Abstract
Olanzapine is a well-known atypical, second-generation, antipsychiatric drug approved by the FDA. Recently, it is being witnessed as a party abusive drug discretely or in combination and as being an antagonist causing seduction. The current study is reported for the first time, and it focuses on a new and sustainable extraction cum detection method to effectively identify the olanzapine drug that has been deliberately infused in two different edible matrices. It entails a new magnetic dispersive microextraction method employing maleimide-coated Fe3O4 nanoparticles to selectively and significantly isolate the drug from the edible matrices, while on the other side, a reliable, efficient, analytical method was developed using AQbD (i.e., Box–Behnken design) in the presence of LC-UV and was also validated. The method’s linearity at an R 2 of 0.9997 for the standard; ∼0.997 for extraction; sensitivity as 1.4 μg/mL for the LOD and 4.9 μg/mL for the LOQ; and accuracy with 97–99% recovery was proved diligently. Complementary to the study, a confirmative analysis with LC-MS was conducted for a more steadfast result. The sustainability of the method has been ensured by opting for recent and innovative evaluating tools. The three major principles of sustainability were assessed by three prominent greenness tools, a blue and red analyzing tool, and more wholly the latest RGBfast software to assess all three principles. The results were indicative of the good eco-friendliness and environmental sustainability of the method.
1. Introduction
Olanzapine (OLZ) is a class of thienobenzodiazepines used to medicate antipsychotic disorders, mainly schizophrenia, bipolar disorder, depression, PTSD (post traumatic stress disorder), and Alzheimer’s disease. It is categorized under second-generation atypical drugs, i.e., a first-line antipsychotic drug with reduced side effects relatively, compared to first-generation ones. OLZ once induced into the human body acts on many neurotransmitters. Predominantly, it acts on dopamine receptors that mainly reduce psychotic diseases and on serotonin receptors, i.e., 5-HT2A and 5-HT2C, that stimulate dopamine secretions. Additionally, it also acts on histamine (H1), α-adrenergic, and muscarinic receptors, leading to side effects such as drowsiness, weight gain, and sedation. As a cause of nonselective binding, OLZ causes euphoria and sedation that has caused it to be eventually taken advantage of by illegally misusing the drug, despite it being a prescribed medication, and it has also been enlisted as a ‘Knockout Drug’. Although it does not have an amnesic effect as effective as common powerful DRDs (date rape drugs) like rohypnol and valium, OLZ at higher doses causes confusion and partial memory loss in victims. , As a result, OLZ as such or in combination with other seductive drugs is misused as a modern date rape drug in DFSA (drug-facilitated sexual assault) and DFC (drug-facilitated crime). It has become the most severe offense to deliberately infuse DRDs into convenient foods and drinks without altering their properties. This eventually causes blackouts in the victim and poses temporary forgetfulness, which makes it easy for the culprits to commit crimes such as sexual assault, robbery, or kidnap. , As a measure, there are many portable detection methods to mention color change/spot tests, colorimetric sensors, and DRD test coasters. , Despite their merits, there are some key drawbacks to focus on. These tests can furnish false positive/negative results and have matrix limitations; to be more specific, the DRD test coaster is not suitable for milk-related or acidic-nature-bearing drinks. For this reason, it is indispensable to develop reliable, sensitive analytical methods using high-end techniques. In spite of literatures with different analytical method developments for the detection of the OLZ drug alone and in the combination form individually or in biological matrices like blood, saliva, and hair, there are no reports on the analytical detection of OLZ from food matrices as other related DRDs (zolpidem, benzodiazepine, ketamine, flunitrazepam) having methods reported in edible matrices. ,
The LC chromatographic separation coupled with varied detectors, such as PDA (photodiode array), DAD (diode array detector), fluorescence, and refractive index depending on application, has prevalently opted for analytical detection of pharmaceutical and forensic-related drugs. Of them, RP-HPLC (reverse-phase high-performance liquid chromatography) with a PDA detector has become more common due to it being easily available, very economic, and yet bringing up reliable results. The method development has been accomplished using a systematic paradigm, AQbD (analytical quality by design), as recommended by the ICH guidelines and the FDA, for an economic and robust method optimization and to obtain effective outcomes. Of several AQbD strategies, Box–Behnken design (BBD) has been opted for the current study due to its significant merits. Moreover, LC-MS is a vivid tool that provides improved specificity, selectivity, and sensitivity, yet has few limitations to mention such as difficulty in availability, high cost, and high energy consumption. Hence, for affordability and greenness, the method development has been carried out with RP-HPLC and confirmation by LC-MS analysis for enhanced results.
Drug detection from food and biological matrices necessitates crucial, efficient sample preparation steps. This is eminent, as these matrices comprise complex compounds, and detection remains challenging. There are numerous extraction procedures for sample preparation. The most common conventional liquid–liquid extraction (LLE) or protein extraction has high drawbacks. To mention, use of large harmful solvents poses a threat to the analyst and the environment. To surpass the issue, solid-phase extraction has come into play that reduces solvent usage and uses solid sorbents for specific isolation of analytes. Yet for more specificity, time efficiency, and drastic reduction of harmful organic solvents, alternative methods are into the field. As well known, nanoparticles have shown their efficacies in multiple fields and, consequently, they have also paved their way into analytical extraction. , In particular, magnetic nanoparticles in extraction have received wide attention in sample preparation due to their unique advantages of magnetism and specificity. − Besides, aiming for a greener and cost-effective method, the trending miniaturization is into the role, which imparts downscaling in each step, aiming to hugely decrease the solvent, sample, and chemical consumption and bring about waste and time control, without compromising the accuracy. This magnetic dispersive microextraction (MDME) renders many advantages like better efficiency and less labor and analysis time, and it is a greener method as it is both recyclable and low-cost. , Hence, the present work includes the MDME method in the efficient extraction of the olanzapine drug from food matrices.
The global challenges, threats, and catastrophes have by now already taken a top seat in our society. In consideration, sustainable development goals have been the target for many years. As part of this, green chemistry by Anastas and Warner took a transformative approach heeding toward sustainability by articulating certain guidelines of 12 principles to be followed in research and industrial fields. Here, the word ‘green’ not only defines environmental safety but also the economic nature. , In recent decades, in the notion to mitigate the toxicity and huge wastages as chemicals and energy, a field like analytical chemistry requires high-energy instruments, huge solvents, and chemical usage, and here is where GAC (green analytical chemistry) is taking its pace. As a matter of need, diverse software applications are currently emerging. More recently, white analytical chemistry has additionally vitalized sustainability by latest software versions and is currently a part of assessing analytical methods. − Of many green and white software, the most preferred, latest software versions have been used in the current study, some of which are Complex MoGAPI (modified green analytical procedure index), ChlorTox, RGBfast (red green blue fast), AGREE (analytical GREEnness), BAGI (blue analytical grade index), RAPI (red analytical performance index), and greenness index by the spider chart and GSST (green solvent selection) tool. The sustainability assessment was performed as a comparative study of the current method with previous methods for better understanding of the difference in sustainability from the present developed method.
2. Materials and Methods
2.1. Chemicals and Instruments Required
All of the solvents and chemicals opted for this experiment are of analytical (HPLC) and reagent grade. The FeCl3.6H2O salt was purchased from MolyChem (Mumbai, India) having 98% purity, while FeCl2.4H2O was purchased from Avra Chemicals (Mumbai, India) with 98.50% purity. 25% aqueous ammonia was obtained from Finar (Ahmedabad, India). Hydrochloric acid (HCl) with 32% purity was bought from Avra Chemicals (Mumbai, India). Tetraethyl orthosilicate (TEOS) and polyethylene imine (PEI) were procured from TCI Chemicals (Hyderabad, India) having 98% purity. Aminopropyl triethoxysilane (APTES) of 98% purity was obtained from Spectrochem. With 99% purity, trifluoroacetic acid (TFA) was taken from Sisco Research Laboratory (SRL) (New Mumbai, India). Glacial acetic acid of 99.7% purity was obtained from Qualigens (Mumbai, India). HPLC-grade methanol and acetonitrile were obtained from Honeywell (New Delhi, India), while HPLC-grade water was obtained from Finar Chemicals (Ahmedabad, India).
The Shimadzu-UV 2600 (Japan) instrument model was opted for UV–visible spectroscopy, while the Shimadzu-IR prestige-21 (Japan) instrument was used to perform Fourier transform infrared (FTIR) spectroscopy analysis. A Waters Alliance 2690 model HPLC system was furnished with a Waters 996 photodiode array (PDA) as the detector (USA) for high-performance liquid chromatography (HPLC) analysis. For working on X-ray diffraction (XRD), a Rigaku SmartLab model provisioned with SmartLab Studio II software (Japan) was preferred. A Zeiss EVO LS15 instrument accompanied by an FE (field emission) source (Germany) was opted for FE-SEM-EDX studies. Transmission electron microscopy was performed using a Tecnai 12, FEI transmission electron microscope (Netherlands), while zeta potential analysis was done using a DelsaMax Pro analyzer (Beckmann Coulter).
2.2. Preparation of Core and Functionalized Magnetic Nanoparticles
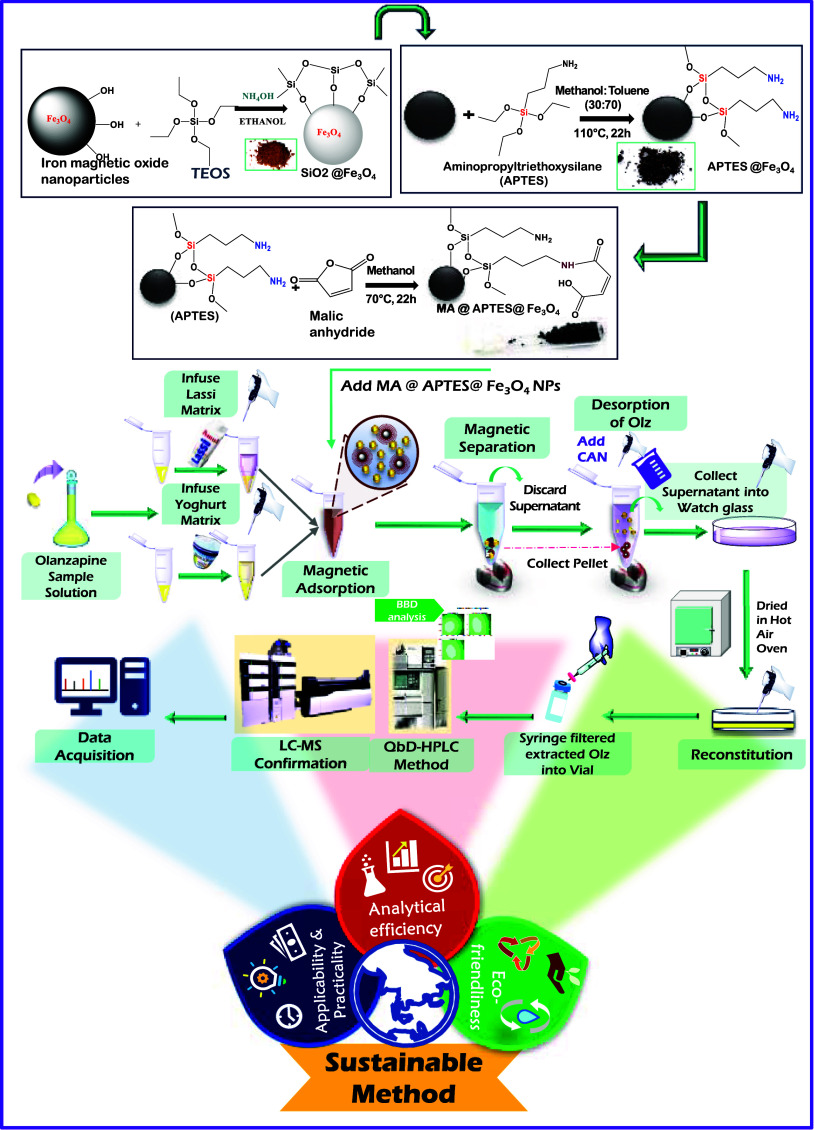
The core Fe3O4 nanoparticles, followed by the silica, PEI, and APTES coating process, have been prepared as previously reported with slight modifications in the method. , Briefly, core Fe3O4 nanoparticles were prepared via coprecipitation of Fe3+ and Fe2+ of chloride salts in 0.1 N HCl under the pressure of nitrogen for an inert nature. After 6 h of magnetic stirring, 25% ammonia is added that changes the orangish brown precipitate to a black precipitate that primarily confirms Fe3O4 NP formation. For the silica coating, the resultant Fe3O4 NPs were refluxed with a few milliliters of TEOS and ethanol, while the PEI coating was done under gentle stirring of distilled water and the PEI reagent. Second, Fe3O4 NPs were refluxed with the APTES reagent under 30 to 70% methanol and toluene to form APTES@Fe3O4 NPs.
After the first layer of the APTES coating, APTES@Fe3O4 nanoparticles were dispersed into a 50 mL round-bottom flask in 30 mL of methanol. To the flask, phthalic anhydride, maleic anhydride, and succinic anhydride were added each in separate round-bottom flasks to procure PA@APTES@Fe3O4 NPs, MA@APTES@Fe3O4 NPs, and SA@APTES@Fe3O4 NPs, respectively. A reflux for 22 h was given with methanol at 70 °C under magnetic stirring at 1000 rpm. Succeeded by the reaction, the NPs were separated from the solution with the aid of a magnet and washed with methanol. The washed NPs were dried in a hot air oven overnight to procure the as-mentioned amide-functionalized Fe3O4 NPs. The synthesis is briefly represented in Scheme .
1. Methodological Representation of the Current Work.
2.3. Selection of Magnetic Nanoparticles for Extraction
To seven different 5 mL vials, about 3 mL of 0.25 mg/mL OLZ stock solution was taken. To each vial, about 2 mg of already synthesized seven different MNPs such as bare Fe3O4, SiO2@Fe3O4, PEI@Fe3O4, APTES@Fe3O4, PA@APTES@Fe3O4 NPs, MA@APTES@Fe3O4 NPs, and SA@APTES@Fe3O4 NPs were added, respectively, and labeled.
2.4. Microextraction Methodology for Olanzapine
Equal amounts of the standard olanzapine tablet solution and matrix sample were added to a 1.5 mL microcentrifuge tube. The mixture was kept aside for 10–15 min. This was followed by the addition of 1.5% TFA and the mixture was set aside for 30–40 min. Accurately weighed 9 mg of the selected MA@APTES@Fe3O4 NPs were added to the solution and ultrasonicated for a min to suspend the NPs. By means of an external magnet, the isolation of MNPs bearing the OLZ drug was done and they were removed from the supernatant. The OLZ-bound MNPs were detached using a diluent, as depicted in Scheme .
2.5. Method Development
According to the solubility, different initial trials were made with different solvents, columns, and other features before proceeding to specific parameters with QbD.
2.5.1. RSM by BBD
In the need to achieve the identification of OLZ with excellent analytical performances, a very suitable method has to be developed. Henceforth, to reach the goal, a statistical analysis, DoE (Design Experiment), was preferred as part of AQbD. , The DoE strategy works based on the correlation of factors and responses in a methodical way. The analysis implicates a series of trials that changes the input factors and analyzes their relation to the significant output results. This interaction-variable study includes different strategy types based on quadratic design. In them, BBD was opted for the current study. BBD is considered more suitable for this type of optimization study for multivariable critical factors. The reason for the choice is its efficiency in comparison to three-level full factorial study, as BBD includes reproducible first- and second-order response surface designs and is better in quadratic designs. Further, it involves the least number of trial runs even for more than three input factors, making the method cost-effective. ,
The BBD analysis was executed with three independent variables, i.e., the mobile phase composition, pH, and flow rate, resulting in output responses of the 3 crucial factors such as RT, theoretical plate, and tailing factor. The analysis was performed in Minitab software that required 15 trial runs alone. ,
2.6. Method Validation
The analytical techniques developed were validated in accordance with the International Council for Harmonization (ICH) criteria or guidelines. The specificity and system suitability were initially checked for the drug along with robustness via QbD. In five consecutive concentrations of 6 to 14 μg/mL OLZ (diluted with methanol), the pure standard linearity was performed. Similarly, extraction linearity for spiked OLZ was performed for the same series of concentrations, taking a constant few mL of both lassi and yogurt matrices separately in all and adding 9 mg of the selected MNP for extraction. Later, we estimated only the adsorbed OLZ for the spiked concentrations, where the LOD and LOQ have been calculated using the signal-to-noise ratio. Further, for accuracy 5, 10, and 15 μg/mL concentrations (diluted with methanol) were performed in three replicas defining 50, 100, and 150% of accuracy , while for the 100% homogeneous concentration, 10 μg/mL OLZ (diluted with methanol) for precision and ruggedness in six replicas was determined.
2.7. Conditions for LC-MS Study
As a confirmatory study, the standard olanzapine solution and extracted solution were injected into an LC-MS instrument of Agilent 1260 Infinity II Prime LC. By maintaining the same developed LC conditions, the mass conditions were applied as follows. The capillary current and voltage were maintained around 4500 to 4600 amp and 3440–3460 V, respectively. A pressure of 60 psi was conditioned in a nebulizer. A gas flow at 13 mL/min and a gas temperature of 350 °C were maintained. The scanning time was 2 ms.
3. Results and Discussion
3.1. Characterization of Synthesized MNPs
The synthesized magnetite nanoparticles with their different suitable coatings have been confirmed by characterizations. Primarily with FTIR spectroscopy through the fingerprint regions and functional groups, the functionalization and their bindings are confirmed, as displayed in Figure S1. Apart from the peaks of the Fe–O bond at ∼580–590 cm–1 and the Si–O bond at ∼1080–1100 cm–1, the formation of amide can be confirmed by the CO–NH bond peak between 1680 and 1630 cm–1 of CO stretching, followed by the presence of N–H stretching at ∼3300–3500 cm–1. This was preceded by FE-SEM-EDX to ensure their purity and particle size. FE-SEM-EDX carries out elemental analysis depicting the weight percentage of elements only present in the respective MNPs explaining the purity of MNPs synthesized, as illustrated in Figure S2. The XRD diffractogram for the Fe3O4 NPs confirms the structure to be a cubic inverse spinel and is a magnetite. The peak angle in correlation with lattice planes shown in Figure S3 matches with the standard data of JCPDS no. 19–0629, and SiO2 peaks match with the standard data of JCPDS no. 46–1045. The amide@APTES@Fe3O4 NPs have shown an amorphous nature from the broad XRD peak at 2θ = ∼30, 35, and 43° with the respective peak signals at (220), (222), and (202) lattice planes, as shown in Figure S3.
3.2. Optimization of Nanoparticles for Extraction
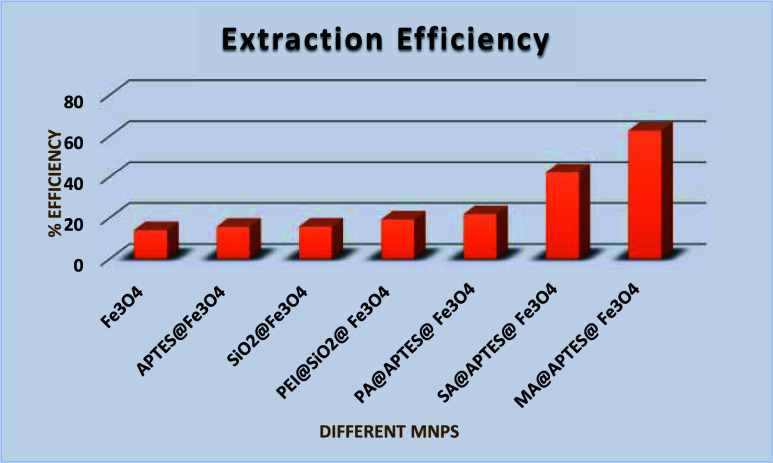
On UV examination of the selected seven MNPs, amide-coated Fe3O4 NPs proved to have better efficiency among others. Among them, MA@APTES@Fe3O4 nanoparticles had greater efficiency in binding toward the OLZ drug, as illustrated in the Figure .
1.
Extraction efficacies of selected MNPs.
3.3. Mechanism for Selection of MA@APTES@Fe3O4 NPs
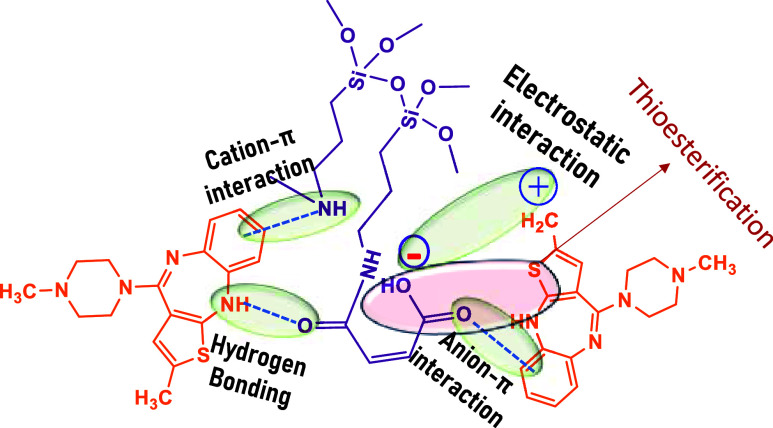
The rationale to opt for amide-based magnetite nanoparticles is that olanzapine is basic and has an overall electron-donating nature due to the presence of piperazine and methyl groups. However, on the other side, we needed an interacting group and hence preferred amides. The selected amides are electron-withdrawing groups. Of the three amide coatings, maleimides showed a higher binding efficiency. The reason is that maleimides have higher reactivity because of having a higher electrophilic nature because of the two carbonyl groups. Though phthalic amide has the same two carbonyl groups, its withdrawing nature is suppressed in the presence of a bulky substituent, a benzene ring that causes steric hindrance, thereby reducing its reactivity. , The feasible mechanism in the binding of the OLZ drug onto MA@APTES@Fe3O4 NPs would be through a few interactions. The main interaction is electrostatic attraction where the drug being positive gets attracted toward the negatively charged MA@APTES@Fe3O4 NPs. The second interaction is thioesterification formed as an interaction between sulfur in OLZ and the hydroxyl group of MA@APTES@Fe3O4 NPs, , as portrayed in Figure . Other possibilities are hydrogen bonding, cation−π bonding (i.e., the NH cation with the π-bond of benzene (OLZ)), and an anion−π interaction between the −COOH group of MA@APTES@Fe3O4 NPs and the π-bond of benzene (OLZ), as shown in Figure .
2.
Plausible mechanism of MNPs binding OLZ.
3.4. FTIR Confirmation Study
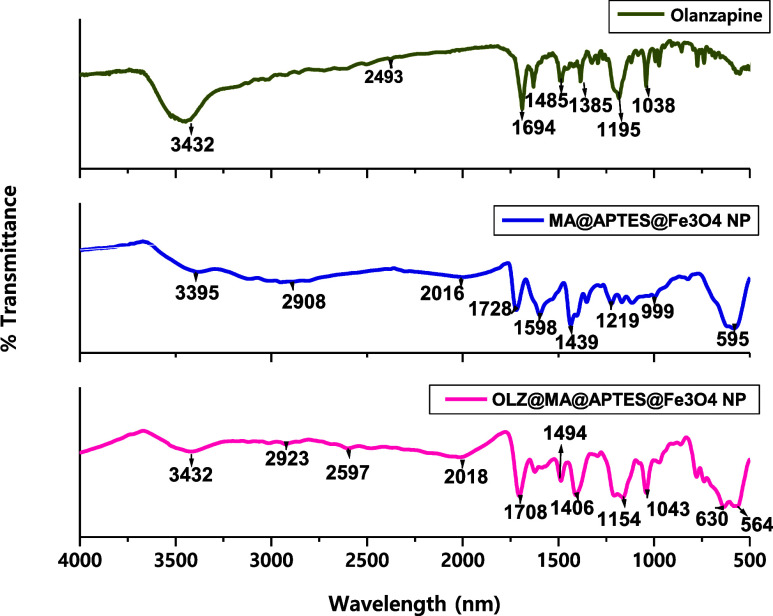
FTIR study on the drug, nanoparticle, and their binding has been performed to ensure their binding and mechanism. As shown in Figure , 3432 cm–1 explains N–H stretching; 2493 cm–1 depicts C–H stretching; 1694 cm–1 specifies CC stretching; 1485 cm–1 depicts CN stretching; 1195, 1038, and 1385 cm–1 depict C–N stretching; and 1385 cm–1 explains C–N bending in aromatic amines. All of these peaks explain the OLZ presence, while the MA@APTES@Fe3O4 NPs represent the ‘Fe–O’ presence at 595 and 999 cm–1. The Si–O bond was observed at 1219 cm–1. The main unsaturated ester formed when maleimides were formed was exhibited at 1598, 1449, and 1728 cm–1 for the CO bond depicting amide formation; at 2016 and 2908 cm–1 for C–H stretching; and at 3395 cm–1 representing N–H stretching.
3.
FTIR spectra of OLZ, MA@APTES@Fe3O4 NPs, and their binding.
The binding of OLZ to MA@APTES@Fe3O4 NPs was confirmed as the peaks of both OLZ and NPs were present. Moreover, the thiol group formation as a result of thioesterfication between OLZ and the NP was exhibited at 2597 cm–1 at low intensity yet at a characteristic peak, , as depicted in Figure .
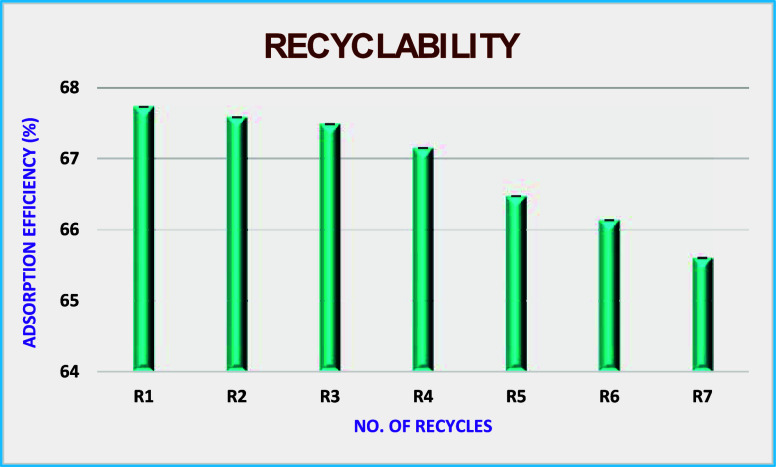
3.5. Recyclability
As a part of green technology, a miniaturization step of recycling saves wastage and the cost of the extraction method. Hence, the recycling capacity of MA@APTES@Fe3O4 NPs was examined for seven consecutive cycles. It was observed that there was good efficiency for 3 to 4 cycles later; a significant reduction from the fifth cycle was seen, but it was around 2.5 to 3.5%, as portrayed in Figure .
4.
Representation of MA@APTES@Fe3O4 NPs’ recyclability in extracting the drug.
3.6. Method Development
A linear regression analysis was done that provided a second-order polynomial equation depicting the association between selected input variables and responses. Later, it was followed by an ANOVA test in order to find the statistical significance of the developed method for the output responses.
The equation explains that on reduction of the buffer composition as the mobile phase, the pH of the buffer increases the RT, while it is vice versa for the flow rate. Hence, for a short analysis time, the buffer composition and pH, i.e., slight acidic nature, prove useful. The interaction between the buffer ratio and pH, the buffer ratio and the flow rate, and pH and the flow rate shows a negative impact on RT.
From ANOVA, the F value for the model was identified to be 303.66 and the P value was observed to be 0.000 that proves the model’s significance as the P value should be below 0.0500. For the linear regression analysis, the goodness of fit was estimated by the correlation coefficient (R2) to be 99.82% and the adjusted R 2 value to be 99.49%, which proves the model to be fit as its R 2 has to be >0.08. However, the nonlinear regression analysis depicts the least-squares score to be 0.0322.
In the equation, it is observed that an inverse relation is seen between the output response and the input variables. In detail, to limit the tailing factor below 2, the buffer composition, buffer pH, and flow rate have to be increased. However, a negative effect is obtained in the interaction between the buffer ratio and pH, the buffer ratio and the flow rate, and pH and the flow rate.
In the statistical analysis for the theoretical plate count, the F value was identified to be 1.20 and the P value was observed to be 0.001 that proves the model’s significance. For the linear regression analysis, R 2 was found to be 99.82% and adjusted R 2 was found to be 99.49%, proving the model to be fit, whereas the nonlinear regression analysis depicts the least-squares score to be 0.0322.
The equation describes that a hike in the buffer composition and pH improves the theoretical plate, while flow rate reduction favors the theoretical plate count. A positive impact is noticed in the mobile phase–flow rate and buffer ratio–pH interactions, while a negative effect is noticed in the case of the buffer ratio–flow rate interaction.
In an ANOVA test, the F value was analyzed as 19.46 and the P value was observed to be 0.002, which proves the model’s significance. For the linear regression analysis, R 2 was found to be 97.22% and adjusted R 2 was found to be 92.23%, proving the model to be fit, whereas the nonlinear regression analysis depicts the least-squares score to be 663.941.
From the statistical analysis, it is deciphered that the experimental results almost reached the predicted outcomes. Further, the analysis represented high accuracy and precision.
Contour plots depict the relationship between interdependent variables and output responses in the form of a graphical visualization. A 2D representation was derived from the 3D data, where for each independent variable, three outcome responses have been noticed. For the RT response shown in Figure a, the increase in the flow rate aids in a short RT, while an increase in the mobile phase composition and pH increases the RT. Henceforth, a decreased pH and mobile phase composition and an increased flow rate provide the requisite short analysis time. For a good chromatogram, peak tailing should not be seen; hence, as per the ICH guidelines, a tailing factor below 2 is preferable. As shown in Figure b, an increase in all three variables (pH, flow rate, and mobile phase composition) results in tailing; hence, a moderate value of these variables proved an excellent tailing factor. As shown in Figure 5c, An increased theoretical plate count, i.e., above 2000, was achieved from the lowest mobile phase composition, flow rate, and a moderate pH value.
5.
BBD contour plots of a) RT b) Flow rate c) Theoretical plateof the responses.
3.6.1. Regression Equation
To obtain a suitable, efficient method, certain trials were made. By changing the columns, mobile phase, flow rate, and a few parameters, a selective condition was opted for QbD studies to have an efficient and specific method for OLZ determination. Under the study, BBD was performed in 15 runs, as listed in Table . From the multiple input response, optimized conditions were predicted that are given below along the output response; the other optimized conditions are jotted. The column chosen was Platisil C18 ODS (Octadecyl Silica) with 250 mm length, 4.6 mm diameter, and 5 μm particle size. An injection volume of 20 μL at 25 °C temperature was conditioned. The detection was carried out with a 996 PDA detector at 232.5 nm wavelength.
1. Optimized Conditions and Their Responses.
| input variable | optimized condition | response variable | output response |
|---|---|---|---|
| mobile phase | 77:33 (0.1% formic acid; methanol) | tailing factor | 1.44 |
| pH | 1.96 | RT | 2.869 |
| flow rate | 1.022 | theoretical plate | 13,232 |
3.7. Method Validation
The correlation coefficient (R 2) of standard linearity was at 0.9997, as plotted in Figure a and in chromatograms in Figure S9, whereas the R 2 for the extraction linearity was 0.9967 for lassi and 0.9975 for the yoghurt matrix, as shown in Figure b, c and in chromatograms in Figure S10. The method proves to be linear as a result of the R 2 being above 0.995. The error bars were calculated from the standard deviation of three replicas of each concentration. The sensitivity of the method for pure standard OLZ was noticed at 1.4 μg/mL for the LOD and 4.9 μg/mL for the LOQ. The LOD and LOQ for lassi were found to be 1.79 and 5.27 μg/mL, while those for extraction from yoghurt were at 2.23 and 5.94 μg/mL, respectively.
6.
Linearity of standard OLZ and OLZ after extraction.
The % recovery and % RSD of accuracy, precision, and ruggedness for standard OLZ, as shown in Table , and the OLZ drug after extraction, as shown in Table S1, were found to be precise and accurate in accordance with the ICH guidelines and chromatograms shown in Figures S5–S8.
2. Results of Accuracy, Precision, and Ruggedness for Pure Standard OLZ.

3.7.1. Selectivity
The developed method meant to be selective, showing only the OLZ peak in tablet samples without any excipient interferences and no endogenous substance interfering from the matrix at 232.5 nm wavelength that is specific to the OLZ drug.
3.7.2. Repeatability
Keeping the same conditions, the same concentration was replicated three times on the same day and another day. It resulted in closeness without much variations from the % RSD that was observed to be below 1, i.e., 0.448 and 0.503, for the same day and the next day.
3.7.3. Robustness
The analysis has been performed to check the developed method’s resistivity toward the deliberate variations made over the optimized factors such as the mobile phase, flow rate, pH, and wavelength. On analysis, it has been observed that even on these mild changes in the parameters, their result did not widely vary from the peak area, tailing factor, and theoretical plates. Moreover, the method’s calculation of % RSD that was below 1, as portrayed in Table S2, has evinced a good robust nature.
3.8. Adsorption Efficiency
The efficiency of NPs to adsorb OLZ was analyzed from the peak area of the standard and extraction linearity concentrations with the following formulas ,
In brief, Q depicts the adsorption efficiency
Co = Initial concentration (pure standard drug conc.)
Ce = Final concentration (drug-adsorbed concentration)
The efficiency was calculated for extraction from both matrices. For the extraction from lassi for concentration, the NPs exhibited ∼60.05 to 64.17% efficiency, while extraction from yoghurt depicted ∼46.99 to 50.06% efficiency, as mentioned in Table S3.
3.9. Green and White Evaluation
GAC acts as a guideline to ensure that our methods meet the sustainability through 12 major green principles in fields specific to quality control and analytical laboratories. This explains the use of mostly green or extremely less toxic solvents and chemicals, possible reduction of waste, minimum energy consumption, and miniaturization to cut down unnecessary wastage. In order to make GAC come in use, a variety of green and white software are set into play for a better and detailed assessment of our methods, and even a comparative study with previously reported methods was performed here in the study. Moreover, the highest scored method’s pictograms among the four tools were circled in their respective colors for easy understanding, as shown in Table .
3. Comparative Study with AGREE, MoGAPI, BAGI, and RAPI Tools.


3.9.1. AGREE and Complex MoGAPI
Out of many of these software, some selective software were opted for the present study. One of the most preferred green metrics is AGREE software, a free user-friendly software. It comprises all 12 principles in its 10 basic criteria and displays in the form of a score between 0 and 1 for easy interpretation. It finally provides a cumulative score that has to be above 0.5 for the method to be green until 1, depending upon the degree of greenness. The AGREE analysis has also been performed for other previous similar methods for comparative studies. Considering that, among the previous work, the current method proved the greenest, scoring 0.75, as shown in Table .
The next preferred software is Complex GAPI, which presents a short overview on the environmental impact and safety of the operator involved at different stages of the analytical methodologies. The representation is done in a simple way, using three different color shades in a pentagram form. Yet the software lacked a proper precise conclusion regarding the analytical methods. Hence, its latest version, Complex MoGAPI, has been very recently introduced into the field. This tool along with the previous features also furnishes a scoring scale for precise analysis and easy conclusion. A score between 50 and 74 is an acceptable range for green, while a score below 50 depicts insufficiency in greenness. An excellent green score has to be above 75, and this has 2 level credits. A score above 89 receives 3 credits, proving outstanding greenness.
The MoGAPI score for the current method was 80, proving excellent greenness, whereas the assessment for previous related methods for comparative study scored between 66 and 73, which was not a good green score but came under the acceptable range, as depicted in Table . −
In relation to the other methods, the reason for the new method to be greener is because it possesses several assets. The present method has microextraction that drastically minimizes solvent, chemical, and sample usage, which is absent in many other methods. The method also uses less-energy-consuming instruments like HPLC rather than high-energy-consuming instruments like GC-MS and LC-MS for method development and validation that makes a significant difference. The other parameter also adds to it, which is the most preferable use of environmentally preferable solvents and the use of very minimal amounts of ACN (quite toxic but unavoidable). The method includes a recyclable magnetic nanoparticle in the extraction procedure, thereby reducing waste.
3.9.2. BAGI
Besides the environmental green assessment, sustainability was also explained in the form of the economic nature and workability of the developed method. In that case, BAGI is a tool to assess the other face of greenness known as the blue principle. It is portrayed in the form of an asteroid color scale with a metric score from the 25 to 100 range, for better understanding.
The present method received 62.5 as a score, while a few previous methods scored above 70 because of high sample throughput and different extraction procedures, i.e., LLE and SPE.
Despite these, the current method encompasses very minimal sample, chemical, or solvent usage as it involves microextraction and better practicality in using HPLC for method development. These pros make the current method to be in a good acceptable score above 60 as per the software shown in Table .
3.9.3. RGBfast
As a holistic perspective, white analytical chemistry (WAC) is also playing an apparent synergistic approach in sustainability. WAC, besides environmental safety, also focuses on two other major principles, i.e., practicality and economic balance. In view of the new principle, the latest tool referred as RGBfast has been developed that is an extended version of the previous RGB 12 model. The tool comprises three colors in representation of three sustainable principles, i.e., analytical efficiency in red, practicality and economic nature in blue, and environmental safety in green. ,
From the comparative assessment study, there are differences in saturation values for each method among three principles whose scores are detailed in Figure S11. To mention, the highest saturation value was secured by the eighth method in the red principle defining the method’s sensitivity. In terms of the blue principle, the fifth method received the highest saturation value, explaining excellent method applicability; the green principle was high for the first and current methods. Even though a few previous methods obtained high scores in a specific principle, the overall aim is to develop a sustainable method. In order to achieve this, there needs to be a balance among all three principles, resulting in a good white score. In that case, our current method has an overall high white score of 56 by having balance among the three saturation values, as displayed in Figure . This depicts the method to be sustainable.
7.
RGBfast assessment of the (a) comparative study and (b) current method.
3.9.4. RAPI
RAPI is a red principle analyzing tool represented in the form of a star-shaped pictogram. It is analogous to the BAGI tool but measures the methods’ analytical performance in terms of validation parameters with respect to concentrations. Depending on the method’s sensitivity, accuracy, precision, robustness, selectivity, and other validation parameters, it displays the score. Here, scoring below 60 indicates a poor analytical performance, and scoring above 60 defines a good to excellent analytical performance based on the score beyond 60. Even the redness is represented in the form of different color shades of red, where dark red depicts a very good red score.
Among the previous similar works, the current method secured the best results in terms of the red principle from RAPI, as shown in Figure . The reason is that though there are a few methods worked on nanolevels till now, they have not included or analyzed repeatability, selectivity, or LOQ that play crucial roles in the analytical performance, along with other validation parameters. The nontested parameters were shaded white by the tool, as represented in Figure . Nevertheless, the current developed method though has worked in the microlevel, yet the results have come very precise, accurate, and robust with excellent % RSD and % recovery levels. The method also includes parameters like repeatability, LOQ, and selectivity that were lacking in few other methods, except reproducibility. A comparative table of the linearity correlation coefficient, LOD, LOQ, and extraction efficiency for the current and previous methods has been reported in Table S4.
3.9.5. Solvent Assessment
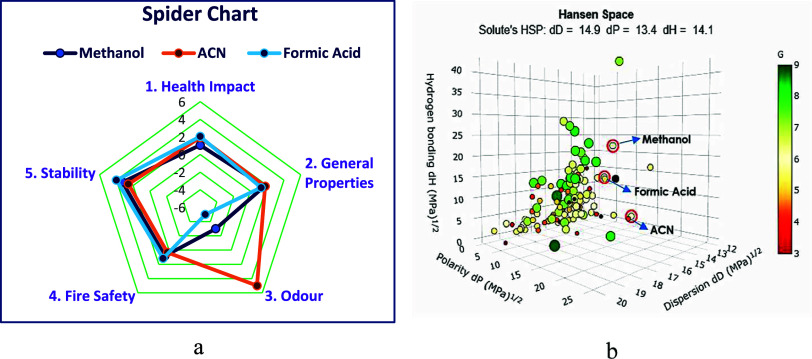
3.9.5.1. Greenness Index from the Spider Chart
It is a hierarchical representation of the solvents’ greenness in the form of a spider chart. There are five categories that describe the solvents’ greenness. These are health impact, general properties, odor, fire safety, and stability. Further, except for the odor class, the other four categories are determined to form their subcategorical parameters as a secondary spider chart that details the SDS data of the solvents at an individual level. The assessment is performed in an Excel sheet by manual feeding of the already set parameters in each category from the available SDS data for each solvent and scored accordingly from 5 to −5, i.e., least safe to very safe. The spider chart greenness assessment has been accomplished by manually gathering most of the information on SDS data of the three chemicals chosen for the work as mentioned in the table. Initially, the evaluation of the secondary spider pictogram of health impact, general properties, fire safety, and stability was carried out, as shown in Figure S12, and the percentage of data availability for the analysis has been mentioned in Table S5 for all three solvents separately with mostly 100% availability. Later, from the interpretation of the spider chart shown in Figure a and Table S6, it can be explained that formic acid and MeOH have a slightly higher greenness index on the whole and are environmentally preferable, whereas ACN has a lower green score, considering its properties.
8.
(a) Spider chart greenness index and (b) GSST analysis for the current method.
3.10. LC-MS Confirmatory Study
The LC-MS confirmatory analysis was performed, and it significantly verifies the presence of olanzapine, quantitates, and looks for any matrix interference in extracted samples. The LC chromatograms for standard OLZ and extracted OLZ from lassi and yoghurt matrices were marked at 2.848, 2.902, and 2.89 min of RT, respectively, as shown in Figure a,c,e. The standard and extracted OLZ samples were passed initially through positive-ion-mode ESI (electron-spray ionization) by keeping the basic nature of OLZ in view. This was preceded by selective ion monitoring (SIM) mode, where OLZ significantly resulted in a single charged protonated precursor via [M + H]+ fragmentation. The resultant m/z ratio was 313.2 for standard and extracted OLZ, as shown in Figure b,d,f. The slight matrix interference in extraction was also scanned for the mass spectrum displayed in Figure S14 & S15. Moreover, the major metabolites of OLZ, such as N-demethylolanzapine, 2-hydroxymethylolanzapine, and olanzapine-N-oxide, can also be identified and differentiated from the parent OLZ drug by the developed LC-MS analysis with the unique m/z ratio posed by each metabolite.
9.

LC-MS analysis of standard OLZ: (a) LC chromatograph and (b) mass spectrum; extracted OLZ from lassi: (c) LC chromatograph and (d) mass spectrum; and extracted OLZ from yoghurt: (e) LC chromatograph and (f) mass spectrum.
4. Conclusions
In the modern decades, there is mounting concern over the deadly misuse of prescribed medications in crimes. Especially, in rave parties, using alcoholic or milk-based beverages as a mode has become common to knock the victims unconscious for the perpetration of hideous crimes. Hence, grounding in to our utmost research findings, it was well noted that there are no preceding studies on the detection methods of OLZ from food/beverage matrices. The work was focused on establishing a new, prominent magnetic dispersive microextraction method that comprises MA@APTES@Fe3O4 NPs and TFA for well isolating the drug and minimizing the matrix interferences. The work was further continued to develop a new, reliable, sustainable analytical method. The QbD-integrated method development and validation were performed by LC-UV with additional LC-MS confirmatory analysis to make the method both efficient and sustainable. The validation parameters were tested for both pure and spiked OLZ samples, proving very good results that have been reflected in the RAPI tool. The sustainability of the current method has been comparatively studied, explaining the current method to be greener and whiter than previous ones with a good score of 62.5 in BAGI while having the highest scores of 80 in MoGAPI, 75 in AGREE, and 72.5 in RAPI. The green solvent assessment has also been carried out using the innovative in-depth spider chart and GSST tools. Moreover, the latest RGBfast tool showed a good score of 56% for the developed method, proving to be whiter than previously reported methods in the comparative study. The newly presented work can also efficiently be applied for other related drugs in milk beverages and other drinks, as TFA can break even complex matrices and also the as-reported NPs prove effective. As forensic crime is an unanticipated genre, it is always a precautionary measure to anticipate it ahead by bringing up the prominently new, advanced technologies and techniques into the play for solving even the typical crimes, especially in the analytical field.
Supplementary Material
Acknowledgments
For the lab and instrumental facilities, we cordially thank the Head, Department of Chemistry, Osmania University and Qualychrome lab. We also show sincere gratitude for the UGC-SRF (bearing ref no. 190520662958) fellowship programme in provision of the financial support. We would also like to thank the MoST (Ministry of Science and Technology Council), Taiwan (contract no. NSTC: 112-2113-M-320-001) and Tzu Chi University and hospital for supporting financially. We acknowledge the partial support from MoE-RUSA 2.0 project of Osmania University for this research.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c03576.
Characterization of the synthesized magnetic nanoparticles (FTIR, FE-SEM, EDX, XRD); BBD-residual plots; accuracy chromatograms; precision chromatograms; standard and extraction linearity chromatograms; validation parameters for spiked olanzapine; robustness with % RSD calculation; adsorption efficiency table; comparative study table on validation parameters; RGBfast; spider greenness secondary chart; and LC-MS drug and matrix mass peaks (PDF)
R.S.M.: Conceptualization, data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, and writingoriginal draft. S.G.: Conceptualization and supervision. A.H.: Funding acquisition. P.M.R. and R.R.: Supervision, project administration, and writingreview and editing.
The authors declare no competing financial interest.
Due to a production error, Table 3 was incomplete in the version of this paper that was published ASAP July 3, 2025. This was corrected and the paper reposted July 3, 2025.
References
- Mauri M. C., Paletta S., Maffini M., Colasanti A., Dragogna F., Di Pace C., Altamura A. C.. Clinical pharmacology of atypical antipsychotics: an update. Excli j. 2014;13:1163–1191. doi: 10.1007/s40262-018-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madea B., Musshoff F.. Knock-out drugs: their prevalence, modes of action, and means of detection. Dtsch Arztebl Int. 2009;106(20):341–347. doi: 10.3238/arztebl.2009.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M., Wezenberg E., Verkes R. J., Hulstijn W., Ruigt G. S., Sabbe B. G.. Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J. Clin Psychopharmacol. 2007;27(1):15–21. doi: 10.1097/jcp.0b013e31802dfff0. [DOI] [PubMed] [Google Scholar]
- Moffat, A. C. ; Osselton, M. D. ; Widdop, B. ; Watts, J. . Clarke’s Analysis of Drugs and Poisons; Pharmaceutical Press: London, 2011; Vol. 3. [Google Scholar]
- Lahane N., Kaur G.. Drug facilitated sexual assault and their analysis. Mater. Today: Proc. 2022;48:1240–1245. doi: 10.1016/j.matpr.2021.08.262. [DOI] [Google Scholar]
- Reeves R. R.. Abuse of olanzapine by substance abusers. J. Psychoactive Drugs. 2007;39(3):297–299. doi: 10.1080/02791072.2007.10400617. [DOI] [PubMed] [Google Scholar]
- Quest D. W., Horsley J.. Field-Test of a Date-Rape Drug Detection Device. Journal of Analytical Toxicology. 2007;31(6):354–357. doi: 10.1093/jat/31.6.354. [DOI] [PubMed] [Google Scholar]
- Son S. U., Jang S., Kang B., Kim J., Lim J., Seo S., Kang T., Jung J., Lee K.-S., Kim H., Lim E.-K.. Colorimetric paper sensor for visual detection of date-rape drug γ-hydroxybutyric acid (GHB) Sens. Actuators, B. 2021;347:130598. doi: 10.1016/j.snb.2021.130598. [DOI] [Google Scholar]
- Meyers J. E., Almirall J. R.. A Study of the Effectiveness of Commercially Available Drink Test Coasters for the Detection of “Date Rape” Drugs in Beverages. J. Analytical Toxicol. 2004;28(8):685–688. doi: 10.1093/jat/28.8.685. [DOI] [PubMed] [Google Scholar]
- Yang H., Liu C., Zhu C., Zheng Y., Li J., Zhu Q., Wang H., Fang X., Liu Q., Liang M., Liu Z.. Determination of ten antipsychotics in blood, hair and nails: Validation of a LC–MS/MS method and forensic application of keratinized matrix analysis. J. Pharm. Biomed. Anal. 2023;234:115557. doi: 10.1016/j.jpba.2023.115557. [DOI] [PubMed] [Google Scholar]
- Sundara Moorthy R., Swetha G., Rondla R., Hu A., Vallakeerthi N., Reddy P. M.. Greener and whiter analytical method development and validation for determining the presence of zolpidem tartrate infused in apple juice using RP-HPLC via magnetic solid-phase extraction followed by LC-MS confirmatory analysis. RSC Adv. 2024;14(38):28168–28181. doi: 10.1039/D4RA04303K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaszczyk P., Gacek E., Wietecha-Posłuszny R.. Green aspects in the procedure of detection ketamine, flunitrazepam, and diazepam in drinks based on dried sample spot analysis. Green Analytical Chem. 2022;3:100029. doi: 10.1016/j.greeac.2022.100029. [DOI] [Google Scholar]
- Hesham N., Hegazy M. A., Wagdy H. A.. Therapeutic drug monitoring of six contraindicated/co-administered drugs by simple and green RP-HPLC-PDA; application to spiked human plasma. BMC Chem. 2024;18(1):66. doi: 10.1186/s13065-024-01161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. J.. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem Rev. 2009;30(1):19–34. [PMC free article] [PubMed] [Google Scholar]
- Merone G. M., Tartaglia A., Rossi S., Santavenere F., Bassotti E., D’Ovidio C., Bonelli M., Rosato E., de Grazia U., Locatelli M., Savini F.. Fast Quantitative LC-MS/MS Determination of Illicit Substances in Solid and Liquid Unknown Seized Samples. Anal. Chem. 2021;93(49):16308–16313. doi: 10.1021/acs.analchem.1c03310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickells S.. Sample Preparation. Analytical Techniques in Forensic Science. 2021:71–108. doi: 10.1002/9781119373421.ch4. [DOI] [Google Scholar]
- Poole C. F.. New trends in solid-phase extraction. TrAC, Trends Anal. Chem. 2003;22(6):362–373. doi: 10.1016/S0165-9936(03)00605-8. [DOI] [Google Scholar]
- Moorthy, R. S. ; Rondla, R. ; Kavitha, M. ; Hima Bindu, P. ; Pasha, C. ; Reddy, P. M. . Potential applications of nanoparticles embedded U-bent fiber optic probe. In AIP Conference ProceedingsAIP Publishing, 2021. [Google Scholar]
- Moorthy, R. S. ; Rondla, R. ; Reddy, P. M. . Nanoparticle Embedded U Bent Fiber Optic Probe and Their Role as a Multi-Sensor Detector. In Research Highlights in Science and Technology 2023; Vol. 7, pp 165–178. [Google Scholar]
- Dmitrienko S. G., Apyari V. V., Tolmacheva V. V., Gorbunova M. V., Furletov A. A.. Dispersive and Magnetic Solid-Phase Extraction of Organic Compounds: Review of Reviews. J. Anal. Chem. 2024;79(2):105–118. doi: 10.1134/S1061934824020060. [DOI] [Google Scholar]
- Wang B., Chen Y., Li W., Liu Y., Xia X., Xu X., Yang Y., Chen D.. Magnetic phytic acid-modified kapok fiber biochar as a novel sorbent for magnetic solid-phase extraction of antidepressants in biofluids. Anal. Chim. Acta. 2024;1296:342295. doi: 10.1016/j.aca.2024.342295. [DOI] [PubMed] [Google Scholar]
- Gandhi A. C., Reddy P. M., Chan T.-S., Ho Y.-P., Wu S. Y.. Memory effect in weakly-interacting Fe3O4 nanoparticles. RSC Adv. 2015;5(103):84782–84789. doi: 10.1039/C5RA14332B. [DOI] [Google Scholar]
- Pena-Pereira F., Pena-Pereira F.. Miniaturization in Sample Preparation. 2014:1. doi: 10.2478/9783110410181.1. [DOI] [Google Scholar]
- Peris-Pastor G., Azorín C., Grau J., Benedé J. L., Chisvert A.. Miniaturization as a smart strategy to achieve greener sample preparation approaches: A view through greenness assessment. TrAC, Trends Anal. Chem. 2024;170:117434. doi: 10.1016/j.trac.2023.117434. [DOI] [Google Scholar]
- Stelmaszczyk P., Białkowska K., Wietecha-Posłuszny R.. supported polystyrene membranes for micro-solid phase extraction of date-rape drugs from urine: a sustainable analytical approach. Anal. Chim. Acta. 2024;1316:342874. doi: 10.1016/j.aca.2024.342874. [DOI] [PubMed] [Google Scholar]
- Anastas P. T.. Green Chemistry and the Role of Analytical Methodology Development. Crit. Rev. Anal. Chem. 1999;29:167–175. doi: 10.1080/10408349891199356. [DOI] [Google Scholar]
- Stumpf, C. The Theory And Practice Of Green Analytical Chemistry. In The White Paper; Waters Corporation Milford: MA, USA. [Google Scholar]
- Gałuszka A., Migaszewski Z., Namieśnik J.. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC, Trends Anal. Chem. 2013;50:78. doi: 10.1016/j.trac.2013.04.010. [DOI] [Google Scholar]
- Nowak P. M., Wietecha-Posłuszny R., Pawliszyn J.. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC, Trends Anal. Chem. 2021;138:116223. doi: 10.1016/j.trac.2021.116223. [DOI] [Google Scholar]
- Płotka-Wasylka J., Namieśnik J.. Green Analytical Chemistry: Past, Present and Perspectives. Green Analytical Chem. 2019 doi: 10.1007/978-981-13-9105-7. [DOI] [Google Scholar]
- Moorthy R. S., Srisailam N., Vallakeerthi N., Marapakala K., Reddy P. M.. Integrative greenness, blueness and whiteness determination of analytical RP-HPLC method for identifying Imipenem, Cilastatin and Relebactam in combined tablet form followed by forced degradation studies. Green Analytical Chem. 2024;11:100176. doi: 10.1016/j.greeac.2024.100176. [DOI] [Google Scholar]
- Nowak P. M., Bis A., Zima A.. ChlorTox Base – a useful source of information on popular reagents in terms of chemical hazards and greenness assessment. Green Anal. Chem. 2023;6:100065. doi: 10.1016/j.greeac.2023.100065. [DOI] [Google Scholar]
- Nowak P. M., Arduini F.. RGBfast – A user-friendly version of the Red-Green-Blue model for assessing greenness and whiteness of analytical methods. Green Anal. Chem. 2024;10:100120. doi: 10.1016/j.greeac.2024.100120. [DOI] [Google Scholar]
- Pena-Pereira F., Wojnowski W., Tobiszewski M.. AGREEAnalytical GREEnness Metric Approach and Software. Anal. Chem. 2020;92(14):10076–10082. doi: 10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manousi N., Wojnowski W., Płotka-Wasylka J., Samanidou V.. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green Chem. 2023;25(19):7598–7604. doi: 10.1039/D3GC02347H. [DOI] [Google Scholar]
- Reddy P. M., Chang K. C., Liu Z. J., Chen C. T., Ho Y. P.. Functionalized magnetic iron oxide (Fe3O4) nanoparticles for capturing gram-positive and gram-negative bacteria. J. Biomed. Nanotechnol. 2014;10(8):1429–1439. doi: 10.1166/jbn.2014.1848. [DOI] [PubMed] [Google Scholar]
- Kannaiah K. P., Chanduluru H. K., Lotfy H. M., Obaydo R. H., El Hamd M. A., Alshehri S., A Mahdi W., Nessim C. K.. Integrative AQbD, up-to-date greenness, and whiteness tools for evaluation of a sustainable RP-HPLC method used for simultaneous separation of triple antihypertensive combination therapy as a model. Sustainable Chem. Pharmacy. 2023;36:101288. doi: 10.1016/j.scp.2023.101288. [DOI] [Google Scholar]
- Al-Kadhi N. S., Mohamed M. A., Ahmed H. A., Nassar H. F.. Facile synthesis and eco-friendly analytical methods for concurrent estimation of selected pharmaceutical drugs in their solutions: application to quality by design, lean six sigma, and stability studies. BMC Chem. 2023;17(1):136. doi: 10.1186/s13065-023-01028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Mullick P., Nandakumar K., Mutalik S., Rao C. M.. Box–Behnken Design-Based Development and Validation of a Reverse-Phase HPLC Analytical Method for the Estimation of Paclitaxel in Cationic Liposomes. Chromatographia. 2022;85(7):629–642. doi: 10.1007/s10337-022-04172-w. [DOI] [Google Scholar]
- Abdel Hamid E. M., Amer A. M., Mahmoud A. K., Mokbl E. M., Hassan M. A., Abdel-Monaim M. O., Amin R. H., Tharwat K. M.. Box-Behnken design (BBD) for optimization and simulation of biolubricant production from biomass using aspen plus with techno-economic analysis. Sci. Rep. 2024;14(1):21769. doi: 10.1038/s41598-024-71266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. L. C., Bruns R. E., Ferreira H. S., Matos G. D., David J. M., Brandão G. C., da Silva E. G. P., Portugal L. A., dos Reis P. S., Souza A. S., dos Santos W. N. L.. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta. 2007;597(2):179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Patel K., Shah U. A., Patel C. N.. Box–Behnken design-assisted optimization of RP-HPLC method for the estimation of evogliptin tartrate by analytical quality by design. Future J. Pharmaceutical Sci. 2023;9(1):57. doi: 10.1186/s43094-023-00509-w. [DOI] [Google Scholar]
- Li Y., Leng T., Lin H., Deng C., Xu X., Yao N., Yang P., Zhang X.. Preparation of Fe3O4@ZrO2 core-shell microspheres as affinity probes for selective enrichment and direct determination of phosphopeptides using matrix-assisted laser desorption ionization mass spectrometry. J. Proteome Res. 2007;6(11):4498–4510. doi: 10.1021/pr070167s. [DOI] [PubMed] [Google Scholar]
- Naidoo C., Kruger C., Abrahamse H.. Targeted photodynamic therapy treatment of in vitro A375 metastatic melanoma cells. Oncotarget. 2019;10:6079–6095. doi: 10.18632/oncotarget.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikuchi E. A., Gaspar R., Silva D., Raimundo I. Jr, Mazali I. O.. PEG size effect and its interaction with Fe3O4 nanoparticles synthesized by solvothermal method: morphology and effect of pH on the stability. Nano Express. 2021;2:020022. doi: 10.1088/2632-959X/ac0596. [DOI] [Google Scholar]
- Jadhav M. N., Kokil G. R., Harak S. S., Wagh S. B.. Direct and Indirect Drug Design Approaches for the Development of Novel Tricyclic Antipsychotics: Potential 5-HT2A Antagonist. J. Chem. 2013;2013(1):930354. doi: 10.1155/2013/930354. [DOI] [Google Scholar]
- Centi, G. ; Pinelli, D. ; Trifiro, F. ; Ungarelli, F. ; Nieto, J. L. . Synthesis of Phthalic and Maleic Anhydrides from n-Pentane: Reactivity of Possible Intermediates and co-Feeding Experiments. In Studies in Surface Science and Catalysis; Centi, G. ; Trifiro, F. , Eds.; Elsevier, 1990; Vol. 55, pp 635–642. [Google Scholar]
- Schnepel C., Pérez L. R., Yu Y., Angelastro A., Heath R. S., Lubberink M., Falcioni F., Mulholland K., Hayes M. A., Turner N. J., Flitsch S. L.. Thioester-mediated biocatalytic amide bond synthesis with in situ thiol recycling. Nature Catalysis. 2023;6(1):89–99. doi: 10.1038/s41929-022-00889-x. [DOI] [Google Scholar]
- Mai W.-P., Zhao Y., Lv M.-X., Zhang W.-R., Xiao Y.-M., Yuan J.-W., Yang L.-R.. Nickel-Catalyzed Synthesis of Thioesters from Amides and Disulfides. Eur. J. Org. Chem. 2024;27(12):e202400026. doi: 10.1002/ejoc.202400026. [DOI] [Google Scholar]
- Dougherty D. A.. The cation-π interaction. Acc. Chem. Res. 2013;46(4):885–893. doi: 10.1021/ar300265y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñonero D., Frontera A., Garau C., Ballester P., Costa A., Deyà P. M.. Interplay between cation-pi, anion-pi and pi-pi interactions. ChemPhysChem. 2006;7(12):2487–2491. doi: 10.1002/cphc.200600343. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V., Nagalingam A., Priya Ranjan Prasad V., Parameshwaran S., George N., Kaliyan P.. Characterization of olanzapine-solid dispersions. Iran J. Pharm. Res. 2011;10(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Körpınar B., Akat H.. A new thiol-based oxidized polyethylene adsorbent for heavy metal ions removal from aqueous solutions. Polym. Bull. 2023;81:1–15. doi: 10.1007/s00289-023-04925-z. [DOI] [Google Scholar]
- Xia, Z. ; Baird, L. ; Zimmerman, N. ; Yeager, M. , Heavy Metal Ion Removal By Thiol Functionalized Aluminum Oxide Hydroxide Nanowhiskers. Appl. Surf. Sci. 2017, 416.565 10.1016/j.apsusc.2017.04.095. [DOI] [Google Scholar]
- Sumanjit, Rani S., Mahajan R. K.. Equilibrium, kinetics and thermodynamic parameters for adsorptive removal of dye Basic Blue 9 by ground nut shells and Eichhornia. Arabian J. Chem. 2016;9:S1464–S1477. doi: 10.1016/j.arabjc.2012.03.013. [DOI] [Google Scholar]
- Sanjeev N. O., Vallabha M. S., Valsan A. E.. Adsorptive removal of pharmaceutically active compounds from multicomponent system using Azadirachta indica induced zinc oxide nanoparticles: analysis of competitive and cooperative adsorption. Water Sci. Technol. 2023;87(1):284–303. doi: 10.2166/wst.2022.428. [DOI] [PubMed] [Google Scholar]
- Abdelrahman, M. M. Green analytical chemistry metrics and life-cycle assessment approach to analytical method development. In Green Chemical Analysis and Sample Preparations: Procedures, Instrumentation, Data Metrics, And Sustainability; Springer, 2022; pp 29–99. [Google Scholar]
- Mansour F. R., Omer K. M., Płotka-Wasylka J.. A total scoring system and software for complex modified GAPI (ComplexMoGAPI) application in the assessment of method greenness. Green Analytical Chemistry. 2024;10:100126. doi: 10.1016/j.greeac.2024.100126. [DOI] [Google Scholar]
- Kantipudi R., Pavuluri S. K.. Bioanalytical method development and validation for the simultaneous estimation of Olanzapine and Samidorphan in rabbit plasma by using HPLC–MS/MS and application to pharmacokinetic study. Future Journal of Pharmaceutical Sciences. 2024;10(1):1. doi: 10.1186/s43094-023-00570-5. [DOI] [Google Scholar]
- Menda J., Chintala V., Kowtharapu L. P., Pydimarry S. P. R., Kanuparthy P. R., Katari N. K.. Quality by design tool evaluated green stability-indicating UPLC content determination method for the Olanzapine and Samidorphan dosage form. Microchem. J. 2024;197:109835. doi: 10.1016/j.microc.2023.109835. [DOI] [Google Scholar]
- Konecki C., Hadjoudj J., Tralongo F., Haudecoeur C., Gozalo C., Fouley A., Marty H., Feliu C., Djerada Z.. Simultaneous quantification of 55 psychotropic drugs and metabolites in human plasma with a fast UPLC-MS/MS method. Biomed Pharmacother. 2023;169:115918. doi: 10.1016/j.biopha.2023.115918. [DOI] [PubMed] [Google Scholar]
- Yang H., Liu C., Zhu C., Zheng Y., Li J., Zhu Q., Wang H., Fang X., Liu Q., Liang M., Liu Z.. Determination of ten antipsychotics in blood, hair and nails: Validation of a LC-MS/MS method and forensic application of keratinized matrix analysis. J. Pharm. Biomed. Anal. 2023;234:115557. doi: 10.1016/j.jpba.2023.115557. [DOI] [PubMed] [Google Scholar]
- Barone R., Giorgetti A., Cardella R., Rossi F., Garagnani M., Pascali J. P., Mohamed S., Fais P., Pelletti G.. Development and validation of a fast UPLC-MS/MS screening method for the detection of 68 psychoactive drugs and metabolites in whole blood and application to post-mortem cases. J. Pharm. Biomed Anal. 2023;228:115315. doi: 10.1016/j.jpba.2023.115315. [DOI] [PubMed] [Google Scholar]
- Mastrogianni O., Orfanidis A., Brousa E., Kevrekidis D.-P., Zagelidou H., Raikos N.. Development and validation of an ultra-performance liquid chromatography–tandem mass spectrometric method for the determination of 25 psychoactive drugs in cerumen and its application to real postmortem samples. Forensic Toxicology. 2023;41(1):94–104. doi: 10.1007/s11419-022-00640-y. [DOI] [PubMed] [Google Scholar]
- Orfanidis A., Krokos A., Mastrogianni O., Gika H., Raikos N., Theodoridis G.. Development and validation of a single step GC/MS method for the determination of 41 drugs and drugs of abuse in postmortem blood. Forensic Sciences. 2022;2(3):473–491. doi: 10.3390/forensicsci2030035. [DOI] [Google Scholar]
- Salem H., Samir E., Mazen D. Z., Madian H., Elkhateeb A. E., Elaraby M., Rasekh M. I., Gamal A.. Spectrofluorimetric first derivative synchronous approach for determination of olanzapine and samidorphan used for treatment of schizophrenia in pharmaceutical formulations and human plasma. Spectrochim Acta A Mol. Biomol Spectrosc. 2022;274:121105. doi: 10.1016/j.saa.2022.121105. [DOI] [PubMed] [Google Scholar]
- Dziurkowska E., Jiménez-Morigosa C., López-Rivadulla M., Wesolowski M.. Development and validation of solid-phase extraction coupled with a liquid chromatography-tandem mass spectrometry method for quantitation of olanzapine in saliva. J. Chromatography B. 2020;1136:121896. doi: 10.1016/j.jchromb.2019.121896. [DOI] [PubMed] [Google Scholar]
- Nowak P. M., Wojnowski W., Manousi N., Samanidou V., Płotka-Wasylka J.. Red analytical performance index (RAPI) and software: the missing tool for assessing methods in terms of analytical performance. Green Chem. 2025;27(19):5546–5553. doi: 10.1039/D4GC05298F. [DOI] [Google Scholar]
- Shen Y., Lo C., Nagaraj D. R., Farinato R., Essenfeld A., Somasundaran P.. Development of Greenness Index as an evaluation tool to assess reagents: Evaluation based on SDS (Safety Data Sheet) information. Miner. Eng. 2016;94:1–9. doi: 10.1016/j.mineng.2016.04.015. [DOI] [Google Scholar]
- Nozawa H., Minakata K., Hasegawa K., Yamagishi I., Miyoshi N., Suzuki M., Kitamoto T., Kondo M., Watanabe K., Suzuki O.. Quantification of olanzapine and its three metabolites by liquid chromatography–tandem mass spectrometry in human body fluids obtained from four deceased, and confirmation of the reduction from olanzapine N-oxide to olanzapine in whole blood in vitro. Forensic Toxicol. 2023;41(2):318–328. doi: 10.1007/s11419-023-00662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.