Abstract
A highly lipophilic polyion complex [Pt(en)2][PtCl2(en)2](1)4 (en, 1,2-diaminoethane) is prepared from one-dimensional mixed valence PtII/PtIV complex and newly designed chiral amphiphile 1. The powdery sample showed purple color, which is a result of the mixed valence absorption of the linear chlorobridged complex (PtII-Cl-PtIV-Cl-)n. When the lipid complex is dispersed in dichloromethane, purple-colored dispersion is obtained at 0°C, whereas the color disappears after heating the solution to 21°C. The observed thermochromism is reversible with respect to the temperature changes and is ascribed to the reversible dissociation and reassembly of the self-assembling inorganic wires. Casting of the 0°C-purple dispersion on solid substrates affords honeycomb nanostructures in addition to the nanowires with the width of about 20 nm. The honeycomb patterns seem to be templated by the condensed water droplets that are formed and aligned on the rapidly evaporating dichloromethane solution. On the other hand, more regular honeycomb structures are exclusively obtained by casting the 21°C-colorless solution. These observations indicate that the ordered honeycomb structures are obtainable on solid surfaces by the self-assembly of molecularly dispersed components [Pt(en)2](1)2 and trans-[PtCl2(en)2](1)2. Very interestingly, formation of double-layered honeycomb nanostructure is observed by scanning electron microscopy. The unit hexagons and pillars of the honeycombs are made of nanowires that are hierarchically assembled from the lipid-packaged PtII/PtIV complexes. The surface self-organization of lipophilic inorganic complexes has a potential to fabricate novel nanoarchitectures with conjugated electronic structures.
The design and formation of metal-ion-directed supramolecular assemblies have been one of the active areas in supramolecular chemistry (1–5). Finite nanosized objects with well defined shapes, such as catenanes (1), knots (1), helicates (3, 4), and nanoboxes (5), have been successfully prepared from the appropriately designed ligands and metal ions. As they usually consist of discrete metal complexes, their structural characterization and structure-related functions such as host–guest binding have been the focus of attention. On the other hand, one of the important challenges in the next nanochemistry includes development of self-assembling molecular- or nanoscale electronic devices. There are two critical issues that need to be addressed for the materialization of such systems. First, self-assembling molecular wires or nanowires are required as the basic structural elements of these devices, with the capability of controlling their electronic states at the molecular level. Second, ability to fabricate technologically useful architectures such as two-dimensional nanopatterns should be developed on the basis of a nonlithographic self-organization process.
We recently have developed soluble nanowires that are self-assembled from the amphiphilic pairs of linear platinum complexes and anionic amphiphiles (6–10). Quasi-one-dimensional, halogen-bridged mixed valence complexes [PtII(en)2][PtIVX2(en)2](ClO4)4 (en = 1,2-diaminoethane; X = Cl, Br, or I) are comprised of parallel-aligned infinite chlorobridged chains of (PtII-Cl-PtIV-Cl-)n and they have been attracting much interest because of the unique physicochemical properties such as intense intervalence charge-transfer (CT; PtII/PtIV→PtIII/PtIII) absorption (11), semiconductivity (12), and large third-order nonlinear optical susceptibilities (13). These linear perchlorate complexes are not soluble in organic solvents and when they are dispersed in water, one-dimensional structures in the solid state are disrupted and dissociate into molecular complexes. In contrast, the lipid-packaged assemblies [Pt(en)2][PtCl2(en)2](lipid)4 are soluble in organic solvents with the maintenance of one-dimensional structures (6–10). They display thermal dissociation into molecular components and reversibly reassemble into the original conjugated nanowires (7, 8). This unique feature satisfies the first condition required for the self-assembling molecular electronics (10). Here, we report that newly developed supramolecular complex [Pt(en)2][PtCl2(en)2](1)4 meets the second criteria and forms pillared honeycomb nanoarchitectures on solid surfaces. Solution characteristics of [Pt(en)2][PtCl2(en)2](1)4 and its influence on the self-assembly of regular stereoarchitectures are also discussed.
Materials and Methods
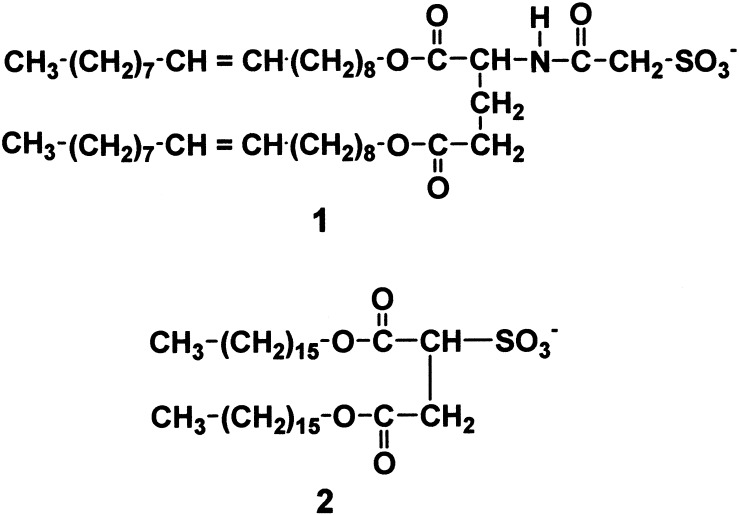
Amphiphile 1 was synthesized by BOP-Cl (N,N-bis(2-oxo-3-oxazolidinyl)-phosphinic chloride), the catalyzed condensation of l-glutamate dioleyl ester and sulfoacetic acid (Fig. 1). l-Glutamate dioleyl ester was synthesized according to the reported procedure (14). The structures of the intermediates and the final product were confirmed by thin layer chromatography, IR, and NMR spectroscopies and elemental analysis. [PtII(en)2]Cl2, trans-[PtIVCl2(en)2]Cl2, and [Pt(en)2][PtCl2(en)2](ClO4)4 were prepared according to the literature (15). Organic solvents used were of spectral grade (Kishida Chemical).
Figure 1.
Chemical structure of sulfonate amphiphiles.
Aqueous dispersion of anionic amphiphile 1 (Na+ salt, 10 mM, 20 ml) was prepared by ultrasonication (Branson Sonifier Model 185, sonic power 45W, 5 min). It was added to equimolar solution containing [Pt(en)2][PtCl2(en)2](ClO4)4 in deionized water ([Pt]total = 10 mM, 4 ml). Purple precipitate formed at room temperature was kept in the aqueous mixture for 12 h. The precipitate was collected by centrifugation (8,000 rpm, 5°C) and washed with pure water to remove sodium perchlorate. The sample was then dried in vacuo. Yield, 140 mg (76%). [Pt(en)2][PtCl2(en)2](1)4, Anal. Calcd. for C180H344N12O32S4Cl2Pt2: C, 56.41; H, 9.05; N, 4.38%. Found: C, 55.87; H, 9.10; N, 4.38%. The colored complexes were dissolved in chloroform and dichloromethane and gave homogeneous solutions (concentration, 0.6 unit mM). These solutions were kept at 0°C for 12 h before the spectral measurements. UV-visible and CD spectra were obtained by using a Jasco (Tokyo) V-570 and a J-725G, respectively. Temperature dependence of the spectra was recorded after keeping the dispersions at a given temperature for 1 h. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were conducted on a Hitachi (Tokyo) H-600 instrument (75 kV) and on an S-5000 instrument (25 kV), respectively. Surface nanostructures were prepared by placing a drop of dichloromethane solution (0.6 mM) on carbon-coated TEM grids. Electron microscopy was conducted without staining.
Results and Discussion
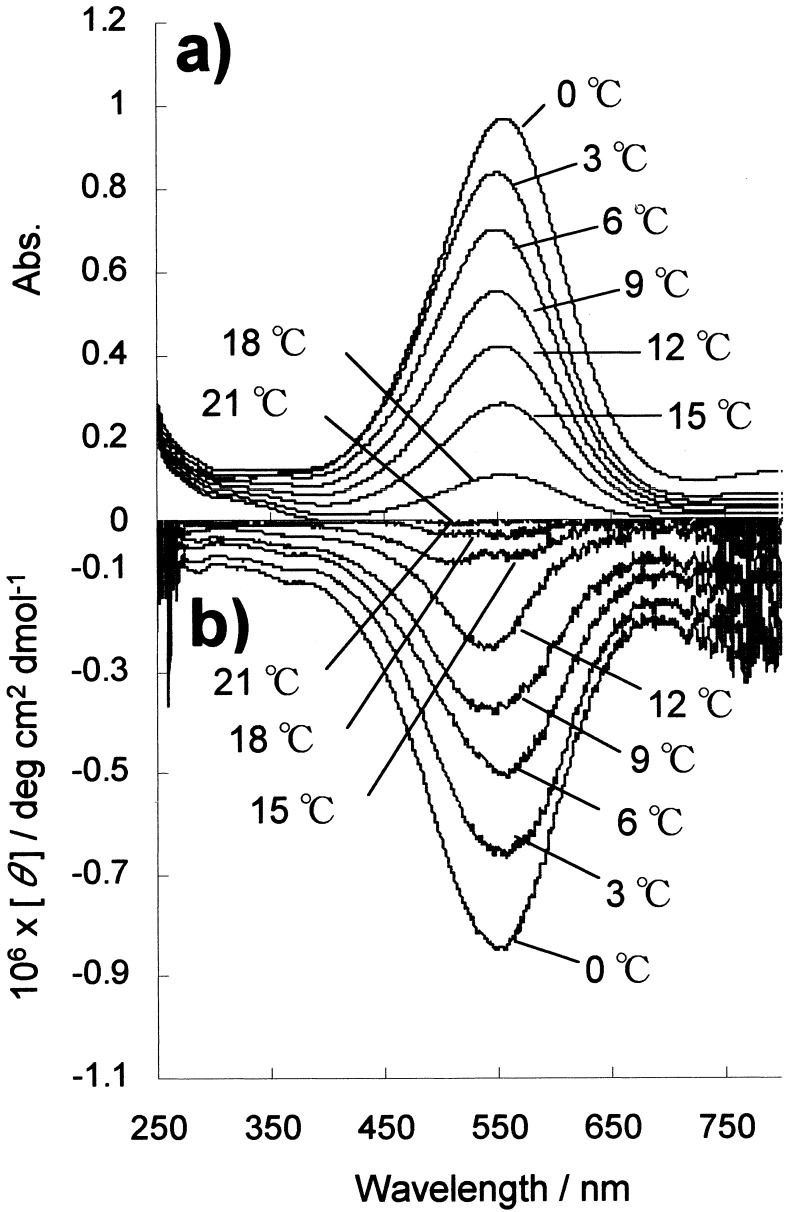
The oleyl chains are introduced in the l-glutamate amphiphile 1, because they act as superior solvophilic groups in organic media (16). The ternary complex of [Pt(en)2][PtCl2(en)2](1)4 showed purple color in the powdery form and it was easily dispersed in organic solvents such as chloroform and dichloromethane. When it was dispersed in these solvents at room temperature (0.6 unit mM), the intense color of the solid disappeared. As the CT transition requires the existence of chlorobridged extended coordination structure (11), the observed fading of the colors at room temperature indicates that the one-dimensional complex structures are not maintained and they are dissociated into component complexes of [Pt(en)2](1)2 and [PtCl2(en)2](1)2. On the other hand, when these solutions were cooled to 0°C, the purple color was restored for the dichloromethane solution. Fig. 2 a and b shows temperature dependence of UV-visible spectra and CD spectra of [Pt(en)2][PtCl2(en)2](1)4 in dichloromethane. The dispersion was first cooled to 0°C, and then the temperature was raised slowly. A typical CT absorption is observed at 550 nm at 0°C (Fig. 2a; ɛ: 16,100 units M−1 cm−1), and this peak is almost comparable to that observed for the nanoassemblies of [Pt(en)2][PtCl2(en)2](2)4 in chloroform (λmax at 580 nm, ɛ: 17,900 units M−1 cm−1, 20°C) (8). The maintenance of the CT absorption indicates that [Pt(en)2][PtCl2(en)2](1)4 is dispersed as amphiphilic polyion complexes with the solvophobic inorganic chain surrounded by the solvophilic oleyl-amphiphiles.
Figure 2.
Temperature dependence of the UV-visible spectra (a) and CD spectra (b) of [Pt(en)2][PtCl2(en)2](1)4 in dichloromethane (0.6 unit mM, 1 mm cell). Spectra were recorded after keeping the dispersion at the given temperatures for 1 h.
These CT absorption maxima are considerably red-shifted compared with that reported for the single crystal of [Pt(en)2][PtCl2(en)2](ClO4)4 (λmax at 456 nm, 2.72 eV) (17). The CT excitation energy of halogen-bridged, mixed valence Pt(en)2 polymers in the crystalline state has been related to the PtII-PtIV distance in a chain and the HOMO-LUMO energy gap (Peierls gap), which is caused by the off-center displacement of halogen ions (11). We have supposed the possibility of increased Pt(II)-Pt(IV) distance as a factor for the red-shifted CT absorption (8), but it seems more reasonable that the observed red shift is ascribed to the enhanced delocalization of the excited Pt(III)-Pt(III) states in the coordination chain, which decreases the energy gap in the one-dimensional electronic structure. This would probably occur as a consequence of the contraction in the PtII-PtIV distance and the decreased off-center displacement of bridging chloride ions (i.e., decrease in Peierls distortion). We are now in preparation of the single crystals to investigate their x-ray crystallography. It is interesting that the lipid anions affect the intervalence electron transfer characteristics by shortening the PtII-PtIV distances. These observations indicate that the lipid counterions not only give solubility to the one-dimensional complex in organic media but also play a decisive role in determining their electronic structures.
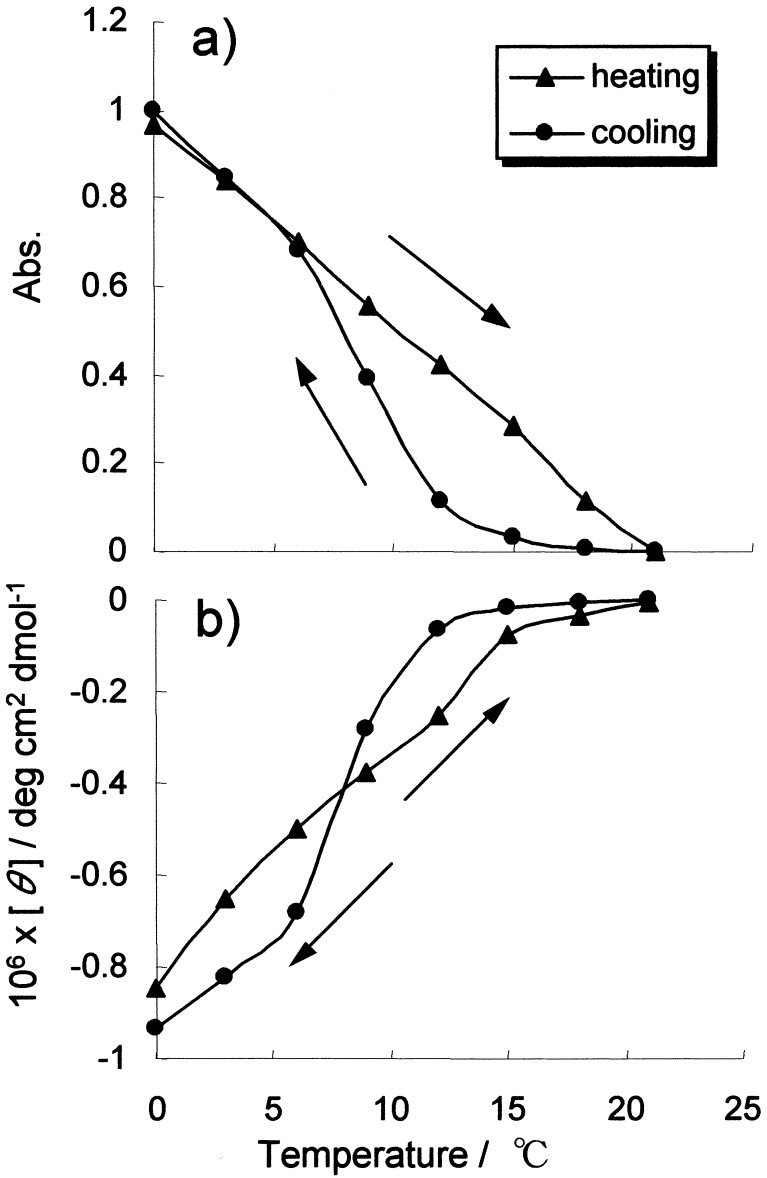
In the CD spectra, an intense cotton effect was observed at the CT absorption band (Fig. 2b, [θ]550 = −8.5 × 105 deg cm2 dmol−1 at 0°C). As the chlorobridged Pt complex is achiral, the observed CD is apparently induced by the associating chiral amphiphiles. After heating the dispersion, intensities of the CT absorption and the induced CD at 550 nm are simultaneously weakened and they disappeared completely at 21°C. The temperature dependence of CT absorption intensity and [θ]550 value are shown in Fig. 3 a and b. These spectral intensities are decreased almost linearly with the increase in temperature, but they reversibly recovered the original intensities after cooling the dispersions to 0°C. The observed thermochromism can be repeated many times, and thus the ternary complex of [Pt(en)2][PtCl2(en)2](1)4 displays reversible self-assembling characteristics in organic media. In the case of [Pt(en)2][PtCl2(en)2](2)4, which contains saturated-alkylchain-lipids, disappearance of the CT absorption peak occurred at the higher temperature of about 60°C in chloroform (8). Apparently, the presence of cis-double bond in the oleyl group provides higher lipophilic nature to the ternary complex and it lowered the thermal stability of the chlorobridged complex in solution.
Figure 3.
Temperature dependence of absorbance (a) and induced CD peak intensity (b) at 550 nm.
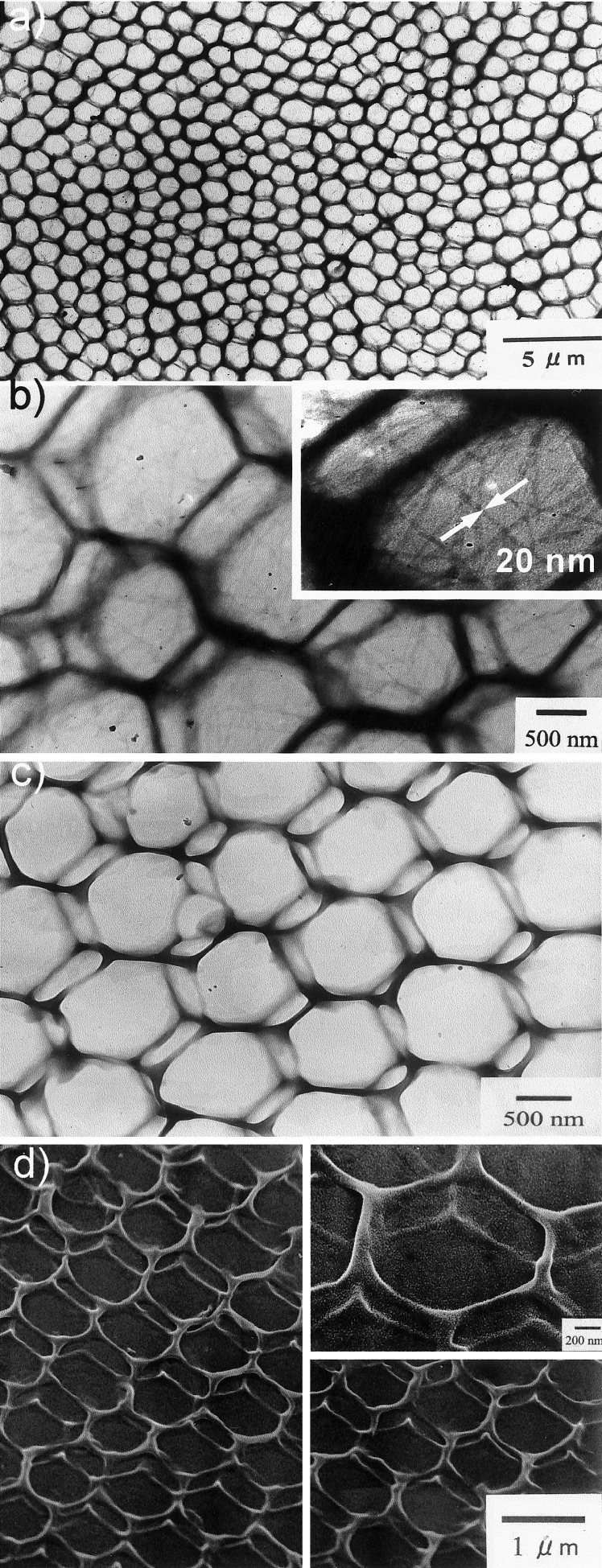
The dichloromethane solutions of [Pt(en)2][PtCl2(en)2](1)4 (temperatures, 0 and 21°C) were dropped on varied solid substrates and were observed by TEM and SEM. Fig. 4 (a–d) shows TEM and SEM images of [Pt(en)2][PtCl2(en)2](1)4 dropped on carbon-coated TEM grids. To our surprise, when the purple dichloromethane dispersion of [Pt(en)2][PtCl2(en)2](1)4 cooled at 0°C was dropped on the TEM grid at room temperature, honeycomb structure was observed in a wide area (Fig. 4a). As the sample is not stained, the dark honeycomb walls are comprised of the chlorobridged mixed valence complex [Pt(en)2][PtCl2(en)2](1)4. The magnified TEM image (Fig. 4b and Inset) shows the presence of nanowires (width, about 20 nm) in the central voids, in addition to the honeycomb structure. The observed nanowire-width is larger than the bimolecular length of amphiphile 1 (about 6 nm by Corey–Pauling–Koltun molecular model), and we presume that the nanowires consist of aggregates of supramolecular polyion complexes [Pt(en)2][PtCl2(en)2](1)4 as discussed later. It is likely that these nanowires have gathered into the honeycomb patterns during the solvent evaporation process.
Figure 4.
TEM (a–c) and SEM (d) images of the cast films of [Pt(en)2][PtCl2(en)2](1)4 dropped from dichloromethane solution (0.6 unit mM). Temperature of the dichloromethane stock solution: 0°C (a and b) and 21°C (c and d). In the preparation of samples (c and d), the TEM grid was cooled to 0°C.
The observed honeycomb morphology is reminiscent of the microporus films formed in the solvent evaporation of copolymers (18–20), conjugated polymers (21, 22), polyion complexes (23), and fluorinated silver nanoparticles (24). In these systems, water droplets condensed from moisture on the evaporating solutions act as the template to direct formation of the honeycomb patterns. A similar mechanism is likely to be involved in this case.
To selectively obtain the regular honeycomb structures, the colorless dichloromethane solution of [Pt(en)2][PtCl2(en)2](1)4 kept at 21°C was dropped on a TEM grid and a quartz plate that were cooled at 0°C. A purple-cast film was formed immediately after evaporation of the solvent on the quartz plate, and the film showed absorption λmax of 554 nm. As the one-dimensional complex is not maintained in the colorless stock solution, polymerization of [Pt(en)2](1)2 and [PtCl2(en)2](1)2 complexes must have proceeded, and the linear mixed valence chains are formed during the solvent evaporation. In TEM, regular honeycomb networks were exclusively observed (Fig. 4c, one side of the hexagons, about 650–750 nm; width of the wires, about 100 nm) with no nanowires in the central voids. Apparently, the honeycomb structure in this case is self-assembled from the molecular complexes of [Pt(en)2](1)2 and [PtCl2(en)2](1)2, and the absence of randomly spread nanowires indicates that the polymerization is highly concerted with the solvent evaporation process.
As the TEM images are indicative of the presence of double-layered honeycomb patterns, the sample TEM grid was observed further by SEM (Fig. 4d). The sample was placed against an electron beam at an angle of 45°, and the observed SEM image clearly shows the presence of regular, double-layered honeycomb architecture. The top honeycomb layer is connected to the basal honeycomb layer by means of perpendicularly oriented pillars (height, about 320–370 nm) at the corner of hexagons (Fig. 4d Inset). Self-assembling characteristics of [Pt(en)2][PtCl2(en)2](1)4 in the course of rapid solvent evaporation seem to be essential factors for the formation of such unique stereo-nanostructures. When purple chlorocyclohexane dispersion of [Pt(en)2][PtCl2(en)2](1)4 is dropped on a TEM grid, only nano- to microcrystalline aggregates originally dispersed in the solution were deposited (data not shown). Apparently, casting of preformed aggregates from slowly evaporating solvents provides no surface architectures. On the other hand, dropping of colorless chloroform solution left irregular honeycomb structures behind (data not shown), and these observations suggest that the mechanism of regular stereoarchitecture formation involves a combination of complicated thermodynamics and kinetically controlled transport and growth of polymeric coordination chains.
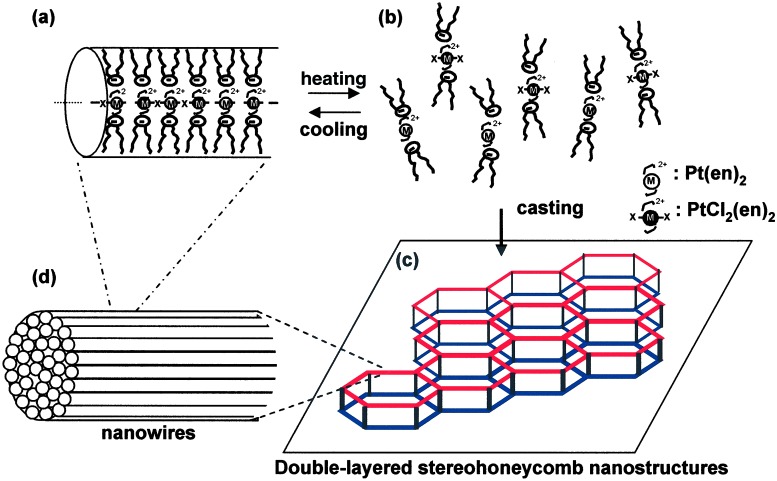
Fig. 5 a and b schematically illustrates the thermal dissociation-reassembly process of the unit supramolecular assembly [Pt(en)2][PtCl2(en)2](1)4 in dichloromethane. At 0°C, lipophilic nanowires with the width of about 20 nm are formed from the aggregates that consist of the chlorobridged mixed valence main-chain and oleyl-amphiphile 1 (Fig. 5a). The high solubility of the supramolecular complex is brought by the surrounding lipophilic oleyl chains. After heating to 21°C, the coordination chains dissociate into the molecular component complexes (Fig. 5b). The casting of dissociated molecular complexes (Fig. 5b) on solid substrates affords the double-layered honeycomb nanoarchitectures (Fig. 5c). The frames of honeycombs consist of nanowires that are hierarchically self-assembled from the molecular wires of [Pt(en)2][PtCl2(en)2](1)4.
Figure 5.
Schematic illustration of the self-assembling characteristics of [Pt(en)2][PtCl2(en)2](1)4 in dichloromethane solution (a and b) and formation of the stereo-nanoarchitectures on the solid surface (c).
It is very interesting that the double-honeycomb networks are formed by the conjugated inorganic complexes. To the best of our knowledge, this is the first example of surface stereoarchitectures self-assembled from the supramolecular assemblies. The directional polymerization of the one-dimensional complex and its tendency to form bundled nanowires during the solvent evaporation process seem to provide such a unique spatial architecture rather than giving the conventional, continuous microporous films.
Conclusion
The present results provide evidence and illustration for the hierarchical self-organization of double-honeycomb nanoarchitectures formed by lipid-packaged mixed valence platinum complexes. Self-assembling molecular wires are thus transformed into the unique three-dimensional architecture that is expended to the level of micrometric dimensions. The present approach bridges the gap among the molecular wire research, inorganic chemistry, and molecular electronic devices. It provides access to the controlled surface nanoarchitectures of the electronically conjugated inorganic materials and opens a new dimension in supramolecular chemistry.
Acknowledgments
We thank Profs. S. Furusaki and M. Goto (Kyushu University) for the use of JASCO J-725G, and Dr. H. Matsune and Mr. T. Nakashima for their skillful technical assistance in the TEM and SEM observations. This work was supported by grant-in-aid for Centers of Excellence research “Design and Control of Advanced Molecular Assembly Systems” (no. 08CE2005) from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese Government.
Abbreviations
- en
1,2-diaminoethane
- CT
charge transfer
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chambron J-C, Buchecker C D, Sauvage J-P. In: Comprehensive Supramolecular Chemistry. Atwood J A, Davies J E D, MacNicol D D, Vögtle F, Lehn J-M, editors. Vol. 9. New York: Elsevier Science; 1996. pp. 43–83. [Google Scholar]
- 2.Fujita M, Ogura K. Bull Chem Soc Jpn. 1996;69:1471–1482. [Google Scholar]
- 3.Lehn J-M. Angew Chem Int Ed Engl. 1990;29:1304–1319. [Google Scholar]
- 4.Hopfgartner G. Chem Rev. 1997;97:2005–2062. doi: 10.1021/cr960053s. [DOI] [PubMed] [Google Scholar]
- 5.Yamanoi Y, Sakamoto Y, Kusukawa T, Fujita M, Sakamoto S, Yamaguchi K. J Am Chem Soc. 2001;123:980–981. doi: 10.1021/ja003043o. [DOI] [PubMed] [Google Scholar]
- 6. Kimizuka, N., Oda, N. & Kunitake, T. (1998) Chem. Lett., 695–696.
- 7.Kimizuka N, Lee S H, Kunitake T. Angew Chem Int Ed Engl. 2000;39:389–391. [PubMed] [Google Scholar]
- 8.Kimizuka N, Oda N, Kunitake T. Inorg Chem. 2000;39:2684–2689. doi: 10.1021/ic000189f. [DOI] [PubMed] [Google Scholar]
- 9.Kimizuka N, Yamada K, Kunitake T. Mol Cryst Liq Cryst. 2000;342:103–110. [Google Scholar]
- 10.Kimizuka N. Adv Mater. 2000;12:1461–1463. [Google Scholar]
- 11.Okamoto H, Yamashita M. Bull Chem Soc Jpn. 1998;71:2023–2039. [Google Scholar]
- 12.Hamaue Y, Aoki R, Yamashita M, Kida K. Inorg Chim Acta. 1981;54:L13–L14. [Google Scholar]
- 13.Iwasa Y, Funatsu E, Hasegawa Y, Koda T, Yamashita M. Appl Phys Lett. 1991;59:2219–2221. [Google Scholar]
- 14. Kimizuka, N., Takasaki, T. & Kunitake, T. (1988) Chem. Lett., 1911–1914.
- 15.Basolo F, Bailar J C, Tarr B R. J Am Chem Soc. 1950;72:2433–2438. [Google Scholar]
- 16.Ishikawa Y, Kuwahara H, Kunitake T. J Am Chem Soc. 1994;116:5579–5591. [Google Scholar]
- 17.Wada Y, Mitani T, Yamashita M, Koda T. J Phys Soc Jpn. 1985;54:3143–3153. [Google Scholar]
- 18.Widawski G, Rawiso M, François B. Nature. 1994;369:387–389. [Google Scholar]
- 19.Jenekhe S A, Chen X L. Science. 1999;283:372–375. doi: 10.1126/science.283.5400.372. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Cho B-K, Ihn K J, Lee W-K, Oh N-K, Zin W-C. J Am Chem Soc. 2001;123:4647–4648. doi: 10.1021/ja004071+. [DOI] [PubMed] [Google Scholar]
- 21.Govor L V, Bashmakov I A, Kiebooms R, Dyakonov V, Parisi J. Adv Mater. 2001;13:588–591. [Google Scholar]
- 22.de Boer B, Stalmach U, Nijland H, Hadziioannou G. Adv Mater. 2000;12:1581–1583. [Google Scholar]
- 23.Karthaus O, Maruyama N, Cieren X, Shimomura M, Hasegawa H, Hashimoto T. Langmuir. 2000;16:6071–6076. [Google Scholar]
- 24.Yonezawa T, Onoue S, Kimizuka N. Adv Mater. 2001;13:140–142. [Google Scholar]