Abstract
Background
The widespread use of low-dose chest computed tomography (CT) has significantly increased the early detection rate of small nodules. Existing localization methods have certain limitations. Preoperative localization using autologous blood combined with methylene blue has garnered attention due to its dual advantages. This study aims to evaluate the safety and pneumothorax risk of CT-guided preoperative localization using this technique and explore the risk factors associated with pneumothorax occurrence.
Methods
This retrospective study included 112 patients who underwent CT-guided preoperative lung nodule localization using autologous blood and methylene blue between November 2019 and November 2024 at The First Hospital of Putian. Patient demographics, imaging characteristics, procedural details, and post-localization complications were collected. Logistic regression was used to analyze the independent risk factors for pneumothorax.
Results
The localization success rate was 90.2%, and the pneumothorax incidence was 16.1%. Multivariate analysis identified white blood cell (WBC) count [odds ratio (OR) 1.43, 95% confidence interval (CI): 1.06–1.96, P=0.02] and the needle-tip-to-visceral-pleura distance (OR 2.36, 95% CI: 1.07–5.44, P=0.04) as independent risk factors for pneumothorax. A predictive nomogram model with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.762 was developed, demonstrating good predictive performance.
Conclusions
Autologous blood combined with methylene blue is a safe and effective localization method for lung nodules. WBC count and needle-tip-to-visceral-pleura distance are independent risk factors for pneumothorax. The nomogram model provides valuable assistance for preoperative risk assessment.
Keywords: Computed tomography-guided localization (CT-guided localization), pulmonary nodules, autologous blood, methylene blue, pneumothorax risk
Highlight box.
Key findings
• This study evaluated the safety and effectiveness of computed tomography (CT)-guided localization using autologous blood combined with methylene blue for pulmonary nodules.
• The localization success rate was 90.2%, with a pneumothorax incidence of 16.1%.
• White blood cell (WBC) count and needle-tip-to-visceral-pleura distance were independent risk factors for pneumothorax.
• A nomogram model predicted pneumothorax risk with an area under the receiver operating characteristic curve of 0.762.
What is known and what is new?
• Current CT-guided localization methods, such as hookwire and methylene blue alone, have limitations, including high complication risks and variable success rates.
• This study demonstrated that combining autologous blood with methylene blue enhances safety by reducing pneumothorax risk. A novel nomogram incorporating WBC count and procedural factors provides personalized risk prediction.
What is the implication, and what should change now?
• The combined technique of autologous blood and methylene blue is effective for preoperative localization, with reduced complication rates.
• The nomogram offers a valuable tool for preoperative risk assessment, enabling tailored clinical decisions. Future studies should validate the model across multiple centers.
Introduction
Lung nodules are a common radiological finding, with solitary pulmonary nodules (SPNs) particularly noteworthy due to their high likelihood of malignancy (1-4). The widespread use of low-dose chest computed tomography (CT) has significantly improved the early detection rate of small nodules. However, accurate preoperative localization remains critical for adequate resection and diagnosis, especially for deep, tiny, or ground-glass nodules (GGNs), where manual palpation is challenging (5-8).
Existing preoperative localization methods, such as hookwire, coil, and methylene blue, have limitations, including high risks of complications like pneumothorax or bleeding and variable success rates (9). Autologous blood alone is associated with diffusion issues, requiring surgery within 12 hours to prevent hematoma dissipation (10). Recently, a technique combining autologous blood and methylene blue has gained attention for its dual advantages: methylene blue for precise localization and autologous blood for sealing the puncture tract, thereby reducing postoperative complications. However, this technique’s safety, efficacy, and influencing factors remain underexplored (11).
Pneumothorax is the most common complication of CT-guided lung nodule localization, and it is influenced by factors such as puncture frequency, nodule size, and individual variability (12,13). Although pneumothorax is generally not considered a severe complication in the preoperative localization of pulmonary nodules, its occurrence can still impact surgical efficiency and patient comfort, as surgery is performed shortly after localization. Current predictive models for pneumothorax primarily focus on conventional factors, often overlooking the potential role of inflammatory markers. This study incorporates white blood cell (WBC) count into pneumothorax risk assessment, developing a more precise predictive model and evaluating its clinical applicability.
This study assesses the safety and pneumothorax incidence of CT-guided preoperative localization using autologous blood and methylene blue. Furthermore, a nomogram predictive model based on risk factors was developed and validated to evaluate its predictive performance and clinical utility. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2286/rc).
Methods
Study population
Between November 2019 and November 2024, patients undergoing preoperative CT-guided localization of pulmonary nodules using autologous blood combined with methylene blue at The First Hospital of Putian were included in this study. Indications for pulmonary nodule puncture localization lack unified standards; current criteria are based on nodule type and the distance to the pleura. Participants met the following inclusion criteria: (I) solid nodules with a maximum diameter ≤1.5 cm and a distance ≥0.5 cm from the visceral pleura; (II) pure GGNs; (III) mixed GGNs with a maximum diameter ≤2 cm and a distance ≥0.5 cm from the pleura. Exclusion criteria included: (I) vascular pulmonary lesions or nodules near the pulmonary hilum or major blood vessels; (II) severe cardiopulmonary dysfunction or bleeding tendencies; (III) history of thoracic surgery (thoracotomy), pleural infection, or ipsilateral pleural thickening and adhesions.
This study enrolled 112 patients with pulmonary nodules. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of The First Hospital of Putian (No. 2018-008) and due to the retrospective cohort design, the requirement for informed consent was waived. Before the CT-guided localization and subsequent video-assisted thoracoscopic surgery (VATS), all patients were informed about the procedure and potential complications and provided written informed consent.
Data collection
Collected data included demographic information, imaging characteristics of the nodules (nodule type, size, location, shortest distance to the visceral pleura, and pleural indentation sign), pre-localization laboratory results [forced vital capacity (FVC), forced expiratory volume in one second/forced vital capacity (FEV1/FVC), maximum voluntary ventilation (MVV), and WBC count], localization site, needle insertion depth, needle-tip-to-lung contact depth and duration, procedure duration, and complications.
Successful marking was defined as the visibility of autologous blood or dye markers under thoracoscopy during surgery (Figure 1). Localization success was defined as the intraoperative removal of lung tissue containing the target nodule, confirmed pathologically to be the intended nodule.
Figure 1.

The markers appeared blue-purple under thoracoscopic observation, indicating successful localization of the pulmonary nodule.
Localization technique
Preoperative localization was performed using a mixture of methylene blue and autologous blood for small lung nodules or masses, followed by thoracoscopic surgery. On the day before or the day of surgery, 5–10 mL of peripheral venous blood was collected and mixed with 1 mL of methylene blue. After the patient was positioned in the CT suite, a suitable position was determined based on the location of the nodule identified in prior CT imaging.
An 18-G PTC needle (Hakko Co., Ltd., Tokyo, Japan) was gradually inserted within 1 cm of the lesion and then withdrawn slightly to confirm that it had not entered a bronchus or blood vessel. Before needle removal, approximately 3 mL of the methylene blue-autologous blood mixture was injected. Post-procedure, a 64-slice spiral CT scanner (Siemens SOMATOM Definition AS, Munich, Germany) was used to confirm the distribution of the marker and check for complications such as pneumothorax or hemothorax.
Construction of the nomogram
In this study, a nomogram was developed to predict the risk of pneumothorax during CT-guided preoperative localization of pulmonary nodules. Independent risk factors were identified using multivariate logistic regression analysis. Significant variables, including WBC count and the needle-tip-to-visceral-pleura distance, were incorporated into the model based on multivariate analysis results.
The nomogram was constructed using R software (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) with the rms and survival packages. The rms package, commonly employed for regression modeling strategies, was used for visualization and calculation of the nomogram (Harrell FE. http://cran.r-project.org/web/packages/rms).
Validation and calibration of the nomogram
Internal validation of the nomogram was conducted using 500 bootstrap resamples from the study cohort. Calibration curves were generated to compare the predicted probabilities of pneumothorax with the observed outcomes, assessing the model’s calibration across the range of predicted risks.
The discrimination performance of the nomogram was evaluated using an area under the receiver operating characteristic (ROC) curve (AUC). AUC values range from 0.5 to 1.0, with higher values indicating better predictive accuracy. Furthermore, decision curve analysis (DCA) was performed to evaluate the clinical utility of the nomogram by quantifying the net benefits across a range of threshold probabilities.
Statistical analysis
Statistical analyses were performed using R software version 4.0.5. The Shapiro-Wilk test was used to assess normality. Continuous variables were expressed as mean and standard deviation (SD) or median and interquartile range (IQR), while categorical variables were presented as frequencies (n) and percentages (%).
Univariate logistic regression analysis was conducted to identify potential risk factors for pneumothorax, with variables yielding a P<0.05 considered significant. Multivariate logistic regression analysis was performed to determine independent risk factors for pneumothorax. Variables identified as significant were included in the final nomogram prediction model. The accuracy of the model was evaluated using the ROC curve, and internal validation was performed using the bootstrap method. Model calibration was assessed with a calibration curve, and clinical utility was tested using DCA.
Results
Baseline characteristics
The baseline characteristics of the included patients, lung nodule imaging features, and localization-related factors are summarized in Table 1. One hundred and twelve patients met the inclusion criteria and were enrolled in the study. The mean age was 54.37±12.63 years, including 63 males and 49 females, with the mean body mass index (BMI) of 23.06±2.75 kg/m2. Laboratory tests revealed a median WBC count of 5.88×109/L [IQR, (5.09–7.04)×109/L], median MVV of 92.63 L/min (IQR, 81.00–106.15 L/min), FVC of 3.02±0.75 L, and FEV1/FVC ratio of 86.14%±9.69%.
Table 1. Baseline characteristics of patients and pulmonary nodules.
| Clinical characteristic | Values (n=112) |
|---|---|
| Age (years) | 54.37±12.63 |
| Sex | |
| Male | 63 (56.25) |
| Female | 49 (43.75) |
| BMI (kg/m2) | 23.06±2.75 |
| WBC (109/L) | 5.88 [5.09, 7.04] |
| MVV (L/min) | 92.63 [81.00, 106.15] |
| FVC (L) | 3.02±0.75 |
| FEV1/FVC (%) | 86.14±9.69 |
| Emphysema | |
| No | 90 (80.36) |
| Yes | 22 (19.64) |
| Pleural stretch sign | |
| No | 93 (83.04) |
| Yes | 19 (16.96) |
| Nodule size (cm) | 0.85 [0.70, 1.10] |
| Nodule type | |
| GGN | 93 (83.04) |
| Solid | 16 (14.29) |
| Cystic | 3 (2.68) |
| Location | |
| No-lower lobe nodules | 76 (67.86) |
| Lower lobe nodules | 36 (32.14) |
| Shortest distance to pleura (cm) | 1.00 [0.30, 1.83] |
| Puncture angle (°) | 71.12±14.18 |
| Puncture time (min) | 5.00 [3.00, 8.00] |
| Positioning time (min) | 15.50 [12.00, 24.00] |
| Lung depth† (cm) | 2.16±0.71 |
| Insertion depth‡ (cm) | 6.25 [5.40, 7.20] |
| Pneumothorax | |
| Yes | 18 (16.07) |
| No | 94 (83.93) |
Values are presented as n (%), mean ± standard deviation, or median [interquartile range]. †, depth from the needle tip to the lung tissue contact site; ‡, total depth of needle insertion from the skin to the target site. BMI, body mass index; FEV1/FVC, forced expiratory volume in the first second/forced vital capacity; FVC, forced vital capacity; GGN, ground-glass nodule; MVV, maximal voluntary ventilation; WBC, white blood cell.
Imaging findings showed a median nodule diameter of 0.85 cm (IQR, 0.70–1.10 cm), including 16 solid nodules, 93 GGNs, and 3 cystic nodules. There were 76 non-lower-lobe nodules and 36 lower-lobe nodules. The shortest distance from the nodules to the visceral pleura had a median of 1.00 cm (IQR, 0.30–1.83 cm). Procedural data included a puncture angle of 71.12°±14.18°, a median needle-lung tissue contact time of 5.00 min (IQR, 3.00–8.00 min), and a median total localization time of 15.5 min (IQR, 12.0–24.0 min). The median distances from the puncture needle to the skin and visceral pleura were 6.25 cm (IQR, 5.40–7.20 cm) and 2.16±0.71 cm, respectively.
Post-localization complications included mild pneumothorax (lung collapse <20%) in 18 patients (16.1%), with no significant respiratory distress or chest tube placement required. The overall localization success rate was 90.2% (101 cases). In our study, inefficient marking was observed in 5.4% (6 cases), including instances where the marker was not visible intraoperatively or excessive dye diffusion resulted in pleural staining. Additionally, minor bleeding occurred in 1.7% (2 cases). All patients returned to their wards without significant postoperative pain. In our study, pathologists did not report any significant interference with diagnosis due to the use of autologous blood combined with methylene blue.
Univariate and multivariate logistic regression analyses
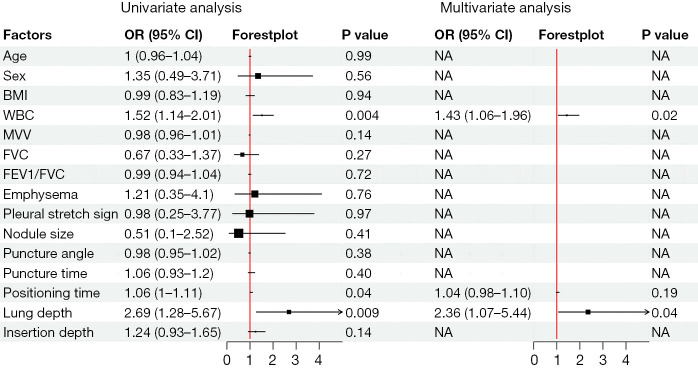
Univariate analysis showed that elevated WBC count significantly increased pneumothorax risk [odds ratio (OR) 1.52, 95% confidence interval (CI): 1.14–2.01, P=0.004]. Prolonged localization time was also associated with pneumothorax (OR 1.06, 95% CI: 1.00–1.11, P=0.04). Additionally, a greater needle-tip-to-visceral-pleura distance significantly increased pneumothorax risk (OR 2.69, 95% CI: 1.28–5.67, P=0.009) (Figure 2).
Figure 2.
Forest plot of univariate and multivariate logistic regression analyses identifying risk factors for pneumothorax. BMI, body mass index; CI, confidence interval; FEV1/FVC, forced expiratory volume in one second/forced vital capacity; FVC, forced vital capacity; MVV, maximum voluntary ventilation; NA, not applicable; OR, odds ratio; WBC, white blood cell.
Multivariate analysis confirmed elevated WBC count (OR 1.43, 95% CI: 1.06–1.96, P=0.02) and greater needle-tip-to-visceral-pleura distance (OR 2.36, 95% CI: 1.07–5.44, P=0.04) as independent risk factors for pneumothorax. Although localization time was significant in univariate analysis, it was not important in multivariate analysis (P>0.05), potentially due to interaction effects or limited sample size. Other variables, such as BMI, nodule size, and puncture angle, showed no significant association (P>0.05) (Figure 2; Table 1).
Development and calibration of the nomogram
Based on multivariate regression results, a nomogram model was developed, incorporating needle-tip-to-visceral-pleura distance and WBC count as predictors (Figure 3). The nomogram assigned point values to each variable, and the total score was used to predict pneumothorax risk.
Figure 3.
Nomogram for predicting pneumothorax risk. The lung depth and WBC count are assigned points based on their respective values. The total score is calculated by summing the individual points, which corresponds to the predicted probability of pneumothorax (Pr). *, P<0.05; **, P<0.01. WBC, white blood cell.
For instance, in one patient, the total score was 78.6, corresponding to a pneumothorax risk of 11.9%. The nomogram demonstrated an AUC of 0.762 in ROC analysis (Figure 4A), indicating acceptable predictive ability. Internal validation using the bootstrap method yielded a 95% CI: for AUC of 0.702–0.771 (Figure 4B). Calibration curves showed good predictive accuracy (Figure 5A), and DCA demonstrated high clinical utility (Figure 5B).
Figure 4.
ROC analysis and bootstrap validation for predicting pneumothorax risk. (A) The ROC curve for the nomogram model, showing an AUC of 0.762 (95% CI: 0.702–0.771). (B) Internal validation using the bootstrap method, illustrating the distribution of t and quantiles of standard normal, confirming the model’s stability. t* represents the statistic (in this case, the AUC) calculated using the bootstrap method. * indicates that this statistic is derived from bootstrap resampling results. AUC, area under the receiver operating characteristic curve; CI, confidence interval; ROC, receiver operating characteristic.
Figure 5.
This figure illustrates the calibration and clinical utility of the nomogram prediction model for pneumothorax risk. (A) Calibration curve of the nomogram prediction model. The dashed line represents the ideal model, the solid line represents the bias-corrected performance, and the dotted line shows the apparent performance. (B) DCA comparing the net benefit of the model against the “intervention for all” (trade-all) and “intervention for none” (repeat-none) strategies. The nomogram demonstrated higher net benefit across the 0–0.8 threshold probability range, indicating good clinical utility. DCA, decision curve analysis.
Discussion
The success rate of lung nodule localization was 90.2%, as the failure rate was relatively high during the early stages of technology application. However, with the advancement of the technique, the failure rate has now decreased to below 5%. Pneumothorax and pulmonary hemorrhage are among the most common complications associated with CT-guided percutaneous lung puncture (14,15). Previous studies have reported complication rates ranging from 9% to 54%, with an average rate of approximately 20%. Severe complications requiring surgical intervention are observed in less than 5% of cases (16,17). In this study, the incidence of pneumothorax following CT-guided preoperative localization using autologous blood and methylene blue was 16.1%, lower than the rates reported in the literature. This reduced incidence may be attributed to the sealing effect of autologous blood and methylene blue in the puncture tract, which effectively minimizes air leakage. Moreover, all pneumothorax cases in this cohort were mild (lung collapse rate <20%), and no patients experienced significant respiratory distress or required invasive intervention, highlighting the safety of this localization technique.
Air embolism is a rare but serious complication that may occur during preoperative puncture of lung nodules under CT guidance. The exact mechanism of air embolism following puncture remains not fully understood. However, most scholars believe that air embolism occurs when the puncture needle penetrates the lung parenchyma, creating a fistula between air-filled spaces and the pulmonary veins. In our study, no cases of air embolism were detected, which may be related to the potential sealing effect of autologous blood injected during the localization procedure, preventing the formation of air fistulas.
Through univariate and multivariate analyses, elevated WBC count and the distance from the needle tip to the visceral pleura were identified as independent risk factors for pneumothorax. Elevated WBC levels significantly increased the risk of pneumothorax, potentially reflecting an underlying inflammatory state. Inflammatory or stress responses may weaken lung tissue integrity, increasing susceptibility to damage and pneumothorax. This finding aligns with previous studies that have linked elevated WBC counts to increased postoperative complications following lung cancer resection (18).
Additionally, the role of systemic inflammation markers, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), in predicting various complications has been previously documented (19). Similarly, the lymphocyte-to-monocyte ratio (LMR) has been associated with spontaneous pneumothorax recurrence (20). These findings suggest that preoperative inflammatory markers, such as WBC count, could serve as valuable tools for risk stratification and patient selection.
This study found that the distance from the needle tip to the visceral pleura is an independent risk factor for pneumothorax. A greater puncture depth significantly increases the risk of pneumothorax. These findings are consistent with related studies in the literature. For example, in a study evaluating complications of percutaneous lung biopsy, a greater puncture path length (>3 cm) was found to significantly increase the likelihood of pneumothorax and was closely associated with lesion size and depth (21). This suggests that a deeper puncture path may lead to increased mechanical damage to lung tissue or local pressure changes, thereby increasing the risk of pneumothorax. Saji et al. analyzed 289 CT-guided lung biopsies and found that the angle of the puncture path was significantly associated with the occurrence of pneumothorax. A larger puncture angle (mean 23.5°) significantly increased the risk of pneumothorax and was one of the independent risk factors for the need for chest tube placement (22). In contrast, this study did not identify puncture angle as an independent risk factor for pneumothorax. Notably, the average puncture angle in our study was 71.2°, much larger than the mean in Saji et al.’s study. This may suggest that when the puncture angle is wide, its impact on pneumothorax risk may be overshadowed by other factors such as puncture depth and patient anatomical characteristics.
A nomogram prediction model for preoperative pneumothorax risk was developed based on independent risk factors identified through multivariate analysis, including WBC count and needle-tip-to-visceral-pleura distance. ROC curve analysis demonstrated satisfactory predictive performance of the model (AUC =0.762), with an internal validation 95% CI of 0.702–0.771, further confirming its stability. The nomogram model developed in this study outperforms models from similar studies (AUC approximately 0.71) (23), likely due to the inclusion of WBC count as a novel risk prediction variable.
There are several limitations in this study. First, its retrospective single-center design and relatively small sample size may limit the generalizability of the findings. Second, external validation of the predictive model in multi-center prospective cohorts is necessary. Lastly, the exclusion of other inflammatory markers beyond WBC count may restrict the comprehensive understanding of factors influencing pneumothorax risk, warranting further investigation in future studies.
Conclusions
CT-guided preoperative localization using autologous blood combined with methylene blue is a safe and effective method for localizing pulmonary nodules. Elevated WBC count and the distance from the needle tip to the visceral pleura are independent risk factors for pneumothorax during localization. The proposed nomogram provides a reliable tool for individualized risk assessment, supporting tailored clinical decision-making. Future research should focus on larger, multi-center prospective studies to validate these findings and explore additional inflammatory markers to refine the risk stratification further.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of The First Hospital of Putian (No. 2018-008). Due to the retrospective cohort design, the requirement for informed consent was waived.
Footnotes
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2286/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2286/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2024-2286/dss
References
- 1.Chan EY, Gaur P, Ge Y, et al. Management of the Solitary Pulmonary Nodule. Arch Pathol Lab Med 2017;141:927-31. 10.5858/arpa.2016-0307-RA [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Li Q, Hao Q, et al. Malignancy risk stratification for solitary pulmonary nodule: A clinical practice guideline. J Evid Based Med 2022;15:142-51. 10.1111/jebm.12476 [DOI] [PubMed] [Google Scholar]
- 3.Harzheim D, Eberhardt R, Hoffmann H, et al. The Solitary Pulmonary Nodule. Respiration 2015;90:160-72. 10.1159/000430996 [DOI] [PubMed] [Google Scholar]
- 4.Kikano GE, Fabien A, Schilz R. Evaluation of the Solitary Pulmonary Nodule. Am Fam Physician 2015;92:1084-91. [PubMed] [Google Scholar]
- 5.Hwang S, Kim TG, Song YG. Comparison of hook wire versus coil localization for video-assisted thoracoscopic surgery. Thorac Cancer 2018;9:384-9. 10.1111/1759-7714.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Sun D, Gao M, et al. Computed tomography-guided localization of pulmonary nodules prior to thoracoscopic surgery. Thorac Cancer 2023;14:119-26. 10.1111/1759-7714.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinohara Y, Oki M. Need for preoperative marking of pulmonary nodules and a more useful technique. J Thorac Dis 2023;15:1548-50. 10.21037/jtd-23-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Chen E. Advances in the localization of pulmonary nodules: a comprehensive review. J Cardiothorac Surg 2024;19:396. 10.1186/s13019-024-02911-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang SF, Liu HR, Ma AL, et al. Preoperative computed tomography-guided localization for multiple pulmonary nodules: comparison of methylene blue and coil. J Cardiothorac Surg 2022;17:186. 10.1186/s13019-022-01941-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. 10.1510/icvts.2011.266932 [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Liao QX, Xie JB, et al. Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses. Open Life Sci 2023;18:20220645. 10.1515/biol-2022-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JY, Tsai SC, Wu TC, et al. Puncture frequency predicts pneumothorax in preoperative computed tomography-guided lung nodule localization for video-assisted thoracoscopic surgery. Thorac Cancer 2022;13:1925-32. 10.1111/1759-7714.14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Wu J, Xiong J, et al. Risk factors of pneumothorax in computed tomography guided lung nodule marking using autologous blood: a retrospective study. J Cardiothorac Surg 2024;19:317. 10.1186/s13019-024-02810-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. 10.1016/j.athoracsur.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 15.Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. 10.3348/kjr.2012.13.6.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809-14. 10.2214/AJR.09.3224 [DOI] [PubMed] [Google Scholar]
- 18.Xiaowei M, Wei Z, Qiang W, et al. Assessment of systemic immune-inflammation index in predicting postoperative pulmonary complications in patients undergoing lung cancer resection. Surgery 2022;172:365-70. 10.1016/j.surg.2021.12.023 [DOI] [PubMed] [Google Scholar]
- 19.Saricam M, Guven O, Ozkan B. Utilizing the lymphocyte-monocyte ratio in predicting the recurrence of spontaneous pneumothorax. Cir Cir 2023;91:725-9. [DOI] [PubMed] [Google Scholar]
- 20.Vunvulea V, Melinte RM, Brinzaniuc K, et al. Blood Count-Derived Inflammatory Markers Correlate with Lengthier Hospital Stay and Are Predictors of Pneumothorax Risk in Thoracic Trauma Patients. Diagnostics (Basel) 2023;13:954. 10.3390/diagnostics13050954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh SE, Wu DD, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singap 2013;42:285-90. 10.47102/annals-acadmedsg.V42N6p285 [DOI] [PubMed] [Google Scholar]
- 22.Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest 2002;121:1521-6. 10.1378/chest.121.5.1521 [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Bao D, Wu W, et al. Development and validation of a prediction model of pneumothorax after CT-guided coaxial core needle lung biopsy. Quant Imaging Med Surg 2022;12:5404-19. 10.21037/qims-22-176 [DOI] [PMC free article] [PubMed] [Google Scholar]