Abstract
Background
Body mass index (BMI) has a complex association with a variety of chronic pain conditions, which may be influenced by sleep apnea syndrome (SAS). However, the role of SAS within the causal pathway linking BMI to chronic pain remains unconfirmed. This study explored whether and to what extent SAS serves as a mediator between BMI and chronic pain using Bayesian-weighted Mendelian randomization (BWMR) techniques.
Methods
This study utilized genome-wide association study (GWAS) summary data for BMI (N=461,460) and SAS (N=476,853; based on ICD-10 code diagnostic registries) from the IEU OpenGWAS database, along with chronic pain GWAS data (ICD-10 code-based diagnostic registries) from the FinnGen database. Utilizing BWMR and inverse-variance weighted (IVW) methods, a two-step, two-sample Mendelian randomization (MR) design was employed to investigate the causal relationships between BMI and various types of chronic pain and to assess the mediating role of SAS in the BMI-chronic pain link. Additionally, a suite of sensitivity analyses, including the use of MR-PRESSO to remove outliers, MR-Egger regression to assess horizontal pleiotropy, and Cochran’s Q test to evaluate heterogeneity, thereby ensuring the robustness and reliability of the MR study findings.
Results
The GWAS data for BMI, SAS, and chronic pain were all derived from European populations, comprising 9,851,867 BMI-associated single-nucleotide polymorphisms (SNPs), 24,183,940 SAS-related SNPs, and 19,680,346 to 19,682,705 chronic pain-linked SNPs in the respective datasets. BWMR revealed significant genetic associations between BMI and various types of chronic pain, including limb pain [odds ratio (OR) =1.34, 95% confidence interval (CI): 1.25–1.44, P<0.001], low back pain (OR =1.34, 95% CI: 1.25–1.43, P<0.001), low back pain with or without sciatica (OR =1.28, 95% CI: 1.21–1.36, P<0.001), and thoracic spine pain (OR =1.18, 95% CI: 1.03–1.36, P=0.02). Additionally, the genetic predisposition of BMI was also strongly associated with the risk of SAS (OR =2.34, 95% CI: 2.12–2.59, P<0.001). Mediation analysis revealed that SAS mediated 15.3% (β=0.045) of BMI’s causal effect on limb pain, 26.8% (β=0.078) on low back pain, and 26.8% (β=0.067) on low back pain with or without sciatica.
Conclusions
There is a causal relationship between BMI and chronic pain, with SAS acting as a mediator for a portion of the causal effects of BMI on conditions such as limb pain, low back pain, and low back pain with or without sciatica. Therefore, clinical strategies should focus on modulating BMI levels to maintain them within the normal range, which may effectively reduce the incidence risk of chronic pain. Furthermore, in patients with elevated BMI and comorbid SAS, targeted SAS treatment could potentially mitigate BMI-associated chronic pain burden.
Keywords: Chronic pain, body mass index (BMI), sleep apnea syndrome (SAS), Mendelian randomization (MR)
Introduction
Chronic pain is clinically defined as persistent or recurrent pain lasting beyond three months, with an estimated global prevalence of approximately 30% (1). An epidemiological survey revealed that approximately half of the interviewees reported pain for at least one day during the preceding month (2). Chronic pain is a significant contributor to the global disease burden, constituting a substantial clinical, social, and economic challenge that affects individuals across all age groups. Chronic pain not only diminishes quality of life and mental well-being but also results in compromised social functioning, elevated economic burden, and a higher likelihood of unemployment (3-7). Recent studies suggest a close positive association between body mass index (BMI) and the occurrence and progression of chronic pain (8,9). Indeed, the impact of BMI on chronic pain is affected by a multitude of factors, and sleep apnea syndrome (SAS) may be one of the contributing factors mediating the link between BMI and chronic pain. A study has demonstrated that patients with SAS experience a series of pathophysiological alterations, including intermittent hypoxia and ischemia-reperfusion injury secondary to sleep fragmentation (10). The systemic oxidative stress and inflammatory response induced by these pathological changes are closely associated with the development and progression of chronic pain (11,12). Observational studies have indicated a strong association between SAS and the development and maintenance of chronic pain as individuals with SAS are often affected by a decreased pain threshold and heightened pain sensitivity (13,14). Additionally, research teams have speculated about potential associations among BMI, SAS, and chronic pain (15). To date, no comprehensive and systematic studies have been conducted to confirm the causal relationships among these three factors.
Mendelian randomization (MR), a genetic variant-based causal inference method, was initially proposed by Katan in 1986 (16). This approach employs single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to simulate natural randomized trials, thereby evaluating the causal effects of exposures on outcomes. Compared to traditional cohort studies, MR analysis, which leverages genetic variants randomly assigned during embryogenesis and independently of postnatal environmental factors, effectively reduces the influence of unmeasured or unknown confounding factors, reverse causation, exposure measurement errors, and selection bias (17). It thereby provides high-quality evidence for causal associations between exposures and outcomes, analogous to randomized controlled trials. In contrast to mediation analysis under structural equation modeling frameworks, MR-based mediation analysis can disentangle direct and indirect (mediated) effects of exposures on outcomes without relying on assumptions of normality, linearity, or completeness of individual-level data, yielding more robust effect estimates (18). However, conventional MR methods remain susceptible to horizontal pleiotropy and weak instrument bias, compromising result reliability. To address the limitations of traditional MR analyses, the Bayesian-weighted Mendelian randomization (BWMR) method integrates Bayesian principles with weighted likelihood, employing probabilistic modeling to reduce the weights of potentially pleiotropic variants, thereby providing enhanced robustness against pleiotropic effects (19). To investigate the genetic-level causal relationships among BMI, SAS, and chronic pain, this study utilized a two-step, two-sample MR design to assess the causal effects of BMI on various chronic pain phenotypes and explore the potential mediating role of SAS in the BMI-chronic pain causal pathway. To further enhance the robustness of causal inference, BWMR analysis was incorporated. We present this article in accordance with the STROBE-MR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-827/rc).
Methods
Study design
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The data curated for this study were obtained from publicly accessible databases that had already received ethical clearance from relevant ethics committees, thereby eliminating the requirement for approval by any local Ethics Committee. During MR, the three pivotal assumptions for genetic instrumental variables (GIVs) to serve as valid tools are as follows: (I) the GIVs must be correlated with the exposure; (II) the GIVs must be independent of confounding factors; and (III) the GIVs should influence the outcome solely through their effects on the exposures (20). We performed a two-step, two-sample MR utilizing a mediation analysis. The research was implemented in three phases: phase 1—determining the causal effect of BMI on chronic pain; phase 2—ascertaining the causal effect of BMI on SAS; and phase 3—exploring and quantifying the mediating role of SAS in the BMI-chronic pain link (Figure 1).
Figure 1.

Schematic diagram of Mendelian analysis in two steps for BMI, SAS, and chronic pain. The total effect is c, the mediation effect is a×b, and the direct effect c’=c−a×b. BMI, body mass index; SAS, sleep apnea syndrome; SNPs, single-nucleotide polymorphisms.
Data sources
The BMI and SAS data from genome-wide association studies (GWAS) were sourced from the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/), which was developed by the Integrative Epidemiology Unit at the University of Bristol, UK, with the GWAS ID being ukb-b-19953 and ebi-a-GCST90018916, respectively. BMI is defined as the ratio of body weight (kg) to height squared (m2). GWAS data for BMI included 461,460 European population samples. SAS was diagnosed using ICD-10 code (G47.30), with GWAS data comprising 13,818 European cases and 463,035 European controls. Chronic pain GWAS data were obtained from the FinnGen database (https://storage.googleapis.com/finngen-public-data-r10). Temporomandibular disorder (TMD)-related pain was diagnosed using ICD-10 code (K07.60, K07.63), with GWAS data comprising 11,592 cases and 400,589 controls. Fibromyalgia-specific TMD-related muscle pain was diagnosed using ICD-10 code (K07.60), with GWAS data comprising 10,243 cases and 401,938 controls. Atypical facial pain was diagnosed using ICD-10 code (G50.1), with GWAS data comprising 1,508 cases and 360,538 controls. Limb pain was diagnosed using ICD-10 code (M79.6), with GWAS data comprising 34,007 cases and 299,606 controls. Low back pain was diagnosed using ICD-10 code (M54.5), with GWAS data comprising 32,845 cases and 294,770 controls. Low back pain with or without sciatica was diagnosed using ICD-10 code (M54.3, M54.4), with GWAS data comprising 46,707 cases and 365,474 controls. Thoracic spine pain was diagnosed using ICD-10 code (M54.6), with GWAS data comprising 5,187 cases and 294,770 controls. All chronic pain phenotypes were derived from European populations. When multiple previously published GWAS datasets were available within a database, the largest or most recently updated dataset with detailed information was selected for further analysis.
Screening of SNPs
After all three pivotal assumptions were met, SNPs reaching a significance threshold of P<5×10−8 were chosen as IVs. A filtering parameter (clump_r2=0.001, clump_kb=10,000) was set to ensure IV independence and avoid linkage disequilibrium (LD). SNPs with allele deletion and palindromic SNPs were excluded from the analysis. The strength of IVs was assessed using the F-statistic, with only those IVs deemed robust (F-statistic >10) being retained for the analysis (21). However, since few IVs met the filtering criteria (P<5×10−8, clump_r2=0.001, clump_kb=10,000), SNPs of SAS meeting new criteria (P<1×10−5, clump_r2=0.001, clump_kb=10,000) were selected to obtain more inclusive results (22).
Statistical analysis and MR methods
All the statistical analyses were conducted utilizing the “TwoSampleM” (version 0.6.6), “RMediation”, and “BWMR” packages within the R software environment (version 4.4.1; R Foundation for Statistical Computing, Vienna, Austria). The inverse-variance weighted (IVW) and BWMR were chosen as the primary analytical approaches to derive the most precise and reliable causal effects, with a significance threshold (P value) <0.05 indicating statistical significance. Considering the potential for biases in the causal estimates produced by IVW, we employed MR-Egger regression, weighted medians, weighted mode, and simple mode as supplementary techniques to validate the robustness of the IVW findings under various assumptions (23,24). Furthermore, we used heterogeneity analysis, pleiotropy analysis, and leave-one-out (LOO) analysis to identify any SNPs with a substantial impact on the outcomes and to evaluate the potential for biases, thereby strengthening the robustness of our findings. The two-step MR approach was utilized to calculate the mediating effect through the following steps (20,25): (I) the total effect (a) was obtained with BMI as the exposure variable and chronic pain as the outcome variable; (II) the exposure-mediator effect (b) was obtained with BMI as the exposure variable and SAS as the outcome variable; (III) the mediator-outcome effect (c) was obtained with SAS as the exposure variable and chronic pain as the outcome variable; and (IV) the mediating effect (c’) was calculated as the product of b×c and the proportion of the mediating effect was quantified as the ratio of c’ to c (Figure 1).
Sensitivity analysis
The Cochran’s Q statistic was employed to assess heterogeneity among the IVs. A P value of >0.05 signified no heterogeneity, for which the fixed-effect IVW model was applied; in the event of detected heterogeneity (P≤0.05), the random-effect model was used (26). The MR-Egger intercept test was utilized to examine the presence of horizontal pleiotropy, with a P value of >0.05 suggesting that there was no horizontal pleiotropy; in addition, the causal effect estimates from the MR-Egger regression were not biased by any pleiotropic effect (27). LOO analysis was employed to test data stability, determining whether there were SNPs that exerted a strong influence when a single SNP was removed (28). Mendelian Randomization Pleiotropy RESidual Sum and Outliers (MR-PRESSO) was utilized to detect outliers among the IVs. We sequentially removed IVs flagged as outliers by using MR-PRESSO, so as to minimize the impact of horizontal pleiotropy. If one or more SNPs were identified as outliers influencing the MR estimates, they would be excluded, and a second MR analysis would be performed (29).
Results
Data characteristics
This study integrated summary data from two major public genomic databases: the IEU OpenGWAS and the FinnGen database. The BMI and SAS data were sourced from the IEU OpenGWAS, with IDs ukb-b-19953 (N=461,460) and ebi-a-GCST90018916 (N=476,853), respectively, as detailed in Table 1. The chronic pain phenotype data were obtained from the FinnGen database, in addition to TMD-related pain (N=412,181), fibromyalgia-specific TMD-related muscle pain (N=412,181), atypical facial pain (N=362,046), limb pain (N=333,613), low back pain (N=327,615), lower back pain with or without sciatica (N=412,181), and thoracic spine pain (N=299,957), as detailed in Table 1.
Table 1. Characteristics of selected genome-wide association studies.
| Trials | Cases (N) | Controls (N) | Sample size (N) | Year of publication | No. of SNPs |
|---|---|---|---|---|---|
| BMI | – | – | 461,460 | 2018 | 9,851,867 |
| SAS | 13,818 | 463,035 | 476,853 | 2021 | 24,183,940 |
| TMD-related pain | 11,592 | 400,589 | 412,181 | 2023 | 19,682,705 |
| TMD muscular pain linked with fibromyalgia | 10,243 | 401,938 | 412,181 | 2023 | 19,682,705 |
| Atypical facial pain | 1,508 | 360,538 | 362,046 | 2023 | 19,681,894 |
| Pain in limb | 34,007 | 299,606 | 333,613 | 2023 | 19,681,161 |
| Low back pain | 32,845 | 294,770 | 327,615 | 2023 | 19,681,164 |
| Lower back pain or/and sciatica | 46,707 | 365,474 | 412,181 | 2023 | 19,682,705 |
| Pain in thoracic spine | 5,187 | 294,770 | 299,957 | 2023 | 19,680,346 |
BMI, body mass index; SAS, sleep apnea syndrome; SNP, single-nucleotide polymorphism; TMD, temporomandibular joint disorder.
A total of 372 SNPs were used for the MR analysis of BMI and TMD-related pain, fibromyalgia-specific TMD-related muscle pain, atypical facial pain, limb pain, and thoracic spine pain; 370 SNPs were used for the MR analysis of BMI and low back pain or lower back pain with or without sciatica; 379 SNPs were used for the MR analysis of BMI and SAS; 52 SNPs were used for the MR analysis of SAS and limb pain or thoracic spine pain; and 50 SNPs were used for the MR analysis of SAS and low back pain or low back pain with or without sciatica. All SNPs had F-statistics greater than 10. The supplementary table (available online: https://cdn.amegroups.cn/static/public/jtd-2025-827-1.xlsx) presents the screening results and genetic features (including chromosome position, effect allele, allele frequency, etc.) of the SNPs used in the final MR analysis of BMI, SAS, and chronic pain.
Impact of BMI on chronic pain
The outcomes from the MR-Egger regression, weighted median, weighted model, and simple model in the MR showed directional consistency with those obtained from the IVW and BWMR approaches, as detailed in the supplementary table (available online: https://cdn.amegroups.cn/static/public/jtd-2025-827-2.xlsx). The IVW analysis revealed that there was a causal relationship between the per 1-standard deviation (SD) increase in genetically predicted BMI and the increased incidence of limb pain [odds ratio (OR) =1.35, 95% confidence interval (CI): 1.26–1.44, P<0.001], low back pain (OR =1.34, 95% CI: 1.25–1.43, P<0.001), low back pain with or without sciatica (OR =1.28, 95% CI: 1.21–1.36, P<0.001), and thoracic spine pain (OR =1.18, 95% CI: 1.03–1.35, P=0.02) (Figures 2,3). Similarly, BWMR showed that per 1-SD increase in genetically predicted BMI had a causal relationship with the elevated incidence of limb pain (OR =1.34, 95% CI: 1.25–1.44, P<0.001), low back pain (OR =1.34, 95% CI: 1.25–1.43, P<0.001), low back pain with or without sciatica (OR =1.28, 95% CI: 1.21–1.36, P<0.001), and thoracic spine pain (OR =1.18, 95% CI: 1.03–1.36, P=0.02) (Figures 2,4). However, no causal effect of BMI on TMD-related pain (IVW: P=0.17, BWMR: P=0.17), fibromyalgia-specific TMD-related muscle pain (IVW: P=0.98, BWMR: P=0.97), or atypical facial pain (IVW: P=0.95, BWMR: P=0.94) was observed (Figure 2, Figures S1-S6). Therefore, we did not proceed with an analysis of the potential mediating role and impact of SAS on these three categories of pain.
Figure 2.
The potential causal relationships among BMI, SAS, and chronic pain. BMI, body mass index; BWMR, Bayesian-weighted Mendelian randomization; CI, confidence interval; IVW, inverse-variance weighting; MR, Mendelian randomization; OR, odds ratio; SAS, sleep apnea syndrome; TMD, temporomandibular disorder.
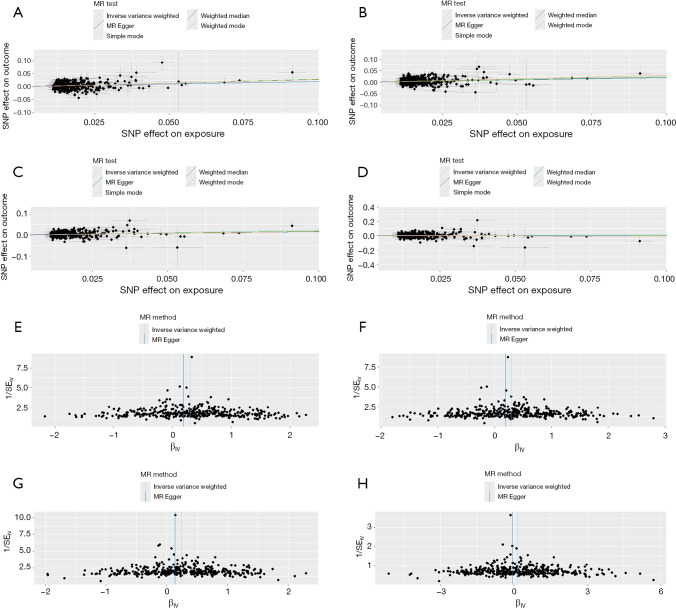
Figure 3.
MR analysis of BMI and pain in limb (A,E), low back pain (B,F), lower back pain or/and sciatica (C,G), and pain in thoracic spine (D,H). (A-D) Scatter plot showing the genetic correlations of BMI and chronic pain. The slopes of the fitted lines (IVW, weighted median, MR-Egger) indicate the causal effect estimates. (E-H) Funnel plots were employed to evaluate potential heterogeneity. BMI, body mass index; IVW, inverse-variance weighting; MR, Mendelian randomization; SNP, single-nucleotide polymorphism; SE, standard error.
Figure 4.
BWMR analysis of BMI and pain in limb (A,E), low back pain (B,F), lower back pain or/and sciatica (C,G), and pain in thoracic spine (D,H). (A-D) Weighted data and regression results illustrating the causal estimates of BMI on each pain condition. (E-H) Changes in ELBO during the iterative process, with the stable ELBO value indicating model performance and higher values suggesting improved model fit. BWMR, Bayesian-weighted Mendelian randomization; SNP, single-nucleotide polymorphism; ELBO, evidence lower bound.
Impact of BMI on SAS
The IVW method indicated a correlation between a rise in BMI and an elevated risk of SAS (OR =2.33, 95% CI: 2.11–2.57, P<0.001). The BWMR approach also revealed a relationship between a higher BMI and an enhanced risk of SAS (OR =2.34, 95% CI: 2.12–2.59, P<0.001) (Figure 2, Figure S7).
Impact of SAS on chronic pain
IVW indicated that SAS had a causal link with limb pain (OR =1.04, 95% CI: 1.00–1.09, P=0.04), low back pain (OR =1.10, 95% CI: 1.05–1.14, P<0.001), and low back pain with or without sciatica (OR =1.08, 95% CI: 1.05–1.11, P<0.001) (Figures S8-S10). However, no causal link was found between SAS and thoracic spine pain (OR =1.07, 95% CI: 0.97–1.17, P=0.17). Furthermore, BWMR revealed that genetically predicted SAS was linked to limb pain (OR =1.06, 95% CI: 1.01–1.10, P=0.02), low back pain (OR =1.10, 95% CI: 1.05–1.14, P<0.001), and low back pain with or without sciatica (OR =1.08, 95% CI: 1.05–1.12, P<0.001) (Figures S11-S13), whereas no significant causal association was observed between SAS and thoracic spine pain (OR =1.05, 95% CI: 0.94–1.16, P=0.39) (Figure 2).
SAS as a mediator in the onset of chronic pain
In the MR mediation analysis, IVW revealed that the impacts of SAS on the causal relationship between the per 1-SD increase in BMI and the increased incidence of limb pain, low back pain, and low back pain with or without sciatica were as follows: total effects: β=0.297, β=0.289, and β=0.249, respectively; direct effects: β=0.260, β=0.212, and β=0.185, respectively; and mediating effects (indirect effects): β=0.037, β=0.077, and β=0.064, respectively. The proportions of the mediating effects accounted for 12.5%, 26.6%, and 25.7%, respectively. Furthermore, BWMR demonstrated that the impacts of SAS on the causal relationship between per 1-SD increase in BMI and the heightened incidence of limb pain, low back pain, and low back pain with or without sciatica were as follows: total effects: β=0.294, β=0.291, and β=0.250, respectively; direct effects: β=0.249, β=0.213, and β=0.183, respectively; and mediating effects (indirect effects): β=0.045, β=0.078, and β=0.067, respectively. The proportions of the mediating effects were 15.3%, 26.8%, and 26.8%, respectively.
Results of sensitivity analysis
For the sensitivity analysis, the MR-Egger regression intercept was employed to examine the horizontal pleiotropy. Additionally, MR-PRESSO was utilized to screen for and exclude outliers, thereby minimizing their impact on the analytic results. In the MR analyses with BMI as the exposure and SAS and chronic pain as outcomes, no horizontal pleiotropy was found. In addition, MR analysis with SAS as the exposure and chronic pain as the outcome also revealed no significant horizontal pleiotropy (Table 2). As some IVs exhibited heterogeneity, the random-effects model was employed to analyze these variables.
Table 2. MR-Egger horizontal pleiotropy analysis results.
| Exposure | Outcome | MR-Egger intercept | SE | P value |
|---|---|---|---|---|
| BMI | Pain in limb | 0.002 | 0.002 | 0.19 |
| Low back pain | 0.002 | 0.002 | 024 | |
| Lower back pain or/and sciatica | 0.002 | 0.001 | 0.14 | |
| Pain in thoracic spine | 0.005 | 0.003 | 0.18 | |
| Sleep apnea syndrome | 0.002 | 0.002 | 0.34 | |
| SAS | Pain in limb | 0.007 | 0.004 | 0.13 |
| Low back pain | 0.007 | 0.005 | 0.16 | |
| Lower back pain or/and sciatica | 0.006 | 0.004 | 0.10 | |
| Pain in thoracic spine | 0.011 | 0.010 | 0.28 |
BMI, body mass index; MR, Mendelian randomization; SAS, sleep apnea syndrome; SE, standard error.
https://cdn.amegroups.cn/static/public/jtd-2025-827-2.xlsx shows the corresponding results of the MR-Egger regression, weighted median, IVW method, and simple model analysis.
Discussion
This study employed MR combined with BWMR to explore the causal relationships among BMI, SAS, and chronic pain. The results revealed significant causal associations between genetically predicted elevated BMI and limb pain, low back pain, and low back pain with or without sciatica. Subsequent mediation analysis indicated that SAS accounted for 15.3% of the causal relationship between BMI and limb pain, 26.8% of the causal relationship between BMI and low back pain, and 26.8% of the causal relationship between BMI and low back pain with or without sciatica. Therefore, there is a strong causal relationship between BMI and chronic pain, wherein SAS mediated high proportions of limb pain, low back pain, and lower back pain with or without sciatica in individuals with elevated BMI. These findings not only offer a novel insight into the mechanisms by which BMI affects chronic pain but also highlight a new pathway for the therapeutic and preventive strategies against chronic pain.
Obesity is a major driver of the global disease burden and a significant high-risk factor for numerous diseases (30). Growing evidence indicates a close association between obesity and the onset and progression of chronic pain, with the prevalence of chronic pain showing a positive correlation with rising BMI (31-33). Additionally, multiple MR studies have demonstrated a causal link between BMI and chronic pain (34-36), and this study further corroborates this association. However, the causal relationship between them can be affected by a range of factors including body fat distribution, vitamin D deficiency, gender, and physical and mechanical stress (37-40). Furthermore, it has been proposed that BMI influences chronic pain through mechanisms involving systemic inflammation, autonomic dysregulation, adipose tissue dysfunction, adipose tissue hypoxia, and psychosocial distress (41). Among these, the link between SAS, as a prevalent condition in patients with obesity (characterized by high BMI), and chronic pain has increasingly been recognized; it may serve as a key mediating factor connecting BMI to chronic pain.
Clinical evidence indicates a significant correlation between SAS and chronic pain, with SAS patients exhibiting a notably higher likelihood of experiencing moderate to severe pain (13). The core pathological mechanism may stem from chronic intermittent hypoxia-induced pathophysiological alterations. Yesildag et al. (10) demonstrated that chronic intermittent hypoxia, a central pathological feature of SAS, may serve as a key pathophysiological hub linking obesity, SAS, and comorbid conditions. Gabryelska et al. (42) further corroborated this perspective, demonstrating that nocturnal intermittent hypoxia in SAS patients resulted in chronic and sustained overexpression of hypoxia-inducible factor-1 (HIF-1). HIF-1 can upregulate the expression of transient receptor potential ankyrin 1 (TRPA1) by binding to the hypoxic response element (HRE) of target genes. TRPA1 is a pivotal ion channel involved in pain signaling, and its abnormal activation is considered a crucial mechanism that mediates chronic pain (43,44). Furthermore, HIF-1 facilitates an increase in reactive oxygen species (ROS) levels by up-regulating the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, thereby activating pain-related ion channels such as TRPA1 and TRPV1 to mediate the development of chronic pain. ROS can also trigger inflammatory pathways [e.g., nuclear factor kappa-B (NF-кB)] to promote the release of pro-inflammatory cytokines such as interleukin (IL)-6 and IL-8, forming a vicious cycle of “oxidative stress-central sensitization-inflammation amplification” (45,46), which mediates the maintenance of the pain trajectory in patients with chronic pain. Therefore, chronic intermittent hypoxia may serve as a critical factor contributing to the development of chronic pain in patients with SAS. Although these findings offer a plausible mechanistic basis for the onset and progression of SAS-mediated chronic pain, whether there is a causal link at the population level remains unclear and needs to be further investigated. Through MR analysis, we have genetically confirmed for the first time a significant causal association between SAS and limb pain, low back pain, and low back pain with or without sciatica. Importantly, MR mediation analysis demonstrated that SAS plays a critical mediating role in the causal pathway linking BMI to these chronic pain conditions. This finding provides a theoretical basis for targeted interventions against SAS to alleviate obesity-associated chronic pain.
In the past, observational studies investigating the associations between SAS or BMI and chronic pain have often been limited by various potential confounding factors, such as selection bias, reverse causation, measurement bias, and unmeasured confounders, which may compromise the reliability of their findings. MR explores the causal relationship between exposures and outcomes at the genetic level. Including data from multiple databases and strictly screening IVs helps to minimize potential biases and measurement errors, thereby yielding more reliable and generalizable results. However, MR is limited by inadequate statistical power and bias in causal effect estimation due to horizontal pleiotropy (47). Therefore, leveraging MR techniques, we have developed a BWMR study to explore the causal link between BMI and various chronic pain conditions and examine the mediating effect of SAS in the BMI-chronic pain link. BWMR represents an innovative technique for causal inference that builds upon the foundation of conventional MR. BWMR provides substantial benefits compared to traditional MR. By employing a Bayesian weighting strategy, BWMR can effectively address the issue of IV assumption violations caused by pleiotropy. In addition, it mitigates the uncertainty of weak effects owing to polygenicity, thereby enhancing the robustness of causal inferences (19).
The strength of our present study lies in its utilization of SAS as a point of departure to delineate the potential mediators in the BMI-chronic pain link from a genetic perspective. The two-sample MR was carried out by utilizing publicly available databases, and the causal relationships of BMI and SAS with chronic pain were investigated at the genetic level, which mitigated the impacts of reverse causality and potential confounding factors (48). Furthermore, on the basis of traditional MR techniques, we innovatively applied the BWMR approach to address the impacts of horizontal pleiotropy, weak IVs, and outliers on the outcomes of conventional MR (19). Nevertheless, our study had some limitations. First, although sensitivity analyses were conducted to control for the horizontal pleiotropy, the potential influence of confounders cannot be entirely ruled out. Second, the data sources for this study were predominantly drawn from European populations, which may constrain the generalizability of our findings to other populations and/or ethnic groups. Third, this study primarily concentrated on SAS as a mediating factor and did not investigate the potential mediating roles of other factors such as body fat distribution and anxiety in the relationship between BMI and chronic pain. Lastly, future research should aim to elucidate the efficacy of SAS interventions in mitigating the detrimental impacts of obesity on chronic pain.
Conclusions
BWMR analysis suggested a genetically predicted causal link between BMI and limb pain, low back pain, and low back pain with or without sciatica, with SAS emerging as a significant mediator in this link. Our study offers novel insights into the prevention and treatment of chronic pain in individuals with abnormal BMI.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/) and the FinnGen database (https://storage.googleapis.com/finngen-public-data-r10) for making the aggregate statistics available for this research.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The data presented in this article were obtained from publicly accessible databases, which have been granted ethical clearance. In line with local legislative and institutional protocols, the research involving human participants is exempt from the need for ethical review and authorization. In adherence to requirements of national laws and policies, the obtaining of written informed consent from the patients/subjects or their legal guardian/close relative is not mandatory for the conduct of this study.
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-827/rc
Funding: This work was supported by the National Natural Science Foundation of China (No. 81860218), Yunnan Province Department of Science Department-Kunming Medical University Joint Research Program (Nos. 202301AY070001-056, 202201AY070001-178, and 202401AY070001-284), Kunming University of Science and Technology Joint Medical Research Project (No. KUST-KH2023026Y), and Open Project of the Clinical Research Center at the First People’s Hospital of Yunnan Province (Nos. 2023YJZX-GK10 and 2024JSKFKT-06).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-827/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Kang Y, Trewern L, Jackman J, et al. Chronic pain: definitions and diagnosis. BMJ 2023;381:e076036. 10.1136/bmj-2023-076036 [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane GJ. The epidemiology of chronic pain. Pain 2016;157:2158-9. 10.1097/j.pain.0000000000000676 [DOI] [PubMed] [Google Scholar]
- 3.Becker N, Thomsen AB, Olsen AK, et al. Pain epidemiology and health related quality of life in chronic non-malignant pain patients referred to a Danish multidisciplinary pain center. Pain 1997;73:393-400. 10.1016/S0304-3959(97)00126-7 [DOI] [PubMed] [Google Scholar]
- 4.Magni G, Marchetti M, Moreschi C, et al. Chronic musculoskeletal pain and depressive symptoms in the National Health and Nutrition Examination. I. Epidemiologic follow-up study. Pain 1993;53:163-8. 10.1016/0304-3959(93)90076-2 [DOI] [PubMed] [Google Scholar]
- 5.Gureje O, Von Korff M, Simon GE, et al. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA 1998;280:147-51. 10.1001/jama.280.2.147 [DOI] [PubMed] [Google Scholar]
- 6.Turk DC, Rudy TE. Toward an empirically derived taxonomy of chronic pain patients: integration of psychological assessment data. J Consult Clin Psychol 1988;56:233-8. 10.1037//0022-006x.56.2.233 [DOI] [PubMed] [Google Scholar]
- 7.Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. Pain 1990;40:279-91. 10.1016/0304-3959(90)91125-3 [DOI] [PubMed] [Google Scholar]
- 8.Wesselink EO, Pool-Goudzwaard A, De Leener B, et al. Investigating the associations between lumbar paraspinal muscle health and age, BMI, sex, physical activity, and back pain using an automated computer-vision model: a UK Biobank study. Spine J 2024;24:1253-66. 10.1016/j.spinee.2024.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuch I, Heuch I, Hagen K, et al. Overweight and obesity as risk factors for chronic low back pain: a new follow-up in the HUNT Study. BMC Public Health 2024;24:2618. 10.1186/s12889-024-20011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeşildağ M, Şentürk Z, Bekci TT, et al. The Usefulness of New Body Indices in Determining the Risk of Cardiovascular Disease in Cases with Obstructive Sleep Apnea Syndrome. Int J Gen Med 2024;17:5523-34. 10.2147/IJGM.S489904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal 2008;10:755-68. 10.1089/ars.2007.1946 [DOI] [PubMed] [Google Scholar]
- 12.Kaushik AS, Strath LJ, Sorge RE. Dietary Interventions for Treatment of Chronic Pain: Oxidative Stress and Inflammation. Pain Ther 2020;9:487-98. 10.1007/s40122-020-00200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athar W, Card ME, Charokopos A, et al. Obstructive Sleep Apnea and Pain Intensity in Young Adults. Ann Am Thorac Soc 2020;17:1273-8. 10.1513/AnnalsATS.201910-750OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahaye C, Miolanne M, Farigon N, et al. Enhanced pain sensitivity in obese patients with obstructive sleep apnoea syndrome is partially reverted by treatment: An exploratory study. Eur J Pain 2023;27:624-35. 10.1002/ejp.2085 [DOI] [PubMed] [Google Scholar]
- 15.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res 2015;8:399-408. 10.2147/JPR.S55598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katan MB. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1986;1:507-8. 10.1016/s0140-6736(86)92972-7 [DOI] [PubMed] [Google Scholar]
- 17.Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1-22. 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 18.Tomarken AJ, Waller NG. Structural equation modeling: strengths, limitations, and misconceptions. Annu Rev Clin Psychol 2005;1:31-65. 10.1146/annurev.clinpsy.1.102803.144239 [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Ming J, Hu X, et al. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics 2020;36:1501-8. 10.1093/bioinformatics/btz749 [DOI] [PubMed] [Google Scholar]
- 20.Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol 2021;36:465-78. 10.1007/s10654-021-00757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BR, Kim G, Jin SP, et al. Causal association between polyunsaturated fatty acids and acne: a two-sample Mendelian randomization study. Br J Dermatol 2025;192:1106-14. 10.1093/bjd/ljaf052 [DOI] [PubMed] [Google Scholar]
- 22.Jia J, Dou P, Gao M, et al. Assessment of Causal Direction Between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis. Diabetes 2019;68:1747-55. 10.2337/db19-0153 [DOI] [PubMed] [Google Scholar]
- 23.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23:R89-98. 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377-89. 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, Li S, Ding Y, et al. Effect of smoking-related features and 731 immune cell phenotypes on esophageal cancer: a two-sample and mediated Mendelian randomized study. Front Immunol 2024;15:1336817. 10.3389/fimmu.2024.1336817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greco M FD, Minelli C, Sheehan NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med 2015;34:2926-40. 10.1002/sim.6522 [DOI] [PubMed] [Google Scholar]
- 27.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512-25. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Dekkers JCM, Fernando RL. Cross-validation of best linear unbiased predictions of breeding values using an efficient leave-one-out strategy. J Anim Breed Genet 2021;138:519-27. 10.1111/jbg.12545 [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Bi Y, Zhu T. Mendelian randomization highlights sleep disturbances mediated the effect of depression on chronic pain. Brain Behav 2024;14:e3596. 10.1002/brb3.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obesity: causes, consequences, treatments, and challenges. J Mol Cell Biol 2021;13:463-5. 10.1093/jmcb/mjab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majedi H, Amini MH, Yousefshahi F, et al. Predicting Factors of Pain Duration in Patients with Chronic Pain: A Large Population-based Study. Anesth Pain Med 2020;10:e95776. 10.5812/aapm.95776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Chen J, Qin Q, et al. Chronic pain and its association with obesity among older adults in China. Arch Gerontol Geriatr 2018;76:12-8. 10.1016/j.archger.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 33.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity (Silver Spring) 2012;20:1491-5. 10.1038/oby.2011.397 [DOI] [PubMed] [Google Scholar]
- 34.Zheng P, Scheffler A, Ewing S, et al. Chronic low back pain causal risk factors identified by Mendelian randomization: a cross-sectional cohort analysis. Spine J 2025;25:1154-66. 10.1016/j.spinee.2024.12.029 [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Mi J, Peng Y, et al. Causal Associations of Obesity With the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front Endocrinol (Lausanne) 2021;12:740200. 10.3389/fendo.2021.740200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z, Qi L, Zhang H, et al. Smoking and BMI mediate the causal effect of education on lower back pain: observational and Mendelian randomization analyses. Front Endocrinol (Lausanne) 2024;15:1288170. 10.3389/fendo.2024.1288170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashani OA, Astita R, Sharp D, et al. Body mass index and distribution of body fat can influence sensory detection and pain sensitivity. Eur J Pain 2017;21:1186-96. 10.1002/ejp.1019 [DOI] [PubMed] [Google Scholar]
- 38.Glover TL, Goodin BR, King CD, et al. A Cross-sectional Examination of Vitamin D, Obesity, and Measures of Pain and Function in Middle-aged and Older Adults With Knee Osteoarthritis. Clin J Pain 2015;31:1060-7. 10.1097/AJP.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes AC, Xie W, Lundberg DJ, et al. Increases in BMI and chronic pain for US adults in midlife, 1992 to 2016. SSM Popul Health 2020;12:100644. 10.1016/j.ssmph.2020.100644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perera RS, Chen L, Hart DJ, et al. Effects of body weight and fat mass on back pain - direct mechanical or indirect through inflammatory and metabolic parameters? Semin Arthritis Rheum 2022;52:151935. 10.1016/j.semarthrit.2021.11.007 [DOI] [PubMed] [Google Scholar]
- 41.Paley CA, Johnson MI. Physical Activity to Reduce Systemic Inflammation Associated With Chronic Pain and Obesity: A Narrative Review. Clin J Pain 2016;32:365-70. 10.1097/AJP.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 42.Gabryelska A, Szmyd B, Szemraj J, et al. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J Clin Sleep Med 2020;16:1761-8. 10.5664/jcsm.8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatano N, Itoh Y, Suzuki H, et al. Hypoxia-inducible factor-1α (HIF1α) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J Biol Chem 2012;287:31962-72. 10.1074/jbc.M112.361139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza Monteiro de Araujo D, Nassini R, Geppetti P, et al. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 2020;24:997-1008. 10.1080/14728222.2020.1815191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan G, Khan SA, Luo W, et al. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 2011;226:2925-33. 10.1002/jcp.22640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kallenborn-Gerhardt W, Schröder K, Del Turco D, et al. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci 2012;32:10136-45. 10.1523/JNEUROSCI.6227-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm FJ, Zhou X. Statistical methods for Mendelian randomization in genome-wide association studies: A review. Comput Struct Biotechnol J 2022;20:2338-51. 10.1016/j.csbj.2022.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu N, Tan JS, Liu L, et al. Roles of obesity in mediating the causal effect of attention-deficit/hyperactivity disorder on diabetes. Epidemiol Psychiatr Sci 2023;32:e32. 10.1017/S2045796023000173 [DOI] [PMC free article] [PubMed] [Google Scholar]