Abstract
Life could not exist without motion induced by a variety of molecular motors. The construction of artificial motors by chemical synthesis, which can power motions that lead to macroscopic detectable effects in a system, is a major endeavor in contemporary science. To move toward this goal, a host–guest system, composed of a nematic liquid crystal film doped with a chiral light-driven molecular motor, is assembled. Irradiation of the film results in unidirectional rotary motion of the molecular motor, which induces a motion of the mesogenic molecules leading to a molecular reorganization and, as a consequence, a change in the color of the film. In this way, by control of the rotary motion at the molecular level, color tuning over the entire visible spectrum is achieved. These findings demonstrate that a molecular motor can exert a visually observable macroscopic change in a material.
Inspired by nature's nanomachinery, there is great current interest in the design of molecular systems that mimic motor functions and are capable of performing linear or rotary movement (1–4). Among the fascinating structures designed to induce mechanical motions are shuttles, muscles, ratches, pseudorotaxanes, and switches (5–12). Recently, the first examples of unidirectional rotary motors were reported (13, 14), and it was shown that the rate of the rotary motion in the light-driven motors could be tuned by structural modification (15). A major challenge is to use a molecular motor for a practical application by exerting a macroscopic change in system (16, 17). Such an application implies that the rotary motion at the molecular level should induce a mechanical effect in the system sufficiently large to allow easy detection by a change in macroscopic properties.
We report here a light-driven rotary motion in liquid crystalline (LC) films that induces a reorganization of the mesogenic molecules, as observed by visual inspection of the change in color of the LC film. Besides a potential information storage system with nondestructive read-out capabilities, these findings demonstrate the possibility of using the light-driven motor to tune the color gradation of an LC device over the entire visible spectrum by light irradiation.
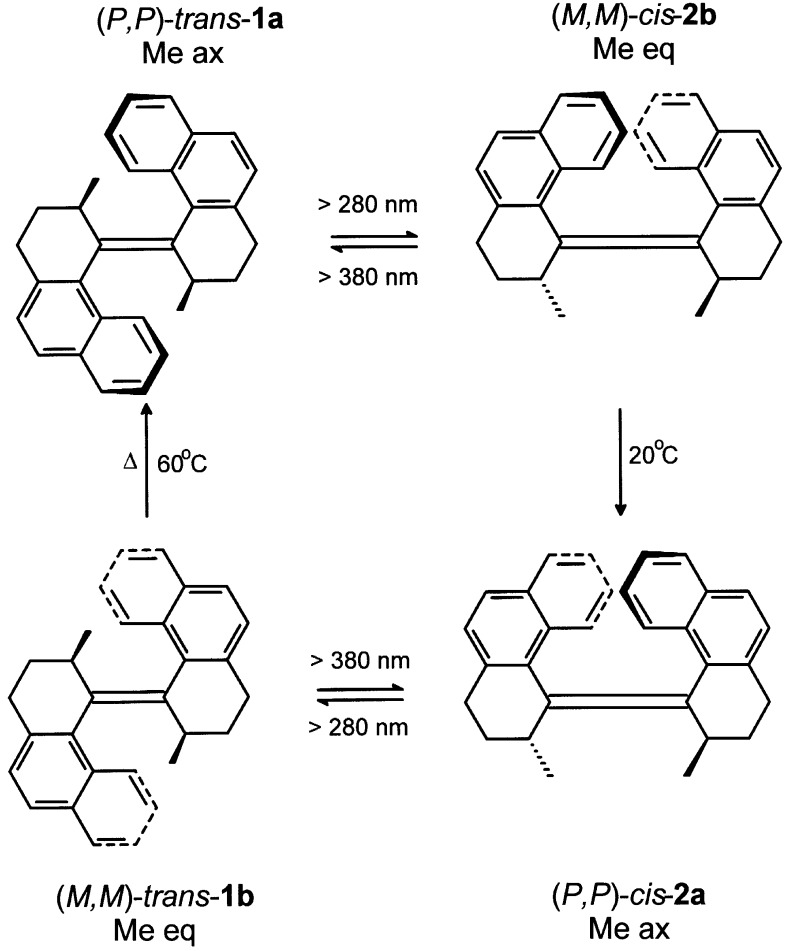
After extensive studies on the thermal and photochemical isomerization processes of biphenanthrylidenes (18–21), we could demonstrate that the intrinsic chirality and dynamic properties associated with chiroptical molecular switches can be used to accomplish unidirectional rotary motion. With sterically overcrowded alkene [(3R,3′R)-(P,P)-trans-1,1′,2,2′,3,3′,4,4′-octahydro-3,3′-dimethyl-4,4′-biphenanthrylidene; 1a], where the two methyl substituents because steric effects adopt an energetically favored axial orientation, it is possible to induce a light-driven full 360° rotation of one (rotor) half of the molecule relative to the other (stator) half in a unidirectional fashion (Scheme S1; refs. l and 13). The complete rotary process involves four steps combining two energetically up-hill photochemically induced isomerization steps with two energetically down-hill thermal helix inversion steps.
Scheme 1.
Four-step unidirectional rotation cycle of molecular motor 1a, 2b, 2a, and 1b.
The control elements that govern the unidirectional rotation are the helicity of the overcrowded alkene, the absolute configuration of the stereogenic centers [(3R,3′R) for clockwise rotation], and the conformational flexibility of the rings in the vicinity of the central olefinic bond. The driving forces in this rotary process are the photoinduced isomerizations (P,P),-trans-1a to (M,M)-cis-2b and (P,P)-cis-2a to (M,M)-trans-1b, where, by the nature of the process, in both cases the methyl substituents are forced to adopt an energetically unfavorable equatorial orientation. Helix inversions of unstable (M,M)-cis-2b and unstable (M,M)-trans-1b release the energy in a unidirectional process to form the stable isomers (P,P),-cis-2a and (P,P)-trans-1a, respectively, with axially oriented methyl groups again. The direction of this rotation is controlled solely by the configuration at the stereogenic centers. This first example of a unidirectional light-driven molecular motor allows repetitive controlled 360° rotations.
In the development of advanced molecular machinery, retention of the photochemical properties when the molecular motor is incorporated in, for example, a polymeric or liquid crystalline matrix, organized on a surface, or becomes part of a supramolecular assembly, is an important issue. In the present study, we focus on using the light-driven motor to control the organization of an LC matrix and, in the reverse sense, employing the changes in an LC matrix to visualize rotary motion at the molecular level.
Photochemically tunable doped cholesteric liquid crystals are promising materials for LCD application (22, 23), and full-color control by adjusting the pitch of a cholesteric phase in a reversible manner by irradiation is particularly challenging (24–26). It is well known that the organization in the LC matrix is usually sensitive to the nature of (chiral) dopants present (27). Based on this property, a variety of photochromic compounds have been used to alter the alignment of LC phases upon irradiation (22, 23, 28, 29). It has been demonstrated that switching between positive and negative cholesteric phases could be accomplished by using chiroptical molecular switches (28–30). Cholesteric LC films show reflection at a specific wavelength (λ) dependent on the pitch (p), the angle of the incident light (α), and the average refractive index (n):
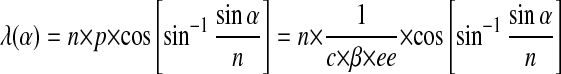 |
The pitch of a doped cholesteric phase is inversely proportional to the concentration (c), enantiomeric excess (ee), and helical twisting power (β) of the chiral dopant. The helical twisting power is an intrinsic property of any chiral dopant, and indicates how efficiently this molecule induces a chiral orientation in the LC material. When the pitch is in the range of the wavelength of visible light, a well defined, bright reflection and a distinct color are observed. Requirements for reversible light-induced color tuning in rewritable color-image formation are stability and reversibility, high helical twisting powers (β) to reach the appropriate pitch, and reflection in the visible spectrum—as well as sufficiently large changes in β-values upon irradiation.
Materials and Methods
Materials.
4-Pentyloxy-4′-biphenylcarbonitrile (M15) was obtained from Aldrich, Zwijndrecht, The Netherlands, and was used without prior purification. This compound shows nematic liquid crystalline behavior in the range of 48–67°C. Nematic liquid crystal E7, which is a mixture of different mesogenic compounds, was obtained from Merck, Darmstadt, Germany, and was used without prior purification. This mixture is designed to be liquid crystalline at room temperature (RT). Molecular motor (3R,3′R)-(P,P)-trans-1,1′,2,2′,3,3′,4,4′-octahydro-3,3′-dimethyl-4,4′-biphenanthrylidene 1a was prepared as reported (20). Enantiomerically pure (P,P)-trans-1a (P,P)-cis-2a and (M,M)-trans-1b were fully characterized and described (13, 20, 31, 32).
Alignment of LC Layers.
For all measurements described here, aligned cholesteric phases were obtained in the following way. A glass surface (typically 6.25 cm2) was spin-coated with commercially available polyimide AL1051 (JSR, Leuven, Belgium). The coated samples were allowed to harden at 170°C in vacuum for 3 h. The surface then was linearly rubbed with a velvet cloth to induce a parallel-alignment in the LC samples. The liquid crystalline material doped with the appropriate amount of enantiomerically pure 1a was dissolved in toluene (typically, 1 ml of toluene was used per 5 mg of sample) and slowly poured onto the aligned surface. For the M15-doped material, the surface was heated to 50°C, whereas for the E7-doped material, RT was sufficient. After slow evaporation of the toluene at the appropriate temperature, an aligned LC film was obtained that was suitable for measurement of reflection wavelengths.
Measurement of Cholesteric Pitches and Reflection Wavelengths.
Cholesteric pitches were determined with the Grandjean-Cano technique (33). For this purpose, the aligned sample was prepared as described above and covered with a plane-convex lens of known radius (Linos Components; Radiometer, The Netherlands). Examination of the samples through a polarization microscope showed concentric rings whose relative radii could be correlated to the pitch of the material. The sign of the cholesteric phases was determined with a contact method (34), where mixing of the samples with a doped cholesteric liquid crystal of known negative screw sense, consisting of dopant ZLI-811 (Merck, Darmstadt, Germany) in the appropriate liquid crystalline host, was tested. Reflection measurements were performed on a Jasco J715 Spectrophotometer (Jasco, Maarssen, The Netherlands) equipped with a fluorescence extension (a photomultiplier perpendicular to the direction of the light). This spectrophotometer was adapted to hold LC-covered glass plates in such a way that both the incident light beam as well as the photomultiplier tube were at an angle of 45° to the surface. Actual color photographs of the aligned cholesteric structures were taken with a Minolta 404Si single-lens reflex camera perpendicular to the LC-covered surface.
Irradiation and Analysis.
Irradiations were performed with a 180-W Oriel Hg-lamp adapted with a Pyrex filter to obtain light with a wavelength longer than 280 nm. Ratios of the different forms of the molecular motor were determined by using HPLC on a silica column (5 μm, 250 × 4.6 mm; Econosphere Silica) and pure n-heptane as eluent, where the three different forms that are observable at RT [(P,P)-trans-1a, (P,P)-cis-2a, and (M,M)-trans-1b] are readily separated {tR [(M,M)-trans-1b] = 11.0 min; tR [(P,P)-trans-1a] = 11.5 min; tR [(P,P)-cis-2a] = 12.2 min} from each other and the LC materials which are eluted only when n-heptane/ethyl alcohol 95/5 is used as the eluent. The ratio of the three forms was checked at their isosbestic point at 305.9 nm [for (P,P)-trans-1a and (P,P)-cis-2a] and 333.2 nm [for (M,M)-trans-1b and (P,P)-cis-2a] by PDA detection with a Waters 996 Diode Array Detector.
Results and Discussion
First, the chiral dopant properties and the helical twisting power of the motor were examined. The different chiral forms observed during a 360° rotation cycle are shown in Scheme S1. Doping of nematic 4-pentyloxy-4′-biphenylcarbonitrile (M15) with only 3.4 weight % of enantiomerically pure (3R,3′R)-(P,P)-trans-1a resulted in a stable cholesteric phase with a pitch of 390 nm. A high helical twisting power (β = +75 μm−1), as determined by the Grandjean Cano technique (33), was found for (3R,3′R)-(P,P)-trans-1a. For comparison, the β-values for the stable cis-isomer (3R,3′R)-(P,P)-cis-2a and the unstable trans-isomer (3R,3′R)-(M,M)-trans-1b in M15 were measured. Furthermore, the β-values of the three stereoisomers were determined in E7, an LC mixture which is nematic at RT. No β-value for the unstable (3R,3′R)-(M,M)-cis-2b could be determined because of the fast helix inversion of this compound forming stable (3R,3′R)-(P,P)-cis-2a at the appropriate temperatures. From the data given in Table 1, it is evident that (P,P)-trans-1a shows a high positive β-value, and a large decrease in helical twisting power is found going to (P,P)-cis-2a and (M,M)-trans-1b. It also should be noted that (P,P)-cis-2a shows positive β-values (right-handed cholesteric), and (M,M)-trans-1b shows negative β-values (left-handed cholesteric).
Table 1.
Helical twisting powers (β-values) of three forms of the molecular motor (1a, 2a, and 1b) in two different liquid crystalline hosts (M15 and E7)
| Host | β-value,
μm−1
|
||
|---|---|---|---|
| (P,P)-trans-1a | (P,P)-cis-2a | (M,M)-trans-1b | |
| M15 (50°C) | +75 | +8 | −18 |
| E7 (20°C) | +69 | +12 | −5* |
Value calculated from helical twisting power for mixtures of known composition.
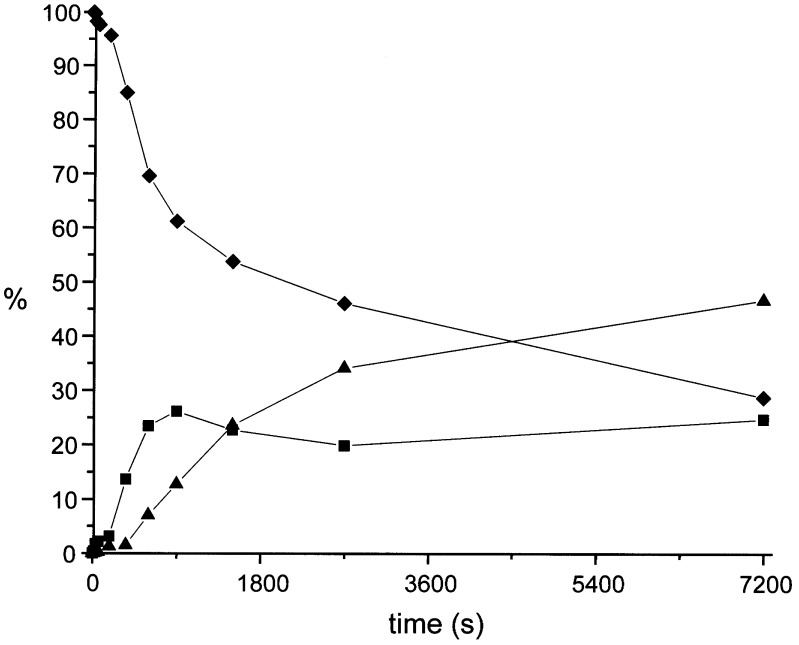
Although the light-driven motor operates efficiently in solution (13), important questions pertain to the unidirectional rotary behavior if it is embedded in an organized LC matrix. A cast thin film of E7, doped with 2.4 weight % of (P,P)-trans-1a, was irradiated at RT with an Hg-lamp at λ > 280 nm, resulting in trans-to-cis isomerization to form unstable (M,M)-cis-2b. In agreement with the observations in solution, the unstable (M,M)-cis-2b readily converts to the stable (P,P)-cis-2a isomer in the LC film at RT. Upon continued irradiation, further isomerization of (P,P)-cis-2a to (M,M)-trans-1b is observed. Although the (M,M)-trans state is unstable compared to the initial (P,P)-trans state, because of the unfavorable equatorial orientation of the methyl substituents, no interconversion takes place at ambient conditions. The progress of the isomerization processes in time in the LC phase, as monitored by chiral HPLC, is shown in Fig. 1.
Figure 1.
Percentage of the three detectable forms of the molecular motor (1a, 2a, 1b) upon irradiation of a doped thin film of 1a (2.4 weight %) in E7 at RT in time. (P,P)-trans-1a (♦), (P,P)-cis-2a (■), and (M,M)-trans-1b (▴).
As expected, the isomerization efficiency strongly depends on the thickness of the LC film. The light-driven process is less efficient under identical experimental conditions in the LC film than in solution, mainly because of the absorption of the E7 material up to 340 nm. The effective wavelength range for the photoisomerization of the dopant is, therefore, >340 nm, which causes a shift in both photoequilibria to the side of stable (P,P)-trans-1a and (P,P)-cis-2a forms. For the (P,P)-trans-1a to (M,M)-cis-2b photoisomerization (step 1 in Scheme S1), this effect is less important, considering the entire rotary process [as unstable (M,M)-cis-2b rapidly converts to (P,P)-cis-2a, shifting the photoequilibrium]. However, the second light-induced isomerization (step 3 in Scheme S1) results in a photostationary state with a 30:70 ratio of (P,P)-cis-2a and (M,M)-trans-1b, compared with a 10:90 ratio in n-hexane solution.
Next, the LC film, now containing (P,P)-trans-1a, (P,P)-cis-2a, and (M,M)-trans-1b, was heated at 60°C to allow the thermal helix inversion of unstable (M,M)-trans-1b to proceed to (P,P)-trans-1a. HPLC analysis showed that unstable (M,M)-trans-1b was quantitatively and stereoselectively converted into stable (P,P)-trans-1a in the LC matrix. This step completes the four-step clockwise rotation, demonstrating that a unidirectional rotary process is possible in an LC matrix. CD analysis of the isomers confirmed the stereochemical change during the rotary cycle.
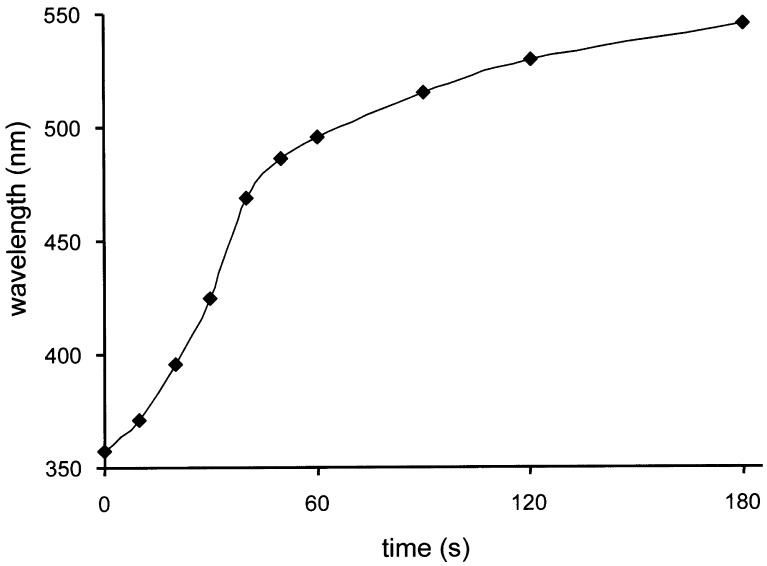
For the color tuning, spin-coated LC films on linearly rubbed polyimide-covered glass plates were used. E7 doped with 6.16 weight % (3R,3′R)-(P,P)-trans-1a results in cholesteric phase with a pitch of 234 nm and a wavelength reflection of 357 nm (for the 45° reflection). The calculated normal reflection is 393 nm, which is confirmed by the violet color of the film. Upon irradiation of this film at λ > 280 nm, a fast bathochromic shift of the reflection wavelength occurs. The time-dependent quantitative change in reflection wavelength measured at a 45° angle is presented in Fig. 2. The change can be fully accounted for by the increase in the amount of both (P,P)-cis-2a and (M,M)-trans-1b upon light-induced isomerization of the dopant together with a slight wavelength-dependent change in net refractive index (n) of the film, as is observed also for undoped E7. The bathochromic shifts reflect the rather low β-values of (P,P)-cis-2a and (M,M)-trans-1b. Accordingly, the color of the film gradually and rapidly changes from violet to red, as can be readily detected by visual inspection (Fig. 3). After heating the sample to 60°C at any time of irradiation, (M,M)-trans-1b is converted to (P,P)-trans-1a, with a concomitant hypsochromic shift of the reflection wavelength.
Figure 2.
Wavelength of reflection at a 45° angle of a molecular motor doped LC phase (6.16 weight % in E7) as a function of time, starting from pure (P,P)-trans-1a upon irradiation with >280 nm light at RT.
Figure 3.

Colors of a molecular motor doped LC phase (6.16 weight % in E7) in time, starting from pure (P,P)-trans-1a upon irradiation with >280 nm light at RT, as taken from actual photographs of the sample. The colors shown from left to right correspond to 0, 10, 20, 30, 40, and 80 s of irradiation time, respectively.
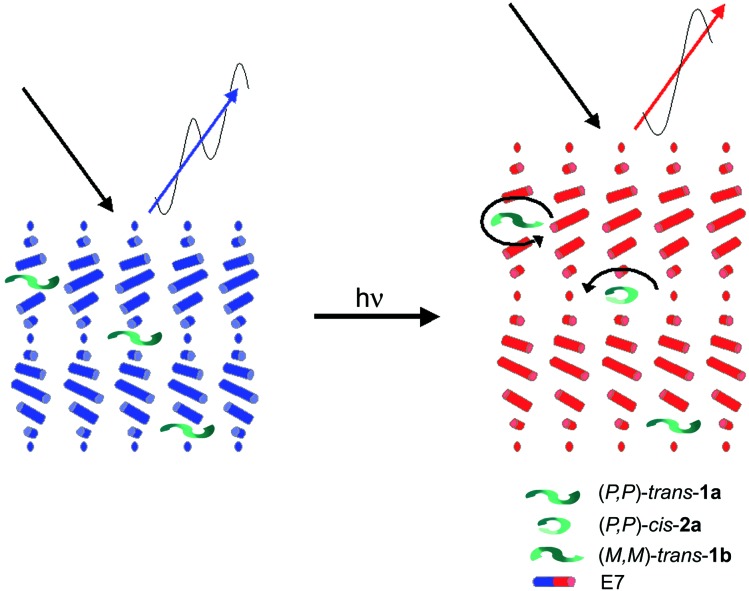
In the host–guest system, comprising a molecular motor (P,P)-trans-1a as dopant in liquid crystal host E7, the chiral guest molecule induces a cholesteric phase with a pitch leading to a reflection wavelength of 357 nm. Upon light induced unidirectional rotary motion, which leads to other stages of the motor with diminished β-values, the cholesteric arrangement expands, resulting in a larger pitch and a red-shift of the reflection wavelength (Fig. 4). By tuning the rotary motion of the molecular motor, all colors of the LC film can be generated.
Figure 4.
Schematic representation of unidirectional rotation of the guest molecular motor 1a, the induced elongation of the pitch of the LC host matrix, and the change in reflection wavelength of the light.
Conclusions
In conclusion, we have shown that unidirectional rotary motion can be performed in an LC matrix. Furthermore, the light-driven motion of the dopant induces the motion of a large ensemble of rod-like molecules during the reorganization in the LC film, which allows direct visual observation of the rotary motion. The high helical twisting power of (P,P)-trans-1a in combination with the large change in β going to the other stages makes it possible that the reflection wavelength can be tuned readily throughout the entire visible spectrum simply by changing the irradiation time. Changing light intensity or irradiation wavelength would have a similar effect. These findings not only demonstrate that a macroscopic effect, i.e., a change of the physical properties of a material (in the present case, an LC film), can be induced by a rotary molecular motor, but also that color pixels in an LC film can be generated by using this supramolecular approach. The mechanism of the pitch increase in the LC phase and, in particular, the intriguing question of whether the molecular motor indeed drives the unidirectional unwinding of the helical packing of several molecules in the LC matrix merits further study.
Abbreviations
- LC
liquid crystalline
- RT
room temperature
- M15
4-pentyloxy-4′-biphenylcarbonitrile
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
To whom reprint requests should be addressed. E-mail: feringa@chem.rug.nl.
References
- 1.Feynman R P. In: Miniaturization. Gilbert H D, editor. New York: Reinhold; 1961. [Google Scholar]
- 2.Goodsell D S. Our Molecular Nature: The Body's Motors, Machines and Messages. New York: Springer; 1996. [Google Scholar]
- 3.Sauvage J-P, Dietrich-Buchecker C, editors. Molecular Catenanes, Rotaxanes and Knots. Weinheim, Germany: Wiley-VCH; 1999. [Google Scholar]
- 4.Bustamante C, Keller D, Oster G, Shipway A N, Willner I, Pease A R, Jeppesen J O, Stoddart J F, Luo Y, Collier C P, et al. Acc Chem Res. 2000;34:409–522. [Google Scholar]
- 5.Balzani V, Gomez-Lopez M, Stoddart J F. Acc Chem Res. 1998;31:405–414. [Google Scholar]
- 6.Sauvage J-P. Acc Chem Res. 1998;31:611–619. [Google Scholar]
- 7.Feringa B L, van Delden R A, Koumura N, Geertsema E M. Chem Rev. 2000;100:1789–1816. doi: 10.1021/cr9900228. [DOI] [PubMed] [Google Scholar]
- 8.Kelly T R, Tellitu I, Sestelo J P. Angew Chem Int Ed Engl. 1997;36:1866–1868. [Google Scholar]
- 9.Balzani V, Credi A, Raymo F M, Stoddart J F. Angew Chem Int Ed Engl. 2000;39:3348–3391. doi: 10.1002/1521-3773(20001002)39:19<3348::aid-anie3348>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez M C, Dietrich-Buchecker C, Sauvage J-P. Angew Chem Int Ed Engl. 2000;39:3284–3287. doi: 10.1002/1521-3773(20000915)39:18<3284::aid-anie3284>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Chia S, Cao J, Stoddart J F, Zink J I. Angew Chem Int Ed Engl. 2001;40:2447–2450. [PubMed] [Google Scholar]
- 12.Feringa B L, editor. Molecular Switches. Weinheim, Germany: Wiley-VCH; 2001. [Google Scholar]
- 13.Koumura N, Zijlstra R W J, van Delden R A, Harada N, Feringa B L. Nature (London) 1999;401:152–155. doi: 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- 14.Kelly T R, De Silva H, Silva R A. Nature. 1999;401:150–152. doi: 10.1038/43639. [DOI] [PubMed] [Google Scholar]
- 15.Koumura N, Geertsema E M, Meetsma A, Feringa B L. J Am Chem Soc. 2000;122:12005–12006. doi: 10.1021/ja012499i. [DOI] [PubMed] [Google Scholar]
- 16.Whitesides G M. Sci Am. 2001;285(3):70–75. doi: 10.1038/scientificamerican0901-78. [DOI] [PubMed] [Google Scholar]
- 17.Taubes G, Pennisi E, Vogel G, Vale R D, Milligan R A, Mahadevan L, Matsudaira P, Dickinson M H, Farley O T, Full R J, et al. Science. 2000;288:79–106. [Google Scholar]
- 18.Harada N, Saito A, Koumura N, Uda H, de Lange B, Jager W F, Wynberg H, Feringa B L. J Am Chem Soc. 1997;119:7241–7248. [Google Scholar]
- 19.Harada N, Saito A, Koumura N, Roe D C, Jager W F, Zijlstra R W J, de Lange B, Feringa B L. J Am Chem Soc. 1997;119:7249–7255. [Google Scholar]
- 20.Harada N, Koumura N, Feringa B L. J Am Chem Soc. 1997;119:7256–7264. [Google Scholar]
- 21.Zijlstra R W J, Jager W F, de Lange B, van Duijnen P T, Feringa B L, Goto H, Saito A, Koumura N, Harada N. J Org Chem. 1999;64:1667–1674. doi: 10.1021/jo982381t. [DOI] [PubMed] [Google Scholar]
- 22.Ichimura K. Chem Rev. 2000;100:1847–1873. doi: 10.1021/cr980079e. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T, Kanazawa A. In: Molecular Switches. Feringa B L, editor. Weinheim, Germany: Wiley-VCH; 2001. pp. 363–398. [Google Scholar]
- 24.Tamaoki N, Song S, Moriyama M, Matsuda H. Adv Mater. 2000;12:94–97. [Google Scholar]
- 25.Broer D J, Lub J, Mol G N. Nature (London) 1995;378:467–469. [Google Scholar]
- 26.Tamaoki N. Adv Mater. 2001;13:1135–1147. [Google Scholar]
- 27.Solladié G, Zimmermann R G. Angew Chem Int Ed Engl. 1984;23:348–362. [Google Scholar]
- 28.Feringa B L, Huck N P M, van Doren H A. J Am Chem Soc. 1995;117:9929–9930. [Google Scholar]
- 29.Huck N P M, Jager W F, de Lange B, Feringa B L. Science. 1996;273:1686–1688. [Google Scholar]
- 30.Sagisaka T, Yokoyama Y. Bull Chem Soc Jpn. 2000;73:191–196. [Google Scholar]
- 31.Koumura N, Harada N. Enantiomer. 1998;3:251–253. [PubMed] [Google Scholar]
- 32. Koumura, N. & Harada, N. (1998) Chem. Lett., 1151–1152.
- 33.Heppke G, Oestreicher F. Mol Cryst Liq Cryst. 1977;41:245–249. [Google Scholar]
- 34.Isaert N, Soulestin B, Malthète J. Mol Cryst Liq Cryst. 1976;37:321–333. [Google Scholar]