Abstract
This study examined 2 samples of adolescents and mothers using a child-based design (Nonshared Environment in Adolescent Development [NEAD] project, N = 395 families) and a parent-based design (Twin Moms [TM] project, N = 236 twin family pairs) to compare genetic and environmental influences on mothering. For both samples, the same measures of positivity, negativity, control, and monitoring were used. The use of matched child-based and parent-based samples enabled passive and nonpassive genotype–environment (GE) correlations to be approximated, providing information about process. Passive GE correlations were suggested for mother’s positivity and monitoring. For mother’s negativity and control, primarily nonpassive GE correlations were suggested. In several cases, both types of GE correlation were indicated. Finally, observer ratings of negativity and monitoring were influenced only by environmental factors.
There is a large body of research that has examined the impact of parenting on child and adolescent adjustment (e.g., Grotevant, 1998; Maccoby, 1992). Because most of this research has examined only one child per family, it has been difficult to interpret this research in terms of the child’s influences on both the way the child is parented and on the child’s own adjustment. It is clear, however, on the basis of studies of twins and siblings, that parenting is not a “pure” environmental measure, a finding that is beginning to be acknowledged by many researchers on parenting (e.g., Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000). When genetic and environmental contributions to parenting have been examined, substantial genetic influences have typically been indicated (see Plomin, 1994, or Towers, Spotts, & Neiderhiser, 2001, for reviews). In other words, genetically influenced characteristics of the children appear to affect the way parents treat their children such that identical twins tend to be parented more similarly than are fraternal twins and full siblings are parented more similarly than are half or stepsiblings. Although these findings are interesting, it is still not clear what genetic influences on measures of parenting mean. In other words, how do genetic factors influence parenting—what are the processes involved?
The majority of studies that have found genetic influences on parenting have examined children or adolescents of varying degrees of genetic relatedness, in other words, “child-based” designs. In a child-based design, the child’s genes are the unit of measurement. Clearly, the child’s genes do not directly influence the way that his or her parents treat him or her, but parents may respond, at least in part, to genetically influenced characteristics in their children. An alternative approach is to examine the parent’s genes using a “parent-based” design. In this case, the parents vary in the degree of their genetic relatedness and the focus is on the influence of the parents’ genes on how they parent their children. The present study used two comparable samples of mothers and their adolescent children, one using a child-based design and the other using a parent-based design. This enabled further specification of how genetic factors influence parenting by allowing comparison of the adolescent’s genetic influences on the mother’s behavior with the influence of the mother’s genes on her own behavior.
Genetic and Environmental Influences on Parenting
There has been a relatively consistent pattern of findings for genetic and environmental influences on parenting in child-based designs that has varied with the specific parenting construct. Measures of parental warmth and support and parental negativity have typically shown substantial genetic and nonshared environmental influences and modest to negligible shared environmental influences (e.g., Elkins, McGue, & Iacono, 1997; Jacobson & Rowe, 1999; Plomin, 1994; Rowe, 1981, 1983). In contrast, primarily shared and nonshared environmental influences have been found for measures of parental monitoring and control, with little evidence of genetic influences (e.g., Elkins et al., 1997; Plomin, 1994; Rowe, 1981, 1983). One exception to this general pattern of findings has been found in the Nonshared Environment in Adolescent Development (NEAD) project (Reiss et al., 1994; Reiss, Neiderhiser, Hetherington, & Plomin, 2000), which used composites across multiple raters and multiple measures. In the NEAD project, genetic, shared environmental, and nonshared environmental influences all contributed significantly to composites of parental positivity, negativity, and monitoring, whereas the findings for parental control were more consistent with previous reports, with only modest genetic influences and substantial shared and nonshared environmental influences (Plomin, Reiss, Hetherington, & Howe, 1994; Reiss et al., 2000).
There have been only a few studies of parenting that used parent-based designs. One set of studies employed a modified version of the Parental Bonding Instrument (PBI) for twin women who were parents (Kendler, 1996). The PBI, an instrument designed to assess remembered attachment during childhood, was modified in several ways for its use in this study, the most relevant change being that the pronouns were changed to allow the twin mothers to report about their own parenting of their children. Kendler (1996) found a pattern of findings similar to that found with child-based designs: Genetic and nonshared environmental influences were important for maternal warmth, and shared and nonshared environmental influences explained all of the variance for maternal protectiveness and authoritarianism. These findings are intriguing but leave many questions unanswered. Because the age and gender of the child the mother reported on were not specified in this report, there is no way of knowing if there were differences in mothering that may have been due to these factors. There are many studies that have found both the age and the gender of the child to have an important impact on parenting (e.g., Cote & Azar, 1997; Dunn, 1991; Dunn & Plomin, 1986; Hughes, Deater-Deckard, & Cutting, 1999). If the child of one twin mother was an adolescent and the child of the second twin mother was a toddler, differences in parental protectiveness within that twin pair, for example, could have been due simply to the child’s age. Nonetheless, these findings are important and novel in that they provide some of the first data on genetic influences on parenting in a parent-based design.
The second parent-based study of parenting examined parents of children younger than 8 years of age (Losoya, Callor, Rowe, & Goldsmith, 1997). This sample consisted of parents who were identical and fraternal twins and of a small subsample of parents who were adoptive siblings. The findings from this study were somewhat different from the findings from studies with child-based designs and from the report by Kendler (1996) in that genetic and nonshared environmental influences were of primary importance for all of the constructs of parenting examined: warmth and support, negative control, and global control as assessed by the Family Environment Scale (Moos & Moos, 1983). For none of the constructs examined were shared environmental influences indicated. Two other studies that used parent-based designs and a global measure of current family environment also found evidence of genetic influences for all of the constructs of parenting assessed, including control (Perusse, Neale, Heath, & Eaves, 1994; Plomin, McClearn, Pedersen, Nesselroade, & Bergeman, 1989). The differences between these findings and those of Kendler (1996) may be due to the different ages of the children and/or the twins and to the fact that the twins and siblings in the three studies described above included both male and female pairs, whereas only female twin pairs were included in the Kendler study. It is also possible that the global measures of parenting may be more likely to show genetic influences across constructs, whereas the more specific parent–child measure used by Kendler and in the child-based designs described above may be more sensitive to differences in genetic and environmental influences that vary depending on the parenting construct.
Genotype–Environment Correlation
An understanding of genotype–environment correlation helps in interpreting genetic influences on parenting, especially when both child-based and parent-based designs are available. Genotype–environment correlation refers simply to a correlation between genotype and environment. Three types of genotype–environment correlation are usually described: passive, active, and evocative (Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1988). Passive genotype–environment correlation arises because parents and children share both genes and environment. In the case of the parenting of adolescents, parents may pass genes related to “difficult temperament” to their children. In parents, these “difficult temperament” genes may be exhibited as irritable and negative parenting, which is also correlated with the child’s difficult temperament. For parenting, this passive genotype–environment correlation would emerge as shared environmental influences in a child-based design and as genetic influences in a parent-based design. Evocative genotype–environment correlation is the result of others (the environment) responding to genetically influenced characteristics of the child. To use the same example of negative parenting, parents may respond to the child’s difficult temperament with harsh and negative parenting. This type of process is likely to be operating in the coercive cycles of parent–child conflict and antisocial behavior that Patterson and his colleagues have described (Patterson, 1982; Patterson, DeBaryshe, & Ramsey, 1989). Finally, active genotype–environment correlation occurs when a child’s genotype and his or her environment are correlated because the child actively selects environments that are correlated with his or her genetically influenced characteristics. This is more difficult to imagine in regard to parenting. A good example of active genotype–environment correlation for an environmental measure other than parenting concerns peers. When genetic influences on peer relationships are found (e.g., Manke, McGuire, Reiss, Hetherington, & Plomin, 1995), one explanation is that adolescents are selecting peers who are like them, and the characteristics that they are selecting on are genetically influenced. Conceptually, these are three distinct processes; however, in terms of function and measurement, evocative genotype–environment correlation and active genotype–environment correlation are indistinguishable outside of a laboratory setting. For this reason, the three types of genotype–environment correlation are described as passive and nonpassive (evocative and/or active).
In order to understand and interpret the processes involved when genetic influences on parenting are found, it is critical to be able to distinguish which type, if any, of genotype–environment correlation is operating. Passive genotype–environment correlations on parenting would result from the parent behaving in a way that was correlated with the child’s characteristics, as a result of genes the parent and the child share. If only parenting was assessed, passive genotype–environment correlation would be indicated by large shared environmental influences on parenting in child-based designs. This is possible because a genetically influenced characteristic of the parent influences the way the parent treats the child, independent of the characteristics of the child (e.g., the child does not seek out or evoke the environment). This could result in parenting that is similar across sibling pairs and that does not vary by the genetic relatedness of the siblings. Finding nonpassive genotype–environment correlations on parenting, on the other hand, would provide evidence for the role of the child in influencing his or her environment. In other words, if nonpassive genotype–environment correlations were indicated, this would suggest that parenting is, at least in part, a response to genetically influenced characteristics in the child. Finally, if no genetic influences are indicated for parenting, as has been the case for several studies of parental monitoring/control, then clearly, genotype–environment correlations are not relevant.
Most studies that have examined genetic and environmental influences on parenting have used child-based designs. When only child-based twin and sibling designs are used, it is impossible to disentangle passive and nonpassive types of genotype–environment correlation. Genetic and/or shared environmental influences on parenting in a child-based design indicate only that some type of genotype–environment correlation is operating. If, on the other hand, a parent-based design is used, parents’ genes become the focus. If genetic contributions are substantial for measures of parenting in a parent-based design, this suggests that some genetically influenced characteristic of the parent, perhaps personality, influences that parent’s parenting. Parent-based designs, when paired with child-based designs, are especially useful for disentangling passive and nonpassive genotype–environment correlations. Table 1 shows the expectations for different patterns of findings for parenting in child-based and parent-based designs representing different types of genotype–environment correlation. For example, genetic influences on parenting in a child-based design are best explained by nonpassive genotype–environment correlation. This type of genotype–environment correlation is further substantiated by also finding little or no evidence of genetic influences on parenting in a parent-based design (see row 2 in Table 1). In other words, the parent’s genes could not be responsible for his or her parenting, indicating that passive genotype–environment correlations do not provide a good explanation for the genetic influences on parenting found in the child-based design. A better explanation for these findings would be that the child evokes a certain type of parenting in response to his or her genetically influenced characteristics (e.g., evocative genotype–environment correlation).
Table 1.
Expectations for Genetic and Environmental Influences on Parenting Given Different Types of Genotype–Environment (GE) Correlation and Child-Based and Parent-Based Designs
| GE correlation | Child-based design | Parent-based design |
|---|---|---|
| Passive | shared environmental | genetic |
| Nonpassive | genetic | shared and/or nonshared environmental |
| None | shared and/or nonshared environmental | shared and/or nonshared environmental |
Passive genotype–environment correlation is most clearly indicated if there are shared environmental influences on parenting in the child-based design and genetic influences in the parent-based design. Specifically, the parents’ genetically influenced characteristics influence their parenting, and those influences are consistent regardless of the genetic relatedness of the sibling pairs. It is important to note that finding shared environmental influences on parenting in a child-based design without complementing these findings with a parent-based design would not necessarily indicate the presence of passive genotype–environment correlation. It is also possible that parents simply treat children in a similar way for purely environmental reasons (e.g., coparenting). This possibility is illustrated in row 3 of Table 1, where there is no evidence of genetic influences on parenting in either a child-based or a parent-based design. This pattern of findings would indicate that only environmental influences can explain variation in parenting and that genotype–environment correlations are not relevant. Given the complexity of most findings in this area, it is unlikely that purely passive or purely nonpassive genotype–environment correlations will be operating in most cases. The patterns of genetic and environmental influences that have been reported for parenting have tended to suggest that both genetic and shared environmental influences contribute to the similarity in twin and sibling treatment, which emphasizes the need to disentangle the types of genotype–environment correlation. This can be achieved through the use of designs with different strengths and designs that enable a focus on different types of genotype–environment correlation.
Understanding whether passive or nonpassive genotype–environment correlation is operating is necessary to advance our understanding of the processes involved in parent–child relationships. There is an increasing acceptance of the view that children influence the way they are treated by others, including their parents (e.g., Belsky & Park, 2000; Deater-Deckard & O’Connor, 2000; Haapasalo & Tremblay, 1994), and that parents tend to respond differentially to their children (e.g., Brody, Stoneman, & McCoy, 1992; Dunn & Plomin, 1986; McHale & Palwetko, 1992), but in many ways this understanding has been rather broad in nature. Identifying the specific aspects of parenting that appear to be more responsive to the child (evocative genotype–environment correlation) and that seem to be more consistently applied by parents (shared environment) is an important step in understanding which parenting behaviors are most likely to be malleable. One design that is ideal for identifying evocative genotype–environment correlations is an adoption design in which information is available about the birth parents. Two studies of adoptees have identified evocative genotype–environment correlation by examining how characteristics in the birth parents (e.g., psychopathology and substance use disorders) increase the risk of behavior problems in the adopted children, which then influences the parenting behavior of the adopted parents (Ge et al., 1996; O’Connor, Deater-Deckard, Fulker, Rutter, & Plomin, 1998). A more recent study that examined young twin–parent mutuality in their relationship and then replicated the findings using an adoption design also provides evidence of evocative genotype–environment correlation (Deater-Deckard & O’Connor, 2000). The findings from these studies are significant in that they provide some of the best evidence to date of the importance of evocative genotype–environment correlations in influencing parenting, thereby underscoring the importance of the role of the child in parent–child relationships. Unfortunately, passive genotype–environment correlations cannot be identified using an adoption design, although the report by Deater-Deckard and O’Connor (2000), which paired a twin study with an adoption study, was a step in that direction.
The present study was designed to provide a better understanding of genotype–environment correlations and how they operate in parent–child relationships. Results from a parent-based study of twin mothers of adolescents were compared and contrasted with results from a child-based twin/sibling study. The use of both child-based and parent-based studies that employed identical measures of parenting and used similarly aged samples of adolescents allows for a more careful examination of genetic influences on parenting as well as for the differentiation of passive and nonpassive types of genotype–environment correlation.
Method
Two samples, matched on many of the inclusion criteria for families and on the majority of the parenting measures, were used for the present study. Each sample is described separately. Because the same parenting measures were used in both projects, the measures are described once, with any differences in the measurement of the two samples noted.
Sample: Nonshared Environment in Adolescent Development (NEAD) Project
Participants were 395 same-sex sibling pairs and their parents who participated in the second wave of data collection in the NEAD project (Reiss et al., 1994). The NEAD project is composed of a nationwide sample of two-parent families, including both nondivorced families and stepfamilies, each with a pair of adolescent siblings no more than 4 years apart in age (M = 1.61 years apart ± 1.29 years). To ensure that none of the stepfamilies were in the unstable early phases of family formation, the families were required to be in existence for at least 5 years prior to the first wave of data collection (M = 8.9 ± 3.7 years of marriage). The second wave sample from the NEAD project was selected as the most comparable to the Twin Moms project sample because of the ages of the adolescents. As detailed below, the adolescents who participated in the Twin Moms project and those in the NEAD project at Time 2 were close to the same age, on average.
The adolescents ranged in age from 13 to 21 years (M = 16.2 ± 2.1), and their siblings ranged in age from 12 to 21 years (M = 14.7 ± 1.9). Participants included in the NEAD project fell into one of six sibling categories in two types of families: 63 monozygotic (MZ) and 75 dizygotic (DZ) twin pairs and 58 full sibling (FI) pairs residing in nondivorced families and 95 full (FS), 60 half (HS), and 44 genetically unrelated (US) sibling pairs residing in stepfamilies. The three stepsibling groups were matched for age of the oldest child and age spacing between the siblings to increase the comparability of these groups.
Sample: Twin Moms (TM) Project
Participants included 326 twin pairs who were mothers of adolescents (150 MZ and 176 DZ twin pairs), their long-term male partners, and one adolescent child of each of the twin mothers. This sample was obtained by using the Swedish Twin Registry. The TM sample was drawn from female–female twin pairs born between 1926 and 1966. Each member of the twin pair was involved in a long-term relationship with a male partner residing in the same home. For inclusion in our sample, each twin was also required to have an adolescent child, ranging in age from 11 to 21 years (mean child age = 15.4 ± 2.2 years), who was the same sex as and no more than 4 years older or younger than her co-twin’s child. Forty-nine percent of the cousin pairs were male, and the average age of the twin mothers was 45.4 years (± 4.5 years; range = 34 to 58 years). Although it was not a requirement for inclusion, 96% of the male partners were biologically related to the adolescents. These inclusion criteria were necessary to ensure that this sample was comparable to the NEAD sample, described above, and to ensure that the current living experience of each of the twin mothers was as comparable to that of their co-twins as possible (see Reiss et al., 2001, for a detailed description of the sample and the study rationale).
One of the objectives of the TM project was to provide a better understanding of the processes through which genetic factors influence parenting. The focus on twin mothers, rather than twin fathers, was both pragmatic and empirical. First, mothers usually rear their own children. By selecting twin mothers, the TM researchers increased the likelihood that the adolescent child in the home was the biological child of the mother. The second reason for the focus on twin mothers was an interest in women’s mental health and how it is influenced by their relationships with their children, their spouses, and their twins. Although these associations are not addressed in the present study, they are of general interest and help to describe the overall logic of the TM project.
Procedures: NEAD and TM Projects
Twins were rated for physical similarity (e.g., eye and hair color) with self-reports and interviewer ratings in the NEAD project and with self-reports in the TM project, using a modified version of a zygosity questionnaire (Nichols & Bilbro, 1966). If any differences in physical characteristics were reported or if respondents reported that people never were confused about the identity of the twins, the twin pair was classified as dizygotic. Questionnaire methods of assigning zygosity have been found to be nearly 100% accurate when compared with DNA assessment of zygosity (Nichols & Bilbro, 1966; Spitz et al., 1996). DNA was also collected from the twins who participated in the TM project. Most of the twin pairs (90%) were assigned zygosity on the basis of these DNA results. The remaining twin pairs either refused to provide a DNA sample or provided a sample that was unusable and were assigned zygosity on the basis of their questionnaire results. Zygosity was unclear for 10 twin pairs in the NEAD sample; therefore, these twin pairs were excluded from all analyses.
Measures
The measures used in both the NEAD and TM projects were derived from stepfamily study. These measures consisted of the mother’s reports of her parenting of her adolescent, each adolescent’s report of his or her mother’s behavior, and videotaped observations of dyadic interactions between each mother and adolescent. For the NEAD project, mothers rated their parenting of each of the two adolescents separately, and the dyadic interactions were coded separately for the mother’s interaction with each of her children. The reliabilities for the measures were in the high to acceptable range and are reported in the following sections describing each measure. The interobserver reliabilities of the ratings of the parent–child interactions were found to be acceptable, with kappas ranging from .50 to .86 for the NEAD sample and from .60 to .79 for the TM sample. A more detailed description of the measures can be found elsewhere (e.g., Henderson, 1999; Hetherington & Clingempeel, 1992; Reiss et al., 1994).
Maternal positivity.
The mother’s positivity toward her adolescent child(ren) was assessed by mother and adolescent reports on the Closeness/Rapport subscale of the Parent–Child Relationships Scale and on the Instrumental and Expressive Affection subscales of the Expression of Affection Inventory (alphas ranged from .78 to .89 for both studies). Observer ratings of the mother’s positive and warm behavior toward her adolescent were derived from the 10-min mother–adolescent dyadic interaction. Composite scores across the two measures of positivity were created for mother and child reports separately (alphas ranged from .71 to .73) and for the observer ratings of the mother’s positivity. Composites were used in order to avoid single-measure biases (Bank, Duncan, & Patterson, 1993; Bank & Patterson, 1992).
Maternal negativity.
The mother’s negativity toward her adolescent was assessed by mother and adolescent reports on the Conflict/Negativity subscale of the Parent–Child Relationships Scale and by the Coercion and Punitiveness subscales of the Parent Discipline Behavior Inventory (alphas ranged from .61 to .92 for both studies). Observer ratings of the mother’s negative behavior toward the adolescent were derived from the videotaped 10-min mother–adolescent dyadic interaction. As for maternal positivity, composite scores for each rater were created: mother report and child reports of maternal negativity (alphas ranged from .74 to .77 for both the NEAD and TM samples) and observer ratings of maternal negativity.
Maternal control and monitoring.
Control and monitoring were also assessed through adolescent, mother, and observer reports. Mothers and adolescents reported on the mother’s Attempted Control, Actual Control, and Knowledge (Monitoring) subscales of the Child Monitoring Scale (alphas ranged from .87 to .92). Mother and adolescent reports on each of these subscales were used to index control and monitoring. Observers also coded the 10-min mother–adolescent dyadic interactions for maternal monitoring, creating one composite: observed monitoring/control. Because this observer rating is likely to be more similar to control than to monitoring, the observer ratings are grouped with the mother and adolescent reports of control rather than monitoring, for the tabular and graphic presentation of the results.
These composites are somewhat different from those previously published for the NEAD project (e.g., Plomin et al., 1994; Reiss et al., 2000). In order to create composites that would be valid and identical for both projects, we factor analyzed the measures that were available for both projects using the data from the TM sample. The most notable difference was that the different reporters were not combined into a single composite across raters. Table 2 presents the correlations across measures and across mother, adolescent, and observer ratings.
Table 2.
Correlations Among Reporters for Mothering Measures From NEAD and Twin Moms Projects
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positivity | |||||||||||||
| 1. Mother | — | .57* | .12* | .04 | .04 | −.07 | .22* | .21* | .28* | .09* | .44* | .25* | .19* |
| 2. Child | .47* | — | .10* | .03 | .05 | −.07 | .20* | .24* | .21* | .10* | .26* | .32* | .16* |
| 3. Observer | .12* | .10* | — | −.02 | −.04 | −.32* | −.06 | .00 | −.03 | .03 | .12* | .05 | .49* |
| Negativity | |||||||||||||
| 4. Mother | −.10* | −.09* | −.12* | — | .44* | .30* | .32* | .16* | .25* | .17* | −.15* | −.13* | .17* |
| 5. Child | −.08* | −.05 | −.13* | .51* | — | .19* | .24* | .25* | .18* | .33* | −.10* | −.26* | .07 |
| 6. Observer | −.18* | −.12* | −.31* | .34* | .25* | — | .08* | −.04 | .05 | .01 | −.14* | −.13* | .06 |
| Control, actual | |||||||||||||
| 7. Mother | .26* | .16* | .00 | .12* | .08* | .06 | — | .24* | .80* | .26* | .12* | −.06 | .10* |
| 8. Child | .17* | .21* | −.01 | .06 | .07 | .00 | .25* | — | .25* | .74* | .05 | .17* | .14* |
| Control, attempted | |||||||||||||
| 9. Mother | .35* | .24* | .01 | −.05 | −.03 | .01 | .72* | .27* | — | .23* | .19* | −.03 | .14* |
| 10. Child | .20* | .31* | −.04 | .03 | .04 | −.01 | .27* | .75* | .35* | — | −.02 | .08* | .10* |
| Monitoring | |||||||||||||
| 11. Mother | .39* | .25* | .09* | −.20* | −.12* | −.10* | .38* | .17* | .52* | .22 | — | .22* | .12* |
| 12. Child | .26* | .36* | .08* | −.15* | −.22* | −.08* | .15* | .37* | .25* | .42* | .34* | — | .03 |
| 13. Observer | −.07* | .00 | .22* | .11* | .07 | .40* | .05 | .09* | .04 | .06 | .00 | .03 | — |
Note. Correlations above the diagonal are from the Twin Moms project, and correlations below the diagonal are from the Nonshared Environment in Adolescent Development (NEAD) project. The intercorrelations across mother, child, and observer ratings are shown in boldface type.
p < .05.
Analyses
Prior to all analyses, raw scores were ranked and then standardized to unit variances using standard techniques in order to avoid bias due to skewness of scales and age trends (Eaves et al., 1997). The NEAD data were then corrected for child age, child sex, and their interaction, and nontwin sibling scores were also corrected for age differences. All TM scores were corrected for the mother’s age, the adolescent’s age, the adolescent’s sex, and the interaction of the adolescent’s age and sex. All age and sex corrections were made by computing standardized partial residuals from the regression of scores on these variables (McGue & Bouchard, 1984).
Intraclass twin and sibling correlations.
Intraclass twin and sibling correlations were computed for each of the mothering constructs in the NEAD and TM projects. The pattern of the intraclass twin and sibling correlations can be examined to estimate the genetic and environmental contributions to the total variation in each measure. Genetic influences are implied if the magnitude of the correlation decreases according to decreasing genetic similarity of the twin and sibling pairs: MZ > DZ = FI = FS > HS > US. Shared environmental influences are indicated if the magnitude of the correlations is substantial and similar across all twin and sibling pairs and by correlations greater than zero for the US sibling pairs. Finally, nonshared environmental contributions are indicated by the difference between MZ twin correlations and 1.0 and include error of measurement. An important distinction between the child-based NEAD study and the parent-based TM project is in the definition of shared environment. Shared environmental influences, in both cases, refer to shared rearing experiences that cause family members to be similar to one another. In the case of the TM project, however, this includes the past rearing environment of the adult twin mothers and any current contact that does not differ for MZ and DZ twins. Current contact between the twin pairs was assessed in this sample. Analyses examining the amount of contact found no significant differences for MZ and DZ twin pairs (Pedersen et al., 1999). Therefore, if current contact influences parenting, it is likely to act as a shared environmental influence.
Univariate model fitting.
Model fitting allows the twin and sibling covariances to be considered simultaneously and facilitates testing of alternative models. A series of nested univariate genetic models were used for all of the measures of parenting from both the NEAD and the TM projects. The models were nested in order to examine a model in which all of the estimates of genetic and environmental influences were constrained to be equal for both samples and a model in which the estimates were allowed to differ for each sample. Figure 1 represents the basic univariate genetic model examined in the current study. Additive genetic (A), shared environmental (C), and nonshared environmental (E) influences on mothering are estimated in this model.
Figure 1.
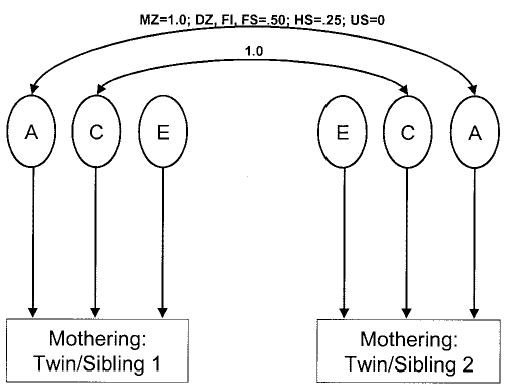
Univariate quantitative genetic model. Genetic influences are represented by A, shared or “common” environmental influences by C, and nonshared environmental influences by E. The correlation between A for the twin/sibling pairs varies based on the degree of genetic relatedness. Because C represents nongenetic influences that make twins and siblings similar to one another, this path is set to 1.0. Nonshared environmental influences (E) are uncorrelated and contain measurement error. MZ = monozygotic; DZ = dizygotic; FI = full siblings in intact families; FS = full siblings in stepfamilies; HS = half siblings in stepfamilies; US = unrelated siblings in stepfamilies.
As can be seen in Figure 1, the double-headed arrow connecting the genetic factor (A) for twin/sibling 1 and twin/sibling 2 is set to be equal to the degree of genetic similarity between the twin/sibling pairs. The double-headed arrow connecting the shared environmental (C) factors is set to be 1.0, because shared environmental influences are all nongenetic influences that make twins and siblings who are reared in the same family similar to one another. Finally, there is no double-headed arrow connecting the nonshared environmental (E) factors because, by definition, nonshared environmental influences make twins and siblings different from one another. This model includes only additive genetic influences (A). We can also examine nonadditive genetic effects, which may be due to either epistasis or dominance, although there is only limited power to detect both nonadditive effects and shared environmental influences in the same model. The decision as to which model is more appropriate (additive or nonadditive genetic effects) is made on the basis of the pattern of twin and sibling correlations. If MZ twin correlations are more than twice as large as DZ twin and full sibling correlations, then nonadditive genetic influences are suggested. Typically, nonadditive genetic influences are not found for models examining genetic and environmental influences on parenting, and we did not anticipate finding such effects in the current study.
Tests of model fit.
The overall fit of each model was tested by chi-square analysis and Akaike’s information criterion (AIC; Akaike, 1987). A nonsignificant chi-square and an AIC that is low and negative indicate that the model provides a good fit to the data. Previous research has found that chi-square is likely to reject a model that fits the data well but imperfectly, is very sensitive to sample size, and improves when more parameters are added (Mulaik et al., 1989; Neale & Cardon, 1992; Tanaka, 1993). AIC considers both the goodness of fit and parsimony, thereby providing a useful fit index to be used in addition to chi-square (Williams & Holahan, 1994). Further discussion of fit indices is available elsewhere (Bollen & Long, 1993; Loehlin, 1992b; Neale & Cardon, 1992). The models estimated in the current study were examined using the Mx statistical package (Neale, 1997).
The model that allows all of the genetic and environmental parameter estimates to vary for the TM and NEAD samples is the “full model” because it is saturated. A constrained model was also tested in which paths were set to be equal for the two samples. The difference in chi-square where the degrees of freedom are equal to the difference in the degrees of freedom for the two models (3, in this case) was used to test whether the constrained model resulted in a significant worsening in fit. If there was not a significantly worse fit, the reduced model was accepted as the best-fitting and most parsimonious model. Otherwise, the full model was considered the best-fitting model.
Model assumptions.
The assumptions of the model are implicit in the figure and are somewhat different for the NEAD and TM samples. For both samples, we assumed that shared and nonshared environmental effects were the same across sibling types and that gene–environment interaction was negligible. For the NEAD sample, we also assumed there was no selective placement of the stepsiblings and no assortative mating. A more detailed discussion of these assumptions for the NEAD sample can be found in Pike, McGuire, Hetherington, Reiss, and Plomin (1996). For a general discussion of the assumptions of quantitative genetic model fitting, see Loehlin (1992b). Most of these assumptions have been tested in previous reports and have been found to be valid (Kendler, Meyers, Prescott, & Neale, 2001; Pike et al., 1996). We also examined the assumptions, where possible, in the current study, as reported below.
The validity of equal twin and sibling environments was tested by examining the impact of remembered parenting as assessed by the PBI (Parker, Tupling, & Brown, 1979) and current contact in the TM sample (e.g., Kendler et al., 2001; Loehlin & Nichols, 1976). When similarity in mothering was associated with similarity in PBI remembered parenting, only two associations, less than expected by chance, were significant—both in the expected direction. The amount of current contact between the adult twin women was not significantly related to any of the parenting dimensions. Another test of the equal twins environment for current environmental influences is possible by examining differences in contact between the twin women by zygosity and then exploring whether any such differences influenced parenting. Although there were some differences in current contact between the twin women that were based on zygosity, these differences were not significantly related to any of the parenting dimensions. For the NEAD sample, the length of cohabitation for the twin and sibling pairs was not significantly related to any of the mothering constructs (Reiss et al., 2000). Both sets of findings indicate that, to the extent that we can test for violations in the TM and NEAD samples, the equal environments assumption is tenable. It is important to note that some studies that have tested for violations of the equal twin environments assumption have found evidence of such violations (e.g., Rose & Kaprio, 1988; Tambs, Harris, & Magnus, 1995). There is an ongoing debate about the viability of this assumption. However, in this sample, with our limited ability to test for such violations, we did not find any evidence that this assumption was violated.
Assortative mating refers to nonrandom mating that could result in effects that are truly genetic emerging as shared environmental effects in the NEAD sample. Sizable correlations between spouses for the same characteristic would indicate assortative mating. These effects can only be tested for the NEAD sample because we do not have data on the parents of the twin women. There is, however, some question as to which factors are likely to have relevant effects on parenting. Personality seems to be a likely candidate; unfortunately, we have no index of personality for parents in the NEAD sample. The only parent-specific characteristics that were measured in the NEAD project were depressive symptoms and vocabulary ability. Neither construct contributes to twin or sibling similarity; therefore, assortative mating for depression or vocabulary ability does not appear to have an impact on these mothering constructs. Any assortative mating effects that we cannot test for in the NEAD sample because the appropriate measures were not included for parents will serve to decrease heritability estimates and increase shared environmental influences.
Results
The results are presented in three parts. First, the intercorrelations among the measures and raters are presented to clarify composite construction. Next, the twin and sibling correlations for the NEAD project and the TM project are reported in Tables 3 and 4, respectively. Finally, the results of the nested univariate genetic models are described separately for mother’s positivity, negativity, control, and monitoring.
Table 3.
Intraclass Twin and Sibling Correlations for Mother–Adolescent Relationships in the Nonshared Environment Adolescent Development (NEAD) Sample
| Nondivorced families
|
Stepfamilies
|
|||||
|---|---|---|---|---|---|---|
| Identical twins (MZ) | Fraternal twins (DZ) | Full siblings | Full siblings | Half siblings | Step siblings | |
| Positivity | ||||||
| Mother report | .94 | .90 | .56 | .70 | .79 | .12 |
| Child report | .49 | .36 | .51 | .08 | .55 | −.22 |
| Observer ratings | .48 | .44 | .70 | .42 | .48 | .56 |
| Negativity | ||||||
| Mother report | .88 | .72 | .70 | .60 | .66 | .43 |
| Child report | .50 | .35 | .41 | .35 | .05 | .10 |
| Observer ratings | .29 | .30 | .36 | .29 | .23 | .18 |
| Control/monitoring | ||||||
| Mother report | ||||||
| Attempted control | .92 | .86 | .84 | .72 | .84 | .70 |
| Actual control | .97 | .92 | .66 | .79 | .78 | .78 |
| Monitoringa | .85 | .88 | .64 | .62 | .61 | .45 |
| Child report | ||||||
| Attempted control | .47 | .36 | .30 | .31 | .26 | .36 |
| Actual control | .53 | .32 | .28 | .27 | .21 | .34 |
| Monitoringa | .38 | .45 | .23 | .28 | .27 | .39 |
| Observer ratings | ||||||
| Mother’s control | .10 | .06 | .18 | .23 | .29 | .14 |
Note. MZ = monozygotic; DZ = dizygotic.
The Child Monitoring Scale (CMS; Hetherington & Clingempeel, 1992) assesses attempted control, actual control and knowledge, which is really monitoring. Therefore, in all tables knowledge from the CMS is referred to as monitoring.
Table 4.
Intraclass Twin Correlations for Mother–Adolescent Relationships in the Twin Moms Sample
| Identical twins | Fraternal twins | |
|---|---|---|
| Positivity | ||
| Mother report | .47 | .19 |
| Child report | .32 | .17 |
| Observer ratings | .24 | .07 |
| Negativity | ||
| Mother report | .41 | .14 |
| Child report | .05 | 06 |
| Observer ratings | .21 | 22 |
| Control/monitoring | ||
| Mother report | ||
| Attempted control | .24 | .25 |
| Actual control | .12 | .13 |
| Monitoring | .39 | .22 |
| Child report | ||
| Attempted control | −.09 | −.03 |
| Actual control | −.10 | .10 |
| Monitoring | .22 | .06 |
| Observer ratings | ||
| Mother’s control | .15 | 06 |
Composite Construction
As described in the Method section, prior to analyses, the mothering constructs were composited separately by reporter. The intercorrelations across mother, adolescent and observer ratings, presented in boldface type in Table 2, indicate that in most cases, although there was some overlap among different raters for the same construct, the correlations were modest, hovering around .30. The largest correlation across raters, .57, was between mother and adolescent reports on positivity in the TM sample. This finding of only modest cross-rater correlations indicates that maintaining distinct composites for each rater is an appropriate strategy for both the NEAD and TM samples. The intercorrelations among the measures that comprise each construct are also reported in Table 2. Most of the correlations across constructs are less than .45. The only exceptions to this are correlations among the two control subscales, Attempted Control and Actual Control. It seems that neither mothers nor adolescents clearly distinguished between attempted and actual control, with within-reporter correlations ranging from .72 to .80. Although the correlations between control and monitoring are also larger than for the other constructs (the largest correlation is .52), they are, in general, more moderate than those for attempted and actual control. Because these two control constructs have been found to provide meaningful distinctions in patterns of associations with child adjustment for the NEAD sample (Reiss et al., 2000), we decided to maintain the distinction for this study.
Intraclass Twin/Sibling Correlations: NEAD Project
The intraclass twin and sibling correlations for the NEAD project are presented in Table 3. For mother reports of her positivity and negativity, mostly shared environmental and genetic influences are indicated. On the other hand, for adolescent reports of positivity, mostly nonshared environmental and genetic influences are suggested, and some shared environmental influences are also indicated for adolescent reports of negativity. For observer ratings of mother’s positivity and negativity, the pattern of correlations indicates that mostly nonshared and shared environmental influences are operating. Finally, for control and monitoring, mostly shared environmental influences and some nonshared environmental influences are indicated for both mother and adolescent reports of control. The pattern of correlations for observer ratings of control suggests mostly nonshared environmental influences, with the possibility of some genetic and shared environmental influences. Mother and adolescent reports of monitoring (knowledge) are similar to the findings for control—mostly shared environmental influences are suggested for mother reports, and mostly nonshared environmental and genetic influences are suggested for adolescent reports.
Intraclass Twin Correlations: Twin Moms Project
The pattern of intraclass twin correlations for the parent-based TM sample, reported in Table 4, suggests that nonshared environmental influences are of primary importance for all of the measures of the mother’s parenting. The largest correlation for MZ twins is .47 for mother reports of positivity, which indicates that approximately 53% of the total variance can be explained by nonshared environmental factors (1.0 − rMZ). For the remaining measures, nonshared environmental factors account for approximately 70% or more of the total variance. Modest genetic influences are also indicated for maternal positivity, for mother reports of her negativity and monitoring, for adolescent reports of monitoring, and for observer ratings of control. For the remaining measures, the pattern of intraclass twin correlations indicates primarily nonshared and modest shared environmental influences, especially for control.
Model-Fitting Results
The standardized parameter estimates for genetic, shared environmental, and nonshared environmental influences, with 95% confidence intervals and fit indices for each model, are presented in Table 5. For each construct, the estimates for the model that constrains the estimates for the NEAD and TM samples to be equal are presented first, and the estimates for the model that allows the estimates to vary for each sample are presented directly below. The best fitting of the two models is indicated in boldface type. In no case did the pattern of twin and sibling correlations suggest that nonadditive genetic influences were likely to be present, so only models including additive genetic effects are reported here. The findings are described for each construct separately below. We also present the parameter estimates from the best-fitting model in Figures 2 through 5. The figures are bar graphs representing 100% of the variance divided into genetic, shared environmental, and nonshared environmental contributions. The bar graphs help to illustrate the similarities and differences in samples and raters that are described below for each construct.
Table 5.
Parameter Estimates (and 95% Confidence Intervals) for Constrained (Equate) and Unconstrained (Differ) Models and Fit Indices From NEAD and Twin Moms Projects
| NEAD
|
Twin Moms
|
Fit indicesa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Reporter | Model | a2 | c2 | e2 | a2 | c2 | e2 | χ2 | AIC |
| Mother’s positivity | |||||||||
| Mom | Equate | .20 (.04–.36) | .40 (.28–.51) | .40 (.33–.48) | 226.16* | 184.16 | |||
| Differ | .46 (.17–.56) | .50 (.00–.21) | .04 (.44–.67) | .45 (.17–.56) | .00 (.00–.21) | .55 (.44–.67) | 55.78* | 19.78 | |
| Child | Equate | .19 (.00–.41) | .17 (.01–.33) | .64 (.54–.75) | 26.58 | −15.42 | |||
| Differ | .27 (.00–.55) | .19 (.00–.36) | .54 (.40–.72) | .30 (.00–.46) | .04 (.00–.32) | .67 (.54–.82) | 22.75 | −13.25 | |
| Observer | Equate | .00 (.00–.12) | .33 (.24–.40) | .67 (.60–.74) | 43.03* | 1.03 | |||
| Differ | .00 (.00–.19) | .50 (.37–.57) | .50 (.39–.58) | .23 (.00–.36) | .00 (.00–.22) | .77 (.64–.91) | 9.67 | −26.33 | |
| Mother’s negativity | |||||||||
| Mom | Equate | .09 (.00–.27) | .43 (.30–.53) | .48 (.40–.57) | 108.53* | 66.53 | |||
| Differ | .40 (.27–.52) | .48 (.37–.57) | .12 (.39–.58) | .39 (.09–.51) | .00 (.00–.22) | .61 (.50–.74) | 10.44 | −25.56 | |
| Child | Equate | .07 (.00–.30) | .14 (.00–.25) | .80 (.68–.89) | 27.74 | −14.26 | |||
| Differ | .43 (.14–.65) | .10 (.00–.27) | .48 (.35–.64) | .05 (.00–.19) | .00 (.00–.14) | .95 (.81–1.0) | 4.58 | −31.42 | |
| Observer | Equate | .00 (.00–.22) | .25 (.10–.32) | .75 (.64–.82) | 3.02 | −38.98 | |||
| Differ | .09 (.00–.39) | .24 (.05–.37) | .67 (.51–.81) | .00 (.00–.35) | .22 (.00–.32) | .78 (.64–.89) | 1.17 | −34.83 | |
| Mother’s attempted control | |||||||||
| Mom | Equate | .00 (.00–.04) | .54 (.48–.59) | .46 (.41–.52) | 216.07* | 174.07 | |||
| Differ | .21 (.13–.29) | .71 (.63–.77) | .08 (.06–.12) | .01 (.00–.37) | .23 (.00–.34) | .76 (.62–.87) | 18.05 | −17.95 | |
| Child | Equate | .00 (.00–.11) | .15 (.06–.22) | .85 (.77–.92) | 33.20* | −8.80 | |||
| Differ | .18 (.00–.48) | .25 (.06–.41) | .57 (.41–.74) | .00 (.00–.08) | .00 (.00–.07) | 1.0 (.92–1.0) | 2.78 | −33.22 | |
| Mother’s actual control | |||||||||
| Mom | Equate | .00 (.00–.03) | .50 (.44–.55) | .50 (.45–.56) | 324.02* | 282.02 | |||
| Differ | .32 (.00–.61) | .16 (.00–.35) | .51 (.37–.71) | .00 (.00–.13) | .02 (.00–.13) | .98 (.86–1.0) | 29.43* | −6.57 | |
| Child | Equate | .00 (.00–.11) | .18 (.09–.25) | .82 (.75–.89) | 16.21 | −25.79 | |||
| Differ | .27 (.23–.36) | .68 (.60–.74) | .03 (.02–.05) | .00 (.00–.27) | .13 (.00–.24) | .87 (.73–.98) | 7.38 | −28.62 | |
| Observer rated control | |||||||||
| Observer | Equate | .00 (.00–.22) | .24 (.08–.31) | .76 (.66–.83) | 16.21 | −25.79 | |||
| Differ | .00 (.00–.24) | .17 (.01–.27) | .83 (.64–.93) | .12 (.00–.26) | .00 (.00–.15) | .88 (.74–1.0) | 8.64 | −27.38 | |
| Mother’s knowledge (monitoring) | |||||||||
| Mom | Equate | .04 (.00–.21) | .48 (.35–.55) | .48 (.40–.56) | 126.49* | 84.49 | |||
| Differ | .33 (.21–.45) | .53 (.43–.63) | .14 (.10–.20) | .40 (.02–.51) | .00 (.00–.30) | .60 (.49–.75) | 29.43* | −6.57 | |
| Child | Equate | .00 (.00–.22) | .24 (.08–.31) | .76 (.66–.83) | 16.21 | −25.79 | |||
| Differ | .05 (.00–.35) | .31 (.12–.42) | .64 (.48–.76) | .20 (.00–.33) | .00 (.00–.22) | .80 (.67–.95) | 6.06 | −29.94 | |
Note. NEAD = Nonshared Environment in Adolescent Development project; AIC = Akaike’s information criterion.
df = 18 for differ models and 21 for equate models. Best-fitting models, as indicated by Δχ2(3), are indicated in bold. A difference greater than 7.82 is significant.
p ≤ .05.
Figure 2.
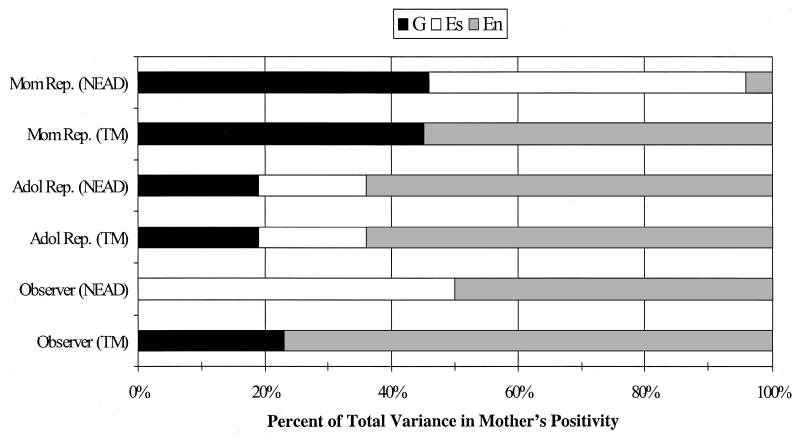
Percentage of variance explained by genetic (G), shared environmental (Es), and nonshared environmental (En) influences from the best-fitting model results for maternal positivity. Mother reports (Mom Rep.) are presented in the top two bars, adolescent reports (Adol Rep.) in the middle two bars, and observer ratings in the bottom two bars. NEAD = Nonshared Environment in Adolescent Development project; TM = Twin Moms project.
Figure 5.
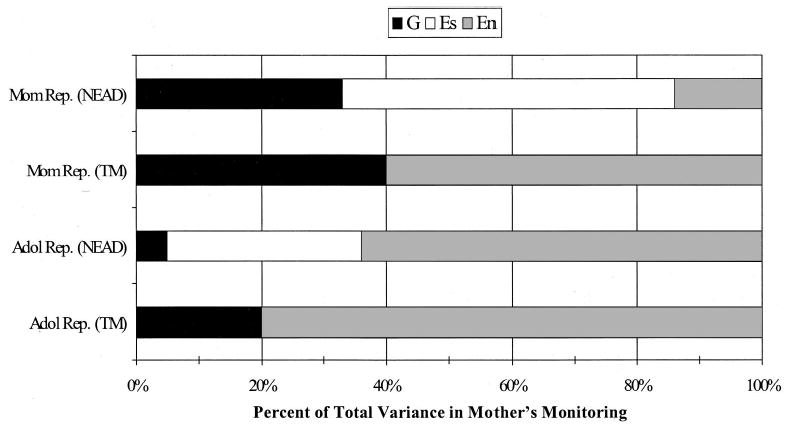
Percentage of variance explained by genetic (G), shared environmental (Es), and nonshared environmental (En) influences from the best-fitting model results for maternal monitoring. Mother reports (Mom Rep.) are presented in the top two bars, and adolescent reports (Adol Rep.) are presented in the bottom two bars. NEAD = Nonshared Environment in Adolescent Development project; TM = Twin Moms project.
Mother’s positivity.
For mother’s positivity, only the estimates for adolescent reports can be constrained to be equal for the NEAD and TM samples. For both mother reports and observer ratings, there is a significantly worse fit when the parameters are not allowed to vary across the two samples. For both mother and observer ratings, the main difference between the findings for the child-based NEAD study and the parent-based TM study is in shared environmental influences. Shared environmental influences are significant and substantial for the NEAD sample but not for the TM sample. The pattern of findings, especially for mother and observer ratings, suggests that passive genotype–environment correlations may be operating in that there are shared environmental influences for the child-based NEAD sample and at least moderate genetic influences for the parent-based TM sample. This finding is especially strong for mother reports, as the bulk of the variance is explained by genetic and shared environmental influences for the child-based NEAD sample and by genetic and nonshared environmental influences for the parent-based TM sample. The presence of nonpassive genotype–environment correlation is also suggested by the moderate genetic influence on adolescent and mother reports of mother’s positivity in the child-based NEAD sample. Figure 2 illustrates these findings and also highlights differences in the patterns of findings for the different raters. Specifically, for both the NEAD and TM samples, mother reports of her positivity show the largest heritability estimates. With that exception, the discrepancies across raters are difficult to discern as a general trend across the two samples, with as many differences emerging by sample as by rater.
Mother’s negativity.
The results for mother’s negativity show a different pattern of findings than those for positivity, suggesting different processes. Specifically, only for observer ratings of mother’s negativity can the estimates for the two samples be equated. The findings for observer ratings suggest no evidence of genotype–environment correlation, with the majority of the variation being due to shared and nonshared environmental influences. Adolescent and mother reports of negativity in the NEAD sample both show significant genetic influences, with more shared environmental influence for mother reports than adolescent reports. For the TM sample, there are also significant genetic influences for mother reports of negativity, whereas for adolescent reports the only significant parameter is nonshared environment (95% of the variance). Taken together, these findings suggest that for mother reports of negativity, both passive and nonpassive genotype–environment correlations may be operating, whereas only nonpassive genotype–environment correlation is suggested for adolescent ratings. These findings are illustrated in Figure 3.
Figure 3.
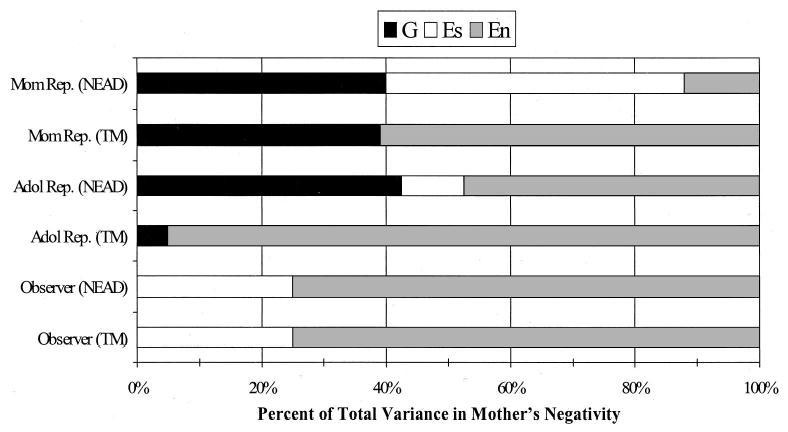
Percentage of variance explained by genetic (G), shared environmental (Es), and nonshared environmental (En) influences from the best-fitting model results for maternal negativity. Mother reports (Mom Rep.) are presented in the top two bars, adolescent reports (Adol Rep.) in the middle two bars, and observer ratings in the bottom two bars. NEAD = Nonshared Environment in Adolescent Development project; TM = Twin Moms project.
Mother’s control and monitoring.
The pattern of findings is very similar for attempted and actual control in both the NEAD and TM projects for mother and adolescent ratings. In all cases, the best-fitting model is the unconstrained model, with the most notable difference in the samples being the shared environmental influences for the NEAD sample and the lack of genetic influences for the TM sample. This pattern of findings suggests that nonpassive genotype–environment correlations are likely to be operating for mother’s attempted and actual control. In contrast, the constrained model provided the best fit for the observer ratings of mother’s control, and there was no evidence of genetic influences. The variance in observer ratings of control was due exclusively to shared and nonshared environmental influences, indicating that genotype–environment correlation was not relevant for this construct. The graphical representation of these findings is presented in Figure 4, which serves to highlight the lack of genetic influences on control for the parent-based TM sample. The most notable differences in the findings by rater for control are between mother and adolescent reports and observer ratings. This difference is especially noticeable in the NEAD sample, where the self-report measures show genetic influences while observer ratings show only environmental influences. There are also differences in mother and adolescent reports, although these differences vary for the two control constructs, with the bulk of the rater differences being due to differences in the size of the shared and nonshared environmental estimates.
Figure 4.
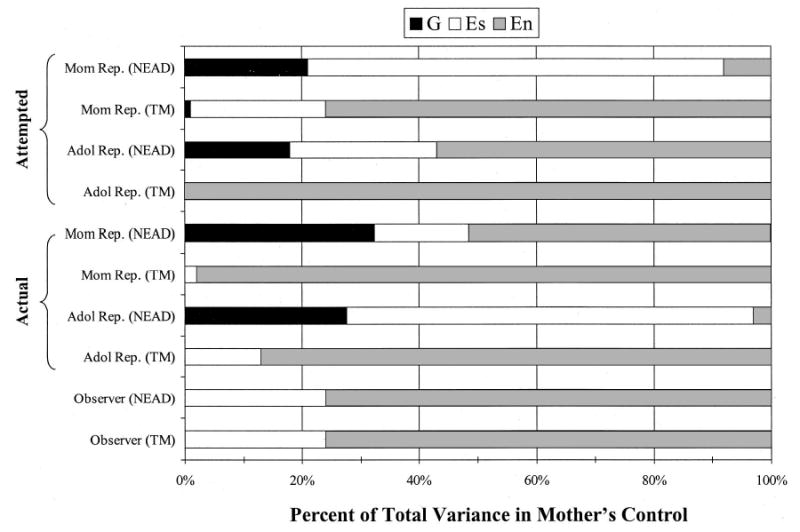
Percentage of variance explained by genetic (G), shared environmental (Es), and nonshared environmental (En) influences from the best-fitting model results for maternal attempted, actual, and observed control. Attempted control is presented in the top section with mother reports (Mom Rep.) above and adolescent reports (Adol Rep.) below. Actual control is similarly presented in the middle section, and observer ratings are presented in the bottom two bars. NEAD = Nonshared Environment in Adolescent Development project; TM = Twin Moms project.
The findings for mother’s knowledge (actual monitoring) are very different from those for mother’s control. Both mother and adolescent reports suggest that passive genotype–environment correlations may be operating. This is indicated by the genetic influences on the parent-based TM sample (although these influences are only modest for adolescent reports) and the shared environmental influences on the child-based NEAD sample. For mother reports of monitoring, there is also some evidence of nonpassive genotype–environment correlation given the significant genetic influences in the child-based NEAD sample. Figure 5 illustrates these findings and helps to highlight the differences in mother and adolescent reports of more genetic influence for mother reports of monitoring than for adolescent reports in both samples.
Discussion
The present study continues an effort to advance our understanding of the processes involved in genetic influences on parenting. Genetic and environmental influences on mothering were estimated for two samples, the child-based NEAD sample and the parent-based TM sample, in an attempt to specify which type of genotype–environment correlation was operating—passive, non-passive/evocative, or none. Four distinct constructs of mothering were examined: positivity, negativity, control, and monitoring. These parenting constructs have been used extensively in other studies and have been found to reliably predict child and adolescent outcome (e.g., Baumrind, 1991; Hetherington & Clingempeel, 1992). The findings from the current study suggest that different types of genotype–environment correlation operate for different mothering constructs. Specifically, passive genotype–environment correlations for mother’s positivity and monitoring and nonpassive genotype–environment correlations for mother’s negativity and control were indicated. Depending on the reporter, there was also evidence that both types of genotype–environment correlation were operating for most of the constructs. For observer ratings of negativity and monitoring, no evidence of genotype–environment correlation was found—that is, all of the variance was explained by environmental influences. Each of these findings and their implications are discussed in turn below.
Although the findings tended to vary by rater, there was some consistency in the patterns of findings for each parenting construct. Specifically, passive genotype–environment correlation seems to operate to influence the mother’s positivity with her adolescent child. This conclusion is supported by genetic influences for the TM sample across the three reporters in combination with shared environmental influences for the NEAD sample. These findings suggest that a mother may interact with her adolescent in a positive way, at least in part, because of her own genetically influenced characteristics. Furthermore, the significant shared environmental influences for the child-based NEAD sample indicate that she is likely to be positive to both of her adolescent children independent of genetically influenced characteristics of the adolescents. For mother reports of positivity, there were also significant genetic influences for the child-based NEAD sample, indicating that children’s genes also have an influence on how positively their mothers behave toward them. The findings for adolescent reports of mother’s positivity also suggest that nonpassive genotype–environment correlations may be present. Because the two samples could not be equated for adolescent reports, it is difficult to distinguish between passive and nonpassive influences. Thus, there was evidence for both passive and nonpassive genotype–environment correlations for mother reports and, to a lesser degree, adolescent reports of positivity, whereas for observer ratings, only passive genotype–environment correlations were indicated.
In contrast, for mother’s negativity, nonpassive genotype–environment correlations were suggested for both mother and adolescent reports, and no genotype–environment correlation was indicated for observer ratings. The clearest example of nonpassive genotype–environment correlation was found for adolescent reports of mother’s negativity. There were substantial and significant genetic influences for the child-based NEAD sample and small and nonsignificant genetic influences for the parent-based TM sample. In other words, mother’s negativity was not influenced by her genotype but was influenced by her adolescent children’s genetically influenced characteristics. For mother reports of her negativity, there was also some indication that passive genotype–environment correlation may be operating. The estimate for shared environmental influences was large and significant for the NEAD sample, and there were also significant genetic influences for the TM sample. These findings indicate that a mother’s negativity is, at least in part, a response of the mother to her adolescent’s genetically influenced characteristics. In other words, a child with a difficult temperament may elicit more negativity from his or her mother than a child who is easygoing, at least according to mother and adolescent reports of the mother’s behavior. These findings are consistent with Patterson’s coercive family interaction model for the development of antisocial behavior (Patterson, 1982). Finally, because there were no significant genetic influences on observer ratings of mother’s negativity for either sample, genotype–environment correlations were not indicated.
The patterns of findings for mother’s attempted control and actual control were very similar, as expected from the sizable correlations across the two constructs. For both mother and adolescent reports, only nonpassive genotype–environment correlations were indicated. In no case were there genetic influences on control for the parent-based TM sample; therefore, passive genotype–environment correlations could not be operating. This suggests, like the findings for mother and adolescent reports of mother’s negativity, that mothers tend to respond to genetically influenced characteristics of their children with varying levels of control. In other words, mothers may not attempt to control their more responsible adolescent children, whereas adolescents who tend to get into trouble may elicit greater control from their mothers. Observer ratings of mother’s control show no evidence of genetic influence; therefore, genotype–environment correlation is not relevant. The bulk of the variance for observer ratings of control is due to nonshared environmental influences, with approximately a quarter of the total variance being due to shared environmental influences.
For mother’s monitoring, the pattern of findings was more complicated: Both passive and nonpassive types of genotype–environment correlation were indicated for mother reports, whereas only passive genotype–environment correlation was indicated for adolescent reports. The finding of only passive genotype–environment correlation for adolescent reports of monitoring is especially interesting given a recent set of findings suggesting that monitoring is not really a measure of how much the parent knows about the child’s activities but more a measure of how much the child discloses to his or her parents (Kerr & Stattin, 2000; Stattin & Kerr, 2000). Passive genotype–environment correlation indicates that genetic influences on mothering are due to the mother and the adolescent sharing genes that influence both the mother’s and the adolescent’s behavior. We would expect that if monitoring was more a function of how much the child disclosed to the parent that it would be better explained by nonpassive rather than passive genotype–environment correlation. In this case, because the findings differed for mother and adolescent reports, and because the measure was not designed to assess adolescent disclosure, we cannot draw any firm conclusions.
Although one needs to keep in mind the variation in findings by rater, there is some consistency in the pattern of findings by mothering construct, especially for mother and adolescent reports. This suggests that the processes involved in mother–adolescent relationships vary for different aspects of the relationship. In other words, mothers may have a generally positive style of interacting, and this positivity is influenced by their own genotype (passive genotype–environment correlation), whereas their negativity and control appear to be evoked, at least in part, by their children’s genetically influenced characteristics (nonpassive genotype–environment correlation). This is consistent with the literature that has found that parents respond differently to their children depending on the children’s characteristics, including age, temperament, and disabled status (e.g., Brody et al., 1992; Dunn & Plomin, 1986; McHale & Palwetko, 1992). The suggestion of primarily passive genotype–environment correlation for mother’s positivity is an important finding and suggests that the level of warmth in the mother–adolescent relationship may be somewhat independent of the adolescent’s own characteristics and behaviors. It may be that a mother’s positivity with her children is influenced by her personality or temperament, both heritable characteristics (e.g., Loehlin, 1992a).
On the other hand, the evidence for nonpassive genotype–environment correlation for mother and adolescent reports of mother’s negativity and control is consistent with a wealth of literature that has identified a coercive parent–child interaction style as an important step down the pathway to antisocial and delinquent behavior (e.g., Patterson, Reid, & Dishion, 1998). These findings suggest that the child elicits negativity from the mother, at least in adolescence. The development of this interaction style over time is likely to be a product of both the parents and the children, perhaps with different contributions at different stages in development. The presence of nonpassive genotype–environment correlation for mother’s negativity is particularly relevant for potential prevention and intervention strategies, because parents can be taught to respond differently to their children (e.g., Hipke, Wolchik, Sandler, & Braver, 2002). If passive genotype–environment correlation had been suggested for both mother’s positivity and negativity, the prospects of modifying a behavior that was more mother-driven than child-driven would be somewhat more daunting, especially if personality or temperament was one of the important mother characteristics that influenced her parenting. We should note, however, that passive genotype–environment correlation was also suggested for mother reports of her negativity and that only environmental influences were present for observer ratings. This discrepancy in the pattern of findings by rater suggests that other factors may be involved in the rater-specific constructs such as perceptions of the rater or rater biases. These potential confounds are discussed in more detail below.
Nonshared environmental influences explained the majority of the variance for most of the mothering constructs for the TM sample. In some ways this is not a surprising finding—as one gains more control of one’s environment, one is likely to experience environments very different from that of one’s twin (or other family members). In the case of the TM sample, an important potential source of nonshared environmental influences on mother’s parenting is the partner. Mothers and fathers may not parent similarly, or may not have the same influences on their children, but it is highly probable that each influences the way the other parents. Another important contributor to the nonshared environmental influences in the TM project is the adolescent child. As more studies examine multiple children in the family, the role of the child in influencing the way his or her parents treat him or her is becoming more obvious (e.g., Dunn & Plomin, 1990; Kandel & Wu, 1995). It is important to note that although the adolescents and their mothers share 50% of their genes, they do not share the other 50%. Thus, nonpassive genotype–environment correlations could emerge as nonshared environmental influences for the TM project. The challenge, in this study and others, is to understand better what these nonshared environmental influences are and how they operate both in influencing the mother’s parenting of her adolescent child and in influencing the mental health of all family members, including parents and children.
The findings from the present study are, for the most part, consistent with previously published findings. Specifically, for the child-based NEAD sample, genetic influences were more consistently indicated for positivity and negativity and were more modest for monitoring and control. Although there was variation by reporter in this pattern of findings, taken as a whole they are consistent with previous work in this area (e.g., Elkins et al., 1997; Plomin, 1994; Rowe, 1981). The findings from the parent-based TM study, on the other hand, differed somewhat from those of other parent-based studies of parenting. The study most consistent with the findings in the present study is that by Kendler (1996), who found a pattern of findings similar to those from child-based designs: Genetic and nonshared environmental influences were important for parental warmth, and shared and nonshared environmental influences explained all of the variance for parental protectiveness and authoritarianism. In the TM project, it was only for mother’s positivity that genetic influences were consistently indicated. If authoritarianism is similar to negativity, and protectiveness to control, the findings from the TM study are consistent with those reported by Kendler. Results from other studies of parenting using parent-based designs have tended to use more global measures of parenting and have consistently found genetic influences on all aspects of parenting assessed (Losoya et al., 1997; Perusse et al., 1994; Plomin et al., 1989). As discussed earlier, it is likely that a more global measure of parenting measures something quite different than do the dyadic relationship-focused measures used in the current study and by Kendler (1996). As there have been few studies that have examined genetic influences on parenting in general, and even fewer that have used a parent-based design, the similarity in the patterns of findings across similar studies is encouraging.
There are several limitations of the present study that should be noted. The most obvious limitation is the lack of consistency across raters within each construct. Although it is possible to draw some general conclusions within construct and across raters, there are notable differences across raters that are fairly consistent from construct to construct. Specifically, genetic influences on mothering tend to be lower for child reports than mother reports for the TM sample, although this is not true for the NEAD sample. Mother reports tend to show more evidence of shared environmental influences for the NEAD sample, with the exception of mother’s actual control. In both cases, these general rater trends make some sense given the nature of the samples. In the TM sample, the mothers are twins and it is possible that some of the genetic influence on mothering found for this sample may be a reflection of a genetically influenced response tendency. In other words, identical twins may tend to report on their mothering more similarly than fraternal twins, and this similarity in response patterns would emerge as genetic influences. In the NEAD sample, the same mother is reporting on two different children, so any response biases would emerge as shared environmental influences for this sample. It is also possible that mothers believe that they treat their two children similarly or wish that they did. This would also be reflected in the shared environment estimate. Another rater difference that is consistent across constructs and samples is the lower estimates of genetic influences for observer ratings of mothering. This is a finding that has been consistent throughout the literature when observer ratings of parenting have been assessed within a genetically sensitive design (e.g., O’Connor, Hetherington, Reiss, & Plomin, 1995). Many studies attempt to address differences across raters by creating composites or using latent constructs. We decided against such correctives because of the relatively modest pattern of intercorrelations among raters. It is possible that patterns of genotype–environment correlation would have been obscured if such methods were used, because genetic influences tend to be higher for composites of adolescent behavior (Reiss et al., 2000).
A second limitation of the present study is that although it is possible to compare and contrast the findings from the TM and NEAD studies, they are not nested. We made a special effort to collect measures of parenting in the TM project that had also been used in the NEAD project. This allowed us to examine patterns of correlations and means for the parenting measures in an effort to detect systematic differences between the two samples. As none were found, examining these two samples together seemed a reasonable step in beginning to disentangle passive and nonpassive types of genotype– environment correlation. It is important to keep in mind, however, that the differences in the patterns of findings for these two samples can only suggest which types of genotype– environment correlation may be operating. It is only by examining a sample that allows for child-based and parent-based analyses within the same study that conclusions can be drawn with confidence. Nonetheless, this study represents another step in advancing our understanding of the processes involved in mothering.
Studies that combine different genetic designs, especially when the measures and/or samples are matched in as many ways as possible, are the most likely to be able to address complicated questions such as those about process. More studies are being conducted in behavior genetics that combine various sibling types (e.g., Reiss et al., 2000), that extend twin studies to other family members (e.g., Heath, Kendler, Eaves & Markell, 1985), and that include careful measurement of the family environment (e.g., Dunn, Deater-Deckard, Pickering, & Golding, 1999; Elkins et al., 1997; Reiss et al., 2001). There are also an increasing number of studies, like the current one, that have combined findings from multiple studies in order to identify processes that would not be discernible if the studies were conducted individually (e.g., Deater-Deckard & O’Connor, 2000; Eley, Lichtenstein, & Stevenson, 1999). As researchers continue their efforts to understand the processes involved in parent–child relationships and in the influence of parenting on child adjustment, such designs will become ever more common. Understanding the processes involved in parenting is important for advancing our understanding of how parenting influences child adjustment. Some studies have already examined this question using genetically informed child-based designs (e.g., Elkins et al., 1997; Neiderhiser, Reiss, Hetherington, & Plomin, 1999; Pike et al., 1996). It is also necessary to examine how a parent’s relationship with his or her child influences the parent’s own mental health and how genetic and environmental factors influence such associations. Finally, understanding which parent characteristics influence parenting will help in understanding passive genotype–environment correlations. It is by examining each of the many processes underlying mental health and the way these processes are interrelated that we will be able to gain an understanding of what can be changed in order to maximize the likelihood of positive adjustment outcomes.
Footnotes
The Twin Moms project was supported by National Institute of Mental Health (NIMH) Grant R01MH54610. The Nonshared Environment in Adolescent Development project was supported by NIMH Grants R01MH43373 and R01MH48825 and by the William T. Grant Foundation.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Bank L, Duncan T, Patterson GR. Parent and teacher ratings in the assessment and prediction of antisocial and delinquent behaviors. Journal of Personality. 1993;61:693–709. doi: 10.1111/j.1467-6494.1993.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Bank, L., & Patterson, G. R. (1992). The use of structural equation modeling in combining data from different types of assessment. In J. Rosen & P. McReynolds (Eds.), Advances in psychological assessment (Vol. 8, pp. 41–73). New York: Plenum Press.
- Baumrind D. The influence of parenting styles on adolescent competence and substance use. Journal of Early Adolescence. 1991;11:56–95. [Google Scholar]
- Belsky J, Park S-Y. Exploring reciprocal parent and child effects in the case of child inhibition in US and Korean samples. International Journal of Behavioral Development. 2000;24:338–347. [Google Scholar]
- Bollen, K. A., & Long, J. S. (Eds.). (1993). Testing structural equation models. Newbury Park, CA: Sage.
- Brody GH, Stoneman Z, McCoy JK. Parental differential treatment of siblings and sibling differences in negative emotionality. Journal of Marriage and the Family. 1992;54:643–651. [Google Scholar]
- Collins W, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. [PubMed] [Google Scholar]
- Cote LR, Azar ST. Child age, parent and child gender, and domain differences in parents’ attributions and responses to children’s outcomes. Sex Roles. 1997;36:23–50. [Google Scholar]
- Deater-Deckard K, O’Connor TG. Parent–child mutuality in early childhood: Two behavioral genetic studies. Developmental Psychology. 2000;36:561–570. doi: 10.1037/0012-1649.36.5.561. [DOI] [PubMed] [Google Scholar]
- Dunn, J. (1991). The developmental importance of differences in siblings’ experiences within the family. In K. A. Pillemer & K. McCartney (Eds.), Parent–child relations throughout life (pp. 113–124). Hillsdale, NJ: Erlbaum.
- Dunn J, Deater-Deckard K, Pickering K, Golding J. Siblings, parents, and partners: Family relationships within a longitudinal community study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:1025–1037. [PubMed] [Google Scholar]
- Dunn J, Plomin R. Determinants of maternal behaviour towards 3-year-old siblings. British Journal of Developmental Psychology. 1986;4:127–137. [Google Scholar]
- Dunn, J., & Plomin, R. (1990). Separate lives: Why siblings are so different. New York: Basic Books.
- Eaves LJ, Silberg JL, Maes HH, Simonoff E, Pickles A, Rutter M, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: Results from two twin studies. Child Development. 1999;70:155–168. doi: 10.1111/1467-8624.00012. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent–son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–353. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, Troughton E, Stewart MA. The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology. 1996;32:574–589. [Google Scholar]
- Grotevant, H. D. (1998). Adolescent development in family contexts. In W. Damon (Series Ed.) & N. Eisenberg (Vol. Ed.), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (5th ed., pp. 1097–1149). New York: Wiley.
- Haapasalo J, Tremblay RE. Physically aggressive boys from ages 6 to 12: Family background, parenting behavior, and prediction of delinquency. Journal of Consulting and Clinical Psychology. 1994;62:1044–1052. doi: 10.1037//0022-006x.62.5.1044. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: Informativeness of different relationships. Behavior Genetics. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Henderson SH. Method. In E M Hetherington, S H Henderson, & D Reiss (Eds), Adolescent siblings in stepfamilies: Family functioning and adolescent adjustment. Monographs of the Society for Research in Child Development. 1999;64(4 Serial No 259):26–49. doi: 10.1111/1540-5834.00046. [DOI] [PubMed] [Google Scholar]
- Hetherington, E. M., & Clingempeel, W. G. (1992). Coping with marital transitions: A family systems perspective. Monographs of the Society for Research in Child Development, 57(2–3, Serial No. 227).
- Hipke K, Wolchik SA, Sandler IN, Braver SL. Predictors of children’s intervention-induced resilience in a parenting program for divorced mothers. Family Relations: Interdisciplinary Journal of Applied Family Studies. 2002;51:121–129. [Google Scholar]
- Hughes C, Deater-Deckard K, Cutting AL. “Speak roughly to your little boy?”. Sex differences in the relations between parenting and preschoolers’ understanding of mind . Social Development. 1999;8:143–160. [Google Scholar]
- Jacobson KC, Rowe DC. Genetic and environmental influences on the relationships between family connectedness, school connectedness, and adolescent depressed mood: Sex differences. Developmental Psychology. 1999;35:926–939. doi: 10.1037//0012-1649.35.4.926. [DOI] [PubMed] [Google Scholar]
- Kandel, D. B., & Wu, P. (1995). Disentangling mother–child effects in the development of antisocial behavior. In J. McCord (Ed.), Coercion and punishment in long-term perspectives (pp. 106–123). New York: Cambridge University Press.
- Kendler KS. Parenting: A genetic–epidemiologic perspective. American Journal of Psychiatry. 1996;153:11–20. doi: 10.1176/ajp.153.1.11. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Meyers J, Prescott CA, Neale MC. The genetic epidemiology of irrational fears and phobias in men. Archives of General Psychiatry. 2001;58:257–265. doi: 10.1001/archpsyc.58.3.257. [DOI] [PubMed] [Google Scholar]
- Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: Further support for a reinterpretation of monitoring. Developmental Psychology. 2000;36:366–380. [PubMed] [Google Scholar]
- Loehlin, J. C. (1992a). Genes and environment in personality development. Newbury Park, CA: Sage.
- Loehlin, J. C. (1992b). Latent variable models: An introduction to factor, path, and structural analysis (2nd ed.). Hillsdale, NJ: Erlbaum.
- Loehlin, J. C., & Nichols, R. C. (1976). Heredity, environment and personality: A study of 850 sets of twins. Austin: University of Texas Press.
- Losoya SH, Callor S, Rowe DC, Goldsmith HH. Origins of familial similarity in parenting: A study of twins and adoptive siblings. Developmental Psychology. 1997;33:1012–1023. doi: 10.1037//0012-1649.33.6.1012. [DOI] [PubMed] [Google Scholar]
- Maccoby EE. The role of parents in the socialization of children: A historical review. Developmental Psychology. 1992;28:1006–1017. [Google Scholar]
- Manke B, McGuire S, Reiss D, Hetherington EM, Plomin R. Genetic contributions to adolescents’ extrafamilial social interactions: Teachers, best friends, and peers. Social Development. 1995;4:238–256. [Google Scholar]
- McGue M, Bouchard TJ., Jr Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McHale SM, Palwetko TM. Differential treatment of siblings in two family contexts. Child Development. 1992;63:68–81. [Google Scholar]
- Moos RH, Moos BS. Adaptation and the quality of life in work and family settings. Journal of Community Psychology. 1983;11:158–170. doi: 10.1002/1520-6629(198304)11:2<158::aid-jcop2290110209>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Mulaik SA, James LR, Alstine JV, Bennett N, Lind S, Stilwell CD. Evaluation of goodness-of-fit indices for structural equation models. Psychological Bulletin. 1989;105:430–445. [Google Scholar]
- Neale, M. C. (1997). Mx: Statistical modeling, 4th edition [Computer software and manual]. Richmond: Medical College of Virginia, Department of Psychiatry.
- Neale, M. C., & Cardon, L. R. (1992). Methodology for genetic studies of twins and families. Boston: Kluwer Academic.
- Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Relationships between parenting and adolescent adjustment over time: Genetic and environmental contributions. Developmental Psychology. 1999;35:680–692. doi: 10.1037//0012-1649.35.3.680. [DOI] [PubMed] [Google Scholar]
- Nichols RC, Bilbro WC., Jr The diagnosis of twin zygosity. Acta Genetica. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype–environment correlations in late childhood and early adolescence: Antisocial behavioral problems and coercive parenting. Developmental Psychology. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Hetherington EM, Reiss D, Plomin R. A twin-sibling study of observed parent–adolescent interactions. Child Development. 1995;66:812–829. [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown L. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Patterson, G. R. (1982). Coercive family process. Eugene, OR: Castalia.
- Patterson GR, DeBaryshe BD, Ramsey E. A developmental perspective on antisocial behavior. American Psychologist. 1989;44:329–335. doi: 10.1037//0003-066x.44.2.329. [DOI] [PubMed] [Google Scholar]
- Patterson, G. R., Reid, J. B., & Dishion, T. J. (1998). Antisocial boys. In J. M. Jenkins & K. Oatley (Eds.), Human emotions: A reader (pp. 330–336). Malden, MA: Blackwell.
- Pedersen NL, Spotts EL, Cederblad M, Lichtenstein P, Hansson K, Neiderhiser JM, Elthammar O, Reiss D. Lack of effects of intrapair contact and aspirations to be similar on twin similarity for personality, marital adjustment, and parenting [Abstract] Behavior Genetics. 1999;29:365–366. [Google Scholar]
- Perusse D, Neale MC, Heath AC, Eaves LJ. Human parental behavior: Evidence for genetic influence and potential implications for gene–culture transmission. Behavior Genetics. 1994;24:327–335. doi: 10.1007/BF01067533. [DOI] [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington EM, Reiss D, Plomin R. Family environment and adolescent depression and antisocial behavior: A multivariate genetic analysis. Developmental Psychology. 1996;32:590–603. [Google Scholar]
- Plomin, R. (1994). Genetics and experience: The interplay between nature and nurture (Vol. 6). Thousand Oaks, CA: Sage.
- Plomin R, DeFries JC, Loehlin JC. Genotype–environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Pedersen NL, Nesselroade JR, Bergeman CS. Genetic influences on adults’ ratings of their current family environment. Journal of Marriage and the Family. 1989;51:791–803. [Google Scholar]
- Plomin R, Reiss D, Hetherington EM, Howe GW. Nature and nurture: Genetic contributions to measures of the family environment. Developmental Psychology. 1994;30:32–43. [Google Scholar]
- Reiss D, Cederblad M, Pedersen NL, Lichtenstein P, Elthammer O, Neiderhiser JM, Hansson K. Genetic probes of three theories of maternal adjustment: Recent evidence and a model. Family Process. 2001;40:247–259. doi: 10.1111/j.1545-5300.2001.4030100247.x. [DOI] [PubMed] [Google Scholar]
- Reiss, D., Neiderhiser, J. M., Hetherington, E. M., & Plomin, R. (2000). The relationship code: Deciphering genetic and social influences on adolescent development. Cambridge, MA: Harvard University Press.
- Reiss, D., Plomin, R., Hetherington, E. M., Howe, G. W., Rovine, M., Tryon, A., & Hagan, M. S. (1994). The separate worlds of teenage siblings: An introduction to the study of the Nonshared Environment and Adolescent Development. In E. M. Hetherington, D. Reiss, & R. Plomin (Eds.), Separate social worlds of siblings: The impact of nonshared environment on development (pp. 63–109). Hillsdale, NJ: Erlbaum.
- Rose RJ, Kaprio J. Shared experience and similarity of personality: Positive data from Finnish and American twins. Behavioral and Brain Sciences. 1988;10:35–36. [Google Scholar]
- Rowe DC. Environmental and genetic influences on dimensions of perceived parenting: A twin study. Developmental Psychology. 1981;17:203–208. [Google Scholar]
- Rowe DC. A biometrical analysis of perceptions of family environment: A study of twins and singleton sibling kinships. Child Development. 1983;54:416–423. [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype–environment effects. Child Development. 1988;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, Carlier M. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behavior Genetics. 1996;26:55–63. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Stattin H, Kerr M. Parental monitoring: A reinterpretation. Child Development. 2000;71:1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- Tambs K, Harris JR, Magnus P. Sex-specific causal factors and effects of common environment for symptoms of anxiety and depression in twins. Behavior Genetics. 1995;25:33–44. doi: 10.1007/BF02197240. [DOI] [PubMed] [Google Scholar]
- Tanaka, J. S. (1993). Multifaceted conceptions of fit in structural equation models. In K. A. Bollen & J. S. Long (Eds.), Testing structural equation models (pp. 10–39). Newbury Park, CA: Sage.
- Towers H, Spotts EL, Neiderhiser JM. Genetic and environmental influences on parenting and marital relationships: Current findings and future directions. Marriage and Family Review. 2001;33:11–29. [Google Scholar]
- Williams LJ, Holahan PJ. Parsimony-based fit indices for multiple-indicator models: Do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]


