The mitotic spindle is an impressive cellular machine, responsible for distributing each set of the duplicated genome into daughter cells. Microtubules, the major component of the spindle, are constantly recycled by two turnover mechanisms: dynamic instability of microtubule plus ends and treadmilling, or flux of tubulin dimers toward the pole. Thus, the critical function of chromosome segregation is based on an intrinsically metastable structure. A nonmicrotubule “spindle matrix” that provides a scaffold for microtubules and/or motors has long been hypothesized. Recent findings have revived the quest for the elusive matrix, and the Saccharomyces cerevisiae gene FIN1 (1) is the latest entry in this small but provocative field.
Chromosome alignment and accurate segregation is no small feat. Duplicated chromosomes attach to microtubules from each spindle pole of the mitotic spindle. Attachment is mediated through kinetochores, a specialized protein complex built at the centromere of eukaryotic chromosomes. Alignment along the metaphase plate occurs through a complicated dance that is no less impressive then the mating rituals of many animals. Microtubule-based motors play key roles in chromosome alignment (2). Motor proteins act at sister kinetochores to “capture” microtubules and align the sister chromatids to opposite poles of the spindle. Sister chromatids move in a coordinated manner; poleward movement of one sister is accompanied by the other sister moving away from its pole (3). The mechanism behind this coordinated movement is poorly understood; however, it is thought to be based on a physical linkage between sister kinetochores. When force is exerted on one sister kinetochore, the motility state of the other sister switches to the direction of the pull, or tension. Underlying the complexity of motor protein–microtubule interactions are the spindle microtubules themselves. The microtubules are dynamically growing and shortening, and individual tubulin dimers within the microtubule polymer are constantly migrating toward the pole. This phenomenon, known as treadmilling or flux, reveals the inherent metastable state of the mitotic spindle. Microtubules can also generate a pushing force against chromosome arms, denoted microtubule “polar winds” (4). Motor proteins contribute to the polar winds and to the fidelity of chromosome distribution by pushing chromosomes that initially associate with one pole (mono-orientation) toward the other pole.
The budding yeast, a single cell eukaryote, exhibits a “closed” mitosis. The mitotic spindle is nucleated from spindle pole bodies embedded in the nuclear envelope (Fig. 1). The spindle is completely contained within the nucleus, which does not break down throughout the cell cycle. The haploid spindle contains ≈8 polar microtubules (pole-to-pole) and 32 kinetochore microtubules (pole-to-chromosome), one for each chromatid. Although chromosomes are not visible by differential interference contrast microscopy, the development of green fluorescent protein (GFP) markers at specific sites in the chromosome relative to the kinetochore has allowed visualization of novel features of chromosome structure and behavior throughout the cell cycle (5). Before anaphase, sister centromeres are separated toward opposite poles (Fig. 2). This separation is important for the maintenance of bipolar chromosome attachment and the functional processes of properly segregating the duplicated genome (5–8). Similar stretching in centromere chromatin has been observed in human tissue culture cells (9) and diatoms (10). Kinetochore stretching depends upon microtubule binding and is indicative of tension across the separated centromeres. Tension is required to stabilize the kinetochore microtubule attachment in, for example, meiotic grasshopper spermatocytes (11) and regulates the direction of chromosome motility (3, 12, 13). The separated sister centromeres oscillate relative to the spindle pole body, in a dynamic sequence exemplified by poleward (P) and away-from-the-pole (AP) movement of vertebrate kinetochores in tissue cells (3). The distance between sister centromeres is also dynamic, albeit reassociation of separated centromeres is infrequent. Yeast centromeres align in a metaphase conformation and, upon anaphase onset, move to the spindle pole (5). The preanaphase and anaphase motility properties of yeast kinetochores are remarkably comparable to motility properties of vertebrate cell kinetochores. Thus the underlying mechanisms and complexity of chromosome segregation in vertebrate cells are likely to be conserved in yeast. The current models, based on a balance of antagonistically acting mitotic motors, kinetochores that can sense their position and direction of force, dynamic microtubules, and polar winds, have the daunting task of explaining how forces are generated on slippery tracks. None of the extant models are completely satisfactory in explaining the diversity of mitotic mechanisms in nature.
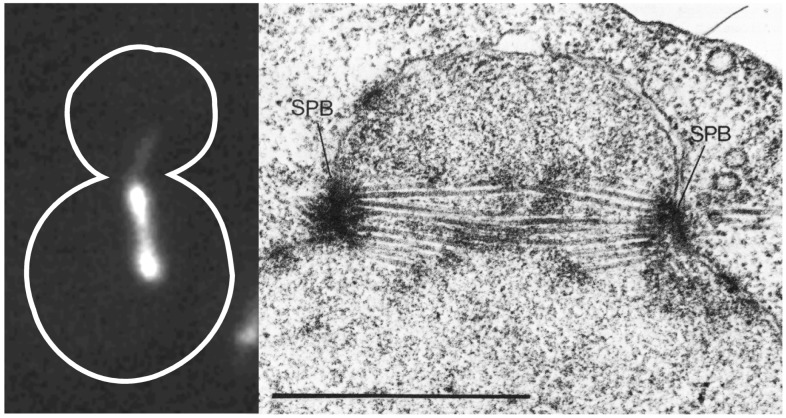
Figure 1.
Spindle structure in yeast. Yeast cells expressing fluorescent Tub1-GFP (Left) (22) and a corresponding electron micrograph of a bipolar spindle (Right; ref. 29). The spindle pole bodies are embedded in the nuclear envelope that does not break down in mitosis. Note the polar microtubules (pole-to-pole) and the discontinuous microtubules (presumptive kinetochore-to-pole microtubules). In fluorescence, the discontinuous kinetochore microtubules appear as tufts of fluorescence emanating from each pole.
Figure 2.
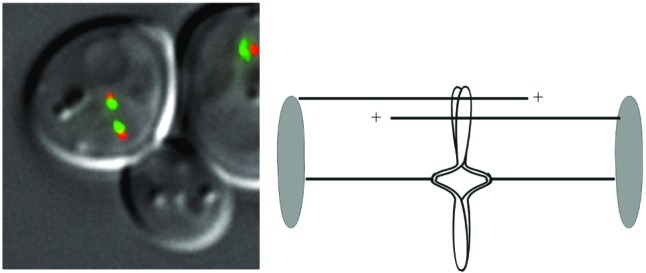
Chromosome structure in yeast. Yeast cells expressing a spindle pole protein fused to cyan fluorescent protein (CFP) (Spc29-CFP, pseudocolored orange) and the centromere-specific histone, Cse4p, fused to GFP (Cse4-GFP, green; ref. 5). The spindle poles are separated by 1.5 μm, indicative of a 1.5-μm mitotic spindle. The centromeres are separated on average by 0.6 μm before anaphase onset. (Right) Schematic representation of the streamlined spindle. The spindle poles (shaded ovals) nucleate polar (pole-to-pole) microtubules (upper lines) and discontinuous microtubules (kinetochore-to-pole; opposing pair of lines). The replicated chromosome illustrates centromere separation and arm cohesion.
An alternative hypothesis invokes the existence of a matrix within or around the mitotic spindle. The evidence for such a matrix includes the identification of spindle microfilaments (14), the shortening response of “cut” spindles (15), the static behavior of a mitotic kinesin (16), and has been the subject of several recent reviews (17, 18). The foundation for this model derives from basic principles of biological structures. The challenge is to understand the dynamic mitotic spindle, independent of scale, built solely with noncompliant structural elements (microtubules). Most biological structures contain tension and compression members, and conserve material relative to volume, issues that are of paramount importance in architectural circles. The architectural beauty of geodesic domes reveals an inherently stable design, spanning unlimited distances with no interior supports and an unprecedented strength-to-weight ratio. The geodesic structure of viruses exemplifies the fact that nature has long since made this discovery. What accounts for the economical elegance in design? Buckminster Fuller coined the term “tensegrity,” a contraction joining the words tension and integrity. “Tensegrity describes a structural-relationship principle in which structural shape is guaranteed by the continuous, tensional behaviors of the system and not by the discontinuous exclusively local compressional member behaviors.” Tensegrity structures maximize the use of tensile materials. The tensile members are continuous, whereas the compression members are discontinuous. The structure is based on rigid struts floating in a sea of tension (19). Is the spindle a geodesic structure? When compression-resisting microtubules of the spindle are severed, the half spindle rapidly shortens, and unbroken microtubules bow outward (15). If compression members of the structure resist tension, removal by cutting results in increased compression on the spindle, resulting in the shortening or bowing of the remaining compression members. The tensegrity feature of the putative spindle matrix predicts that tension elements provide the anchor for motor proteins to translocate chromosomes along rigid microtubule struts.
A nonmicrotubule foundation in the spindle would have a profound influence on understanding the mechanisms of chromosome movement. In the latest chapter of the spindle matrix, a new player, FIN1 (1), has been found in an unsuspecting organism. Within the yeast nucleus lies a newly discovered filament, based on the Fin1p protein. FIN1 was discovered by virtue of its interaction with a 14–3-3 protein, Bmh2p. Fin1p is present throughout the nucleus of small budded cells in a diffuse pattern. In large budded cells, Fin1p-GFP forms linear arrays that extend from the spindle poles and that span the pole-to-pole distance from mother to daughter cell. These arrays form in apparent concert with spindle assembly and remain until spindle disassembly, at which time the Fin1p arrays disassemble to their diffuse nuclear position. Most remarkable is the ability of Fin1p to assemble into filamentous structures in vitro. Fin1p-6XHis isolated from yeast forms a 10-nm filamentous structure, as determined by both electron and atomic force microscopy. Thus, the streamlined mitotic spindle in budding yeast belies an underlying filamentous structure.
Is Fin1p the founding member of the spindle matrix? Fin1p is certainly not the first candidate. Skeletor, a matrix candidate identified in Drosophila, assembles into a spindle-like form before spindle assembly (20). Skeletor does not require microtubules to form or to maintain its fusiform structure. Likewise, Fin1p does not require microtubules to assemble filaments in vitro. However, it is not known whether Fin1p assembly in vivo requires microtubules. Both Fin1p and Skeletor are novel proteins, without any distinctive features indicative of their biologic function. Furthermore, there is no functional evidence that either of these proteins contributes to mitosis or the fidelity of chromosome segregation. FIN1 is not essential for growth. The community anxiously awaits a more detailed analysis of the phenotype, particularly with respect to chromosome dynamics (see above) and the fidelity of chromosome segregation.
What would constitute definitive evidence for the spindle matrix? The localization of Fin1p and Skeletor are suggestive but certainly not definitive. An elegant study from Kapoor and Mitchison provides recent functional evidence for a spindle matrix (16, 18). These investigators took advantage of fluorescent imaging methods to establish differential behavior of a microtubule-based motor protein, Eg5, relative to spindle microtubules. Fluorescent speckle microscopy is a visual assay for protein dynamics in live cells (ref. 21; Fig. 3). Fluorescent speckles in microtubules, for example, are produced by limited expression of labeled tubulin (e.g., <5% Tub1-GFP of total tubulin) in the cell. Speckles represent the stochastic incorporation of fluorescent tubulin relative to the majority of unlabeled tubulin. The speckles serve as fiducial marks, allowing one to determine the sites of tubulin incorporation relative to the microtubule plus or minus end. For vertebrate cell mitotic spindles, this technology revealed that tubulin speckles move continuously poleward by flux (21). Kapoor and Mitchison used this method to examine the dynamics of Eg5 in Xenopus extract meiotic spindles (16). They discovered that Eg5 is relatively static, whereas microtubules flux poleward. The data are consistent with the hypothesis that a nonmicrotubule matrix tethers Eg5 differentially from the “slippery microtubule tracks.”
Figure 3.
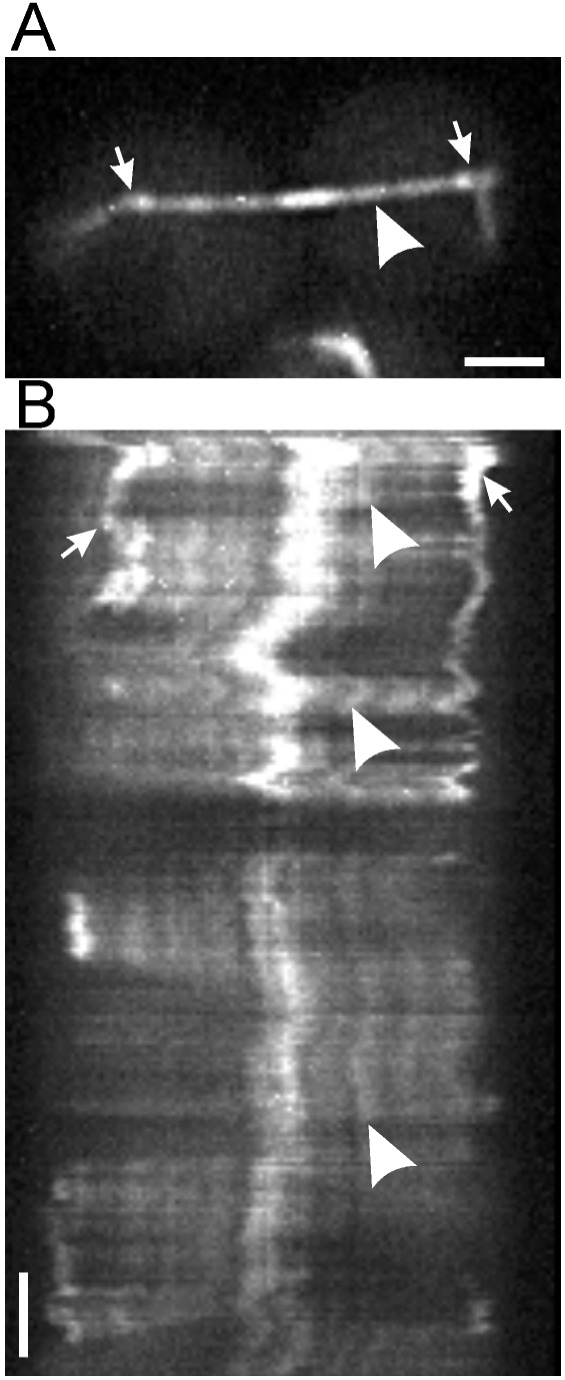
Fluorescence speckle microscopy. Yeast cells expressing limiting amounts of Tub1-GFP contain speckled spindles. Arrows mark the spindle pole bodies, and the arrowhead marks a selected fluorescent speckle (A). The kymograph (B) shows that the speckles, marked by the indicated speckle (arrowheads), do not move relative to the spindle pole bodies. The bright region in the middle of the spindle is the site of overlap of the pole-to-pole microtubules (see Schematic in Fig. 2; adapted from ref. 22).
Fluorescent speckle microscopy has recently been applied to yeast microtubules. Low-level expression of Tub1-GFP results in speckled cytoplasmic and spindle microtubules, revealing that astral and spindle microtubules grow and shorten from their plus-ends (ref. 22; Fig. 3). There is no evidence for poleward movement of tubulin dimers in yeast. However, the ability to determine protein dynamics with speckle microscopy in fin1 mutants will enable investigators to determine whether any motor proteins (like Eg5) are tethered to the Fin1p matrix.
What are the prospects for the matrix and other functions toward which Fin1p filaments may contribute? Two-hybrid analysis reveals two intriguing candidates. Fin1p interacts with two proteins, Reb1p (Ribosomal DNA Enhancer Binding protein; ref. 23) and Fir1p (Factor Interacting with Ref2), involved RNA metabolism and the type II phosphatase Glc7 (24, 25). Ribosomal RNA metabolism is of particular interest in light of recent findings that proteins regulating mitotic exit are sequestered in the nucleolus (26). The mitotic exit network (MEN) regulates mitotic exit and the timing of spindle disassembly with respect to spindle position. Although many of the components of the MEN have been identified, less is known about the signals from a properly positioned spindle to the release of proteins from the nucleolus. Fin1p localization to the spindle poles and spindle could contribute to the signaling pathway of mitotic exit. Interestingly, the Fin1p filament elongation begins at about 12 min and filament disassembly occurs at 81 min in van Hemert et al. (1). If Fin1p filaments mirror the timing of mitotic spindle elongation and disassembly, the 69 min of elongation are greatly exaggerated relative to wild-type. The delayed kinetics of spindle elongation/disassembly may be preliminary indications of a mitotic exit defect in cells expressing Fin1p-GFP.
The interaction with Glc7p also provides an interesting clue to Fin1p's cellular role. Glc7p has many cellular functions, one of which is to regulate the phosphorylation state of kinetochore proteins (24, 25). The other Fin1p interacting protein, Fir1p, interacts with the kinetochore protein Spc24p (27). Spc24p is part of the Ndc80 kinetochore complex in yeast. The Ndc80 complex interacts with microtubules (27), providing a potential link between Fin1p, kinetochores, and microtubules. Unfortunately, as the molecular linkage between kinetochores and microtubules remains a mystery, the significance of these interactions is unclear. That yeast kinetochores attach to a single microtubule seems particularly precarious. The Fin1p filaments may function to back-up the microtubule cytoskeleton in instances of kinetochore detachment. In this regard, it is interesting that yeast kinetochores cluster into a rosette around the spindle pole (28), and these rosettes can be visualized in live cells with the pan-centromere histone, Cse4-GFP (ref. 5; Fig. 2). Centromeric clustering depends on essential kinetochore proteins (Ndc10p) and microtubules (28); however, the significance of this biological structure remains elusive. From an architectural viewpoint, centromere clustering in yeast may release the dependency of kinetochore attachment on a single microtubule. Whether Fin1p promotes centromere clustering, or provides anchors for motors within the spindle as the proposed spindle matrix are questions for future studies. The description of this novel filamentous structure coincident with the mitotic spindle fuels speculation regarding the spindle matrix and should foster discussions and experiments to test the validity of cellular tensegrity.
Footnotes
See companion article on page 5390.
References
- 1.van Hemert M J, Lamers G E M, Klein D C G, Oosterkamp T H, Steensma H Y, van Heusden G P H. Proc Natl Acad Sci USA. 2002;99:5390–5393. doi: 10.1073/pnas.072556099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaar B T, Chan G K, Maddox P, Salmon E D, Yen T J. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skibbens R V, Rieder C L, Salmon E D. J Cell Sci. 1995;108:2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- 4.Rieder C L, Salmon E D. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson C G, Maddox P S, Salmon E D, Bloom K. J Cell Biol. 2001;152:1255–1266. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X, Asthana S, Sorger P K. Cell. 2000;101:763–775. doi: 10.1016/s0092-8674(00)80888-0. [DOI] [PubMed] [Google Scholar]
- 7.Goshima G, Yanagida M. Cell. 2000;100:619–633. doi: 10.1016/s0092-8674(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Fuchs J, Loidl J, Nasmyth K. Nat Cell Biol. 2000;2:492–499. doi: 10.1038/35019529. [DOI] [PubMed] [Google Scholar]
- 9.Shelby R D, Hahn K M, Sullivan K F. J Cell Biol. 1996;135:545–557. doi: 10.1083/jcb.135.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tippit D H, Pickett-Heaps J D, Leslie R. J Cell Biol. 1980;86:402–416. doi: 10.1083/jcb.86.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicklas R B, Koch C A. J Cell Biol. 1969;43:40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicklas R B. In: Mitosis Facts and Questions. Little M, editor. Berlin: Springer; 1977. pp. 150–155. [Google Scholar]
- 13.Inoue S, Salmon E D. Molecular Biology of the Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickett-Heaps J D, Carpenter J. Eur J Cell Biol. 1993;60:300–307. [PubMed] [Google Scholar]
- 15.Pickett-Heaps J D, Forer A, Spurck T. Cell Motil Cytoskeleton. 1997;37:1–6. doi: 10.1002/(SICI)1097-0169(1997)37:1<1::AID-CM1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor T M, Mitchison T J. J Cell Biol. 2001;154:1125–1134. doi: 10.1083/jcb.200106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholey J M, Rogers G C, Sharp D J. J Cell Biol. 2001;154:261–266. doi: 10.1083/jcb.200101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells W A. J Cell Biol. 2001;154:1102–1104. doi: 10.1083/jcb.200108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingber D E. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- 20.Walker D L, Wang D, Jin Y, Rath U, Wang Y, Johansen J, Johansen K M. J Cell Biol. 2000;151:1401–1412. doi: 10.1083/jcb.151.7.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterman-Storer C M, Salmon E D. Biophys J. 1998;75:2059–2069. doi: 10.1016/S0006-3495(98)77648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddox P, Bloom K, Salmon E D. Nat Cell Biol. 2000;2:36–41. doi: 10.1038/71357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow B E, Johnson S P, Warner J R. J Biol Chem. 1989;264:9061–9068. [PubMed] [Google Scholar]
- 24.Bloecher A, Tatchell K. Genes Dev. 1999;13:517–522. doi: 10.1101/gad.13.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sassoon I, Severin F F, Andrews P D, Taba M R, Kaplan K B, Ashford A J, Stark M J, Sorger P K, Hyman A A. Genes Dev. 1999;13:545–555. doi: 10.1101/gad.13.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shou W, Seol J H, Shevchenko A, Baskerville C, Moazed D, Chen Z W, Jang J, Charbonneau H, Deshaies R J. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 27.Wigge P A, Kilmartin J V. J Cell Biol. 2001;152:349–360. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Q W, Fuchs J, Loidl J. J Cell Sci. 2000;113:1903–1912. doi: 10.1242/jcs.113.11.1903. [DOI] [PubMed] [Google Scholar]
- 29.Kubai D F. Int Rev Cytol. 1975;43:167–227. doi: 10.1016/s0074-7696(08)60069-8. [DOI] [PubMed] [Google Scholar]