Abstract
Self-assembly is a process in which components, either separate or linked, spontaneously form ordered aggregates. Self-assembly can occur with components having sizes from the molecular to the macroscopic, provided that appropriate conditions are met. Although much of the work in self-assembly has focused on molecular components, many of the most interesting applications of self-assembling processes can be found at larger sizes (nanometers to micrometers). These larger systems also offer a level of control over the characteristics of the components and over the interactions among them that makes fundamental investigations especially tractable.
Molecular synthesis is a technology that chemists use to make molecules by forming covalent bonds between atoms. Molecular self-assembly is a process in which molecules (or parts of molecules) spontaneously form ordered aggregates and involves no human intervention; the interactions involved usually are noncovalent. In molecular self-assembly, the molecular structure determines the structure of the assembly (1). Synthesis makes molecules; self-assembly makes ordered ensembles of molecules (or ordered forms of macromolecules). The structures generated in molecular self-assembly are usually in equilibrium states (or at least in metastable states).
Molecular self-assembly is ubiquitous in chemistry, materials science, and biology and has been so long before self-assembly emerged as a discrete field of study and as a synthetic strategy (2, 3). The formation of molecular crystals (4), colloids (5), lipid bilayers (6), phase-separated polymers (7), and self-assembled monolayers (8) are all examples of molecular self-assembly, as are the folding of polypeptide chains into proteins (9) and the folding of nucleic acids into their functional forms (10). Even the association of a ligand with a receptor is a form of self-assembly (11); the semantic boundaries between self-assembly, molecular recognition, complexation, and other processes that form more ordered from less ordered assemblies of molecules expand or contract at the whim of those using them.
Self-assembly is scientifically interesting and technologically important for at least four reasons. The first is that it is centrally important in life. The cell contains an astonishing range of complex structures such as lipid membranes, folded proteins, structured nucleic acids, protein aggregates, molecular machines, and many others that form by self-assembly (12). The second is that self-assembly provides routes to a range of materials with regular structures: molecular crystals (13), liquid crystals (14), and semicrystalline and phase-separated polymers (15) are examples. Third, self-assembly also occurs widely in systems of components larger than molecules, and there is great potential for its use in materials and condensed matter science (16). Fourth, self-assembly seems to offer one of the most general strategies now available for generating nanostructures. Thus self-assembly is important in a range of fields: chemistry, physics, biology, materials science, nanoscience, and manufacturing. There is an exciting opportunity for self-assembly to develop through the interchange of concepts and techniques among these fields.
Self-Assembly Is Not Limited to Molecules
Although the concepts of self-assembly were developed with molecules, and self-assembling processes currently are best understood and most highly developed for molecules, components of any size (from molecules to galaxies) can self-assemble in a permissive environment (17). The focus on self-assembly as a strategy for synthesis has been confined largely to molecules, because chemists are professionally concerned with manipulating the structure of matter at the molecular scale. The expanding contact of chemistry with biology and materials science and the direction of technology toward nanometer- and micrometer-scale structures, however, has begun to broaden this focus to include matter at scales larger than the molecular. There are now three ranges of sizes of components for which self-assembly is important: molecular, nanoscale (colloids, nanowires and nanospheres, and related structures), and meso- to macroscopic (objects with dimensions from microns to centimeters). The rules for self-assembly in each of these ranges are similar but not identical. Because new types of aggregates, especially those with potential for application in microelectronics (18, 19), photonics (20, 21), near-field optics (22), and the emerging field of nanoscience (23–25), have become increasingly important technologically, interest in self-assembly as a route to aggregates of components larger than molecules has grown. There are many opportunities for fabrication of useful structures of nano- and macroscale components using self-assembly; ultimately, self-assembly may prove to be more important in these areas than in molecular science!
Principles of Molecular Self-Assembly
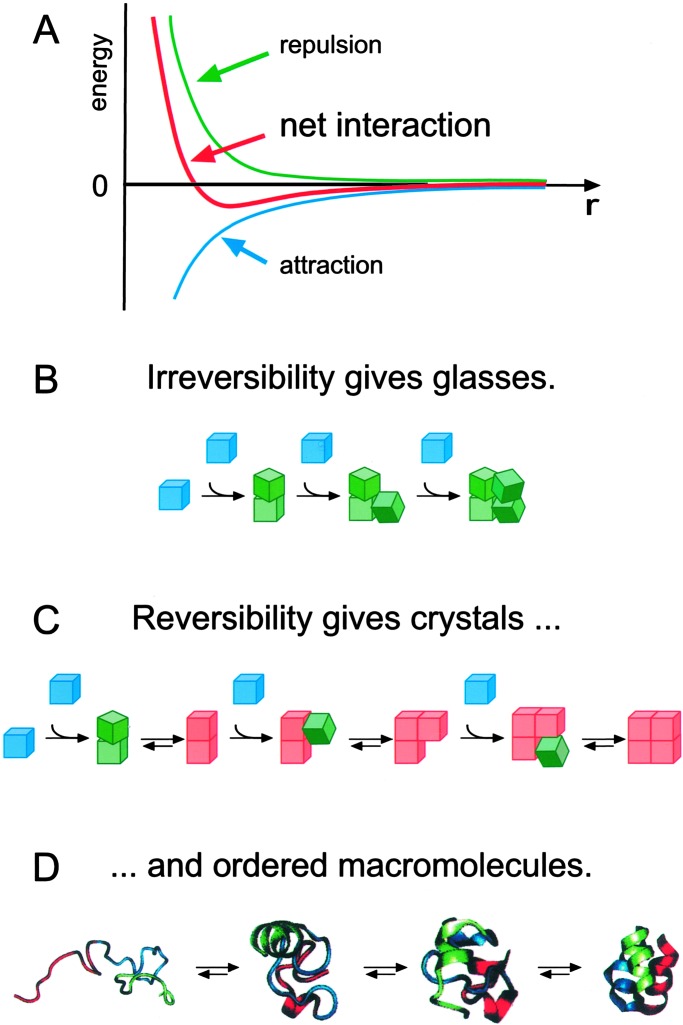
The concepts of self-assembly historically have come from studying molecular processes. The success of self-assembly in a molecular system is determined by five characteristics of the system (Fig. 1).
Figure 1.
(A) Aggregation occurs when there is a net attraction and an equilibrium separation between the components. The equilibrium separation normally represents a balance between attraction and repulsion. These two interactions are fixed in molecular self-assembly but can be engineered independently in macroscopic self-assembly. (B and C) Schematic illustration of the essential differences between irreversible aggregation and ordered self-assembly. (B) Components (shown in blue) that interact with one another irreversibly form disordered glasses (shown in green). (C) Components that can equilibrate, or adjust their positions once in contact, can form ordered crystals if the ordered form is the lowest-energy form (shown in red). (D) Biology provides many examples of self-assembly (here, the formation of a protein, an asymmetric, catalytically active nanostructure); these examples will stimulate the design of biomimetic processes.
Components.
A self-assembling system consists of a group of molecules or segments of a macromolecule that interact with one another. These molecules or molecular segments may be the same or different. Their interaction leads from some less ordered state (a solution, disordered aggregate, or random coil) to a final state (a crystal or folded macromolecule) that is more ordered.
Interactions.
Self-assembly occurs when molecules interact with one another through a balance of attractive and repulsive interactions. These interactions are generally weak (that is, comparable to thermal energies) and noncovalent (van der Waals and Coulomb interactions, hydrophobic interactions, and hydrogen bonds) but relatively weak covalent bonds (coordination bonds) are recognized increasingly as appropriate for self-assembly (26, 27). Complementarity in shapes among the self-assembling components is also crucial.
Reversibility (or Adjustability).
For self-assembly to generate ordered structures, the association either must be reversible or must allow the components to adjust their positions within an aggregate once it has formed. The strength of the bonds between the components, therefore, must be comparable to the forces tending to disrupt them. For molecules, the forces are generated by thermal motion. Processes in which collision between molecules leads to irreversible sticking generate glasses, not crystals.
Environment.
The self-assembly of molecules normally is carried out in solution or at an interface to allow the required motion of the components. The interaction of the components with their environment can strongly influence the course of the process.
Mass Transport and Agitation.
For self-assembly to occur, the molecules must be mobile. In solution, thermal motion provides the major part of the motion required to bring the molecules into contact.
In nanoscale, mesoscopic, and macroscopic self-assembly systems, the components interact in ways that are analogous to those involving molecules. In designing such systems, the first challenge often is assuring the mobility of the components; as they become larger than molecules, Brownian motion rapidly becomes irrelevant, and gravity and friction become important. The choice of interactions between the components (that is, the choice of interactions allowing the system to approach equilibrium) is also important.
Why Think About Nonmolecular (Nanoscale and Macroscale) Self-Assembly?
There are six reasons. First, nonmolecular systems allow tests of hypotheses about self-assembly that cannot be carried out with molecules and extend our understanding of the fundamental, abstract concepts of self-assembly. The characteristics of atoms determine the interactions between molecules. It is difficult to adjust the potential function between, say, two chlorine atoms to determine the influence of that change on self-assembly; chlorine atoms are what they are. Chemistry has great control over the structure of molecules, but little control over the characteristics of atoms. By contrast, it is possible to choose among a wide range of interactions (van der Waals, ionic, steric, entropic, magnetic, gravitational, electrostatic, and others) when using components larger than molecules, and often it is possible to adjust these interactions over wide ranges of strength, range, and selectivity. Nonmolecular systems are, thus, in many ways more flexible in their design than molecular systems. The second reason is experimental convenience. It often is easier to fabricate nonmolecular components than it is to synthesize molecules, and easier to observe the process and products of self-assembly using these large components. Third, self-assembly offers routes to ordered states of matter (for example, ordered arrays of nanospheres for photonic crystals; ref. 21) that probably cannot be generated practically by any other type of process; it thus has specific application in important problems in materials science, condensed matter science, and engineering (28). Fourth, self-assembly shows every promise of playing a key role in nanoscience and nanotechnology. Assembling nanometer-scale components into ordered arrays probably will be possible only by self-assembly (29). Fifth, self-assembly offers a possible route to the fabrication of three-dimensional (3D) microstructures (30). Photolithography, the technique used most widely to fabricate microstructures, is intrinsically a planar technology, and its extension to 3D structures is limited to the stacking of planar sheets. Self-assembly may offer a more flexible alternative. Sixth, a number of problems in manufacturing including problems in robotic assembly may be aided by self-assembly (31). For macroscopic objects (those sufficiently large that conventional robotic pick-and-place might in some circumstances be practical and economical), self-assembly may offer the opportunity to form structures in regions inaccessible to robotic arms. It may even be an interesting strategy for the assembly of large structures in environments (for example the microgravity of space or the ocean) where lateral mobility is relatively unhindered by the effects of gravity and friction.
Designing New, Self-Assembling Systems
We believe that the design of systems of components with nano- to macroscale dimensions for self-assembly can be aided enormously by considering analogies with molecular systems (32). To test this belief, we have explored one of many imaginable systems of self-assembling macroscopic components: systems based on capillary interactions (Fig. 2). These studies have demonstrated that it is practical to design new systems of self-assembling components essentially de novo and suggest that such systems can find rapid application. The objective of this program has been more to demonstrate the usefulness of transferring concepts from molecular systems to these larger systems than to solve practical problems, but the progression from fundamental studies to applications has been astonishingly rapid.
Figure 2.
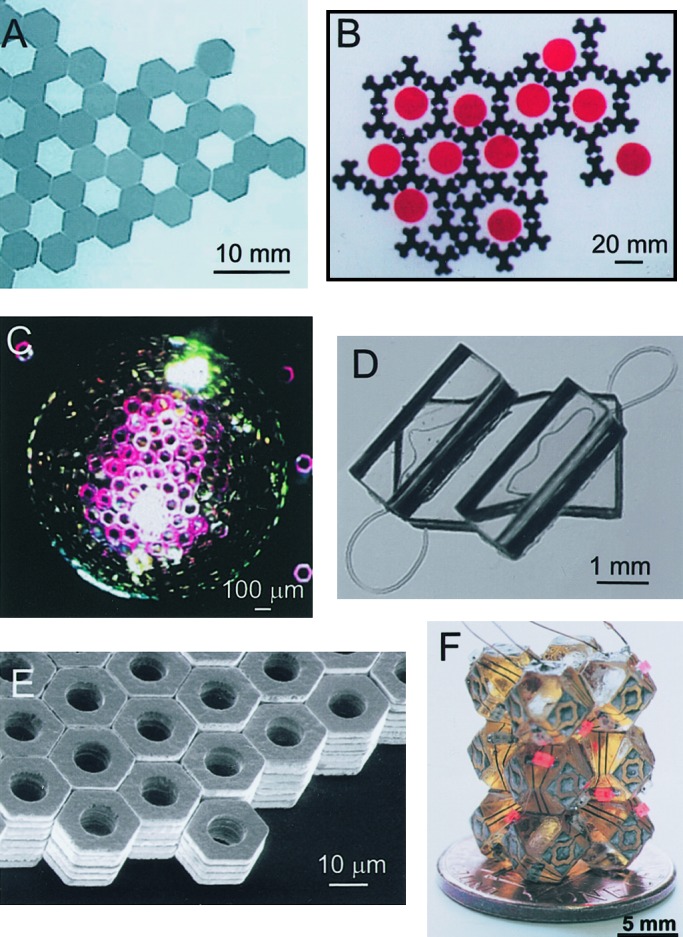
Examples of two-dimensional (A and B) and 3D (C–F) structures, self-assembled in systems of macroscopic components interacting via capillary interactions. Open hexagonal array (A; reprinted with permission from ref. 33, copyright 1997 American Association for the Advancement of Science) and hexagonal lattice formed around circular templates (B; reprinted with permission from ref. 48, copyright 2000 American Chemical Society) self-assembled from poly(dimethylsiloxane) plates floating at the interface between perfluodecalin and water. (C) Spherical structure formed by self-assembly of hexagonal metal plates on the surface of a drop of perfluodecalin in water (reprinted with permission from ref. 49, copyright 1998 American Chemical Society). (D) Compact 3D structure formed by self-folding of a string of tethered, polymeric polyhedra (reprinted with permission from ref. 54, copyright 2002 American Chemical Society). (E) Large crystal self-assembled from micrometer-sized hexagonal metal plates (reprinted with permission from ref. 36, copyright 2001 American Chemical Society). (F) Aggregate with electrical connectivity self-assembled from polyhedral, polymer components bearing solder patterns of wires and dots (reprinted with permission from ref. 42, copyright 2000 American Association for the Advancement of Science).
Our work has involved millimeter-scale components either floating at a fluid–fluid interface (33, 34) or suspended in an approximately isodense fluid medium (35–37). Capillary interactions (that is, forces resulting from minimizing the contribution of interfaces to free energies by minimizing interfacial areas) are particularly useful for these sizes and in these environments (38). For components floating at a liquid–liquid or liquid–vapor interface, the nature of the capillary interactions can be tailored by controlling the shape of the menisci at the interface between the components and the liquid (39). For components in suspension, capillary interactions between drops of liquid with high interfacial free energy provide the attractive interactions. Molten solder is one particularly useful type of liquid in these systems (40, 41). It has a high interfacial free energy and thus provides a strong interaction between components; when it solidifies below its melting point, it provides a mechanically strong and electrically conducting connection between components. Electrical conductivity is crucial to building self-assembling microelectronic systems (42).
The most complex structures that we have prepared from millimeter-sized components by self-assembly are still too primitive to be useful. However, another form of macroscopic self-assembly, the fluidic self-assembly pioneered by Jeh and Smith (43) and Howe and coworkers (44), is being developed commercially (Alien Technology, Morgan Hill, CA, www.alientechnology.com/technology/overview.html). In this technique, a suspension of small (70–180-μm) polyhedral components in a fluid is allowed to flow across a templating surface having a series of indentations complementary in shape to these components. When one of these components falls into an indentation in the correct orientation, its surface is flush with the surface of the template, and it escapes the shear of the flowing fluid; when the component is not correctly oriented with respect to the cavity, it is not flush with the surface and is removed from the cavity by shear. Another component then has the opportunity to fill the cavity correctly.
Defects, Designed Asymmetries, Constrained Self-Assembly, and Templating
One important and still unanswered question in self-assembly (at all scales, from molecular to macroscopic) is what range of structures can be formed, what are the extent and perfection of these structures, and what is the nature of their defects? The character of defects, in principle, might be quite different in molecular and macroscopic self-assembly. A molecular crystal, for example, has many opportunities to minimize its free energy. The characteristic on- and off- rates describing a macroscopic component entering and leaving an ordered, macroscopic aggregate will be orders of magnitude lower than those for molecular components, and the rate at which a macroscopic system can approach a minimum in free energy will therefore also be very low. Macroscopic self-assembly, however, typically involves much smaller numbers of components than does molecular self-assembly, and the degree of perfection required to generate a particular material also may be lower than that required of a molecular crystal. The compromise between numbers of particles, rates of equilibration, and number of defects in all forms of self-assembly, from molecular to macroscopic, remains to be established (45, 46).
Biology offers important hints about how to limit defects. One is the involvement of templates (e.g., chaperonins; ref. 47) to ensure the correct folding of proteins in competition with other possible processes (e.g., intermolecular aggregation and precipitation). Chaperonin-guided folding suggests the value of templates (e.g., geometrical constraints) in limiting defects in this type of self-assembly. Geometrical templating also has proved valuable in nonbiological self-assembly (48–51).
A second general concern in self-assembly is the generation of asymmetry in self-assembling structures. The simplest form of self-assembly, the ordered aggregation of identical components, is the one most commonly studied; this type of process, for molecular components, leads to the formation of molecular crystals or discrete, structurally defined aggregates, usually with high symmetries. For self-assembly to have broad applications (especially in microelectronics and photonics), it must be able to generate asymmetrical structures: analogs of proteins rather than analogues of crystals. The strategy used to generate globular proteins, which have unsymmetrical 3D structures, again provides an example of a successful biological solution to this problem. In the biosynthesis of proteins, the precursor polypeptide is generated, by using covalent chemistry, as a linear string; this string folds spontaneously into a compact, 3D, globular, functional structure. The sequence of amino acids in the polypeptide, of course, is fixed, and this sequence enormously restricts the range of structures that can be formed from that set of amino acids. The strategy of allowing a string of components to fold into a compact, functional structure is successful also at the macroscopic scale; we have used it to make a prototype microelectronic system (52). Understanding constrained self-assembly (of which this demonstration is an example) in sufficient detail to be able to use it by design is a challenge for the future; the basic biophysics of protein folding is still incompletely understood, and a fundamental understanding of folding as a process leading to macroscopic self-assembly is essentially nonexistent.
Studying biology has identified many processes that use templated self-assembly; these strategies will provide stimuli for nonmolecular self-assembly for many years. Biological strategies, however, may be constrained in ways that are not relevant in nonbiological systems; we cannot assume that biology demonstrates all strategies for self-assembly. The study of biology thus may also suggest new, useful strategies for self-assembly that are not used in the cell. The folding of proteins and RNAs provides an example. Both processes generate sophisticated 3D structures by spontaneous folding of linear precursors. The fact that the precursors are linear reflects the ease with which linear macromolecules are synthesized through repetitive formation of covalent bonds. In nonbiological, macroscopic self-assembly, the constraints on synthesis that exist in the cell need not apply, and one could ask what might happen if there was no constraint favoring the formation of linear strings. How, for example, would a flexible, two-dimensional sheet bearing patterns of attractive and repulsive surfaces crumple spontaneously?
It seems that templating (that is, providing constraints whether physical boundaries or sequence in a chain) may be an important way to bring order and asymmetric structure to self-assembled aggregates. This area of strategy for self-assembly is just beginning to be explored (53–55).
Components for Self-Assembly
It is possible now to synthesize many nanoscale structures, colloids, quantum dots, buckytubes, nanotubes, and nanowires, but it remains difficult to induce their self-assembly into functional structures (24, 56–58). Macroscopic objects can be fabricated that self-assemble well (32), but scaling the fabrication of these structures into dimensions of microns, much less nanometers, remains an unsolved problem. In fact, the crux of mesoscale self-assembly ultimately may lie in the fabrication of components. Self-assembly of appropriate components as a strategy for generating ordered aggregates seems likely to prove reliable and versatile. Designing and fabricating these components, especially those with micro- and nanometer dimensions, is difficult, and there is no certain pathway to make them (17). In this respect, molecular self-assembly backed by the enormous power of molecular synthesis has an advantage. Unfortunately molecular synthesis is not yet capable of making structures that form the basis for nanoelectronic systems, although research pointed in that direction is very active (59–62).
The first issue in designing components whose self-assembly will generate functional systems is to understand the trade-offs between ease of fabrication (easier with large components) and function (often higher with small components). The shape of the components is also important: for 3D microelectronic devices, for example, self-assembly must probably generate a structure with continuous voids, to allow for external cooling (63). The more complicated the shape, the more difficult it is to fabricate, especially in a form functionalized for self-assembly. Most methods for 3D microfabrication are based on photolithography (30, 64). A number of techniques (some very clever but none very simple or easily extended to the parallel fabrication of very large numbers of components) have been used to convert 100-μm-scale two-dimensional structures produced by photolithography into 3D structures (65–68). The most versatile of these at present seems to be self-folding, which is another form of self-assembly. This technique uses conventional lithographic technology to make planar precursors to the 3D microstructures. These structures then fold spontaneously into 3D shapes via capillary forces (69, 70) or electrical activation of conjugated polymers (71). The processes used in this type of fabrication involve multiple steps of fabrication, however, and the folding processes are early in their development. Hierarchical self-assembly, the self-assembly of simple components such as microspheres into structured aggregates such as triangles or tetrahedra, followed by the self-assembly of those structures into more complex structures is another promising approach (72–74).
Equilibrium and Nonequilibrium Self-Assembly
Molecular self-assembly has focused almost exclusively on equilibrium systems. These types of systems have the advantage that they tend to form highly ordered arrays (crystals) and tend to be self-healing if damaged. These characteristics unquestionably are useful. It is, however, important to remember that the most interesting self-assembling systems, including many centrally important to the life of the cell, are dynamic; that is, they are out-of-equilibrium systems, and they form their characteristic order only when dissipating energy (75). Understanding and controlling dynamic, self-assembling systems is a difficult problem, but the solution of which is required both to maximize the value of self-assembly as a strategy for synthesis and fabrication and to understand the role of self-assembly in biology. Work in this area is just beginning (76, 77).
Conclusions
Self-assembly has the potential to provide the basis for a new form of molecular synthesis. Classical, covalent synthesis now is so accomplished and successful as an art and a technology that it can make most target molecules. One new class of targets now facing organic synthesis that cannot be made by classical, covalent organic synthesis is large aggregates of structured matter: aggregates with structural complexity comparable to that of biological macromolecules or structures such as virus particles. Synthesis of structures of this complexity will require new strategies that rely heavily on noncovalent synthesis (78). Self-assembly is, in some sense, the core of noncovalent, molecular synthesis.
The potential of self-assembly as a strategy for forming useful and interesting structures, however, extends far beyond molecules. It offers a very promising route for making crystals of nanometer- and micrometer-scale components. It may provide a way of assembling electrically or optically functional components. The range of practical problems in fabrication (photonic crystals, 3D microelectronic systems, displays, or sensors) probably is broader and better defined than that facing molecular self-assembly (where much of the activity in research still remains exploratory and in which new applications of self-assembly are emerging only slowly). Based on admittedly limited experience, we would judge that the greatest challenge in using self-assembly to make complex electrically or optically functional assemblies is that of fabricating the precursor components, not carrying out the self-assembly once these components are available. Self-assembly as a strategy for fabrication of systems and synthesis of materials seems flexible and robust to changes in the characteristics of the components.
To those attacking these new, challenging, and complex problems in self-assembly, biology offers a wonderful array of successful examples. The cell exists as a result of processes that generate complex, multicomponent, functional structures by self-assembly. To the extent that those interested in fabricating complex structures using components at all scales can understand the strategies used in biology, there seems limitless opportunity for the development of self-assembly as a strategy for the fabrication of complex systems. Using biomimetic strategies to expand the horizons of self-assembly beyond static systems into dynamic self-assembly promises to open an entire new chapter in this field.
The objectives of self-assembly are to make structures that cannot be made by other means and to understand one aspect of life. Self-assembly is an area that is engaging many of the classical disciplines of science and engineering in pursuing these objectives. Integrating concepts and techniques from across this range of disciplines promises to be enormously interesting and ultimately very useful.
Acknowledgments
This work was supported by National Science Foundation Grants CHE-0101432 and CHE-9901358 and grants from the Defense Advanced Research Projects Agency/Office of Naval Research and Department of Energy.
References
- 1.Whitesides G M, Simanek E E, Gorman C B. In: NATO Advanced Study Institute on Chemical Synthesis: Gnosis to Prognosis. Chatgilialoglu C, Snieckus V, editors. Dordrecht, the Netherlands: Kluwer; 1996. pp. 565–588. [Google Scholar]
- 2.Philip D, Stoddart J F. Angew Chem Int Ed Engl. 1996;35:1155–1196. [Google Scholar]
- 3.Lehn J M, Ball P. In: The New Chemistry. Hall N, editor. Cambridge, U.K.: Cambridge Univ. Press; 2000. pp. 300–351. [Google Scholar]
- 4.Desiraju G R. Crystal Engineering: The Design of Organic Solids. New York: Elsevier; 1989. [Google Scholar]
- 5.Evans D F, Wennerstrom H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. New York: Wiley; 1999. [Google Scholar]
- 6.Jones M N, Chapman D. Micelles, Monolayers, and Biomembranes. New York: Wiley–Liss; 1995. [Google Scholar]
- 7.Thomas E L. Science. 1999;286:1307. [Google Scholar]
- 8.Kumar A, Abbott N A, Kim E, Biebuyck H A, Whitesides G M. Acc Chem Res. 1995;28:219–226. [Google Scholar]
- 9.Grantcharova V, Alm E J, Baker D, Horwich A L. Curr Opin Struct Biol. 2001;11:70–82. doi: 10.1016/s0959-440x(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 10.Neidle S. Oxford Handbook of Nucleic Acid Structure. Oxford, U.K.: Oxford Univ. Press; 1999. [Google Scholar]
- 11.Bongrand P. Rep Prog Phys. 1999;62:921–968. [Google Scholar]
- 12.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular Biology of the Cell. New York: Garland; 1994. [Google Scholar]
- 13.Schwiebert K E, Chin D N, MacDonald J C, Whitesides G M. J Am Chem Soc. 1996;118:4018–4029. [Google Scholar]
- 14.Schmidt-Mende L, Fechtenkotter A, Mullen K, Moons E, Friend R H, MacKenzie J D. Science. 2001;293:1119–1122. doi: 10.1126/science.293.5532.1119. [DOI] [PubMed] [Google Scholar]
- 15.De Rosa C, Park C, Thomas E L, Lotz B. Nature (London) 2000;405:433–437. doi: 10.1038/35013018. [DOI] [PubMed] [Google Scholar]
- 16.Whitesides G M. Sci Am. 1995;273(3):146–149. [Google Scholar]
- 17.Isaacs L, Chin D N, Bowden N, Xia Y, Whitesides G M. In: Supramolecular Technology. Reinhoudt D N, editor. New York: Wiley; 1999. pp. 1–46. [Google Scholar]
- 18.Sirringhaus H, Kawase T, Friend R H, Shimoda T, Inbasekaran M, Wu W, Woo E P. Science. 2000;290:2123–2126. doi: 10.1126/science.290.5499.2123. [DOI] [PubMed] [Google Scholar]
- 19.Rogers J A, Bao Z, Baldwin K, Dodabalapur A, Crone B, Raju V R, Kuck V, Katz H, Amundson K, Ewing J, Drzaic P. Proc Natl Acad Sci USA. 2001;98:4835–4840. doi: 10.1073/pnas.091588098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenekhe S A, Chen L X. Science. 1999;283:372–375. doi: 10.1126/science.283.5400.372. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Gates B, Yin Y, Lu Y. Adv Mater. 2000;12:693–713. [Google Scholar]
- 22.Wu M H, Whitesides G M. Appl Phys Lett. 2001;78:2273–2275. [Google Scholar]
- 23.Lieber C M. Sci Am. 2001;285(3):58–64. doi: 10.1038/scientificamerican0901-58. [DOI] [PubMed] [Google Scholar]
- 24.Klimov V I, Mikhailovski A A, Xu S, Malko A, Hollingsworth J A, Leatherdale C A, Eisler H-J, Bawendi M G. Science. 2000;290:314–317. doi: 10.1126/science.290.5490.314. [DOI] [PubMed] [Google Scholar]
- 25.Sun S H, Murray C B, Weller D, Folks L, Moser A. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 26.Olenyuk B, Whiteford J A, Fechtenkotter A, Stang P J. Nature (London) 1999;398:796–799. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- 27.Lehn J-M. NATO ASI Ser Ser E. 1996;320:511–524. [Google Scholar]
- 28.Whitesides G M. Angew Chem Int Ed Engl. 1990;29:1209–1218. [Google Scholar]
- 29.Whitesides G M. Sci Am. 2001;285(3):78–83. doi: 10.1038/scientificamerican0901-78. [DOI] [PubMed] [Google Scholar]
- 30.Madou M. Fundamentals of Microfabrication. Boca Raton, FL: CRC; 1997. [Google Scholar]
- 31.Boehringer K F, Fearing R S, Goldberg K Y. In: The Handbook of Industrial Robotics. Nof S, editor. New York: Wiley; 1999. pp. 1045–1066. [Google Scholar]
- 32.Bowden N B, Weck M, Choi I S, Whitesides G M. Acc Chem Res. 2001;34:231–238. doi: 10.1021/ar0000760. [DOI] [PubMed] [Google Scholar]
- 33.Bowden N, Terfort A, Carbeck J, Whitesides G M. Science. 1997;276:233–235. doi: 10.1126/science.276.5310.233. [DOI] [PubMed] [Google Scholar]
- 34.Bowden N, Oliver S, Whitesides G M. J Phys Chem. 2000;104:2714–2724. [Google Scholar]
- 35.Jackman R J, Brittain S T, Adams A, Prentiss M G, Whitesides G M. Science. 1998;280:2089–2091. doi: 10.1126/science.280.5372.2089. [DOI] [PubMed] [Google Scholar]
- 36.Clark T D, Tien J, Duffy D C, Paul K, Whitesides G M. J Am Chem Soc. 2001;123:7677–7682. doi: 10.1021/ja010634l. [DOI] [PubMed] [Google Scholar]
- 37.Oliver S R J, Bowden N, Whitesides G M. J Colloid Interface Sci. 2000;224:425–428. doi: 10.1006/jcis.1999.6695. [DOI] [PubMed] [Google Scholar]
- 38.Bowden N, Choi I S, Grzybowski B, Whitesides G M. J Am Chem Soc. 1999;121:5373–5391. [Google Scholar]
- 39.Grzybowski B A, Bowden N, Arias F, Yang H, Whitesides G M. J Phys Chem B. 2001;105:404–412. [Google Scholar]
- 40.Syms R R A, Yeatman E M. Electronics Lett. 1993;29:662–664. [Google Scholar]
- 41.Harsh K F, Bright V M, Lee Y C. Sens Actuators A. 1999;77:237–244. [Google Scholar]
- 42.Gracias D H, Tien J, Breen T L, Hsu C, Whitesides G M. Science. 2000;289:1170–1172. doi: 10.1126/science.289.5482.1170. [DOI] [PubMed] [Google Scholar]
- 43.Jeh H-J J, Smith J S. IEEE Photon Technol Lett. 1994;6:706–708. [Google Scholar]
- 44.Srinivasan U, Liepmann D, Howe R T. J Microelectromech Syst. 2001;10:17–24. [Google Scholar]
- 45.Hosokawa K, Shimoyara I, Miura H. Artif Life. 1995;1:413–427. [Google Scholar]
- 46. Boehringer, K. F., Srinivasan, U. & Howe, R. T. (2001) Proc. IEEE Conf. Microelectromech. Syst., 369–374.
- 47.Frydman J. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 48.Choi I S, Weck M, Xu B, Jeon N L, Whitesides G M. Langmuir. 2000;16:2997–2999. [Google Scholar]
- 49.Huck W T S, Tien J, Whitesides G M. J Am Chem Soc. 1998;120:8267–8268. [Google Scholar]
- 50.Velev O D, Furusawa K, Nagayama K. Langmuir. 1996;12:2374–2384. [Google Scholar]
- 51.Kim E, Whitesides G M. Chem Mater. 1995;7:1257–1264. [Google Scholar]
- 52.Boncheva M, Gracias D H, Jacobs H O, Whitesides G M. Proc. Natl. Acad. Sci. USA. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi I S, Weck M, Jeon N L, Whitesides G M. J Am Chem Soc. 2000;122:11997–11998. [Google Scholar]
- 54.Clark T D, Boncheva M, German J M, Weck M, Whitesides G M. J Am Chem Soc. 2002;124:18–19. doi: 10.1021/ja0120633. [DOI] [PubMed] [Google Scholar]
- 55. Clark, T. D., Ferrigno, R., Tien, J., Paul, K. & Whitesides, G. M. (2002) J. Am. Chem. Soc., in press. [DOI] [PubMed]
- 56.Lu Y, Yin Y, Xia Y. Adv Mater. 2001;13:409–413. [Google Scholar]
- 57.Bachtold A, Hadley P, Nakanishi T, Dekker C. Science. 2001;294:1317–1320. doi: 10.1126/science.1065824. [DOI] [PubMed] [Google Scholar]
- 58.Duan X, Huang Y, Cui Y, Wang J, Lieber C M. Nature (London) 2001;409:66–69. doi: 10.1038/35051047. [DOI] [PubMed] [Google Scholar]
- 59.Derycke V, Martel R, Appenzeller J, Avouris P. Nano Lett. 2001;1:453–456. [Google Scholar]
- 60.Reed M A, Tour J M. Sci Am. 2000;282(6):86–93. doi: 10.1038/scientificamerican0600-86. [DOI] [PubMed] [Google Scholar]
- 61.Davis W B, Svec W A, Ratner M A, Wasielewski M R. Nature (London) 1998;396:60–63. [Google Scholar]
- 62.Schon J H, Meng H, Bao Z. Nature (London) 2001;413:713–716. doi: 10.1038/35099520. [DOI] [PubMed] [Google Scholar]
- 63.Breen T L, Tien J, Oliver S R J, Hadzic T, Whitesides G M. Science. 1999;284:948–951. doi: 10.1126/science.284.5416.948. [DOI] [PubMed] [Google Scholar]
- 64.Campbell S A. The Science and Engineering of Microelectronic Fabrication. New York: Oxford Univ. Press; 1996. [Google Scholar]
- 65.Qin D, Xia Y, Rogers J A, Jackman R J, Zhao X-M, Whitesides G M. Top Curr Chem. 1998;194:1–20. [Google Scholar]
- 66.Paul K E, Breen T L, Aizenberg J, Whitesides G M. Appl Phys Lett. 1998;73:2893–2895. [Google Scholar]
- 67.Xia Y, Rogers J A, Paul K E, Whitesides G M. Chem Rev. 1999;99:1823–1848. doi: 10.1021/cr980002q. [DOI] [PubMed] [Google Scholar]
- 68. Jacobs, H. O., Tao, A. R., Schwartz, A., Gracias, D. H. & Whitesides, G. M. (2002) Science, in press. [DOI] [PubMed]
- 69.Syms R R A, Gormley C, Blackstone S. Sens Actuators A. 2000;2839:1–11. [Google Scholar]
- 70.Gracias D H, Kavthekar V, Love J C, Paul K E, Whitesides G M. Adv Mater. 2002;14:235–238. [Google Scholar]
- 71.Jager E W H, Smela E, Inganäs O. Science. 2000;290:1540–1545. doi: 10.1126/science.290.5496.1540. [DOI] [PubMed] [Google Scholar]
- 72.Vlasov Y A, Yao N, Norris D J. Adv Mater. 1999;11:165–169. [Google Scholar]
- 73.Yin Y, Lu Y, Gates B, Xia Y. J Am Chem Soc. 2001;123:8718–8729. doi: 10.1021/ja011048v. [DOI] [PubMed] [Google Scholar]
- 74.Lopes W A, Jaeger H M. Nature (London) 2001;414:735–738. doi: 10.1038/414735a. [DOI] [PubMed] [Google Scholar]
- 75.Ball P. The Self-Made Tapestry: Pattern Formation in Nature. Oxford, U.K.: Oxford Univ. Press; 1999. [Google Scholar]
- 76.Grzybowski B, Stone H A, Whitesides G M. Nature (London) 2000;405:1033–1036. doi: 10.1038/35016528. [DOI] [PubMed] [Google Scholar]
- 77.Grzybowski B A, Whitesides G M. J Phys Chem B. 2001;105:8770–8775. [Google Scholar]
- 78.Simanek E E, Mathias J P, Seto C T, Chin D, Mammen M, Gordon D M, Whitesides G M. Acc Chem Res. 1995;28:37–44. [Google Scholar]