Abstract
Salmonella infections cause significant economic losses in poultry and livestock production. Salmonella infection reprograms the proteomic and miRNA profiles of intestinal macrophage-derived exosomes, which subsequently modulates the inflammatory responses of surrounding recipient cells. However, the mechanisms underlying the selective packaging of specific miRNAs into exosomes during inflammation are still incompletely understood. In this study, we employed Tandem Mass Tag (TMT)-based quantitative proteomics to analyze exosomes derived from macrophages following Salmonella infection versus uninfected controls. Furthermore, RNA immunoprecipitation (RIP) assays were conducted to investigate potential interactions between a candidate exosomal protein and miR-27a-5p. The proteomic analysis revealed that Salmonella infection induced significant remodeling of the exosomal protein cargo secreted by RAW 264.7 macrophage-like cells. Quantitative comparison with uninfected controls showed 383 proteins were significantly upregulated and 666 were downregulated in exosomes at 12 h post-infection, demonstrating infection-dependent reprogramming of exosomal composition. Limited differential protein expression was observed at 3 h post-Salmonella infection group. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis indicated that the target genes are mainly associated with bacterial infectious diseases, translation, and molecular binding. Notably, the RNA binding protein heterogeneous nuclear ribonucleoprotein A/B (hnRNP A/B) was significantly upregulated in exosomes derived from Salmonella-infected macrophages. RIP assays confirmed specific binding of hnRNP A/B to miR-27a-5p. Furthermore, knockout (KO) of hnRNP A/B markedly decreased exosomal miR-27a-5p export while increasing its cellular retention. These findings establish hnRNP A/B as a critical regulator of miR-27a-5p sorting during Salmonella infection, suggesting its potential as a therapeutic target to interfere with bacterial pathogenesis and host immune evasion mechanisms in poultry and livestock medicine.
Keywords: Salmonella: Macrophage, Exosome, miR-27a-5p, hnRNP A/B
Introduction
Salmonella infections pose a significant threat to both the livestock breeding industry and human health. In livestock farming, this results in reduced productivity. Infected animals often show symptoms such as diarrhoea, weight loss and reduced growth rate, resulting in financial losses for farmers (Knodler and Elfenbein, 2019). In humans, Salmonella can cause foodborne illness, with symptoms ranging from abdominal pain, vomiting and fever to more severe cases that can lead to long-term health problems and hospitalization (Gilchrist and MacLennan, 2019; Scallan et al., 2015). Understanding the process of Salmonella infection and its immune escape mechanism is of great value. By studying the infection process, we can identify the key stages at which the bacteria invade and multiply in the host, enabling the development of targeted preventive measures in livestock farming, such as improved hygiene practices and vaccination strategies.
Exosomes, with diameters ranging from 30 nm to 150 nm, play an important role in intercellular communication (Buzas, 2023; Kalluri and LeBleu, 2020). They act as natural shuttles, transporting a diverse cargo of biomolecules, including proteins, lipids and nucleic acids, between cells (Qu et al., 2022). This transfer of materials allows cells to influence the behavior and function of neighboring and even distant cells, contributing to processes such as tissue repair, immune response modulation, and the regulation of normal physiological development (Hui et al., 2018). Among the various components carried by exosomes, microRNAs (miRNAs) have attracted significant attention. MiRNAs are small, non-coding RNA molecules, typically 20-24 nucleotides in length. In the context of exosomes, exosomal miRNAs are not only protected from degradation in the extracellular environment, but also gain the ability to be delivered to the recipient cells (Gong et al., 2022; Ro et al., 2018; Wang et al., 2021b; Xu et al., 2021). Once inside the recipient cells, exosomal miRNAs can regulate gene expression by binding to complementary sequences in mRNAs, leading to either translational repression or mRNA degradation. This unique function of exosomal miRNAs has implications for numerous biological processes and diseases, including cancer metastasis, neurodegenerative disorders, and cardiovascular diseases.
Our previous study revealed an interesting phenomenon in the context of Salmonella infection. When Salmonella infects macrophages, the expression of miR-27a-5p, secreted by these infected macrophages, is significantly elevated (Qu et al., 2025). This up-regulated miR-27a-5p has a profound effect on the immune response. It has the ability to suppress the activation of surrounding immune cells, which in turn delays the development of inflammation and the process of eliminating Salmonella. This suggests that Salmonella may exploit miR-27a-5p to facilitate immune evasion. In light of this discovery, it becomes paramount to uncover the mechanisms involved in packing miR-27a-5p into the exosome. Studies have shown that some miRNAs are highly expressed in the exosome and some are highly expressed in the cell, and that they have specific sorting signals that determine whether to be secreted into the exosome or retained in the cytoplasm (Garcia-Martin et al., 2022; Groot and Lee, 2020). RNA-binding proteins (RBPs) such as hnRNPA2B1, synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP), and Fragile X Messenger Ribonucleoprotein 1 (FMR1) are reported to recognize specific motifs of different miRNAs, bind to them, and help the miRNAs to be wrapped into exosomes, which are then secreted into the cell (Villarroya-Beltri et al., 2013; Wozniak et al., 2020). Identifying distinct RBPs is crucial for elucidating the molecular mechanisms underlying miRNA sorting into exosomes. The objective of this study is to explore possible mechanisms by which miR-27a-5p is packed into exosomes. Identifying these proteins could potentially provide new targets for developing strategies to effectively block the immune escape of Salmonella. Interfering with the packaging process of miR-27a-5p into the exosome may be able to disrupt the pathogen's immune evasion mechanism, thereby enhancing the body's ability to fight Salmonella infection.
Materials and methods
Cell culture, bacterial strain and infection
RAW264.7 cells or HEK 293T cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; VivaCell, Shanghai, China) along with 10 % fetal bovine serum (FBS; Gibco, CA, USA). The Salmonella enterica serovar Typhimurium bacteria ATCC 14028 (ST14028) were obtained from American Type Culture Collection (ATCC, VA, USA). Wild-type Salmonella typhimurium 173 and Salmonella enteritidis GL228 were from Shandong Yisheng livestock and poultry breeding company (Yantai, China). Salmonella was subsequently cultured overnight in Luria-Bertani medium (LB broth; Solarbio, Beijing, China) without antibiotics at 37°C while being shaken. After that, it was subcultured in LB for 12 h (with an optical density of 0.7 to 1 at 600 nm) and then harvested by centrifugation at 6,000 g for 20 min. The bacterial inoculates were diluted in DMEM without antibiotics and then added to the cells at a multiplicity of infection (MOI) of 20:1 (Qu et al., 2025). Following an incubation period of 1 h, the extracellular bacteria were washed twice using PBS. Afterward, the culture media was replaced with a medium containing 200 μg/mL gentamicin sulfates. RAW264.7 cells were then incubated at 37°C under 5 % CO2 for another 1 h. After that, the medium was replaced with DMEM that was supplemented with 10 % exosome-free FBS (VivaCell, Shanghai, China) and also contained 20 μg/mL gentamicin. The infected cells were further incubated at 37°C in a 5 % CO2 incubator for subsequent experiments.
Exosomal protein extraction and TMT/Isobaric tag for relative absolute quantitation (iTRAQ) protein sequencing
Extracellular vesicles in culture media were derived from RAW 264.7 cells left uninfected or infected with ST 14028 as previously described (Qu et al., 2025). Briefly, after exosomes were isolated by using differential ultracentrifugation and Umibio® exosome isolation kit (Umibio, Shanghai, China), total proteins were extracted using a protease inhibitor cocktail (Thermo Fisher Scientific, MA, USA). The samples were ultrasonically treated on ice for 2 min and then lysed for 10 min. The protein supernatant was obtained after 12,000 g centrifugation at 4°C for 30 min. After quantification by the Bicinchoninic Acid (BCA) Protein Assay Kit (Pierce, Rochford, IL), the protein mass was detected by SDS-PAGE. TMT high-throughput sequencing of protein was carried out by Majorbio company (Shanghai, China) (Qu et al., 2023). Briefly, the reduced alkylation treatment was performed on a protein sample of qualified quality. An equal amount of protein was taken from each sample for trypsin digestion. TMT tags were used to label peptide fragments. The labelled peptide fragments were mixed in equal amounts and pre-separated using a C18 inversion column (Waters, MA, USA). The liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis was performed.
Bioinformatic analysis of exosomal protein
The ProteomeDiscovererTM Software 2.4 was used for database search using the Sequest or Mascot modules. Data statistics and bioinformatics analysis were performed on the obtained database search results including Salmonella enterica serovar Typhimurium protein and murine protein (https://www.uniprot.org/proteomes). The differently expressed proteins (DEPs) were defined using a fold change (|log2FC| ≥ 0.263) and P value (< 0.05). The UniProt GOA database (https://geneontology.org/) was used for GO annotation. Pathway enrichment was performed using KEGG database (https://www.genome.jp.kegg/). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository (Chen and Ma, 2022; Ma et al., 2019) with the dataset identifier PXD062445. Project Webpage: https://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD062445.
RNA extraction, reverse transcription, and quantitative real-time PCR (RT-qPCR)
The miRNeasy Serum/Plasma Advanced kit (Qiagen, DUS, GER) was applied to isolate total RNA from exosomes, while the miRNeasy mini Kit (Qiagen, DUS, GER) was used for the isolation of total RNA from RAW264.7 cells respectively. Thereafter, either the Hairpin-itTM miRNAs qPCR Quantitation Kit (Genepharma, Shanghai, China) or the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, USA) was utilized to perform reverse transcription of the RNA. Then, a quantitative real-time PCR assay was carried out on an Applied Biosystems QuantStudioTM 6 Real-Time PCR System. All procedures were carried out in strict accordance with the manufacturer's instructions for the reagents. The relative expression of the gene miR-27a-5p was determined using the 2−△△Ct method (Lai et al., 2015). Here, Data were normalized to levels of U6 (cellular) or cel-miR-39 (exosome) for miRNA. The sequences of primers are as follow: mmu-miR-27a-5p: 5’-AGACTGAGGGCTTAGCTGCTTG-3’ and 5’-TATGGTTGTTGACGACTGGTTGAC-3’, U6: 5’-CAGCACATATACTAAAATTGGAACG-3’ and 5’-ACGAATTTGCGTGTCATCC-3’, and cel-miR-39-3p: 5’- ATATCATCTCACCGGGTGTAAATC-3’ and 5’- TATGGTTTTGACGACTGTGTGAT-3’.
Western blot aassay
Total proteins were extracted from the exosomes of RAW264.7 cells. The extraction process involved the use of a radio immunoprecipitation assay (RIPA) lysis buffer (Sangon Biotech, Shanghai, China) that was supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF). Subsequently, the concentration of the obtained proteins was determined by applying a BCA protein assay kit (Pierce, Rochford, IL). For each sample, an equal quantity of protein, specifically 30 μg per lane, was subjected to separation through 10 % SDS-PAGE. Following the separation, the proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA) on ice. Then, the PVDF membrane was cut into strips according to the protein markers (Thermo Scientific, Mass, USA) and blocked with 5 % skimmed milk (Cell Signalling Technology, Mass, USA) in Tris Buffered Saline Tween (TBST) buffer containing for 1 h at room temperature. After the blocking step, the membrane was then incubated overnight at 4 °C with primary antibodies against hnRNP A/B (1:1000, Abcam, MA, USA), FXR1 (from Santa Cruz Biotechnology, CA, USA) or β-actin (1:1000, CST, MA, USA). After the overnight incubation with the primary antibodies, the proteins were detected by incubating the membrane with a horseradish peroxidase (HRP)-conjugated secondary antibody (at a dilution ratio of 1:2500, obtained from Cell Signalling Technology, Mass, USA) for 1 h at room temperature. Finally, all the protein bands were visualized using an enhanced chemiluminescence (ECL) kit of SuperSignal™ West Pico PLUS (Thermo Scientific, Mass, USA) in conjunction with a gel imaging analytic system (Bio-Rad, CA, United States). Finally, the visualized protein bands were analyzed using ImageJ software.
Interaction of RNA Binding Proteins and MiRNA Assay
To obtain additional evidence for the interaction between RBPs and miRNA, hnRNP A/B proteins were immunoprecipitated from the lysates of RAW264.7 cells. This was achieved by employing an anti-hnRNP A/B antibody (Santa Cruz Tech, CA, USA). The procedure was carried out in strict accordance with the guidelines provided in the instruction manual of the PureBinding® RNA Immunoprecipitation kit (Gene Seed, Guangzhou, China) (Liang et al., 2022). In short, RAW264.7 cells were homogenized in a lysis buffer containing both protease and RNase inhibitors. Subsequently, the homogenized cell mixture was split into two groups. One group was used for capturing anti-hnRNP A/B-coated protein A/G magnetic Beads. This capture process was performed using a rotating mixer at 4 °C overnight. For the other group, anti-IgG (Santa Cruz Tech, CA, USA) was used as negative controls. The miR-27a-5p/hnRNP A/B complex was then immunoprecipitated and pooled for RT-qPCR analysis to measure miR-27a-5p levels. Moreover, HEK 293T cells were transfected with a clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) knockout hnRNP A/B plasmids (Santa Cruz Tech, CA, USA). The aim of this transfection was to detect the expression of miR-27a-5p in both cells and exosomes after hnRNP A/B had been interfered with.
Statistical analysis
All the data were processed and analyzed using GraphPad Prism version 8.0 (GraphPad Inc., San Diego, CA, USA) and showed as the mean ± SD from three independent repeats. A Student's t-test was used to assess the difference between the two groups. In contrast, when analyzing multiple groups, a one-way ANOVA was carried out. Statistical significance was determined based on the P value ≤ 0.05.
Results
Macrophages secrete exosomes after being uninfected or infected with Salmonella
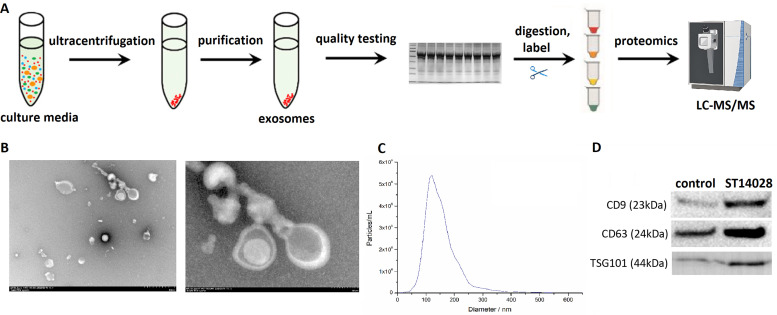
As mentioned in previous studies, macrophages treated with ST 14028 at MOI 20 for 12 h were able to differentiate into M1-type phenotypes, which secrete exosomes with different expression of miRNA (Qu et al., 2025). MiR-27a-5p enriched in exosomes of Salmonella-infected macrophage attenuates Toll-like receptor 7/ nuclear factor kappa-B (TLR7/NF-κB) signaling, which seems to reprogram the inflammation in recipient cells to help Salmonella immune escape. Identifying the key proteins that facilitate the packing of miR-27a-5p into the exosome can provide a reference for preventing immune escape from Salmonella. To uncover the mechanism by which miRNA is packaged into exosomes, naïve RAW 264.7 cells were treated with or without ST14028 for 12 h at a MOI of 20 and macrophage culture media was collected to obtain exosomes for high-throughput proteomic analysis (Fig. 1A). It was used to identify host and bacterial proteins carried by these host-released EVs. Afterward, TEM images revealed that the exosome has a cup-like appearance with a diameter of approximately 30 nm to 200 nm (Fig. 1B, C). Common exosomal markers CD9, CD81 and TSG101 were confidently identified in vesicles obtained from both groups of macrophages (Fig. 1D). These data suggest that exosomes have been successfully obtained and refined from macrophages exposed to Salmonella, and that they are suitable for follow-up in-depth investigations.
Fig. 1.
Work flow and exosome identification from infected RAW 264.7 cells. A. Schematic analysis of the proteomic content of exosomes. Exosomes derived from Salmonella-infected RAW264.7 macrophages were isolated. Equal protein samples of each group were subjected to SDS-PAGE for protein extraction and separation. Protein excised from each lane was denatured, reduced, alkylated and tryptic digested with trypsin. TMT tags were used to label peptide fragments. Labelled peptide pools were desalted by C18 inversion column and analyzed by LC-MS/MS. B. Electron microscopy revealed the cup-like character of the exosome isolated from RAW 264.7 cells in culture media. Scale bar, 500 nm or 100 nm. C. NTA determined exosome size distribution in RAW 264.7 cells with or without ST 14028 infection. D. Exosomal markers were tested by Western blot assay.
Salmonella alters the profile of RAW264.7 Cell-derived Exosomal Proteins
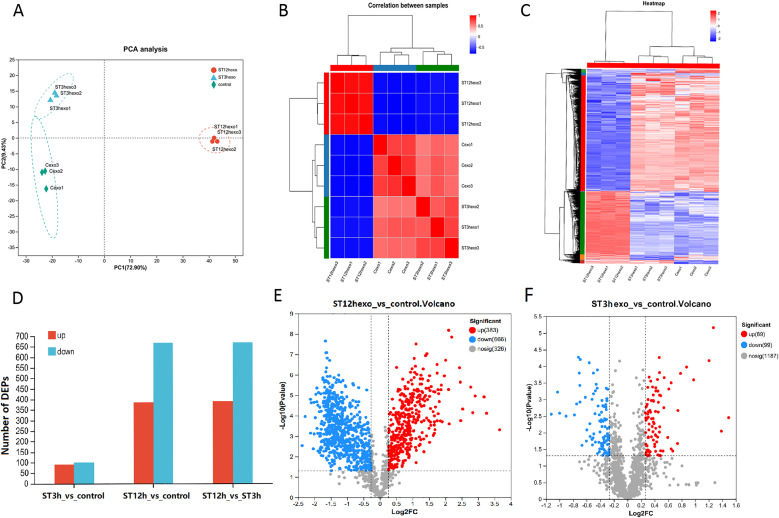
Previous studies have suggested that RBPs are typically responsible for packing of miRNA into exosomes. In order to systematically analyze the role of the RBPs within the packing of miR-27a-5p into the exosome during the inflammatory response induced by Salmonella, RAW 264.7 cells were left untreated or treated with ST14028 for 3 h or 12 h. The protein composition of the secreted exosome was assessed using a TMT proteomic analysis. Principal component analysis (PCA) results showed an obvious difference between the 3 h and 12 h Salmonella stimulation compared with the unstimulated group (Fig. 2A). There was also a distinct difference in Salmonella stimulus between 3 h and 12 h. The correlation heat map showed that the difference was most significant in the Salmonella infected group at 12 h compared to the untreated group (Fig. 2B). The heat map showed the DEPs, where some were up-regulated in the exosome from Salmonella-exposed macrophages, while others were down-regulated (Fig. 2C). In contrast to the unstimulated group, 383 exosomal proteins were over-expressed and 666 were under-expressed in the 12 h of Salmonella infected macrophages (Fig. 2D, E). The top 20 proteins with higher differential expression in the exosome were shown in Table 1. In contrast to the Salmonella stimulus at 3 h, there were 389 upregulated proteins and 669 downregulated proteins at 12 h. Nonetheless, the number of different proteins in the Salmonella stimulated 3 h group and the unstimulated group is relatively small (Fig. 2F). Furthermore, Salmonella proteins have been also detected in exosomes secreted by macrophages infected with Salmonella at 12 h, as shown in Table 2. Since the largest difference was found in the Salmonella stimulated group at 12 h compared to the unstimulated group, the follow-up trial mainly compared protein differences between the treated and untreated Salmonella groups at 12 h.
Fig. 2.
Salmonella alters the profile of RAW264.7 cell-derived exosomal proteins. A, B. PCA and heat maps indicating the correlations in Salmonella-infected macrophages compared to the resting cells. C, D. Heat maps and histograms showing changes in the exosomal proteins from RAW 264.7 cells with or without ST 14028 infection for 3 h or 12 h. E, F. Volcano plots showing the DEPs in the exosome of RAW 264.7 cells infected with Salmonella for 12 h or 3 h compared to uninfected cells.
Table 1.
List of 20 up-regulated proteins in exosomes secreted by macrophages infected with Salmonella at 12 h.
| Accession | Symbol | Description | Fold change (FC) | P_value |
|---|---|---|---|---|
| Q8CGP7 | H2A T1-K | Histone H2A type 1-K | 12.5954198473 | 0.0004886 |
| Q8BFU2 | H2A T3 | Histone H2A type 3 | 9.06957250629 | 0.00001198 |
| Q64522 | H2A T2-B | Histone H2A type 2-B | 5.7745773325 | 0.00004711 |
| Q52KG9 | CCT6 | chaperonin containing Tcp1, subunit 6A (Zeta) | 4.56704980843 | 0.00000001412 |
| A2BFF8 | DYNC1I | Dynein cytoplasmic 1 intermediate chain 2 | 4.3280584297 | 0.0002504 |
| P80316 | CCT5 | T-complex protein 1 subunit epsilon | 4.28152492669 | 0.000000006562 |
| Q9CS06 | CCT6 | T-complex protein 1 subunit theta (Fragment) | 4.01992409867 | 0.000000193 |
| Q9CYA6 | ZCCHC8 | Zinc finger CCHC domain-containing protein 8 | 3.91342412451 | 0.000001115 |
| D3YZJ1 | SQSTM1 | Sequestosome 1 | 3.73195084485 | 0.0001964 |
| Q3TET0 | CCT7 | T-complex protein 1 | 3.669921875 | 0.000003222 |
| Q3UDN2 | VPS53 | Vacuolar protein sorting-associated protein 53 homolog | 3.63271302644 | 0.000005346 |
| A0JLN9 | GBF1 | Gbf1 protein (Fragment) | 3.51451187335 | 0.00002021 |
| A0A1W2P6G5 | MYL6 | Myosin, light polypeptide 6, alkali, smooth muscle and non-muscle | 3.46364494806 | 0.000008276 |
| Q3U2P1 | SEC24 | Protein transport protein Sec24A | 3.42947667431 | 0.0000798 |
| Q3UPL0 | SEC31 | Protein transport protein Sec31A | 3.42199856219 | 0.00000532 |
| A0A494B990 | LPXN | Leupaxin | 3.36270022883 | 0.0001778 |
| P80314 | CCT2 | T-complex protein 1 subunit beta | 3.36082474227 | 0.0000008152 |
| Q01853 | VCP | Transitional endoplasmic reticulum ATPase | 3.35188679245 | 0.0000003137 |
| Q99020 | HNRNPAB | Heterogeneous nuclear ribonucleoprotein A/B | 3.24532019704 | 0.00003709 |
| Q8BXC6 | COMMD2 | COMM domain-containing protein 2 | 3.20837124659 | 0.00000684 |
Table 2.
Salmonella proteins in exosomes secreted by Salmonella-infected macrophages at 12 h.
| Accession | Symbol | Description | Fold change (FC) | P_value |
|---|---|---|---|---|
| A0A606SXK5 | DUF1471 domain-containing protein | 7.50777202073 | 0.0000108 | |
| A0A5W0WAN2 | OsmY | Molecular chaperone OsmY | 9.54940282302 | 0.00007596 |
| A0A5W0KNK6 | SOD1 | Superoxide dismutase [Cu-Zn] | 7.13246753247 | 0.00007053 |
| A0A5W0DKI3 | SitA | Iron/manganese ABC transporter substrate-binding protein SitA | 6.83713692946 | 0.00000399 |
| A0A602NHW0 | OmpA | Outer membrane protein A | 5.38938848921 | 0.0000004489 |
| A0A5W0U4C1 | SlyB | Outer membrane lipoprotein SlyB | 3.59568073027 | 0.0001801 |
| A0A5W0 × 7E4 | PagC | Virulence membrane protein PagC | 5.53682170543 | 0.0001737 |
| A0A5W0WX84 | PAP2 | Phosphatase PAP2 family protein | 5.48808290155 | 0.00000227 |
| A0A5W0VGR5 | CynT | Lipoprotein | 2.15034168565 | 0.01973 |
| A0A5W0 × 624 | YncE | Carbonic anhydrase | 3.20653907496 | 0.0004819 |
| A0A601PNG1 | PgtE | YncE family protein | 2.82717872969 | 0.0004138 |
| A0A5W4DAX8 | PotB | Omptin family outer membrane protease | 4.27497945286 | 0.000005215 |
| A0A5W0RTD5 | GroEL | Putrescine-binding periplasmic protein | 2.29831516353 | 0.0000198 |
| A0A602HLN6 | ZnuA | Chaperonin GroEL | 2.20020325203 | 0.00001296 |
| A0A5Z6S4I1 | RfbJ | High-affinity zinc uptake system protein | 1.60404624277 | 0.0008785 |
| A0A5W0W543 | PrmA | CDP-abequose synthase | 0.302813067151 | 0.000278 |
| A0A5Z5Q4W7 | DAPAL | Ribosomal protein L11 methyltransferase | 0.323774283071 | 0.000532 |
| A0A5W0RNN3 | EcnB | Diaminopropionate ammonia-lyase | 0.321303501946 | 0.00002957 |
| A0A5W0WCQ2 | YebC | Lipoprotein toxin entericidin B | 0.383062200957 | 0.0003237 |
| A0A602I8D9 | UgpC | Probable transcriptional regulatory protein | 0.421516587678 | 0.0004378 |
| A0A5W0WXF0 | YdiV | sn-glycerol-3-phosphate import ATP-binding protein UgpC | 0.474025974026 | 0.0006588 |
| A0A5W0 × 4H8 | Anti-FlhC(2)FlhD(4) factor YdiV | 0.432728921124 | 0.00806 |
GO Enrichment and KEGG Pathway Analysis
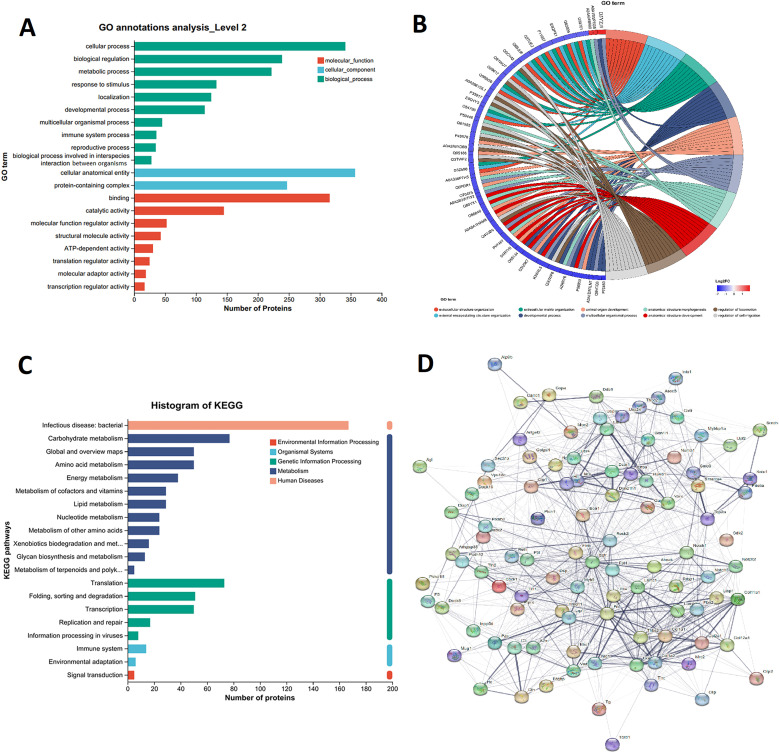
Exosomes carrying over-expressed proteins are phagocytosed by neighboring immune cells, which can cause changes in cell function. To clarify the role of DEPs in exosomes after Salmonella infection at 12 h, GO enrichment analysis was performed (Fig. 3A), which was classified into three specific GO categories. In terms of the biological process enriched in the Salmonella treatment group, the most important pathways were response to cellular process (GO: 0009987), biological regulation (GO: 0065007), metabolic process (GO: 0008152), and response to stimulus (GO: 0050896). As for the cellular component, cellular anatomical entity (GO: 0110165) and protein-containing complex (GO: 0140776) were the major modules of DEPs distribution. Binding (GO: 0005488) and catalytic activity (GO: 0003824) were the most two representatives in molecular function. The chord diagram of GO enrichment suggests that after endocytosis of the exosome, naïve macrophages may remodel the cell function through three processes: developmental process, multicellular organismal process, and extracellular structural organization to combat bacterial invasion (Fig. 3B). During the KEGG pathway analysis, DEPs were predominantly concentrated in the areas of bacterial-induced infectious disease, carbohydrate metabolism, amino acid metabolism, and processes related to translation, folding, sorting, and degradation (Fig. 3C). Based on the intact database, network diagrams of the interactions of DEPs in the exosome were obtained, which clearly showed the possible interactions of different proteins (Fig. 3D). These results suggest that macrophages may initiate various mechanisms involved in information exchange and communication when it comes to their antimicrobial function.
Fig. 3.
GO functional enrichment analysis and KEGG pathway analysis of DEPs. A. Histograms showing GO annotations for DEPs. B. GO enrichment string diagrams showing the correspondence between the set of target proteins and the enrichment of the GO Term. C. Histogram showing KEGG pathway enrichments for DEPs. D. Interaction network of exosomes DEPs in exosomes secreted by macrophages infected with Salmonella. The thickness of the line indicates the data support of the interaction, that is, the above evidence is integrated to obtain the final score, with higher scores indicating thicker lines.
The RNA binding protein hnRNP A/B is over expressed in the exosome of Salmonella-infected cells
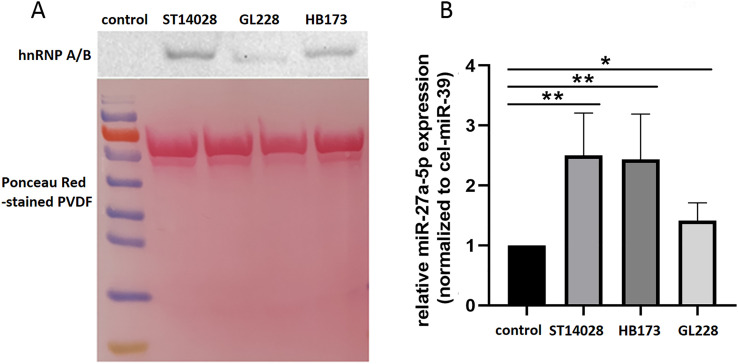
There are considerable evidences that RBPs (i.e. hnRNP A2B1, SYNCRIP, Ago-2 and FMR1) are key players in the regulation of RNA transport and post-transcriptional processes (Garcia-Martin et al., 2022; Villarroya-Beltri et al., 2013; Wozniak et al., 2020). Although RBPs recognize and bind to specific motifs of RNA sequences, facilitating their inclusion in EVs including exosomes, it has not been able to provide an uncontested conclusion as different RBPs bind to distinct miRNAs. Among the highly expressed proteins in the exosome of Salmonella-infected cells. There are a few proteins, such as hnRNP A/B, FXR1, that may be responsible for the delivery of miRNA packages into the exosome, and since it has been reported that hnRNP A/B and FXR1 are among the most abundant RBPs (He and Smith, 2009; Wang et al., 2021a). Then the expressions of hnRNP A/B and FXR1 were detected by western blot assay. It was shown that the expression of hnRNP A/B was higher in groups of ST 14028, HB173 and GL228 activated exosomes compared to untreated cells (Fig. 4A), while FXR1 could not be examined clearly for the low expression. At the same time, enrichment of miR-27a-5p was detected in the exosome of macrophages infected with three strains of Salmonella, including ST 14028, HB173 and GL228, compared to uninfected cells (Fig. 4B). In other words, hnRNP A/B expression is also increased in exosomes secreted by Salmonella-infected macrophages, while miR-27a-5p is highly expressed, suggesting a possible interaction between them.
Fig. 4.
HnRNAP A/B and miR-27a-5p in exosome of Salmonella infection cells. A. Western blot analysis of hnRNP A/B expression in exosomes from RAW 264.7 cells at 12 h post-infection with Salmonella. Ponceau-stained PVDF shows equal amounts of exosomal protein loaded in each lane. B. RT-qPCR analysis of miR-27a-5p expression in the exosome of RAW 264.7 cells left uninfected or infected with Salmonella. Cel-miR-39: external control. Data are shown as the mean ± SD of three independent experiments. * P ≤ 0.05; ** P ≤ 0.01.
Hn RNP A/B is responsible for the sorting of miR-27a-5p into exosomes
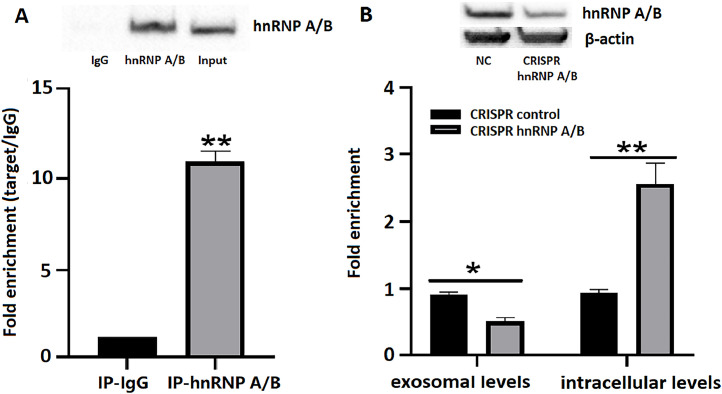
To explore the involvement of hnRNP A/B in miR-27a-5p sorting into exosomes after Salmonella infection, RIP assays were performed to examine the relationship between hnRNP A/B and miR-27a-5p. The results indicated that miR-27a-5p expression can be significantly detected in beads conjugated with hnRNP A/B antibodies compared to beads conjugated with IgG antibodies (Fig. 5A). These demonstrate that hnRNP A/B can specifically bind miR-27a-5p. To further elucidate the effect of the hnRNP A/B content on the packing of miR-27a-5p into the exosome, a gene knockout vector CRISPR was constructed for hnRNP A/B and the expression changes of miR-27a-5p were detected in both the intracellular and exosome after transfection. RT-qPCR analysis verified that once hnRNP A/B was interfered with, miR-27a-5p was significantly less represented in the exosome, notably, but the intracellular miR-27a-5p content increased (Fig. 5B). These results confirm that hnRNP A/B is responsible for the packaging of miR-27a-5p into exosomes during Salmonella infection.
Fig. 5.
HnRNP A/B helps miR-27a-5p package into exosome. A. RIP experiments with anti-hnRNP A/B antibodies (or IgG as negative control) were performed on cellular lysates. The level of hnRNP A/B was detected by using western blot assay and miR-27a-5p in the immunoprecipitated sample was determined by RT-qPCR and quantified as a percentage relative to the input sample ( % input). B. The expression of hnRNP A/B was analyzed by Western blot assay following gene knockout. β-actin was used as an internal control. RT-qPCR analysis of miR-27a-5p levels (exosomal and intracellular) in hnRNP A/B-KO vs. scramble control cells. Data are shown as the mean ± SD of three independent experiments. * P ≤ 0.05; ** P ≤ 0.01.
Discussion
Salmonella employs sophisticated strategies to establish infection and evade host immune responses. Upon entering the host, Salmonella invades macrophages, where it manipulates cellular processes to survive and replicate (Herrero-Fresno and Olsen, 2018; Lu et al., 2025; Reuter et al., 2020). One key mechanism involves the secretion of virulence factors, such as effector proteins, through type III secretion systems (T3SS) (Bohn et al., 2019; Ramsden et al., 2007). These effectors modulate host signaling pathways, including NF-κB and mitogen-activated protein kinase (MAPK), to suppress pro-inflammatory cytokine production and inhibit immune cell activation (Rahman and McFadden, 2011; Yang et al., 2021). Additionally, Salmonella promotes the formation of specialized vacuoles (SCVs) that protect it from lysosomal degradation (Göser et al., 2023). To further evade immune detection, Salmonella-infected macrophages also release exosomes containing anti-inflammatory microRNAs (e.g., miR-146a, miR-155) and proteins, which dampen the immune response in neighboring cells (Essandoh et al., 2016; Yu et al., 2020). This immune suppression facilitates bacterial persistence and systemic dissemination (Göser et al., 2023). Furthermore, Salmonella alters its surface antigens, such as lipopolysaccharide (LPS), to avoid recognition by host antibodies (Maldonado et al., 2016). These combined mechanisms enable Salmonella to evade both innate and adaptive immunity, contributing to chronic infection and posing challenges for effective treatment and vaccine development (Jia et al., 2020).
In this study, exosomes secreted by Salmonella-infected cells were found to be abnormally enriched with distinct protein profiles as revealed by high throughput proteomics analysis. A total of 14 % and 76 % of the proteins with significantly altered extracellular abundance were annotated as present in extracellular vesicles after 3 h and 12 h of infection, respectively. These demonstrate that as the infection progressed, exosomal protein profiles displayed a growing number of differentially expressed species over time. GO and KEGG analyses revealed that the candidate target genes are predominantly associated with immune response, microbial infection, and signal transduction, consistent with the observed antibacterial activity. The exosomal proteins secreted by macrophages include not only macrophage-derived proteins but also Salmonella-derived proteins. Furthermore, the abundance of Salmonella-derived proteins in exosomes showed a time-dependent increase, with significantly higher levels detected at 12 h compared to 3 h post-infection. Several Salmonella proteins, including OmpA, OsmY, and PagC (as listed in Table 2), were detected in the cell culture supernatants of infected cells, thereby reinforcing the validity of this proteomic study. Most Salmonella effector proteins have been shown to either: (1) mediate lysosomal evasion and autophagy suppression in macrophages, or (2) facilitate bacterial replication within Salmonella-containing vacuoles (Roy Chowdhury et al., 2025; Zheng et al., 2015). Certain proteins can also serve as biomarkers for detecting Salmonella contamination that have been detected in pork, milk, and other agricultural products (Wang et al., 2018). Prior research indicates that proinflammatory exosomes, formed early in Salmonella-infected macrophages, transfer cargo to naïve cells and trigger their activation (Hui et al., 2018). Besides lipopolysaccharide, several virulence factors were identified, including the T1SS-secreted RTX toxin, the invasion-associated protein SipC, and phase 2 flagellin. Additionally, studies have shown that different culture conditions for Salmonella can lead to significant variations in the RNA content of either bacterial exosomes or intracellular compartments (Malabirade et al., 2018). These differences may consequently alter the composition of exosomes derived from infected cells. Our previous study showed that miR-27a-5p accumulates in exosomes secreted by macrophages at 12 h post Salmonella infection, and these exosomes suppress the proinflammatory response in recipient naïve macrophages. This objective was to identify the key protein(s) responsible for miR-27a-5p packaging into exosomes from the pool of DEPs.
RBPs have been identified as key components in the post-transcriptional regulation of gene expression and represent a broad family of characteristic RBPs (Geuens et al., 2016; Wozniak et al., 2020). They are highly expressed in mammalian cells and consist of approximately 30 members, including 20 major hnRNP types from A to U, as well as several minor hnRNP types. HnRNP A/B is considered as one of the abundant proteins that may be involved in the RNA sorting function of cells (He and Smith, 2009; Lu et al., 2022). In this study, proteomic analysis and western blot assays showed that hnRNP A/B is highly expressed in exosomes secreted by Salmonella-induced inflammatory macrophages. We then investigated whether hnRNP A/B facilitates the transport of miR-27a-5p from Salmonella-infected cells to exosomes. As summarized, RIP assays showed that hnRNP A/B binds to the proinflammatory suppressor miR-27a-5p, forming the hnRNP A/B protein miR-27a-5p complex. These observations imply a potential interaction between hnRNP A/B and miR-27a-5p. We hypothesize that this complex is selectively packaged into exosomes and secreted, resulting in elevated miR-27a-5p levels within exosomes and a concomitant decrease in cytoplasmic miR-27a-5p. We then performed hnRNP A/B knockout experiments, which caused intracellular accumulation of miR-27a-5p in exosome donor cells while reducing its exosomal export. The results confirmed that hnRNP A/B mediates miR-27a-5p packaging into exosomes. In other words, hnRNP A/B-dependent exosomal sorting of miR-27a-5p promotes an anti-inflammatory state in recipient cells, consequently suppressing Salmonella clearance. Targeting hnRNP A/B could potentially inhibit the packaging and subsequent secretion of immunosuppressive miR-27a-5p in exosomes. Future studies should investigate whether hnRNP A/B similarly regulates other anti-inflammatory miRNAs, such as miR-146a or miR-155, during Salmonella infection.
In summary, Salmonella infection activates the TLR7/NF-κB pathway in macrophages, inducing inflammatory responses that promote pathogen clearance. Interestingly, hnRNP A/B mediates miR-27a-5p packaging into exosomes. These exosomes, when taken up by adjacent naïve macrophages, attenuate TLR7/NF-κB signaling and inflammatory responses, enabling Salmonella immune evasion. Our results reveal that infection-derived exosomes contain both inflammatory and immunosuppressive components. Targeting these immunosuppressive factors may provide new strategies to block Salmonella evasion and improve poultry safety.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bohn E., Sonnabend M., Klein K., Autenrieth I.B. Bacterial adhesion and host cell factors leading to effector protein injection by type III secretion system. Int. J. Med. Microbiol. 2019;309:344–350. doi: 10.1016/j.ijmm.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Buzas E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023;23:236–250. doi: 10.1038/s41577-022-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T., Ma, J., 2022. iProX in 2021: connecting proteomics data sharing with big data. 50, D1522-d1527. [DOI] [PMC free article] [PubMed]
- Essandoh K., Li Y., Huo J., Fan G.C. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock (Augusta Ga,) 2016;46:122–131. doi: 10.1097/SHK.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göser V., Sander N., Schulte M., Scharte F., Franzkoch R., Liss V., Psathaki O.E., Hensel M. Single molecule analyses reveal dynamics of Salmonella translocated effector proteins in host cell endomembranes. Nat. Commun. 2023;14:1240. doi: 10.1038/s41467-023-36758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martin R., Wang G., Brandão B.B., Zanotto T.M., Shah S., Kumar Patel S., Schilling B., Kahn C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens T., Bouhy D., Timmerman V. The hnRNP family: insights into their role in health and disease. Hum. Genet. 2016;135:851–867. doi: 10.1007/s00439-016-1683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J.J., MacLennan C.A. Invasive nontyphoidal Salmonella disease in Africa. EcoSal. Plus. 2019;8 doi: 10.1128/ecosalplus.esp-0007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Xiao J., Yi J., Xiao J., Lu F., Liu X. Immunomodulatory effect of serum exosomes from Crohn Disease on macrophages via let-7b-5p/TLR4 signaling. Inflamm. Bowel Dis. 2022;28:96–108. doi: 10.1093/ibd/izab132. [DOI] [PubMed] [Google Scholar]
- Groot M., Lee H. Sorting mechanisms for MicroRNAs into extracellular vesicles and their associated diseases. Cells. 2020;9 doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Smith R. Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell. Mol. Life Sci. 2009:1239–1256. doi: 10.1007/s00018-008-8532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Fresno A., Olsen J.E. Salmonella typhimurium metabolism affects virulence in the host - A mini-review. Food microbiol. 2018;71:98–110. doi: 10.1016/j.fm.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Hui W.W., Hercik K., Belsare S., Alugubelly N., Clapp B., Rinaldi C., Edelmann M.J. Salmonella enterica serovar typhimurium alters the extracellular proteome of macrophages and leads to the production of proinflammatory exosomes. Infect. Immun. 2018;86 doi: 10.1128/IAI.00386-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., McWhorter A.R., Andrews D.M., Underwood G.J., Chousalkar K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines. 2020;8 doi: 10.3390/vaccines8040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L.A., Elfenbein J.R. Salmonella enterica. Trends Microbiol. 2019;27:964–965. doi: 10.1016/j.tim.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Lai N.S., Wu D.G., Fang X.G., Lin Y.C., Chen S.S., Li Z.B., Xu S.S. Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer. 2015;112:1241–1246. doi: 10.1038/bjc.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Zhu Y., Li J., Zeng J., Wu L. ALKBH5-mediated m6A modification of circCCDC134 facilitates cervical cancer metastasis by enhancing HIF1A transcription. J. Exp. Clin. Cancer Res. 2022;41:261. doi: 10.1186/s13046-022-02462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Wu H., Wu S., Wang S., Fan H., Ruan H., Qiao J., Caiyin Q., Wen M. Salmonella: infection mechanism and control strategies. Microbiol. Res. 2025;292 doi: 10.1016/j.micres.2024.128013. [DOI] [PubMed] [Google Scholar]
- Lu Y., Wang X., Gu Q., Wang J., Sui Y., Wu J., Feng J. Heterogeneous nuclear ribonucleoprotein A/B: an emerging group of cancer biomarkers and therapeutic targets. Cell Death Discov. 2022;8:337. doi: 10.1038/s41420-022-01129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Chen T., Wu S., Yang C., Bai M., Shu K., Li K., Zhang G., Jin Z., He F., Hermjakob H., Zhu Y. iProX: an integrated proteome resource. Nucleic Acids Res. 2019;47:D1211–d1217. doi: 10.1093/nar/gky869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabirade A., Habier J., Heintz-Buschart A., May P., Godet J., Halder R., Etheridge A., Galas D., Wilmes P., Fritz J.V. The RNA complement of outer membrane vesicles from Salmonella enterica serovar typhimurium under distinct culture conditions. Front. Microbiol. 2018;9:2015. doi: 10.3389/fmicb.2018.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R.F., Sá-Correia I., Valvano M.A. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Liu Y., Deng J., Ma X., Fan D. Ginsenoside Rk3 is a novel PI3K/AKT-targeting therapeutics agent that regulates autophagy and apoptosis in hepatocellular carcinoma. J. Pharm. Anal. 2023;13:463–482. doi: 10.1016/j.jpha.2023.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M., Su S., Jiang L., Yu X., Zhang J., Zhu H., Han K., Zhang X. Exosomal miR-27a-5p attenuates inflammation through toll-like receptor 7 in foodborne Salmonella infections. Vet. Microbiol. 2025;302 doi: 10.1016/j.vetmic.2025.110394. [DOI] [PubMed] [Google Scholar]
- Qu M., Zhu H., Zhang X. Extracellular vesicle-mediated regulation of macrophage polarization in bacterial infections. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., McFadden G. Modulation of NF-κb signalling by microbial pathogens. Nat. Rev., Microbiol. 2011;9:291–306. doi: 10.1038/nrmicro2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden A.E., Holden D.W., Mota L.J. Membrane dynamics and spatial distribution of Salmonella-containing vacuoles. Trends Microbiol. 2007;15:516–524. doi: 10.1016/j.tim.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Reuter T., Vorwerk S., Liss V., Chao T.C., Hensel M., Hansmeier N. Proteomic analysis of Salmonella-modified membranes reveals adaptations to macrophage hosts. Mol. Cell. Proteom. 2020;19:900–912. doi: 10.1074/mcp.RA119.001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro Y.T., Jo G.H., Jung S.A., Lee E.H., Shin J., Lee J.H. Salmonella‑induced miR‑155 enhances necroptotic death in macrophage cells via targeting RIP1/3. Mol. Med. Rep. 2018;18:5133–5140. doi: 10.3892/mmr.2018.9525. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury A., Hajra D., Mukherjee D., Nair A.V., Chakravortty D. Functional OmpA of Salmonella typhimurium provides protection from lysosomal degradation and inhibits autophagic processes in macrophages. J. Infect. Dis. 2025;231:716–728. doi: 10.1093/infdis/jiae376. [DOI] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Mahon B.E., Jones T.F., Griffin P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015;143:2795–2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Yin H., Zhang H., Wang T. circNRIP1 facilitates keloid progression via FXR1‑mediated upregulation of miR‑503‑3p and miR‑503‑5p. Int, J. Mol. Med. 2021;47 doi: 10.3892/ijmm.2021.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li Y., Chen J., Hua D., Li Y., Deng H., Li Y., Liang Z., Huang J. Rapid detection of food-borne Salmonella contamination using IMBs-qPCR method based on pagC gene. Braz. J. Microbiol. 2018;49:320–328. doi: 10.1016/j.bjm.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang H., Guo R., Li X., Liu H., Wang Z., Du Q., Tong D., Huang Y. MicroRNA-223 modulates the IL-4-medicated macrophage M2-type polarization to control the progress of sepsis. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107783. [DOI] [PubMed] [Google Scholar]
- Wozniak A.L., Adams A., King K.E., Dunn W., Christenson L.K., Hung W.T., Weinman S.A. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201912074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Ye T., Wang Y., Pan L., Ye Y., Ding Z., Bao D. MicroRNA-139-5p inhibits inflammatory and oxidative stress responses of Salmonella-infected macrophages through modulating TRAF6. Pathog. Dis. 2021;79 doi: 10.1093/femspd/ftab018. [DOI] [PubMed] [Google Scholar]
- Yang F., Sheng X., Huang X., Zhang Y. Interactions between Salmonella and host macrophages - dissecting NF-κb signaling pathway responses. Microb. Pathog. 2021;154 doi: 10.1016/j.micpath.2021.104846. [DOI] [PubMed] [Google Scholar]
- Yu H., Qin L., Peng Y., Bai W., Wang Z. Exosomes derived from hypertrophic cardiomyocytes induce inflammation in macrophages via miR-155 mediated MAPK pathway. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Ji Y., Weng X., Huang X. RpoS-dependent expression of OsmY in Salmonella enterica serovar typhi: activation under stationary phase and SPI-2-inducing conditions. Curr. Microbiol. 2015;70:877–882. doi: 10.1007/s00284-015-0802-1. [DOI] [PubMed] [Google Scholar]