Abstract
OBJECTIVE
Use axial magnetic resonance imaging to test the null hypothesis that no difference exists in apparent vaginal thickness between women with and those without prolapse.
METHODS
Magnetic resonance imaging studies of 24 patients with prolapse at least 2 cm beyond the introitus were selected from an ongoing study comparing women with prolapse with normal control subjects. The magnetic resonance scans of 24 women with prolapse (cases) and 24 women without prolapse (controls) were selected from those of women of similar age, race, and parity. The magnetic resonance files were imported into an experimental modeling program, and 3-dimensional models of each vagina were created. The minimum transverse plane cross-sectional area, mid-sagittal plane diameter, and transverse plane perimeter of each vaginal model were calculated.
RESULTS
Neither the mean age (cases 58.6 years ± standard deviation [SD] 14.4 versus controls 59.4 years ± SD 13.2) nor the mean body mass index (cases 24.1 kg/m2± SD 3.3, controls 25.7 kg/m2± SD 3.7) differed significantly between groups. Minimum mid-sagittal vaginal diameters did not differ between groups. Patients with prolapse had larger minimum vaginal cross-sectional areas than controls (5.71 cm2± standard error of the mean [SEM] 0.25 versus 4.76 cm2± SEM 0.20, respectively; P = .005). The perimeter of the vagina was also larger in the prolapse group (11.10 cm ± SEM 0.24) compared with controls (9.96 cm ± SEM 0.22) P = .001. Subgroup analysis of patients with endogenous or exogenous estrogen showed prolapse patients had larger vaginal cross-sectional area (P = .030); in patients without estrogen group differences were not significant (P = .099).
CONCLUSION
Vaginal thickness is similar in women with and those without pelvic organ prolapse. The vaginal perimeter and cross-sectional areas are 11% and 20% larger in prolapse patients, respectively. Estrogen status did not affect differences found between groups.
Pelvic organ prolapse is a remarkably common problem, yet the disease mechanism resulting in its occurrence remains poorly understood. There are competing hypotheses that have been proposed to explain its cause. For example, 2 differing mechanisms have been used to explain the formation of a cystocele: vaginal wall stretching versus vaginal wall detachment. The first focuses on stretching of the fibromuscular vaginal tube (fascia) as the disease mechanism of prolapse. This has also been referred to as a distention cystocele.1 The distention cystocele implies that the vagina becomes thin and attenuated in the formation of a cystocele. The other focuses more on the connections of the vaginal tube relative to the pelvic sidewall.2,3 When these connections break, a paravaginal defect or a displacement cystocele occurs.1,4 Similar considerations have been voiced concerning support of the posterior vaginal wall.5
Magnetic resonance imaging is a tool that can be used to study pelvic floor structures. On axial magnetic resonance images, the vagina is a discreet structure with visible boundaries. The outer border of the vagina is delineated by a dark line that represents the fibromuscular wall of the vagina. Because the vagina is a potential space that is not filled with fluid or air, the structure inside the dark fibromuscular circumference is made up of the coapted walls of the vagina. This allows the overall thickness of the vaginal wall to be measured and quantified.
To help gain insight regarding the role of each of the above hypotheses in explaining pelvic organ prolapse, we used magnetic resonance imaging to test the null hypothesis that no difference exists in apparent vaginal thickness between women with and those without prolapse.
MATERIALS AND METHODS
Axial magnetic resonance scans of 25 women with prolapse of a vaginal wall or cervix at least 2 cm beyond the introitus were selected from an ongoing institutional review board–approved study comparing women with prolapse with normal control subjects. The prolapse patients were recruited through the University of Michigan Urogynecology Clinic. The controls were recruited mainly through advertisements, as well as through the Women’s Health Registry, a list of women who expressed interest in participating in women’s health projects. Patients were excluded if they had previous surgery for prolapse or incontinence, had genital anomalies, or had delivered in the past year. Patients were enrolled between June 2001 and September 2003. The magnetic resonance scans of 25 women with prolapse (cases) and 25 women without prolapse (controls) were selected from those of women of similar age, race, and parity. One patient in whom the prolapse was protruding at the time of magnetic resonance was excluded because this created a double layer of vagina, which would have confounded results. A control subject was therefore also excluded to produce 24 patients in each group for analysis. Multiplanar 2-dimensional fast-spin proton density magnetic resonance images (echo time 15 ms, repetition time 4,000 ms) of all pelvises were obtained by use of a 1.5 T superconducting magnet (Signa Horizon LX, General Electric, Milwaukee, WI) with version 9.1 software. The fields of view in both axial and coronal images were 16 × 16 cm, and the field of view in the sagittal images was 20 × 20 cm. All 3 views had slice thicknesses of 4 mm, with a 1-mm gap between slices.
The outer margin of the vagina was traced to include the fibromuscular wall by using 3-dimensional computer graphics and modeling software (Slicer 2b1; Massachusetts Institute of Technology, Cambridge, MA). In subjects with a uterus and cervix, the vagina was traced until the cervix could be seen (Fig. 1) to avoid including the cervix in the vaginal wall measurements. The vagina was traced in all of the axial slices to the level of the introitus, noted by the visibility of the vestibular bulbs (Fig. 1). Blinding of the investigator was attempted but often not possible because of the visible nature of the prolapse in many of the magnetic resonance images. Three-dimensional models based on the axial magnetic resonance tracings were then constructed. The models were imported into I-DEAS 9.0 (EDS, Hook, UK), an engineering computer-aided analysis and design program (Fig. 2), to make measurements perpendicular to the long axis of the vagina to avoid 2-dimensional measurement errors (Fig. 3A).6 The longitudinal axis of the 3-dimensional vaginal models was determined as the midline of the vagina contours in the mid-sagittal plane (Fig. 3B). Five equally spaced points were selected along the longitudinal axis of each model, and cross sections of the vagina perpendicular to the longitudinal axis were made (Fig. 3C). The minimum diameter in mid-sagittal plane, cross-sectional area, and length of the perimeter of the vagina were calculated at each cross section (Fig. 3D). The perimeter of the vaginal wall was measured as the circumferential length of the outer vaginal wall margin on each cross section.
Fig. 1.
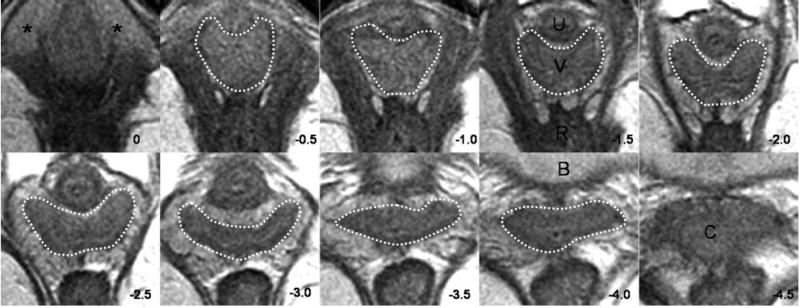
Axial slices at 5-mm intervals arranged caudal to cephalad starting from the image in the upper left (image 0). Vaginal tracings were made from above the level of the vestibular bulbs (VB), represented by asterisks (*), caudally (image 0) to below where the cervix (C) could be seen (image -4.0). U, urethra; V, vagina; R, rectum; B, bladder. © DeLancey 2004.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
Fig. 2.
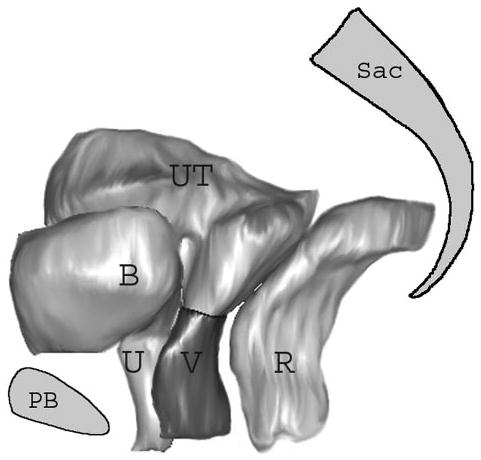
Selected 3-dimensional model as it appears in I-DEAS 9.0. The sacrum (Sac), pubic bone (PB), and pelvic organs have been shown for orientation. B, bladder; U, urethra; UT, uterus; V, vagina; R, rectum. © DeLancey 2004.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
Fig. 3.
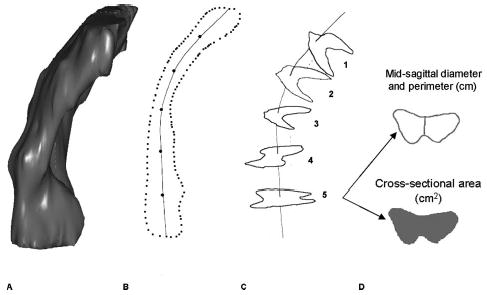
Steps in obtaining vaginal measurements: A. Three-dimensional I-DEAS 9.0 vaginal model. B. Longitudinal axis determined in the mid-sagittal plane, with 5 equally spaced locations along the longitudinal axis marked. C. Sample cross sections: Location 1 is near the vaginal apex, and location 5 is near the hymen. D. Mid-sagittal diameter, perimeter, and cross-sectional area were calculated for each axial cross section. © DeLancey 2004.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
Descriptive statistics were generated, including the mean and standard error of the mean (SEM). The measurements at each cross section were summed for the groups, and an aggregated average was calculated. A repeated-measures analysis of variance was used to test for group and level effects. Two-sided, 2-group, t tests were used to test group differences. P < .05 was considered significant.
RESULTS
Subject groups had similar mean age and body mass index (Table 1). The analysis of variance showed that mid-sagittal vaginal diameters did not differ by group. There was, however, a significant difference by location (Table 2, Fig. 4). The vaginal cross-sectional areas differed significantly by group but not by level (Table 2). The case group had mean cross-sectional areas that were 20% larger than those of controls (5.71 cm2± SEM 0.25 versus 4.76 cm2± SEM 0.20, respectively; P = .005; Fig. 5). The perimeter of the vagina was significantly different by group as well as location (Table 2). The perimeter was significantly longer in the case group than in the controls (11.10 cm ± SEM 0.24 versus 9.96 cm ± SEM 0.22, respectively; P = .001; Fig. 6). Post hoc tests of statistical power were calculated for group differences in mid-sagittal diameter, cross-sectional area, and perimeter and were found to be 5%, 83%, and 92%, respectively.
Table 1.
Patient Characteristics
| Prolapse (n = 24) | Controls (n = 24) | |
|---|---|---|
| Age (y)* | 58.6 ± 14.4 | 59.4 ± 13.2 |
| Body mass index (kg/m2)† | 24.1 ± 3.3 | 25.7 ± 3.7 |
| Estrogen | ||
| Yes | 16 | 14 |
| No | 8 | 10 |
Data are expressed as mean ±standard deviation or number.
P = .844.
P = .12.
Table 2.
ANOVA Results in Terms of F Statistics and P Values
| Mid-Sagittal Diameter
|
Cross-Sectional Area
|
Vaginal Perimeter
|
||||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Group | 0.02 | .88 | 8.91 | .005 | 12.03 | .001 |
| Location | 7.21 | < .001 | 0.81 | .52 | 5.52 | < .001 |
| Group × location | 0.72 | .58 | 1.12 | .35 | 0.95 | .44 |
ANOVA, analysis of variance.
Fig. 4.
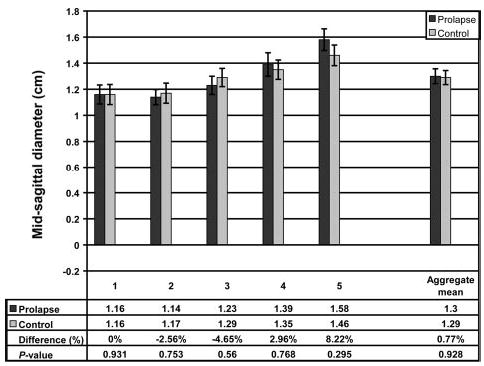
Vaginal mid-sagittal diameter at each of the 5 vaginal locations. Refer to Figure 3 for vaginal locations. The aggregate mean of the 5 individual segments is also shown at right. Error bars indicate standard error of the mean.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
Fig. 5.
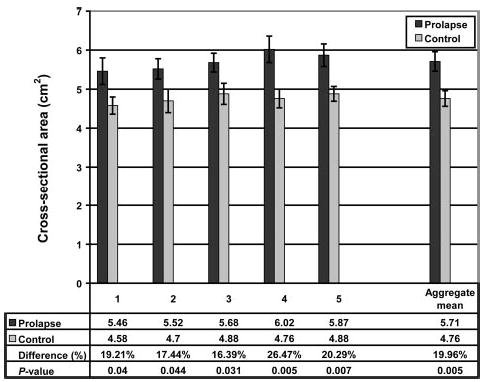
Cross-sectional area at each of the 5 vaginal locations. The aggregate mean of the 5 individual segments is shown at right. Error bars indicate standard error of the mean.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
Fig. 6.
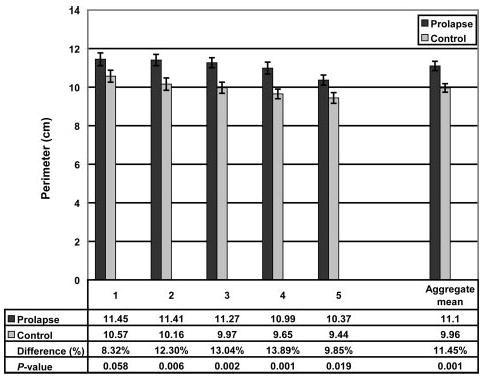
Vaginal perimeter. The aggregate mean of the 5 individual segments is shown at right. Error bars indicate standard error of the mean.
Hsu. Vaginal Thickness and Pelvic Prolapse. Obstet Gynecol 2005.
A subanalysis was performed to examine the effect of estrogen status on vaginal cross-sectional area and vaginal thickness. In the case group, 16 patients were either premenopausal or on hormone replacement therapy, compared with 14 in the control group. Eight patients in the case group and 10 in the control group were post-menopausal and not on hormone replacement therapy. Comparing cases and controls who were estrogenized, the case patients had 19% larger mean cross-sectional areas (5.90 cm2± SEM 0.33) than the controls (4.96 cm2± SEM 0.25; P = .030). Among women without estrogen, there was a trend toward the case group having larger mean cross-sectional areas (case 5.33 cm2± SEM 0.34, controls 4.48 cm2± SEM 0.34; P = .099), but the difference was not statistically significant. When mean mid-sagittal diameter was examined based on hormonal status, there were no group differences in either women with estrogen (case 1.31 cm ± SEM 0.08, control 1.35 cm ± SEM 0.07; P = .699) or those without estrogen (case 1.28 cm ± SEM 0.09, control 1.21 cm ± SEM 0.09; P = .614). The mean mid-sagittal vaginal diameters in the controls with (n = 14) and those without (n = 10) estrogen were compared, but no significant differences were found.
DISCUSSION
As judged by its appearance on magnetic resonance scans, vaginal thickness in women with prolapse was not different from that in women with normal support. There was less than a 1% difference in the mid-sagittal diameter between the groups. Because the mid-sagittal diameter is the sum of the anterior and the posterior vaginal walls, women with prolapse do not appear to have thinner vaginas.
Women with prolapse do have a 20% larger mean vaginal cross-sectional area. Because the mid-sagittal diameters of the 2 groups were similar, the larger cross-sectional area of the prolapse group must be due to a larger vaginal width, which is represented by the 11% larger vaginal perimeter. These data indicate that there is more vaginal tissue in women with prolapse than in women without prolapse. This contradicts the clinical assumption that the vagina in women with prolapse is thin.
The fact that the same trends in cross-sectional area and mid-sagittal vaginal diameter were maintained regardless of estrogen status confirms that hormonal status was not a confounding variable in this study. Although estrogen status did not change the trends in mean cross-sectional area and mid-sagittal diameter between the groups, it has been well established that the presence of estrogen positively affects vaginal maturation.7,8 Indeed, the 10% larger mid-sagittal vaginal diameter in control patients with estrogen compared with controls without estrogen suggests that, with a larger sample size, the difference in thickness might become statistically significant.
Historically, pelvic surgeons have focused on attenuation of the “endopelvic fascia” as the cause of prolapse. In 1954, Ricci performed definitive anatomical studies on pelvic connective tissue and provided an excellent review of the origin of the myth of the endopelvic fascia.8,9 Repeated histologic examination has failed to demonstrate a clear “fascial” layer10–13. After performing a literature review and performing their own histologic studies, Weber and Walters12 described the vagina as being made up of 3 layers: mucosa, muscularis, and adventitia. The adventitial layer is a connective tissue layer of collagen and elastin that separates the muscular wall of the vagina and the paravaginal tissues. The adventitia is variably discrete and does not form a complete capsule surrounding the vagina as a true fascial layer.12 Because of the lack of discrete fascial covering, our tracing outside the fibromuscular wall includes all the important structural layers of the vagina. In a study of 13 women with enteroceles, vaginal wall muscularis was found between vaginal epithelium and peritoneum in all cases; this also suggests that it is not a site-specific defect in the fibromuscular wall that leads to prolapse.14
Although the site-specific defect theory for prolapse has become less popular because of the lack of histologic evidence, a significant body of research remains focused on the vaginal tube as the source of the problem. Boreham et al13 found that the morphometry of anterior vaginal wall tissue obtained from women with prolapse was significantly altered compared with that from women with normal support. Women with prolapse had decreased fractional area of smooth muscle and disorganized smooth muscle bundles with decreased α-actin staining.13 In a separate study,15 they found that the decreased fractional area of smooth muscle was also present in the posterior vaginal wall and that women with prolapse had smaller and fewer nerve bundles in the muscularis compared with controls. Boreham et al16 also found that caldesmon, a protein that inhibits smooth muscle contractility, had increased expression in the vaginal smooth muscle of women with prolapse. A number of other studies have focused on collagen differences in the vaginal tube of women with prolapse compared with controls.17–19 Although differences in collagen content have been found, it is difficult to determine whether these differences resulted in or are a result of prolapse.
The mechanical strength of the vaginal wall is determined by its composition, tissue architecture, and thickness, all of which influence its material properties. Few studies have examined the thickness of the vaginal wall in prolapse compared with controls. Tulikangas et al14 found that the muscularis layer was thicker in women with enteroceles compared with controls. Shrinkage due to formalin fixation and differences between excised tissue and in situ tissues may help explain why vaginal thickness in that study was approximately half that found in the present study. We are not aware of any other studies that have quantified vaginal wall thickness in patients with prolapse or controls. Our findings that vaginal wall thickness appears similar in both groups suggest that it is not the fibromuscular tube of the vagina that is defective. However, we are not able to comment on the vaginal tissue architecture or composition, both of which can influence the material properties of the vagina.
A strength of this study is the development of a method to quantitatively describe the anatomy of prolapse compared with normal women. Measurements done on 2-dimensional axial magnetic resonance images are inaccurate because they are not usually acquired perpendicular to the longitudinal axis of the vagina. This is due to the position of the patient in the magnetic resonance scanner as well as the natural sagittal curvature of the vagina. The variations in slice angle can lead to systemic errors in the measurements of the 2-dimensional magnetic resonance images.6 By varying the slice angle of magnetic resonance images, Hoyte and Ratiu6 found that 2-dimensional measurements can differ by up to 15%. To circumvent this error, we created 3-dimensional models of the vaginas of our subjects. This allowed for accurate comparisons of the vaginal tube of women with prolapse and that of normal controls.
Although we found that the thicknesses of the vaginas between groups were not statistically different, we cannot preclude the possibility that the material properties of the vaginal wall might differ in their functional capacities. It has also been observed that women with prolapse have dilated venules in the vaginal wall.13 This may have led to errors in measurements of vaginal wall thickness in the present study. In addition, the study was performed on women in the supine posture when the gravity vector acts posteriorly rather than caudally, a difference that may affect the tensile loading of the vaginal wall in women with prolapse. This might have led to an underestimation of group differences in the supine posture.
To date, a unifying theory explaining the cause of pelvic organ prolapse based on objective scientific data does not exist. Different theories have been proposed, but data to resolve these differences are not available. Magnetic resonance imaging allows us to gather data that can be used to test the hypotheses concerning these long-debated issues. Although the development of prolapse is likely the common end point of different disease mechanisms, our study suggests that more attention should be focused on the connections of the vagina to the pelvic sidewall rather than the vagina itself.
Footnotes
Supported by National Institutes of Health grant R01 HD038665.
References
- 1.Nichols DH, Randall CL. Vaginal surgery. 4th ed. Baltimore (MD): Williams & Wilkins; 1996.
- 2.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 3.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 4.Richardson AC. Paravaginal repair. In: Hurt WG, editor. Urogynecologic surgery. 2nd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2000. p. 71–80.
- 5.Richardson AC, DeLancey JOL. Anatomy of genital support. In: Hurt WG, editor. Urogynecologic surgery. 2nd ed. Philadephia (PA): Lippincott Williams & Wilkins; 2000. p. 21–35.
- 6.Hoyte L, Ratiu P. Linear measurements in 2-dimensional pelvic floor imaging: the impact of slice tilt angles on measurement reproducibility. Am J Obstet Gynecol. 2001;185:537–44. doi: 10.1067/mob.2001.116751. [DOI] [PubMed] [Google Scholar]
- 7.Blakeman PJ, Hilton P, Bulmer JN. Cellular proliferation in the female lower urinary tract with reference to oestrogen status. BJOG. 2001;108:813–6. doi: 10.1111/j.1471-0528.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Vardy MD, Landsay R, Scotti RJ, Mikhail M, Richart RM, Nieves J, et al. Short-term urogenital effects of raloxifene, tamoxifen and estrogen. Am J Obstet Gynecol. 2003;189:81–8. doi: 10.1067/mob.2003.374. [DOI] [PubMed] [Google Scholar]
- 9.Ricci JV, Thom CH. The myth of a surgically useful fascia in vaginal plastic reconstructions. Q Rev Surg. 1954;11:253–61. [PubMed] [Google Scholar]
- 10.Ricci JV, Lisa JR, Thom CH, Kron WL. The relationship of the vagina to adjacent organs in reconstructive surgery: a histologic study. Am J Surg. 1947;74:387–410. doi: 10.1016/0002-9610(47)90131-1. [DOI] [PubMed] [Google Scholar]
- 11.Gitsch E, Palmrich AH. Gynelogical operative anatomy. Berlin, Germany: De Gruyter; 1977.
- 12.Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311–8. doi: 10.1016/S0029-7844(96)00322-5. [DOI] [PubMed] [Google Scholar]
- 13.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187:56–63. doi: 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 14.Tulikangas PK, Walters MD, Brainard JA, Weber AM. Enterocele: is there a histologic defect? Obstet Gynecol. 2001;98:634–7. doi: 10.1016/s0029-7844(01)01524-1. [DOI] [PubMed] [Google Scholar]
- 15.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187:1501–9. doi: 10.1067/mob.2002.130005. [DOI] [PubMed] [Google Scholar]
- 16.Boreham MK, Miller RT, Schaffer JI, Word RA. Smooth muscle heavy chain and caldesmon expression in the anterior vaginal wall of women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:944–52. doi: 10.1067/mob.2001.117342. [DOI] [PubMed] [Google Scholar]
- 17.Norton P, Boyd C, Deak S. Collagen synthesis in women with genital prolapse or stress urinary incontinence. Neurourol Urodyn. 1992;11:300–1. [Google Scholar]
- 18.Takano CC, Girao MJ, Sartori MG, Castro RA, Arruda RM, Simoes MJ, et al. Analysis of collagen in parametrium and vaginal apex of women with and without uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:342–5. doi: 10.1007/s001920200076. [DOI] [PubMed] [Google Scholar]
- 19.Liapis A, Bakas P, Pafiti A, Frangos-Plemenos M, Arnoyannaki N, Creatsas G. Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol. 2001;97:76–9. doi: 10.1016/s0301-2115(00)00478-4. [DOI] [PubMed] [Google Scholar]


