochemistry and Pharmacology. Curr Med Chem. 2012;19(18):2992–3030. [DOI] [PubMed] [Google Scholar]
- 25.Wen J, Okyere SK, Wang S, Wang J, Xie L, Ran Y, Hu Y. Endophytic fungi: an effective alternative source of Plant-Derived bioactive compounds for Pharmacological studies. J Fungi (Basel). 2022;8(2). [DOI] [PMC free article] [PubMed]
- 26.Aly AH, Debbab A, Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol. 2011;90(6):1829–45. [DOI] [PubMed] [Google Scholar]
- 27.Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK. Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech. 2014;4(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuklinsky-Sobral J, Araújo WL, Mendes R, Pizzirani-Kleiner AA, Azevedo JL. Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil. 2005;273(1–2):91–9. [Google Scholar]
- 29.Morales A, Cornell, University. Cornell University. 2008. Available from: https://blog.mycology.cornell.edu/2008/01/10/a-simple-way-to-preserve-fungal-cultures/
- 30.Navi SS, Bandyopadhyay R, Hall AJ, Bramel-Cox PJ. A pictorial guide for the identification of mold fungi on sorghum grain. Patancheru 502 324, Andhra Pradesh. India:: International Crops Research Institute for the Semi-Arid Tropics 1999. [Google Scholar]
- 31.Pitt JI, Hocking AD. Fungi and Food Spoilage 2022.
- 32.Prabavathy D, Nachiyar CV. Cytotoxicity of Ethyl acetate extract of endophytic Aspergillus fumigatus on A549 cell lines. Biosci Biotechnol Res Asia. 2014;11(2):797–802. [Google Scholar]
- 33.Rumidatul A, Rahmawati N, Sunarya S. Production of secondary metabolites and its antibacterial and antioxidant activity during the growth period of endophytic Fungi isolated from gall rust Sengon plants. Pharmacognosy J. 2020;13(2):325–31. [Google Scholar]
- 34.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16. [DOI] [PubMed] [Google Scholar]
- 35.Sebola TE, Ndinteh DT, Niemann N, Mavumengwana V. Metal Analysis, Phytochemical and Antibacterial Investigation of Crinum Macowanii Bulb. International Conference on Advances in Science, Engineering, Technology and Natural Resources (ICASETNR-16) Nov 24–25, 2016 Parys (South Africa)2016.
- 36.Pelo S, Mavumengwana V, Green E. Diversity and antimicrobial activity of culturable fungal endophytes in Solanum mauritianum. Int J Environ Res Public Health. 2020;17(2). [DOI] [PMC free article] [PubMed]
- 37.Sebola TE, Uche-Okereafor NC, Tapfuma KI, Mekuto L, Green E, Mavumengwana V. Evaluating antibacterial and anticancer activity of crude extracts of bacterial endophytes from Crinum macowanii Baker bulbs. Microbiologyopen. 2019;8(12):e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogofure AG, Sebola T, Green E. Antibacterial and anticancer properties of Solanum mauritianum fruit components analyzed using LC-QTOF-MS/MS. Sci Rep. 2025;15(1):16698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCauley J, Zivanovic A, Skropeta D. Bioassays for anticancer activities. Methods Mol Biol. 2013;1055:191–205. [DOI] [PubMed] [Google Scholar]
- 40.Handayani D, Rasyid W, Rustini, Zainudin EN, Hertiani T. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: hela, widr, T47D and Vero. J App Pharm Sci. 2018;8(01):055–8. [Google Scholar]
- 41.Pelo SP, Adebo OA, Green E. Chemotaxonomic profiling of fungal endophytes of Solanum mauritianum (alien weed) using gas chromatography high resolution time-of-flight mass spectrometry (GC-HRTOF-MS). Metabolomics. 2021;17(5):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tapfuma KI, Mekuto L, Makatini MM, Mavumengwana V. The LC-QTOF-MS/MS analysis data of detected metabolites from the crude extract of Datura stramonium leaves. Data Brief. 2019a;25:104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blazenovic I, Kind T, Ji J, Fiehn O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 2018;8(2). [DOI] [PMC free article] [PubMed]
- 44.R-Core Team. R: A Language and environment for statistical computing. 4.3.3 ed. Vienna, Austria: R Foundation for Statistical Computing 2023. [Google Scholar]
- 45.Kolde R, pheatmap. Pretty Heatmaps version 1.0.12 from CRAN. 2019.
- 46.Wickham H. Data analysis. ggplot2. 2016;Use R:189–201. [Google Scholar]
- 47.Ogofure AG, Green E. Bioactivity and metabolic profiling of crude extracts from endophytic bacteria linked to Solanum mauritianum scope: discovery of antibacterial and anticancer properties. Heliyon. 2025;11(2):e40525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebola TE, Uche-Okereafor NC, Mekuto L, Makatini MM, Green E, Mavumengwana V. Antibacterial and anticancer activity and untargeted secondary metabolite profiling of crude bacterial endophyte extracts from Crinum macowanii Baker leaves. Int J Microbiol. 2020;2020:8839490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdalla MA, Aro AO, Gado D, Passari AK, Mishra VK, Singh BP, McGaw LJ. Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases. South Afr J Bot. 2020;134:336–42. [Google Scholar]
- 51.Faeth SH, Fagan WF. Fungal endophytes: common host plant symbionts but uncommon mutualists. Integr Comp Biol. 2002;42(2):360–8. [DOI] [PubMed] [Google Scholar]
- 52.Morare RF, Ubomba-Jaswa E, Serepa-Dlamini MH. Isolation and identification of bacterial endophytes from Crinum macowanii Baker. Afr J Biotechnol. 2018;17(33):1040–7. [Google Scholar]
- 53.Grabka R, d’Entremont TW, Adams SJ, Walker AK, Tanney JB, Abbasi PA, Ali S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants (Basel). 2022;11(3). [DOI] [PMC free article] [PubMed]
- 54.Khan MS, Gao J, Munir I, Zhang M, Liu Y, Moe TS, Xue J, Zhang X. Characterization of endophytic fungi, acremonium sp., from Lilium Davidii and analysis of its antifungal and plant Growth-Promoting effects. Biomed Res Int. 2021;2021:9930210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Efendi MR, Rusdi MS, Dinda A. Antibacterial activity of ethyl acetate extracts of fungal endophytes isolated from leaf gambir leaves (Uncaria gambir (Hunter) Roxb). Media Farmasi: Jurnal Ilmu Farmasi. 2022;19(1).
- 56.Caruso G, Golubkina N, Tallarita A, Abdelhamid MT, Sekara A. Biodiversity, ecology, and secondary metabolites production of endophytic Fungi associated with Amaryllidaceae crops. Agriculture. 2020;10(11).
- 57.Gomes JVD, Tosta CL, Fagg NÁC, Silva CW, Gomes-Copeland CAG, Magalhães KKP, Fonseca-Bazzo PO, Jamal YM, Silveira CM. Chemical profile and biological activity of Crinum americanum L. (Amaryllidaceae). South Afr J Bot. 2022;146:25–35. [Google Scholar]
- 58.Varli M, Pham HT, Kim SM, Tas I, Gamage CDB, Zhou R, Pulat S, Park SY, Sesal NC, Hur JS, Kang KB, Kim H. An acetonic extract and secondary metabolites from the Endolichenic fungus nemania sp. EL006872 exhibit immune checkpoint inhibitory activity in lung cancer cell. Front Pharmacol. 2022;13:986946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Wu X, Ma Y, Zhang W, Hu L, Feng X, Li X, Tang X. Endophytic fungi from Mangrove inhibit lung cancer cell growth and angiogenesis in vitro. Oncol Rep. 2017;37(3):1793–803. [DOI] [PubMed] [Google Scholar]
- 60.Taritla S, Kumari M, Kamat S, Bhat SG, Jayabaskaran C. Optimization of physicochemical parameters for production of cytotoxic secondary metabolites and apoptosis induction activities in the culture extract of a marine Algal-Derived endophytic fungus Aspergillus Sp. Front Pharmacol. 2021;12:542891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamat S, Kumari M, Taritla S, Jayabaskaran C. Endophytic Fungi of marine Alga from Konkan coast, India—A rich source of bioactive material. Front Mar Sci. 2020;7.
- 62.Weng W, Jiang S, Sun C, Pan X, Xian L, Lu X, Zhang C. Cytotoxic secondary metabolites isolated from penicillium sp. YT2019-3321, an endophytic fungus derived from Lonicera Japonica. Front Microbiol. 2022;13:1099592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tapfuma KI, Uche-Okereafor N, Sebola TE, Hussan R, Mekuto L, Makatini MM, Green E, Mavumengwana V. Cytotoxic activity of crude extracts from Datura stramonium’s fungal endophytes against A549 lung carcinoma and UMG87 glioblastoma cell lines and LC-QTOF-MS/MS based metabolite profiling. BMC Complement Altern Med. 2019b;19(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui L, Lu H, Lee YH. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom Rev. 2018;37(6):772–92. [DOI] [PubMed] [Google Scholar]
- 65.You Y, Hu Q, Liu N, Xu C, Lu S, Xu T, Mao X. Metabolite analysis of Alternaria Mycotoxins by LC-MS/MS and multiple tools. Molecules. 2023;28(7). [DOI] [PMC free article] [PubMed]
- 66.Fang N, Yu S, Ronis MJ, Badger TM. Matrix effects break the LC behavior rule for analytes in LC-MS/MS analysis of biological samples. Exp Biol Med (Maywood). 2015;240(4):488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawikowska A, Piasecka A, Kachlicki P, Krajewski P. Separation of chromatographic Co-Eluted compounds by clustering and by functional data analysis. Metabolites. 2021;11(4). [DOI] [PMC free article] [PubMed]
- 68.Zhou J, Liu Z, Wang S, Li J, Li Y, Chen WK, Wang R. Fungal endophytes promote the accumulation of Amaryllidaceae alkaloids in Lycoris radiata. Environ Microbiol. 2020;22(4):1421–34. [DOI] [PubMed] [Google Scholar]
- 69.Akinyele ST, Elusiyan CA, Omisore NO, Adewunmi CO. ANTIMALARIAL activities and alkaloids from Crinum Jagus (Thomps) DANDY. J Ethnopharmacol. 2022;296:115359. [DOI] [PubMed] [Google Scholar]
- 70.Cheesman L, Nair JJ, van Staden J. Antibacterial activity of Crinane alkaloids from boophone disticha (Amaryllidaceae). J Ethnopharmacol. 2012;140(2):405–8. [DOI] [PubMed] [Google Scholar]
- 71.Khumkhrong P, Piboonprai K, Chaichompoo W, Pimtong W, Khongkow M, Namdee K, Jantimaporn A, Japrung D, Asawapirom U, Suksamrarn A, Iempridee T. Crinamine induces apoptosis and inhibits proliferation, migration, and angiogenesis in cervical Cancer SiHa cells. Biomolecules. 2019;9(9). [DOI] [PMC free article] [PubMed]
- 72.Iannello C, Bastida J, Bonvicini F, Antognoni F, Gentilomi GA, Poli F. Chemical composition, and in vitro antibacterial and antifungal activity of an alkaloid extract from Crinum angustum Steud. Nat Prod Res. 2014;28(10):704–10. [DOI] [PubMed] [Google Scholar]
- 73.Dapar MLG, Demayo CG, Senarath WTPSK. Antimicrobial and cellular metabolic inhibitory properties of the ethanolic extract from the bark of ‘Lunas-Bagon’ (Lunasia sp). Digital Repository) Library, University of Sri Jayewardenupura 2019.
- 74.Macabeo AP, Aguinaldo A. Chemical and phytomedicinal investigations in Lunasia amara. Pharmacogn Rev. 2008;2(4):317–22. [Google Scholar]
- 75.Prescott TA, Sadler IH, Kiapranis R, Maciver SK. Lunacridine from lunasia amara is a DNA intercalating topoisomerase II inhibitor. J Ethnopharmacol. 2007;109(2):289–94. [DOI] [PubMed] [Google Scholar]
- 76.Zubair MS, Anam S, Lallo S. Cytotoxic activity and phytochemical standardization of lunasia amara Blanco wood extract. Asian Pac J Trop Biomed. 2016;6(11):962–6. [Google Scholar]
- 77.Kianfé BY, Kühlborn J, Tchuenguem RT, Tchegnitegni BT, Ponou BK, Groß J, Teponno RB, Dzoyem JP, Opatz T, Tapondjou LA. Antimicrobial secondary metabolites from the medicinal plant Crinum glaucum A. Chev. (Amaryllidaceae). South Afr J Bot. 2020;133:161–6. [Google Scholar]
- 78.Qing Z-X, Yang P, Tang Q, Cheng P, Liu X-B, Zheng Y-j, Liu Y-S, Zeng J-G. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and Structure-activity relationship. Curr Org Chem. 2017;21(18).
- 79.Chen MX, Huo JM, Hu J, Xu ZP, Zhang X. Amaryllidaceae alkaloids from Crinum latifolium with cytotoxic, antimicrobial, antioxidant, and anti-inflammatory activities. Fitoterapia. 2018;130:48–53. [DOI] [PubMed] [Google Scholar]
- 80.Silva LC, Correia AF, Gomes JVD, Romao W, Motta LC, Fagg CW, Magalhaes PO, Silveira D, Fonseca-Bazzo YM. Lycorine alkaloid and Crinum americanum L. (Amaryllidaceae) extracts display antifungal activity on clinically relevant Candida species. Molecules. 2022;27(9). [DOI] [PMC free article] [PubMed]
- 81.Herrera-Mata H, Rosas-Romero AV, Oscar C. Biological activity of Sanguinaria (Justicia secunda) extracts. Pharm Biol. 2008;40(3):206–12. [Google Scholar]
- 82.Nunes TRS, Cordeiro MF, Beserra FG, de Souza ML, da Silva WAV, Ferreira MRA, Soares LAL, Costa-Junior SD, Cavalcanti IMF, Pitta M, Pitta IDR, Rego M. Organic extract of Justicia pectoralis jacq. Leaf inhibits Interferon-gamma secretion and has bacteriostatic activity against Acinetobacter baumannii and Klebsiella pneumoniae. Evid Based Complement Alternat Med. 2018;2018:5762368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grewal AS. Isatin derivatives with several biological activities. Int J Pharm Res. 2014;6(1):1–7. [Google Scholar]
- 84.Sharma A, Bhardwaj V, Singh S. Putative role of Isatin derivatives synthesis and their biological applications- a review. Int J Pharm Sci Res. 2023;14(6).
- 85.Nath R, Pathania S, Grover G, Akhtar MJ. Isatin containing heterocycles for different biological activities: analysis of structure activity relationship. J Mol Struct. 2020;1222.
- 86.Pathak A, Soni N, Pandey DD, Verma D. Recent advances, future challenges, Sar and antimicrobial activities of isatin: a Breaf review. J Pharm Negat Results. 2023;13(9):7285–307.
- 87.Avci FG, Atas B, G TG, Gurer C. A.B. S. Antibacterial and antifungal activities of isoquinoline alkaloids of the Papaveraceae and Fumariaceae families and their implications in structure–activity relationships. Bioactive Natural Products. Studies in Natural Products Chemistry 2021;87–118.
- 88.Verma VA, Synthesis. Antimicrobial, and antioxidant studies of some new Indolo[3,2-c]isoquinoline derivatives. Russ J Gen Chem. 2019;88(12):2628–45. [Google Scholar]
- 89.Chen M-S, Qiu W-Y, Lin Z-L, Wu Y-P, Li W, Gong D-P, Dong M, Wang W-G, Li Y-K, Yao H, Hu Q-F. Two new isoquinolines from cigar Tobacco-Derived endophytic Fungi Aspergillus puniceus and their antibacterial activity. Chem Nat Compd. 2023;59(6):1142–6. [Google Scholar]
- 90.de-Amorim MR, Paz TA, Pinto LDS, Hilario F, Zanini CL, Aguiar ACC, Silva DES, Furlan M, Guido RVC, Bauab TM, Netto AVG, Dos Santos LC. New isoquinoline alkaloids from paraphaeosphaeria sporulosa F03, a fungal endophyte isolated from Paepalanthus planifolius. Planta Med. 2022;88(12):994–1003. [DOI] [PubMed] [Google Scholar]
- 91.Nord C, Levenfors JJ, Bjerketorp J, Sahlberg C, Guss B, Oberg B, Broberg A. Antibacterial isoquinoline alkaloids from the fungus penicillium spathulatum Em19. Molecules. 2019;24(24). [DOI] [PMC free article] [PubMed]
- 92.Guzman MA, Rugel AR, Tarpley RS, Alwan SN, Chevalier FD, Kovalskyy DP, Cao X, Holloway SP, Anderson TJC, Taylor AB, McHardy SF, LoVerde PT. An iterative process produces oxamniquine derivatives that kill the major species of schistosomes infecting humans. PLoS Negl Trop Dis. 2020;14(8):e0008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hess J, Panic G, Patra M, Mastrobuoni L, Spingler B, Roy S, Keiser J, Gasser G. Ferrocenyl, ruthenocenyl, and benzyl oxamniquine derivatives with Cross-Species activity against Schistosoma mansoni and Schistosoma haematobium. ACS Infect Dis. 2017;3(9):645–52. [DOI] [PubMed] [Google Scholar]
- 94.Na SH, Jeon H, Kim YJ, Kwon HI, Selasi GN, Nicholas A, Yun CS, Lee SH, Lee JC. Antimicrobial activity of novel 4H-4-oxoquinolizine compounds against extensively drug-resistant Acinetobacter baumannii strains. Int J Antimicrob Agents. 2017;49(1):107–11. [DOI] [PubMed] [Google Scholar]
- 95.Sabutski YE, Menchinskaya ES, Shevchenko LS, Chingizova EA, Chingizov AR, Popov RS, Denisenko VA, Mikhailov VV, Aminin DL, Polonik SG. Synthesis and evaluation of antimicrobial and cytotoxic activity of Oxathiine-Fused Quinone-Thioglucoside conjugates of substituted 1,4-Naphthoquinones. Molecules. 2020;25(16). [DOI] [PMC free article] [PubMed]
- 96.Ghosh S, Samanta A, Mandal NB, Bannerjee S, Chattopadhyay D. Evaluation of the wound healing activity of methanol extract of Pedilanthus tithymaloides (L.) poit leaf and its isolated active constituents in topical formulation. J Ethnopharmacol. 2012;142(3):714–22. [DOI] [PubMed] [Google Scholar]
- 97.Joshi B, Panda SK, Jouneghani RS, Liu M, Parajuli N, Leyssen P, Neyts J, Luyten W. Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evidence-Based Complement Altern Med. 2020;2020(1):1043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava R, Soni N. An updated review on phytopharmacological profile of Euphorbia tithymaloides (L.) poit. Pharma Innov J. 2019;8(5):109–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.