Abstract
The approach to LDL-C management in primary prevention has shifted from lipid-centric strategies to personalized, risk-based frameworks. However, the binary distinction between primary and secondary prevention may overlook individuals with advanced subclinical atherosclerosis. Coronary artery calcium (CAC) scoring has become central in identifying such patients, especially those whose risk exceeds that implied by traditional risk calculators.
The 2022 ACC Expert Consensus Decision Pathway (ECDP) incorporated CAC into LDL-C goal-setting, advocating for LDL-C < 70 mg/dL when CAC >100 or >75th percentile. Emerging evidence suggests that CAC ≥300 portends risk comparable to clinical ASCVD, warranting even lower targets (<55 mg/dL). This editorial highlights how Figure 6 from the 2022 ECDP offers a framework for reclassifying high-risk individuals and recommends that future guidelines recognize CAC >300 as equivalent to secondary prevention.
Keywords: LDL-C management, Coronary artery calcium scoring, Primary prevention, Secondary prevention, Cardiovascular risk stratification, Subclinical atherosclerosis
1. Brief history of LDL management guidelines
Early guidelines (1970s–1980s) focused on lifestyle changes without specific LDL-C targets [1]. The 1988 National Cholesterol Education Program Adult Treatment Panel (ATP) I guidelines prioritized dietary therapy to lower saturated fat and cholesterol intake, set an LDL-C goal of <130 mg/dL, and recommended drug therapy if levels remained elevated after six months [2]. The 1993 ATP II guidelines prioritized LDL-C management, introduced CHD risk stratification, recognized HDL-C as a risk factor, and emphasized lifestyle interventions before drug therapy [3]. The 2001 ATP III guidelines maintained LDL-C as the primary treatment target, introducing Framingham Risk Score-based stratification with LDL-C goals of <100 mg/dL for high-risk patients, <130 mg/dL for moderate-risk, and <160 mg/dL for low-risk individuals, with an optional <70 mg/dL target for very high-risk patients [4,5]. The FRS estimates 10-year CHD risk using age, sex, blood pressure, cholesterol, smoking, and diabetes, guiding risk-based treatment decisions. The 2013 ACC/AHA guidelines abandoned specific LDL-C targets, instead recommending -intensity of statin therapy based on ASCVD risk assessment using the Pooled Cohort Equations [6]. The 2018 ACC/AHA guidelines reintroduced an LDL-C threshold of 70 mg/dL for secondary prevention, emphasizing aggressive management for very high-risk individuals, including those with multiple ASCVD events or high-risk conditions like diabetes, CKD, hypertension, or persistently elevated LDL-C (≥100 mg/dL) despite therapy [7]. The 2019 European Society of Cardiology (ESC) guidelines established more explicit targets, from <100 mg/dL for moderate risk in primary prevention to <55 mg/dL for very high-risk secondary prevention [8].
2. Coronary artery calcium and LDL-C management
Coronary artery calcium (CAC) emerged in the 1990s as a powerful marker of subclinical coronary atherosclerosis, outperforming traditional risk factors in Atherosclerotic Cardiovascular Disease (ASCVD) prediction [[9], [10], [11]]. The 2010 ACCF/AHA guidelines recommended CAC scoring as a Class IIa indication (Level of Evidence B) for refining risk assessment in intermediate-risk asymptomatic adults [12]. By 2018, the ACC/AHA guidelines reaffirmed the role of CAC in guiding statin decisions, Class IIa indication (Level of Evidence B) [7].
CAC scoring refines LDL-C management in primary prevention, with a CAC score of 0 supporting statin deferral and significant CAC (>100), or above 75th percentile warranting aggressive LDL-C lowering. It is especially helpful in reclassifying risk for those whose risk status is uncertain, thereby aiding in more personalized management decisions [7]. In addition, CAC scoring enhances personalized preventive strategies by informing more tailored BP and aspirin management decisions [13,14].
3. Reframing high CAC as secondary prevention
Previously, guidelines for cardiovascular risk management were bifurcated into two distinct categories: primary prevention (for individuals without known ASCVD) and secondary prevention (for those with established ASCVD). Each approach had its own set of recommendations, particularly regarding the intensity of lipid-lowering therapy, aspirin use, and other preventive interventions.
However, this binary framework overlooks a critical subset: individuals with substantial subclinical coronary atherosclerosis, often revealed by CAC scoring or other imaging. Although they have not experienced a clinical event, their high atherosclerotic burden places them at risk comparable to, or greater than, many secondary prevention patients [15].
As a result, many of these patients are undertreated when relying solely on traditional risk scores (e.g., the Pooled Cohort Equations), which do not incorporate CAC findings [16].
Recent insights, particularly from studies on CAC scoring have blurred the line between primary and secondary prevention, suggesting the need for a more risk-driven, individualized approach rather than strict categorical assignment [17,18]. Thus, modern preventive cardiology is shifting toward a spectrum-based approach, where the presence of significant atherosclerosis (e.g., CAC >100, or other imaging findings) can and should warrant more intensive intervention, even in those without prior ASCVD events.
Advanced subclinical atherosclerosis introduces a crucial intermediate risk category that transcends the traditional primary vs. secondary prevention divide. Individuals with substantial yet nonobstructive atherosclerotic plaque, detected via imaging techniques such as CAC scoring, coronary computed tomography angiography (CCTA), or carotid ultrasound, remain asymptomatic but face cardiovascular event rates akin to those with established disease, indicating that conventional risk assessment tools may underestimate their true risk [19,20]. Focusing on plaque burden as a critical indicator of disease severity enables more accurate risk stratification, supporting the initiation of intensive lipid-lowering treatments and lifestyle changes in high-risk patients earlier than traditional primary prevention strategies, while adopting a less aggressive approach in low-risk individuals. This perspective reframes cardiovascular risk management as a ‘see disease, treat disease’ approach, prioritizing early detection and direct treatment of subclinical atherosclerosis to prevent overt cardiovascular events [21].
4. Why CAC should drive treatment targets
CAC is a direct marker of coronary atherosclerosis, reflecting the cumulative burden of calcified plaque within the coronary arteries. Higher CAC scores are strongly associated with an increased risk of cardiovascular events, independent of traditional risk factors [11]. The 2024 ESC Guidelines for Chronic Coronary Syndromes (CCS) introduce a significant shift by integrating primary and secondary prevention strategies based on plaque burden, including asymptomatic coronary stenosis. This plaque burden-focused model represents an important step toward more personalized cardiovascular care. By aligning LDL-C reduction goals with the severity of atherosclerosis, these guidelines bridge traditional prevention categories [22].
CAC scoring is a valuable tool not only for determining the need for statin therapy in intermediate-risk individuals but also for guiding the addition of non-statin lipid-lowering agents. Beyond statin eligibility, CAC scoring aids in risk stratification for more intensive lipid-lowering therapy. In individuals with CAC, particularly those with higher scores or progressive calcification the addition of non-statin agents such as ezetimibe, PCSK9 inhibitors, or bempedoic acid may be warranted, especially when LDL-C levels remain above guideline-recommended thresholds despite maximally tolerated statins. This tailored approach ensures aggressive therapy for high-risk patients, while sparing low-risk individuals with a CAC of zero from unnecessary treatment [23].
5. The 2022 ACC expert consensus decision pathway (ECDP) on non-statin use
The 2022 ACC ECDP advances the role of CAC in ASCVD prevention, introducing the important Figure 6 to establish LDL-C targets based on CAC scores, aligning treatment intensity to be more similar to secondary prevention strategies. By integrating CAC into LDL-C target setting, Figure 6 provides a structured, risk-based approach to lipid management, an advancement in personalized cardiovascular care that has been largely overlooked. In addition to providing a structured approach to incorporating CAC scores into risk assessment and treatment for adults without clinical ASCVD, diabetes, or LDL-C ≥ 190 mg/dL, Figure 6 enhances risk-based decision-making by refining LDL-C targets based on CAC burden. It personalizes treatment strategies based on plaque burden, with recommendations ranging from statin deferral for CAC = 0 to aggressive LDL-C lowering for CAC >1000. By emphasizing advanced subclinical atherosclerosis, Figure 6 highlights how patients with significant CAC scores can have risk levels comparable to secondary prevention populations, warranting intensive interventions.
The Fig. 6 approach bridges traditional prevention categories and aligns treatment with the progressive nature of atherosclerosis [23]. For patients with CAC 100–999 AU or CAC 1–99 at ≥75th percentile, Fig. 6 recommends moderate- to high-intensity statin therapy as the initial approach. If LDL-C reduction >50 % is not achieved or LDL-C remains ≥70 mg/dL, adding ezetimibe is recommended. For CAC >1000 AU, if LDL-C remains above target despite maximally tolerated statin and ezetimibe, a PCSK9 monoclonal antibody is recommended. A CAC score ≥1000 represents a threshold at which primary prevention merges with secondary prevention in both risk and treatment intensity., not observation, justifying intensive preventive strategies in this highest-risk group [24,25].
Despite its clinical relevance, Fig. 6 appears to be either unknown or underutilized among clinicians. Based on our anecdotal experience, even within specialized academic medical centers, awareness of this ACC-endorsed framework remains limited. Moreover, to our knowledge, no studies have examined the rates of LDL goal achievement stratified by CAC score, highlighting a gap in both implementation and evidence. Increasing awareness of this approach is essential to optimizing prevention strategies, particularly in adults without clinical ASCVD, diabetes, or LDL-C ≥ 190 mg/dL, where CAC can refine risk assessment and treatment decisions [23]. This awareness is particularly important before the looming introduction of the next set of ACC/AHA Cholesterol and Prevention Guidelines.
6. Moving guidelines beyond the 2022 ECDP Figure 6: treating CAC >300 as established ASCVD
Using Fig. 6 as a guide, this editorial review seeks to move our next set of ACC/AHA Cholesterol and Prevention guidelines further and examines the rationale, approach, and clinical implications of targeting LDL-C < 55 mg/dL in individuals with CAC >300 AU Given the significant cardiovascular risk associated with CAC >300, we propose LDL-C < 55 mg/dL as a necessary and aggressive target to prevent future cardiovascular events. A CAC score >300 reflects advanced coronary atherosclerosis, indicating that the patient likely harbors a significant volume of both calcified and non-calcified plaque. Thus, for such patients, an aggressive LDL-C reduction strategy is warranted to stabilize plaque, reduce progression, and lower the likelihood of plaque rupture, thrombosis, and downstream ischemic events. CAC >1000 remains an important distinct group, with risk equivalent to high-risk secondary prevention populations, that should be eligible for immediate upfront combination lipid lowering therapy as well as a host of other “secondary prevention” measures beyond LDL-lowering [24,25].
Recent research by Budoff et al. highlights the prognostic significance of a CAC score >300 in persons without established ASCVD, suggesting that their risk of major adverse cardiovascular events (MACE) is equivalent to those with established ASCVD [26], specifically those patients with obstructive CAD on CCTA imaging. Thus, patients with CAC > 300 overlap traditional concepts of secondary prevention. Recognizing CAC >300 as representative of significant coronary artery disease supports a more aggressive LDL-C lowering strategy, with intensified lipid-lowering therapy aimed at achieving at least LDL-C < 70 mg/dL, or optimally <55 mg/dL, as outlined in Fig. 6 [27] (Fig. 1).
Fig. 1.
Patients with CAC>300 have a similar MACE risk to those with established ASCVD (Figure reproduced from: Budoff MJ, Kinninger A, Gransar H, Achenbach S, Al-Mallah M, Bax JJ, et al. When Does a Calcium Score Equate to Secondary Prevention? Insights From the Multinational CONFIRM Registry).
Aggressive LDL-C reduction in patients with CAC score >300 is based on the recognition that calcified plaque is only part of the atherosclerotic disease process. By setting a more aggressive LDL-C target, clinicians can also address dynamic mixed plaques and non-calcified, lipid-rich plaques, which are particularly prone to rupture and thrombotic events. Lowering LDL-C to <55 mg/dL has been shown to slow plaque progression, promote stabilization, and reduce the risk of MI and other acute coronary events [[29], [30], [31]].
Multiple clinical trials, such as the IMPROVE-IT, ODYSSEY OUTCOMES and FOURIER studies, have demonstrated that intensive LDL-C lowering in high-risk patients which can be safely achieved through high-intensity statin therapy and the addition of ezetimibe or PCSK9 inhibitors, significantly reduces cardiovascular events. These trials provide the foundation for setting LDL-C targets <55 mg/dL in select high-risk populations. The evidence supports the hypothesis that driving LDL-C levels as low as possible offers protective benefits for patients with substantial coronary artery disease burden, minimizing residual risk [[29], [30], [31]]. These findings, coupled with the observational epidemiology on CAC, reinforce the need to reconsider LDL-C targets based on plaque burden rather than traditional primary vs. secondary prevention categories (Fig. 2).
Fig. 2.
Treating CAC >300 as Established ASCVD.
7. Who should get a CAC scan in the era of the prevent risk equations?
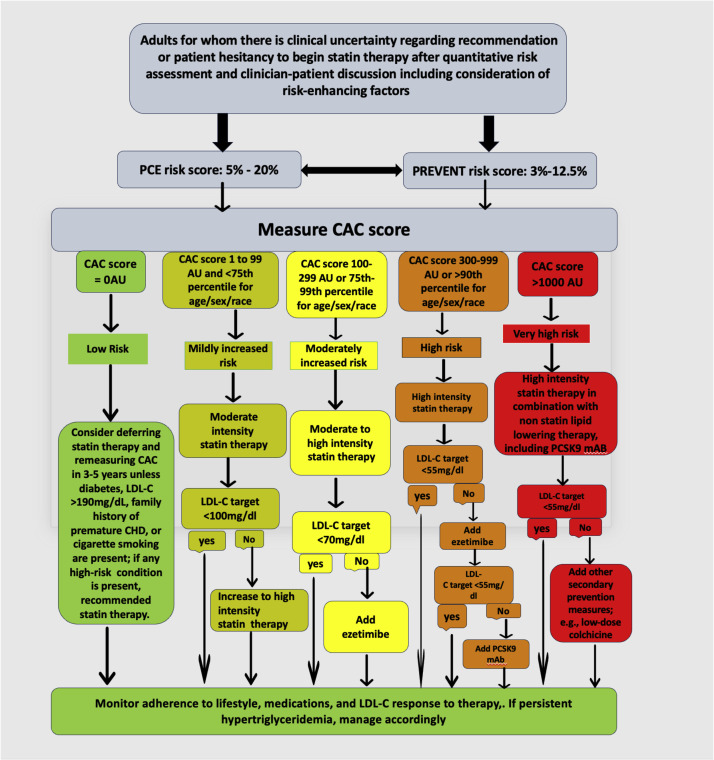
The Predicting Risk of CVD EVENTs (PREVENT) calculator, developed by the American Heart Association (AHA), is a newly designed cardiovascular disease (CVD) risk assessment tool aimed at improving upon the Pooled Cohort Equations (PCEs) by incorporating a larger, more diverse dataset and factoring in additional health conditions such as chronic kidney disease (CKD) and metabolic disorders. Unlike PCE, PREVENT eliminates race-based adjustments and instead includes social determinants of health, making it a more inclusive and equitable risk model [[32], [33], [34]]. A key advantage of PREVENT is its improved calibration, which reduces the overestimation of risk seen in PCE, particularly in men [35]. However, PREVENT may underestimate risk in certain individuals, potentially missing high-risk patients who would have been identified for treatment under PCE [36,37]. To address this shift in calibration and preserve a similarly broad borderline-to-intermediate risk category as used in prior guidelines, we strongly recommend routine consideration of CAC imaging in individuals with a PREVENT risk score between 3 % and 12.5 %. More broadly, we advocate for CAC assessment in any individual with a PREVENT risk >3 %, without imposing an upper threshold. Traditional risk factors alone do not reliably identify individuals with advanced subclinical atherosclerosis or established ASCVD. A particular concern is that classifying all individuals with a PREVENT risk >10 % as “high risk” may lead to uniform recommendations for high-intensity statin therapy, even in older adults without additional risk factors, without offering further risk stratification, which CAC scoring enables. This approach may obscure clinically relevant distinctions, such as between a younger individual with multiple risk factors and an older patient with none, and may limit opportunities for individualized LDL-C goal setting. Therefore, integrating CAC in this broader risk range supports a more personalized, evidence-informed approach to preventive cardiovascular care.
Recently, Maron et al. presented a five‐stage CAC framework, ranging from CAC = 0 (no statin unless very high risk) through CAC ≥1000 (intensive lipid‐lowering plus emerging therapies), to align preventive interventions with escalating atherosclerotic burden. Their published table provides a concise, static summary of each stage’s score range, representative imaging, and broad treatment principles [28]. Our manuscript translates those identical CAC cutoffs into a stepwise “if–then” decision pathway, which critically begins by assessing PCE or PREVENT risk to determine whether CAC measurement is warranted, and then guiding sequentially toward the appropriate statin intensity, aspirin use, and adjunctive non-statin therapies based on the patient’s CAC category (e.g., “if CAC = 0, evaluate diabetes/LDL/ASCVD risk and consider deferring statin; if CAC 1–99, initiate moderate‐intensity statin,” etc.). Uniquely, we proposed consideration of CAC for anyone with PREVENT >3 %, discarding the notion of a “high risk patient” by risk factors alone that would not benefit from further risk stratification and goal setting. Importantly, our algorithm additionally addresses the important CAC >90th percentile group, which is a an extremely high lifetime risk group that might not achieve high absolute CAC scores at young ages, but deserves the most intensive interventions nonetheless. By channeling each patient through precisely the right intervention at each juncture, we believe that the flowchart’s sequential algorithmic layout is practical for real‐time clinical decision‐making (Fig. 3).
Fig. 3.
Enhanced Risk Assessment and Management for Adults Without Clinical ASCVD, Diabetes, or LDL-C ≥ 190 mg/dL: Proposed update to 2022 ACC ECDP Figure 6, incorporating an LDL-C target <55 mg/dL for those with CAC >300 or >90th percentile by age/sex/race, using subclinical atherosclerosis imaging to guide therapy.
8. Conclusion
Over the past decades, LDL-C targets have become increasingly aggressive, particularly for secondary prevention, driven by robust evidence from trials involving non-statin therapies such as PCSK9 inhibitors. CAC scoring has further disrupted the traditional primary vs. secondary prevention dichotomy, revealing that individuals with CAC >300 or >90th percentile for age/sex/race or >1000 have cardiovascular risk profiles that rival, or exceed, those with established ASCVD.
The time has come for widespread adoption of the 2022 ACC ECDP Figure 6 framework, and for future guidelines to go even further. We strongly advocate for an LDL-C target of <55 mg/dL in individuals with CAC >300 or >90th percentile for age/sex/race, recognizing that these patients deserve the same intensity of preventive care as those with known disease. A plaque-driven, risk-aligned approach to LDL-C lowering will not only personalize care but also prevent events before they happen.
Authors agreement
Originality confirmation
Authors declare that the manuscript is original, has not been published before, and is not currently under consideration elsewhere.
Funding acknowledgment
None.
CRediT authorship contribution statement
Semenawit Burka: Writing – review & editing, Writing – original draft, Conceptualization. Ehimen Aneni: Writing – review & editing. Thorsten Leucker: Writing – review & editing. Michael J. Blaha: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Semenawit Burka, Email: Sburka3@jh.edu.
Ehimen Aneni, Email: ehimen.aneni@yale.edu.
Thorsten Leucker, Email: tleucke1@jhmi.edu.
Michael J. Blaha, Email: mblaha1@jhmi.edu.
References
- 1.The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251(3):365–374. [PubMed] [Google Scholar]
- 2.Report of the national cholesterol education program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. The Expert Panel. Arch Intern Med. 1988;148(1):36–69. [PubMed] [Google Scholar]
- 3.Summary of the second report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel II) JAMA. 1993;269(23):3015–3023. [PubMed] [Google Scholar]
- 4.Pasternak R.C. Report of the adult treatment panel III: the 2001 national cholesterol education program guidelines on the detection, evaluation and treatment of elevated cholesterol in adults. Cardiol Clin. 2003;21(3):393–398. doi: 10.1016/s0733-8651(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 5.National Cholesterol Education Program Expert Panel on Detection E Treatment of High Blood Cholesterol in A. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 6.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 7.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood Cholesterol: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Detrano R., Guerci A.D., Carr J.J., Bild D.E., Burke G., Folsom A.R., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 11.Budoff M.J., Shaw L.J., Liu S.T., Weinstein S.R., Mosler T.P., Tseng P.H., et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P., Alpert J.S., Beller G.A., Benjamin E.J., Budoff M.J., Fayad Z.A., et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 13.Cainzos-Achirica M., Miedema M.D., McEvoy J.W., Al Rifai M., Greenland P., Dardari Z., et al. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019: the MESA study (Multi-Ethnic Study of Atherosclerosis) Circulation. 2020;141(19):1541–1553. doi: 10.1161/CIRCULATIONAHA.119.045010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orringer C.E., Blaha M.J., Blankstein R., Budoff M.J., Goldberg R.B., Gill E.A., et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33–60. doi: 10.1016/j.jacl.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 16.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: executive Summary: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e563–ee95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golub I.S., Termeie O.G., Kristo S., Schroeder L.P., Lakshmanan S., Shafter A.M., et al. Major global coronary artery calcium guidelines. JACC Cardiovasc Imaging. 2023;16(1):98–117. doi: 10.1016/j.jcmg.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Blaha M.J., Mortensen M.B., Kianoush S., Tota-Maharaj R., Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging. 2017;10(8):923–937. doi: 10.1016/j.jcmg.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Blaha M.J., Abdelhamid M., Santilli F., Shi Z., Sibbing D. Advanced subclinical atherosclerosis: a novel category within the cardiovascular risk continuum with distinct treatment implications. Am J Prev Cardiol. 2023;13 doi: 10.1016/j.ajpc.2022.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erbel R., Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: subclinical atherosclerosis: the memory of lifetime risk factor exposure. Eur Heart J. 2012;33(10):1201–1213. doi: 10.1093/eurheartj/ehs076. [DOI] [PubMed] [Google Scholar]
- 21.Blaha M.J., DeFilippis A.P. Multi-Ethnic Study of Atherosclerosis (MESA): JACC Focus Seminar 5/8. J Am Coll Cardiol. 2021;77(25):3195–3216. doi: 10.1016/S0735-1097(21)04550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrints C., Andreotti F., Koskinas K.C., Rossello X., Adamo M., Ainslie J., et al. 2024 ESC guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024;45(36):3415–3537. doi: 10.1093/eurheartj/ehae177. [DOI] [PubMed] [Google Scholar]
- 23.Writing C., Lloyd-Jones D.M., Morris P.B., Ballantyne C.M., Birtcher K.K., Covington A.M., et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80(14):1366–1418. doi: 10.1016/j.jacc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Peng A.W., Dardari Z.A., Blumenthal R.S., Dzaye O., Obisesan O.H., Iftekhar Uddin S.M., et al. Very high coronary artery calcium (>/=1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation. 2021;143(16):1571–1583. doi: 10.1161/CIRCULATIONAHA.120.050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzaye O., Razavi A.C., Michos E.D., Mortensen M.B., Dardari Z.A., Nasir K., et al. Coronary artery calcium scores indicating secondary prevention level risk: findings from the CAC consortium and FOURIER trial. Atherosclerosis. 2022;347:70–76. doi: 10.1016/j.atherosclerosis.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budoff M.J., Kinninger A., Gransar H., Achenbach S., Al-Mallah M., Bax J.J., et al. When does a calcium score equate to secondary prevention?: insights from the multinational CONFIRM registry. JACC Cardiovasc Imaging. 2023;16(9):1181–1189. doi: 10.1016/j.jcmg.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maron D.J., Budoff M.J., Sky J.C., Bommer W.J., Epstein S.D., Fisher D.A., et al. Coronary artery calcium staging to guide preventive interventions: a proposal and call to action. JACC Adv. 2024;3(11) doi: 10.1016/j.jacadv.2024.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 30.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 31.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Theroux P., et al. Ezetimibe added to Statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 32.Khan S.S., Matsushita K., Sang Y., Ballew S.H., Grams M.E., Surapaneni A., et al. Development and validation of the American Heart Association's PREVENT equations. Circulation. 2024;149(6):430–449. doi: 10.1161/CIRCULATIONAHA.123.067626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razavi A.C., Kohli P., McGuire D.K., Martin S.S., Polonsky T.S., McEvoy J.W., et al. PREVENT equations: a new era in cardiovascular disease risk assessment. Circ Cardiovasc Qual Outcomes. 2024;17(4) doi: 10.1161/CIRCOUTCOMES.123.010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larkin H. What to know about PREVENT, the AHA's new cardiovascular disease risk calculator. JAMA. 2024;331(4):277–279. doi: 10.1001/jama.2023.25115. [DOI] [PubMed] [Google Scholar]
- 35.Scheuermann B., Brown A., Colburn T., Hakeem H., Chow C.H. Ade C. External validation of the American Heart Association PREVENT cardiovascular disease risk equations. JAMA Netw Open. 2024;7(10) doi: 10.1001/jamanetworkopen.2024.38311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty N.S., Gaonkar M., Patel N., Vekariya N., Li P., Arora G., et al. PREVENT and pooled cohort equations in mortality risk prediction: national Health and Nutrition Examination Survey. JACC Adv. 2024;3(12) doi: 10.1016/j.jacadv.2024.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diao J.A., Shi I., Murthy V.L., Buckley T.A., Patel C.J., Pierson E., et al. Projected changes in statin and antihypertensive therapy eligibility with the AHA PREVENT cardiovascular risk equations. JAMA. 2024;332(12):989–1000. doi: 10.1001/jama.2024.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]