Abstract
Background
Psychosis occurs in a wide spectrum of mental and somatic disorders, with autoimmune processes being a potentially underdiagnosed cause. Clinical warning-signs can help identifying autoimmune encephalitis (AIE) or psychosis (AIP). Here we evaluated warning-signs and biomarkers in patients experiencing acute psychotic episodes who were admitted to inner-city sectorized care with a focus on identifying autoimmune causes of psychosis.
Methods
We analyzed data obtained from routine clinical care, including blood, urine, CSF, EEG, and MRI when available. CSF-analysis included screening for antineuronal autoantibodies using commercial antibody screening (CAS) and indirect immunofluorescence (IFT). Origin of psychosis was defined according to patients’ discharge diagnosis (ICD-10 criteria).
Results
Within 39 months, 352 participants were included, 114 of them experienced their first episode of psychosis (FEP). In 139 patients, psychotic symptoms were attributed to exogenous origin (F0: N = 90; F1: N = 48), the others were diagnosed with categories F2, F3 and F4. Among the 139 patients, 3 patients had pleocytosis or other CSF abnormalities. CAS was positive in two patients in CSF, leading to a confirmed diagnose of AIP in only one case while evaluated as unspecific in the other. IFT determined the prevalence of IgG-autoantibodies in CSF in four patients, who had FEP. Symptoms improved following immunotherapy in three of the four diagnosed patients.
Conclusion
CSF analysis suggested four cases with AIP, with only one detected through commercial assays. Despite the rather low prevalence of AIP in this community sample, the availability of specific treatment options underscores the importance of further research regarding in-depth diagnostic evaluation for autoimmune processes in patients with acute psychosis.
1. Background
Psychotic symptoms may meet the diagnostic criteria for schizophrenia or other non-affective psychoses, but can also occur in conditions auch as organic hallucinosis or delusional disorders (Marneros, 1988; World Health, 1992). Psychotic symptoms are characterized by hallucinations, self-disorders, and delusions, and may be accompanied by changes in affect, motivation, and higher cognitive functions (International Classification of Diseases and Eleventh Revision (ICD-11), World Health Organization (WHO) 019/2021; World Health, 1992; International Classification of Diseases and Eleventh Revision (ICD-11), World Health Organization (WHO) 019/2021; McCleery and Nuechterlein, 2019). Psychotic disorders such as schizophrenia may be associated with a significant reduction in life expectancy and potentially severe limitations in quality of life (Charlson et al., 2018; Marneros, 2003). They typically begin in early adulthood, with a pooled incidence of approximately 26.6 cases per 100.000 person-years (Jongsma et al., 2019); the worldwide incidence of schizophrenia was reported to be 11–20 per 100.000 individuals worldwide (Saha et al., 2006). The etiology of schizophrenia is not yet fully understood, and there is a long-standing tradition of discussing the origins and progression of psychotic symptoms (Bleuler, 1911; Kraepelin, 1896; Marneros, 2006). Within a multifactorial model of pathogenesis, genetic, neurodevelopmental, and environmental factors are implicated (Birnbaum and Weinberger, 2024) and can include autoimmune processes (Pollak et al., 2020a; Endres et al., 2020a; Najjar et al., 2018a). Comprehensive diagnostic evaluation is essential in first-episode psychosis (FEP), and a diagnosis of schizophreniform psychosis should only be made after organic causes have been thoroughly excluded (Marneros, 1988; World Health, 1992; Association, 2013). Psychotic symptoms may occur in multiple disorders such as non-affective disorders and affective disorders.
Recent discussions have highlighted that diagnostic criteria are particularly important in distiguishing autoimmune processes, which can clinically mimic acute schizophrenia or acute and transient psychotic disorders (Heinz et al., 2016; Pollak et al., 2017). Autoimmune processes can manifest as autoimmune encephalitis (AIE) with psychotic symptoms, potentially accompanied by various neurological symptoms, as well as isolated autoimmune psychosis (AIP) that clinically resembles the schizophrenia spectrum (Pollak et al., 2020b), especially in drug resistant courses (Najjar et al., 2018a). Accordingly, there could be a relevant group of patients clinically diagnosed with acute psychotic episodes, in whom autoimmune-mediated processes might be associated with clinical symptoms responsive to immunotherapy (Pollak et al., 2017; Ellul et al., 2017; Al-Diwani et al., 2017; Pavăl et al., 2024). To reliably identify and treat this sub-group, clinical guidelines recommend using possible and probable warning signs (referred to as yellow and red flags) including psychopathological symptoms and biomarkers, especially in the routine assessment of individuals with FEP (see Fig. 1). Yellow flags have a low pre-test probability, while red flags have a high probability for AIE (Pollak et al., 2020b; Najjar et al., 2018b; Herken and Pruss, 2017). Even though AIP may be rare (Endres et al., 2015), a systematic evaluation in this regard is important to prevent misdiagnosis potentially leading to chronic courses with long-term use of neuroleptic medication and its respective side effects (Murray et al., 2016; Ho et al., 2011).
Fig. 1.
Clinical criteria for AIE and AIP.
To estimate the frequency of AIP/AIE in a community sample, we included all patients with acute psychotic symptoms who were admitted to one of our two hospitals responsible for the catchment area during the recruitment period. We thus included patients regardless of age or potential organic causes for their illness. Accordingly, following inpatient diagnostic procedures and treatment, patients were discharged with diagnoses spanning the F0 - F4 chapters of ICD-10. ICD-10-category F0 includes patients with organic, including somatic, mental disorders, F1 includes mental, behavioral disorders due to psychoactive substance use, F2 schizophrenia, schizotypical, delusional and other non-mood psychotic disorders, F3 affective and F4 anxiety, dissociative, stress-related, somatoform disorders (World Health, 1992)).
Diagnostic procedures followed a standardized approach including in depth psychopathological assessment (Stieglitz et al., 2017), EEG, and structural MRI, commercial antibody screening for serum and cerebrospinal fluid samples (CSF) as well as indirect immunofluorescence in murine brain tissue (Kreye et al., 2020), see methods section. In this study, we present data of the Berlin-Psychosis-Register (REPOSE), which includes 352 patients admitted with acute psychotic disorders, 139 of which were cateroisedas exogenous origin. We report clinical warning signs and biomarkers in all 139 patients with clinically diagnosed exogeneous psychosis (ExP; ICD-10 F0 and F1 diagnoses) and describe four clinical cases with AIP who received immunotherapy.
2. Materials & methods
2.1. Berlin REPOSE register study
The Berlin REPOSE-register (registered 2020; DRKS-ID DRKS00023222, ethics approval EA1/0170/20) is a project of NEUROCURE, a Cluster of Excellence at the Charité – Universitätsmedizin Berlin, and builds on a cooperation between the Department of Psychiatry and Neurosciences and the Department of Neurology and Experimental Neurology. Recruitment for REPOSE took place in two hospitals serving the inner-city caresector of Berlin (Departments of Psychiatry & Neurosciences and Neurology & Experimental Neurology, both Charité Campus Mitte, as well as Department of Psychiatry and Psychotherapy, St. Hedwig's Hospital, Berlin, Germany). The study was approved by the local ethics committee, and procedures were implemented as part of standard clinical care for persons with acute psychotic disorders. Patients were informed and included in the study after regaining the ability to consent. Only after this point were clinical data, collected as part of routine diagnostics, evaluated.
We examined a naturalistic setting in sectorized care, where every person living in Berlin's central inner-city district has the right to be treated in one of the two hospitals listed above. We aimed at including all inpatients with psychotic episodes in the two departments as well as intermediate care unit within the period September 2020 and December 2023. All clinical ratings and examinations of diagnostic criteria according to ICD-10 were independently carried out by two experienced psychiatrists (MB and FM), and supervised by a board-qualified psychiatrist (AH). Our assessment included a detailed exploration based on a standard rating of psychopathology (Broome et al., 2018).
In addition to a detailed medical history, basic blood and urine tests as well as cranial computer tomography (CCT), magnetic resonance tomography (MRI) and EEG data were acquired whenever possible. Whenever patients agreed, a lumbar puncture was performed, which routinely included extended screening for antineuronal autoantibodies (CSF commercial antibody screening-panel (CAS): aquaporin-4, contactin-associated protein-2 (CASPR2), Hu, Ri, Yo, Delta/Notch-like Epidermal Growth Factor-Released-receptores (Tr/DNER), myelin, paraneoplastic antigen 2 (Ma2/Ta), glutamic acid decarboxylase 65 (GAD65), collapsin response mediator protein5 (CV2/CRMP5), amphiphysin, α-amino-3-hydroxy-5-hydroxy-5-methyl-4-isoxazolproprionsäure-receptor (AMPAR 1/2), γ-aminobutyric-acid A + B (GABA-A and B receptor), late gestation lung protein 1(LGI1), Dipeptidy-petidase-Protein (DPPX), dopamine-2-Ak, glycine (GlyR), N-methyl-D-aspartic acid (NMDA) and metabotropic glutamate receptor (mGluR5); see glossary/supplement for further explanation. Every CSF-sample was also tested by indirect immunofluorescence (IFT). Autoimmune markers were also routinely tested in serum (CASPR2,DPPX,GABA-B; AMPA1/2, LGl1, NMDA; see glossary), even in the absence of signs of inflammation.
We compared patients with so called exogenous (ExP) vs. endogenous psychosis (EnP), operationalized as diagnosis at discharge (after full clinical and laboratory examination) belonging to ICD-10-categories F0 and F1 (exogenous psychosis) vs. F2, F3 and F4 (endogenous psychosis). We used the chi-square test to assess significant differences in pathological MRI findings, CSF and blood markers as well as cognitive impairments.
2.2. CSF analysis – commercial ELISAs and mouse brain reactivity screening (IFT)
A total of 47 CSF samples of the subgroup of patients with EP were screened for autoantibodies using CAS including tissue immunofluorescence assay, line blots and enzyme linked immunosorbent assays (ELISAs) of EUROIMMUN test kits by the certified laboratory “Labor Berlin” (Berlin, Germany) (Berlin). For example, NMDAR-abs, provided by EUROIMMUN, test CSF with a sensitivity and specificity up to 100 % (see glossary for additional information).
As a second and highly sensitive screening, IFT was conducted to determine the prevalence of IgG-autoantibodies to mouse brain sections. CSF was screened in the Department of Experimental Neurology (Prof. Prüss, Charité & DZNE, Berlin, Germany), on 20 μm cryostat-cut unfixed sagittal mouse brain sections (C57BL/6 mice), using an in-house diagnostic assay with unfixed murine brain sections. In order to avoid a loss of certain antigenic epitopes, this method works without methanol, PFA (paraformadehyd) or acetone. (Kreye et al., 2020; Endres et al., 2020b). Tissue reactivity was examined by two independent researchers regarding intensity and anatomical pattern of binding. Only strongly positive (+++/++++) and reproducible IgG antibody binding patterns were included in the analysis. Samples counted as technically positive were discussed and evaluated within the clinical symptom complex by an interdisciplinary board. According to our experience and a current literature research, there are no indications that psychiatric medication influences the results of the IFT.
2.3. Statistical analyses
All statistical analyses were conducted using SPSS, version 29.00. We compared patients with ExP and EnP, using the chi-square test to assess significant differences in pathological MRI findings, CSF and blood markers as well as cognitive impairments (see Table 1a).
Table 1a.
Sociodemographic and paraclinical data of the REPOSE cohort.
| N | Mean age (SD) |
Number of PE (%) |
Duration of PE until admission |
CSF |
Counts of pathological CSF and blood tests |
Brain MRI |
EEG |
ICD Diagnose groups |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | >2 | mis-sing | <24 | 2-7d | 7-30d | >30d | un-known | Avail-able | CSF Cell-count |
OCB in CSF |
Hb | CRP | Hypo-natre-mia | con-ducted | patho-logical finding | con-duc-ted | patholo-gical | F0 | F1 | F2 | F3 | F4∗ | |||
| Wo-man | 131 | 49,04 (19,01) |
47 36 % |
17 13 % |
60 46 % |
7 5 % |
4 3.1 % |
24 18 % |
28 21 % |
61 47 % |
14 11 % |
49 37 % |
4 3.1 % |
3 2.3 % |
31 24 % |
16 36 % |
11 8.5 % |
77 59 % |
25 32 % |
31 45 % |
5 19 % |
35 27 % |
11 8 % |
73 56 % |
12 9 % |
1 0,8 % |
| Men | 221 | 46,03 (17,47) | 67 31 % |
26 12 % |
111 55 % |
17 7 % |
10 4.5 % |
45 21 % |
44 22 % |
88 40 % |
34 15 % |
52 24 % |
6 2.7 % |
7 3.2 % |
58 27 % |
30 32 % |
8 3.6 % |
104 47 % |
31 29 % |
59 36 % |
14 27 % |
56 26 % |
37 17 % |
104 47 % |
23 11 % |
0 |
| total | 352 | 47,15 (18,0) | 114 32 % |
43 12 % |
171 49 % |
24 7 % |
14 4 % |
69 20 % |
72 21 % |
149 42 % |
48 14 % |
101 29 % |
10 2.8 % |
10 2.8 % |
89 26 % |
46 34 % |
19 5.4 % |
181 52 % |
56 31 % |
91 39 % |
19 24 % |
91 26 % |
48 14 % |
177 50 % |
35 10 % |
1 0,8 % |
PE = psychotic episode. tested basic blood parameters with regards to warning signs for AIE/AIP: hemoglobin (Hb), C-reactive protein (CRP), sodium. OCB: olicoclonal bands. N(%). Chi-square-test: number of PE; duration of PE until admission, CSF and MRI:p = 0,56. F4 - one patient was diagnosed with dissociative disorder.
3. Results
During the observation period, we registered an incidence of FEP of 9 per 100.000 inhabitants per year, within a total population of 380.917 individuals registered in the Berlin Mitte-district, who have the right to be treated in our hosipitals in case of need (sectorized care) (Bibliothek, 2020). These findings are within the lower spectrum of worldwide incidence and suggest that we were able to recruit a representative cohort within our catchment area (sector).
Altogether, 352 participants were included, 221 men and 131 women (see Table 1a). The majority of psychoses were of endogenous origin (ICD-10-categories F2: N = 177, F3: N = 35, F4: N = 1). In 139 patients, psychotic symptoms were attributed to ExP detected during treatment in our university hospital, resulting in ICD-10 F0 (n = 91) and F1 (n = 48) diagnoses at discharge. There were no significant differences between these two groups with respect to age and gender.
Warning signs of AIE or AIP were systematically assessed in all patients and included pathological changes in blood parameters, specifically hyponatremia (ExP: N = 10 vs. EnP: N = 9), changes in hemoglobin (ExP N = 49; EnP N = 40) as well as signs of inflammation with elevated CRP (ExP N = 46; EnP N = 0) (see Table 1a, Table 1ba and b). Clinial warnings signs, especially neurological deficits found in our sample included epileptic seizures, headache, movement and autonomic disorders (see Table 2a, Table 2ba and b); while they can indicate autoimmune processes, in our sample they manifested in individuals in whom these symptoms had been present for many years and belonged to underlying diseases such as Parkinson's disease, atypical Parkinson's syndromes, migraine or idiopathic epilepsy and did therefore neither discriminate between Exp and EnP nor pointed out AIP.
Table 1b.
Sociodemographic and paraclinical data within the sample of patients with psychotic episodes of exogenous origin (ExP).
| Count | Mean age in years (SD) |
1.PE | MRI conducted total |
MRI noteworthy changes total |
Counts of pathological blood test total Hb CRP hypo- natre- mia |
CSF samples available Total |
pathologic CSF total (%): cell albumin OKB positive count quotients in CSF |
EEGs Conducted pathological |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | 46 35 % |
58 (20,8) |
27 | 25 56 % |
14 54 % |
30 71 % |
15 33 % |
16 36 % |
6 13.6 % |
22 48 % |
2 4,3 % |
6 27 % |
2 4.3 % |
21 47 % |
3 16 % |
| Men | 93 42 % |
52 (20,0) |
47 | 36 39 % |
18 49 % |
58 64 % |
34 37 % |
30 33 % |
4 4.3 % |
25 27 % |
5 5.4 % |
8 31 % |
7 7.5 % |
35 38 % |
8 22 % |
| total | 139 | 54 | 74 | 61 44 % |
32 51 % |
88 67 % |
49 36& |
46 34 % |
10 7.3 % |
47 34 % |
7 5 % |
14 29 % |
9 6.5 % |
56 41 % |
17 30 % |
∗PE = psychotic episode; tested basic blood parameters with regards to warning signs for AIE/AIP: hemoglobin (Hb), C-reactive protein (CRP), sodium. Pathological CSF parameters do overlap in patients. N(%). Chi-square-test:p = 0,56.
Table 2a.
Frequency of clinical positive yellow and red flags within the total cohort.
| Reduced level of conscious-ness |
epileptic seizures | headache | rigor | paresthesia | nystagmus | ataxia | hypokinesia | dyskinesia | tremor | Cognitive dysfunction | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | 13 10 % |
2 9.2 % |
10 67 % |
8 36 % |
5 23 % |
1 4.5 % |
4 18 % |
0 | 1 4.5 % |
4 18 % |
84 68 % |
| Men | 26 12 % |
4 14 % |
10 53 % |
10 36 % |
2 7.1 % |
1 3.6 % |
5 18 % |
1 3.6 % |
6 21.4 % |
11 39 % |
149 70 % |
| Total (%) | 39 11 % |
6 12 % |
20 59 % |
18 36 % |
7 14 % |
1 4 % |
9 18 % |
1 2 % |
7 14 % |
15 30 % |
233 70 % |
∗ The presence of the following symptoms was examined by Chi-Square-Test:p = 0.65; yellow flags: reduced level of consciousness, abnormal postures or movements, autonomic instability, focal neurological deficits, aphasia or dysarthria, hyponatremia (see Tale1a), catatonia, headache, other autoimmune diseases:N = 0; and as red flags: CSF lymphocytic pleocytosis or oligoclonal bands (see Table 1a), MRI abnormalities (see Table 1a); epileptic seizures, faciobrachial dystonic seizures: N = 0, suspected malignant neuroleptic syndrome:N = 0, For AIP, we additionally examined the presence of cognitive dysfunction.
Table 2b.
Frequency of clinical positive yellow and red flags within the cohort of patients with ExP.
| Reduced level of consciousness | epileptic seizures | paresthesia | nystagmus | ataxia | hypokinesia | dyskinesia | tremor | rigor | headache | cognitive dysfunction | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | 9 20 % |
2 15 % |
2 15 % |
1 8 % |
3 22 % |
5 39 % |
1 7,7 % |
4 31 % |
4 31 % |
3 60 % |
34 77 % |
| Men | 19 21 % |
3 14 % |
2 9.5 % |
0 | 3 14 % |
2 9.5 % |
4 19 % |
6 29 % |
9 43 % |
5 46 % |
73 79 % |
| Total | 28 20 % |
5 15 % |
4 12 % |
1 2.9 % |
6 18 % |
7 21 % |
5 15 % |
10 29 % |
13 38 % |
8 50 % |
107 79 % |
∗ The presence of the following symptoms was examined by Chi-Square-Test:p = 0.65; yellow flags: reduced level of consciousness, abnormal postures or movements, autonomic instability, focal neurological deficits, aphasia or dysarthria, hyponatremia (see Tale1b), catatonia, headache, other autoimmune diseases:N = 0; and as red flags: CSF lymphocytic pleocytosis or oligoclonal bands (see Table 1b), MRI abnormalities (see Table 1b); epileptic seizures, faciobrachial dystonic seizures: N = 0, suspected malignant neuroleptic syndrome:N = 0, For AIP, we additionally examined the presence of cognitive dysfunction.
To diagnose cognitive dysfunctions relevant for AIP, we applied a standardized psychopathological rating (Stieglitz et al., 2017) for attention, concentration and memory and found impairments in the majoritiy of our participants (ExP:79 %; EnP:70 %). Altogether among patients with ExP, cognitive impairments, neurological symptoms and common blood parameter alterations were widespread and not limited to a specific diagnostic category; the four patients diagnosed with AIP due to CSF findings had unremarkable basic blood results but presented with cognitive impairment.
MRI data were available in 66 of 139 patients with ExP, of which 51 % showed changes that were assessed as “unspecific, but noteworthy” by neuroradiologists (Table 1b). We observed mainly leukoencephalopathic changes classified as unspecific (9 %); further findings belonged to diagnose group F0 and included general atrophy (5 %), temporo-mesial atrophy (4 %), ischemia/bleeding (2 %), space occupying lesion (5 %) or traumatic lesions (1 %). However, we did not observe edema, MRI hyperintensity or contrast-agent uptake in any case, classically be indicative of AIP (Graus et al., 2016). In summary, the available MRIs did not point out patients with AIP.
With respect to EEG findings (available in 91 patients), patients with F1 diagnoses usually showed physiological alpha activity with a 10 Hz-peak, while persons with F0 diagnoses had a tendency to slowing of baseline-activity (Fig. 2). Given these widespread, nonspecific alterations, routine EEG data did not indicate potential AIP in persons with psychosis.
Fig. 2.
Changes in EEG baseline activity depending on the diagnosis.
In serum, autoimmune diagnostics were carried out in 105 patients of EnP and in 64 patients with ExP using CAS by “Labor Berlin” (see Table 3a) (Berlin). In EnP we observed 10 subjects with antibodies (IgG-type) against myelin, but all an a very low titer and only in serum, as can be also observed in healthy persons (Genain et al., 1999), and therefore no immunotherapy was administered.
Table 3a.
Frequency of autoantibodies in EnP.
| NMDA S |
Myelin S |
Rings &rods CSF |
Epen- dym CSF |
ANA CSF |
AIS CSF |
|
|---|---|---|---|---|---|---|
| Woman | 1 | 6 | 1 | 0 | 2 | 0 |
| Men | 0 | 4 | 1 | 1 | 8 | 1 |
| Total | 1 | 10 | 2 | 1 | 10 | 1 |
∗all following antibodies were assessed in CSF (N = 54) and serum (N = 105): aquaporin-4, contactin-associated protein-2 (CASPR2), Hu, Ri, Yo, Delta/Notch-like Epidermal Growth Factor-Released”-Rezeptoren (Tr/DNER), myelin, paraneoplastic antigen 2(Ma2/Ta), glutamic acid decarboxylase 65 (GAD65), collapsin response mediator protein5 (CV2/CRMP5), amphiphysin, α-amino-3-hydroxy-5-hydroxy-5-methyl-4-isoxazolproprionsäure-receptor (AMPAR 1/2), γ-aminobutyric-acid A + B (GABA-A and B receptor), late gestation lung protein 1(LGI1), DPPX, dopamine-2-Ak, GlyR, N-methyl-D-aspartic acid (NMDA) and metabotropic glutamate receptor (mGluR5).AIS = axoninitialsegment.ANA:Antinuclear-antibodies.S=Serum. CSF = cerebrospinal fluid.
In ExP, using CAS we found serum antibodies against myelin four times, and antibodies against amphiphysin and CV2 were each found in one patient (see Table 3b). Amphiphysin and CV2 antibodies belonged to cases with suspected paraneoplastic syndromes. Since onconeural autoantibodies targeting intracellular structures usually do not respond well to immunotherapy (Heine et al., 2023), no such treatment was administered. The anti-myelin antibodies showed low titres and were discussed as unspecific.
Table 3b.
Frequency of autoantibodies in the cohort of ExP (N = 47).
| NMDA S CSF |
Myelin S CSF |
CV2 S |
Amphiyphysin S |
Neuropil CSF |
Vessel CSF |
Astrocytes | Rings &rods CSF |
Myelin CSF |
Nucle-oli CSF |
Epen- Dym CSF |
Fibers CSF |
Purkinye cells CSF |
ANA CSF |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Woman | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 3 | 2 | 0 | 1 | 2 | 6 |
| Men | 1 | 2 | 3 | 1 | 1 | 1 | 2 | 3 | 3 | 1 | 1 | 2 | 1 | 4 | 7 | 7 |
| Total | 0 | 2 | 4 | 2 | 1 | 1 | 4 | 4 | 4 | 1 | 4 | 4 | 1 | 5 | 9 | 13 |
∗all following antibodies were assessed in CSF (N = 47) and serum (N = 64), only positive results are reported: aquaporin-4, contactin-associated protein-2 (CASPR2), Hu, Ri, Yo, Delta/Notch-like Epidermal Growth Factor-Released”-Rezeptoren (Tr/DNER), myelin, paraneoplastic antigen 2(Ma2/Ta), glutamic acid decarboxylase 65 (GAD65), collapsin response mediator protein5 (CV2/CRMP5), amphiphysin, α-amino-3-hydroxy-5-hydroxy-5-methyl-4-isoxazolproprionsäure-receptor (AMPAR 1/2), γ-aminobutyric-acid A + B (GABA-A and B receptor), late gestation lung protein 1(LGI1), DPPX, dopamine-2-Ak, GlyR, N-methyl-D-aspartic acid (NMDA) and metabotropic glutamate receptor (mGluR5).
CSF data were available in 54 of patients with EnP and of 47 with ExP (Table 3a/b). CAS in CSF was negative in all patients with EnP. IFT showed more positive results (rings&rods:N = 2; ependym:N = 1, ANA:N = 10, nucleoli:N = 4; fibers:N = 5; purkinye-cells:N = 9; myelin:N = 10), but since these patterns are not well established yet, again, no immutherapy was administered.
Regarding ExP, basic parameters such as cell count, protein and oligoclonal bands were pathological in 61 % of the CSF samples examined. The number of positive findings of CAS in CSF was lower as in blood, with NMDA-receptor antibodies (IgG-type) that were specific for the diagnosis of NMDA-receptor-encephalitis, found in one case. In addition, anti-myelin antibodies were detected in CSF of two cases; as the relationship between not further specified anti-myelin antibodies and psychosis is not yet clear, these findings were evaluated as unspecific and these two patients did not receive immunotherapy (Guasp and Dalmau, 2023).
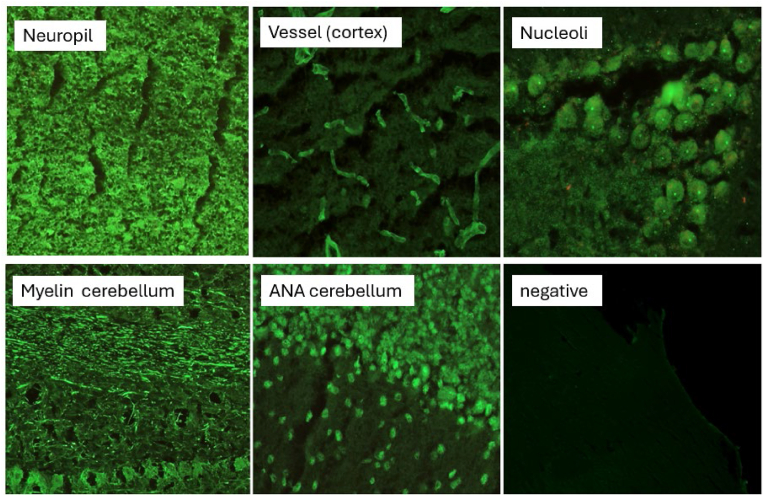
Furthermore, 13 out of 30 samples showed antineuronal-autoantibodies of IgG-type and were directed against neuropil, vessels, nucleoli, astrocytes and/or myelin (4 patients each) according to their binding pattern (Fig. 3). Some patients showed overlapping patterns; some of these patterns are already known from studies with neuropsychiatric symptom complexes (Endres et al., 2022; Ances et al., 2005).
Fig. 3.
Tissue based antibody screening, patterns found within the cohort.
All positive findings were discussed by a board of clinicians and scientists from neurology and psychiatry. Taking into account the severity of symptoms, clinical comorbidities and individual patient wishes, immunotherapy was recommended and carried out in 1 case with NMDA-R-antibodies and 3 further cases, identified by IFT. Their clinical courses are described within the following panels and findings are summarized in Table 4. Other patients with technically positive IFT results were not treated in the absence of further clinical and paraclinical findings fully meeting criteria for AIE or AIP.
Table 4.
Paraclinical findings within the case serie.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| cell count (number/μl) | 8 | 17 | 2 | 3 |
| protein (mg/l) |
381.6 | 201.7 | 252 | 311.0 |
| oligoclonal bands | positive | positive | negative | negative |
| Tau (pg/ml) |
NA | NA | 968 | NA |
| pTau181 (pg/ml) |
NA | NA | 130.0 | NA |
| Beta-amyloid-ratio (42/40) | NA | NA | 0.042 | NA |
| Nfl (pg/ml) |
739 | NA | 1346 | 231 |
| Commercial antineuronal autoantibodies∗ | Anti-NMDAR 1:3 | negative | negative | negative |
| IFT Murine brain section | NMDAR | Neuropil | Bergmann glia | Astrocytes |
| Blood | ||||
| CRP (mg/l) |
1.4 | <0.6 | <0.6 | <0.6 |
| TSH (mU/l) |
1.29 | 1.45 | 0.6 | 0.82 |
| oligoclonal bands in CSF | negative | negative | negative | negative |
| Sodium (mmol/l) |
140 | 140 | 139 | 139 |
| potassium (mmol/l) |
3.5 | 4.2 | 4.3 | 4.0 |
| haemoglobin (g/dl) |
15.2 | 13.8 | 13.0 | 13.4 |
| Commercial antineuronal autoantibodies∗ | negative | negative | Anti-Caspr2-Ab (Type IgG) 1:32 | negative |
| Clinical parameters | ||||
| Age | 36 | 18 | 44 | 26 |
| Diagnosis | Autoimmune encephalitis (NMDAR) | Autoimmune encephalitis (Neuropil) | Delirium due to Early onset Alzheimer Dementia |
Autoimmune encephalitis (Astrocytes) |
| Immunological Treatment Approach |
oral prednisolone (4 weeks) |
immunoadsorption (7 cycles) |
i.v. prednisolone (5 days) oral prednisolone (2 weeks) |
i.v. prednisolone (5 days) oral prednisolon (6 weeks) |
| Treatment 6m follow up drug [mg/d] duration [weeks] |
Rituximab | Rituximab (1000 mg/ |
Donepezil 10 Cariprazine 6 |
Rituximab |
| Improvement | Yes, fully recovered | Yes | No, but stabilization | Yes |
Results in bolt are pathological. ∗ Commercial antineural antobodies: aquaporin-4, contactin-associated protein-2, Hu, Ri, Yo, Tr/DNER, myelin, Ma2/Ta, GAD65, CV2, amphiphysin, glutamate receptor type AMPAR 1/2, GABA-A and B receptor, LGI1, Caspr2, DPPX, dopamine-2, glycine receptor (GlyR), NMDA and metabotropic glutamate receptor 5.
Panel 1.
A 36-year-old patient contacted our emergency department on his own initiative and reported insomnia, cognitive impairments regarding concentration and memory, scenic hallucinations and depressed mood. There were also delusions of control and other delusional ideas, developing within the last four weeks.
MRI showed leukoencephalopathic alterations that were classified as unspecific by radiological examination, blood and urine test were unremarkable and the patient was initially diagnosed with a first episode of an acute polymorph psychotic disorder (F23.0). CAS in blood was negative, but CSF analysis and autoimmune screening revealed acute inflammation by pleocytosis (of 8 WBC/μl, mainly lymphocytes) and positive oligoclonal bands in CSF. Furthermore, anti-NMDA-autoantibodies (type IgG) in CSF (1:3) were detected as well as a matching pattern in IFT tissue-based testing (neuropil signal in hippocampus region; Fig. 6), leading to the diagnosis of NMDAR-encephalitis presenting as AIP.
Fig. 6.
Tissue based antibody screening in patient 3.
Treatment with haloperidol (2 mg) was initially administered and led to significant improvement of psychotic symptoms and sleep disturbances over a course of two weeks. After diagnosing AIP, the patient received oral prednisolone therapy for four weeks due to the mild clinical course (starting with 40 mg/day). Rituximab (1000 mg/infusion) was administered as maintenance therapy, under which psychotic symptoms remitted completely, antipsychotic medication could be terminated after 12 weeks. In the six- and nine-month follow-up, no psychotic symptoms or cognitive impairments were observed. The patient had also subjectively recovered his prior condition, and returned to work again.
Panel 2.
A 18-year-old female patient reached out to our outpatient clinic with a first episode of paranoid-hallucinatory syndrome. For four months she had been experiencing persistent thought echoes and -thought withdrawal, commenting phonemes, tactile hallucinations, and delusions of control. Furthermore, concentration problems and a depressed affect with inner tension were reported. In the absence of neurological symptoms, the patient met criteria for paranoid schizophrenia (F20.0). Antipsychotic medication (5 mg aripiprazole) was initiated, leading to a cessation of psychotic symptoms. However, cognitive impairment persisted, limiting her ability to work to around 30 min a day and indicating a putative AIP. Yet MRI, blood and urine tests were unremarkable. EEG was also unremarkable and showed regular power spectrum with 10 Hz peak and no slowing or epileptic activity.
CSF analyses revealed a pathological increase in cell count (of 17 WBC/μl, mainly lymphocytes) and positive oligoclonal bands. Infectious causes (e.g. Herpes-Virus, Borrelia) were ruled out and no antineuronal autoantibody within the CAS was detected in blood or CSF. IFT antibody screening on murine brain sections revealed a strong binding pattern to neuropil over all brain areas (Fig. 4). This finding has been described earlier with comparable symptoms (psychiatric-behavioral and cognitive) and showed a good response to immunotherapy (Ances et al., 2005). Hence, we interpreted the acute psychotic episode and persistent cognitive impairment together with CSF-results as AIP.
Fig. 4.
Tissue based antibody screening in patient 1.
Immunoabsorption (7 cycles) was conducted according to current treatment recommendations (Fassbender et al., 2017). Subsequently, the patient also received B-cell depleting therapy with rituximab in standard dosage (1000 mg/infusion). This led to a significant improvement of the cognitive impairment and enabled the patient to partially return to her prior work. The effect of treatment will continue to be evaluated as part of our standard follow-up care.
Panel 3.
A 44-year-old female patient with a FEP reported thought deprivation and echo, occasional commenting phonemes, and vaguely described scenic hallucinations. There were signs of disorientation and impaired attention as well as delusions of persecution with a moderate degree of delusional systematization. In addition, psychomotor slowing was registered. With an unsuspicious MRI, basic blood and urine tests, the patient fulfilled criteria for paranoid schizophrenia (F20.0), and treatment with olanzapine was initiated. Although symptoms improved
To some extent, there were still paranoid symptoms even at a daily maximum dose of 20 mg of olanzapine in the presence of severe side effects (weight gain and parkinsonoid).
In blood, a CASPR2 antibody (Type IgG) was detected at a low titer level (1:32). Since it did not match the phenotype of our patient and was only found in serum, it was not considered pathogenic. CSF analysis revealed a mild pleocytosis (of 6 WBC/μl, mainly lymphocytes), but CAS was negative (also in blood). IFT on murine brain sections showed strong IgG binding against Bergmann glia (Fig. 5). AIP was diagnosed in the absence of response to antipsychotic medication, persistent cognitive impairment, inflammatory CSF syndrome and positive IFT.
Fig. 5.
Tissue based antibody screening in patient 2.
Immunotherapy with intravenous prednisolone (500 mg daily for five days) was initiated, followed by oral administration of prednisolone (0.5 mg per kg body weight for 6 weeks). A temporary stabilization was achieved that allowed a transfer to an open neurological ward, although the psychotic symptoms persisted. Therefore, antipsychotic treatment was supplemented with amisulpride (up to 400 mg daily), and due to insufficient clinical response and prolonged QT-time finally switched to cariprazine (up to 6 mg daily) as monotherapy. The patient showed further improvement in psychotic symptoms, but still no complete remission. Because of clinically recognizable and persistent cognitive impairments, further CSF diagnosis assessed neurodegeneration markers and revealed an Alzheimer's-typical profile A + T + N+ (Jack et al., 2018). The association of autoantibodies in the context of neurodegenerative diseases is attracting increasing scientific attention and represents a new potential treatment approach (Bastiaansen et al., 2021; Long and Day, 2018). In this case, the IFT result was interpreted as an indication of potential autoimmune contribution in both, the neurodegenerative and psychotic course, although no conclusive statement on causality could be made. Further investigations revealed a spontaneous (non-genetic cause) early-onset Alzheimer's disease, leading to the initiation of 5 mg donepezil. In the 12-month follow-up, the patient still exhibited delusions of thought, a blunted affect and mild parkinsonoid. Furthermore, impairments of memory and executive functions became more apparent requiring external care. Altogether, the therapy-refractory paranoid-hallucinatory syndrome in this case was neither typical for early-onset Alzheimer's disease nor for an onset of schizophrenia. We assume that an autoimmune process occurred as part of the underlying neurodegenerative process. The attempt at immunotherapy was not successful in this case, although positive effects have been described for autoimmune mediated dementias (Rössling and Prüss, 2020) and may therefore be undertaken after individual consideration.
Panel 4.
A 26-year-old woman sought psychiatric care due to subjectively significant sleep disorders. After a severe flu-like infection months ago, she noted headache for two days but also reported low-frequent migraine as a chronic condition. We observed cognitive impairments regarding concentration and memory as well as increasingly suspicious behavior with experiences of thought withdrawal and delusions of persecution. There were unremarkable blood, urine and MRI results, also EEG was without any pathological findings and showed regular alpha-activity (Fig. 7). According to ICD-10 criteria, she was diagnosed with a first episode of paranoid schizophrenia (F20.0). Antipsychotic medication was initiated (aripiprazole 5 mg and olanzapine 10 mg) as well as lorazepam pro re nata (PRN). Sleep disturbances improved somewhat but psychotic symptoms remained, the patient reported ongoing subjective cognitive impairment.
Fig. 7.
Tissue based antibody screening in patient 4.
CAS in blood was negative, in line with basic blood parameters. CSF parameters, including CAS, were completely unremarkable. However, IFT detected a strong IgG signal against astrocytes, which most closely corresponded to anti-GFAP antibodies (type IgG, Fig. 7). Thus, we diagnosed AIP even in the absence of signs of inflammation in basic CSF parameters.
The patient was treated with intravenous prednisolone (1000 mg daily for five days), resulting in rapid clinical improvement. This improvement allowed for the reduction and eventual discontinuation of antipsychotic medication. Maintenance treatment with rituximab (1000 mg/infusion) was initiated, and within the three and six-months-follow up, the patient was fully recovered, stable and able to continue her university studies.
4. Discussion
The key finding of our study is that about 1 % of all persons with psychotic symptoms in an inner city catchment area showed signs of autimmune psychosis (AIP) upon analysis of CSF samples. Our results provide a comprehensive representation of the clinical care landscape within a European inner-city catchment area. Our cohort displayed an incidence of psychotic disorders within the lower worldwide spectrum (Saha et al., 2006), suggesting that our sample adequately reflects psychotic disorders in the evaluated district. The rather low incidence may be due to the generally long period of undetected psychosis/schizophrenia diagnosis (Fusar-Poli et al., 2021), and a relatively lower number of compulsory admission to psychiatry care in Berlin compared to other regions in Germany, thus patients may choose other treatment options (Darsow-Schutte and Muller, 2001; Kunz and Heinz, 2007). The rather old mean age, especially whithin the participants experiencing their first psychotic episode, maybe due to the fact that 56 % of the participants with FEP belonged to group F0 and 9 % to group F1. Since the typical age spectrum of organic hallucinations is clearly in the second half of life and is primarily based on primary neurodegenerative diseases, this results in a relatively high average age. Nevertheless, the representativeness of our cohort with regard to mean age may be questioned.
The presence of clinical warning signs (red and yellow flags) should help identify patients with underlying AIE and AIP more reliably, facilitating prompt diagnosis, especially in those with FEP. However, these warning signs include symptoms like “cognitive impairments”, which are commonly observed in patients with psychosis (Lee et al., 2024). In fact, about 79 % of our patients with ExP displayed cognitive impairments, which was thus not a clinically useful indicator. A noteworthy limitation is the nature of the cognitive assessment. Systematic neuropsychological testing was not feasible during the acute phase of psychosis, and only a clinical assessment was applied. A more comprehensive evaluation of various cognitive domains would be valuable in identifying persons with AIP.
Neurological symptoms were also common and attributable to already known neurological diseases including Parkinsons Syndromes or epilepsy. MRI and EEG findings in our sample were also too unspecific to indicate AIE or AIP as well as endogeneous or exogeneous origin. None of the patients showed “adverse repsonse to antipsychotics”, which would be considered a yellow flag. The resistance to antipsychotic treatment in contrast was discussed in other studies as a potential indicator for autoimmune origin (Dalmau et al., 2021) and was detected in two of the 4 treated patients. From our experience this factor is of more use than the current criterion of adverse affects.
While pathological basic CSF parameters were observed in more than half of patients examined, only four patients showed specific autoimmune markers in CSF, which led to immunotherapy. Interestingly, only one of them was detected by commercial antibody screening (CAS) regarding NMDAR antibodies, while three other patients were detected via indirect immunofluorescence (IFT) (Kreye et al., 2020). Autoimmune findings in blood and CSF show that encephalitis-associated autoantibodies occurred rather rarely within our cohort. It may therefore be helpful to evaluate an adjusted screening panel for the suspected diagnosis of AIP, which contains autoantibodies more frequently associated with the phenotype than the conventional AIE panel used here, which – except for NMDAR encephalitis – did not add additional knowledge. In contrast, IFT was more sensitive and revealed autoimmune processes against yet unknown neuronal target structures. Therefore, we suggest to use commercial autoantibody-screenings in psychosis that are narrowed down to anti-metabolic glutamate receptor 5 (mGluR5), contactin-associated protein-like 2 (CASPR2), leucine-rich glioma-inactivated 1 (LGl1), anti N-Methyl-D-Aspartat-receptor (NMDAR), anti gamma-aminobutyric acid (GABA(A)R) and anti thyroid peroxidase or thyroglobulin antibodies (TPO/TG) (Hansen and Timäus, 2021; Spatola et al., 2020; van Sonderen et al., 2016; Wang et al., 2022), because all of them are clearly known to be clinically associated. In case of negative screening but conspicuous CSF basic parameters like cell count elevations or CNS-specific oligoclonal bands, we suggest carrying out additional screening with IFT (Pollak et al., 2020b; Endres et al., 2022). Additionally, we believe that elevated protein levels in CSF or detection of isolated oligoclonal bands in CSF should always prompt further investigation (Endres et al., 2015) with an extended diagnostic assessment including the involvement of a neuropsychiatric board. In view of the sensitivity and specificity of IFT in CSF analyses (Pollak et al., 2020b; Najjar et al., 2018b), we established this as a standard operating procedure, especially for patients with FEP.
Regarding IFT, antibodies against ANA in CSF were detected quite frequently (EnP:10; ExP:13) but did not elicit immunotherapy in this study. Even though the test result was technically positive, the findings were assessed as negative due to the low specificity. Anti-ANA antibodies do not bind specifically against neuronal structures (but nucleus structures), therefore occur more often and might imply underlying rheumatologic diseases (Endres et al., 2020a; Leypoldt et al., 2015). In none of these patients a neuropsychiatric Lupus erythematodes or other rheumatological diseases could be confirmed.
Altogether, our observations support the recommendation to perform CSF analysis in all patients with acute psychosis. Regarding clinical presentation, three of the four patients described above, who responded to immunotherapy, would have fulfilled criteria for schizophrenia or schizophrenia spectrum disorder in the absence of CSF diagnostics. Particularly in view of the broader diagnostic criteria set out in the new ICD-11 (Fusar-Poli et al., 2021) and the insufficiently specificity of clinical warning signs, diagnosis should be supplemented by EEG, MRI and CSF including IFT.
One limitation of our study is the rather low rate of lumbar punctures and MRI data. Despite our standardized protocol requiring this diagnostic workup, the rate of MRIs and lumbar punctures was far from complete. This was mainly due to patients rejecting imaging or lumbar puncture due to assumed adverse effects, alternative explanatory models for medical diagnosis or limited insight, although these issues can also be assumed for some practitioners (Buchbinder, 2013). Some comparable studies found higher numbers of positive findings in IFT (Endres et al., 2020b, 2022), which may be due to elective patient recruitment and higher rates of lumbar punctures performed within such settings, while studies from routine care also show lower frequencies of positive IFT (Endres et al., 2015). The results of our study thus emphasize the need of comprehensive diagnostics including IFT in CSF. In fact, all of our patients with AIP were of young age and they would otherwise have missed an opportunity for causal treatment and risk long term impairments (Ho et al., 2011). Further limitations are due to the clincial setting of our study: although the participating hospitals have the care mandate in the inner city sector of Berlin with its 380.917 inhabitants (Bibliothek, 2020), the freedom of choice allows patients to seek care at other hospitals. Consequently, a significant number of individuals with acute psychotic episodes residing within the sector may have been missed. Finally, patients were recruited from psychiatry, neurological and intensive care wards; regarding clinical assessment and diagnosis at discharge, we tried to reduce variability by applying two study physicians to monitor all diagnostic procedures.
Another limitation of our study is the reliance on the response to non-randomized treatment as a confirmation of AIP/AIE diagnosis. Persons with acute psychosis are known to have spontaneous remissions and clinical courses characterized by waxing and waning symptoms. Therefore, controlled trials are warranted.
In conclusion, within a cohort of 352 patients with acute psychosis, we observed AIP in four patients who then received immunotherapy and improved clinically in three cases. One conclusion is that there is no clinical or para-clinical feature that reliably indicates whether a patient has an autoimmune etiology. Therefore, CSF should be assessed when patients present with psychotic symptoms, particularly when experiencing a FEP. The rather high rejection rates in our sample emphasize the importance to promote CSF analysis among persons with psychotic symptoms. Regarding red and yellow flags, our study suggests that they were indicative but not sufficient to identify cases of AIP.
Considering potential benefits for patients and the increasing focus on autoimmunity in various neuropsychiatric disorders (Long and Day, 2018; Hansen and Timäus, 2021; Bastiaansen et al., 2023), we recommend that comprehensive biomarker analysis is routinely included in diagnostics of patients diagnosed within the ICD-11 F2 or DSM-5 schizophrenia categories, particularly when psychotic symptoms are manifested and clinically resemble for the first time. Altogether, we feel that our findings suggest that while cases with AIP were rare in our sample (about 1 %), they responded to specific immunological treatment, and we therefore recommend extended CSF analysis. Our results therefore underline the importance of CSF diagnostics to identify an autoimmune etiology of psychotic experiences.
CRediT authorship contribution statement
Maria Buthut: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. David Haslacher: Data curation, Formal analysis, Methodology, Writing – review & editing. Surjo R. Soekadar: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Felix Machleid: Data curation, Investigation, Writing – review & editing. Jakob Kaminski: Data curation, Software, Writing – review & editing. Philipp Reber: Formal analysis, Writing – review & editing. Johanna Schoener: Data curation, Project administration, Writing – review & editing. Anna Pichler: Data curation, Writing – review & editing. Moritz Thiele: Data curation, Writing – review & editing. Jochen Michely: Data curation, Writing – review & editing. Helle Foverskov-Rasmussen: Data curation, Writing – review & editing. Irina Baskow: Writing - review & editing. Verena Rösgen-Petzold: Data curation, Project administration, Writing – review & editing. Harald Prüss: Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – review & editing. Matthias Endres: Funding acquisition, Resources, Supervision, Writing – review & editing. Lasse Brandt: Methodology, Formal analysis, Writing - review & editing. Andreas Heinz: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Ethical standard statement
This case report was performed according to the international ethical guidelines and followed our institutional ethical tenets. Permission was obtained from the patient and legal representatives for the publication of this report.
Declaration of competing interest
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article. ME reports grants from Bayer and fees paid to the Charité from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Pfizer, all outside the submitted work.
Other (Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid): European Academy of Neurology (Board of directors, unpaid), German Society of Neurology (Member, unpaid), International Society of Cerebral Blood Flow Metabolism (Member, unpaid), American Heart Association/American Stroke Association (Member, unpaid), World Stroke Organization (Member, unpaid), European Stroke Organization (Fellow, unpaid), German Center of Neurodegenerative Diseases (personal contract, paid).
Acknowledgements
M.E. received funding from DFG under Germany's Excellence Strategy – EXC-2049 – 390688087, Collaborative Research Center ReTune TRR 295–424778381, Clinical Research Group KFO 5023 BeCAUSE-Y, project 2 EN343/16-1. BMBF, DZNE, DZHK, DZPG, EU, Corona Foundation, and Fondation Leducq
H.P. received funding from DFG (grants FOR3004, PR1274/9-1, clinical research unit 5023/1 ‘BECAUSE-Y’ [project number 504745852]), from the Helmholtz Association (HIL-A03 BaoBab), and the German Federal Ministry of Education and Research (Connect-Generate 16GW0279K).
S.R.S. received funding from the European Research Council (ERC) under the project NGBMI (759370) and BNCI2 (101088715), the Deutsche Forschungsgemeinschaft (DFG SO932/7-1) and the Einstein Stiftung Berlin.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2025.101033.
Abbreviations
- AIE
autoimmune encephalitis
- AIP
autoimmune psychosis
- CAS
commercial autoantibody screening
- CSF
cerebrospinal fluid
- EP
exogeneous psychosis
- FEP
first time episode of psychosis
- IFT
indirect immunofluorescence
- WBC
white blood cell count
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Al-Diwani A., et al. Synaptic and neuronal autoantibody-associated psychiatric syndromes: controversies and hypotheses. Front. Psychiatr. 2017;8:13. doi: 10.3389/fpsyt.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances B.M., et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128(Pt 8):1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.P. 5th ed. 2013. Diagnostic and Statistical Manual of Mental Disorders - DSM V. Arlington. [DOI] [PubMed] [Google Scholar]
- Bastiaansen A.E.M., et al. Autoimmune encephalitis resembling dementia syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(5) doi: 10.1212/NXI.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen A.E.M., et al. Antibodies associated with autoimmune encephalitis in patients with presumed neurodegenerative dementia. Neurol Neuroimmunol Neuroinflamm. 2023;10(5) doi: 10.1212/NXI.0000000000200137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, L., List of Services.
- Bibliothek S. Statistischer Bericht - Einwohnerinnen und Einwohner im Land Berlin am 30. Juni 2020. 2020. https://www.statistischebibliothek.de/mir/servlets/MCRFileNodeServlet/BBHeft_derivate_00022651/SB_A01-05-00_2020h01_BE.pdf Available from:
- Birnbaum R., Weinberger D.R. The genesis of schizophrenia: an origin story. Am. J. Psychiatr. 2024;181(6):482–492. doi: 10.1176/appi.ajp.20240305. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Vol. 4. 1911. (Dementia praecox, oder Gruppe der Schizophrenien). Deuticke. [Google Scholar]
- Broome Matthew R., B R., Rösler Michael, Stieglitz Rolf-Dieter. the AMDP System Manual for Assessment and Documentation of Psychopathology in Psychiatry. 9th. Hogrefe; 2018. ISBN: 9780889375420. [Google Scholar]
- Buchbinder M. The canon - 5. Patients and healers in the context of culture: an explorationof the borderland between anthropology, medicine, and psychiatry, by Arthur Kleinman. Anthropol. Med. 2013;20(1):109–111. doi: 10.1080/13648470.2012.762337. [DOI] [PubMed] [Google Scholar]
- Charlson F.J., et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull. 2018;44(6):1195–1203. doi: 10.1093/schbul/sby058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J., Guasp M., Graus F. Author response: clinical, neuroimmunologic, and CSF investigations in first episode psychosis. Neurology. 2021;97(21):1010. doi: 10.1212/WNL.0000000000012899. [DOI] [PubMed] [Google Scholar]
- Darsow-Schutte K.I., Muller P. [Number of hospitalizations according to German "PsychKG" legislation has doubled in 10 years] Psychiatr. Prax. 2001;28(5):226–229. doi: 10.1055/s-2001-15575. [DOI] [PubMed] [Google Scholar]
- Ellul P., et al. The clinical challenge of autoimmune psychosis: learning from anti-NMDA receptor autoantibodies. Front. Psychiatr. 2017;8:54. doi: 10.3389/fpsyt.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., et al. Immunological findings in psychotic syndromes: a tertiary care hospital's CSF sample of 180 patients. Front. Hum. Neurosci. 2015;9:476. doi: 10.3389/fnhum.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., et al. Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur. Arch. Psychiatr. Clin. Neurosci. 2020;270(7):803–818. doi: 10.1007/s00406-020-01113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., et al. Cerebrospinal fluid, antineuronal autoantibody, EEG, and MRI findings from 992 patients with schizophreniform and affective psychosis. Transl. Psychiatry. 2020;10(1):279. doi: 10.1038/s41398-020-00967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., et al. Spectrum of novel anti-central nervous system autoantibodies in the cerebrospinal fluid of 119 patients with schizophreniform and affective disorders. Biol. Psychiatry. 2022;92(4):261–274. doi: 10.1016/j.biopsych.2022.02.010. [DOI] [PubMed] [Google Scholar]
- Fassbender C., Klingel R., Kohler W. Immunoadsorption for autoimmune encephalitis. Atherosclerosis Suppl. 2017;30:257–263. doi: 10.1016/j.atherosclerosissup.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr. Res. 2021;228:547–554. doi: 10.1016/j.schres.2020.11.031. [DOI] [PubMed] [Google Scholar]
- Genain C.P., et al. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999;5(2):170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Graus F., et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasp M., Dalmau J. Searching for neuronal antibodies in psychiatric diseases: uncertain findings and implications. Neurology. 2023;101(15):656–660. doi: 10.1212/WNL.0000000000207486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N., Timäus C. Autoimmune encephalitis with psychiatric features in adults: historical evolution and prospective challenge. J. Neural Transm. 2021;128(1):1–14. doi: 10.1007/s00702-020-02258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J., et al. [Autoimmune encephalitis-An update] Nervenarzt. 2023;94(6):525–537. doi: 10.1007/s00115-022-01411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., et al. Shall we really say goodbye to first rank symptoms? Eur. Psychiatry. 2016;37:8–13. doi: 10.1016/j.eurpsy.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Herken J., Pruss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front. Psychiatr. 2017;8:25. doi: 10.3389/fpsyt.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B.C., et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Classification of Diseases, Eleventh Revision (ICD-11), World Health Organization (WHO) 019/2021. . Licensed under Creative Commons Attribution-NoDerivatives 3.0 IGO Licence (CC BY-ND 3.0 IGO). WHO.
- Jack C.R., Jr., et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma H.E., et al. International incidence of psychotic disorders, 2002-17: a systematic review and meta-analysis. Lancet Public Health. 2019;4(5):e229–e244. doi: 10.1016/S2468-2667(19)30056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Barth; Leipzig: 1896. Lehrbuch der psychiatrie. [Google Scholar]
- Kreye J., et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell. 2020;183(4):1058–1069 e19. doi: 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D.P.S., Heinz A. Compulsory psychiatric admissions in two districts of Berlin – how open are the psychiatric services? Dtsch Arztebl International. 2007;104(18) A-1232. [Google Scholar]
- Lee M., et al. Cognitive function and variability in antipsychotic drug-naive patients with first-episode psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81(5):468–476. doi: 10.1001/jamapsychiatry.2024.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypoldt F., Armangue T., Dalmau J. Autoimmune encephalopathies. Ann. N. Y. Acad. Sci. 2015;1338(1):94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.M., Day G.S. Autoimmune dementia. Semin. Neurol. 2018;38(3):303–315. doi: 10.1055/s-0038-1660480. [DOI] [PubMed] [Google Scholar]
- Marneros A. Schizophrenic first-rank symptoms in organic mental disorders. Br. J. Psychiatry. 1988;152:625–628. doi: 10.1192/bjp.152.5.625. [DOI] [PubMed] [Google Scholar]
- Marneros A. The schizoaffective phenomenon: the state of the art. Acta Psychiatr. Scand. Suppl. 2003;(418):29–33. doi: 10.1034/j.1600-0447.108.s418.7.x. [DOI] [PubMed] [Google Scholar]
- Marneros A. Beyond the Kraepelinian dichotomy: acute and transient psychotic disorders and the necessity for clinical differentiation. Br. J. Psychiatry. 2006;189:1–2. doi: 10.1192/bjp.bp.106.024257. [DOI] [PubMed] [Google Scholar]
- McCleery A., Nuechterlein K.H. Cognitive impairment in psychotic illness: prevalence, profile of impairment, developmental course, and treatment considerations Dialogues. Clin. Neurosci. 2019;21(3):239–248. doi: 10.31887/DCNS.2019.21.3/amccleery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.M., et al. Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics? Br. J. Psychiatry. 2016;209(5):361–365. doi: 10.1192/bjp.bp.116.182683. [DOI] [PubMed] [Google Scholar]
- Najjar S., et al. A clinical approach to new-onset psychosis associated with immune dysregulation: the concept of autoimmune psychosis. J. Neuroinflammation. 2018;15(1):40. doi: 10.1186/s12974-018-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S., et al. A clinical approach to new-onset psychosis associated with immune dysregulation: the concept of autoimmune psychosis. J. Neuroinflammation. 2018;15(1):1–8. doi: 10.1186/s12974-018-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavăl D., et al. Neural antibodies in first-episode psychosis patients with warning signs for autoimmune encephalitis. Clin Psychopharmacol Neurosci. 2024;22(3):520–530. doi: 10.9758/cpn.24.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak T.A., Al-Diwani A.A., Lennox B. Neuronal surface autoantibodies, encephalitis, and psychosis: from neurology to psychiatry. Adv Clin Neurosci Rehabil. 2017;17(2):6–10. [Google Scholar]
- Pollak T.A., et al. Autoimmune psychosis - authors' reply. Lancet Psychiatry. 2020;7(2):123–125. doi: 10.1016/S2215-0366(19)30527-9. [DOI] [PubMed] [Google Scholar]
- Pollak T.A., et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93–108. doi: 10.1016/S2215-0366(19)30290-1. [DOI] [PubMed] [Google Scholar]
- Rössling R., Prüss H. Apheresis in autoimmune encephalitis and autoimmune dementia. J. Clin. Med. 2020;9(9) doi: 10.3390/jcm9092683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., et al. Incidence of schizophrenia does not vary with economic status of the country: evidence from a systematic review. Soc. Psychiatr. Psychiatr. Epidemiol. 2006;41(5):338–340. doi: 10.1007/s00127-006-0041-7. [DOI] [PubMed] [Google Scholar]
- van Sonderen A., et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87(5):521–528. doi: 10.1212/WNL.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatola M., et al. Clinical features, prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology. 2020;95(22):e3012–e3025. doi: 10.1212/WNL.0000000000010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz R.D., et al. Comprehensive psychopathological assessment based on the association for methodology and documentation in psychiatry (AMDP) system: development, methodological foundation, application in clinical routine, and research. Front. Psychiatr. 2017;8:45. doi: 10.3389/fpsyt.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.Y., et al. Clinical characteristics of anti-N-methyl-D-aspartate receptor encephalitis in patients with a long-term history of mental disorders. Eur. J. Med. Res. 2022;27(1):38. doi: 10.1186/s40001-022-00664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health O. WHO; 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines: Clinical Description and Diagnostic Guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.