Abstract
Current clinical guidelines suggest methylphenidate (MPH) as first-line option for treatment of attention-deficit/hyperactivity disorder (ADHD). However, the Lombardy ADHD Registry initiative has recently raised some concerns about discrepant therapeutic approaches between different clinical centers. In this naturalistic observational study, we report the experience of a third-level referral center in monitoring care services for young ADHD patients. We described the clinical characteristics of the children/adolescents who were diagnosed with ADHD from 2017 and prescribed or not with MPH. We further followed-up ADHD patients who were taking MPH for 12 weeks to assess any clinical amelioration using the Clinical Global Impression—Severity scale, controlling for other outcome predictors. One fourth of patients received a MPH prescription after the ADHD diagnosis. Those children/adolescents showed more complex clinical manifestations, with greater ADHD difficulties and neuropsychiatric comorbidities. ADHD patients displayed significant improvements of functioning after 12 weeks of the initiation of MPH use, but not after 4 weeks. IQ level and the presence of co-occurring autism predicted ADHD severity at baseline. After controlling for other predictors’ effect, the severity at the first visit predicted ADHD severity after 4 weeks of MPH use, which in turn predicted the clinical functioning at the 12-week visit.
Keywords: Attention-deficit/hyperactivity disorder, Autism spectrum disorders, Comorbidity, Methylphenidate, Childhood, Adolescents
Subject terms: Psychology, Health care, Medical research
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a heterogeneous neurodevelopmental condition characterized by difficulty in maintaining attention and/or hyperactive-impulsive behaviors. Those symptoms should have manifested before 12 years of age and persisted for a minimum of 6 months1. Although the clinical features are commonly recognized in childhood, significant difficulties persist into adulthood for 70% of individuals2. In 2019 Global Burden Disease (GBD) study indicated a global age-standardized incidence and prevalence of ADHD across the life span at 0.061% (95%UI = 0.040–0.087) and 1.13% (95%UI = 0.831–1.494), respectively. ADHD accounts for 0.8% of the global mental disorder DALYs, with mortality set at zero by the GBD. Both incidence and prevalence are approximately 2.5 higher in males than females3. ADHD is also associated with an increased likelihood of having other coexisting psychiatric disorders. Comorbidity rate ranges from 65 to 85% in clinically referred children4. The most frequent co-occuring conditions are learning disorders (70%), autism (59%), tic disorders (55%) (for an in-depth discussion on comorbidities, see Gnanavel et al., 2019)5.
Current clinical guidelines suggest pharmacotherapy as first-line option for treatment of ADHD, although non-pharmacological interventions such as behavioral therapy are recommended in younger patients6,7. The mainstay pharmacological treatments for ADHD are stimulants, particularly methylphenidate (MPH) and amphetamines. Their beneficial effects have been known for more than 70 years and they are safe and well-tolerated in most cases8. Systematic reviews and network meta-analyses of double-blind randomized controlled trials recommend MPH in children and adolescents, and amphetamines in adults, as preferred first-choice medications for the short-term treatment of ADHD9,10. These medications indeed lead to a rapid and considerable improvement in the patient’s core symptoms and behavior11. Several neuropsychological studies documented additional positive effects of pharmacotherapy on cognition and motivation12,13. Notwithstanding those benefits, adverse events have also been reported, with insomnia, decreased appetite, dysphoria, irritability, and a slight increase in heart rate being the most common7.
Despite the recommendations of both national and international ADHD guidelines6,14,15, access to diagnostic and treatment services in Italy is still limited16,17. In an attempt to overcome these shortcomings, a network of 18 Lombardy clinical centers activated in June 2011 the Regional ADHD Registry in order to gather systematic data about the therapeutic services for patients who access the centers18. Ten-year results of Lombardy Registry4,19 indicated a significant amelioration in half of the patients after one year of treatment, in line with earlier evidence on the efficacy of ADHD treatments10,20 and with results coming from other naturalistic observational studies21–24. Nonetheless, no studies have explored in the Lombardy sample the short-term efficacy of MPH. With this respect, a 3-year follow up of the longitudinal study of Kaalund-Brok and colleagues has recently shown that positive responses to MPH treatment after 3 and 12 weeks significantly predict less hyperactivity/impulsivity symptoms at long term25.
Given these premises, we report in the present work the experience of a single center of the Lombardy ADHD registry—Scientific Institute, IRCCS Eugenio Medea (Bosisio Parini, Italy)—with two aims. First, we were interested in exploring the characteristics of the children/adolescents who were diagnosed with ADHD at our clinical center and were prescribed or not with MPH. We focused on MPH prescriptions only on the basis of the strong evidence indicating this medication as preferred first-choice option for the short-term treatment of ADHD. We also limited this analysis to patients who have accessed our Institute from 2017, when comorbid diagnoses of autism spectrum disorder (ASD) were made allowable under DSM-51. Despite still unclear clinical implications, ADHD+ASD could indeed represent a distinct phenotype with higher rates of cognitive, adaptive, and mental health problems compared to ADHD without co-occurring autism26. Furthermore, a recent research in an Italian sample highlights how the presence of significant non-ADHD psychopathology should be considered as a clinical factor associated with the need for MPH prescription in children and adolescents with ADHD27. The second aim of this study was to measure any improvement of these patients on their Clinical Global Impression—Severity scale (CGI-S) scores 4 and 12 weeks after the initiation of MPH use. We hypothesize that patients who received the MPH drug prescription in our sample will present more complex clinical manifestations—both in terms of symptom severity and of number of co-occurring conditions—than not-prescribed children. We also expect to find a significant clinical amelioration as indicated by lower CGI-S scores 12 weeks after the initiation of MPH, even after controlling for known predictors, such as gender, age, IQ, and comorbidities.
Results
Demographic and clinical characteristics of prescribed versus not-prescribed participants
Table 1 describes the demographic and clinical characteristics of the two samples of participants (children/adolescents who were prescribed with MPH vs children/adolescents who were not prescribed). The first group included 105 children/adolescents prescribed with MPH (24.4% of all the participants; 83 males, 22 females), the second group 326 children/adolescents who were not prescribed (75.6% of the participants; 276 males, 50 females).
Table 1.
Demographic and clinical description of the sample of children with and without MPH prescription.
| Variable | Participants prescribed with MPH (N 105, 24.4%) | Participants NOT prescribed with MPH (N 326, 75.6%) | Statistical comparison (p-value) |
|---|---|---|---|
| Gender (M:F) | 83:22 | 276:50 | 0.180a |
| Adoptive (Y/N) | 8:97 | 9:317 | 0.026a |
| Age (years ± SD) | 9.7 ± 2.7 | 8.7 ± 2.1 | 0.000b |
| SES (Mean ± SD) | 47.7 ± 10.7 | 50.9 ± 11.5 | 0.039b |
| IQ (Mean ± SD) | 83.5 ± 20.7 | 95.8 ± 14.9 | 0.000b |
| ADHD subtype (C:H:I) | 87:1:17 | 241:18:67 | 0.068a |
| ASD comorbidity | 38:67 | 35:291 | 0.000a |
| ID comorbidity | 31:74 | 32:294 | 0.000a |
| ODD comorbidity | 20:85 | 9:317 | 0.000a |
| SLD comorbidity | 18:87 | 119:207 | 0.000a |
| AD comorbidity | 5:100 | 28:298 | 0.200a |
| MD comorbidity | 7:98 | 13:313 | 0.256a |
| SWD comorbidity | 9:96 | 39:287 | 0.337a |
| Tic comorbidity | 5:100 | 12:314 | 0.621a |
| DCD comorbidity | 5:100 | 6:320 | 0.099a |
| CD comorbidity | 2:103 | 6:320 | 0.966a |
| LD comorbidity | 10:95 | 17:309 | 0.113a |
| CPRS-R Oppositional (Mean ± SD) | 70.2 ± 15.9 | 63.8 ± 15.9 | 0.010b |
| CPRS-R Cognitive Problems (Mean ± SD) | 78.8 ± 12.5 | 74.3 ± 12.5 | 0.023b |
| CPRS-R Hyperactivity–Impulsivity (Mean ± SD) | 74.1 ± 14.3 | 70.3 ± 14.1 | 0.082b |
| CPRS-R ADHD Index (Mean ± SD) | 80.0 ± 11.3 | 76.4 ± 11.5 | 0.044b |
| CPRS-R Global Index Emotional Liability (Mean ± SD) | 65.1 ± 15.5 | 59.0 ± 15.7 | 0.011b |
| CGI-S (Mean ± SD) | 5.2 ± 0.6 | 3.9 ± 0.7 | 0.000b |
AD, anxiety disorders; ASD, autism spectrum disorder; CD, conduct disorder, CGI-S, clinical severity and global functioning; CPRS-R, Conners’ parent rating scales-revised; DCD, developmental coordination disorder; ID, intellectual disabilities; IQ, intelligence quotient; LD, language disorder; MD, mood disorders; MPH, methylphenidate; N, number; ODD, oppositional defiant disorder; SD, standard deviation; SWD, sleep–wake disorders; SES, socio-economic status; SLD, specific learning disorders.
aChi-square test.
bt test.
Significant values are in bold.
No statistically significant differences were found in gender between the two subsamples (p > 0.05). In contrast, there were statistically significant between-group differences in age and IQ (both p < 0.001), with participants prescribed with MPH being older and scoring lower on standardized tests of cognitive functioning; prescribed children also presented slightly lower SES and were more likely to be adopted than not prescribed children (p = 0.039). Furthermore, co-occurring autism spectrum disorder, intellectual disabilities, specific learning disorders, and oppositional defiant disorder were higher in children/adolescents who were prescribed with MPH (all p < 0.001), whereas the two groups did not differ significantly for ADHD subtypes. Lastly, prescribed children presented higher scores on CGI-S (p < 0.001) and on several CPRS-R subscales (with significance values ranging between p = 0.010 and p = 0.044).
The group of MPH prescription subjects took an average of 9.07 (± 3.41) mg/die of MPH at prescription and 17.17 (± 10,79) mg/die by the 12th week.
Participants who were not prescribed with MPH mostly received psychological interventions such as child training or parent training or, less frequently, were prescribed with other medications (e.g. antidepressants, anxiolytics, antipsychotics)28,29.
Treatment response
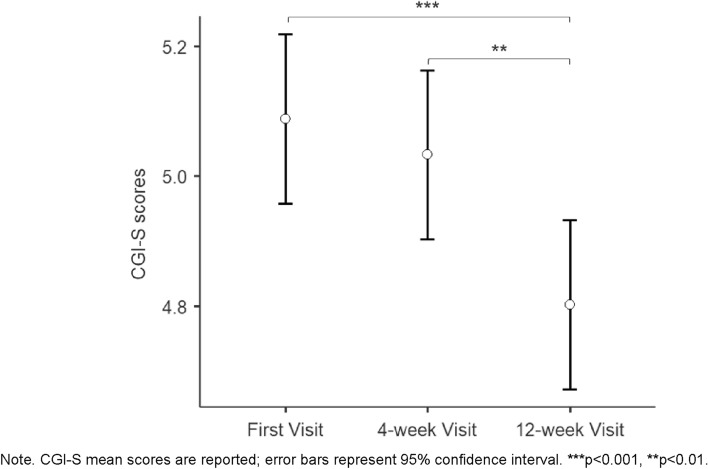
Repeated-measure analysis resulted in a significant effect of time over the study period, with children/adolescents showing ameliorations (p < 0.001) between the first visit (CGI-S score = 5.09 ± 0.55) and the visit after 12 weeks (CGI-S score = 4.80 ± 0.75), and between the 4-week (CGI-S score = 5.03 ± 0.57) and the 12-week visit (p = 0.001). No differences were found between CGI-S scores at the baseline and those at the 4-week visit (p > 0.05). Figure 1 represents CGI-S scores of MPH prescribed participants at different time points.
Fig. 1.
CGI-S scores of MPH prescribed participants at first visit, 4-week visit, and 12-week visit. Figure 1 shows the mean of CGI-S scores of MPH prescribed participants at the different time points.
Over the course of the 12-week, mild ARs were reported in 12 participants, medium severe effects were described in 11 participants, and severe effects in 5 participants. Most frequent mild ARs were inappetence, nausea, irritability, headache, or asthenia, whereas most often medium severe ARs were tic, insomnia, agitation or unstable mood. Lastly, most subjects who experienced severe adverse effects experienced inappetence, nausea, irritability, agitation or impulsivity. Mild adverse effects led to no treatment modification or level changes in dosage. The medium Rs may have led to treatment modification or suspension of treatment, whereas the severe ARs may have led to suspension of the treatment or level changes in dosage. Eighteen out of 105 (17.14%) prescribed patients either suspended (n = 6) or changed (n = 12) their therapeutic plan according to the nature and the severity of participants’ adverse events and needs. The removal of these children from the repeated-measures analysis did not change the outcome results.
With respect to combined treatment, 79 children/adolescents received MPH-only, 15 MPH+parent training, 1 MPH+child training, 10 MPH+child training+parent training.
As shown in Table 2, of the considered potential outcome predictors, only IQ and co-occurring autism (yes/no) were significantly associated with CGI-S scores over the time. Namely, higher IQ scores were associated with lower CGI-S scores at baseline (r = − 0.450, p < 0.001), at the 4-week visit (r = − 0.362, p < 0.001), and at the 12-week visit (r = − 0.283, p = 0.006), while the autism comorbidity was associated with higher CGI-S scores at baseline (r = + 0.323, p = 0.001), at the 4-week visit (r = + 0.332, p = 0.001), and at the 12-week visit (r = + 0.210, p = 0.039).
Table 2.
Pearson’s correlation analysis for the association between CGI-S scores and other outcome predictors.
| gender | age | IQ | SES | comorbidities (Y/N) | co-occuring autism (Y/N) | ||
|---|---|---|---|---|---|---|---|
| CGI-S first visit | Pearson r | 0.027 | − 0.023 | − 0.450** | 0.006 | 0.164 | 0.323** |
| p value | 0.786 | 0.816 | 0.000 | 0.959 | 0.094 | 0.001 | |
| N | 105 | 105 | 103 | 68 | 105 | 105 | |
| CGI-S 4-week visit | Pearson r | 0.083 | − 0.079 | − 0.362** | − 0.0.076 | 0.193 | 0.332** |
| p value | 0.424 | 0.443 | 0.000 | 0.545 | 0.059 | 0.001 | |
| N | 96 | 96 | 94 | 65 | 96 | 96 | |
| CGI-S 12-week visit | Pearson r | − 0.052 | − 0.091 | − 0.283** | − 0.091 | 0.178 | 0.210* |
| p value | 0.613 | 0.373 | 0.006 | 0.470 | 0.081 | 0.039 | |
| N | 97 | 97 | 95 | 65 | 97 | 97 |
CGI-S, clinical severity and global functioning; IQ, intelligence quotient; N, number; Pearson r, Pearson Correlation coefficient; SES, socio-economic status; Y/N, yes/no variable.
Figure 2 shows the path coefficients for the associations between CGI-S scores at first visit, 4 weeks, and 12 weeks, and the associations between IQ and comorbid ASD and the three CGI-S scores, respectively.
Fig. 2.
Path analysis model showing the effect of adjusting the associations between CGI-S scores over the study period for the significantly associated predictors. Figure 2 shows the path coefficients for the associations between CGI-S scores at first visit, 4 weeks, and 12 weeks, and the associations between IQ and comorbid autism and the three CGI-S scores, respectively.
This model estimates the level of association between CGI-S scores over the study period, adjusting the effect for alternative outcome predictors. The model was saturated (fit indices were therefore not informative) and accounted for the 34.8% of the variance of CGI-S scores at the 12-week visit. After controlling for IQ and comorbid autism, the path model demonstrated a stability effect between CGI-S scores at baseline and 4 weeks (β = 0.604, p < 0.001), and between CGI-S scores at 4 and 12 weeks (β = 0.493, p < 0.001); in contrast, CGI-S scores at baseline were not associated with those at 12 weeks (β = − 0.006, p > 0.05). IQ and comorbid autism were significantly associated with CGI-S scores at baseline (β = − 0.381, p < 0.001 and β = + 0.192, p = 0.036, respectively), but not with those at either 4 or 12 weeks (p > 0.05).
Discussion
The overarching goal of this naturalistic observational study was to describe the clinical experience of a single center (Scientific Institute, IRCCS Eugenio Medea, Bosisio Parini, Italy) in monitoring diagnostic and therapeutic services for young ADHD patients within the network of the Lombardy ADHD registry. In doing so, we explored the demographic and clinical characteristics of the children/adolescents who have accessed our Institute from 2017, were diagnosed with ADHD, and prescribed or not with MPH. Secondly, we followed-up ADHD patients who were taking MPH for 12 weeks, to assess any clinical amelioration, after controlling for other outcome predictors.
With respect to the first aim, approximately one fourth of the children/adolescents received a MPH prescription after being diagnosed with ADHD. This observation is in line with other studies in Italian settings, which indicated that patients who access health facilities for suspected ADHD and need pharmacological treatment can range from 1530 to 37%27. Nonetheless, those frequencies are considerably higher than the comprehensive national average of approximately 1% according to data gathered between 2007 and 201631. This discrepancy could be associated with referral bias, as our Institute is a third-level clinical center specialized in early detection and diagnosis of both ADHD and autism in Lombardy region.
In line with recent literature5,27,32, our subsample who received MPH prescription showed more complex clinical manifestations, in terms of more extensive ADHD difficulties as quantitatively rated by clinicians and parents and of a greater number of neuropsychiatric comorbidities. Furthermore, we extend previous evidence22,25,26,33 indicating that the comorbidity between ADHD and autism presents a more challenging psychopathological picture, with the consequence of an increased likelihood of being prescribed with MPH.
The prescribed individuals were also slightly older and with a lower socioeconomic status than not prescribed children/adolescents. Both national27 and international32 studies found similar differences, indicating more severe symptomatology, lower IQ, and older ages in patients who were prescribed. The findings about lower IQ in prescribed children are also consistent with other existing literature34. Conversely, the higher mean age of prescribed patients could be explained by the fact that most severe individuals enter the pharmacological treatment pathway after other non pharmacological interventions27, or by other biases, such as parental treatment compliance. Lastly, with respect to socioeconomic status’ findings, previous research underlined that lower area-level SES is associated with an increased likelihood of being prescribed ADHD medication35,36, whereas in other cases SES was identified as a significant predictor of incidence of MPH use37. Those evidence are also consistent with the correlation we observed between lower SES and more severe clinical patterns of children with ADHD.
With regard to the second goal, ADHD children/adolescents in our sample displayed statistically significant improvements of their functioning—as rated by clinicians at CGI-S—after 12 weeks of the initiation of MPH use, but not after 4 weeks. Although different in several methodological characteristics, such as the measures used, the informants, and the defined response criteria, the present result is in line with findings from a recent, ecological study by the DANISH INDICES consortium22. Our results are also consistent with other studies from the Lombardy ADHD Registry reporting significant ameliorations in half of the patients followed after 1 year19. Taken together, those findings therefore substantiated the effectiveness of MPH even after 12 weeks of use, apart from the well-known longer positive effect on ADHD functioning.
Furthermore, we explored whether previously known factors, such as gender, age, IQ, socioeconomic level, and the presence of comorbidities, would predict the degree of response to MPH in our sample in combination with the level of functioning at baseline38–40. Our models of predictive variables explained nearly 35% of the variance in the 12-week outcome as estimated by clinicians as CGI-S score. Interestingly, we found that, after having controlled for other predictors’ effect, only the severity at the first visit predicted ADHD severity after 4 weeks of MPH use, which in turn predicted the degree of clinical functioning at the 12-week visit. While not having a significant impact on later CGI-S scores, IQ level and the presence of co-occurring autism predicted the severity of baseline. This latter result is in line with previous evidence reporting an additive effect of symptom severity in children with both ADHD+intellectual disability and ADHD+ASD22,41. With respect to the intervention adherence, 17.14% of participants in our sample either suspended or changed their therapeutic plan according to the nature and the severity of their adverse events. This result is in line with previous literature about adherence rate in naturalistic studies22,25.
As for other ecologically valid studies22, a strength of the present work was the inclusion of a sample of help-seeking children consecutively recruited at a single clinical center. This limited potential concerns about the clinical routine and diagnostic evaluation, which could have a significant impact on treatment outcomes42. This study also showed several limitations. The lack of a control group of ADHD children not taking MPH followed-up for 12 weeks did not allow us to disentangle the effect of the medication from a more general placebo effect. In addition, clinicians who scored the CGI-S throughout the study were also in charge of the participants’ therapeutic plans; thus, we cannot exclude that CGI-S scores could be biased to some extent. Lastly, the present work was a short-term study and its duration may be too short to effectively detect greater changes in clinical functioning and the potential effects of concurring factors. Future data analyses or new research could therefore take into consideration longer-term efficacy data.
In summary, the present study provides further evidence about the short-term effectiveness of pharmacological MPH treatment in naturalistic observational settings. Patients with ADHD in our sample showed improvements in functioning 12 weeks after starting MPH, and the severity of ADHD manifestation after 4 weeks of MPH use was the only significant predictor of the clinical outcome. Altogether, these findings strengthen the importance of carefully monitoring ADHD patients during the first weeks of medication treatment.
Methods
This research is a naturalistic, observational retrospective study. The medical records of patients who accessed the Scientific Institute, IRCCS Eugenio Medea for an in-depth diagnostic examination between January 2017 and May 2023 were identified from the Regional ADHD Registry database and retrieved in June 2023. As a part of the Regional ADHD Registry, the research was approved by the Institutional Review Board of the IRCCS—Istituto di Ricerche Farmacologiche “Mario Negri” (Milan, Italy), and all of the participants’ parents or legal guardians gave informed written consent before participation.
As explained in previous works18,43, the main purpose of the Regional ADHD Registry is to ensure appropriate care and safety of drug use in ADHD children. In our clinical center, as well as in the other 17 centers, an ad hoc, multidisciplinary assessment team was created, including a medical doctor specialized in child neuropsychiatry with experience in ADHD and an experienced child psychologist. This first clinical evaluation consists of an anamnestic and clinical interview, the semi-structured interview “Development and Well-Being Assessment (DAWBA)”44, the neurological examination, the cognitive evaluation, and a multi-informant evaluation of ADHD symptoms through clinician, parental, and teachers reports. In case of a diagnosis of ADHD, the Regional ADHD Registry includes follow-up visits at 3 and 6 months after the diagnosis, and every 6 months afterward, to monitor the clinical outcome of the treatment strategies. Patients prescribed with MPH are additionally monitored at the prescription of pharmacological treatment, after 4 weeks and after 12 weeks, and then over time according to individual therapeutic programs.
In the present study, we focused our analysis on data of patients who were eventually diagnosed with ADHD between January 2017 and May 2023. We first investigated the individual characteristics of these patients to disclose any clinical/functional difference between those prescribed with MPH and those not prescribed, who in turn may have received or no treatment beyond the MPH. Then, we measured the improvements of children/adolescents given MPH on their clinical global impression—severity scale (CGI-S) scores at the 4-week and at 12-week follow-up visits, after controlling for other predictors. In doing so, it is important to note that the number of clinical evaluations throughout the time window of interest was significantly limited by the COVID-19 pandemic and its related public health restrictions.
Dosing of MPH
As part of the standard national procedure for MPH administration, prescribed participants received the first dose of MPH in a controlled clinical setting to monitor for possible adverse events during the first hours after the administration. During this first visit, participants received an initial immediate release (IR) MPH dose based on body weight, on average 0.3–0.6 mg/Kg, either two or three times a day according to participants’ individual needs and guidelines45. The dosage could be increased depending upon the subject’s clinical response and tolerability up to a maximum of 60 mg/day. The total dose could be administered in two or three doses/day. After one month of titration, the IR-MPH was generally replaced with extended release-MPH. The dosing was individually titrated based on weekly assessments of effect and adverse reactions (AR).
Before the single-dose MPH challenge, all the patients who were eligible to receive MPH treatment underwent an electrocardiogram (ECG) and blood exams to exclude any other medical condition associated with ADHD.
Measures
At the first clinical evaluation, the intelligence quotient (IQ) was estimated by the Wechsler Intelligence Scale for Children-III or -IV (WISC-III or -IV)46,47 for all children/adolescents. Clinical and behavioral profiles were assessed through Conners’ Parent Rating Scales-Revised (CPRS-R)48,49. The CPRS-R is one of the most widely used instruments for assessing symptomatology in children with ADHD, offering a parental report for inattention, hyperactivity, and other behavioral domains. In this work we included as dependent variables the 7 factorial-derived subscales—cognitive problems, oppositional, hyperactivity–impulsivity, anxious–shy, perfectionism, social problems, and psychosomatic—and the ADHD index score. Participants’ socioeconomic status has been evaluated according to the Hollingshead four factor index of socioeconomic status (SES)50. The Hollingshead four factor index of SES is a survey designed to measure social status of an individual based on four domains: marital status, retired/employed status, educational attainment, and occupational prestige.
Outcome measure
The degree of the clinical presentation was assessed also by the clinician through the CGI-S51, a one-item measure evaluating the severity of psychopathology from 1 to 7 (1 = not at all ill; 7 = among the most extremely ill patients). According to the comprehensive diagnostic evaluation, we further recorded the prevalence of ADHD subtypes and the co-occurrence of other neurodevelopmental disorders within our sample.
CGI-S scores were also recorded 4 and 12 weeks after the initiation of MPH use to examine the response to the drug administration. Side effects were also strictly monitored by the clinician throughout the follow-up visits.
Data analysis
Statistical analyses were performed using SPSS Statistics Software (Version 21.0, IBM Corporation, Armonk, NY, USA). The alpha level was set to 0.05 for all data analyses.
After splitting the data into two samples (participants who were prescribed with MPH vs participants who were not), chi-square analysis or independent-samples t test were used to compare the two groups on gender distribution, age, IQ, ADHD subtypes, clinical measures, and SES, according to the distributional nature of the data.
Secondly, a repeated-measures analysis of variance (ANOVA), with time as within subject factor (first visit, 4 weeks, 12 weeks), was used to explore changes in CGI-S scores only in participants given MPH. The analysis was further repeated also after having excluded participants who either presented side effects after the MPH assumption or who took supplemental medications during the 12 weeks. The frequency of other treatments (i.e., MPH administration in combination with either parent or child training) were also checked, although the short time window of the study did not permit exploring potentially combined effects.
Last, to test the degree of association with potential outcome predictors, Pearson’s bivariate correlation matrices were computed between CGI-S scores at first visit, 4 weeks, and 12 weeks and gender, age, IQ, and the presence of co-occurring autism or any other neuropsychiatric comorbidity. We then used path analysis as implemented in the MPLUS software package52,53 to test the effect of adjusting the raw associations obtained with the repeated-measures ANOVA for the predictors significantly associated with CGI-S scores through time.
Acknowledgements
Lombardy ADHD Group.
Author contributions
M.M. had the idea for the study. S.T., A.S., A.V., M.N., V.M collected the data. A.C. and E.R. managed and analyzed the data and drafted the initial version. All authors participated in study design, contributed to the interpretation of data, critical review and revision of the report, and approved the final report as submitted. All authors reviewed the manuscript.
Funding
This work was supported by grants from the Italian Ministry of Health (Ricerca Corrente 2024–2025).
Data availability
The dataset analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alessandro Crippa and Eleonora Rosi.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. (American Psychiatric Association, 2022).
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet396, 1204–22. 10.1016/S0140-6736(20)30925-9 (2020). [DOI] [PMC free article] [PubMed]
- 3.Cortese, S. et al. Incidence, prevalence, and global burden of ADHD from 1990 to 2019 across 204 countries: Data, with critical re-analysis, from the Global Burden of Disease study. Mol. Psychiatry28, 4823–4830. 10.1038/s41380-023-02228-3 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Reale, L. et al. Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur Child Adolesc Psychiatry26(12), 1443–1457. 10.1007/s00787-017-1005-z (2017). [DOI] [PubMed] [Google Scholar]
- 5.Gnanavel, S., Sharma, P., Kaushal, P. & Hussain, S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J. Clin. Cases7(17), 2420–2426. 10.12998/wjcc.v7.i17.2420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. https://www.nice.org.uk/guidance/NG87. (2018). [PubMed]
- 7.Faraone, S. V. et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers10, 11. 10.1038/s41572-024-00495-0 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Caye, A., Swanson, J. M., Coghill, D. & Rohde, L. A. Treatment strategies for ADHD: An evidence-based guide to select optimal treatment. Mol. Psychiatry24(3), 390–408. 10.1038/s41380-018-0116-3 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Cortese, S. et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry5(9), 727–738. 10.1016/S2215-0366(18)30269-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintero, J., Gutiérrez-Casares, J. R. & Álamo, C. Molecular characterisation of the mechanism of action of stimulant drugs lisdexamfetamine and methylphenidate on ADHD neurobiology: A review. Neurol. Ther.11, 1489–1517. 10.1007/s40120-022-00392-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serrano-Troncoso, E., Guidi, M. & Alda-Díez, J. Á. Is psychological treatment efficacious for attention deficit hyperactivity disorder (ADHD)? Review of non-pharmacological treatments in children and adolescents with ADHD. Actas Esp Psiquiatr.41(1), 44–51 (2013). [PubMed] [Google Scholar]
- 12.Volkow, N. D., Fowler, J. S., Wang, G. J. & Swanson, J. M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry9(6), 557–569. 10.1038/sj.mp.4001507 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Coghill, D. R. et al. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: Evidence from a systematic review and a meta-analysis. Biol. Psychiatry76(8), 603–615. 10.1016/j.biopsych.2013.10.005 (2014). [DOI] [PubMed] [Google Scholar]
- 14.SINPIA—Italian Society of Neuropsychiatry of Childhood and Adolescence. Linee-guida per la diagnosi e la terapia farmacologica del Disturbo da Deficit Attentivo con Iperattività (ADHD) in età evolutiva. http://www.iss.it/binary/wpop/cont/SINPIA_L.g.ADHD.1116940207.pdf (2002).
- 15.Signorini, G. et al. Architecture and functioning of child and adolescent mental health services: A 28-country survey in Europe. Lancet Psychiatry4(9), 715–724. 10.1016/S2215-0366(17)30127-X (2017). [DOI] [PubMed] [Google Scholar]
- 16.Clavenna, A. & Bonati, M. Pediatric pharmacoepidemiology—safety and effectiveness of medicines for ADHD. Expert Opin Drug Saf.16(12), 1335–1345. 10.1080/14740338.2017.1389894 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bonati, M., Cartabia, M. & Zanetti, M. Waiting times for diagnosis of attention-deficit hyperactivity disorder in children and adolescents referred to Italian ADHD centers must be reduced. BMC Health Serv. Res.19, 1–10. 10.1186/s12913-019-4524-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonati, M. et al. A Regional ADHD center-based network project for the diagnosis and treatment of children and adolescents with ADHD. J. Atten. Disord.22, 1173–1184. 10.1177/1087054715599573 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Bonati, M., Scarpellini, F., Cartabia, M., Zanetti, M., & Lombardy ADHD Group. Ten years (2011–2021) of the Italian Lombardy ADHD register for the diagnosis and treatment of children and adolescents with ADHD. Children. 8(7), 598. 10.3390/children8070598 (2021). [DOI] [PMC free article] [PubMed]
- 20.MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry56(12), 1073–1086. 10.1001/archpsyc.56.12.1073 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Chermá, M. D. et al. Methylphenidate for treating ADHD: A naturalistic clinical study of methylphenidate blood concentrations in children and adults with optimized dosage. Eur. J. Drug Metab. Pharmacokinet.42(2), 295–307. 10.1007/s13318-016-0346-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaalund-Brok, K. et al. Outcomes of a 12-week ecologically valid observational study of first treatment with methylphenidate in a representative clinical sample of drug naïve children with ADHD. PLoS ONE16(10), e0253727. 10.1371/journal.pone.0253727 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura, P. et al. Methylphenidate use for emotional dysregulation in children and adolescents with ADHD and ADHD and ASD: A naturalistic study. J. Clin. Med.11(10), 2922. 10.3390/jcm11102922 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, Y. et al. Efficacy and safety of methylphenidate and atomoxetine in medication-naive children with attention-deficit hyperactivity disorder in a real-world setting. Drugs R&D24(1), 29–39. 10.1007/s40268-023-00445-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houmann, T. B. et al. Early treatment response as predictor of long-term outcome in a clinical cohort of children with ADHD. Eur. Child. Adolesc. Psychiatry33(2), 357–367. 10.1007/s00787-023-02158-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosello, R. et al. Cognitive, social, and behavioral manifestations of the co-occurrence of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review. Autism26(4), 743–760. 10.1177/13623613211065545 (2022). [DOI] [PubMed] [Google Scholar]
- 27.De Rossi, P. et al. Clinical characteristics of children and adolescents with ADHD with or without methylphenidate prescription at their first diagnostic assessment. Eur. Arch. Psychiatry Clin. Neurosci.272(8), 1437–1442. 10.1007/s00406-022-01386-9 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Vanzin, L. et al. The effectiveness of coping power program for ADHD: An observational outcome study. J. Child Fam. Stud.27(11), 3554–3563. 10.1007/s10826-018-1207-0 (2018). [Google Scholar]
- 29.Vanzin, L. et al. Does ACT-group training improve cognitive domain in children with attention deficit hyperactivity disorder? A single-arm, open-label study. Behav. Chang.37(1), 33–44. 10.1017/bec.2020.3 (2020). [Google Scholar]
- 30.Reale, L., Zanetti, M., Cartabia, M., Fortinguerra, F. & Bonati, M. Due anni di attività del Registro ADHD della Regione Lombardia: Analisi dei percorsi di cura diagnostici e terapeutici. Ric&Pra30(5), 198–211. 10.1707/1664.18220 (2014). [Google Scholar]
- 31.Germinario, E.A.P., Arcieri, R., Marzi, M., Panei, P., Vella, S. Registro nazionale ADHD (Attention-Deficit/Hyperactivity Disorder): Dati dal 2007 al 2016. Rapporti ISTISAN 16/37. https://www.iss.it/documents/20126/45616/16_37_web.pdf/c8e4e341-238f-ad08-545b-928b0255aded?t=1581095636154 (Istituto Superiore di Sanità, 2016).
- 32.Man, K. K. C. Long-term safety of methylphenidate in children and adolescents with ADHD: 2-year outcomes of the attention deficit hyperactivity disorder drugs use chronic effects (ADDUCE) study. Lancet Psychiatry10(5), 323–333. 10.1016/S2215-0366(23)00042-1 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Antshel, K. M. & Russo, N. Autism spectrum disorders and ADHD: Overlapping phenomenology, diagnostic issues, and treatment considerations. Curr. Psychiatry Rep.21(5), 34. 10.1007/s11920-019-1020-5 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Fraizer, T. W., Demaree, H. A. & Youngstrom, E. A. Meta-analysis of intellectual and neuropsychological test performance in attention deficit/hyperactivity disorder. Neuropsychology18(3), 543–555. 10.1037/0894-4105.18.3.543 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Efron, D. et al. Prevalence and predictors of medication use in children with attention-deficit/hyperactivity disorder: Evidence from a community-based longitudinal study. J. Child Adolesc. Psychopharmacol.29(1), 50–57. 10.1089/cap.2018.0095 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Sawyer, M. G. et al. The prevalence of stimulant and antidepressant use by Australian children and adolescents with attention-deficit/hyperactivity disorder and major depressive disorder: A national survey. J. Child Adolesc. Psychopharmacol.27, 177–184. 10.1089/cap.2016.0017 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Brownell, M. D., Mayer, T. & Chateau, D. The incidence of methylphenidate use by Canadian children: What is the impact of socioeconomic status and urban or rural residence?. Can. J. Psychiatry51(13), 847–854. 10.1177/070674370605101306 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Cheung, C. H. et al. Childhood predictors of adolescent and young adult outcome in ADHD. J. Psychiatr. Res.62, 92–100. 10.1016/j.jpsychires.2015.01.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowlem, F. D. et al. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry28, 481–489. 10.1007/s00787-018-1211-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Lieshout, M. et al. A 6-year follow-up of a large European cohort of children with attention-deficit/hyperactivity disorder-combined subtype: Outcomes in late adolescence and young adulthood. Eur. Child Adolesc Psychiatry25, 1007–1017. 10.1007/s00787-016-0820-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grazioli, S. Exploring telediagnostic procedures in child neuropsychiatry: Addressing ADHD diagnosis and autism symptoms through supervised machine learning. Eur. Child. Adolesc. Psychiatry33(1), 139–149. 10.1007/s00787-023-02145-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cartabia, M., Finazzi, S. & Bonati, M. Differences between centers in functional outcome of patients with ADHD after 1 year from the time of diagnosis. Sci. Rep.13(1), 18738. 10.1038/s41598-023-45714-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonati, M. & Reale, L. Reducing overdiagnosis and disease mongering in ADHD in Lombardy. BMJ347, f7474. 10.1136/bmj.f7474 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Goodman, R., Ford, T., Richards, H., Gatward, R. & Meltzer, H. The development and wellbeing assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry41, 645–655 (2000). [PubMed] [Google Scholar]
- 45.AIFA-Ministero della Salute. Disturbo da deficit di attenzione con iperattività (ADHD): Le tappe per un uso razionale dei farmaci. Bollettino di Informazione sui Farmaci13, 197–203 (2006). [Google Scholar]
- 46.Wechsler, D. Wechsler Intelligence Scale for Children–III (WISC-III), 3th ed. (Organizzazioni Speciali, 2006).
- 47.Wechsler, D. Wechsler Intelligence Scale for Children–IV (WISC-IV), 4th ed. (Organizzazioni Speciali, 2012).
- 48.Conners, C. K., Sitarenios, G., Parker, J. D. & Epstein, J. N. The revised Conners’ parent rating scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm Child. Psychol.26, 257–268. 10.1023/a:1022602400621 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Nobile, M., Alberti, B., Zuddas, A. CRS-R. Conners’ Rating Scales. Revised. Manuale. (Giunti Editore, 2007).
- 50.Hollingshead, A.B. Four Factor Index of Social Status. (Yale University, 1975).
- 51.Busner, J. & Targum, S. D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry4, 28–37 (2007). [PMC free article] [PubMed] [Google Scholar]
- 52.Fritz, M. S. & Mackinnon, D. P. Required sample size to detect the mediated effect. Psychol Sci.18(3), 233–239. 10.1111/j.1467-9280.2007.01882.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muthén, B., & Muthén, L. Mplus. In Handbook of Item Response Theory. 507–518. (Chapman and Hall/CRC, 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed during the current study are available from the corresponding author upon reasonable request.