Abstract
The enclosure of functional entities within a protective boundary is an essential feature of biological systems. On a molecular scale, free-standing capsules with an internal volume sufficiently large to house molecular species have been synthesized and studied for more than a decade. These capsules have been prepared by either covalent synthesis or self-assembly, and the internal volumes have ranged from 200 to 1,500 Å3. Although biological systems possess a remarkable degree of order within the protective boundaries, to date only steric constraints have been used to order the guests within molecular capsules. In this article we describe the synthesis and characterization of hexameric molecular capsules held together by hydrogen bonding. These capsules possess internal order of the guests brought about by hydrogen bond donors within, but not used by, the framework of the capsule. The basic building blocks of the hexameric capsules are tetrameric macrocycles related to resorcin[4]arenes and pyrogallol[4]arenes. The former contain four 1,3-dihydroxybenzene rings bridged together by -CHR- units, whereas the latter contain four 1,2,3-trihydroxybenzene rings bridged together. We now report the synthesis of related mixed macrocycles, and the main focus is on the macrocycle composed of three 1,2,3-trihydroxybenzene rings and one 1,3-dihydroxybenzene ring bridged together. The mixed macrocycles self-assemble from a mixture of closely related compounds to form the hexameric capsule with internally ordered guests.
A key feature of biological systems is the encapsulation of entities within a structure (1). The cell is a beautifully complex example of this enclosure of chemical space. The term chemical space is used to specify that volume within a molecular capsule that may be used to house a guest. On a somewhat simpler scale, nature also has used proteins and polypeptide chains as the building blocks for assemblies that contain and protect guests (2). In well-studied systems the guests may be the nucleic acid of viruses in the rhinovirus (3), poliovirus (4), or cowpea chlorotic mottle virus (5), or the iron-containing core in the iron storage protein ferritin (6). Until now, these biological capsules have been beyond the synthetic grasp of the chemist owing to the vast size and complexity of the enclosure. A further complication that has not often been addressed in the chemical literature is the very high level of organization found on the interior of enclosures of biological importance.
The encapsulation of chemical space on the scale of simple molecules has been a topic of considerable interest for more than a decade. For the formation of capsules, two strategies have emerged: covalent synthesis and self-assembly. Cram et al. (7), Gabard and Collet (8), and Chapman and Sherman (9) have synthesized carcerands capable of encapsulating up to three small molecular guests (10). Rebek and colleagues (11–14) have pioneered the use of self-assembly to produce a variety of entities seamed together by hydrogen bonds. Multicomponent systems also have been assembled by means of transition metal-based coordinate bonds (15–20). The synthesis of hosts capable of housing a wide variety of guests has further been achieved by the use of the principles of crystal engineering (21, 22). Based on this wide variety of work focused on the enclosure of chemical space, we recently put forward a set of general principles for the design of discrete spherical molecular host capsules (23–25).
Once the enclosure of space has been accomplished, the organization of the guests contained within becomes a key issue. Rebek and colleagues (26) have used steric constraints to organize two guests within a tubular dimer, but for those assemblies with large enclosed volumes, both discrete and infinite, the guests are most often disordered (23, 27–29). An example of significant order within an enclosure is the ionic core consisting of 30 water molecules and two sodium ions in the (p-sulfonatocalix[4]arene)12 assembly (30). We report herein an example of a molecular capsule in which the guests are ordered by hydrogen bond donors within the framework of the enclosure.
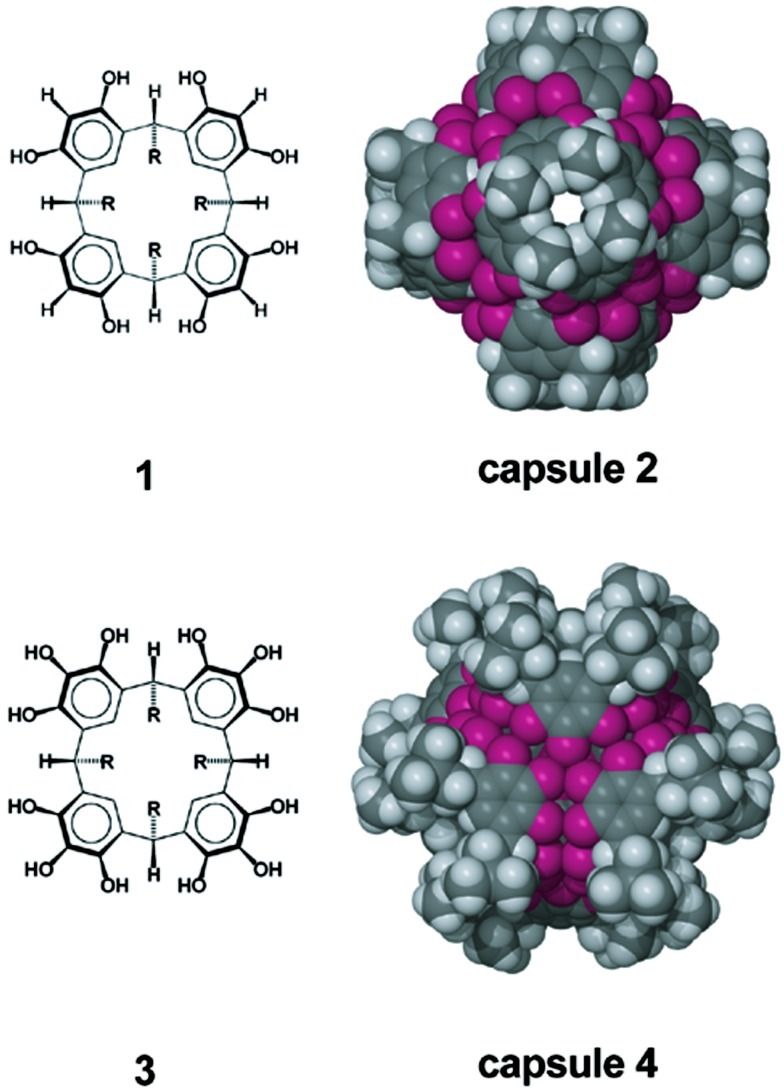
We have previously reported that the macrocycle C-methylresorcin[4]arene, 1 (R = methyl), may be used as a building block, which, along with water, self-assembles to form the capsule [(C-methylresorcin[4]arene)6(H2O)8], 2 (Scheme S1). Capsule 2 possesses an excess of four hydrogen bond donors, but these donors are positioned such that they project outward from the surface of the enclosure (23). They are, therefore, incapable of effecting organization of the guests within the capsule.
Scheme 1.
The macrocycle C-isobutylpyrogallol[4]arene, 3 (R = isobutyl), self-assembles to form a hexameric capsule [(C-isobutylpyrogallol[4]arene)6], 4 (Scheme S1). In capsule 4, all 72 of the hydrogen bond donors, 12 from each macrocycle, are used in completing the hydrogen bond arrangement that forms the capsule. Therefore, the guests within are not ordered (27–29). It should be noted that hexamer 4 is held together by 48 intermolecular hydrogen bonds (resulting in eight hydrogen bonds per monomer), and the capsule is stable even in polar media (28). A macrocyclic building block was sought that would self-assemble into a capsule possessing a sufficient degree of hydrogen bonding to afford solution stability, at least in nonpolar media, but without the rather perfect match of hydrogen bond donors and acceptors found in 4.
Methods
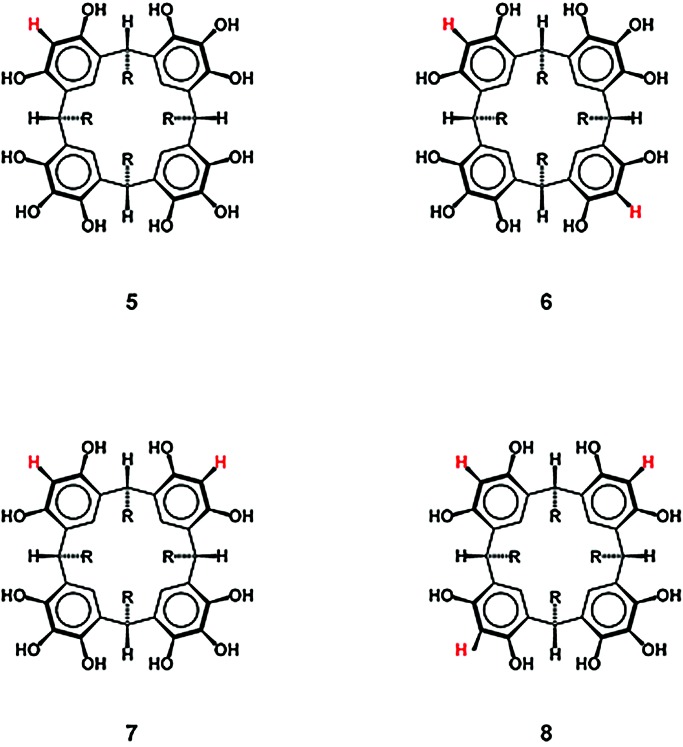
Pyrogallol[4]arenes, 3, are prepared by the acid-catalyzed condensation of aldehydes with pyrogallol in 95% ethanol at room temperature over a period of minutes to hours, depending on the aldehyde. Similarly, resorcin[4]arenes, 1, are prepared by the acid-catalyzed condensation of aldehydes with resorcinol in 95% ethanol at reflux over a period of hours to days, depending on the aldehyde. Pyrogallol is therefore seen to be considerably more reactive than is resorcinol under the same condensation conditions. It seemed reasonable to expect that conditions could be found in which pyrogallol and resorcinol could be joined to yield mixed macrocycles consisting of pyrogallol and resorcinol units as in 5–8 (Scheme S2). For mixed macrocycle 5, for example, we could not model a capsule in which all hydrogen bond donors and acceptors were paired as in the pyrogallol[4]arene hexamers. Indeed, we expected that 5 might form a capsule with interesting properties, perhaps even the desired property of an excess of hydrogen bond donors, together with an orientation of some of these hydrogen donors into the capsule.
Scheme 2.
A facile synthesis of macrocycle 5 has now been discovered. Macrocycle 5 was synthesized, along with other related macrocycles, from the acid-catalyzed condensation of equimolar amounts of resorcinol and pyrogallol with isovaleraldehyde. In a typical experiment, 0.02 mol (2.2 g) resorcinol and 0.02 mol (2.5 g) pyrogallol were dissolved at room temperature in 30 ml of 95% ethanol at room temperature. The catalyst, 6 ml of HClconc, was then added. A quantity of 0.04 mol (4.3 ml) isovaleraldehyde was added dropwise with stirring. The reaction mixture was stirred at room temperature for 48 h, at which time the solution was filtered and the colorless precipitate was collected. Macrocylce 5 was purified by recrystallization from diethyl ether.
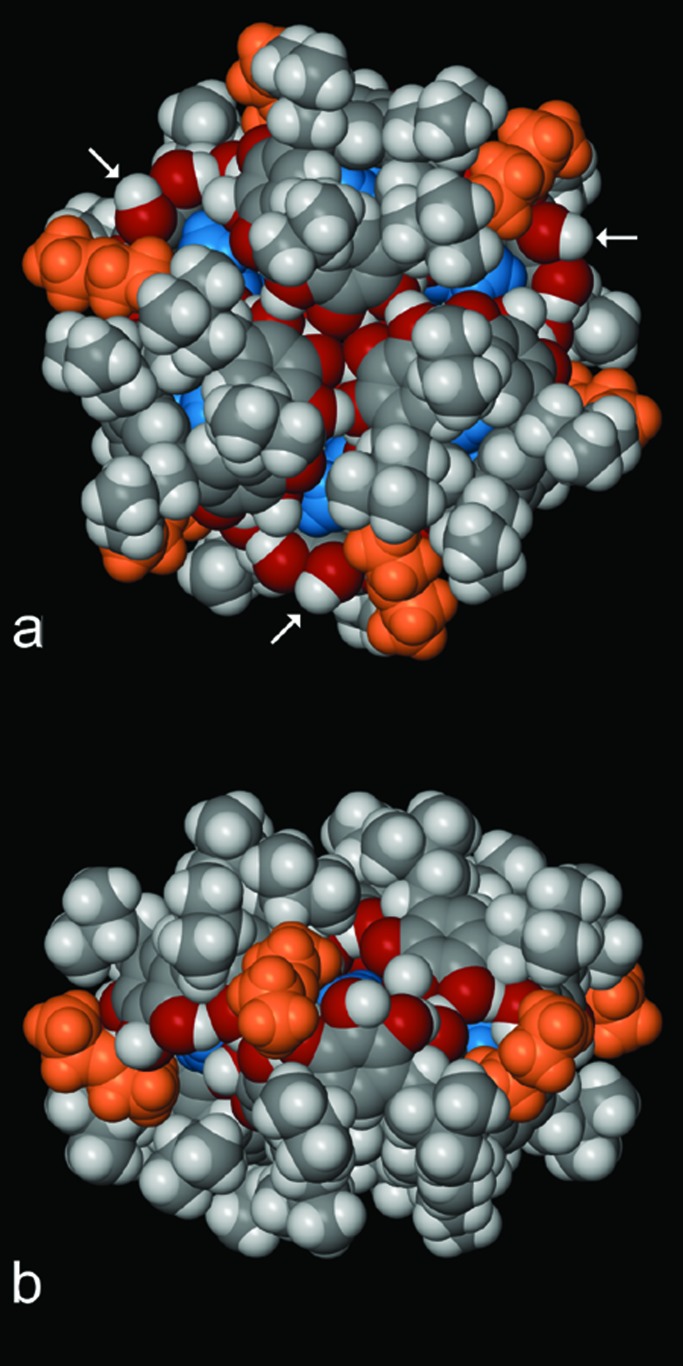
Recrystallization from diethyl ether afforded the remarkable structure shown in Fig. 1. The structure of hexameric assembly 9 was determined by x-ray crystallographic methods. Hexamer 9 crystallizes in the monoclinic, space group R3bar, unit cell parameters a = b = 37.815(4), c = 19.988(3) Å, cell volume V = 24,752 Å3, formula units per cell Z = 18, calculated density ρcalcd = 1.128 g⋅cm−3, reliability index (for data collected at 173 K) R = 0.124 [intensity Inet > 2.0 σ(Ι)]; standard errors in the last decimal place are given in parentheses. Details of the x-ray structure determination are available from the Cambridge Crystallographic Data Centre (CCDC) (deposition number CCDC 178706).
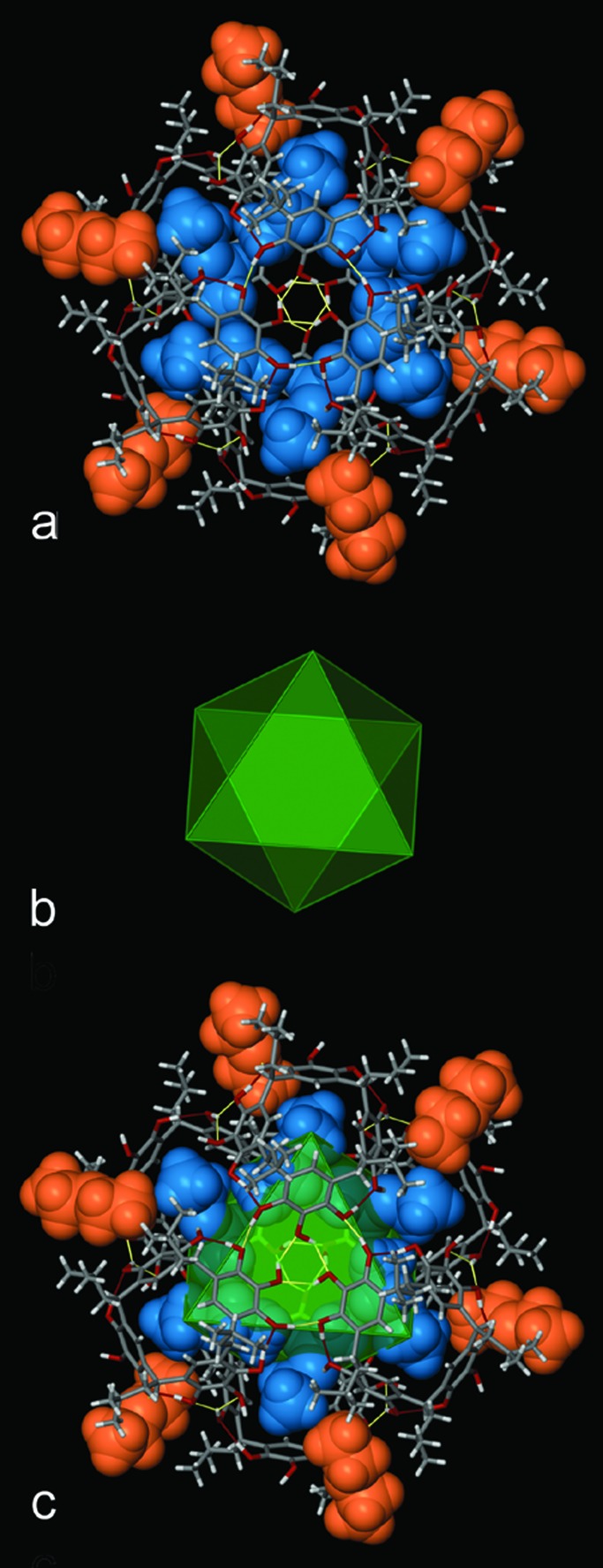
Figure 1.
(a) Space-filling representation of hexamer 9, of mixed macrocycle 5, viewed along the 3bar axis of the capsule. The six diethyl ether molecules bound to the hydrogen bond donors oriented toward the interior of the capsule are shown in blue; the six diethyl ether molecules bound to hydrogen bond donors oriented toward the exterior of the capsule are shown in orange. Host oxygen atoms are given in red. (b) Capsule 9 viewed perpendicular to the 3bar axis. Three of the six hydrogen bond donors that are not involved in the O—H⋅⋅⋅O hydrogen bonding scheme are indicated by the white arrows. The remaining three are rotated by 60o and hidden from view on the bottom half of a.
Results and Discussion
This hexameric assembly, 9, with six hydrogen bond donors positioned toward the interior of the capsule, possesses an internal volume of 860 Å3. The six diethyl ether molecules on the inside of the capsule are thus ordered by these six hydrogen bond donors. There are an additional six hydrogen bond donors oriented toward the outside, and these donors bind six additional diethyl ether molecules on the outside of the hexamer, further sealing the capsule (Fig. 1).
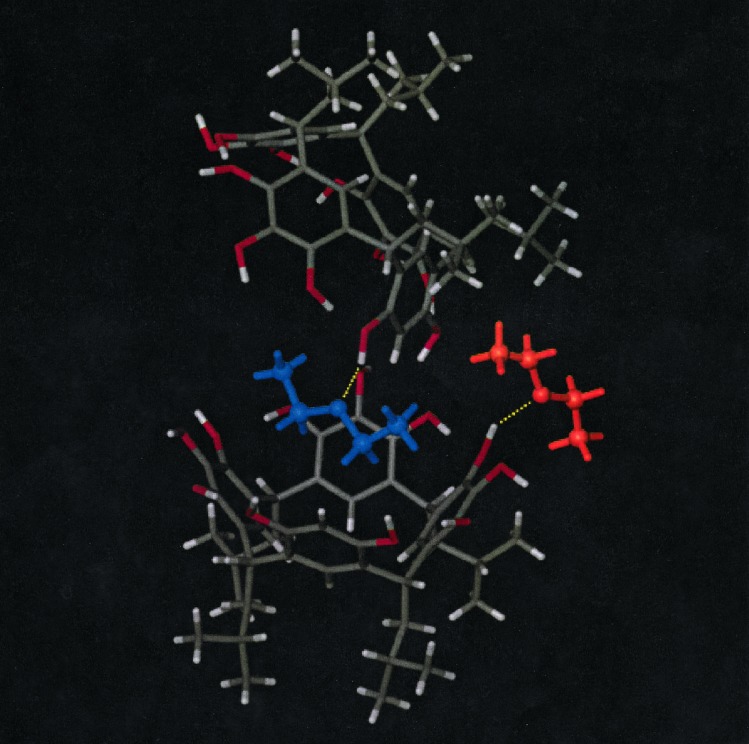
To understand the complicated hydrogen bonding pattern, the fulfillment of which leads to the hexamer of 5, it is useful to account for the hydrogen bond donors and acceptors compared with those of the hexamer of 3, capsule 4. In capsule 4, each macrocycle is rimmed with 12 hydroxyl groups, thus providing 12 potential hydrogen bond donors. As we have noted, in the hexamer 48 of the 72 potential hydrogen bond donors are used in intermolecular hydrogen bonds to seam the capsule together. The remaining 24 hydrogen bond donors are used in intramolecular hydrogen bonds between adjacent rings in the macrocyclic building block 3. In the capsule of mixed macrocycle 5, each mixed macrocycle is rimmed with 11 hydroxyl groups, providing 66 potential hydrogen bond donors in the hexamer. A detailed study of Figs. 1 and 2 shows that 24 hydrogen bond donors are used in intermolecular hydrogen bonds and 24 are used in intramolecular hydrogen bonds (as in capsule 4). Of the 66–48 = 18 remaining hydrogen bond donors, six are oriented toward the inside and six toward the outside, bonding the diethyl ether molecules. Fig. 2 more clearly illustrates the hydrogen bond donors used to bind the internal diethyl ether molecules (blue) and the external diethyl ether molecules (orange). The internal diethyl ether molecules are bound by a hydrogen bond to one of the resorcinol ring OH groups, whereas the external diethyl ether molecules are bound by a hydrogen bond to one of the 1-OH groups of a pyrogallol ring. As might be expected, the thermal motion of both the external hydrogen bond donor oxygen atoms and the external diethyl ether molecules is higher than that of the internal ones. The thermal parameters for the external diethyl ether carbon and oxygen atoms are 50% higher than those of the internal diethyl ether carbon and oxygen atoms.
Figure 2.
View of two mixed macrocycles, 5, displaying the manner in which the diethyl ether molecules are bound on the inside (blue) and on the outside (orange) of the capsule. The O—H⋅⋅⋅O hydrogen bond distance for the diethyl ether molecules bound on the inside of capsule 9 is 2.69 Å, while that for those bound to the outside is 2.68 Å.
The accounting of the hydrogen bond donors discussed above may be completed by noting that the hexamer in Fig. 1 has 24 intermolecular hydrogen bonds, 24 intramolecular hydrogen bonds, and 12 hydrogen bonds to diethyl ether molecules for a total of 60 hydrogen bonds. The remaining six hydrogen bond donors, which are not involved in the O—H⋅⋅⋅O hydrogen bonding scheme, are located external to the capsule, as may be seen in Fig. 1.
It is interesting to note that capsule 9 assumes the shape of a trigonal antiprism with the centers of 5 at its corners (24). Fig. 3 emphasizes this geometrical similarity. Fig. 3a presents a view of capsule 9 with the framework in stick bond representation and the diethyl ether molecules as space-filling models. The orientation of the capsule is identical to that of Fig. 1a. Fig. 3b displays the trigonal antiprism constructed from the centroids of the centers of the rings of mixed macrocycle 5. The superposition of capsule 9 and the trigonal antiprism is shown in Fig. 3c. It has been common practice for decades to refer to inorganic complexes in geometrical terms, i.e., octahedral, trigonal bipyramidal, trigonal antiprismatic. It is also instructive to understand the rather complicated structures of large supramolecular assemblies in terms of their solid geometric shapes. Indeed, capsule 2 has the shape of a snub cube and capsule 4, a small rhombicuboctahedron. As Fig. 3 shows, hexamer 9 has the shape of a trigonal antiprism.
Figure 3.
(a) Capsule 9 shown in stick bond representation with the diethyl ether guests given in space-filling representation. The orientation of the capsule is identical to that given in Fig. 1a. (b) The trigonal antiprism that results from connection of the centroids of the centers of the aromatic rings of macrocycle 5. The top (and bottom) equilateral triangular faces possess edge lengths of 12.43 Å, while the edge lengths of the isosceles side triangles is 9.51 Å. (c) Superposition of the trigonal antiprism and capsule.
In addition to the main theme of this contribution, the internal order of guests exhibited by capsule 9, mixed macrocycle 5 possesses remarkable molecular recognition properties. In the initial synthesis of 5, electrospray mass spectrometry revealed that the first precipitate contains at least 10 different compounds with the macrocycles in approximately the following percentages: 1 (3%), 3 (5%), 5 (30%), 6 and 7 (33%) (6 and 7 are isomers and together appear at 33%), 8 (17%), 10 (5%), 11 (3%), 12 (3%), and 13 (1%). Compounds 1, 3, 5, 6, 7, and 8 are macrocycles as previously defined. Compounds 10–13 are noncyclized pyrogallol and/or resorcinol-containing products.
Conventional wisdom would hold that the probability of isolating a pure compound by crystallization from such a closely related mixture is near zero. Yet, we have repeatedly crystallized pure 5 as the hexamer from syntheses involving different aldehydes. This finding implies that macrocycle 5 is programmed with enough information to allow it to assemble the pure hexamer in solution, and then for the hexamers to come together in the crystallization process. This process of molecular recognition exhibited by macrocycle 5 in solution must be a dynamic one. Many low energy paths for macrocycles 1, 3, 6, 7, and 8 to bind to the upper rim of macrocycle 5 may be envisioned. Mistakes in recognition are surely made in solution, but, as discovered by the x-ray structural studies, these mistakes are corrected and hexamers of 5 crystallize.
The enclosure of chemical space can be effected by using existing supramolecular strategies. In this article we have shown that internal order can be enforced by hydrogen bonding between the framework and the guests. It is now possible to envision molecular capsules as nano-scale “reaction vessels” in which extraordinary control over the reactants can be obtained.
Acknowledgments
We are grateful for funding from the National Science Foundation.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Avers C J. Molecular Cell Biology. Reading: Addison–Wesley; 1986. pp. 768–770. [Google Scholar]
- 2.Caspar D, Klug A. Cold Spring Harbor Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- 3.Branden C, Tooze J. Introduction to Protein Structure. New York: Garland; 1991. pp. 161–177. [Google Scholar]
- 4.Hogle J M, Chow M, Filman D J. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 5.Douglas T, Young M. Nature (London) 1998;191:152–155. [Google Scholar]
- 6.Douglas T. In: Biomimetic Approaches to Materials Science. Mann S, editor. New York: VCH; 1996. pp. 91–115. [Google Scholar]
- 7.Cram D J, Karbach S, Kim Y H, Baczynskyj L, Kalleymeyn G W. J Am Chem Soc. 1985;107:2575–2576. [Google Scholar]
- 8. Gabard, J. & Collet, A. (1981) Chem. Commun., 1137–1138.
- 9.Chapman R G, Sherman J C. J Am Chem Soc. 1995;117:9081–9082. [Google Scholar]
- 10.Chopra N, Sherman J C. Angew Chem Int Ed Engl. 1999;38:1955–1957. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1955::AID-ANIE1955>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Wyler R, de Mendoza J, Rebek J., Jr Angew Chem Int Ed Engl. 1993;32:1699–1701. [Google Scholar]
- 12.Conn M M, Rebek J., Jr Chem Rev. 1997;97:1647–1668. doi: 10.1021/cr9603800. [DOI] [PubMed] [Google Scholar]
- 13. Rebek, J., Jr. (2000) Chem. Commun., 637–643.
- 14. Shivanyuk, A. & Rebek, J., Jr. (2001) Chem. Commun., 2374–2375. [DOI] [PubMed]
- 15.Fujita M, Kwon Y J, Washirzu S, Ogura K. J Am Chem Soc. 1994;116:1151–1152. [Google Scholar]
- 16.Takeda N, Umemoto K, Yamaguchi K, Fujita M. Nature (London) 1999;398:794–796. [Google Scholar]
- 17.Olenyuk B, Whiteford J A, Fechtenkotter A, Stang P J. Nature (London) 1999;398:796–799. doi: 10.1038/19740. [DOI] [PubMed] [Google Scholar]
- 18.Fox O D, Dalley N K, Harrison R G. J Am Chem Soc. 1998;120:7111–7112. [Google Scholar]
- 19. Hardie, M. J. & Raston, C. L. (2000) Dalton Trans., 2483–2492.
- 20.Atwood J L, Barbour L J, Hardie M J, Raston C L. Coord Chem Rev. 2001;222:3–32. [Google Scholar]
- 21.Biradha K, Seward C, Zaworotko M J. Angew Chem Int Ed Engl. 1999;38:492–495. doi: 10.1002/(SICI)1521-3773(19990215)38:4<492::AID-ANIE492>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22. Rose, K. N., Barbour, L. J., Orr, G. W. & Atwood, J. L. (1998) Chem. Commun., 407–408.
- 23.MacGillivray L R, Atwood J L. Nature (London) 1997;389:469–472. [Google Scholar]
- 24.MacGillivray L R, Atwood J L. Angew Chem Int Ed Engl. 1999;38:1019–1033. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1018::AID-ANIE1018>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.MacGillivray L R, Atwood J L. In: Advances in Supramolecular Chemistry. Gokel G W, editor. Stamford, CT: JAI; 2000. pp. 157–183. [Google Scholar]
- 26.Heinz T, Rudkevich D M, Rebek J., Jr Nature (London) 1998;394:764–766. [Google Scholar]
- 27. Gerkensmeier, T., Iwanek, W., Agena, C., Frohlich, R., Kotila, S., Nather, C. & Mattay, J. (1999) Eur. J. Org. Chem., 2257–2262.
- 28. Atwood, J. L., Barbour, L. J. & Jerga, A. (2001) Chem. Commun., 2376–2377. [DOI] [PubMed]
- 29. Atwood, J. L., Barbour, L. J. & Jerga, A. (2002) J. Supramol. Chem., in press.
- 30.Orr G W, Barbour L J, Atwood J L. Science. 1999;285:1049–1052. doi: 10.1126/science.285.5430.1049. [DOI] [PubMed] [Google Scholar]