Remarkable progress in the synthetic chemistry of supramolecular assemblies has created many new structural types. With the proliferation of new structures has come increasing interest in the properties and behavior of these assemblies. Does the cooperativity of constituent components lend an assembly new properties distinct from the components themselves? Do the properties of an assembly make it suitable for application as a nanovessel for new chemistries? The latter question may lead to the synthetic targeting of assemblies for specific applications. Physical study of supramolecular systems informs the evolution of new structures, which express more complex supramolecular functionality.
Jean-Marie Lehn aptly described supramolecular chemistry as an “information science”: the instruction set for the creation of a large complex assembly is contained within its constituent components (1). Nature provides the most spectacular examples of discrete molecular assemblies. Supermolecules such as the octahedral iron storage vessel ferritin (2) are assembled from many smaller repeated subunits that contain precise information for their correct integration into the larger structure (1, 3). For the synthetic chemist, self-assembly represents a powerful synthetic methodology in the creation of large, discrete, ordered structures from relatively simple synthons. A number of such synthons have been developed, particularly organic hydrogen-bond donors and acceptors (4, 5) or metal (M) and ligand (L) components (6–10).
In this article we ask: How are these clusters assembled? What are the consequences of the supramolecular instruction set in the dynamic behavior of the product assemblies? Supramolecular assemblies demonstrate cooperativity. This cooperativity affects both the stability of the cluster and the mechanism of its formation and rearrangement. Here we will concentrate on the formation and rearrangement of supramolecular assemblies composed of metals and ligands.
Supramolecular Dynamics
Detailed understanding of the dynamic processes becomes crucial to use supramolecular assemblies to influence reaction chemistry, selectively encapsulate small molecules, or create new nanodevices. Increasingly, the focus is on application of these molecules to other chemistry problems: selective substrate binding, trapping reactive intermediates or protecting unstable species, and influencing reaction chemistry within assembly cavities. Design of assemblies for specific applications may require full understanding and control of the solution dynamic behavior exhibited by these systems.
The mechanisms of formation or ligand exchange for mononuclear metal–ligand complexes are well understood, with individual reaction types categorized and described (11). In contrast, the description of the dynamic exchange and rearrangements of metal–ligand assemblies presents new challenges in coordination chemistry, will have important impact in the development of supramolecular chemistry, and ultimately may allow for predictable incorporation of desired properties and functionality within complex assemblies.
One of the limiting factors in the study of supramolecular assemblies is their characterization. Rigorous identification of the structures themselves can be difficult because of their large size and extended connectivity (8). Therefore, study of their dynamic behavior can prove particularly challenging.
Self-Assembly
Kinetically labile metal–ligand interactions are essential for the self-correction of initially formed, random, oligomeric structures, for which the number of possible initial structures is very large. How do these structures self-assemble? Can the spontaneous formation of the observed product be predicted or simply described?
The synthesis of clusters from nonlabile components provides a view of the stepwise assembly of complex structures (12). It is unlikely, however, that labile systems under thermodynamic control exhibit a single, simple route of assembly. The complexity of these systems depends on the number and configuration of available interaction sites and therefore the number of possible modes of assembly. Assembly processes also should be expected to be highly concentration-dependent, with smaller discrete species entropically favored over extended oligomers in dilute solution.
The dynamic assembly of helicates has been investigated by Albrecht-Gary, Lehn, and coworkers (13, 14). Spectrophotometric kinetic studies of tricuprous double-stranded helicates (Cu3L2) led to the development of a proposed stepwise mechanism involving several kinetic intermediates (14). Although the formation of extended random oligomers is not inferred from these studies, the kinetic intermediates do not correspond to complexes identified in the corresponding thermodynamic studies. Instead they seem to have altered coordination geometries with respect to the Cu(I) metal centers and coordinated solvent molecules. Rearrangement of misaligned components, structural self-correction, is highlighted as an important mechanistic process.
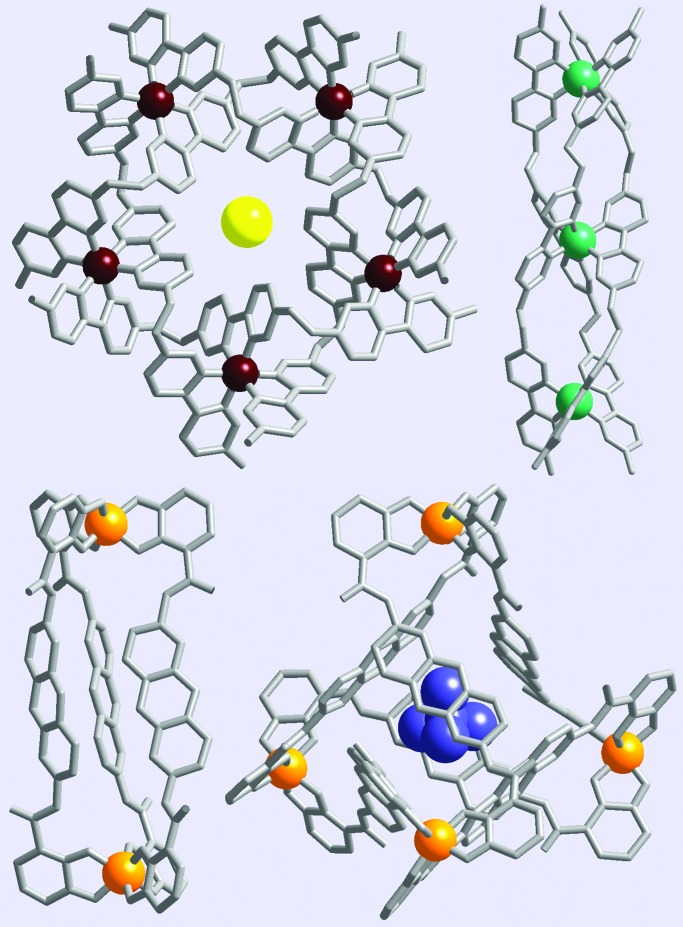
In certain metallosupramolecular systems, the addition of an external agent or a change in solution conditions prompts the conversion of one structure to another. Supramolecular transformations of this type may provide access to assembly processes, because they may occur more slowly and involve fewer intermediate species. We reported the slow conversion of a dinuclear triple-stranded helicate (M2L3) to a tetranuclear tetrahedron (M4L6) with the addition of an appropriate guest molecule (ref. 15; Fig. 1). Although the conversion takes place over 24 h at 70°C, no intermediate species were detected. The helicate might be thought of as an intermediate in the stepwise construction of the tetrahedron, although it is not clear that the same mechanism of assembly is operable in the de novo assembly of the tetrahedron from metal, ligand, and guest components.
Figure 1.
(Upper) M5L5 circular helicate and M3L3 triple-stranded helicate (15). (Lower) M2L3 triple-stranded helicate and M4L6 tetradedron (16).
Lehn and coworkers (16) described the conversion of a triferrous linear triple helicate to a pentaferrous circular helicate with the addition of chloride as a guest for the larger structure (Fig. 1). In this system, the helicate is observed as a kinetic product in the formation of the circular helicate even when the guest is included in the synthesis. The study highlights the role of kinetic control in self-assembly processes and the complexity of possible mechanistic interpretations.
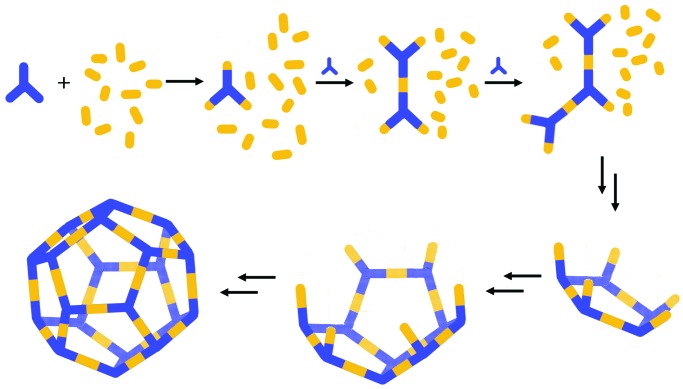
In their description of the formation of a dodecahedron from 50 metal and ligand components, Levin and Stang (17) invoke kinetic control, when the ratio of vertex to linear units is limited. Initial formation of syn ring fragments (rings in which the growing ends extend in the same direction) templates further ring closures and leads kinetically to the convex structure (Scheme S1). The nucleation of the structure by initial ring formation seems to inhibit assembly of the multitudes of unintended assemblies.
Scheme 1.
Growth of a dodecahedral coordination assembly from 50 components as the ratio of vertex to linear units is increased (17).
Cooperativity
The solution dynamic behavior of metallosupramolecular structures often reflects the integration of constituent components, meaning that properties of the assembled components of a structure may not be independent.
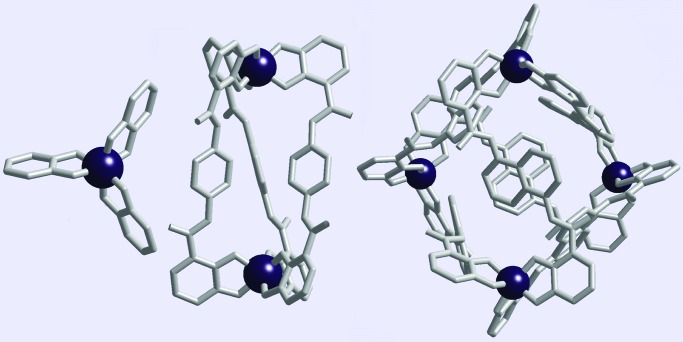
We have studied labile tris–catecholato complexes of octahedral metal centers. These complexes adopt a propeller-like geometry making them chiral (Δ or Λ configuration). When linked together with sufficiently rigid ligands, multinuclear, tris–catecholato complexes form only homochiral structures. We have described M2L3 helicates and M4L6 tetrahedra of this type (Fig. 2; ref. 18). A mononuclear tris–catecholato complex (ML3) racemizes rapidly in basic solution by way of a trigonal twist mechanism (19). When two tris–catecholate complexes are linked in a helicate, the racemization of the M2L3 structure (from ΔΔ to ΛΛ) slows by a factor of 100, demonstrating the mechanical coupling between the two metal centers (20). In the absence of this coupling, racemization of the helicate would be expected to occur at the same rate as that of the mononuclear complex.
Figure 2.
Tris–catecholate structures: mononuclear ML3, dinuclear M2L3 helicate, and tetranuclear M4L6 tetrahedron. Racemization (ΔΔ to ΛΛ) of the helicate is slowed by the mechanical coupling of the metal centers, whereas the homochiral tetrahedron is inert to racemization (19, 20).
Linkage of four of these complexes in a tetrahedral M4L6 structure imposes an even stronger coupling. As a consequence, the racemization (ΔΔΔΔ to ΛΛΛΛ isomerization) of the structure is not observed (21). Nonetheless, ligand and guest (small molecules can be encapsulated within the tetrahedron) exchange experiments attest to the dynamic nature of this resilient assembly (M. Ziegler, A.V.D., D. W. Johnson, and K.N.R., unpublished data). Although the components of the tetrahedron are labile, the chiral tetrahedral structure itself displays unique structural integrity.
Williams and coworkers (22) also have investigated the racemization processes of dinuclear helicates as compared with mononuclear analogs. Working with Co2+ benzimidazole complexes, they found the racemization rate of [Co2L3]4+ to be 6 orders of magnitude slower that that of the mononuclear [CoL3]2+ analog. In these systems, the researchers concluded that racemization occurs by way of a dissociative mechanism that is impeded by the rigid supramolecular structure of the helicate.
Host-Guest Chemistry and Nanoscale Reaction Vessels
Many larger supramolecular structures contain cavities capable of binding small molecules, and the encapsulation of a variety of molecules has been reported (23). Perhaps some of the most intriguing applications of supramolecular assemblies exploit host-guest chemistry in attempts to redefine solution reaction chemistry. In parallel with the function of natural supramolecular clusters, the goal for functional application of synthetic supramolecular clusters is to protect a valuable guest molecule or to provide an environment that makes possible chemistry not possible in the bulk environment. We emphasize again that the dynamic behavior of these systems impacts their performance as chemical vessels and must be considered as their functionality is pursued.
The rate and mechanism of guest exchange is of interest if assemblies are to be used as trapping containers for reactive species or as small molecule reaction vessels. For example, Rebek and coworkers (24) have examined guest exchange in hydrogen-bonded capsules. Taking into account the unzipping of hydrogen-bonded capsule seams, they proposed a mechanism for guest exchange reactions.
We have studied the exchange of encapsulated species in the M4L6 tetrahedron discussed previously (25, 26). The cavity of this structure is isolated from the external solution because of the tight packing of the ligand edges. We are evaluating a trial mechanism that accounts for partial dissociation of the structure in the release and capture of guest species.
The residence time of different guest molecules within an assembly and the mechanism of entry and exit may determine the effectiveness with which such cavities can be exploited for other chemical functions. For example, we have demonstrated the synthesis and stabilization of a reactive phosphonium-acetone adduct within the M4L6 cavity in aqueous solution (27). The free adduct degrades rapidly in aqueous solution and would not be observed to form in water without the protection of the hydrophobic cavity of the molecular tetrahedron. The extent of the kinetic stabilization of this product depends on the dynamics of the host-guest interaction.
The use of supramolecular assemblies as reaction vessels is emerging as a new direction in the field (27–32). The host-guest properties of each assembly dictate which chemical reactions will be suited for transfer from macroscale to nanoscale containers. The size, shape, and chemical environment of an assembly cavity determine the binding affinity for particular guest substrates.
Remarkable studies by Fujita and coworkers (32) demonstrate the influence of assembly cavities on reaction products. The synthesis of labile silanol oligomers can be “cavity-directed”: the nuclearity of the silanol products is determined by the size and shape of the supramolecular cavity within which it was synthesized. This result reveals an important concept in the initial stages of chemistry within molecules, the tailoring of nanoscale flasks for reaction chemistry. Assemblies can be designed not only to selectively bind particular guest molecules but also to accommodate and influence specified reaction chemistry. The host assembly serves as a mold for product synthesis. In this system, lability of the alkoxysilane condensation reaction with respect to host-guest exchange enables the observed product discrimination.
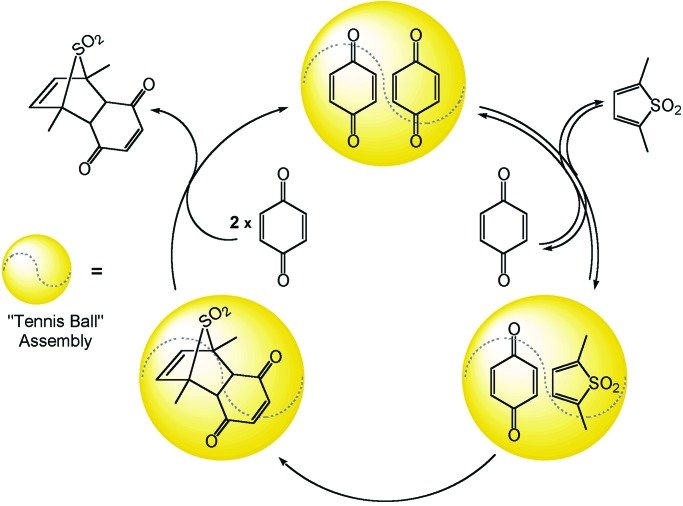
Rebek and coworkers (28–30) have made significant progress in the development of encapsulated reaction chemistry by using organic hydrogen-bond-assembled capsules as reaction containers for Diels–Alder transformations of encapsulated diene–dienophile pairs. Here the assembly was observed to accelerate product formation. Encapsulated Diels–Alder reactions exhibited faster rates than the corresponding nonencapsulated reactions. Effectively, reactants were concentrated within the nanoscale reaction flask. A limitation in some of these reactions proved to be the affinity of the reaction product for the flask; essentially, it stuck to the walls of the flask. As the researchers point out, the assembly should preferentially recognize the transition state of the reaction in order for it to act as a catalyst. When a Diels–Alder product was found that had a weak affinity for the capsule, the encapsulated reaction became catalytic (ref. 30; Scheme S2).
Scheme 2.
Catalytic acceleration of a Diels–Alder reaction within a hydrogen-bonded “tennis ball” assembly (30).
Here the capsule acts as a real catalyst, accelerating an irreversible reaction. The system dynamics balance to produce the catalytic cycle. This work demonstrates the complexity of encapsulated chemistry. Thermodynamic considerations play a key role in defining an effective catalytic system. The relative kinetic rates of the reaction chemistry itself and of the host-guest chemistry also are an important factor in the success of this type of system. If guest exchange were too fast in relation to the organic transformation, the concentration effect of encapsulation of the substrates might be negated.
The encapsulation of catalysts is a promising application of assembly chemistry. Nguyen, Hupp, and coworkers (33) describe what they call an artificial enzyme formed by binding a Mn3+ porphyrin epoxidation catalyst within a molecular square. In comparison to the free catalyst, their system demonstrated enhanced catalyst stability and substrate selectivity. Here the researchers speculated that the dynamic nature of the host–guest complex lead to the eventual deactivation of the catalyst. More weakly bound catalysts resulted in fewer catalyst turnovers. Inert host-guest behavior serves the reaction chemistry best, because the host acts as a porous yet protective housing for the catalyst.
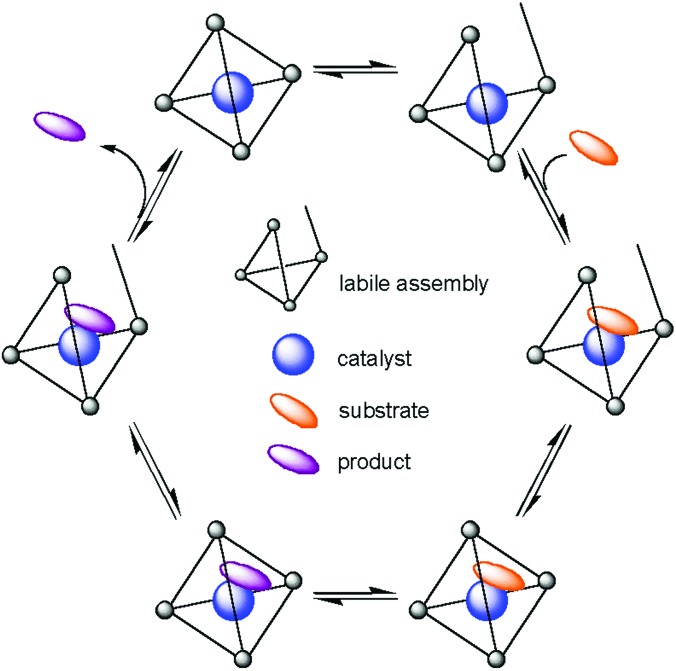
We have synthesized M4L6 assemblies, which are water-soluble and yet have hydrophobic cavity interiors completely isolated from the bulk solution. Because these cavities are chiral, could an achiral catalyst effect asymmetric synthesis in a chiral cavity? Many possible effects of encapsulation on catalyst activity can be imagined. However, if such a system is to work, the solution dynamics must be favorable. A hypothetical mechanistic scheme is presented in Scheme S3. The scheme demonstrates the intertwining of dynamic events and illustrates the critical balance of competing reactions. How do the rates of guest (guest catalyst, for example) entry and exit compare with the rate of the chemical transformation of interest? As seen in the Diels–Alder chemistry described previously, encapsulation may alter the rate of a chemical transformation. The relative thermodynamic stabilities of each proposed host–guest complex again would be crucial. Although such a system might not prove to be catalytic, it could allow for the stabilization of a reactive intermediate, providing for greater insight into the catalysis chemistry itself.
Scheme 3.
Representation of chemical catalysis within a labile assembly.
Conclusion
Although self-assembly represents a simple and efficient route to the construction of large, complex structures, understanding how this process works is not so straightforward. Synthetic chemists have acknowledged the inspiration provided by biological structures in preparing supramolecular assemblies. Yet, elucidation of the mechanisms of formation and guest exchange of nature's nanovessel assemblies remains largely unknown. For example, how does ferritin, the elegant, octahedral protein capsule designed to solubilize small iron oxide particles in vivo, absorb and dispense its valuable guest, Fe(III)? Description of the behavior of this bioassembly has proven to be a formidable challenge (34).
Supramolecular chemists are gaining new insight into the motion of supramolecular assemblies. What do they do and how do they do it? Understanding this dynamic process is sure to shape the design and application of assembly chemistry. Reaction chemistry inside nanovessels, for example, may be increasingly thought of as a complete system, designed in an integrated fashion. It must be remembered that the lability of supramolecular components essential to their efficient self-assembly also imparts dynamic solution properties. Harnessing the full functionality of these nanostructures will require control over their intricate molecular dynamics.
References
- 1.Lehn J-M. Supramolecular Chemistry: Concepts and Perspectives. Weinheim, Germany: VCH; 1995. [Google Scholar]
- 2.Proulxcurry P M, Chasteen N D. Coord Chem Rev. 1995;144:347–368. [Google Scholar]
- 3.Steed J W, Atwood J L. Supramolecular Chemistry. Chichester, U.K.: Wiley; 2000. [Google Scholar]
- 4.Prins L J, Reinhoudt D N, Timmerman P. Angew Chem Int Ed Engl. 2001;40:2382–2426. doi: 10.1002/1521-3773(20010702)40:13<2382::aid-anie2382>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Rebek J., Jr Acc Chem Res. 1999;32:278–286. [Google Scholar]
- 6. Fujita, M., Kazuhiko, U., Yoshizawa, M., Fujita, N., Kusukawa, T. & Biradha, K. (2001) Chem. Commun., 509–518.
- 7.Swiegers G F, Malefeste T J. Chem Rev (Washington, DC) 2000;100:3483–3538. doi: 10.1021/cr990110s. [DOI] [PubMed] [Google Scholar]
- 8.Leininger S, Olenyuk B, Stang P J. Chem Rev. 2000;100:853–907. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]
- 9.Saalfrank R W, Demleitner B. In: Perspectives in Supramolecular Chemistry. Sauvage J-P, editor. Vol. 5. Chichester, U.K.: Wiley; 1999. [Google Scholar]
- 10. Caulder, D. L. & Raymond, K. N. (1999) J. Chem. Soc. Dalton Trans., 1185–1200.
- 11.Basolo F, Pearson R G. Mechanisms of Inorganic Reactions. New York: Wiley; 1967. [Google Scholar]
- 12.Klausmeyer K K, Rauchfuss T B, Wilson S R. Angew Chem Int Ed Engl. 1998;37:1694–1696. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1694::AID-ANIE1694>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Fatin-Rouge N, Blanc S, Van Dorsselaer A, Baret P, Pierre J-L, Albrecht-Gary A-M. Inorg Chem. 2000;39:5771–5778. doi: 10.1021/ic000229f. [DOI] [PubMed] [Google Scholar]
- 14.Fatin-Rouge N, Blanc S, Pfeil A, Rigault A, Albrecht-Gary A-M, Lehn J-M. Helv Chim Acta. 2001;84:1694–1711. [Google Scholar]
- 15.Scherer M, Caulder D L, Johnson D W, Raymond K N. Angew Chem Int Ed Engl. 1999;38:1588–1592. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1587::AID-ANIE1587>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Hasenknopf B, Lehn J-M, Boumediene N, Dupont-Gervais A, Van Dorsselaer A, Kneisel B, Fenske D. Angew Chem Int Ed Engl. 1998;37:3265–3268. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3265::AID-ANIE3265>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Levin M J, Stang P J. J Am Chem Soc. 2000;122:7428–7429. [Google Scholar]
- 18.Caulder D L, Raymond K N. Acc Chem Res. 1999;32:975–982. [Google Scholar]
- 19.Kersting B, Telford J R, Meyer M, Raymond K N. J Am Chem Soc. 1996;118:5712–5721. [Google Scholar]
- 20.Kersting B, Meyer M, Powers R E, Raymond K N. J Am Chem Soc. 1996;118:7221–7222. [Google Scholar]
- 21.Terpin A J, Ziegler M, Johnson D W, Raymond K N. Angew Chem Int Ed Engl. 2001;40:157–160. [PubMed] [Google Scholar]
- 22.Charbonniere L J, Williams A F, Frey U, Merbach A E, Kamalaprija P, Schaad O. J Am Chem Soc. 1997;119:2488–2496. [Google Scholar]
- 23.Johnson D W, Raymond K N. Supramolecular Chemistry. 2001;13:639–659. [Google Scholar]
- 24.Santamaría J, Martín T, Hilmersson G, Craig S L, Rebek J. Proc Nat Acad Sci USA. 1999;96:8344–8347. doi: 10.1073/pnas.96.15.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caulder D L, Powers R E, Parac T N, Raymond K N. Angew Chem Int Ed Engl. 1998;37:1840–1843. [Google Scholar]
- 26.Parac T N, Caulder D L, Raymond K N. J Am Chem Soc. 1998;120:8003–8004. [Google Scholar]
- 27.Ziegler M, Brumaghim J L, Raymond K N. Angew Chem Int Ed Engl. 2000;39:4119–4121. doi: 10.1002/1521-3773(20001117)39:22<4119::aid-anie4119>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Kang J, Rebek J., Jr Nature (London) 1997;385:50–52. doi: 10.1038/385050a0. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Hilmersson G, Santamaria J, Rebek J., Jr J Am Chem Soc. 1998;120:3650–3656. [Google Scholar]
- 30.Kang J, Santamaria J, Hilmersson G, Rebek J., Jr J Am Chem Soc. 1998;120:7389–7390. [Google Scholar]
- 31.Yoshizawa M, Kusukawa T, Fujita M, Yamaguchi K. J Am Chem Soc. 2000;122:6311–6312. doi: 10.1021/ja010875t. [DOI] [PubMed] [Google Scholar]
- 32.Yoshizawa M, Kusukawa T, Fujita M, Sakamoto S, Yamaguchi K. J Am Chem Soc. 2001;123:10454–10459. doi: 10.1021/ja010875t. [DOI] [PubMed] [Google Scholar]
- 33.Merlau M L, Mejia M P, Nguyen S T, Hupp J T. Angew Chem Int Ed Engl. 2001;40:4239–4242. doi: 10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Theil E C, Takagi H, Small G W, He L, Tipton A R, Danger D. Inorg Chim Acta. 2000;297:242–251. [Google Scholar]