Since their introduction in 1985 by Tomalia et al. (1) and Newkome et al. (2), dendrimers have attracted much attention because of their fascinating structure and unique properties (3, 4). Dendrimers are globular, size monodisperse macromolecules in which all bonds emerge radially from a central focal point or core with a regular branching pattern and with repeat units that each contribute a branch point. Not all regularly branched molecules are dendrimers because properties of the dendritic state (4), such as core encapsulation (5, 6) and unusually low intrinsic viscosity in solution (7), are reached only when globularity is achieved at a certain generation or size threshold. Therefore, many low-generation dendrons or the early cascade molecules of Vögtle and coworkers (8) are too small to exhibit the properties of dendrimers, but they are frequently used as branched oligomeric building blocks in their construction, and have a size relationship to dendrimers somewhat akin to that between oligomers and polymers.
Two distinct synthetic methodologies have been used for the preparation of dendrimers: the divergent approach (1, 2), in which growths starts at the core and proceeds radially outward toward the dendrimer periphery, and the convergent approach, (9, 10) in which growth starts at what will become the periphery of the dendrimer proceeding inward. The two methodologies are complementary and neither is generally better, the choice of synthetic approach being usually justified by the features desired for the target molecule, the chemistry available for growth, and the specific building blocks used in the construction of the dendritic framework. In general, the convergent approach provides better overall structural control, in part as a result of its enhanced potential for purification at intermediate stages of growth, and, in part, as a result of its innate ability to introduce differentiated functionalities at the focal point and the periphery of the dendrimer. In contrast, purity and structural uniformity are harder to maintain in the divergent approach, because the number of reactions that must be completed at each step of growth increases exponentially requiring large excesses of reagents, but the process is better suited not only for syntheses on a larger scale but also for the preparation of high-generation dendrimers. Although the majority of the dendrimers prepared to-date have been built of covalent bonds (3, 10), many noncovalent dendrimers (3, 11) have also been prepared by a variety of self-assembly processes involving, for example, hydrogen bonding (12) or supramolecular coordination chemistry (13).
Relating the concepts of dendrimers and supramolecular chemistry (14) in this article requires more that just a consideration of molecules resulting from supramolecular construction. The unique layered architecture of dendrimers, their globular shape, highly controlled size, radially controlled chemical make-up, multivalent periphery, variable inner volume, and controlled intramolecular dynamics endow dendrimers with unique features and provide them with the ability to morph into a variety of virtual supramolecular arrangements in response to external stimuli. Unlike collections of small molecules, which might require supramolecular assembly to deliver function, dendrimers can simply use internal dynamics to arrange their multiple and interconnected components in ways that minimize free energy (15) and afford specific functions. Such intramolecular reorganizations may lead to shape or volume changes, the creation of internal microenvironments, the cooperative organization of surface or inner functionalities, the concentration or exclusion of substrate from the molecular “cavity” of dendrimers, or the formation of defined multimolecular assemblies.
Supramolecular Assembly of Dendrimers
The use of metal branching centers, such as ruthenium and osmium, and multidentate ligands for the construction of early metallodendrimers centers was described a decade ago by Balzani and coworkers (16, 17) Since this early work, a large number of self-assembled dendritic structures have been prepared by using mainly metal ligand complexes (Fig. 1A), hydrogen bonding moieties (Fig. 1B), or ionic interactions (Fig. 2) for the assembly. The topic of self-assembly of dendrimers has been thoroughly reviewed (11, 17–24).
Figure 1.
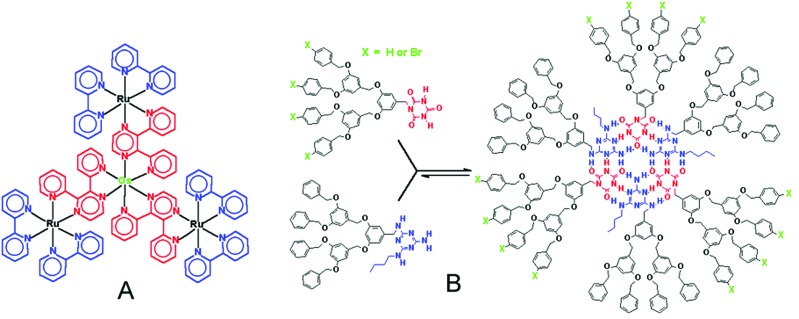
(A) Self-assembled metallodendrimer. (B) Self-assembly of dendrimer by hydrogen-bonding.
Figure 2.
Schematic representation of self-assembled ionic dendrimer.
In their seminal work, Balzani and coworkers (16) demonstrated the formation of dendritic polynuclear species by using, for example, 2,3-bis(2-pyridyl)pyrazine as bridging ligands, bipyridines as terminal ligands, and metals such as Ru(II) or Os(II). Fig. 1A shows a very small bimetallic dendrimer obtained with a modular strategy that has been used to prepare many larger dendrimers with a variety of arrangements of the internal and peripheral metal centers (17). These polynuclear complexes, which are redox-active and have luminescent properties, have been suggested for use as photochemical molecular devices although many current structures are clearly not well suited for practical applications. Coordination chemistry has also been used to prepare a great variety of other dendrimers or dendritic hybrids such as the “lock and key” structure of Newkome et al. (25). The use of coordination chemistry to prepare dendrimers is synthetically accessible and, with appropriate choices of metals and ligands, can afford durable structures. Supramolecular dendrimers containing both metal centers and complementary functional moieties, as well as supramolecular assemblies of nanocrystals and dendrimers, may well prove useful in areas such as energy harvesting and conversion, signaling, and diagnostics.
Following the early work of Kato and Fréchet (26, 27) and of Lehn and coworkers (28) on the use of hydrogen bonding for the assembly of supramolecular polymer structures, a remarkable family of dendrimers was prepared by Zimmerman et al. (12) by using the H-bond-mediated self-assembly of six Fréchet-type polyether dendrons (9) fitted with tetracarboxylic acid moieties at their focal point. Analogous but unsymmetrical dendritic structures were also obtained by Freeman et al. (29) with the self-assembly of two types of convergent polyether dendrons with complementary derivatives of melamine and cyanuric acid at their focal points (Fig. 1B). In this instance, however, the self-assembled structure had an even lower stability than Zimmerman's dendrimers as fewer H-bonds contributed to its assembly. Reinhoudt and coworkers (30) have combined palladium-centered coordination chemistry and melamine-cyanuric acid H-bonding to prepare a variety of metallodendrimers. Although such structures are indeed beautiful, their synthesis is even more demanding than that of covalent analogs, and the molecules remain little used.
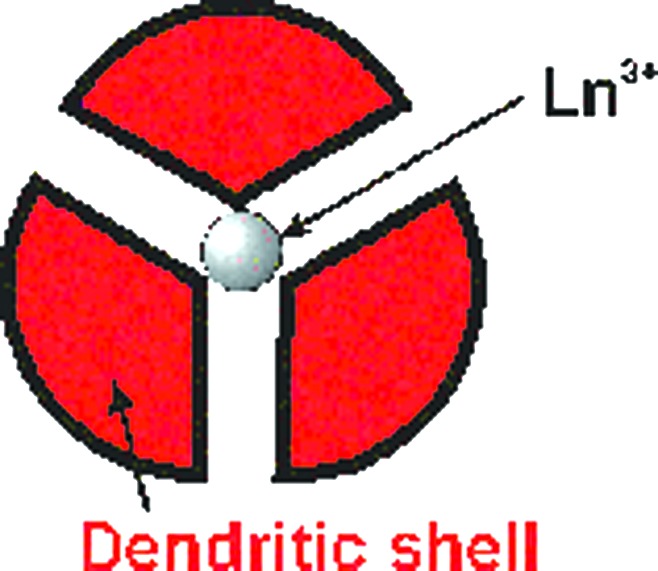
A third type of self-assembled dendrimer results from ionic interactions as exemplified by the lanthanide-core dendrimers of Kawa and Fréchet (31) (Fig. 2). In this approach, convergent polyether dendrons with a carboxylic acid focal point are assembled around a central trivalent lanthanide cation via metathesis by using, for example, Er(OAc)3, Tb(OAc)3, or Eu(OAc)3. In this case the dendrons perform the function of shielding the lanthanide ions from one another. This site-isolation (vide infra) eliminates the self-quenching between metal atoms, enabling the use of such structures for optical amplification, an area of practical significance for fiber optics communication.
Among the numerous other self-assembling dendritic systems that have been described, those involving the columnar assembly of dendrons or of dendronized polymers are particularly intriguing. In their seminal work Percec et al. (32, 33) have used van der Waals interactions and H-bonding to self-assemble functionalized polyether dendrons into cylindrical columnar or spherical assemblies that mimic the shape and architecture of certain types of viruses. Aida and coworkers (34) have used an analogous strategy involving weak metal–metal interactions to effect the hierarchical self-organization of small dendrimers into luminescent superhelical fibers (Fig. 3). With a rigid dendronized polymer consisting of a poly(phenylene ethynylene) backbone substituted with Fréchet-type polyether dendrons, evaporation of the solvent led to the formation of very large donut-shaped assemblies (35). Given their sizes, optical and electronic properties, such assemblies could well find display, storage, or other applications in micro- and nanoelectronics.
Figure 3.
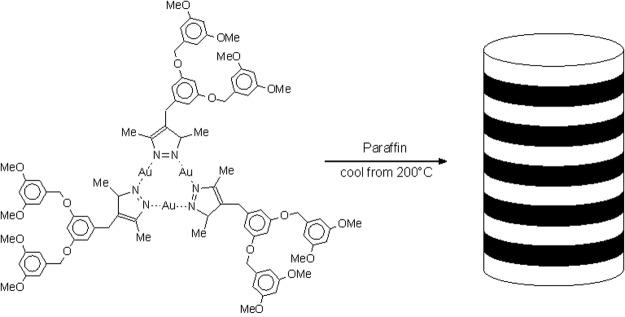
Organization of self-assembled dendrimers into supramolecular fibers.
Overall, it is clear that truly supramolecular dendrimers and dendritic systems can be obtained by a variety of approaches, but the supramolecular character of dendrimers extends far beyond their preparation. The following paragraphs will therefore examine characteristics and properties of dendrimers that result from intramolecular dynamics and interactions involving the various building blocks of dendrimers with solvents or other types of external environments, including surfaces.
Shape and Conformations of Dendrimers
There has been significant and exaggerated controversy about the shape of dendrimers, and the placement of their chain ends either at the “periphery” of the globular macromolecule or back-folded within its building blocks. Indeed, a variety of calculations and measurements have suggested back-folding of the chain ends whereas others have ascertained their peripheral arrangement (36).
Many dendrimers have been shown to be flexible, whereas a few of the largest seem to be fairly rigid. In general, it may be said that true dendrimers—such as those that exhibit an unusual intrinsic viscosity to molecular weight relationship (7)—are globular macromolecules that acquire significant rigidity only at high generation. Small dendrimers, and especially those that involve long and flexible connectors between branch points, are generally quite malleable and may even collapse into high-aspect ratio ovoids or flattened pancake-like shapes once solvent is evaporated after spreading on a surface.
The question of the existence of a “cavity” within dendrimers has also been raised frequently. Indeed, molecules have been encapsulated in noncovalent fashion within dendrimers but this does not mean that dendrimers have a permanent and rigid cavity. Most dendrimers are flexible enough to accommodate inclusion guests—indeed solvent molecules are generally thought to freely penetrate dendrimers—but they are also capable of rearranging themselves with significant volume collapse when solvent is removed. This collapse may leave guest molecules trapped inside the dendrimer, especially if favorable interactions exist, as in some “dendritic micelles” (37–41), or if the dendrimer structure has been rigidified to prevent their escape as in the “dendritic box” of Meijer and coworkers (42, 43).
Overall, and as might be expected, free energy reigns supreme, and dendrimers react to their environments to minimize their free energy. In a good solvent, a dendrimer may be fully extended, reaching a volumetric maximum and an almost spherical shape. In the absence of solvent, the volume of a dendrimer collapses while its final shape is determined by its flexibility, the interactions of its various components (core, building blocks, chain-ends), and the interactions with its near neighbors. Similarly, the location of chain-ends (peripheral or back-folded) in all but the most rigid structures is dictated by free energy. If the chain-ends possess favorable interactions (e.g., H-bonding or π-stacking) with the inner building blocks, back-folding may be expected to occur, a phenomenon that may be exacerbated or mitigated by solvent. In the absence of a favorable enthalpic contribution, entropy considerations will usually disfavor the mixing of chain-ends with dissimilar inner building blocks. In the case of dendrimer monolayers, molecular interactions between the various components of the dendrimer and with the surface also affect their shape (44–46).
Dendritic Unimolecular Micelles
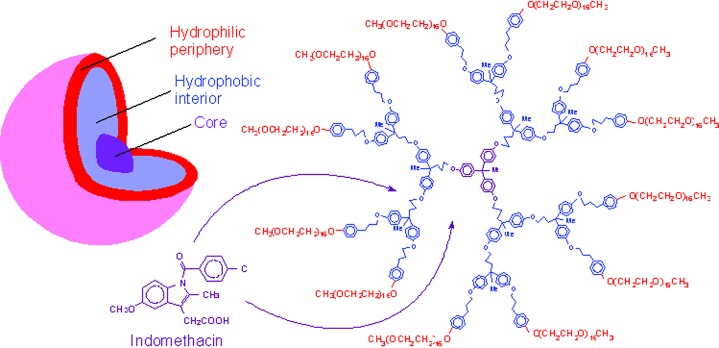
Specially designed dendrimers with a hydrophobic interior and a hydrophilic periphery are not only capable of molecular inclusion but they can also solubilize hydrophobic molecules, such as pyrene in aqueous solution, to an extent at least equal to traditional micelles while not displaying any critical micelle concentration (37–41). In contrast to classical micelles that are thermodynamic aggregates of amphiphilic molecules and therefore dynamic assemblies of small molecules, these “unimolecular micelles” are static and retain their cohesion regardless of concentration. Similarly, dendritic molecules with a polar interior and a nonpolar periphery behave as unimolecular reverse micelles capable of extracting and concentrating polar molecules from their solution in nonpolar solvents (44). The shape of such molecules, dissolved in a solvent that matches the polarity of their periphery, is expected to be spherical with chain-ends extended toward the solvent and an interior that may be collapsed to minimize the unfavorable interactions that might result from solvent penetration.
As a result of their inherent stability, unimolecular micelles may be used to encapsulate guest molecules with a simple precipitation approach. For example, we have used dendritic molecules with a hydrophobic interior and an oligoethylene glycol periphery to entrap significant amounts of a hydrophobic drug, such as indomethacin, within their collapsed interior as a model for a slow-release drug-delivery agent (41) (Fig. 4).
Figure 4.
Dendritic “unimolecular micelle” used for the slow release of indomethacin.
Site Isolation and Dendritic Encapsulation
Numerous biological systems make use of the concept of site isolation in which an active center or catalytic site is encapsulated, frequently within a protein, leading to properties that would not be encountered in the bulk state. The inherent topological features of a dendrimer in which a core is surrounded by a branched shell that carries peripheral functionalities may, of course, be used to similarly encapsulate functional core moieties and to create specific site-isolated nanoenvironments (6), thereby affecting molecular properties. However, for all but the largest and most rigid dendrimer structures, the inherent ability of the various structural subunits of a dendrimer to undergo molecular motions and intramolecular self-organization requires a careful design of both the encapsulated and encapsulating components.
Following the early demonstration of encapsulation by Hawker et al. (5), who used a solvatochromic probe placed at the focal point of a homologous series of Fréchet-type dendrons, a number of researchers, using the distinct properties of the dendrimer architecture, have placed active sites that have photophysical, photochemical, electrochemical, or catalytic functions at the core of dendrimers. For example, there have been numerous reports of dendrimer-encapsulated porphyrins designed to mimic some of the features found in cytochromes. Both the redox potential and the accessibility of the porphyrin cores are greatly affected by their environment and the characteristics and size of the encapsulating layer. In other systems, encapsulation of photoactive units in dendritic shells leads to enhanced luminescence behavior and reduced self-quenching (6, 18, 22, 31, 47).
In an early report of dendrimer-mediated catalysis, Moore and coworkers (48) have used a manganese porphyrin (Fig. 5) and iodobenzene to effect the shape-selective epoxidation of alkenes. In this case, dendrimer encapsulation of the active heme-like site contributed to both its enhanced stability and selectivity.
Figure 5.
Dendritic manganese porphyrin oxidation catalyst.
In all catalytic systems, turnover is an important issue requiring good mass transfer for the substrate and rapid removal of the product from the catalytic site to avoid inhibition of catalytic activity. Given that the size of dendrimers is roughly comparable to that of many proteins, and that, just like enzymes, dendrimers are able to create a special microenvironment within their structure, we have recently exploited this feature of dendrimers to construct “nanoreactors” in which catalytic action is combined with rapid and directional transfer of reagents and products (49, 50). For example, a dendrimer with a nonpolar aliphatic periphery and polar inner functionalities (Fig. 6) can be used to catalyze the E1 elimination of a solution of a tertiary alkyl halide (49) in a nonpolar aliphatic solvent. As the alkyl halide has some polarity, it becomes concentrated within the polar dendrimer as the system minimizes its free energy. The polar medium within the dendrimers favors the formation of polar transition states and intermediates, and an equilibrium is established in which some free alkene is formed. As a result of its low polarity and the existence of a gradient of polarity between the dendrimer interior and its exterior, the alkene product is rapidly expelled from the dendrimer back into the nonpolar solvent. Overall, the reaction is driven to completion by using as little as 0.01% of dendrimer in the presence of an auxiliary acid acceptor such as solid potassium carbonate. In a more recent example we have demonstrated a similar synergistic combination of catalytic and pumping actions with a dendrimer designed with a reversed polarity gradient (50) surrounding a photocatalytic core.
Figure 6.
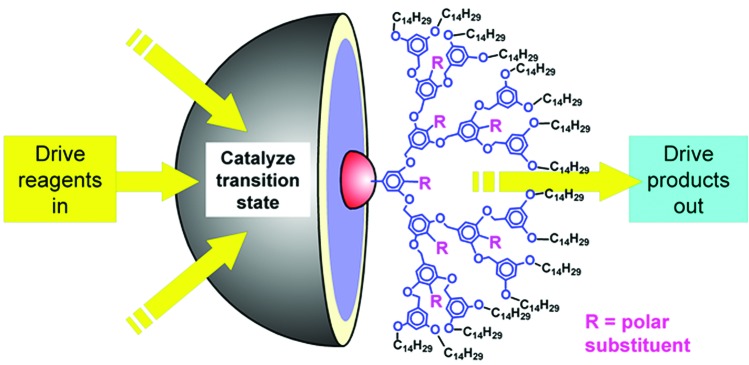
A dendritic nanoreactor providing both catalysis and rapid mass transfer.
A different type of encapsulation, involving the formation of metal nanoparticles within dendrimers, has been used to prepare inorganic–organic composite structures that are also useful in catalytic applications (51). The initial driving force for encapsulation of the metals in poly(propylene imine) dendrimers with inert or noncomplexing peripheral groups usually involves complexation of a metal ion with reactive inner tertiary amine functionalities present in the dendrimer framework itself. This is followed by chemical reduction within the dendrimer to afford the final dendrimer-encapsulated zerovalent metal nanoparticles. In this application, the dendrimers serve both as templating hosts for the preparation of the catalytic nanoparticles and as nanoporous stabilizers preventing their aggregation. Crooks et al. (51) have shown that substrates can readily penetrate the dendrimers to access the catalytic sites and undergo simple reactions such as hydrogenations. In this application, dendrimer encapsulation can lead to materials with substantial activities, as the surface of the metal nanoparticles remains largely unpassivated whereas the dendrimer layers themselves can also contribute a sieving or gating effect on approaching substrates.
From Dendritic Antennae to Monolayers
The highly compact and globular shape of dendrimers, coupled to their uniform size, restricted interpenetration, plurifunctional character, and controlled arrangement of functional groups, makes them ideal for applications in energy harvesting and storage, or as functional surfaces or interfaces. For example, dendrimers with different chromophores placed at their periphery and focal point have been use as light-harvesting antennae (52) in which geometry and chromophore selection control intramolecular energy transfer. In such applications, the size of dendrimers is a clear asset as its range roughly matches the sort of distances over which energy transfer by the Förster mechanism can operate with high effectiveness. With appropriate design, the undesired interchromophoric phenomena that might arise from interactions of close neighbors can be avoided, and extremely efficient transfer of energy can be achieved. The concept is especially attractive for designs in which multiple peripheral chromophores funnel their energy to a central chromophore located at the focal point of the dendrimer, as demonstrated again recently by Aida and coworkers (53), with a very large dendrimer containing an array of 28 zinc porphyrin donor chromophores radially emanating from a central free-base porphyrin acceptor. These early mimics of natural photosystems show great promise and their combination with moieties capable of charge separation and electron injection might contribute to lessen our dependence on energy from fossil fuels. In more technological areas, such as nanoscale photonics, dendrimers show excellent promise because, to be used for devices, the materials should have a well defined macromolecular architecture with a large cross section for energy absorption, extremely high quantum yields of fluorescence, as well as good solubility and processability characteristics. It is clear that dendrimers have all of these assets and also have the built-in structural, chemical, and physical versatility that will enable the tuning of each of these properties for the specific intended application.
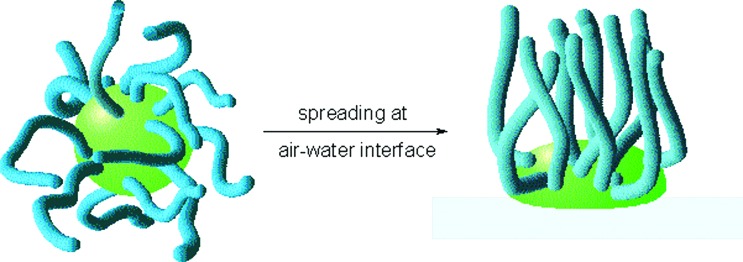
The many unique structural features of dendrimers also make them attractive for the modification of surfaces and interfaces (44). A number of studies have explored the ability of dendrimers to form self-assembled monolayers. Although dendrimer molecules are significantly larger than the classical surfactants used for most monolayers, they can interact with surfaces through the cooperative effect of multiple peripheral (54, 55) or inner structural (45, 46, 56, 57) functionalities, or even through their single focal point (58, 59). In the latter case, surface-binding is generally rather weak unless strong ionic (60) or covalent (61) linkages are used. The flexible nature of lower generation dendrimers is frequently an asset because molecular interactions can be maximized for strong binding by distortion of the globular framework of the dendrimer. This was demonstrated by Meijer and coworkers with a study of functionalized dendrimers at the air–water interface (45). This involved a poly(propylene imine) dendrimer with chain-ends modified with long-chain alkyl substituents, which could only interact with the water surface through their hydrophilic internal components and not through their hydrophobic chain-ends. As a result, the shape of the dendrimers changed from a sphere with extended chain-ends—the conformation favored in a nonpolar solvent—to a flattened conformation in which the hydrophilic dendrimer interior maximizes its association with the water surface, while the hydrophobic end groups are forced upward and away from the water surface (Fig. 7). These findings correlate well with the earlier work of Saville et al. (58, 59) on deuterated Fréchet-type poly(benzyl ether) dendrons and those of Tomalia and coworkers (56) and Hawker and coworkers (57) on end-modified poly(amido amine) dendrimers.
Figure 7.
Shape change of alkyl-modified poly(propylene imine) dendrimer after spreading at air–water interface.
Because of their unique properties, dendrimer monolayers are finding a number of applications from surface-confined chemical sensor arrays (54, 55) or affinity biosensors (62, 63) to resists for nanolithography (60, 61). Kim and coworkers' (62, 63) electrochemical biosensing device uses cyclic voltammetry to detect the avidin–biotin interaction by monitoring the redox properties of free glucose oxidase in a glucose-containing electrolyte solution. Self-assembled monolayers of amine-terminated poly(amido amine) dendrimers were prepared on gold electrodes, and the periphery was functionalized randomly with both ferrocenyl groups and biotin analogues. In the absence of avidin, an electrochemical signal is generated by the enzymatic activity of glucose oxidase. As the concentration of avidin in the electrolyte solution is increased, the electrochemical signal is decreased because of the steric blockage by the avidin adlayer formed on the modified electrode. Once used, the biosensing device can be regenerated by treatment with excess biotin to desorb avidin from the biotinylated dendrimer surface.
Dendrimer self-assembled monolayers, attached either covalently or ionically onto a silicon surface, have been used successfully as resist materials for nanolithography (60, 61) (Fig. 8). Following imaging that has used a scanning probe, appropriately designed dendrimer monolayers are capable of withstanding the harsh conditions of an aqueous fluoride wet-etching process and both negative- and positive-tone images can be obtained. This approach would still require significant process optimization and extensive tip multiplexing (64) to become practical.
Figure 8.
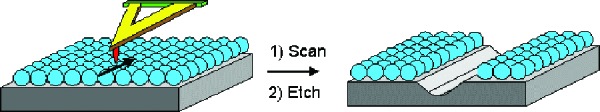
Scanning-probe lithography on dendrimer monolayer used as resist.
Assembling Dendrimers into Megamers
It is appropriate to conclude this brief perspective on dendrimers and supramolecular chemistry with a few words on megamers, molecules that are multimolecular assemblies of dendrimers. It is clear that some of the dendrimeric clusters, self-assembled monolayers, or multilayer gels mentioned earlier constitute supramacromolecular assemblies of dendrimers. Similarly, simple covalent oligomeric species in which two or three dendrimers are joined together have also been known for some time, as they are frequently obtained as products of coupling side reactions during the divergent synthesis of poly(amido amine) dendrimers. However, dendrimers can also be used as reactive building blocks for the rapid construction of larger structures possessing both complexity and dimensions beyond the dendrimer itself. Tomalia et al. (65–67) have combined preformed poly(amido amide) dendrimers of different generations to obtain well defined core-shell-type megamers in which the central, and usually larger, “core” dendrimer is surrounded by a well defined number of smaller dendrimers (Fig. 9). Although much remains to be done to obtain size-monodisperse megamers and avoid clustering into larger, ill-defined entities, megamers and analogous assemblies such as those obtained from nanocrystals and dendrimers are likely to draw much attention in the coming years, as they may be expected to provide access to new properties and phenomena.
Figure 9.
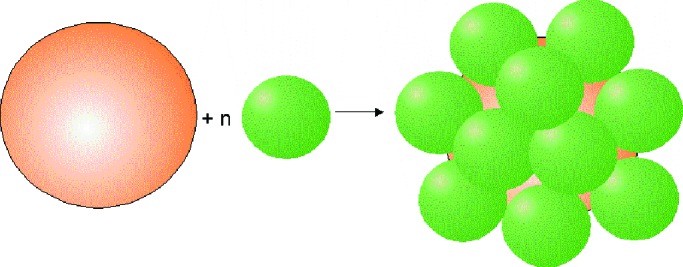
Assembly of a megamer from two different types of dendrimers.
As outlined in this article, dendrimers, with all their interesting architectural features and unusual properties, extend the concept of supramolecular chemistry far beyond Lehn's original definition. Dendrimers come in many forms and sizes; a few are rigid whereas many others have the ability to react to their environment, modifying their shape and the arrangement of their various constituting blocks to minimize overall free energy even as their internal hierarchical order is preserved. Although a number of possible areas of application of dendrimers resulting from their unconventional supramolecular character has been outlined in the above text, there is little doubt that rich additional and more refined findings are likely to emerge within the next decade.
Acknowledgments
Thanks to all my excellent coworkers and collaborators whose names appear in the references. This research was supported by the Department of Energy (Basic Energy Sciences), the Air Force Office of Scientific Research, and by the National Science Foundation (Division of Materials Research). The Army Research Office (Multi-University Research Initiative program) is acknowledged with thanks.
References
- 1.Tomalia D A, Baker H, Dewald J, Hall J M, Kallos G, Martin R, Ryder J. Polym J. 1985;17:117–132. [Google Scholar]
- 2.Newkome G R, Yao Z, Baker G R, Gupta V K. J Org Chem. 1985;50:2003–2004. [Google Scholar]
- 3.Newkome G R, Moorefield C N, Vögtle F. Dendritic Molecules: Concepts, Syntheses, Perspectives. Weinheim, Germany: VCH; 1986. [Google Scholar]
- 4.Fréchet J M J, Tomalia D A, editors. Dendrimers and Other Dendritic Polymers. Chichester, U.K.: Wiley; 2001. [Google Scholar]
- 5.Hawker C J, Wooley K L, Fréchet J M J. J Am Chem Soc. 1993;115:4375–4376. [Google Scholar]
- 6.Hecht S, Fréchet J M J. Angew Chem Int Ed Engl. 2001;40:74–91. doi: 10.1002/1521-3773(20010105)40:1<74::aid-anie74>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Mourey T H, Turner S R, Rubinstein M, Fréchet J M J, Hawker C J, Wooley K L. Macromolecules. 1992;25:2401–2406. [Google Scholar]
- 8. Buhleier, E., Wehner, W. & Vögtle, F. (1978) Synthesis, 155–163.
- 9.Hawker C J, Fréchet J M J. J Am Chem Soc. 1990;112:7638. [Google Scholar]
- 10.Grayson S, Fréchet J M J. Chem Rev. 2001;101:3819–3868. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]
- 11.Zeng F, Zimmerman S C. Chem Rev. 1997;97:1681–1712. doi: 10.1021/cr9603892. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman S C, Zeng F W, Reichert D E C. Science. 1996;271:1095–1098. doi: 10.1126/science.271.5252.1095. [DOI] [PubMed] [Google Scholar]
- 13.Ward D M. Annu Rep Prog Chem Sect A. 2000;96:345–385. [Google Scholar]
- 14.Lehn J M. Angew Chem Int Ed Engl. 1988;27:89–112. [Google Scholar]
- 15.Fréchet J M J. Science. 1994;263:1710–1715. doi: 10.1126/science.8134834. [DOI] [PubMed] [Google Scholar]
- 16.Denti G, Campagna S, Serroni S, Ciano N, Balzani V. J Am Chem Soc. 1992;114:2944–2950. [Google Scholar]
- 17.Balzani V, Juris A, Venturi M, Campagna S, Serroni S. Chem Rev. 1996;96:759–833. doi: 10.1021/cr941154y. [DOI] [PubMed] [Google Scholar]
- 18.Newkome G R, He E, Moorefield C N. Chem Rev. 1999;99:1689–1746. doi: 10.1021/cr9800659. [DOI] [PubMed] [Google Scholar]
- 19.Emrick T, Fréchet J M J. Curr Opin Colloid Interface Sci. 1999;4:15–23. [Google Scholar]
- 20.Moore J S. Curr Opin Colloid Interface Sci. 1999;4:108–115. [Google Scholar]
- 21.Astruc D, Blais J-C, Cloutet E, Djakovitch L, Rigaud S, Ruiz J, Sartor V, Valério C. Top Curr Chem. 2000;210:229–259. [Google Scholar]
- 22.Zimmerman S C, Lawless L J. Top Curr Chem. 2001;217:95–120. [Google Scholar]
- 23.Smith D K, Diederich F. Top Curr Chem. 2000;210:183–227. [Google Scholar]
- 24.van Manem H-J, van Veggel F C J M, Reinhoudt D N. Top Curr Chem. 2001;217:121–162. [Google Scholar]
- 25.Newkome G R, Güther R, Moorefield C N, Cardullo F, Echegoyen L, Pérez-Cordero E, Luftmann H. Angew Chem Int Ed Engl. 1995;34:2023–2026. [Google Scholar]
- 26.Kato T, Fréchet J M J. Macromolecules. 1989;22:3818–3819. [Google Scholar]
- 27.Kato T, Kihara H, Kumar U, Uryu T, Fréchet J M J. Angew Chem Int Ed Engl. 1994;33:1644–1645. [Google Scholar]
- 28.Fouquey C, Lehn J M, Levelut A M. Adv Mat. 1990;2:254–257. [Google Scholar]
- 29.Freeman A W, Vreekamp R H, Fréchet J M J. Polym Mat Sci Eng. 1997;77:138–139. [Google Scholar]
- 30.Huck W T S, Hulst R, Timmerman P, van Veggel F C J M, Reinhoudt D N. Angew Chem Int Ed Engl. 1997;36:1006–1008. [Google Scholar]
- 31.Kawa M, Fréchet J M J. Chem Mater. 1998;10:286–296. [Google Scholar]
- 32.Percec V, Cho W D, Mosier P E, Ungar G, Yeardley D J P. J Am Chem Soc. 1998;120:11061–11070. [Google Scholar]
- 33.Hudson S D, Jung HY, Percec V, Cho W D, Johansson G, Ungar G, Balagurusamy V S R. Science. 1997;278:449–452. [Google Scholar]
- 34.Enomoto M, Kishimura A, Aida T. J Am Chem Soc. 2001;123:5608–5609. doi: 10.1021/ja010426t. [DOI] [PubMed] [Google Scholar]
- 35.Masuo S, Yoshikawa H, Asahi T, Masuhara H, Sato T, Jiang D L, Aida T. J Phys Chem B. 2001;105:2885–2889. [Google Scholar]
- 36.Bauer B J, Amis E J. In: Dendrimers and Other Dendritic Polymers. Fréchet J M J, Tomalia D A, editors. Chichester, U.K.: Wiley; 2001. pp. 271–274. [Google Scholar]
- 37. Hawker, C. J., Wooley, K. L. & Fréchet, J. M. J. (1993) J. Chem. Soc. Perkin. Trans. 1, 1287–1297.
- 38.Newkome G R, Moorefield N, Baker G R, Saunders M J, Grossman S H. Angew Chem Int Ed Engl. 1991;30:1178–1180. [Google Scholar]
- 39.Mattei S, Seiler P, Diederich F, Gramlich V. Helv Chim Acta. 1995;78:1904–1912. [Google Scholar]
- 40.Stevelmans S, van Hest J C M, Jansen J F G A, van Boxtel D A F J, de Brabander-van den Berg E M M, Meijer E W. J Am Chem Soc. 1996;118:7398–7399. [Google Scholar]
- 41.Liu M, Kono K, Fréchet J M J. J Controlled Release. 2000;65:121–131. doi: 10.1016/s0168-3659(99)00245-x. [DOI] [PubMed] [Google Scholar]
- 42.Jansen J F G A, de Brabander-van den Berg E M M, Meijer E W. Science. 1994;266:1226–1229. doi: 10.1126/science.266.5188.1226. [DOI] [PubMed] [Google Scholar]
- 43.Jansen J F G A, Meijer E W, de Brabander-van den Berg E M M. J Am Chem Soc. 1995;117:4417–4418. [Google Scholar]
- 44. Tully, D. C. & Fréchet, J. M. J. (2001) Chem. Commun., 1229–1239.
- 45.Schenning A P H J, Elissen-Roman C, Weener J W, Baars M W P L, van der Gaast S J, Meijer E W. J Am Chem Soc. 1998;120:8199–8208. [Google Scholar]
- 46.Wiener J W, Baars M W P L, Meijer E W. In: Dendrimers and Other Dendritic Polymers. Fréchet J M J, Tomalia D A, editors. Chichester, U.K.: Wiley; 2001. pp. 387–424. [Google Scholar]
- 47.Gorman C B, Smith J C. Acc Chem Res. 2001;34:60–71. doi: 10.1021/ar000044c. [DOI] [PubMed] [Google Scholar]
- 48.Bhyrappa P, Young J K, Moore J S, Suslick K S. J Am Chem Soc. 1996;118:5708–5711. [Google Scholar]
- 49.Piotti M E, Rivera F, Bond R, Hawker C J, Fréchet J M J. J Am Chem Soc. 1999;121:9471–9472. [Google Scholar]
- 50.Hecht S, Fréchet J M J. J Am Chem Soc. 2001;123:6959–6960. doi: 10.1021/ja003304u. [DOI] [PubMed] [Google Scholar]
- 51.Crooks R M, Zhao M, Sun L, Chechik V, Yeung L K. Acc Chem Res. 2001;34:181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 52. Adronov, A. & Fréchet, J. M. J. (2000) Chem. Commun., 1701–1710.
- 53.Choi M S, Aida T, Yamazaki T, Yamazaki I. Angew Chem Int Ed Engl. 2001;40:3194–3198. doi: 10.1002/1521-3773(20010903)40:17<3194::AID-ANIE3194>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 54.Wells M, Crooks R M. J Am Chem Soc. 1996;118:3988–3989. [Google Scholar]
- 55.Crooks R M, Ricco A J. Acc Chem Res. 1998;31:219–227. [Google Scholar]
- 56.Sayed-Sweet Y, Hedstrand D M, Spinder R, Tomalia D A. J Mater Chem. 1997;7:1199–1205. [Google Scholar]
- 57.Kampf J P, Frank C W, Malmström E E, Hawker C J. Langmuir. 1999;15:227–233. [Google Scholar]
- 58.Saville P M, White J W, Hawker C J, Wooley K L, Fréchet J M J. J Phys Chem. 1993;97:293–294. [Google Scholar]
- 59.Saville P M, Reynolds P A, White J W, Hawker C J, Fréchet J M J, Wooley K L, Penfold J, Webster J R P. J Phys Chem. 1995;99:8283–8289. [Google Scholar]
- 60.Tully D C, Trimble A R, Fréchet J M J, Wilder K, Quate C F. Chem Mater. 1999;11:2892–2988. [Google Scholar]
- 61.Tully D C, Wilder K, Fréchet J M J, Trimble A R, Quate C F. Adv Mater. 1999;11:314–318. [Google Scholar]
- 62.Yoon H C, Hong M Y, Kim H S. Anal Biochem. 2000;282:121–128. doi: 10.1006/abio.2000.4608. [DOI] [PubMed] [Google Scholar]
- 63.Yoon H C, Hong M Y, Kim H S. Langmuir. 2001;17:1234–1239. [Google Scholar]
- 64.Minne S C, Adams J D, Yaralioglu G, Manalis S R, Atalar A, Quate C F. Appl Phys Lett. 1998;73:1742–1744. [Google Scholar]
- 65.Tomalia D A, Swanson D R. In: Dendrimers and Other Dendritic Polymers. Fréchet J M J, Tomalia D A, editors. Chichester, U.K.: Wiley; 2001. pp. 617–629. [Google Scholar]
- 66.Uppuluri S, Piehler L T, Li J, Swanson D R, Hagnauer G L, Tomalia D A. Adv Mater. 2000;12:796–800. [Google Scholar]
- 67.Li J, Swanson D R, Qin D, Brothers H M II, Piehler L T, Tomalia D A, Meier D J. Langmuir. 1999;15:7347–7350. [Google Scholar]