Abstract
Background
Lung cancer, especially non-small cell lung cancer (NSCLC), is a leading cause of cancer mortality. Epidermal growth factor receptor (EGFR) mutations drive NSCLC progression but also sensitize tumors to EGFR-tyrosine kinase inhibitors (TKIs). However, the response rate to targeted therapy is only 70%, and most patients experience disease progression 9 to 14 months after first- or second-generation EGFR-TKI treatment. This study aims to examine the association between super-amplification refractory mutation system (ARMS)-derived ΔCt values [mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value] and EGFR mutation (EGFRm) abundance in predicting the efficacy and prognosis of EGFR-TKIs in NSCLC patients.
Methods
The present retrospective research encompassed 139 patients with stage IIIB–IV NSCLC treated with EGFR-TKIs. Patients were categorized based on super-ARMS ΔCt values and Kaplan-Meier, and Cox regression models were used to evaluate the outcomes in survival and independent influencing factors, thus establishing the optimal ΔCt value for EGFR-TKIs response.
Results
High mutation abundance, defined by ΔCt ≤3.76, was correlated with increased objective response rate (ORR) (61.2% vs. 36.8%, P=0.003) and longer median progression-free survival (mPFS) (20.9 vs. 15.8 months, log-rank P=0.005) compared to low abundance. The optimal ΔCt cut-off predictive of EGFR-TKIs response was 4.335. Patients with ΔCt ≤4.335 demonstrated superior ORR (64.6% vs. 28.1%, P<0.001) and mPFS (20.9 vs. 13.5 months, log-rank P<0.001) compared to those with ΔCt >4.335. Multivariate Cox analysis identified median ΔCt value group (ΔCt ≤3.76 or ΔCt >3.76), the optimal ΔCt cut-off value group (ΔCt ≤4.335 or ΔCt >4.335), brain metastasis, liver metastasis, EGFRm status, performance status (PS) score, and the generation of EGFR-TKIs as independent predictors of PFS in first-line EGFR-TKIs-treated patients.
Conclusions
Stratification based on ΔCt values derived from the super-ARMS system can predict the efficacy and clinical prognosis of first-line EGFR-TKI treatment in NSCLC patients. Additionally, higher mutation abundance may contribute to the superior efficacy and prognosis of EGFR-TKIs in patients with exon 19 deletions compared to those with the 21L858R mutation.
Keywords: Non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), tyrosine kinase inhibitors (TKIs), circulating tumor DNA (ctDNA), super-amplification refractory mutation system (super-ARMS)
Highlight box.
Key findings
• Stratification based on ΔCt [mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value] values derived from the Super-Amplification refractory mutation system (ARMS) can predict the efficacy and clinical prognosis of first-line epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) treatment in non-small cell lung cancer (NSCLC) patients.
What is known and what is new?
• Quantitative assessment of EGFR mutation status in plasma ctDNA collected from NSCLC patients can predict the efficacy and prognosis of first-line EGFR-TKIs treatment.
• High mutation abundance, defined by ΔCt ≤4.335, was correlated with increased objective response rate (ORR) and longer median progression-free survival (mPFS) compared to low abundance.
What is the implication, and what should change now?
• Stratification of patients with EGFR-mutation-positive NSCLC based on ΔCt values derived from the super-ARMS system is of significant value in predicting the efficacy of first-line EGFR-TKI treatment.
• Utilizing ΔCt values to guide first-line EGFR-TKI therapy may help improve the prognosis of NSCLC patients.
Introduction
Lung cancer continues to be the primary contributor of cancer-related mortality globally, with non-small cell lung cancer (NSCLC) contributing to over 80% of all instances (1), in which nearly 60% of cases were diagnosed at an advanced stage (2). The detection of the epidermal growth factor receptor (EGFR) mutations has initiated a new phase in customized treatment for NSCLC. These mutations are associated with enhanced cell proliferation and survival, and the sensitivity of EGFR-mutated NSCLC patients to EGFR tyrosine kinase inhibitors (TKIs) leads to tremendous therapeutic progress in advanced NSCLC, enhancing both long-term survival and quality of life (3). However, the response rate to targeted therapy is suboptimal at 70%, with a majority of patients experiencing progression in their disease around 9 to 14 months after receiving first-generation or second-generation EGFR-TKI treatment (4). The FLAURA study demonstrated that treatment with the third-generation EGFR-TKI osimertinib upgraded median progression-free survival (mPFS) to 18.9 months (5). Variability in the efficacy and prognosis of EGFR-TKIs between different groups of patients suggests that factors such as mutation types, co-mutations, mutation abundance, and other clinical characteristics may influence treatment outcomes (6-8). The concept of mutation abundance, represented as the ratio of the number of mutant alleles to the total number of alleles, was proposed by Yung et al. in 2009 using droplet digital polymerase chain reaction (ddPCR) technology (9). Zhou et al. found that EGFR mutation (EGFRm) abundance in NSCLC patients could predict the efficacy of gefitinib, with higher abundance associated with significantly longer progression-free survival (PFS) (10). Although multiple studies have validated the significance of EGFRm abundance in treatment response and prognosis, there is still no unified standard for high, medium, and low abundance, because statistically significant abundance values were defined based on the data in each specific study. Amplification refractory mutation system (ARMS), ddPCR, and next-generation DNA sequencing (NGS) have been used for detecting EGFRms (11-13). ARMS, a qualitative test approved for clinical use, is cost-effective but lacks sensitivity and the ability to quantify mutation abundance (14). ddPCR and NGS are both highly sensitive and precise techniques capable of quantitatively analyzing the status of circulating tumor DNA (ctDNA) in plasma but face challenges in clinical adoption due to the complexity of ddPCR assay development and the high cost and complexity of NGS data analysis (15,16). The super-ARMS, based on ARMS technology, has been approved by the China Food and Drug Administration (CFDA) for qualitative evaluation of EGFRm status in plasma ctDNA owing to its superior selectivity, sensitivity, and price-effectiveness (17).
This study utilized the super-ARMS technique to evaluate EGFRm status in plasma ctDNA collected from NSCLC patients and explored the potential of using ΔCt values [mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value] (18) by trying to transform the qualitative analysis into a semi-quantitative polymerase chain reaction (PCR) detection method. Patients before subjecting to first-line EGFR-TKIs treatment were divided into high and low mutation abundance groups, as defined by ΔCt values. They were then treated with EGFR-TKIs to evaluate the efficiency and prognosis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-97/rc).
Methods
Clinical data
In this retrospective cohort analysis, executed from January 2020 to March 2023, we enrolled 139 patients with stage III or IV NSCLC at the Affiliated Hospital of Guangdong Medical University, who were pathologically diagnosed with adenocarcinoma, adenosquamous carcinoma or squamous cell carcinoma and initiated EGFR-TKI therapy. The inclusion criteria included: (I) histopathologically confirmed NSCLC; (II) clinical staging from IIIB to IV; (III) detected EGFR gene mutations: exon 19 deletion or exon 21L858R mutation; (IV) first-line EGFR-TKI treatment; (V) newly diagnosed or recurrence after surgery; (VI) no severe concomitant systemic diseases. The technical flowchart of the study is depicted in Figure 1. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics board of the Affiliated Hospital of Guangdong Medical University (No. PJKT2024-070) and individual consent for this retrospective analysis was waived.
Figure 1.
Flowchart of patient enrollment in this study. A total of 139 patients met the inclusion and exclusion criteria, of whom 65 patients received first- or second-generation EGFR-TKIs and 74 patients received third-generation EGFR-TKIs. DCR, disease control rate; EGFR, epidermal growth factor receptor; mPFS, median progression-free survival; NSCLC, non-small cell lung cancer; ORR, objective response rate; qPCR, quantitative polymerase chain reaction; ΔCt, mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value; super-ARMS, super-Amplification Refractory Mutation System; TKIs, tyrosine kinase inhibitors.
Experimental methods
Specimen collection and DNA extraction
Peripheral venous blood (10 mL) was dispensed into an ethylenediaminetetraacetic acid (EDTA)-coated tube, and plasma was separated within 2 hours after collection. ctDNA was separated from blood plasma using the AmoyDx kit (Amoy Diagnostics, Xiamen, China), and stored at −80 ℃ for further analysis. DNA purity was evaluated using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Corporation, Wilmington, USA), with A260/A280 ratio between 1.8 and 2.0 suitable for analysis. The purified DNA was then used for the molecular identification of EGFRms.
Detection of EGFRms in plasma ctDNA
The study employed the super-ARMS EGFRm detection kit, which is capable of detecting 41 frequent somatic EGFRms from exons 18 to 21. Real-time fluorescence quantitative PCR was executed by following the manufacturer’s instructions and employing the SLAN-96S real-time PCR apparatus (Shanghai Hongshi, China). By calculating mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value, this assay could reliably detect the presence of EGFRms with a sensitivity exceeding 0.2%. ΔCt values were automatically derived from the fluorescence PCR amplification curves and corresponding cycle numbers. ΔCt cut-offs were established at 11 (19del/L858R/20ins), 12 (G719X/L861Q/S768I) and 8 (T790M). Samples with ΔCt values below these thresholds were classified as positive for the respective EGFRm types, while those above these thresholds were considered as negative.
Efficacy assessment
An efficacy evaluation was performed one month after the initiation of EGFR-TKI targeted therapy, with subsequent CT and other imaging evaluations performed every 2 months for patients showing stable or effective responses. The efficacy was evaluated by adopting the Remission Evaluation Criteria in Solid Tumors (RECIST), and classifying responses into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The objective response rate (ORR) was determined utilizing the formula (CR + PR)/(CR + PR + SD + PD) ×100%, while the disease control rate (DCR) was calculated as (CR + PR + SD)/(CR + PR + SD + PD) ×100%.
Follow-up and survival analysis
This study utilized a combination of outpatient and telephone follow-up, with the final follow-up date set for November 15, 2024. Overall survival (OS) was defined as the interval from the initiation of EGFR-TKI targeted therapy to the patient’s death or last follow-up. PFS was defined as the interval from the initiation of EGFR-TKI targeted therapy to documented disease progression or the patient’s death from any cause.
Statistical analysis
Statistical analysis was conducted utilizing IBM SPSS software version 27.0. The Chi-squared test was used for the comparative examination of categorical variables concerning clinical features and treatment results. The Kaplan-Meier analysis of survival and Cox proportional risk models was used for prognosis analysis of PFS and OS, with graphical representations generated using Prism software. A statistically significant limit of P<0.05 was set. The test of Pearson’s correlation was applied to evaluate the relationship between ΔCt values and PFS. The analysis of the receiver operating characteristic (ROC) curve was performed to identify the best cut-off value of ΔCt associated with the treatment efficacy of EGFR-TKIs.
Results
Clinical features of participating patients
The median ages among the 139 enrolled patients were 66.0 years. Utilizing the ΔCt values generated from the super-ARM system and based on a median ΔCt value of 3.76, we classified them into high and low mutation abundance groups. We also employed the ROC curve to determine the optimal ΔCt cut-off value of 4.335 associated with the effectiveness of EGFR-TKIs. Based on the median ΔCt value and the optimal ΔCt cut-off value, we analyzed the clinical features among different groups, including gender, median age at diagnosis, smoking history, performance status (PS) score, EGFRm status, clinical stage, existence of brain or liver metastases, and generation of EGFR-TKIs. No significant statistical differences were observed in baseline clinical features between groups with different ΔCt values of ctDNA EGFRm, with the exception of EGFRm status. Significant statistical differences were noted between exon 19 deletion (19Del) and 21L858R mutation, whether categorized by the median ΔCt value of 3.76 (79% vs. 21%, P<0.001) or by the ideal ΔCt cut-off value of 4.335 (76% vs. 24%, P<0.001). This suggests that the percentage of patients with the 19Del in the high mutation abundance group was markedly greater than that of patients with the 21L858R mutation. The comparison of clinical characteristics between groups with different ΔCt values of ctDNA EGFRm is shown in Table 1.
Table 1. Comparison of clinical characteristics between groups with different ΔCt values of ctDNA EGFRm.
| Factors | Baseline ctDNA EGFRm ΔCt values | Baseline ctDNA EGFRm ΔCt values | |||||
|---|---|---|---|---|---|---|---|
| ≤3.76 (n=71) | >3.76 (n=68) | P | ≤4.335 (n=82) | >4.335 (n=57) | P | ||
| Gender | 0.69 | >0.99 | |||||
| Female | 41 [58] | 37 [54] | 46 [56] | 32 [56] | |||
| Male | 30 [42] | 31 [46] | 36 [44] | 25 [44] | |||
| Median age at diagnosis (years) | 0.19 | 0.16 | |||||
| <65 | 35 [49] | 26 [38] | 40 [49] | 21 [37] | |||
| ≥65 | 36 [51] | 42 [62] | 42 [51] | 36 [63] | |||
| Smoking history | 0.23 | 0.40 | |||||
| No | 60 [85] | 52 [76] | 68 [83] | 44 [77] | |||
| Yes | 11 [15] | 16 [24] | 14 [17] | 13 [23] | |||
| Performance status | 0.23 | 0.67 | |||||
| 0–1 | 63 [89] | 62 [91] | 73 [89] | 52 [91] | |||
| >1 | 8 [11] | 6 [9] | 9 [11] | 5 [09] | |||
| EGFR mutation status | <0.001 | <0.001 | |||||
| Exon 19 deletion | 56 [79] | 29 [43] | 62 [76] | 23 [40] | |||
| 21L858R mutation | 15 [21] | 39 [57] | 20 [24] | 34 [60] | |||
| Clinical stage | 0.93 | 0.85 | |||||
| III | 8 [11] | 8 [12] | 8 [10] | 5 [09] | |||
| IV | 63 [89] | 60 [88] | 74 [90] | 52 [91] | |||
| Brain metastases | 0.48 | 0.79 | |||||
| No | 45 [63] | 47 [69] | 55 [67] | 37 [65] | |||
| Yes | 26 [37] | 21 [31] | 27 [33] | 20 [35] | |||
| Liver metastases | 0.96 | 0.51 | |||||
| No | 53 [75] | 51 [75] | 63 [77] | 41 [72] | |||
| Yes | 18 [25] | 17 [25] | 19 [23] | 16 [28] | |||
| Generation of EGFR-TKIs | 0.79 | 0.42 | |||||
| 1st or 2nd generation EGFR-TKIs | 34 [48] | 31 [46] | 36 [44] | 29 [51] | |||
| 3rd generation EGFR-TKIs | 37 [52] | 37 [54] | 46 [56] | 28 [49] | |||
Data are presented as n [%]. ΔCt, mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value; ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EGFRm, epidermal growth factor receptor mutation; TKIs, tyrosine kinase inhibitors.
Efficacy response
Among the 139 patients treated with EGFR-TKIs, 69 showed PR, with a total ORR of 49.6% (69/139), and an overall disease control rate (DCR) of 88.5% (123/139).
In terms of ORR, patients with exon 19 deletion exhibited a superior ORR with respect to individuals with 21L858R mutations (58.8% vs. 35.2%, P=0.007). Patients lacking liver metastasis exhibited more favorable ORR than individuals with liver metastasis (57.7% vs. 25.7%, P=0.001). Patients receiving third-generation EGFR-TKIs exhibited a markedly superior ORR compared to those receiving first- or second-generation EGFR-TKIs (58.1% vs. 40.0%, P=0.03).
Regarding DCR, patients with the PS score of 0–1 demonstrated an increased DCR than those with the PS score >1 (91.2% vs. 64.3%, P=0.003); patients devoid of brain metastasis displayed an improved DCR over individuals with brain metastasis (92.4% vs. 80.9%, P=0.044), and patients receiving third-generation EGFR-TKIs demonstrated a significantly better DCR compared to those receiving first- or second-generation EGFR-TKIs (98.6% vs. 76.9%, P<0.001). The efficacy response assessment for each clinical characteristic group is shown in Table 2.
Table 2. Evaluation of objective response rates and disease control rates of EGFR-TKIs across various clinical characteristic groups.
| Features | N | Objective response rate | Disease control rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | χ2 | P | N | % | χ2 | P | |||
| Overall rate | 139 | 69 | 49.6 | – | – | 123 | 88.5 | – | – | |
| Gender | 0.608 | 0.44 | 1.172 | 0.28 | ||||||
| Female | 78 | 41 | 52.6 | 67 | 85.9 | |||||
| Male | 61 | 28 | 45.9 | 56 | 91.8 | |||||
| Median age at diagnosis (years) | 2.603 | 0.11 | 2.619 | 0.11 | ||||||
| <65 | 61 | 35 | 57.4 | 57 | 93.4 | |||||
| ≥65 | 78 | 34 | 43.6 | 66 | 84.6 | |||||
| Smoking history | 1.062 | 0.30 | 0.359 | 0.55 | ||||||
| No | 112 | 58 | 51.8 | 100 | 89.3 | |||||
| Yes | 27 | 11 | 40.7 | 23 | 85.2 | |||||
| Performance status | 1.208 | 0.27 | 8.954 | 0.003 | ||||||
| 0–1 | 125 | 64 | 51.2 | 114 | 91.2 | |||||
| ≥2 | 14 | 5 | 35.7 | 9 | 64.3 | |||||
| EGFR mutation status | 7.381 | 0.007 | 0.014 | 0.91 | ||||||
| Exon 19 deletion | 85 | 50 | 58.8 | 75 | 88.2 | |||||
| 21L858R mutation | 54 | 19 | 35.2 | 48 | 88.9 | |||||
| Clinical stage | 2.202 | 0.14 | 0.827 | 0.36 | ||||||
| III | 13 | 9 | 69.2 | 13 | 100.0 | |||||
| IV | 126 | 60 | 47.6 | 110 | 87.3 | |||||
| Brain metastases | 3.654 | 0.056 | 4.067 | 0.044 | ||||||
| No | 92 | 51 | 55.4 | 85 | 92.4 | |||||
| Yes | 47 | 18 | 38.3 | 38 | 80.9 | |||||
| Liver metastases | 10.712 | 0.001 | 3.310 | 0.07 | ||||||
| No | 104 | 60 | 57.7 | 95 | 91.3 | |||||
| Yes | 35 | 9 | 25.7 | 28 | 80.0 | |||||
| Generation of EGFR-TKIs | 4.539 | 0.03 | 16.035 | <0.001 | ||||||
| 3rd generation EGFR-TKIs | 74 | 43 | 58.1 | 73 | 98.6 | |||||
| 1st or 2nd generation EGFR-TKIs | 65 | 26 | 40.0 | 50 | 76.9 | |||||
EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
Survival analysis
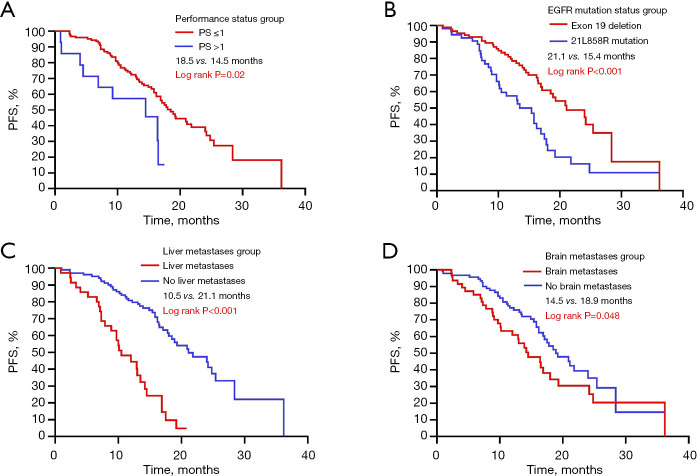
The median progression-free survival (mPFS) period among 139 participants was 14.3 months. During the median follow-up period of 19.3 months, 54.0% (75/139) of the patients experienced disease progression, and only 12.2% (17/139) of the patients died without reaching the median OS (mOS). The incidence of adverse reactions was 13.4%, with no treatment-related deaths. Kaplan-Meier analysis demonstrated that patients with the PS score in 0–1 had a longer mPFS than those with the PS score >1 (18.5 vs. 14.5 months, log-rank P=0.02); patients with exon 19 deletion exhibited a markedly extended mPFS compared with individuals with 21L858R mutations (21.1 vs. 15.4 months, log-rank P<0.001); patients lacking liver metastasis exhibited a prolonged mPFS over individuals with liver metastasis (21.1 vs. 10.5 months, log-rank P<0.001); patients lacking brain metastasis exhibited a prolonged mPFS over individuals with brain metastasis (18.9 vs. 14.5 months, log-rank P=0.048). The PFS prognosis analysis for these four clinical characteristics is shown in Figure 2. No significant variation in mPFS was detected concerning clinical stage, smoking history, age, and gender (Figure 3). Additionally, Kaplan-Meier analysis demonstrated that third-generation EGFR-TKIs exhibited a markedly extended mPFS in comparison with first- and second-generation EGFR-TKIs (20.9 vs. 14.5 months, log-rank P<0.001). The enhanced capacity of third-generation EGFR-TKIs to penetrate the blood-brain barrier, in contrast to first and second-generation TKIs, allows for superior efficacy for treating patients with brain metastases, particularly in controlling intracranial disease progression (19). Consequently, we examined the influence of brain metastases on PFS in treatment cohorts receiving first/second or third-generation EGFR-TKIs. The results indicated that following treatment with the first/second-generation EGFR-TKIs, the mPFS of the cohort without brain metastases was significantly longer than that of the cohort with brain metastases (16.9 vs. 7.3 months, log-rank P=0.04). Conversely, following treatment with the third-generation EGFR-TKIs, no significant difference in mPFS was observed between the cohort without brain metastases and the cohort with brain metastases (25.4 vs. 16.9 months, log-rank P=0.07) (Figure 4).
Figure 2.
Analysis of PFS curves for NSCLC patients treated with EGFR-TKIs, categorized by performance status (A), EGFR mutation status (B), and the presence of liver (C) and brain (D) metastases. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; PS, performance status; TKIs, tyrosine kinase inhibitors.
Figure 3.
Analysis of PFS curves for NSCLC patients receiving EGFR-TKI therapy, categorized by smoking history (A), clinical stage (B), gender (C), and age (D). EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
Figure 4.
Analysis of PFS curves for NSCLC patients receiving first- and second-generation EGFR-TKIs versus third-generation EGFR-TKIs (A), with further stratification of the PFS curves based on the presence or absence of brain metastases between treatment groups of first- or second-generation EGFR-TKIs (B) and third-generation EGFR-TKIs (C). The analysis of PFS curves is also stratified based on the median ΔCt value (ΔCt ≤3.76 or ΔCt >3.76) (D) and the optimal ΔCt cut-off value (ΔCt ≤4.335 or ΔCt >4.335) (E). ΔCt, mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
Prediction of EGFR-TKI efficacy and prognosis based on ΔCt value grouping
Utilizing the ΔCt values generated from the super-ARM system, we classified 139 patients into high (ΔCt <3.76, n=71) and low (ΔCt >3.76, n=68) mutation abundance groups based on a median ΔCt value of 3.76. The efficacy evaluation showed that the high mutation abundance group exhibited a markedly superior ORR than the low abundance group (61.2% vs. 36.8%, P=0.003). While no significant difference in DCR was detected between the two groups (91.5% vs. 88.2%, P=0.52). Patients in the high mutation abundance cohort experienced substantially prolonged median PFS relative to those in the low mutation abundance cohort (20.9 vs. 15.8 months, log-rank P=0.005) (Figure 4). Additionally, Pearson correlation analysis confirmed a negative correlation between ΔCt values and patient PFS (Pearson’s correlation coefficient, −0.306, P<0.001), indicating a weak but statistically significant association. The optimal ΔCt cut-off value associated with EGFR-TKI treatment response, determined by the Youden index from the ROC curve, was 4.335. Patients were categorized into two separate groups according to this threshold value: ΔCt ≤4.335 (n=82) and ΔCt >4.335 (n=57). Efficacy evaluation and prognosis analysis for these two groups showed that patients with ΔCt ≤4.335 exhibited a significantly higher ORR than those with ΔCt >4.335 (64.6% vs. 28.1%, P<0.001), while no significant disparity was observed in DCR (91.5% vs. 84.2%, P=0.19). However, the mPFS for the ΔCt ≤4.335 group was substantially longer than that for the ΔCt >4.335 group (20.9 vs. 13.5 months, log-rank P<0.001) (Figure 4). Table 3 presents the efficacy response assessment results for patients stratified by ΔCt values.
Table 3. Objective response rate and disease control rate in EGFR-TKIs-treated patients grouped by median ΔCt value and optimal ΔCt cut-off value.
| ctDNA EGFRm ΔCt values | ORR (%) | P | DCR (%) | P | mPFS (m) | P |
|---|---|---|---|---|---|---|
| ΔCt value ≤3.76 | 61.2 | 0.003 | 91.5 | 0.52 | 20.9 | 0.005 |
| ΔCt value >3.76 | 36.8 | 88.2 | 15.8 | |||
| ΔCt value ≤4.335 | 64.6 | <0.001 | 91.5 | 20.9 | <0.001 | |
| ΔCt value >4.335 | 28.1 | 84.2 | 0.19 | 13.5 |
ΔCt, mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value; ctDNA, circulating tumor DNA; DCR, disease control rate; EGFR, epidermal growth factor receptor; EGFRm, epidermal growth factor receptor mutation; m, months; mPFS, median progression-free survival; ORR, objective response rate; TKIs, tyrosine kinase inhibitors.
All clinical features, as well as the median ΔCt value and the optimal ΔCt cut-off value, were included in the univariate analysis. The results indicated that median age at diagnosis, smoking history, PS score, EGFRm status, existence of brain or liver metastases, the generation of EGFR-TKIs, the median ΔCt value, and the optimal ΔCt cut-off value were statistically significant in the univariate analysis. We then incorporated variables with statistical significance from the univariate analysis into the multivariate analysis. Due to the potential collinearity derived from the grouping based on median ΔCt value and optimal ΔCt cut-off value, which may lead to unstable regression coefficient estimates, we addressed this issue by variable selection (20). This approach involved incorporating the median ΔCt value and the optimal ΔCt cut-off value separately with seven other clinical characteristics (brain metastases, liver metastases, PS score, EGFRm status, treatment methods, median age at diagnosis, and smoking history) in the multivariate COX regression analysis. This analysis identified median ΔCt value group (ΔCt ≤3.76 or ΔCt >3.76), the optimal ΔCt cut-off value group (ΔCt ≤4.335 or ΔCt>4.335), brain metastases, liver metastasis, PS score, and EGFRm status and treatment methods as independent factors influencing PFS in patients treated with EGFR-TKIs. Finally, we identified ΔCt ≤3.76, ΔCt ≤4.335, the presence of exon 19 deletion status and third-generation EGFR-TKIs as favorable factors for PFS, but brain metastases, liver metastases and PS >1 were deemed unfavorable factors for PFS. The results of the multivariate analysis are shown in Figure 5.
Figure 5.
Multivariate Cox regression analysis examining the association of various stratification methods, including ΔCt median value group (ΔCt ≤3.76 or ΔCt >3.76), the optimal ΔCt cut-off value group (ΔCt ≤4.335 or ΔCt >4.335), brain metastases (yes or no), liver metastases (yes or no), EGFR mutation status (exon 19 deletion or 21L858R), performance status score (>1 or 0–1), generation of EGFR-TKIs (1st and 2nd, or 3rd), median age at diagnosis (≥65 or <65 years), and smoking history (yes or no), with PFS in patients treated with EGFR-TKIs. HR exceeding 1 signifies a positive factor for PFS, whereas an HR below 1 indicates an unfavorable factor for PFS. ΔCt, mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value; CI, confidence interval; EGFR, epidermal growth factor receptor; PFS, progression-free survival; TKIs, tyrosine kinase inhibitors.
Discussion
Liquid biopsy, by assessing plasma ctDNA for EGFRms, has been established in a previous study as a means to guide targeted therapy in NSCLC, offering a non-invasive approach to genomic analysis that complements traditional tissue biopsy (21). In this study, we leveraged ΔCt values obtained from the super-ARMS testing process to semi-quantitatively assess plasma ctDNA for EGFRm status and further predict the impact of ΔCt values defined by high and low mutation abundance on the efficacy and prognosis of patients treated with first-line EGFR-TKIs. Our study indicates that the higher mutation abundance group (ΔCt ≤3.76 or ΔCt ≤4.335) was associated with an elevated response rate to first-line EGFR-TKI therapy. Previously, Park et al. also found that the corrected ΔCt value, which refers to EGFRm quantification by PNA-mediated PCR clamping, can predict a better clinical response to EGFR-TKI therapy. Furthermore, there was a significant inverse correlation between the corrected ∆Ct values and the disease response (r=−0.184, P=0.02). Their study also investigates quantitative ctDNA testing for predicting targeted therapy efficacy but differs from our liquid biopsy approach. Obtaining small tissue samples via bronchoscopy or needle aspiration is challenging, and the study by Park et al. further highlights the clinical limitations of tissue biopsy compared to liquid biopsy (22). Other studies have shown that patients with higher levels of EGFRm abundance exhibit a significantly improved objective response to first-line EGFR-TKI treatment (23,24). Importantly, the cohort with higher mutation abundance (ΔCt ≤3.76 or ΔCt ≤4.335) also exhibited substantially superior mPFS in comparison with the low abundance cohort. Liu et al. also employed ΔCt values obtained from the super-ARMS assay to evaluate the influence of different baseline plasma ctDNA EGFRm levels and the changes in ctDNA EGFRms after one month of targeted therapy on the therapeutic outcomes and prognoses of patients undergoing EGFR-TKI treatment. Their result indicated that ΔCt values ≤8.11 for EGFRm may be associated with longer OS (without reaching 11.0 months, log-rank P=0.02) compared to ΔCt values >8.11, but no significant difference was observed in mPFS (22.2 vs. 27.5 months; log-rank P=0.91). This study also indicated that a ΔCt value change >4.89 after one month of targeted therapy may predict better OS (without reaching 11.0 months, log-rank P=0.01) compared to a ΔCt value change ≤4.89, while there was no significant difference in mPFS (27.5 vs. 11.0 months; log-rank P=0.20). However, the study by Liu et al. featured a smaller sample size (n=41) and did not control for other clinical characteristics or potential confounding factors that could impact the results. Moreover, their findings were restricted to demonstrating the ΔCt cut-off value associated with OS (25). Wang et al. utilized the circulating single-molecule amplification and resequencing technology (cSMART) assay to prospectively evaluate the baseline plasma EGFRm status and to monitor the dynamic changes in EGFRms during EGFR-TKI therapy. They discovered that patients with EGFRm abundance >0.1% exhibited significantly longer mPFS (9.5 vs. 5.0 months, log-rank P=0.01) when treated with EGFR-TKIs compared to those with mutation abundance between 0.01% and 0.1%. However, the number of participants with EGFRms was limited (n=54), and comparisons between different subgroups may be subject to certain statistical errors (24). Another study has also shown that the concurrent detection of EGFRms in tissue and ctDNA (B+/T+) indicates a high level of EGFRm abundance, with the high mutation abundance group exhibiting better mPFS (18.8 vs. 9.4 vs. 6.9 months, log-rank P=0.003) than in the (B−/T+) or (B+/T−) groups. However, the technology employed in this study requires concurrent EGFRm testing of both tissue and blood samples to establish the high mutation abundance group, which limits its further clinical application (26). These findings align with the conclusions drawn from our research. In addition, Yan et al. demonstrated that for patients with low EGFRm abundance, the mPFS (7.9 vs. 5.9 months, log-rank P=0.01) in the group treated with EGFR-TKI combined with chemotherapy was significantly longer than in the group treated with EGFR-TKI monotherapy (27).
Therefore, quantitative stratification of EGFRms in ctDNA not only facilitates the selection of patients who are more likely to benefit from EGFR-TKI therapy but also aids in formulating more effective treatment strategies for patients with lower EGFRm abundance. Compared to previous relevant studies, the strengths of our study include an ample number of EGFR-mutation-positive participants (n=139), the determination of an optimal ΔCt cut-off value (4.335) via ROC curve analysis for predicting the best response rate and PFS to EGFR-TKIs, exclusion of other common independent influencing factors that might bias the results and employs the cost-effective super-ARMS technology. Super-ARMS, compared to ddPCR and NGS, offers the advantages of cost-effectiveness and ease of operation, and its sensitivity and specificity in detecting EGFRm status are close to that of ddPCR (28). These advantages make it more suitable for clinical promotion and guidance of targeted therapy.
Exon 19 deletion has been demonstrated to confer greater clinical benefits compared to the L858R mutation in patients treated with different generations of EGFR-TKI, a finding that has been substantiated by multiple studies (29-31). There may be several potential mechanisms contributing to the therapeutic efficacy differences between the two mutations. First, due to the differences in the activation mechanisms and states of their kinase domains, there is a disparity in the affinity and sensitivity to EGFR-TKIs (32,33). Second, the higher proportion of co-occurring mutations in patients with the 21L858R mutation compared to those with exon 19 deletions is also one of the primary reasons (34-36). This study indicates that the prevalence of exon 19 deletion in the group exhibiting greater gene mutation abundance was markedly higher than that of the 21L858R mutation. This indicates that, alongside the previously described factors, a higher mutation abundance may also be one of the reasons why exon 19 deletion has better efficacy and prognosis with EGFR-TKIs compared to the 21L858R mutation. Li et al. utilized the ARMS+ method to detect EGFRm abundance in tumor tissue/ctDNA samples, and found that the exon 19 mutation was more prevalent in the group with high mutation abundance (23). Employing the ARMS plus technology, Liu et al. revealed a higher prevalence of exon 19 deletion in the high mutation abundance group, which may correlate with superior survival outcomes compared to the 21L858R mutation (37). Nevertheless, some related studies may have overlooked this aspect, potentially due to limitations in sample size or different directions in statistical analysis.
The presence of brain metastases and the generation of EGFR-TKIs as independent prognostic factors for PFS in patients receiving first-line targeted therapy have been confirmed in this study. A series of pivotal trials, namely FLAURA, FURLONG, and AENEAS, have unequivocally established the superior PFS and OS benefits conferred by the third-generation EGFR-TKIs over the first-generation counterparts in the management of patients with brain metastases from NSCLC (31,38,39). A previous study by Ju et al. also demonstrated that the status of brain metastases and the generation of EGFR-TKIs have a combined impact on PFS in patients treated with TKIs (40). Therefore, we conducted a Kaplan-Meier analysis of PFS based on different generations of EGFR-TKIs for brain metastasis subgroups. In patients treated with first- and second-generation EGFR-TKIs, those without brain metastases demonstrated significantly longer mPFS compared to those with brain metastases. However, no significant difference in mPFS was observed between patients with and without brain metastases when treated with third-generation EGFR-TKIs. These findings suggest that, in NSCLC patients with brain metastases carrying EGFRm and undergoing first-line targeted therapy, the utilization of third-generation EGFR-TKIs may yield superior clinical benefits.
Liver metastasis has been recognized as an independent prognostic indicator of worse outcomes in EGFR-TKI therapy in several retrospective studies (41,42). Our investigation revealed that patients with liver metastasis had markedly inferior ORR and mPFS compared to those lacking liver metastasis. In the tumor microenvironment of liver metastasis, the expression level of vascular endothelial growth factor (VEGF) is increased compared to other metastatic sites (43). VEGF not only induces tumor angiogenesis but also promotes the proliferation of EGFR-mutated cancer cells and influences the immunosuppressive network (44,45). Insulin-like growth factor 1 (IGF-1) serves as a ligand for the IGF-1 receptor (IGF-1R) and is highly expressed in the microenvironment of liver metastatic sites. Signaling from IGF-1R promotes the resistance of EGFR-mutated tumors to osimertinib (46). Combined anti-angiogenic therapy may enhance OS rates in patients with liver metastases (47), but there is a scarcity of data on the best systemic treatment for liver metastatic tumors (48,49). Future clinical studies are needed to explore the efficacy and survival benefits of EGFR-TKIs combined with systemic or local treatment modalities in lung cancer patients with liver metastasis, thereby advancing the development of personalized precision therapy.
However, there are some limitations in this study. Firstly, while our study focused on the most common EGFRms, prior research has demonstrated that compound mutations, such as those involving TP53 mutations (50), KRAS mutations (51), PIK3CA mutations and MET amplification (8), may affect the treatment response rate to EGFR-TKIs and the prognosis of patients. Future studies should incorporate comprehensive screening for compound mutations to better understand their impact on treatment outcomes. Secondly, the data were sourced from a single-center database, thus restricting the generalizability of the findings, necessitating multi-center investigations for validation. Additionally, we were unable to dynamically re-evaluate changes in ΔCt values after treatment, thus preventing us from understanding the role of ΔCt changes in EGFR-TKI treatment. Future research utilizing a larger sample size and dynamic super-ARMS testing is required to examine the effect of baseline ΔCt levels and alterations in ctDNA EGFRms on the efficacy of targeted therapies for patients. For the efficacy prediction of targeted drugs, it is not sufficient to evaluate the mutation abundance of the EGFR gene alone. An exhaustive assessment of diverse clinical factors is essential to formulate more rational clinical study designs and mitigate the influence of confounding variables on the outcomes.
Conclusions
Stratification based on ΔCt values derived from the super-ARMS system can predict the efficacy and clinical prognosis of first-line EGFR-TKI treatment in patients with NSCLC. However, the assessment of potential benefits from EGFR-TKIs should also consider other independent prognostic factors. Moreover, higher mutation abundance may contribute to the superior efficacy and prognosis of EGFR-TKIs in patients with exon 19 deletions compared to those with the 21L858R mutation.
Supplementary
The article’s supplementary files as
Acknowledgments
This study was supported by the Big Data Platform of Affiliated Hospital of Guangdong Medical University.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics board of the Affiliated Hospital of Guangdong Medical University (No. PJKT2024-070) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-97/rc
Funding: This research was supported by the National Natural Science Foundation of China (NSFC) (No. 82073388 to W.S.), the Natural Outstanding Youth Fund of Guangdong Province (No. 2022B1515020090 to W.S.), the Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-Communicable Diseases (No. 2022B1212030003 to W.S.), the Affiliated Hospital of Guangdong Medical University Clinical Research Program (Nos. LCYJ2020B005 and LCY2022DL002 to W.S.), the Affiliated Hospital of Guangdong Medical University Clinical Research Program (Nos. LY-2024-08-010 and LY-2024-11-006 to W.S.), and Zhanjiang Key Laboratory of Tumor Microenvironment and Organoid Research, Beijing Kechuang Medical Development Foundation (No. KC2023-JK-0288-PM30).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-97/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-97/dss
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Hendriks LEL, Remon J, Faivre-Finn C, et al. Non-small-cell lung cancer. Nat Rev Dis Primers 2024;10:71. 10.1038/s41572-024-00551-9 [DOI] [PubMed] [Google Scholar]
- 3.Corvaja C, Passaro A, Attili I, et al. Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging biological insights and therapeutic development. Cancer Treat Rev 2024;130:102824. 10.1016/j.ctrv.2024.102824 [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. 10.1093/annonc/mdv276 [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, He Y, Li W, et al. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target Oncol 2021;16:165-76. 10.1007/s11523-021-00794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY, Myung JK, Kim HR, et al. Factors that Predict Clinical Benefit of EGFR TKI Therapy in Patients with EGFR Wild-Type Lung Adenocarcinoma. Tuberc Respir Dis (Seoul) 2019;82:62-70. 10.4046/trd.2018.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gieszer B, Megyesfalvi Z, Dulai V, et al. EGFR variant allele frequency predicts EGFR-TKI efficacy in lung adenocarcinoma: a multicenter study. Transl Lung Cancer Res 2021;10:662-74. 10.21037/tlcr-20-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Song J, Wang Y, et al. Concurrent Genetic Alterations and Other Biomarkers Predict Treatment Efficacy of EGFR-TKIs in EGFR-Mutant Non-Small Cell Lung Cancer: A Review. Front Oncol 2020;10:610923. 10.3389/fonc.2020.610923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung TK, Chan KC, Mok TS, et al. Single-molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PCR in non-small cell lung cancer patients. Clin Cancer Res 2009;15:2076-84. 10.1158/1078-0432.CCR-08-2622 [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Zhang XC, Chen ZH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:3316-21. 10.1200/JCO.2010.33.3757 [DOI] [PubMed] [Google Scholar]
- 11.Sorber L, Zwaenepoel K, Deschoolmeester V, et al. A Comparison of Cell-Free DNA Isolation Kits: Isolation and Quantification of Cell-Free DNA in Plasma. J Mol Diagn 2017;19:162-8. 10.1016/j.jmoldx.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 12.De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol Sci 2019;40:172-86. 10.1016/j.tips.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Zhao J, Zhao X, et al. Detection of EGFR gene mutations in 100 non-small cell lung cancer clinical samples by a real-time polymerase chain reaction method using amplification refractory mutation system specific primers and Taqman fluorescence probes. Zhongguo Fei Ai Za Zhi 2013;16:25-32. 10.3779/j.issn.1009-3419.2013.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma M, Shi C, Qian J, et al. Comparison of plasma and tissue samples in epidermal growth factor receptor mutation by ARMS in advanced non-small cell lung cancer. Gene 2016;591:58-64. 10.1016/j.gene.2016.06.053 [DOI] [PubMed] [Google Scholar]
- 15.Mirza M, Goerke L, Anderson A, et al. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health 2024;27:1300-9. 10.1016/j.jval.2024.04.023 [DOI] [PubMed] [Google Scholar]
- 16.Mirabile A, Sangiorgio G, Bonacci PG, et al. Advancing Pathogen Identification: The Role of Digital PCR in Enhancing Diagnostic Power in Different Settings. Diagnostics (Basel) 2024;14:1598. 10.3390/diagnostics14151598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui S, Ye L, Wang H, et al. Use of SuperARMS EGFR Mutation Detection Kit to Detect EGFR in Plasma Cell-free DNA of Patients With Lung Adenocarcinoma. Clin Lung Cancer 2018;19:e313-22. 10.1016/j.cllc.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 18.Yuan JS, Reed A, Chen F, et al. Statistical analysis of real-time PCR data. BMC Bioinformatics 2006;7:85. 10.1186/1471-2105-7-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Pang X, Zhang S, et al. First-line treatment of EGFR-mutated non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Sci Rep 2024;14:22901. 10.1038/s41598-024-74496-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauerbrei W, Perperoglou A, Schmid M, et al. State of the art in selection of variables and functional forms in multivariable analysis-outstanding issues. Diagn Progn Res 2020;4:3. 10.1186/s41512-020-00074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YZ, Kong SN, Liu YP, et al. Can Liquid Biopsy Based on ctDNA/cfDNA Replace Tissue Biopsy for the Precision Treatment of EGFR-Mutated NSCLC? J Clin Med 2023;12:1438. 10.3390/jcm12041438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HY, Oh HJ, Kim KH, et al. Quantification of epidermal growth factor receptor (EGFR) mutation may be a predictor of EGFR-tyrosine kinase inhibitor treatment response. Thorac Cancer 2016;7:639-47. 10.1111/1759-7714.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Cai W, Yang G, et al. Comprehensive Analysis of EGFR-Mutant Abundance and Its Effect on Efficacy of EGFR TKIs in Advanced NSCLC with EGFR Mutations. J Thorac Oncol 2017;12:1388-97. 10.1016/j.jtho.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Liu Y, Meng Z, et al. Plasma EGFR mutation abundance affects clinical response to first-line EGFR-TKIs in patients with advanced non-small cell lung cancer. Ann Transl Med 2021;9:635. 10.21037/atm-20-7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XL, Bai RL, Chen X, et al. Correlation of circulating tumor DNA EGFR mutation levels with clinical outcomes in patients with advanced lung adenocarcinoma. Chin Med J (Engl) 2021;134:2430-7. 10.1097/CM9.0000000000001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Duan J, Chen H, et al. Analysis of EGFR mutation status in tissue and plasma for predicting response to EGFR-TKIs in advanced non-small-cell lung cancer. Oncol Lett 2017;13:2425-31. 10.3892/ol.2017.5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan X, Wang H, Li P, et al. Efficacy of first-line treatment with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) alone or in combination with chemotherapy for advanced non-small cell lung cancer (NSCLC) with low-abundance mutation. Lung Cancer 2019;128:6-12. 10.1016/j.lungcan.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 28.Feng WN, Gu WQ, Zhao N, et al. Comparison of the SuperARMS and Droplet Digital PCR for Detecting EGFR Mutation in ctDNA From NSCLC Patients. Transl Oncol 2018;11:542-5. 10.1016/j.tranon.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel N, Wu P, Zhang H. Comparison of gefitinib as first- and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 or 19 del epidermal growth factor receptor mutation. Cancer Manag Res 2017;9:243-8. 10.2147/CMAR.S138643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 31.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 32.Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9:52. 10.3390/cancers9050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamirat MZ, Koivu M, Elenius K, et al. Structural characterization of EGFR exon 19 deletion mutation using molecular dynamics simulation. PLoS One 2019;14:e0222814. 10.1371/journal.pone.0222814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S, Gao F, Fu S, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:739-42. 10.1001/jamaoncol.2018.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao XD, He X, Qin BD, et al. The prognostic value of tumor mutation burden in EGFR-mutant advanced lung adenocarcinoma, an analysis based on cBioPortal data base. J Thorac Dis 2019;11:4507-15. 10.21037/jtd.2019.11.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan J, Xu J, Wang Z, et al. Refined Stratification Based on Baseline Concomitant Mutations and Longitudinal Circulating Tumor DNA Monitoring in Advanced EGFR-Mutant Lung Adenocarcinoma Under Gefitinib Treatment. J Thorac Oncol 2020;15:1857-70. 10.1016/j.jtho.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wang H, Yang S, et al. EGFR mutation types and abundance were associated with the overall survival of advanced lung adenocarcinoma patients receiving first-line tyrosine kinase inhibitors. J Thorac Dis 2022;14:2254-67. 10.21037/jtd-22-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med 2022;10:1019-28. 10.1016/S2213-2600(22)00168-0 [DOI] [PubMed] [Google Scholar]
- 39.Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol 2022;40:3162-71. 10.1200/JCO.21.02641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju JS, Huang AC, Tung PH, et al. Brain metastasis, EGFR mutation subtype and generation of EGFR-TKI jointly influence the treatment outcome of patient with EGFR-mutant NSCLC. Sci Rep 2023;13:20323. 10.1038/s41598-023-45815-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang T, Cheng R, Zhang G, et al. Characterization of Liver Metastasis and Its Effect on Targeted Therapy in EGFR-mutant NSCLC: A Multicenter Study. Clin Lung Cancer 2017;18:631-639.e2. 10.1016/j.cllc.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 42.Wu KL, Tsai MJ, Yang CJ, et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer 2015;88:187-94. 10.1016/j.lungcan.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 43.Chen DS, Hurwitz H. Combinations of Bevacizumab With Cancer Immunotherapy. Cancer J 2018;24:193-204. 10.1097/PPO.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 44.Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol 2021;16:205-15. 10.1016/j.jtho.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun 2019;10:259. 10.1038/s41467-018-08074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You L, Zheng X, Deng D, et al. The benefit of anti-angiogenic therapy in EGFR exon 21 L858R mutant non-small cell lung cancer patients: a retrospective study. Sci Rep 2022;12:14624. 10.1038/s41598-022-18889-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castañón E, Rolfo C, Viñal D, et al. Impact of epidermal growth factor receptor (EGFR) activating mutations and their targeted treatment in the prognosis of stage IV non-small cell lung cancer (NSCLC) patients harboring liver metastasis. J Transl Med 2015;13:257. 10.1186/s12967-015-0622-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantelmo AR, Dejos C, Kocher F, et al. Angiogenesis inhibition in non-small cell lung cancer: a critical appraisal, basic concepts and updates from American Society for Clinical Oncology 2019. Curr Opin Oncol 2020;32:44-53. 10.1097/CCO.0000000000000591 [DOI] [PubMed] [Google Scholar]
- 50.Lan B, Zhao N, Du K, et al. Concurrent TP53 mutations predict a poor prognosis of EGFR-mutant NSCLCs treated with TKIs: An updated systematic review and meta-analysis. Oncol Lett 2022;24:384. 10.3892/ol.2022.13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rachiglio AM, Fenizia F, Piccirillo MC, et al. The Presence of Concomitant Mutations Affects the Activity of EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Non-Small Cell Lung Cancer (NSCLC) Patients. Cancers (Basel) 2019;11:341. 10.3390/cancers11030341 [DOI] [PMC free article] [PubMed] [Google Scholar]