Abstract
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapies have transformed the management of retinal diseases such as diabetic macular edema and neovascular age-related macular degeneration. However, concerns about their systemic absorption and potential adverse effects on overall health remain. In this review, we systematically aggregate existing literature on the systemic impact of anti-VEGF therapy, with a detailed analysis of the pharmacodynamics and pharmacokinetics of individual drugs. By examining their metabolism, clearance, and systemic exposure, we aim to clarify the extent of their effects beyond the eye. We further explore their influence on renal and cardiovascular health, with evidence suggesting a generally safe profile in the short term but potential risks in high-risk patients, particularly those with preexisting kidney or heart conditions. Additionally, this review addresses the critical concerns surrounding anti-VEGF use in special populations, including pregnant and lactating women and neonates with retinopathy of prematurity (ROP). We discuss the potential risks, the safest options available, and precautionary measures that should be taken when administering these therapies in these groups. While anti-VEGFs remain an essential tool in ophthalmology, careful patient selection, monitoring, and individualized treatment approaches are necessary to mitigate potential systemic risks. Further research is needed to refine our understanding of long-term safety.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-025-01157-4.
Keywords: Anti-VEGF therapy, Pharmacokinetics of anti-VEGF, Pharmacodynamics of anti-VEGF, Systemic safety, Renal effects, Cardiovascular risks, Anti-VEGF use in pregnancy and lactation, Retinopathy of prematurity (ROP)
Plain Language Summary
Anti-vascular endothelial growth factor (anti-VEGF) injections are widely used to treat eye diseases such as diabetic macular edema and age-related macular degeneration by blocking abnormal blood vessel growth in the retina. Although these drugs are injected into the eye, small amounts can enter the bloodstream, raising concerns about their effects on other organs, especially the kidneys, heart, and brain. This review explores whether anti-VEGF therapy affects kidney function, increases the risk of heart disease, or impacts the nervous system. Most studies suggest that these drugs are generally safe for the kidneys, but some research indicates a possible risk of kidney damage in patients with preexisting conditions. Similarly, while there is no strong evidence that anti-VEGFs cause heart problems, certain patients—such as those with a history of heart disease—may be at higher risk of complications such as stroke or heart attacks. There are also concerns about potential effects on brain health, with some studies linking anti-VEGFs to an increased risk of stroke and cognitive decline. Special precautions are needed for pregnant and breastfeeding women, as well as premature infants receiving these treatments for retinopathy of prematurity. Overall, anti-VEGF therapy remains a crucial treatment for vision-threatening diseases, but doctors should monitor patients carefully, especially those with underlying health conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-025-01157-4.
Key Summary Points
| While short-term use of anti-vascular endothelial growth factor (anti-VEGF) agents appears safe for most patients, those with preexisting kidney disease may be at higher risk of kidney dysfunction, particularly with prolonged treatment. |
| There is no definitive evidence linking anti-VEGF therapy to major heart problems, but patients with prior cardiovascular disease may face a slightly increased risk of stroke or heart attacks, necessitating close monitoring. |
| Pregnant women, breastfeeding mothers, and neonates with retinopathy of prematurity require careful evaluation before receiving anti-VEGF therapy, as its systemic effects may impact fetal and infant development. |
| By synthesizing available literature, our review not only discusses potential systemic risks but also offers practical recommendations on patient selection, monitoring protocols, and risk mitigation strategies. This adds a real-world clinical perspective, helping ophthalmologists and other specialists make informed treatment decisions. |
Introduction
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections have transformed the treatment of retinal diseases such as diabetic macular edema and neovascular age-related macular degeneration by targeting VEGF, a key mediator of pathological angiogenesis and vascular permeability. However, many elderly patients and those with diabetes receiving these therapies have systemic comorbidities, including cardiovascular and renal diseases, raising concerns about potential systemic effects. While some studies suggest minimal risk, others indicate possible adverse outcomes, highlighting the need for further research. Despite low intravitreal doses, systemic absorption and fellow eye effects suggest a plausible risk, warranting closer investigation.
In this review article, we provide a comprehensive assessment of the pharmacokinetics and pharmacodynamics of these drugs to better understand their safety profile. Additionally, we review existing literature to summarize known systemic effects and potential risks. We also discuss their use in special populations, including pregnant women, breastfeeding mothers, and neonates.
Methods
This comprehensive review describes the pharmacokinetics, pharmacodynamics, and reported systemic side effects of intravitreal anti-VEGF administration with special emphasis on renal, cardiovascular, and neuronal health. A systematic literature search was conducted using the MEDLINE database accessed through the PubMed search engine. The search strategy included the terms “pharmacodynamics”, “pharmacokinetics”, “renal effects”, “cardiovascular effects”, “central nervous system effects” of individual anti-VEGF agents available on the market at present, “anti-VEGF” and “pregnancy” or “breastfeeding” or “lactation” or “fetal development” or “Retinopathy of prematurity”. The review focused on articles published in English up until January 2025. A total of approximately 105 articles were identified initially; however, 22 articles were removed owing to de-duplication, commentaries, animal studies not relevant to systemic safety concerns, and non-English articles (Supplementary Material).
Abstracts of all relevant articles were initially screened by two authors (M.B. and S.K.P.), and the full texts of the articles were carefully examined for further synthesis. Non‑English articles and conference papers were excluded from the analysis. The review process followed the guidelines recommended by the Enhancing Transparency in Reporting the Synthesis of Qualitative Research (ENTREQ) checklist for qualitative studies, ensuring a systematic and rigorous approach to data synthesis. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Pharmacokinetics and Pharmacodynamics of Individual Anti-VEGFs
Pharmacokinetics is defined as the study of the time course of drug absorption, distribution, metabolism, and excretion. In other words, it describes what the body does to the drug administered [26]. Pharmacodynamics explores the relationship between the concentration of a drug at its site of action and the resulting effects. It explains how the drug influences the body, providing insights into its mechanism of action, therapeutic efficiency, and potential toxicity. The pharmacodynamic and pharmacokinetic parameters of anti-VEGF agents are summarized in Table 1.
Table 1.
Pharmacokinetic and pharmacodynamic parameters of intravitreal anti–vascular endothelial growth factor agents
| Distribution | Primarily confined to the vitreous cavity, with minimal distribution to other ocular structures (lens, retina, ciliary body, and iris) due to high molecular weight, steric hindrance, and hydrophilic properties [24, 25]. |
| Vitreal clearance | Cleared from the vitreous mainly via passive diffusion into surrounding ocular tissues and aqueous humor outflow. Intravitreal biologics are predominantly eliminated through the anterior route. Minimal intraocular metabolism or degradation occurs [26]. |
| Metabolism | Proteolytic degradation of the macromolecular structure into smaller peptides and amino acids [26]. |
| Excretion | Metabolites are excreted primarily through the renal system. Larger molecules (e.g., bevacizumab) are mainly excreted via the hepatobiliary route. |
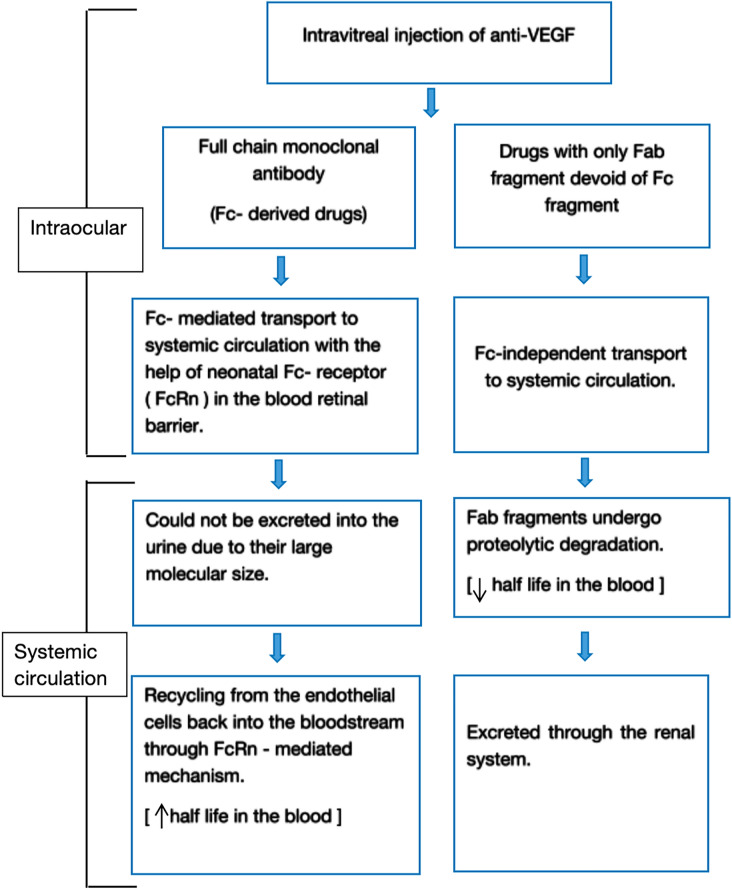
The pharmacokinetic model for intravitreal anti-VEGFS is illustrated in the flowchart in Fig. 1 [27]. The structural, pharmacodynamic, and pharmacokinetic parameters of the routinely used antiangiogenic drugs are presented in Table 2 [28–31].
Fig. 1.
Systemic pharmacokinetics of anti-VEGF agents following intravitreal injection. The diagram illustrates the differential transport and clearance mechanisms of full-length monoclonal antibodies (Fc-derived drugs) versus antibody fragments lacking the Fc region (Fab-only drugs). Fc-derived drugs undergo Fc receptor (FcRn)-mediated transport across the blood–retina barrier into systemic circulation, where their large molecular size prevents renal excretion. They are recycled via endothelial FcRn receptors, prolonging their half-life in blood. In contrast, Fab-only drugs enter systemic circulation through Fc-independent mechanisms, are subject to proteolytic degradation, and are cleared via renal excretion, resulting in a shorter systemic half-life. VEGF vascular endothelial growth factor, Fc fragment crystallizable, Fab fragment antigen-binding, FcRn neonatal Fc receptor
Table 2.
Structural, pharmacodynamic, and pharmacokinetic properties of commonly used antiangiogenic drugs
| Agent | Structure | Target (s) | Mol. weight (kDa) | Fc portion | Affinity (Kd, pM) | Potency (IC50, pM) | t1/2 (vitreous, days) | t1/2 (plasma, days) | Dose |
|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab | Humanized monoclonal full-length antibody | VEGF-A | 149 | Yes | 58–4456 | 500–1476 | 6/7–10 | 21 | 1.25 mg (0.05 mL) |
| Ranibizumab | Humanized recombinant Fab | VEGF-A | 48 | No | 9.2–179 | 88–1140 | 7.1 | 0.25 | 0.5 mg (0.05 mL) |
| Aflibercept | Recombinant fusion protein | VEGF-A, VEGF-B, PlGF | 115 | Yes | 0.49–9263 | 16–90 | 4.5–4.7 (rabbits) | 18 | 2 mg (0.05 mL) |
| Brolucizumab | Humanized single-chain antibody fragment | VEGF-A | 26 | No | 28.4 (for VEGF₁₆₅) | 860 | 4.4–5.6 | 6 | 6 mg (0.05 mL) |
| Faricimab | Bispecific monoclonal antibody | VEGF-A, Ang-2 | 150 | Modified Fc region to reduce FcγR and FcRn binding | 46–60 (VEGF-A), 22–30 (Ang-2) | 490 (VEGF-A), 240 (Ang-2) | 6.7–12 | 12 | 6 mg (0.05 mL) |
Abbreviations: VEGF Vascular endothelial growth factor, PlGF placental growth factor, Ang-2 Angiopoietin-2, Fab fragment antigen-binding, Fc fragment crystallisable, FcγR Fc gamma receptor, FcRn neonatal Fc receptor, Kd dissociation constant (affinity), IC₅₀ half maximal inhibitory concentration (potency), t1/2 half-life
Role of VEGF in Renal and Cardiovascular System
VEGF and Renal System
VEGF plays a pivotal role in the renal system by stimulating endothelial cell proliferation and differentiation, facilitating endothelium-dependent vasodilatation, inducing microvascular hyperpermeability, and contributing to interstitial matrix remodeling [1].
Distribution of VEGF and Its Receptors in the Kidney
VEGF is expressed most prominently in the glomerular podocytes and tubular epithelial cells [1]. In the tubules of the normal human kidney, VEGF expression has been observed to be confined to the distal and collecting duct epithelium [2]. The expression of the VEGF in the distal tubule was found to be weaker than in the glomerulus [3]. VEGF receptors VEGFR-1 and VEGFR-2 are found on preglomerular, glomerular, and peritubular endothelial cells [2, 4, 5].
Functional role of VEGF in the Kidney
-
I.
Renal development
VEGF is responsible for renal vasculogenesis, glomerulogenesis, and tubulogenesis. It guides endothelial cell proliferation and migration during kidney morphogenesis [6].
-
II.
Glomerular function
VEGF secreted by the podocytes is postulated to be involved in the induction and maintenance of the fenestrae in the glomerular capillary endothelial cells [7]. Thus, it facilitates high rate of glomerular ultrafiltration, maintaining the selective permeability of the glomerular capillaries, preventing leakage of protein in the urine. Caveolae, the plausible structures responsible for the increase in the endothelial permeability, are seen to organize into elongated cell-spanning structures in vitro after exposure to VEGF [8]. VEGF is necessary for glomerular and tubular hypertrophy and proliferation in response to reduction in nephrons [1].
-
III.
Hypoxia and ischemic response
VEGF expression is upregulated by hypoxia, leading to an angiogenic response [9]. This helps in tissue recovery after ischemic injury. This is crucial in acute kidney injury, where VEGF upregulation prevents endothelial cell apoptosis and supports vascular repair.
Role of VEGF in Diabetic Nephropathy
In diabetes, overexpression of VEGF-A has been noted, which triggers the activation of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF). This promotes mesangial and endothelial cell proliferation, foot process effacement, and thickening of the glomerular basement membrane, ultimately resulting in renal fibrosis [10–12].
VEGF maintains vascular integrity through a nitric oxide (NO)-dependent pathway but contributes to vascular injury, endothelial overactivation, vascular smooth muscle cell proliferation, and macrophage activation via an NO-independent pathway. In diabetes, reduced endothelial NO bioavailability amplifies the harmful effects of VEGF through the NO-independent pathway, exacerbating diabetic nephropathy [13].
In summary, the pathological changes in diabetic nephropathy caused by overexpressed VEGF-A include increased vascular permeability leading to proteinuria, glomerular hypertrophy, basement membrane thickening, podocyte injury, and capillary rarefaction.
VEGF and Cardiovascular System
VEGF plays a crucial role in the human heart by regulating angiogenesis, vascular permeability, inflammation, lipid metabolism, and resisting oxidative stress.
Distribution of VEGF and Its Receptors in the Heart
VEGF-A is expressed in the pericytes, endothelial cells, and angioblasts of the cardiovascular system [14]. In patients with congestive heart disease, the myocardium secretes VEGF in response to local inflammation, mechanical stress, and cytokines. This leads to myocardial deformation, impaired contraction, and hindered recovery [15]. VEGF-B is expressed in myocardium, coronary artery smooth muscle cells, and endothelial cells [16]. VEGF-C and VEGF-D are also reported to be expressed in the heart [17].
VEGFR-1 and VEGFR-2 are expressed in endothelial cells, inflammatory cells, vascular smooth muscle cells, and cardiac fibroblasts. VEGFR-1 is a member of the receptor tyrosine kinase family with high affinity for VEGF-A, but weaker kinase activity compared with VEGFR-2. The combination of VEGF-A and VEGFR-1 and VEGFR-2 controls angiogenesis, vascular permeability, and inflammation.
Functional Role of VEGF in Human Heart
-
I.
Angiogenesis
VEGF-A stimulates the proliferation, migration, and invasion of endothelial cells into surrounding tissues, facilitating the formation of lumen structures and neovascularization, which ensures an adequate blood supply to cardiac tissues.
After myocardial infarction (MI), VEGF contributes to repairing damaged heart tissue by stimulating angiogenesis, which restores blood flow to ischemic areas.
-
II.
Vascular permeability
VEGF-165 promotes synthesis of endogenous endothelial platelet activating factor and nitric oxide, leading to vascular permeability [18]. This ensures proper oxygen and nutrient delivery to the cardiac tissues.
-
III.
Inflammation
VEGF facilitates the recruitment of inflammatory cells, primarily neutrophils and macrophages, and is a marker of local inflammatory response [19].
-
IV.
Antioxidant
Oxidative stress is a significant risk factor for developing congestive heart disease. VEGF-B has been shown to exert antioxidant effects by binding to VEGFR-1 and upregulating various antioxidant genes, such as Gpx1 [20].
-
V.
Anti-apoptosis
VEGF-B and VEGF-C play an anti-apoptotic effect on the myocardial cells after myocardial infarction (MI). VEGF-B induces specific gene expression profiles of compensatory hypertrophy, preventing the loss of myocardial mass and maintaining myocardial contractility [21].
-
VI.
Fibrinogenesis
VEGF-C/VEGFR-3 promotes proliferation, migration, and collagen synthesis of cardiac fibroblasts, promoting myocardial fibrosis after MI [22].
-
VII.
Lymphangiogenesis
Lymphangiogenesis is the cardinal biological effect of VEGF-C and VEGF-D. They bind to VEGFR-3 expressed on lymphatic endothelial cells, promoting their proliferation, migration, and differentiation. After acute MI, the cardiac lymphatic vessels transport immune cells to the mediastinal lymph nodes, promoting the clearance of the acute inflammatory response and aids in cardiac repair. It also enhances fluid drainage from the interstitial space, reducing myocardial edema and restoring tissue fluid balance [23].
In summary, VEGF-induced angiogenesis compensates for hypoxia and ischemia by restoring blood supply, while lymphangiogenesis helps reduce inflammation by providing an exit pathway for accumulated inflammatory cytokines. Its anti-apoptotic effects protect the myocardium from damage following MI, and its role in fibrinogenesis contributes to myocardial fibrosis after MI.
Renal Effects of Anti-VEGF
The effect of intravitreal anti-VEGF agents on the renal system is an area of growing interest owing to their systemic absorption and potential off-target effects.
The 50% inhibitory concentration required to “maximally inhibit systemic VEGF,” known as the IC50, helps to determine the systemically measured serum drug levels after the injection of standard doses.
The average systemic level after intravitreal injection of aflibercept was found to be much higher than the IC50 of aflibercept for weeks (up to 30 days), while that of bevacizumab closely approximated the IC50 and decreased below that level 2 weeks post injection [32, 33]. Ranibizumab had initial post injection levels near the IC50, but it decreases quickly within days and is eliminated more rapidly from the serum than other agents [32, 33]. These pieces of evidence have raised concerns regarding the systemic safety of these drugs.
VEGF secreted by podocytes is believed to regulate the proliferation, migration, and survival of glomerular endothelial, mesangial, and peritubular capillary cells through paracrine signaling [34, 35]. Depletion of VEGF due to anti-VEGF therapy can harm these cells, resulting in renal vascular and parenchymal damage. This damage may lead to impaired kidney filtration, proteinuria, hypertension, and thrombotic microangiopathy [36].
The cumulative evidence on the renal effects of multiple doses of different anti-VEGF agents is summarized in Table 3 on the basis of a total of 12 studies. Table 4 highlights recent case reports on the renal effects of anti-VEGF therapy.
Table 3.
Summary of studies assessing renal safety of intravitreal anti-VEGF agents
| Author, year | Study type | Anti-VEGF agent(s) | Key results | Inference |
|---|---|---|---|---|
| Bagheri et al. 2018 [37] | Prospective observational study of 40 patients with diabetic nephropathy and PDR and/or significant DME | Bevacizumab | ↑ Diastolic BP at 1 month (p = 0.002); no significant change in ACR, serum creatinine, or eGFR at 1 month | Bevacizumab not associated with renal dysfunction or proteinuria in diabetic nephropathy patients |
| Glassman et al. 2018 [38] | Secondary analysis of Protocol T (DRCR.net), 660 DME patients | Bevacizumab, ranibizumab, aflibercept | No significant changes in mean arterial pressure or UACR among groups at 1–2 years | No difference in renal impact between agents |
| Neill et al. 2019 [39] | Retrospective review over 2.6 years, assessing eGFR and ACR trends in patients with DME receiving multiple injections | Ranibizumab, aflibercept | Mean of 26.8 injections per patient; no association between injection frequency and eGFR/ACR change | Long-term VEGF inhibition not associated with progressive renal impairment |
| Yang et al. 2022 [40] | Retrospective review (2000–2017); anti-VEGF versus non-injection group with same retinal diagnoses | Ranibizumab, aflibercept | ↑ Dialysis risk in anti-VEGF users, especially those treated for DME | Anti-VEGF may increase dialysis risk, particularly in DME |
| Chen et al. 2023 [41] | Retrospective review: patients > 20 years receiving versus not receiving injections | Ranibizumab | ↑ Risk of CKD in patients receiving Ranibizumab | Ranibizumab exposure is an independent CKD risk factor |
| Cai et al. 2024 [42] | Retrospective cohort of patients ≥ 18 years receiving 3 monthly anti-VEGF injections | Bevacizumab, ranibizumab, aflibercept | Kidney failure incidence: 742/100,000 person-years; no significant differences in hazard ratio between agents | Risk of kidney failure present; no preference among agents based on current evidence |
| Bunge et al. 2024 [43] | Retrospective study of patients with PDR or DME receiving anti-VEGF versus controls | Bevacizumab, ranibizumab, aflibercept | No overall ↑ kidney risk; ↑ risk in patients with baseline eGFR > 30 mL/min/1.73 m [2] (HR: 1.86); > 12 injections not associated with decline | Anti-VEGF generally safe for kidney function, though caution advised in some subgroups |
Abbreviations: VEGF vascular endothelial growth factor, DME diabetic macular edema, PDR proliferative diabetic retinopathy, BP blood pressure, eGFR estimated glomerular filtration rate, ACR albumin–creatinine ratio, UACR urine albumin–creatinine ratio, CKD chronic kidney disease, HRhazard ratio, CI confidence interval
Table 4.
Case reports highlighting renal effects of intravitreal anti-VEGF therapy
| Author, year | Age/gender | Retinal disease | Anti-VEGF agent(s) | Renal symptoms | Renal diagnosis | Management |
|---|---|---|---|---|---|---|
| Shye et al. 2020 [44] |
59 years/M 58 years/M 46 years/M |
DR with DME (all cases) | Bevacizumab and ranibizumab | Proteinuria, hypertension, worsening renal function | Collapsing FSGS, diabetic glomerulosclerosis, interstitial nephritis | Dialysis |
| Hanna et al. 2020 [45] | 37 years/F | DR with DME | Bevacizumab | Proteinuria, hypertension | Not specified | Renal replacement therapy |
| Morales et al. 2021 [46] | 56 years/M | DR with DME | Ranibizumab | Proteinuria, worsening renal function | Not specified | Renal replacement therapy |
| Ahmed et al. 2021 [47] | 41 years/M | DR with DME | Bevacizumab | Proteinuria, hypertension, worsening renal function | Diabetic nephropathy | Not specified |
| Gan et al. 2022 [48] | 57 years/M | CRVO | Ranibizumab | Proteinuria, hypertension, worsening renal function | Membranoproliferative GN with nephroangiosclerosis | Discontinuation of injections |
Abbreviations: DR diabetic retinopathy, DME diabetic macular edema, CRVO central retinal vein occlusion, FSGS focal segmental glomerulosclerosis, GN glomerulonephritis, y years, M/F male/female, VEGF vascular endothelial growth factor
Current evidence supports the short- to medium-term renal safety of intravitreal anti-VEGF agents, even in patients with diabetes and chronic kidney disease (CKD). However, data on long-term cumulative effects and subtle renal changes remain limited, warranting cautious optimism. Until larger, more comprehensive studies provide further clarity, clinicians should carefully assess individual patient risks and monitor renal function, particularly in high-risk groups receiving anti-VEGF therapy.
While no definitive evidence links anti-VEGF therapy to significant renal deterioration, patients with preexisting borderline kidney dysfunction may have a higher risk of renal adverse effects following injections. According to existing literature, anti-VEGF agents can be used safely even in patients with CKD [37–39]. However, in cases where baseline renal function is significantly compromised (e.g., eGFR < 30 mL/min/1.73m2), additional caution is advised, with close monitoring to detect any potential renal impact [43].
Cardiovascular Effects of Anti VEGFs
Intravitreal anti-VEGF therapy has been widely used for retinal diseases in recent times, which has raised concerns about its systemic effects, particularly on the heart. Systemic absorption of anti-VEGFs has been reported to be associated with arterial thromboembolic events (ATEs), hypertension, heart failure, and arrhythmias [49].
The cumulative evidence on the cardiovascular effects of repeated doses of various anti-VEGF agents is reviewed in Table 5 to understand the potential impact of intravitreal anti-VEGF therapy on the heart.
Table 5.
Cardiovascular effects of repeated intravitreal anti-VEGF therapy
| Author, year | Type of study | Anti-VEGF agent(s) | Results | Inference |
|---|---|---|---|---|
| Kwon et al. 2018 [50] | Retrospective study investigating MI within 2 months of anti-VEGF injections | Bevacizumab | No difference in MI prevalence by retinal disease; MI associated with prior MI/CVA | Caution advised before administering anti-VEGF in patients with MI risk factors |
| Dalvin et al. 2019 [51] | Population-based cohort study of patients with exudative ARMD (2004–2013) | Pegaptanib, bevacizumab, ranibizumab, aflibercept | Anti-VEGF therapy was not associated with increased MI risk versus controls | Cardiac events not attributable to anti-VEGF therapy in exudative ARMD |
| Chen et al. 2021 [52] | Retrospective population-based study comparing IVI versus non-IVI groups in patients with prior MI/stroke | Not specified | IVI group had significantly higher mortality, especially when injections occurred within 1 year of prior MI/stroke | Increased mortality risk associated with anti-VEGF in post-MI/stroke patients |
| Chou et al. 2023 [49] | Nationwide population-based cohort study analyzing ATE risk | Ranibizumab, aflibercept | 38 ATEs in 6 months post-injection; IVR had lower ATE risk than IVA; higher ATE risk in patients with recent MI/IS or CAD | IVR may be safer than IVA in patients with recent cardiovascular events; caution recommended |
| Lai et al.. 2024 [53] | Retrospective study of diabetic patients without prior CVD; compared anti-VEGF versus laser/steroid cohort | Bevacizumab, ranibizumab, aflibercept, faricimab, brolucizumab | No acute MI events at 1 month; similar MI incidence at 6 and 12 months across groups | Anti-VEGF therapy appears safe in diabetic patients without baseline CVD |
| Sui et al. 2024 [54] | Case report of elderly patient with 10 years of anti-VEGF therapy | Ranibizumab, conbercept, aflibercept | Unexpected cardiac dysfunction observed; improvement after discontinuation of anti-VEGF | Suggests importance of cardiac monitoring in elderly and long-term anti-VEGF users |
Abbreviations: MI myocardial infarction, CVA cerebrovascular accident, ARMD age-related macular degeneration, IVI intravitreal injection, ATE arterial thromboembolic events, CAD coronary artery disease, IS ischemic stroke, IVR intravitreal ranibizumab, IVA intravitreal aflibercept, CVD cardiovascular disease, VEGF vascular endothelial growth factor
Systematic reviews and meta-analyses of intravitreal anti-VEGF agents, including bevacizumab, ranibizumab, and aflibercept, have found no significant increase in the risk of major adverse cardiovascular events (MACEs) such as myocardial infarction (MI), stroke, or cardiovascular death compared with controls [55]. Similarly, overall mortality was not significantly elevated; however, subgroup analysis indicated a potential concern in patients with diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR), where mortality risk appeared higher. Notably, no increased risk was observed for nonocular hemorrhages, venous thromboembolism, arterial hypertension, or heart failure across studies. Sensitivity analyses confirmed the robustness of these findings, with low heterogeneity, though slight asymmetry in funnel plots suggested minor publication bias, which was not statistically significant.
A systematic review and meta-analysis by Lees et al. found that the incidence of hypertension and new or worsening heart failure was comparable between patients receiving intravitreal anti-VEGF therapy and control groups [56].
A pharmacovigilance study using the VigiBase database analyzed 23,129 adverse drug reactions (ADRs) associated with intravitreal anti-VEGF therapy and found a significantly higher reporting rate of cardiovascular and cerebrovascular ADRs compared with the full database [57]. Reported cardiovascular ADRs included myocardial infarction, angina pectoris, arrhythmias (such as atrial fibrillation, atrial flutter, and ventricular fibrillation), hypertension, and hypertensive crisis. Interdrug comparisons showed that aflibercept had lower reporting odds ratios for myocardial infarction, atrial fibrillation, and cerebrovascular accidents compared with ranibizumab. These findings emphasize the increased reporting of cardiovascular and cerebrovascular ADRs following anti-VEGF therapy, with ranibizumab exhibiting a higher reporting rate relative to bevacizumab and aflibercept. This underscores the importance of careful monitoring, especially in patients at higher risk for systemic adverse events.
The apparent discrepancy between these studies could stem from differences in methodology. Systematic reviews and meta-analyses synthesize controlled trial data, whereas pharmacovigilance studies reflect real-world reporting, which may include biases and confounders. Nonetheless, the increased reporting in pharmacovigilance data emphasizes the need for heightened vigilance, especially for high-risk groups such as those with preexisting cardiovascular or cerebrovascular conditions.
These conclusions advocate for personalized treatment approaches, careful patient selection, and post-marketing surveillance to mitigate systemic risks while ensuring the therapeutic benefits of anti-VEGF therapies in retinal diseases. Further research is needed to explore the biological and pharmacokinetic mechanisms underlying these observed ADRs and their variation across different anti-VEGF agents. While intravitreal anti-VEGF therapies are generally safe, systemic risks, particularly cardiovascular and cerebrovascular ADRs, should not be overlooked in high-risk populations. The choice of anti-VEGF agent should be personalized, with heightened vigilance for patients with diabetes or preexisting cardiovascular conditions. Bridging the gap between controlled trials and real-world data remains essential to optimize patient safety.
To summarize, while concerns exist regarding systemic absorption of anti-VEGF agents following intravitreal administration, current evidence from the literature generally indicates no significant adverse effects on the heart. Our research findings suggest that the proximity of a prior stroke or MI, particularly within 6–12 months, is a stronger predictor of increased cardiovascular risk than prolonged q4 injections. While long-term frequent injections (e.g., over 10 years) may contribute to gradual cardiac dysfunction, the immediate post-stroke/MI period presents the highest risk.
From an extensive review of the nine documented studies, we summarize that a thorough cardiac evaluation before and after injections is essential for patients with cardiovascular risk factors.
Central Nervous System (CNS) Effects of Anti-VEGFs
The systemic absorption of intravitreal anti-VEGF agents raises concerns about their potential impact on the nervous system. The blood–brain barrier (BBB) is a highly selective barrier that typically prevents anti-VEGF molecules from crossing under normal conditions. However, in pathological states such as stroke, neuroinflammatory disease, or tumors, a compromised BBB may allow small amounts of these agents to enter the CNS. Additionally, minimal levels may reach the CNS through systemic circulation.
The BHAM study highlighted that neuronal health and cognitive function in the central nervous system are linked to the maintenance of normal physiological VEGF expression levels [58].
The cumulative evidence on the nervous system effects of repeated doses of various anti-VEGF agents is reviewed in Table 6 on the basis of an extensive analysis of eight recent studies, to understand the potential impact of intravitreal anti-VEGF therapy on the CNS.
Table 6.
Neurological and cerebrovascular effects of repeated intravitreal anti-VEGF therapy
| Author, year | Type of study | Anti-VEGF agent(s) | Results | Inference |
|---|---|---|---|---|
| Starr et al. 2019 [59] | Retrospective study with 2-year follow-up; control group with no anti-VEGF injections | Not specified | 5.8% had stroke post anti-VEGF; 71.1% ischemic, 15.8% embolic, 13.2% hemorrhagic; stroke types similar to control group | No increased stroke subtype risk due to anti-VEGF; no predilection toward infarcts or hemorrhages |
| Dalvin et al. 2019 [60] | Population-based retrospective cohort study (2004–2013) in exudative ARMD patients | Pegaptanib, bevacizumab, ranibizumab, aflibercept | No increased stroke risk in anti-VEGF group versus controls | Intravitreal anti-VEGF not linked to increased stroke risk in ARMD |
| Sultana et al. 2020 [61] | Retrospective review of individual case safety records (ICSRs) for anti-VEGF drugs (2010–2016) | Bevacizumab, ranibizumab, pegaptanib, aflibercept | 59.88% of ICSRs from > 65 y/o; potential signal linking intravitreal ranibizumab with Parkinson’s disease | VEGF inhibition might impair neural signalling and plasticity, potentially contributing to Parkinson’s disease |
| Chen et al. 2021 [51] | Population-based retrospective study comparing patients with stroke/MI who received versus did not receive anti-VEGF | Not specified | Higher mortality in IVI group, especially if injections given within 1 year of MI/stroke | Increased mortality risk with anti-VEGF in patients with recent stroke/MI |
| Yoshimoto et al. 2021 [62] | Case report of 70-year-old male with DME | Aflibercept | Hypertensive cerebral hemorrhage 1-month post-bilateral injections; plasma VEGF dropped below detection and remained low for 2 months; recovered to 41 pg/ml after 2 months | Persistent VEGF suppression can trigger hypertension and cerebral hemorrhage; caution in elderly with comorbidities |
| Ray et al. 2021 [57] | Prospective study of ARMD patients (age 65–85); used iPad-based brain health test versus injection number | Not specified | > 20 injections linked with increased risk of mild cognitive impairment | Possible cumulative effect of anti-VEGF on cognitive function; brain health monitoring may be warranted |
| Fugara et al. 2022 [63] | Prospective study of diabetic patients: laser/conservative (Ggroup 1) versus anti-VEGF (group 2) | Bevacizumab | Non-arteritic ischemic optic neuropathy (NAION) more common in group 2, especially with ≥ 3 injections | Repeated bevacizumab may increase NAION risk in diabetic eyes |
| Yang et al. 2025 [57] | Pharmacovigilance study using VigiBase for cardiovascular/cerebrovascular ADRs with anti-VEGF | Ranibizumab, bevacizumab, aflibercept | Higher reporting of cerebral infarction, carotid stenosis, cerebral and subarachnoid hemorrhage; underreporting with aflibercept; higher rates with ranibizumab | Intravitreal anti-VEGF associated with cerebrovascular ADRs; ranibizumab showed higher cerebrovascular ADR reports than other agents |
IVI intravitreal injection, DME diabetic macular edema, ARMD age-related macular degeneration, ICSR individual case safety record, ADRs adverse drug reactions, NAION non-arteritic ischemic optic neuropathy, VEGF vascular endothelial growth factor, MI myocardial infarction
The impact of anti-VEGF therapy on CNS health remains a subject of ongoing debate. While some studies suggest a decline in neuronal health with repeated injections and an increased risk of cerebrovascular adverse events, many others have demonstrated a favorable safety profile compared with controls. However, there is a broad consensus on exercising caution when administering anti-VEGF injections in patients with a recent history of stroke, as repeated injections may elevate the risk of stroke. Further research is needed to clarify these concerns, but until then, patient management should be approached on a case-by-case basis.
Safety Concerns of Anti-VEGFs in Special Situations
Pregnancy
The administration of intravitreal anti-VEGFs in sight-threatening conditions during pregnancy raises significant concerns. This is primarily due to the systemic absorption of these drugs and the potential risk of placental transmission, which raises questions about possible teratogenic effects on the fetus. In this context, it is essential to examine the concerns, review the available evidence, and explore the major consensus on the use of anti-VEGFs during pregnancy.
Concerns about Intravitreal Anti-VEGF Use during Pregnancy
Anti-VEGF drugs are classified as category C medications and are generally not recommended for use in pregnant women [64]. However, pregnancy can trigger or worsen retinal conditions that may necessitate treatment with these agents. Therefore, a thorough evaluation of their safety is crucial for making an informed clinical decision. Since VEGF plays a vital role in maintaining fetal and placental vasculature, the antiangiogenic effects of these drugs raise significant concerns [65, 66].
Evidence on the Safety Concerns of Anti-VEGF Use in Pregnancy
Table 7 presents the latest evidence on the safety of intravitreal anti-VEGF use during pregnancy.
Table 7.
Latest evidence on the safety of intravitreal anti-VEGF use during pregnancy
| Study | Study design | Anti-VEGF agent(s) | Patient details and results | Inference |
|---|---|---|---|---|
| Polizzi et al. 2015 [67] | Case series (n = 3) | Bevacizumab |
All injections in second or third trimester No drug-related adverse events Healthy full-term infants One patient had miscarriage risk factors |
Anti-VEGF may be used in later trimesters when benefit outweighs fetal risk Exercise caution, especially in the first trimester |
| Fossum et al. 2018 [68] | Case series (n = 3) | Ranibizumab |
Injections at 8, 10, and 21 weeks post-LMP All delivered healthy babies No developmental malformations |
Ranibizumab did not show adverse effects, even in early pregnancy exposure |
| Ong et al. 2022 [69] | Retrospective study (n = 42 pregnancies) | Ranibizumab, aflibercept |
40% (16/41) unaware of pregnancy during injection 81% live births (34/42) 12% miscarriages (5/42), 7% stillbirths (3/42), mostly in high-risk pregnancies |
Anti-VEGF may not significantly affect obstetric outcomes Recommend routine pregnancy screening before injections in women of child-bearing age |
n number of subjects or patients in the study, VEGF vascular endothelial growth factor, LMP last menstrual period
Isolated case reports have documented instances of spontaneous abortion occurring within days of intravitreal bevacizumab administration, particularly in high-risk patients [70, 71]. Additionally, Sullivan et al. reported a case of preeclampsia following intravitreal bevacizumab treatment [72] .
Major Consensus till Date Based on Available Evidence
Among anti-VEGF agents, bevacizumab has been linked to the highest systemic exposure, while ranibizumab exhibits the least. Furthermore, no drug accumulation has been observed between the first and third doses of ranibizumab, in contrast to the persistent accumulation seen with bevacizumab and aflibercept. On the basis of this evidence, ranibizumab may be considered a potentially safer intravitreal option for pregnant patients [73]. Given the inherently high risk of spontaneous miscarriage in the first trimester, establishing a direct causal relationship between early pregnancy loss and anti-VEGF injections is challenging. However, the short interval between injection administration and abortion raises concern. Therefore, it is advisable to avoid intravitreal anti-VEGF treatment during the first trimester, particularly in high-risk cases [73].
The transplacental passage rate of ranibizumab is lower than bevacizumab owing to the inability of the Fab fragment to cross the placenta [68]. So, ranibizumab seems to be a safer option than other anti-VEGFs in pregnancy. Many patients are unaware of their pregnancy when injected with anti-VEGFs. Conducting a routine urinary pregnancy test in the child-bearing age group can be considered before treatment to prevent unwanted complications [69]. To conclude, intravitreal anti-VEGFs can be given during pregnancy only when the potential benefit to the woman justifies the potential risks to the fetus.
Lactating Women
The systemic absorption of anti-VEGF agents and their potential secretion into breast milk during the postpartum period have raised concerns regarding their safety.
Concerns about Intravitreal Anti-VEGF Use during Breastfeeding
VEGF-A regulates local mammary gland development in the lactating mother and is an essential growth factor in infancy. It is present in high concentration in breast milk [74]. VEGF-A also plays an important role in the development of the digestive system, neurogenesis, renal medullary microcirculation expansion, and lung angiogenesis and alveolarization in infants [74].
Intravitreal anti-VEGF administration has been reported to be associated with a reduction in VEGF-A levels in breast milk [74]. This raises concern about the safety of these drugs.
Evidence on Safety Concerns of Anti-VEGF Use during Breastfeeding
There are very limited data on breastfeeding in the context of intravitreal anti-VEGFs. McFarland et al. [75] reported a reduction of 35% in VEGF-A levels in breast milk 2 weeks following bevacizumab injection. A transient drop in VEGF-A levels in breast milk has been reported within 6–12 h (about 20–30% lower), after an intravitreal ranibizumab injection, and increasing to pre-injection levels by 24 h after injection [76]. It has been observed that discontinuation of breast feeding after injection can lead to accumulation of the drug in breast milk.
Table 7 illustrates the effects of anti-VEGF therapy on pregnancy on the basis of findings from three recent comprehensive studies.
Major Consensus till Date Based on Available Evidence
Considering the small number of cases in the literature, it is challenging to draw definite conclusions regarding the clinical impact of anti-VEGF injections during breastfeeding. On the basis of available data, ranibizumab appears to be a safer option than aflibercept for breastfeeding women, as aflibercept has been associated with a greater reduction in plasma VEGF levels compared with ranibizumab [77].
Basilious et al. recommended a 3-day “pump and dump” breastfeeding strategy for lactating mothers after an intravitreal injection [64]. In this approach, nursing mothers pump and discard their breast milk for 3 days following the injection, then resume breastfeeding on demand after the third day. Preliminary results from this study suggest that this strategy effectively minimizes the infant’s exposure to the drug. This approach resulted in undetectable levels of ranibizumab in the infant’s serum, with plasma VEGF-A levels remaining comparable to those observed in control infants.
Retinopathy of Prematurity (ROP)
The use of anti-VEGF treatment has revolutionized the management of ROP by promoting progressive vascularization of the avascular retina, offering the potential for a broader visual field development. However, this has raised concerns about its safety in newborns.
Concerns about Intravitreal Anti-VEGF Use in ROP
Inhibiting VEGF bioactivity in preterm neonates may disrupt normal growth and development in multiple organs. VEGF is crucial for neuronal and endothelial cell survival, with neuroprotective and neurotrophic properties in the brain. It also supports blood–brain barrier maintenance, lung alveolar development, surfactant synthesis, and kidney glomerular development, while playing a role in skeletal growth. Blocking VEGF could therefore impact various developing organs in neonates [78, 79].
Evidence on the Safety Concerns of Anti-VEGF Use in ROP
Table 8 presents the safety concerns of anti-VEGF use in ROP on the basis of an evaluation of four recent studies.
Table 8.
Evidence on the safety concerns of anti-VEGF use in retinopathy of prematurity (ROP)
| Author, year | Study design | Anti-VEGF | Results | Inference |
|---|---|---|---|---|
| Arima et al. 2020 [80] | Retrospective study | Bevacizumab (IVB) | Significant association between IVB and neurodevelopmental delay in language-social scores, even after adjusting for GA and BW | IVB may affect the development of verbal/social skills in ROP infants |
| Zayek et al. 2020 [81] | Retrospective study | Bevacizumab (IVB) | No significant association between IVB and neurodevelopmental delay | IVB was not associated with adverse neurodevelopment |
| Abrishami et al. 2021 [82] | Retrospective study | Bevacizumab | No significant difference between treatment groups in developmental milestones (motor, personal–social, etc.) at 18 months | No growth or neurodevelopmental differences between treatment-naïve infants and those receiving bevacizumab for ROP |
| Chen et al. 2023 [83] | Retrospective study | Ranibizumab (IVR), Aflibercept (IVA) | BW gain for the first week after treatment was significantly lower than pre-treatment week (P < 0.05). No decrease in BW gain in the second week | IVR and IVA could have a short-term inhibitive effect on body weight gain in infants after ROP treatment |
IVB intravitreal bevacizumab, IVR intravitreal ranibizumab, IVA intravitreal aflibercept, GA gestational age, BW birth weight, VGEF vascular endothelial growth factor
Major Consensus till Date Based on Available Evidence
Infants treated with intravitreal injections are often significantly more premature and have lower birth weights compared with those treated with laser. Therefore, it is challenging to establish a direct causal association between anti-VEGF treatment and potential adverse effects.
Most of the literature does not report significant long-term systemic side effects or neurological impairment associated with anti-VEGF use in ROP. Thus, to conclude, anti-VEGF therapy can be considered for vision-threatening ROP cases after carefully weighing the potential risks and benefits [84].
Table 9 summarizes the key clinical guidelines for anti-VEGF use across different systems.
Table 9.
Clinical guidelines for anti-VEGF use across different systems
| System | Guidelines |
|---|---|
| Renal | Exercise caution in patients with significantly compromised renal function (eGFR < 30 mL/min/1.73m2) |
| Close monitoring is advised to detect potential renal impact | |
| Cardiovascular (CVS) | Prior stroke or MI within 6–12 months is a stronger predictor of cardiovascular risk than prolonged q4 injections |
| Long-term frequent injections (> 10 years) may contribute to gradual cardiac dysfunction | |
| — | |
| Central nervous system (CNS) | Most of the literature does not report significant long-term systemic side effects or neurological impairment associated with anti-VEGF use |
| Caution is advised when administering anti-VEGFs to patients with a recent history of stroke, as repeated injections may elevate stroke risk | |
| Pregnancy | Anti-VEGFs should be used only when the potential benefit to the woman justifies the potential fetal risks |
| A routine urinary pregnancy test can be considered before treatment in women of childbearing age | |
| Ranibizumab appears safer than other anti-VEGFs in pregnancy | |
| Avoid intravitreal anti-VEGF treatment during the first trimester, particularly in high-risk cases | |
| Breastfeeding | Ranibizumab appears to be a safer option than aflibercept for breastfeeding women |
| A 3-day “pump and dump” strategy is recommended after intravitreal injections | |
| Retinopathy of prematurity (ROP) | Anti-VEGF therapy can be considered for vision-threatening ROP cases after carefully weighing risks and benefits |
VEGF vascular endothelial growth factor, eGFR estimated glomerular filtration rate, MI myocardial infarction
Conclusions
While intravitreal anti-VEGF therapies have revolutionized the treatment of various retinal conditions, understanding their systemic side effects is crucial for optimizing patient care. Current evidence supports the renal safety of anti-VEGF agents in the short to medium term, with cautious monitoring advised for patients with preexisting renal dysfunction, particularly those with significantly impaired kidney function. Cardiovascular and cerebrovascular risks, though not conclusively linked to anti-VEGF therapy, warrant vigilance, especially in high-risk patients, as some studies suggest a slight increase in adverse events such as myocardial infarction, stroke, and arrhythmias. As further studies are conducted, a more refined understanding of the long-term and subtle systemic impacts of anti-VEGF therapy will be essential. Clinicians must continue to assess individual risks, monitor patients closely, and provide personalized care to ensure the safe use of these therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Mousumi Banerjee conducted the literature search and drafted the manuscript. Sikshya Moharana contributed to drafting the section on pregnancy and anti-VEGF. Srikanta Kumar Padhy assisted with conceptualization, study design, and manuscript editing. All authors reviewed and approved the final version of the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Declarations
Conflict of Interest
Srikanta Kumar Padhy, Sikshya Moharana, and Mousumi Banerjee have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003–17. [DOI] [PubMed] [Google Scholar]
- 2.Simon M, Gröne HJ, Jöhren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268(2 Pt 2):F240–50. [DOI] [PubMed] [Google Scholar]
- 3.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A. 1998;95(26):15809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gröne HJ, Simon M, Gröne EF. Expression of vascular endothelial growth factor in renal vascular disease and renal allografts. J Pathol. 1995;177(3):259–67. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S, Vanuystel J, Gruden G, Rodríguez V, et al. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11(7):1236–43. [DOI] [PubMed] [Google Scholar]
- 6.Crivellato E. The role of angiogenic growth factors in organogenesis. Int J Dev Biol. 2011;55(4–5):365–75. [DOI] [PubMed] [Google Scholar]
- 7.Eremina V, Sood M, Haigh J, Nagy A, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palade GE, Simionescu M, Simionescu N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl. 1979;463:11–32. [PubMed] [Google Scholar]
- 9.Kim BS, Goligorsky MS. Role of VEGF in kidney development, microvascular maintenance and pathophysiology of renal disease. Korean J Intern Med. 2003;18(2):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinashi H, Falke LL, Nguyen TQ, Bovenschen N, Aten J, Leask A, Ito Y, Goldschmeding R. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int. 2017;92(4):850–63. [DOI] [PubMed] [Google Scholar]
- 11.Veron D, Aggarwal PK, Velazquez H, Kashgarian M, et al. Podocyte-specific VEGF-a gain of function induces nodular glomerulosclerosis in eNOS null mice. J Am Soc Nephrol. 2014;25(8):1814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baelde HJ, Eikmans M, Lappin DW, Doran PP, et al. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71(7):637–45. [DOI] [PubMed] [Google Scholar]
- 13.Doi K, Noiri E, Fujita T. Role of vascular endothelial growth factor in kidney disease. Curr Vasc Pharmacol. 2010;8(1):122–8. [DOI] [PubMed] [Google Scholar]
- 14.Melincovici CS, Bosca AB, Susman S, Marginean M, Mihu C, Istrate M, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59:455–67. [PubMed] [Google Scholar]
- 15.Braile M, Marcella S, Cristinziano L, Galdiero MR, et al. VEGF-A in cardiomyocytes and heart diseases. Int J Mol Sci. 2020;21(15):5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv YX, Zhong S, Tang H, Luo B, et al. VEGF-A and VEGF-B coordinate the arteriogenesis to repair the infarcted heart with vagus nerve stimulation. Cell Physiol Biochem. 2018;48(2):433–49. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Zhu X, Cui H, Shi J, et al. The role of the VEGF family in coronary heart disease. Front Cardiovasc Med. 2021;8: 738325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100(3):727–37. [DOI] [PubMed] [Google Scholar]
- 19.Lucarini G, Zizzi A, Rubini C, Ciolino F, et al. VEGF, microvessel density, and CD44 as inflammation markers in Peri-implant healthy mucosa, peri-implant mucositis, and peri-implantitis: impact of age, smoking, ppd, and obesity. Inflammation. 2019;42(2):682–9. [DOI] [PubMed] [Google Scholar]
- 20.Arjunan P, Lin X, Tang Z, Du Y, et al. VEGF-B is a potent antioxidant. Proc Natl Acad Sci U S A. 2018;115(41):10351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zentilin L, Puligadda U, Lionetti V, Zacchigna S, et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2010;24(5):1467–78. [DOI] [PubMed] [Google Scholar]
- 22.Zhao T, Zhao W, Meng W, Liu C, et al. Vascular endothelial growth factor-C: its unrevealed role in fibrogenesis. Am J Physiol Heart Circ Physiol. 2014;306(6):H789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira JM, Norman S, Villa Del Campo C, Cahill TJ, et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J Clin Invest. 2018;128(8):3402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamminsalo M, Urtti A, Ranta VP. Quantitative pharmacokinetic analyses of anterior and posterior elimination routes of intravitreal anti-VEGF macromolecules using published human and rabbit data. Exp Eye Res. 2022;222: 109162. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser PK, Kodjikian L, Korobelnik JF, Winkler J, Torri A, Zeitz O, et al. Systemic pharmacokinetic/pharmacodynamic analysis of intravitreal aflibercept injection in patients with retinal diseases. BMJ Open Ophthalmol. 2019;4(1): e000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semeraro F, Morescalchi F, Duse S, Gambicorti E, Cancarini A, Costagliola C. Pharmacokinetic and pharmacodynamic properties of Anti-VEGF drugs after intravitreal injection. Curr Drug Metab. 2015;16(7):572–84. [DOI] [PubMed] [Google Scholar]
- 27.Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond). 2018;32(6):1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyake T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ogasawara K, Ohji M. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Invest Ophthalmol Vis Sci. 2010;51(3):1606–8. [DOI] [PubMed] [Google Scholar]
- 30.Gaudreault J, Gunde T, Floyd HS, Ellis J, Tietz J, Binggeli D, et al. Preclinical pharmacology and safety of ESBA1008, a single-chain antibody fragment, investigated as potential treatment for age related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(14):3025. [Google Scholar]
- 31.Diack C, Gibiansky L, Jaminion F, Gibiansky E, Gaudreault J, Bogman K, et al. Ocular pharmacokinetics of faricimab following intravitreal administration in patients with retinal disease. Transl Vis Sci Technol. 2024;13(11):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna RM, Barsoum M, Arman F, Selamet U, et al. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 2019;96(3):572–80. [DOI] [PubMed] [Google Scholar]
- 34.Eremina V, Cui S, Gerber H, et al. Vascular endothelial growth factor A signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17(3):724–35. [DOI] [PubMed] [Google Scholar]
- 35.Dimke H, Sparks MA, Thomson BR, Frische S, et al. Tubulovascular cross-talk by vascular endothelial growth factor A maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2015;26(5):1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado FG, Kuriki PS, Fujihara CK, et al. Chronic VEGF blockade worsens glomerular injury in the remnant kidney model. PLoS One. 2012;7(6): e39580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagheri S, Dormanesh B, Afarid M, Sagheb MM. Proteinuria and renal dysfunction after intravitreal injection of bevacizumab in patients with diabetic nephropathy: a prospective observational Study. Galen Med J. 2018;7: e1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glassman AR, Liu D, Jampol LM, Sun JK, Diabetic Retinopathy Clinical Research Network. Changes in blood pressure and urine albumin-creatinine ratio in a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(3):1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill RA, Gallagher P, Douglas T, Little JA, et al. Evaluation of long-term intravitreal anti-vascular endothelial growth factor injections on renal function in patients with and without diabetic kidney disease. BMC Nephrol. 2019;20(1):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang SF, Su YC, Lim CC, Huang JY, Hsu SM, Wu LW, Chang YS, Hung JH. Risk of dialysis in patients receiving intravitreal anti-vascular endothelial growth factor treatment: a population-based cohort study. Aging (Albany NY). 2022;14(12):5116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CH, Lim PS, Wu TK, Chuang WL, Yu TS, Tsai FJ, Chen CM, Chang KH. Intravitreal ranibizumab injection is associated with an increased risk of chronic kidney disease: a population-based study in Taiwan. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(7):4799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai CX, Nishimura A, Bowring MG, Westlund E, et al. Similar risk of kidney failure among patients with blinding diseases who receive ranibizumab, aflibercept, and bevacizumab: an observational health data sciences and informatics Network Study. Ophthalmol Retina. 2024;8(8):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunge CC, Dalal PJ, Gray E, Culler K, Brown JJ, Quaggin SE, et al. The association of intravitreal Anti-VEGF injections with kidney function in diabetic retinopathy. Ophthalmol Sci. 2023;3(4):100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shye M, Hanna RM, Patel SS, et al. Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin Kidney J. 2020;13(6):969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna RM, Tran NT, Patel SS, et al. Thrombotic microangiopathy and acute kidney injury induced after intravitreal injection of vascular endothelial growth factor inhibitors VEGF blockade-related TMA after intravitreal use. Front Med. 2020;7(7): 579603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales E, Moliz C, Gutierrez E. Renal damage associated to intravitreal administration of ranibizumab. Nefrol Engl Ed. 2017;37(6):653–5. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M, Alouch N, Ahmed A, Jagadesh SK. Worsening of renal function and uncontrolled hypertension from intravitreal bevacizumab injections. Bayl Univ Med Cent Proc. 2021;34(4):527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gan G, Michel M, Max A, Sujet-Perone N, Zevering Y, Vermion JC, Zaidi M, Savenkoff B, Perone JM. Membranoproliferative glomerulonephritis after intravitreal vascular growth factor inhibitor injections: a case report and review of the literature. Br J Clin Pharmacol. 2023;89(1):401–9. [DOI] [PubMed] [Google Scholar]
- 49.Chou YI, Chang HY, Lin MY, Tseng CH, Wang TJ, Lin IC. Risk analysis for patients with arterial thromboembolic events after intravitreal ranibizumab or aflibercept injections. Sci Rep. 2023;13(1):7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon JW, Jee D, La TY. The association between myocardial infarction and intravitreal bevacizumab injection. Medicine (Baltimore). 2018;97(13): e0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen YY, Lai YJ, Yen YF, Chou P. Increased mortality after intravitreal injections of anti-VEGF for neovascular AMD among patients with prior stroke or acute myocardial infarction. Eye (Lond). 2022;36(1):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou YI, Chang HY, Lin MY, Tseng CH, Wang TJ, Lin IC. Risk analysis for patients with arterial thromboembolic events after intravitreal ranibizumab or aflibercept injections. Sci Rep. 2023;13(1):7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai JYM, Riley DR, Anson M, Henney A, Cuthbertson DJ, Hernadez G, et al. Cardiovascular outcomes with intravitreal anti-vascular endothelial growth factor therapy in patients with diabetes: a real-world data analysis. Diabetes Ther. 2024;15(4):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui Y, Zhao Y, Zhou N, Sun H, Sun Y, Liu J. Case report: heart failure related to intravitreal injection of anti-VEGF. BMC Cardiovasc Disord. 2024;24(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngo Ntjam N, Thulliez M, Paintaud G, Salvo F, Angoulvant D, Pisella PJ, et al. Cardiovascular adverse events with intravitreal anti-vascular endothelial growth factor drugs: a systematic review and meta-analysis of Randomized Clinical Trials. JAMA Ophthalmol. 2021;139(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lees JS, Dobbin SJH, Elyan BMP, Gilmour DF, Tomlinson LP, Lang NN, Mark PB. A systematic review and meta-analysis of the effect of intravitreal VEGF inhibitors on cardiorenal outcomes. Nephrol Dial Transplant. 2023;38(7):1666–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang JM, Jung SY, Kim MS, Lee SW, Yon DK, Shin JI, et al. Cardiovascular and cerebrovascular adverse events associated with intravitreal Anti-VEGF monoclonal antibodies: a World Health Organization Pharmacovigilance Study. Ophthalmology. 2025;132(1):62–78. [DOI] [PubMed] [Google Scholar]
- 58.Ray SK, Manz SN. Brain health assessment in macular degeneration patients undergoing intravitreal anti-vascular endothelial growth factor injections (The Bham Study): an Interim analysis. Retina. 2021;41(8):1748–53. [DOI] [PubMed] [Google Scholar]
- 59.Starr MR, Dalvin LA, AbouChehade JE, Damento GM, Garcia MD, Shah SM, Hodge DO, Meissner I, Iezzi R, Bakri SJ. Classification of strokes in patients receiving intravitreal anti-vascular endothelial growth factor. Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):e140–57. [DOI] [PubMed] [Google Scholar]
- 60.Dalvin LA, Starr MR, AbouChehade JE, et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol. 2019;137(5):483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sultana J, Scondotto G, Cutroneo PM, Morgante F, Trifirò G. Intravitreal anti-VEGF drugs and signals of dementia and Parkinson-like events: analysis of the VigiBase database of spontaneous reports. Front Pharmacol. 2020;11:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshimoto M, Takeda N, Yoshimoto T, Matsumoto S. Hypertensive cerebral hemorrhage with undetectable plasma vascular endothelial growth factor levels in a patient receiving intravitreal injection of aflibercept for bilateral diabetic macular edema: a case report. J Med Case Rep. 2021;15(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fugara NA, Shawareb ZA, Rakkad NK, Barhoum ML, Shawareb BA, Al-Madani MM, Al-Madani MV. The risk of non-arteritic ischemic optic neuropathy post-intravitreal bevacizumab injection. Cureus. 2022;14(10): e30185. 10.7759/cureus.30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basilious A, Muni RH, Juncal VR. Anti-VEGF therapy in pregnancy and breastfeeding. Can Eye Care Today. 2023;2(2):15–9. [Google Scholar]
- 65.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280(6):C1358–66. [DOI] [PubMed] [Google Scholar]
- 66.Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med. 2009;22(5):371–8. [DOI] [PubMed] [Google Scholar]
- 67.Polizzi S, Mahajan VB. Intravitreal Anti-VEGF injections in pregnancy: case series and review of literature. J Ocul Pharmacol Ther. 2015;31(10):605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fossum P, Couret C, Briend B, Weber M, Lagarce L. Safety of intravitreal injection of ranibizumab in early pregnancy: a series of three cases. Eye (Lond). 2018;32(4):830–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong AY, Kiire CA, Frise C, Bakr Y, de Silva SR. Intravitreal anti-vascular endothelial growth factor injections in pregnancy and breastfeeding: a case series and systematic review of the literature. Eye (Lond). 2024;38(5):951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petrou P, Georgalas I, Giavaras G, et al. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88(4): e136. [DOI] [PubMed] [Google Scholar]
- 71.Kianersi F, Ghanbari H, Naderi Beni Z, Naderi BA. Intravitreal vascular endothelial growth factor (VEGF) inhibitor injection in patient during pregnancy. J Drug Assess. 2021;10(1):7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sullivan L, Kelly SP, Glenn A, Williams CP, McKibbin M. Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye (Lond). 2014;28(4):492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98(12):1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozgurtas T, Aydin I, Turan O, Koc E, Hirfanoglu IM, Acikel CH, Akyol M, Serdar M, Erbil KM. Soluble vascular endothelial growth factor receptor 1 in human breast milk. Hormone Res Paediat. 2011;76(1):17–21. [DOI] [PubMed] [Google Scholar]
- 75.McFarland TJ, Rhoads AD, Hartzell M, Emerson GG, Bhavsar AR, Stout JT. Bevacizumab levels in breast milk after long-term intravitreal injections. Retina. 2015;35(8):1670–3. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y, Zhou R, Sun Z, Zheng Y, Lin B. Vascular endothelial growth factor-A level in human breast milk after intravitreal injection of ranibizumab: a case report. Int Breastfeed J. 2022;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Avery R, Castellarin A, Steinle N, Dhoot D, Pieramici D, See R, et al. Systemic pharmacokinetics and pharmacodynamics of afibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78..Hapsari D, Sitorus RS. Intravitreal bevacizumab in retinopathy of prematurity: inject or not? Asia Pac J Ophthalmol. 2014;3(6):368–78. [DOI] [PubMed]
- 79.Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F170–4. [DOI] [PubMed] [Google Scholar]
- 80.Arima M, Akiyama M, Fujiwara K, Mori Y, Inoue H, Seki E, Nakama T, Tsukamoto S, Ochiai M, Ohga S, Sonoda KH. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS One. 2020;15(3): e0230678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81..Zayek M, Parker K, Rydzewska M, Rifai A, Bhat R, Eyal F. Bevacizumab for retinopathy of prematurity: 2-year neurodevelopmental follow-up. Am J Perinatol. 2021;38(11):1158–66. [DOI] [PubMed]
- 82.Abrishami M, Boskabadi H, Abrishami M, Shekarchian F, Khadem-Rezaiyan M, Shoeibi N. Growth and neurodevelopmental status in patients with retinopathy of prematurity treated with intravitreal bevacizumab: a case-control study. Int J Retina Vitreous. 2021;7(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Hao Q, Zhang J, Du Y, Chen H, Cheng X. Short-term effects of intravitreal anti-vascular endothelial growth factor agents on body weight and multiple systems after treatment for retinopathy of prematurity. Front Pediatr. 2023;10:1077137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalvin LA, Starr MR, AbouChehade JE, Damento GM, Garcia M, Shah SM, et al. Association of intravitreal anti-vascular endothelial growth factor therapy with risk of stroke, myocardial infarction, and death in patients with exudative age-related macular degeneration. JAMA Ophthalmol. 2019;137(5):483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.