Abstract
The synthesis and characterization of two cytosine-substituted calix[4]pyrrole conjugates, bearing the appended cytosine attached at either a β- or meso-pyrrolic position, is described. These systems were tested as nucleotide-selective carriers and as active components of nucleotide-sensing ion-selective electrodes at pH 6.6. Studies of carrier selectivity were made using a Pressman-type model membrane system consisting of an initial pH 6.0 aqueous phase, an intervening dichloromethane barrier containing the calix[4]pyrrole conjugate, and a receiving basic aqueous phase. Good selectivity for the Watson–Crick complementary nucleotide, 5′-guanosine monophosphate (5′-GMP), was seen in the case of the meso-linked conjugate with the relative rates of through-membrane transport being 7.7:4.1:1 for 5′-GMP, 5′-AMP, and 5′-CMP, respectively. By contrast, the β-substituted conjugate, while showing a selectivity for 5′-GMP that was enhanced relative to unsubstituted calix[4]pyrrole, was found to transport 5′-CMP roughly 4.5 times more quickly than 5′-GMP. Higher selectivities were also found for 5′-CMP when both the β- and meso-substituted conjugates were incorporated into polyvinyl chloride membranes and tested as ion selective electrodes at pH 6.6, whereas near-equal selectivities were observed for 5′-CMP and 5′-GMP in the case of unsubstituted calix[4]pyrroles. These seemingly disparate results are consistent with a picture wherein the meso-substituted cytosine calix[4]pyrrole conjugate, but not its β-linked congener, is capable of acting as a ditopic receptor, binding concurrently both the phosphate anion and nucleobase portions of 5′-GMP to the calixpyrrole core and cytosine “tails” of the molecule, respectively, with the effect of this binding being most apparent under the conditions of the transport experiments.
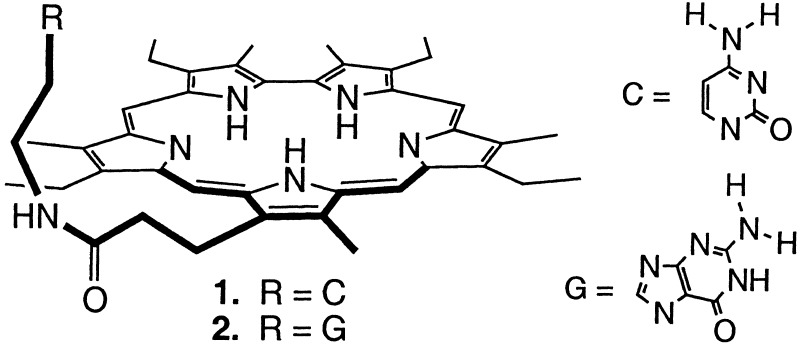
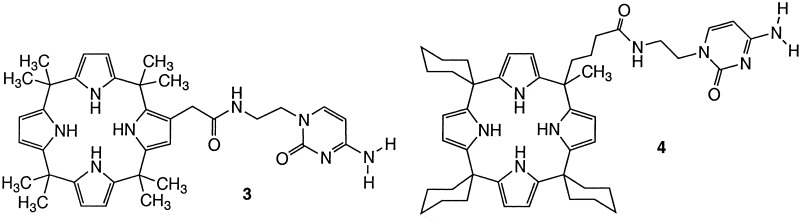
The design and synthesis of receptors that can be used to recognize, sense, or transport mononucleotides constitutes a current challenge for supramolecular and analytical chemists (1–18). Much of this challenge derives from the fact that mononucleotides are complex substrates, containing both anionic phosphorylated “ends” and species-specific nucleic acid base “tails.” The selective recognition of these water-soluble materials can thus only be achieved under conditions where (i) their inherently high energy of hydration is overcome and (ii) Watson–Crick or other ancillary selectivity-inducing interactions are used. Recently, we introduced a successful approach to nucleotide binding that was predicated on the use of nucleobase-substituted monoprotonated sapphyrins [e.g., structures l and 2 (Scheme S1)]. Here, complementary base-pairing effects were used to enhance the basic phosphate binding chemistry of sapphyrins such that selective recognition (19) and transport (4, 5, 8) of mononucleotides could be achieved at neutral pH. In this paper we report an extension of this approach that is based on the use of a non-sapphyrin phosphate binding core. Specifically, we describe the synthesis and characterization of two cytosine substituted calixpyrroles, systems 3 and 4 (Scheme S2), and detail how one of these, the meso-linked system 4, acts as a moderately selective receptor for its Watson–Crick complement, 5′-GMP, when tested as a through-CH2Cl2 model membrane carrier but not when incorporated into a poly(vinyl chloride) (PVC)-based ion-selective electrode (ISE).
Scheme 1.
Scheme 2.
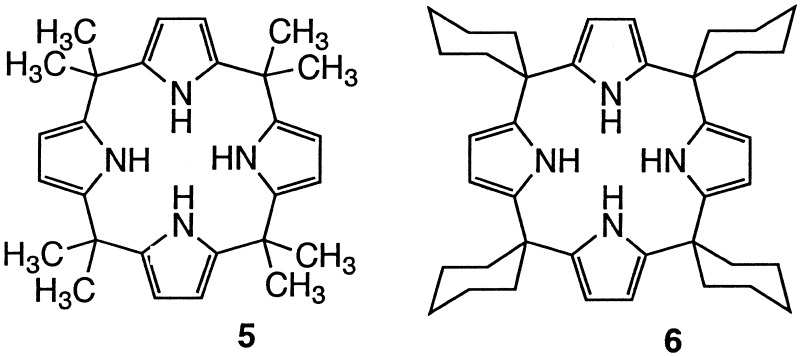
The calixpyrroles [e.g., 5 and 6 (Scheme S3)] are polypyrrole-based anion binding agents that differ from the sapphyrins in several important ways. First, they are neutral receptors that, in marked contradistinction to the sapphyrins, bind phosphate anions but weakly even in organic media (20). The question we sought to address, therefore, was whether calix[4]pyrroles, with or without ancillary nucleobase recognition units, would bind mononucleotides with sufficient affinity that they could be used to recognize these or other phosphorylated substrates under the aqueous–organic interfacial conditions associated with potentiometric measurements using ISE or through-model-membrane transport experiments.
Scheme 3.
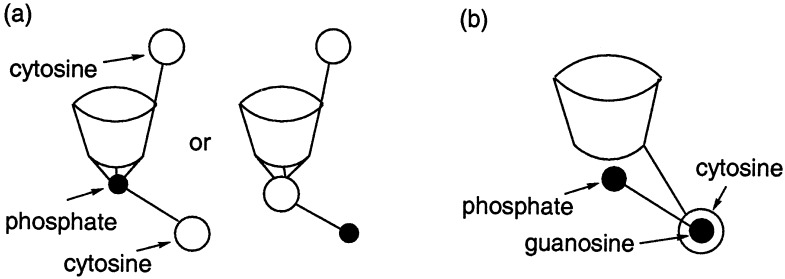
Apart from charge and reduced inherent for-phosphate anion affinity, the calix[4]pyrroles differ from the sapphyrins in terms of both shape and rigidity. Whereas the sapphyrins are flat, the calixpyrroles are three-dimensional objects that can exist in a number of different conformations (cone, partial cone, 1,2-alternate, or 1,3-alternate; ref. 20). What this means in practical terms is that a nucleobase substituent, when connected to the calix[4]pyrrole framework via a meso-tethered linker (e.g., 4), should allow for the cooperative recognition of a complementary mononucleotide substrate as shown in schematically in Fig. 1a. By contrast, an analogous system, bearing a nucleobase recognition subunit tethered via a short β-pyrrolic linkage (e.g., 3), should be unable to effect such ditopic binding. In this instance, nucleotide substrates would be expected to interact via a combination of nonspecific calixpyrrole NH-phosphate oxyanion attractions, pyrrole NH-nucleobase hydrogen bonds, and nucleobase–nucleobase interactions, as shown schematically in Fig. lb. In any case, it would be predicted that β-linked systems such as 3 would be less selective for Watson–Crick complementary targets such as 5′-GMP than the corresponding meso-linked analogues (e.g., 4). Nonetheless, they might display selectivities for nucleotides that are enhanced, or at least modified, relative to substituent free systems such as 5 and 6. To test this hypothesis we have synthesized the modified calixpyrroles 3 and 4, constructed ISEs based on 3-6, and have carried out competitive through-CH2Cl2 model membrane transport studies using compounds 3, 4, and 5.
Figure 1.
Schematic representation showing how receptor 3 can bind 5′-CMP and other nucleotides via two different “one point” binding modes (a), while its congener 4 can bind 5′-GMP in a complementary ditopic manner (b).
Materials and Methods
Synthesis: General Procedure for Preparing Cytosine-Calixpyrrole Conjugates.
The appropriate calix[4]pyrrole carboxylic acid (0.1 mmol; refs. 20 and 21) was dissolved in dry dichloromethane (25 ml) and cooled to 0°C. In accord with the generalized conjugation procedures published previously (8, 23, 24), diisopropylcarbodiimide (1.3 molar eq) was added, followed by 1-hydroxybenzotriazole (HOBt) and dimethylaminopyridine (DMAP) (both 3 mg), and 1-(2-aminoethyl)-4[(triphenylmethyl)amino]pyrimidin-2-one (0.12 mmol) (25). The reaction mixture was stirred for 20 h and then washed with water, and the organic phase dried over magnesium sulfate, filtered, and after removal of volatile components on the rotary evaporator, purified by column chromatography on silica gel, using dichloromethane–methanol (1–10%, gradient) as the eluent. The yields of 7 and 8 were 76 and 85%, respectively. Stirring with HOBt-trifluoroethanol for 3–4 days served to effect detritylation. Purification of the final products 3 and 4 by column chromatography [silica gel, dichloromethane–methanol (5–20%, gradient) eluent] afforded the final products in yields of 94 and 89%, respectively.
Trityl Protected β-linked Calix[4]pyrrole Cytosine Conjugate 7.
1H NMR (500 MHz, CDCl3) δ: 1.46–1.68 (overlapping singlets, 24H, CH3), 3.23 (t, 2H, CONHCH2CH2), 3.65 (CH2CONH), 3.83 (t, 2H, CONHCH2CH2), 5.03 (d, 1H, C5H), 5.62–5.88 (m, 6H, pyrrole CH), 5. 99 (m, 1H, pyrrole CH), 6.64 (s, 1 H, NHTr), 6.75 (d, 1H, C6H), 7.16–7.30 (m, 15H, TrH), 7.58 (s, 1H, CONH), 8.30, 8.40, 9.15, 9.30 (s 4H, NH pyrrole). 13C NMR (125 MHz, CDCl3 with 5% CD3OD) δ: 14.9, 18.9, 25.7, 26.8, 30.7, 30.8, 31.2, 31.9, 32.4, 40.6, 42.1, 43.8, 45.9, 46.3, 52.7, 53.42, 70.8, 94.2, 101.4, 102.9, 103.4, 107.5, 127.6, 128.4, 128.8, 134.4, 137.5, 138.5, 139.3, 140.6, 143.9, 145.8, 147.6, 156.4, 159.6, 165.8, 168.4, 172.9. HRMS: fast atom bombardment (FAB) HR: For C55H61N8O2 [MH+]: calculated 865.491749; found 865.490519.
β-Linked Calix[4]pyrrole Cytosine Conjugate 3.
1H NMR (500 MHz, CDCl3) δ: 1.52–1.82 (overlapping singlets, 24H, CH3), 3.15 (t, 2H, CONHCH2CH2), 3.88 (CH2CONH), 4.13 (t, 2H, CONHCH2CH2), 5.43 (d, 1H, C5H), 5.78–5:81 (m, 6H, pyrrole CH), 5.91 (m, 1H, pyrrole CH), 6.65 (d, 1H, C6H), 7.58 (s, 1H, CONH), 6.19, 6.27, 7.05, 7.57 (s 4H, NH pyrrole). 1H NMR (500 MHz, CDCl3 with 5% CD3OD) δ: 1.42–1.72 (overlapping singlets, 24H, CH3), 3.10 (t, 2H, CONHCH2CH2), 3.77 (CH2CONH), 4.03 (t, 2H, CONHCH2CH2), 5.05 (d, 1H, C5H), 5.68–5:85 (m, 6H, pyrrole CH), 5.95 (m, 1H, pyrrole CH), 6.67 (d, 1H, C6H). 13C NMR (125 MHz, CDCl3 with 5% CD3OD) δ: 14.5, 18.2, 25.5, 26.7, 30.4, 30.7, 30.9, 31.2, 31.9, 32.2, 32.4, 42.0, 43.9, 45.9, 46.1, 46.3, 53.42, 101.4, 102.9, 103.4, 107.5, 134.2, 137.5, 138.5, 139.3, 140.4, 147.6, 159.6, 168.4, 172.6. HRMS: FAB HR: For C36H46N8O2 [MH+]: calculated 622.3744; found 622.3751; for noncovalent dimer, C72H93N16O4, [MH+]: calculated 1245.756571; found 1245.755032.
Trityl Protected meso-Linked Calix[4]pyrrole Cytosine Conjugate 8.
1H NMR (500 MHz, CDCl3) δ: 1.02–1.18 (m, 18H, CH2), 1.22–1.56 (m, 12H, CH3), 1.88 (m, 2H, CH2), 2.05 (m, 2H, CH2), 3.25 (t, 2H, CONHCH2CH2), 3.65 (CH2CONH), 3.89 (t, 2H, CONHCH2CH2), 5.08 (d, 1H, C5H), 5.62–5.91 (m, 8H, pyrrole CH), 6.64 (s, 1 H, NHTr), 6.85 (d, 1H, C6H), 7.16–7.33 (m, 15H, TrH), 7.58 (s, 1H, CONH), 7.20 (s 1H, NH pyrrole), 7.25 (s 1H, NH pyrrole), 7.55 (s 1H, NH pyrrole), 7.62 (s 1H, NH pyrrole). HRMS: FAB HR: For C65H74N8O2 [MH+]: calculated 999.593474; found 999.593324.
meso-Linked Calix[4]pyrrole Cytosine Conjugate 4.
1H NMR (500 MHz, CDCl3) δ: 1.06–1.16 (m, 18H, CH2), 1.20–1.58 (m, 12H, CH2), 1.58 (s, 3H, CH3), 1.88 (m, 2H, CH2), 2.05 (m, 2H, CH2), 3.20 (t, 2H, CONHCH2CH2), 3.58 (m, 2H CH2CONH), 3.98 (t, 2H, CONHCH2CH2), 5.05 (d, 1H, C5H), 5.68–5.88 (m, 8H, pyrrole CH), 6.75 (d, 1H, C6H), 7.09 (s, 2H, NH pyrrole), 7.27(s, 1H, NH pyrrole), 7.57 (s, 1H, NH pyrrole). 13C NMR (125 MHz, CDCl3 with 5% CD3OD) δ: 12.7, 18.1, 24.3, 25.5, 26.8, 28.9, 29.4, 30.6, 30.9, 31.3, 31.8, 33.2, 38.3, 40.9, 45.1, 46.4, 47.8, 52.6, 53.5, 93.1, 94.7, 96.2, 96.4, 127.3, 128.0, 129.4, 133.4, 141.4, 146.2, 151.4, 156.352, 157.7, 165.8, 166.1, 173.1. HRMS: FAB HR: For C46H60N8O2 [MH+]: calculated 756.48389; found 756.484584; for noncovalent dimer, C92H121N16O4 [MH+]: calculated 1513.975673; found 1513.974559.
Electrode Preparation and ISE Measurements.
Ion-selective membranes were prepared in accord with the procedure used to prepare ISEs containing calix[4]pyrrole 5 (12). In the present study, ≈0.7 ml THF was used to dissolve approximately 100 mg of a mixture composed of 3 wt % of the receptor in question, 22 wt % PVC, and 75 wt % o-NPOE. The resulting membrane, obtained following evaporation as before, was mounted on an electrode body (Crytur, Czech Republic). Control electrodes, containing just TDDMACl, were prepared as reported (12).
EMF measurements were performed with a digital voltammeter, Model
M1T330 (Metra s.p., Blansko, Czech Republic) with the following cell
assembly: Hg | Hg2Cl2 | 3 M KCl ∥ 0.1 M
HEPES–NaOH pH 6.6 ∥ sample | modified PVC-membrane | 0.1 M KCl
| AgCl | Ag. All potentiometric analyses were carried out at
ambient temperature. The pH was monitored using glass electrode Type
01–29 B (Labio Prague, Czech Republic) on pH-Meter Type OP–205/1
(Budapest). In the studies of potentiometric response and anion
selectivity, working solutions of the analytes in question were
prepared by diluting concentrated stock solutions with 0.1 M HEPES
adjusted to pH 6.6 with NaOH. Calibration curves were constructed by
plotting the potential vs. logarithm of concentration of the anion
present in the buffer solution. Anion concentrations rather than
activities were used because it is difficult to estimate activity
coefficients in the zwitterionic buffer. Before starting the studies,
the electrodes were soaked overnight in 0.1 M HEPES pH 6.6 solution in
the absence of analyte. Potentiometric selectivity coefficients
(K ) were then
determined by the separate solution method (26), with the primary and
interfering ion concentrations being 1.0 ×
10−2 for the PVC membranes derived from
3 and 4, which were conditioned overnight in 0.01
M 5′-UMP and 5′-CMP, respectively. The selectivity sequence reported
here corresponds to the observed position of the tested nucleotides on
the potentiometric curve and the selectivity sequence determined by the
matched potential method (27).
) were then
determined by the separate solution method (26), with the primary and
interfering ion concentrations being 1.0 ×
10−2 for the PVC membranes derived from
3 and 4, which were conditioned overnight in 0.01
M 5′-UMP and 5′-CMP, respectively. The selectivity sequence reported
here corresponds to the observed position of the tested nucleotides on
the potentiometric curve and the selectivity sequence determined by the
matched potential method (27).
Transport Studies.
Transport studies were performed as described (8) with the exception that tetrabutylammonium perchlorate (0.1 mM) was added to the lipophilic phase, which contained the carrier at the same concentration. The pH of the initial phase, containing the nucleotide at a concentration of 10 mM, was adjusted to a value of 6.0 by the addition of NaOH or H2SO4, as necessary. The extent of nucleotide transport was quantified using an HPLC-based analysis as described (28).
Results and Discussion
Synthesis and Characterization.
The β- and meso-linked calixpyrroles 3 and 4 were prepared using a strategy analogous to that used to prepare the cytosine-functionalized sapphyrin 1 (4, 8). This chemistry, illustrated in Scheme S4 for the specific case of 3, involves reacting the appropriate β- and meso-“hook” carboxylic acids (21, 22) with trityl-protected aminoethylcytosine (25) under standard peptide coupling conditions [diisopropylcarbodiimide (DIPC), 1-hydroxybenzotriazole (HOBt), dimethylaminopyridine (DMAP)] and then subjecting the resulting intermediate species 7 (cf. Scheme S4) and 8 (structure not shown) to deprotection. Because of the sensitivity of the calix[4]pyrrole ring to acid, trifluoroacetic acid was not used to effect this latter transformation. Rather, more neutral conditions (HOBt; CF3CH2OH) were used. Purification by column chromatography (silica gel; methanol (5–20%) in dichloromethane, eluent) than gave the desired products, 3 and 4, in overall yields of 75–79%.
Scheme 4.
Compounds 3 and 4 both gave NMR spectral and mass spectrometric data consistent with their proposed structures. However, in addition to the dominant peak expected for the monomeric form of these conjugates, small peaks (ca. 10% intensity) corresponding to twice the expected mass were observed in the high-resolution FAB mass spectra of 3 and 4, but not their trityl protected precursors 7 and 8. Broad peaks were also observed when the 1H NMR spectra of 3 and 4 were recorded in pure CDCl3, but not when recorded in CDCl3 containing 5% CD3OD. On the other hand, for the spectra recorded in CDCl3, the position of the NH proton signals in 3 and 4 were found to be only slightly shifted (Δδ generally less than 0.5 ppm) as compared with what is seen for various simple amide and ester derivatives of the calix[4]pyrrole carboxylic acids from which they were prepared (20–22) or for the free calix[4]pyrroles 5 and 6 (20). Such observations are consistent with the formation, at least as minor equilibrium species, of “head-to-tail” narcissistic dimers or possibly higher order aggregates, wherein the cytosine “tail” is bound to the calix[4]pyrrole “head” of a second conjugate.¶ To the extent that such dimers are formed they would support the notion that direct interactions between the calix[4]pyrrole NH hydrogen bond donors and nucleobase portion of 5′-CMP and other nucleotides are possible and that interactions such as these, as well as direct calix[4]pyrrole phosphate binding, might need to be considered in analyzing the results of the transport and ISE experiments. Unfortunately, efforts to quantify the extent of dimerization by NMR spectroscopic methods were thwarted by the low solubility of 3 and 4 in pure chloroform or dichloromethane. Similar considerations limited our ability to measure the affinity constants for the interaction of 5′-GMP and 5′-CMP with 5 or 6. However, previous work has served to show that small carbonyl containing species [e.g., dimethylformamide (DMF)] can be bound to simple calix[4]pyrroles with affinity constants of ≈10–15 M−1 in C6D6 at room temperature (29).
Transport Studies.
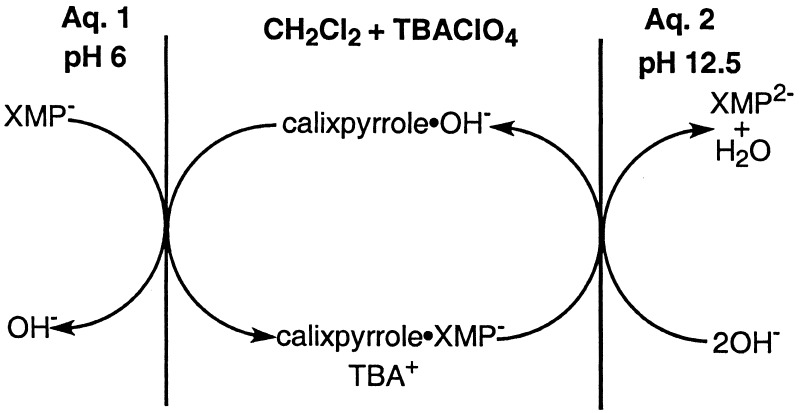
Once in hand, systems 3 and 4 were tested as carriers for 5′-GMP and analogous nucleotides. This test was done using a Pressman-type model-membrane set up similar to that used previously to test carriers 1 and 2 (4). This model membrane, shown schematically in Scheme S5, consists of (i) an initial pH 6.0 aqueous phase (Aq. 1) containing a mixture of nucleotide monophosphates (5′-XMP; X = A, G, and C; 10 mM each), (ii) an intervening dichloromethane layer containing a 1:1 mixture (0.1 mM each) of the calix[4]pyrrole carrier in question (i.e., 3, 4, or 5) and tetrabutylammonium perchlorate (TBAClO4), and (iii) an aqueous 10 mM NaOH (pH ≈12.5) receiving phase, Aq. 2. The transport experiment itself thus consists of monitoring the rate of transport of a given substrate through the “membrane” by determining its build-up in Aq. 2 as a function of time. Therefore, to the extent that it is observed, selective transport can reflect a range of factors, including selective binding and enhanced extraction out of Aq. 1, improved organic solubility of the specific receptor-substrate supramolecular complex in question, and hence augmented rates of through-dichloromethane transport, as well as, possibly, more effective substrate release at the “membrane”–Aq. 2 interface. Although the need to consider these and other factors introduces a complexity into the analysis of transport-related results, at the most fundamental level the observation of enhanced transport for a given species within a series of like substrates does at least imply a degree of recognition specificity. As a result, we and others have used model membrane transport studies to provide a quick read as to whether a given system can or cannot act as a specific receptor (30).
Scheme 5.
The present model membrane differs in one important way from that we have used previously: it contains TBAClO4 in the organic “membrane” phase. The presence of this additive reflects the fact that, in contrast to what proved true for the sapphyrin-based carriers 1 and 2, species that are monoprotonated at neutral pH (31), little or no transport was seen using calix[4]pyrroles as putative carriers unless this organic soluble salt was added to the dichloromethane phase. Presumably, the TBAClO4 serves as a source of hydrophobic tetrabutylammonium counter cations that neutralize the charge of the complex anion formed between monobasic 5′-XMP and neutral calix[4]pyrrole, thus facilitating transport (cf. Scheme S5). Also required for transport is a basic receiving phase (kT < 10−13 mol⋅cm2⋅h when the pH of Aq. 2 is 7); it presumably serves to convert the nucleotide into its more hydrophilic dianionic form, thereby facilitating release.
Under the above conditions, good selectivity for 5′-GMP was seen for the meso-substituted carrier 4 (cf. Fig. 2 Left), although the absolute rate of transport (kT = 9.26 × 10−10 mol⋅cm2⋅h) proved to be an order of magnitude lower than what was found when the corresponding sapphyrin-based carrier, 1, was used (kT = 1.42 × 10−8 mol⋅cm2⋅h) (8). Replacing 4 by its β-linked analogue 3 ([TBAClO4] = [3] = 0.1 mM) served to lower further the transport rate and produce a system that was selective for 5′-CMP, rather than 5′-GMP (kT = 4.4 × 10−10, 9.8 × 10−11, and 5.6 × 10−11 mol⋅cm2⋅h for 5′-CMP, 5′-GMP, and 5′-AMP, respectively). No appreciable change in 5′-CMP transport rate was seen when the control system 5 was used. However, this “cytosine-free” analogue of 3 was found to be even more selective for 5′-CMP over 5′-GMP, displaying a kT ratio on the order of 31:1.
Figure 2.
Results of model through-membrane transport experiments conducted using the β- and meso-substituted cytosine functionalized calix[4]pyrrole carriers 3 (Left) and 4 (Right). See text for details.
Taken together, the above findings are consistent with carrier 4 acting as a ditopic receptor and binding both the phosphate “head” and purine “tail” of 5′-GMP in a “two-point”-like fashion as shown schematically in Fig. 1a and more explicitly in structure 9 (see Scheme S6). The present results also support the notion that the β-linked system 3 acts as a mixed receptor binding both the phosphate and nucleobase portions of nucleotide monophosphates at the calix[4]pyrrole NH hydrogen bond donor sites and the guanosine portion of 5′-GMP with its cytosine “tail” (cf. Fig. 1b). Although the latter interactions are potentially significant, they do not overcome the high inherent selectivity for 5′-CMP displayed by simple unsubstituted calix[4]pyrroles, such as 5. The net result is a monotopic (i.e., “single-point”) carrier that transports 5′-GMP more effectively than a control calix[4]pyrrole system lacking a cytosine “tail” but still far less efficiently than its congener 4, in which this “tail” is properly oriented so as to allow for the concurrent complexation of both the nucleobase and phosphate portions of 5′-GMP.
Scheme 6.
Ion-Selective Electrode Studies.
Carrier-based ISEs provide another means of testing whether a given
receptor displays selectivity for a targeted analyte (1, 3, 6, 7, 9,
11–13). Like the bulk membrane transport studies described above, this
method can provide insight into recognition events that take place at
an aqueous–organic interface but does not involve a direct monitoring
of binding (and/or release) per se. Rather, what is
studied is the change in membrane potential observed on exposure of a
liquid or polymeric electrode to solutions of various putative
analytes. Read-out parameters thus include response (total emf change
engendered by a given concentration of analyte), sensitivity (change in
emf as function of analyte concentration), and selectivity, often
expressed in relative terms as a selectivity coefficient
K , where
I and J represent the two competing analytes in
question, and the linear range over which the response, Nernstian or
otherwise, is seen.
, where
I and J represent the two competing analytes in
question, and the linear range over which the response, Nernstian or
otherwise, is seen.
In previous work, we demonstrated that calix[4]pyrroles, such as 5, show near-Nernstian responses to a range of anionic analytes, including phosphates, when incorporated into PVC–ortho-nitrophenyl octyl ether (o-NPOE) membranes and tested as ISEs (12). We were thus keen to see whether appending a cytosine “tail” onto the calixpyrrole skeleton would lead to the generation of nucleotide-specific ISEs and, to the extent this proved true, whether or not the choice of linkage (meso vs. β) would effect the response selectivity.
Before analyzing the “tailed” systems 3 and
4, the unfunctionalized calix[4]pyrroles 5 and
6 were tested as ISE sensor elements. These species were
thus incorporated into PVC–o-NPOE membranes and the
potential response as a function of analyte concentration was measured
using a standard Hg | Hg2Cl2 | 3 M KCl ∥
0.1 M HEPES–NaOH, pH 6.6. ∥ sample | modified PVC membrane | 0.1
M KCl | AgCl | Ag cell assembly. In both cases, little in the way
of pH-dependent behavior was seen at or near neutral pH. On the other
hand, an inherent selectivity for 5′-AMP < 5′-GMP ≈
5′-CMP < 5′-UMP ≈ 5′-TMP was observed at pH 6.6 (a value
chosen to ensure a significant concentration of the dianionic
form)‖ for both
systems as judged from the extent of the anionic (negative)
potentiometric response. (The total emf change at an analyte
concentration of 10−2 M was, relative to no
analyte, −6.3, −20, −20, −24, −24 and −15, −51, −52, −72, −77
mV for 5′-AMP, 5′-GMP, 5′-CMP, 5′-UMP, and 5′-TMP in the case of
5 and 6, respectively.) By contrast, a slight
selectivity for 5′-GMP and 5′-CMP was seen in the case of
“control” electrodes made up from the hydrophobic cation,
tridodecylmethylammonium chloride (TDDMACl): 5′-AMP (0.00) <
5′-UMP (0.07) < 5′-CMP (0.24) < 5′-GMP (0.76), where the
values in parentheses refer to the selectivity coefficients
(logK ) calculated as
detailed in Materials and Methods.
) calculated as
detailed in Materials and Methods.
Taken together, these findings are consistent with the conclusion that unfunctionalized calixpyrroles mediate their observed ISE response for nucleotides by acting more as specific, nucleobase-dependent molecular recognition elements than as pure anion extractants, as is known to be true for membranes made up from TDDMACl. Support for this conclusion comes from the observation that across the board a greater response is observed in the case of the more hydrophobic cyclohexyl-substituted system 6 than in the octamethyl system 5 and that the selectivity pattern correlates with the number of accessible hydrogen bond acceptor elements (i.e., carbonyl groups) present in the nucleobase portion of the mononucleotides being studied [i.e., 5′-UMP ≈ 5′-TMP (two carbonyls) > 5′-GMP ≈ 5′-CMP (one carbonyl) > 5′-AMP (no carbonyls)]. This latter observation also rules out a response process that is dominated by direct phosphate-calixpyrrole “anion chelation” and leads rather to the inference that under the interfacial conditions of the ISE experiment, it is the strength and specificity of the nucleobase-calix[4]pyrrole NH interactions that dominates the selectivity, even if it is the presence of the negatively charged phosphate groups that leads to the actual observation of an anionic potentiometric response. Unfortunately, PVC membranes of the type used here often contain anionic impurities that can act as inherent cation exchangers. This adds a complicating factor that makes direct comparisons between the ISE and transport results difficult.
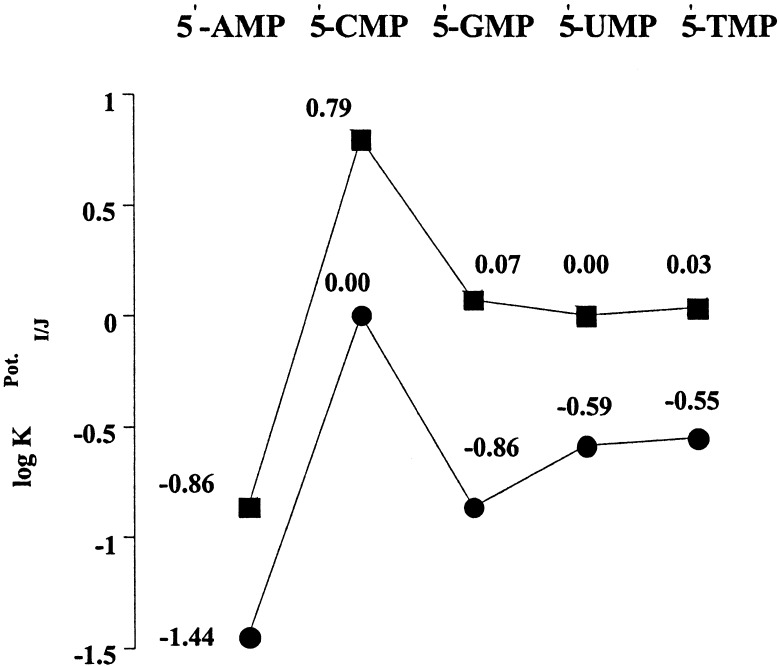
The above considerations provide a framework for what would otherwise be a set of difficult to comprehend results; namely that both cytosine “tailed” calix[4]pyrrole derivatives, 3 and 4, show a greater specificity for 5′-CMP than 5′-GMP. As can be seen by inspection of Fig. 3, this selectivity is actually somewhat greater in the case of the meso-substituted system 4.** Such an observation is consistent with the overall selectivity for 5′-GMP being reduced in both 3 and 4 as compared with 5 and 6. Specifically, it is proposed that in the functionalized systems 3 and 4, base-pairing between the purine moiety present in 5′-GMP with the cytosine “tail” competes with the “normal” pyrrole NH-nucleobase carbonyl interactions invoked in the case of 5 and 6.‡‡ These base-pairing interactions would have the effect, more so in the case of 4 than in 3, of orienting the nucleotide phosphate group such that it can interact with the calix[4]pyrrole NH core (vide supra). This, in turn, would reduce the amount of “bare” anionic charge that could serve to mediate a potentiometric response. By contrast, in the case of 5′-CMP, base-pairing effects are likely to be small for both 3 and 4, with the consequence that the same high level of anionic response seen in the case of the control systems 5 and 6 should be observed, as is indeed seen by experiment.
Figure 3.
Comparison of potentiometric selectivities of PVC membranes based on
β- and meso-substituted cytosine-functionalized
calix[4]pyrroles 3 (■,
logK ) and
4 (●,
logK
) and
4 (●,
logK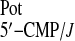 ).
).
Consistent with the idea that base-pairing effects are more important
in the case of 4 than 3 is the finding that
adding 50 mol% TDDMACl to the PVC membranes containing these receptors
did not effect appreciably the selectivity of the ISEs derived from
3 but gave rise to selectivities in the case of 4
that approximate those seen in the transport experiments. Specifically,
at pH 6.6 logK values of
0.00, 1.53, and 2.23 were seen for 5′-CMP, 5′-AMP, and 5′-GMP,
respectively. Unfortunately, the inherent selectivity for 5′-GMP seen
in membranes containing just TDDMACl (vide supra)
complicates analysis of these findings.
values of
0.00, 1.53, and 2.23 were seen for 5′-CMP, 5′-AMP, and 5′-GMP,
respectively. Unfortunately, the inherent selectivity for 5′-GMP seen
in membranes containing just TDDMACl (vide supra)
complicates analysis of these findings.
Conclusion
The present study serves to show that neutral anion recognition systems, although lacking the high inherent anion affinities characteristic of charged receptors (e.g., sapphyrins), can nonetheless be made to act as specific nucleotide carriers, provided that they are appropriately functionalized. Here, severe design constraints are imposed that reflect both the structure of both the targeted substrate, 5′-GMP in the present instance, and the structure and conformation of the flexible calix[4]pyrrole skeleton. However, if these are considered, good selectivities can be achieved.
The design paradigm that allows for the construction of effective and selective nucleotide carriers, at least as judged from the present model membrane studies, breaks down when it comes to the construction of ISEs. In this case, the presence of a complementary “tail,” in a Watson–Crick base-pairing sense, is seen to engender a “negative selectivity” in the absence of TDDMACl that was found to favor 5′-CMP in the present instance. Nonetheless, the fact that the inherent specificity of a PVC-derived ISE can be altered by the use of functionalized calix[4]pyrroles augurs well for the generation of electrodes that can recognize and sense selectively a range of targeted analytes. Work along these lines is currently in progress.
Acknowledgments
Support for this project came from National Institutes of Health Grant GM 58907 (to J.L.S.), Texas Advanced Research Program Grant 0059 (to J.L.S.), Ministry of Education of the Czech Republic Grant CEZ J19/98:223400008 (to V.K.), and the Grant Agency of the Czech Republic (301/98/KO42 to V.K.). P.A.G. thanks the Royal Society for a University Research Fellowship.
Abbreviations
- ISE
ion-selective electrode
- PVC
poly(vinyl chloride)
- FAB
fast atom bombardment
- TDDMACl
tridodecylmethylammonium chloride
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Precedent for the formation of “head-to-tail” dimers exists in the case of calix[4]pyrroles bearing stronger hydrogen bond acceptor groups (see ref. 22).
For pKa's values of the nucleotides studied in this work, see ref. 32.
In the case of 4, a good Nernstian response for 5′-CMP of (−29.5 mV/decade) was seen over the range of 1.0 × 10−4 M to 1.0 × 10−2 M, whereas in the case of 3 the best Nernstian behavior was seen in the case of 5′-GMP (−26.0 mV/decade) and 5′-UMP (−27.5 mV/decade) over a range of 1.0 × 10−3 M to 1.0 × 10−2 M and 1.0 × 10−5 M to 1.0 × 10−2 M, respectively. Because of the lack of Nernstian response seen for the other nucleotides in question, comparisons of selectivity were made using the matched potential method. With 5′-AMP at 10−6 M as the background, the concentration of the interfering anion was varied (up to n ×10−8 M).
Support for the notion that Watson–Crick molecular recognition processes can be important under the aqueous–organic conditions of the ISE experiments comes from previous studies of PVC membranes incorporating lipophilic cytosine derivatives; these displayed greater selectivity for 5′-GMP over 5′-AMP (see ref. 6).
References
- 1.Umezawa Y, Kataoka M, Takami W, Kimura E, Koike T, Nada H. Anal Chem. 1988;60:2392–2396. doi: 10.1021/ac00172a014. [DOI] [PubMed] [Google Scholar]
- 2.Furuta H, Furuta K, Sessler J L. J Am Chem Soc. 1991;113:4706–4707. [Google Scholar]
- 3.Tohda K, Tange M, Odashima K, Umezawa Y, Furuta H, Sessler J L. Anal Chem. 1992;64:960–964. doi: 10.1021/ac00032a023. [DOI] [PubMed] [Google Scholar]
- 4.Král V, Sessler J L, Furuta H. J Am Chem Soc. 1992;114:8704–8705. [Google Scholar]
- 5.Sessler J L, Furuta H, Král V. Supramolec Chem. 1993;1:209–220. [Google Scholar]
- 6.Tohda K, Naganawa R, Lin X M, Tange M, Umezawa K, Odashima K, Umezawa Y, Furuta H, Sessler J L. Sensors Actuators B. 1993;13–14:669–672. [Google Scholar]
- 7.Odashima K, Naganawa R, Radecka H, Kataoka M, Kimura E, Koike T, Tohda K, Tange M, Furuta H, Sessler J L, Yagi K, Umezawa Y. Supramolec Chem. 1994;4:101–113. [Google Scholar]
- 8.Král V, Sessler J L. Tetrahedron. 1995;51:539–554. [Google Scholar]
- 9.Amemiya S, Bühlmann P, Tohda K, Umezawa Y. Anal Chim Acta. 1997;341:129–139. [Google Scholar]
- 10.Aoki S, Honda Y, Kimura E. J Am Chem Soc. 1998;120:10018–19926. [Google Scholar]
- 11.Bühlmann P, Amemiya S, Nishizawa S, Xiao K P, Umezawa Y. J Inclusion Phenom Mol Recognit Chem. 1998;32:151–163. [Google Scholar]
- 12.Král V, Sessler J L, Shishkanova T V, Gale P A, Volf R. J Am Chem Soc. 1999;121:8771–8775. [Google Scholar]
- 13.Picioreanu S, Poels I, Frank J, van Dam J C, van Dedem G W K, Nagels L J. Anal Chem. 2000;72:2029–2034. doi: 10.1021/ac991294d. [DOI] [PubMed] [Google Scholar]
- 14.Sirish M, Schneider H-J. J Am Chem Soc. 2000;122:5881–5882. [Google Scholar]
- 15.Sirish M, Schneider H-J. J Am Chem Soc. 2000;122:11274–11274. [Google Scholar]
- 16.Aoki S, Kimura E. J Am Chem Soc. 2000;122:4542–4548. [Google Scholar]
- 17.Anda C, Llobet A, Salvado V, Reibenspies J, Motekaitis R J, Martell A E. Inorg Chem. 2000;39:2986–2999. doi: 10.1021/ic990818p. [DOI] [PubMed] [Google Scholar]
- 18.Sebo L, Diederich F, Gramlich V. Helv Chim Acta. 2000;83:93–113. [Google Scholar]
- 19.Sessler J L, Genge J W, Král V, Iverson B L. Supramolec Chem. 1996;8:45–52. [Google Scholar]
- 20.Gale P A, Anzenbacher P, Jr, Sessler J L. Coord Chem Rev. 2001;222:57–102. [Google Scholar]
- 21. Gale, P. A., Sessler, J. L., Allen, W. E., Tvermoes, N. A. & Lynch, V. (1997) Chem. Commun. 665–666.
- 22.Sessler J L, Andrievsky A, Gale P A, Lynch V. Angew Chem Int Ed Engl. 1997;35:2782–2785. [Google Scholar]
- 23.Sessler J L, Gale P A, Genge J W. Chem Eur J. 1998;4:1095–1099. [Google Scholar]
- 24.Sessler J L, Gebauer A, Gale P A. Gazz Chim Ital. 1997;127:723–726. [Google Scholar]
- 25.Sessler J L, Magda D, Furuta H. J Org Chem. 1992;57:818–826. [Google Scholar]
- 26.Umezawa Y, Umezawa K, Sato H. Pure Appl Chem. 1995;67:507–518. [Google Scholar]
- 27.Bakker E, Pretsch E, Bühlmann P. Anal Chem. 2000;72:1127–1133. doi: 10.1021/ac991146n. [DOI] [PubMed] [Google Scholar]
- 28.Furuta H, Cyr M J, Sessler J L. J Am Chem Soc. 1991;113:6677–6678. [Google Scholar]
- 29.Allen W E, Gale P A, Brown C T, Lynch V M, Sessler J L. J Am Chem Soc. 1996;118:12471–12472. [Google Scholar]
- 30.Allen W E, Sessler J L. ChemTech. 1999;29:16–24. [Google Scholar]
- 31.Iverson B L, Shreder K, Král V, Sansom P, Lynch V, Sessler J L. J Am Chem Soc. 1996;118:1608–1616. [Google Scholar]
- 32.Dawson R M C, Elliott D C, Elliott W H, Jones K M. Data for Biochemical Research. 3rd Ed. Oxford: Clarendon; 1986. [Google Scholar]