Summary
Persistent chronic inflammation is a hallmark of ankylosing spondylitis (AS), with cytotoxic T cells (CTLs) increasingly implicated in its pathogenesis. Ordinarily, T cell exhaustion follows sustained, persistent T cell activation to limit collateral tissue damage. Using mass cytometry and single-cell RNA sequencing (scRNA-seq), we identified a clonally expanded CTL subset in AS synovial fluid that expresses inhibitory receptors (PD-1, TIGIT, LAG-3) yet retains its effector capacity to express granzymes, perforin, TNF-α, and IFN-γ. Gene expression profile of this CTL subset shows the downregulation of canonical exhaustion markers. At the protein level, TOX, a critical transcription factor regulating CTL exhaustion, is downregulated in PD-1+TIGIT+LAG-3+CTLs. In-silico trajectory analyses suggest that these cells may differentiate into other effector CTL subsets. Our findings reveal a checkpoint-expressing CTL population in AS that resists exhaustion and retains an activated, effector phenotype. We propose that failure to undergo exhaustion may be a fundamental mechanism sustaining AS chronic inflammation.
Subject areas: Immunology, Immune response, Transcriptomics
Graphical abstract

Highlights
-
•
CyTOF reveals CD8+ T cells with inhibitory receptors enriched in AS synovial fluid
-
•
scRNA-seq reveals the clonal CTL expansion and downregulation of exhaustion markers
-
•
AS synovial CTLs with inhibitory receptors retain an effector-not exhausted-state
-
•
CTL resistance to exhaustion may sustain activation, driving AS's chronic inflammation
Immunology; Immune response; Transcriptomics
Introduction
Ankylosing spondylitis (AS) is a chronic, inflammatory disease characterized by back pain, spinal ankylosis, peripheral arthritis, and in many cases extra-articular inflammatory manifestations.1 Chronic, persistent inflammation in the spine and the sacroiliac joints is a hallmark of the disease and is a precursor to later spinal ankylosis.2 The strong genetic association between HLA-B27 and AS is well established.3 The predominant physiological role of HLA-B27, a MHC class I molecule, is to bind and present antigenic peptides on the surface of antigen-presenting cells for recognition by CD8+ cytotoxic T lymphocytes (CTLs). Recent discoveries of pathogenic CTLs enriched in AS synovial fluid (SF) have reinvigorated studies addressing their contributions to AS inflammation.4,5 We recently reported a population of mature CD103+ CD49a+ CTLs residing in AS synovial fluid which express a unique set of surface integrins, implicating gut-joint trafficking of pathogenic CD8+ T cells.4 RNA-sequencing of synovial CTLs revealed that these cells possess both cytotoxic and regulatory properties, which may contribute to persistent inflammation in AS.
T cell exhaustion refers to a distinct immune state whereby CTLs are rendered unresponsive to further T cell receptor (TCR) stimulation.6,7 Chronic antigen exposure and persistent TCR stimulation in the absence of T cell co-stimulation are central to this process. Exhausted CD8+ T cells (Tex) often develop in the setting of chronic viral infections or cancer, facilitating viral persistence and impairment of anti-tumour immunity, respectively.8 Physiologically, exhaustion functions as a host homeostatic mechanism to limit excessive collateral tissue damage caused by over-activated T cells in the setting of chronic inflammation.6,7 T cell exhaustion is characterized by reduced proliferative potential, progressive loss of cytotoxic effector functions (e.g., production of IFNγ and TNFα, as well as release of perforin and granzyme), and a transcriptional and epigenetic state distinct from effector, memory, and anergic T cells (e.g., upregulated TOX expression).7 Another hallmark of T cell exhaustion is sustained, elevated the expression of co-inhibitory receptors (e.g., PD-1, TIGIT, LAG-3, 2B4, and CD39). However, mere expression of these checkpoint receptors does not necessarily define an exhausted CTL.7,9 Activated effector CTLs within a proinflammatory microenvironment can also express elevated levels of co-inhibitory receptors.9 In autoimmunity, the enrichment of Tex cells has been implicated in patients with less severe disease,10 suggesting that targeted manipulation of the exhaustion process could represent new therapeutic opportunities. Chronic antigen exposure and persistent inflammation are central in AS. These conditions are conducive to the development of Tex. In this study, we sought proteomic and transcriptomic evidence to determine whether CTLs in AS are truly immunologically exhausted or behave aberrantly as activated effector cells. Delineating the complex heterogeneity of exhausted and “non-exhausted” CTLs through single-cell analyses could provide new insights for manipulating pathogenic CTLs in AS.
A recent study from Yang et al. provided supportive evidence of pathogenic TRBV9+ CTLs in blood and synovial fluid (SF) of patients with AS that may recognize self and microbial antigens.11 In the inflammatory milieu of AS, CTLs at sites of inflammation are perpetually stimulated. We hypothesize that chronically activated CTLs in this environment play a key pathogenic role in sustaining AS chronic inflammation. In this study, we applied high dimensional immune profiling strategies to characterize subsets of pathogenic CTLs in AS blood and SF. Combining mass cytometry profiling with single cell RNA-sequencing, we have identified a population of SF CD127- CTLs that express classical co-inhibitory receptors: PD-1, TIGIT, and LAG-3. These CTLs deviate from the canonical properties of Tex, such as the absence of TOX and other inhibitory receptors (e.g., 2B4 and CD39) expression, yet retain the capacity to produce inflammatory cytokines and CTL effector molecules.
Results
Expression patterns of checkpoint inhibitory receptors on ankylosing spondylitis cytotoxic cells
Our central hypothesis is that a subset of chronically activated CTLs is expanded in patients with AS and reflects a loss of immune homeostasis that normally limits excess effector CTL functions during inflammation. To address this, we designed a mass cytometry time-of-flight (CyTOF) panel (Table S3) to specifically measure frequencies of CTL memory subsets, to measure CTL activation and immune checkpoint receptor expression, and to evaluate expressions of markers related to cytolytic capacities (e.g., granzymes and perforin) of CTLs from peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) of patients with AS. PD-1 and TIGIT are well characterized inhibitory receptors (IR) that function as immune checkpoints to restrain T cell activation and effector functions. To characterize the overall immune checkpoint landscape of CTLs in AS, we first measured PD-1 and TIGIT expression on CD45RO+ CTLs from a cohort of patients with AS (n = 18) with active disease (Table S1). Compared to age- and sex-matched healthy controls, we discovered that surface PD-1 and TIGIT expression are significantly downregulated on peripheral blood CTLs of patients with AS with active disease (Figure 1A), suggesting that peripheral CTLs in patients with AS may not effectively restrain effector functions, and downregulation of these IRs could enhance short-term effector functions.12 These CTLs may migrate toward the joint as potent activated effector T cells.
Figure 1.
Dysregulation of immune checkpoint expression on peripheral cytotoxic cells (CTLs) in patients with ankylosing spondylitis
(A) PD-1 and TIGIT expression on peripheral CTLs from age and sex-matched healthy control subjects (n = 10) and patients with active axial spondyloarthritis (axSpA/ankylosing spondylitis/AS) (n = 18). ∗p < 0.05, calculated by Mann-Whitney U test.
(B) Uniform manifold approximation and projection (UMAP) plot shows CTL PhenoGraph clusters in AS and healthy control (HC) PBMCs. Clusters are annotated by cluster number (Right).
(C) CTL clusters with the proportions significantly increased in AS PBMC versus HC PBMC (red, Log2 FC > 0) or significantly decreased (blue, Log2 FC < 0). Clusters in gray: no significant proportion difference.
(D) Bar Graph shows the log2 fold proportional change (Log2 FC) for each cluster between the two groups (AS PBMC vs. HC PBMC). ∗∗p < 0.005, ∗p < 0.05. The p values are calculated by the diffcyt R package.
(E) For each PhenoGraph cluster, the colors represent the row Z score values of the median expression values of the indicated markers. Heatmap is generated by the ComplexHeatmap R package.
(F) Stacked bar plot shows the frequencies of each PhenoGraph cluster as a percentage of total CD8+ T cells for 10 HC PBMCs and 18 AS PBMCs.
(G) Frequency of biaxially gated- CD38 expressing effector CTLs defined as CD3+ CD8+ CD45RA- CCR7- CD27+ CD28+ for 10 HC PBMCs and 18 AS PBMCs. ∗p < 0.05, calculated by Mann-Whitney U test.
To further understand the immune alterations of CTLs in PBMCs of patients with AS, we performed clustering analysis of total peripheral blood CTLs from patients with AS and healthy controls using PhenoGraph.13 In total, 31 clusters were identified (Figure 1B), comprising of major CD8+ T cells memory subsets, as defined based on the expression of CD45RA, CCR7, CD62L, CD27, CD28 and CD95. Compared with age- and sex-matched healthy control subjects, PhenoGraph clusters c20, c27, c29, and c18 were most represented in patients with active AS (Figures 1C and 1D). Cluster c20 defines a subset of CD38+ naive T cells (CD45RA+ CCR7+CD62L+) that do not express checkpoint receptors (PD-1, TIGIT, and LAG-3), while cluster c27 defines a population of cells expressing CX3CR1 and cytolytic markers (PRF+, GZMB+, GZMA+) expressing terminally differentiated memory cells (TEMRA, CD45RA+ CCR7- KLRG1+) (Figure 1E). Cluster c29 defines a population of activated (HLA-DR+) memory CTLs expressing classical resident memory T cell markers (CD103+, CD49a+, and CD69+) as well as PD-L1 (Figure 1E). Through differential abundance analyses, we observed that although clusters c20, c27, and c29 were significantly overrepresented in patients with AS, these clusters were not uniformly present in all patients with AS (Figure 1F). Interestingly, cluster c29 was completely absent in healthy control subjects but was exclusively found in 8 out of 18 patients with AS with active disease (Figure 1F). To investigate whether the presence of clusters c20, c27, and c29 relates to certain clinical characteristics, we performed correlation analysis using the frequencies of clusters (% of all CD8 cells) and key clinical parameters: age, ESR, CRP, BASDAI (Bath AS Disease Activity Index), and ASDAS-CRP. The presence of cluster c20 (CD38+ naive CTLs) negatively correlated (r = −0.53, p = 0.0237) with age (Figure S1A), suggesting that CD38 expression on naive CTLs of younger patients with AS could be clinically informative. The presence of cluster c27 (PRF+ GZMB+ GZMA+ expressing TEMRA) negatively correlated (r = −0.4833, p = 0.0494) with BASDAI values (Figure S1C), suggesting that patients with higher disease activity have a reduced frequency of perforin- and granzyme-expressing CTLs, consistent with our prior observations.5
In contrast to clusters c20, c27, and c29, cluster c18, a population of CD38+ effector memory CTLs (CCR7- CD45RA- CD27+) was uniformly present in all patients with AS (Figure 1F), indicating that CD38 expression on effector memory CTLs could serve as an informative biomarker in AS. Biaxial gating of CD8+ T cells revealed that CD38+ CD95+ expressing CCR7- CD45RA- effector memory T cells were significantly enriched in the PBMCs (>10% of CD3+ CD8+ cells) of patients with AS compared to healthy control subjects (Figure 1G).
Our CyTOF analysis also revealed that clusters c26 and c19 were significantly underrepresented in AS PBMCs (Figure 1D). These clusters represent terminally effector memory CTL subsets, CCR7- CD45RA+, (Figure 1E) and were enriched with cytolytic markers: perforin, granzyme B, granzyme A, and granzyme K (Figure 1E). The downregulation of perforin and granzyme expressing CTL subsets was consistent with our previous observation that suggest patients with AS have an altered CTL profile.5 In summary, our CyTOF analysis confirmed that CTLs are dysregulated in AS. We also identified a distinct CTL signature present in the peripheral blood of patients with active AS.
Cytotoxic cells in ankylosing spondylitis synovial fluid are highly enriched for checkpoint receptors
Our previous publications suggested that activated CTLs are recruited to the joint.4,5 To gain insight into CTL dysregulation at sites of inflammation in patients with AS, we next dissected CTL phenotypes in AS SFMCs. In a previous CyTOF study, we identified a distinctive CTL phenotype expressing a unique set of integrin markers, exclusively in AS SFMCs.4 In the present study, we assembled a discovery cohort of 8 patients with AS (Table S1) active disease to investigate CTL memory subsets and IR expression on CTLs from SFMCs and matched PBMCs. Similar to the PBMC analysis above, we applied the same CyTOF panel to analyze surface PD-1, TIGIT, and LAG-3 expression of CTLs from 8 matching pairs of SFMCs-PBMCs. Uniform Manifold Approximation and Projection (UMAP) analysis of the CyTOF experiment revealed that the surface expression of PD-1, TIGIT, and LAG-3 was significantly upregulated on a cluster of CTLs from SFMC of patients with AS (Figure S2A). Notably, this cluster of IR-expressing CTLs was absent in the PBMCs of these patients (Figures S2A and S2B). Manual gating of live SF CD3+ CD8+ CD45RO+ cells revealed that PD-1 was simultaneously co-expressed with TIGIT and LAG-3 in AS SF CTLs. Additionally, this cluster of CTLs co-expressed markers that are indicative of tissue-residency: CD103, CD69, and CD49a (Figure S2C). PhenoGraph clustering analysis of AS CTLs from SFMCs and matched PBMCs identified 31 total clusters (Figure 2A). Compared to matched AS PBMCs, the following clusters were significantly enriched in AS SFMCs (p < 0.001, Log2 FC > 2, Figures 2B and 2C): (i) c25 (Resident memory CTLs expressing IRs), (ii) c24 (GZMK+ GZMA+ IR+), (iii) c15 (GZMB+ GZMK+ CD103+ IR+), (iv) c31 (CD49a+ PRF- GZM- IR+), and (v) c22 (CD38 mid PRF- GZMA+ GZMK+ IR+). IR expression, particularly PD-1 and LAG-3, was uniformly expressed in all 5 of these clusters, with the varying co-expression of other cytolytic markers and resident memory markers (Figure 2D). All 5 clusters were antigen-experienced CTLs with high expression of CD45RO. Conversely, naive T cells (clusters c3 and c7) were noticeably absent (Log2FC < 5, Figures 2B and 2C) in SFMCs. Differential abundance analysis of the PhenoGraph clusters confirmed that these clusters were uniformly expressed in AS SFMCs (Figures 2D and 2E). In summary, we observed a distinctive pattern of IR expression on AS SF CTLs. In the chronic inflammatory milieu of the joint, PD-1, TIGIT, and LAG-3 are highly upregulated in a subset of tissue resident memory-like CTLs from AS SFMC.
Figure 2.
Inhibitory receptor expressing CTLs are highly enriched in the synovial fluid of patients with ankylosing spondylitis
(A) Uniform manifold approximation and projection (UMAP) plot shows CTL PhenoGraph clusters in AS SFMCs and matched AS PBMCs. Clusters are annotated by cluster numbers (Right).
(B) CTL clusters with the proportions significantly increased in AS PBMC versus HC PBMC (red, Log2 FC > 0) or significantly decreased (blue, Log2 FC < 0). Clusters in gray: no significant proportion difference.
(C) Bar Graph shows the log2 fold proportional change (Log2 FC) for each cluster between the two groups (AS SFMC vs. matched PBMC). ∗∗∗p < 0.0005, ∗∗p < 0.005, ∗p < 0.05. The p values are calculated by the diffcyt R package.
(D) For each PhenoGraph cluster, the colors represent the row Z score values of the median expression values of the indicated markers. Heatmap is generated by the ComplexHeatmap R package.
(E) Stacked bar plot shows the frequencies of each PhenoGraph cluster as a percentage of total CD8+ T cells for 8 pairs of SFMCs-PBMCs.
(F) Mass cytometric analyses to quantify proportions of CD127- PD-1+ TIGIT+ CTLs from synovial fluid of patients with AS. ∗p < 0.05, calculated by Friedman repeated measures test with Bonferroni correction.
Bifurcation of CD127+ and CD127- PD-1+ TIGIT+ co-expressing cytotoxic cells in blood and synovial fluid
The co-expression of the inhibitory receptor PD-1 and the IL7R α-chain (CD127) has been proposed to discriminate between antigen-specific CTLs that are exhausted due to the persistent recognition of antigenic epitopes (PD-1+ CD127-) and inexhausted antigen-specific CTLs (PD-1+ CD127+) that are self-renewable with a memory phenotype.14 Considering our previous observation that CTLs in AS SFMCs highly co-expressed PD-1, TIGIT, and LAG-3, we hypothesized that this subset of CTLs contains key elements of CTL exhaustion, such as the downregulation of CD127. To address this, we performed co-expression biaxial gating analysis of PD-1, TIGIT, and CD127 by flow cytometry (Figure S2D). Indeed, CD127-PD-1+TIGIT+ CTLs are highly enriched in AS SFMCs, compared to matched PBMCs (Figure 2F). CD127+PD-1+TIGIT+ CTLs co-exist with the CD127-fraction, albeit at a lower frequency in AS SFMCs compared to matched PBMCs. UMAP and PhenoGraph clustering analysis of the CyTOF data corroborated this finding. Specifically, CD127 expression was completely absent in the subset of CTLs that highly expressed PD-1, TIGIT, and LAG-3 (Figure 2D). CD127 expression was notably absent in all 5 clusters (c25, c24, c15, c31, and c22) identified by the PhenoGraph analysis (Figure 2D). The combination of high IR expression and CD127 downregulation suggests that this subset of CTLs in AS SF exhibits dual features of immune exhaustion and activated effector CD8+ T cells. On the other hand, the co-expression of CD127 with PD-1 on CTLs in a setting of chronically activated immunity suggests that these CTLs retain memory-like survival characteristics, such as those found in patients with chronically infected HCV.14 High expression of the transcription factor TCF-1 (T cell transcription factor-1) is known to be required for the maintenance and persistence of CD127+ PD-1+ memory CTLs. Therefore, we examined TCF-1 expression in AS SF CTLs by intracellular flow cytometry. As expected, TCF-1 is expressed primarily in CD127+ PD-1+ CTLs found in AS SFMCs and PBMCs (Figure S3). However, we observed that TCF-1 is downregulated in CD127- PD-1+ CTLs, consistent with the hypothesis that this subset of CTLs do not carry self-renewal capacity. To summarize, we have identified a subset of CD127- CTLs that highly express PD-1, TIGIT, and LAG-3 enriched in SFMCs of patients with AS. The expression patterns of IRs in these CTLs are consistent with activated effector CTLs with features of immune exhaustion typically found in patients with cancer and chronic viral infection.
Identification of CD127- PD-1+ TIGIT+ LAG-3+ activated effector cytotoxic cells through single cell RNA-sequencing (scRNA-seq)
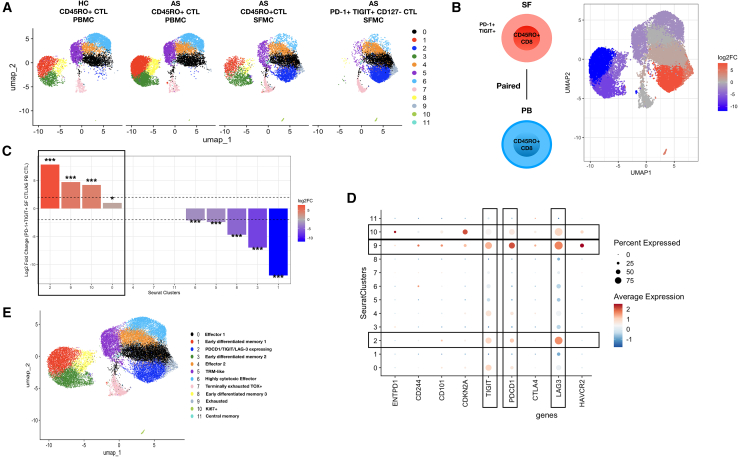
The identification of a CTL subset that closely resembles canonically exhausted CTLs commonly found in settings of cancer and chronic infection prompted us to deeply characterize expression patterns of transcripts related to CTL exhaustion in CD127- PD-1+ TIGIT+ CTLs. To delineate the transcriptomic heterogeneity of CTLs in AS SFMCs and PBMCs, we performed single-cell RNA sequencing (scRNA-seq). We FACS-sorted CD45RO+ memory CTL subsets to prioritize disease-relevant cells. Additionally, to further resolve any potential transcriptomic heterogeneity of synovial fluid CD127- PD-1+ TIGIT+ CTLs, we performed FACS sorting of these CTLs from three patients (Figure S4A). In total, we assembled a dataset of 31,184 scRNA-seq profiles of CTLs FACS sorted from paired PBMCs and SFMCs from patients with AS (n = 4) and PBMCs from healthy controls (HC, n = 4), in addition to FACS-sorted CD127- PD-1+ TIGIT+ CTLs from SFMCs (n = 3). A summary of subject demographics is displayed in Table S2.
Seurat-based clustering of AS and HC PBMCs/SFMCs resolved 12 clusters of CTLs (Figure 3A). Clusters 1, 3, and 8 represent three different early differentiated CTLs (Figure S5). Collectively, these CTLs highly express IL7R, a transcript for CD127. As expected, clusters 1, 3, and 8 are absent in CD127- PD-1+ TIGIT+ CTLs (Figure 3A) because we performed the negative selection of CD127+ CTLs during library preparation. Clusters 1, 3, and 8 also express moderate levels of TCF7, which encodes for TCF-1, an essential transcription factor for the commitment, maintenance, and differentiation of memory T cell subsets.15 Cluster 11 represents central memory CTLs reflected in the high expression of CCR7 and LEF1. In contrast to early differentiated memory clusters, which expressed minimal levels of cytotoxic effector genes, clusters 0, 2, 4, 6, 9, and 10 were observed to express varying levels of cytotoxic effector genes, including GZMA, GZMB, GZMK, PRF1, and NKG7 (Figure S6). Among these clusters, CTLs from cluster 6 express high levels of GZMB, GZMH, GNLY, PRF1, and NKG7 (Figure S6). NKG7, which is essential for cytotoxic degranulation in CTLs, is uniformly expressed at various levels among all identified cytotoxic effector clusters. We also observed the co-expression of GZMB and GZMK in the majority of the cytotoxic effector clusters, especially for cells belonging to cluster 9 (Figure S6). Notably, cluster 9 is enriched primarily in AS SF CTLs in comparison to peripheral blood CTL (Figure 3A), reminiscent of a recent study reporting the clonal expansion of GZMB+GZMK+ in inflamed synovial tissue from patients with rheumatoid arthritis.16
Figure 3.
Single cell RNA sequencing (scRNA-seq) of AS synovial fluid CTLs
(A) Uniform manifold approximation and projection (UMAP) plots display the transcriptomics profiles of sorted CD45RO + CTLs from the indicated groups. HC- Healthy control CD45RO + CTL from PBMC; AS CD45RO + CTL from PBMC; AS CD45RO + CTL from SFMC; PD-1+ TIGIT+ CD127- CTL from SFMC.
(B) CTL clusters with the proportions significantly increased (red, Log2 FC > 0) or significantly decreased (blue, Log2 FC < 0) in PD-1+ TIGIT+ CD127- CD45RO + CD8+ T cells versus matched peripheral blood (from the same patient) CD45RO + CD8+ T cells. Clusters in gray: no significant proportion difference.
(C) Bar graph shows the log2 fold proportional change (Log2 FC) for each scRNA-seq cluster between PD-1+ TIGIT+ CD127- SF CTL and matched peripheral blood CD45RO + CTL. ∗∗∗p < 0.001, ∗p < 0.05. p-values are calculated by the diffcyt R package.
(D) Dot plot shows the relative, scaled expression of the indicated exhaustion-related genes (ENTPD1, CD244, CD101, CDKN2A, TIGIT, PDCD1, CTLA4, LAG3, and HAVCR2; x axis) for each Seurat cluster (y axis).
(E) UMAP clustering of CTLs captured by sequencing mature CD45RO + CTLs from PBMCs of healthy control (n = 4) and patients with AS (n = 4); SFMCs of patients with AS (n = 4) and PD-1+ TIGIT+ CD127- CTL sorted from SFMCs of patients with AS (n = 3).
To further characterize the transcript expression patterns of CD127- PD-1+ TIGIT+ CTLs identified in our CyTOF studies, we compared scRNA-seq profiles of FACS sorted SF CD127- PD-1+ TIGIT+ CTLs with matching CD45RO + CTLs from peripheral blood. CTL clusters 2, 9 and 10 were significantly enriched in CD127- PD-1+ TIGIT+ CTLs (p < 0.001, Log FC > 2, Figures 3B and 3C) compared to matching CTLs from peripheral blood. Notably, clusters 2, 9, and 10 uniformly expressed high levels of PDCD1 (encoding for PD-1), TIGIT, and LAG3 (Figure 3D). Differentially expressed gene (DEG) analysis confirmed that LAG3 is highly expressed in CD127- PD-1+ TIGIT+ CTLs compared to matching mature CTLs from PBMCs (Figure S7A). To further characterize the transcriptional profile of CD127- PD-1+ TIGIT+ CTLs, we asked whether these cells expressed canonical transcripts that are associated with T cell exhaustion. Transcriptional data from our scRNA-seq dataset indicated that CD127- PD-1+ TIGIT+ CTLs highly express LAG3 (Figure S4B), corroborating our CyTOF profiling data. HAVCR2, which encodes for TIM-3, an inhibitory receptor commonly expressed in exhausted T cells17 was notably absent in cluster 2 but was expressed in clusters 9 and 10 (Figure 3D). The expression of other exhaustion-related inhibitory receptors, such as ENTPD1 (encodes for CD39), CD160, and NT5E (encodes for CD73) was notably absent in CD127- PD-1+ TIGIT+ CTLs (Figure S4B). The expression of inhibitory receptors: ENTPD1 (CD39), CD244 (encodes for 2B4), and CDKN2A was markedly diminished in cluster 2 compared to clusters 9, and 10 (Figure 3D). Together, these data suggest that cluster 2 represents a subset of CTLs expressing high levels of PD-1, TIGIT, and LAG-3, corresponding to the CTLs identified in our CyTOF analyses. In contrast to cluster 9, which corresponds to a small subset of exhausted CTLs (Figure 3E) identified primarily in SF CTLs, CTLs from cluster 2 do not express other canonical exhaustion markers. Furthermore, transcripts for GZMK, GZMA and GZMB were highly expressed on CD127- PD-1+ TIGIT+ CTLs (Figure S4B), suggesting these cells retain a key CTL effector phenotype despite expressing exhaustion-related inhibitory receptors. DEG analysis comparing CD127- PD-1+ TIGIT+ CTLs versus matching peripheral blood CD45RO + CTLs revealed that type 1 interferon signature genes- ISG15, IFITM1, IFI6, and MX1 were abundantly expressed on CD127- PD-1+ TIGIT+ CTLs (Figure S7A), indicating that a type I interferon signaling pathway is involved in the development and maintenance of these CTLs. Collectively, our single cell transcriptomic data of CD127- PD-1+ TIGIT+ CTLs suggest that these cells are not completely exhausted as would be expected in a chronically inflamed microenvironment, but rather retain a proinflammatory, overactivated CTL phenotype.
Notably, CD74 (encoding for HLA Class II invariant chain) was also abundantly expressed on CD127- PD-1+ TIGIT+ CTLs (Figure S4B), specifically in clusters 0, 2, 4, and 9 (Figure S7B). DEG analysis comparing PD-1+ TIGIT+ CTLs versus matching peripheral blood CD45RO + CTLs also revealed that CD74 transcript is abundantly expressed (Figure S7A). To confirm this finding at the protein level, we used flow cytometry to compare the expression of the intracellular domain of CD74 (CD74-ICD) in SF CTLs and PB CTLs (Figure S7C). The mean fluorescent intensity of CD74-ICD staining was significantly higher among CD127- PD-1+ TIGIT+ CTLs from SFMCs compared to CD45RO + CTLs from PBMCs (Figure S7C). These findings confirm that CD74 is uniquely expressed in SF mature CTLs and remains elevated in the PD-1+ TIGIT+ CD127- CTL subset.
We further investigated whether the CD127- PD-1+ TIGIT+ CTL subset might be associated with the SFMC resident memory T (Trm) cell subset, owing to the observation in our CyTOF profiling that these cells highly express CD103 and CD69 (Figure S2C). ITGAE (CD103) and CD69 are expressed in CD127- PD-1+ TIGIT+ CTLs as well as mature CD45RO + CTLs, whereas ITGA1 (CD49a) is expressed only in mature CD45RO + CTLs (Figure S8). Two Trm-associated genes, PRDM1 (Blimp-1) and RUNX3, are expressed in both mature CD45RO + CTLs and in CD127- PD-1+ TIGIT+ CTLs, whereas ZNF683 (Hobit) is expressed only in CD45RO + CTLs (Figure S8). Trm surface markers involved in Trm homing and activation (CXCR3, CXCR6 and CD27) are expressed in both CD45RO + CTLs and CD127- PD-1+ TIGIT+ CTLs (Figure S8). The downregulation of S1PR1 and CCR7 in CD127- PD-1+ TIGIT+ CTLs, as compared to PB CTLs, further indicates that a subset of CD127- PD-1+ TIGIT+ CTLs resembles some characteristics consistent with tissue-resident effector CTLs.
Constructing the developmental trajectories of cytotoxic cells in the AS synovial fluid microenvironment
To depict the developmental relationships across different subsets of mature CD45RO + CTLs identified in our single cell transcriptomics analyses, we performed pseudo-time analysis to establish developmental trajectories across CTL subsets using the Slingshot (Figure 4A) and monocle3 (Figure 4B) algorithms. The inferred trajectories generated by Slingshot and monocle3 (Figures 4A and 4B) contain two lineages branching from PD-1+ TIGIT+ LAG-3+ CD127- CTLs (Cluster 2), representing a bifurcation of these CTLs toward either effector CTLs (Cluster 0) or exhausted CTLs (Cluster 9). Another trajectory was also observed using the monocle3 algorithm that depicts the differentiation of effector CTLs (Cluster 0) toward terminally exhausted CTLs (Cluster 7) which express the highest level of TOX transcript among all CTL clusters (Figure 4C). We then asked whether CD127- PD-1+ TIGIT+ LAG-3+ CTLs (Cluster 2) represent a terminally differentiated state in comparison to other CTL subsets. To address this, we ordered all CTL clusters according to their assigned pseudo-time values generated by the monocle3 algorithm (Figures 4D and 4E). As expected, CTL clusters annotated as early differentiated memory clusters (Clusters 1,3, 8) ranked the lowest in pseudo-time values, suggesting an earlier developmental stage. Whereas CTL clusters annotated as exhausted and highly cytotoxic effector CTLs (Clusters 9, 6) ranked the highest (Figure 4E), suggesting a later developmental stage. CD127- PD-1+ TIGIT+ LAG-3+ CTLs (Cluster 2) ranked beneath the highly cytotoxic effector CTLs (Cluster 6), suggesting that these CTLs may not be as terminally differentiated compared to exhausted CTLs (Cluster 9) and highly cytotoxic effector CTLs (Cluster 6). In summary, our in-silico trajectory analyses suggest that CD127- PD-1+ TIGIT+ LAG-3+ CTLs are not fully terminally differentiated cells and may differentiate into other effector CTL subsets or into other exhausted CTLs.
Figure 4.
Trajectory analyses depicting the developmental relationship between CTL clusters identified by scRNA-seq
(A) UMAP plot displays Slingshot trajectory inferences generated by “GEX_lineage_trajectories” function from the Platypus R package.
(B) UMAP plot displays Monocle3 trajectory inferences. The principal roots of the trajectories were defined from early differentiated memory CTL clusters- 1, 3, and 8. Rectangular box depicts possible developmental trajectories within cluster 2.
(C) UMAP plot (left) displays scaled expression of the TOX transcript. Dot plot (right) representation of scaled expression of TOX within each indicated Seurat cluster (y axis).
(D) Pseudo-time analysis of the development trajectories. UMAP plot shows Monocle3 trajectory inferences overlaying with pseudo-time values generated by Monocle3.
(E) Boxplot representation of average pseudo-time values for each indicated cell type, defined in the cluster identification analysis.
CD127- PD-1+ TIGIT+ cytotoxic cells are clonally expanded in ankylosing spondylitis synovial fluid
In the absence of a known autoantigen in AS, we next investigated whether the inflammation-associated enrichment of CD127- PD-1+ TIGIT+ CTL in AS synovial fluid was due to a local clonal expansion of CD8+ T cells or a passive influx of CTLs from the periphery and other sites of inflammation. Therefore, we performed single cell TCR (T cell receptor) sequencing and RNA sequencing on paired SF CD127- PD-1+ TIGIT+ CTL and CD45RO + CTL from PBMC from 3 patients to interrogate the TCR repertoire of these CTL subsets. Clonotype diversity analysis of the most dominant and abundant clonotypes indicates that the TCR repertoire of CD127- PD-1+ TIGIT+ CTLs from SF-AS was less diverse compared to CD45RO + mature CTLs from paired PBMCs (Figure S9A). Examination of the relative abundance of specific clonotypes revealed the presence of hyperexpanded CTL clones in SF CD127- PD-1+ TIGIT+ CTLs FACS-sorted from two patients with HLA-B27+ AS (Figure S9B). Paired PB-SF TRBV CDR3 amino acid sequencing analysis showed that several clonotypes, as defined by identical CDR3 amino acid sequences on the TRBV chain, are significantly expanded in the SFMCs compared with PBMCs (Figure S9C), indicative of clonal expansion in the joint. For example, in patient SF81, >20% of the SF CTL clones were represented by the CDR3 amino acid sequence CSVEDPSSFSYEQYF in the TRBV chain, whereas this clonotype was only represented by <2% of PB CTL clones. Additionally, we found overlapping TCR gene usage between SF CD127- PD-1+ TIGIT+ CTLs and mature CD45RO + CTLs from PB (Figure S9D). TRBV15 chain (pairing with TRAV17) was preferentially used in patient SF76, while TRBV29-1 chain (pairing with TRAV3) was preferentially used in patient SF81. Notably, we did not observe any common TRBV and TRAV gene usage among the patients with AS. To further confirm that CD127- PD-1+ TIGIT+ are clonally expanded in the SF, we examined the transcript expression of TNFRSF9, encoding for 4-1BB, a known regulator of CTL cell clonal expansion and recent TCR activation.18 From our scRNAseq dataset, SF CD127- PD-1+ TIGIT+ CTLs showed higher TNFRSF9 transcript expression compared to AS and healthy control PB CD45RO + mature CTLs (Figure S4B). In summary, we found that CD127- PD-1+ TIGIT+ CTLs have a restricted TCR repertoire and have shared features with their blood CD45RO + CTL counterparts, suggesting that CD127- PD-1+ TIGIT+ CTLs are clonally expanded in the inflammatory milieu of the joint in AS.
Altered cytotoxicity profiles of CD127- PD-1+ TIGIT+ cytotoxic cells in ankylosing spondylitis
Our previous studies indicated that patients with AS have an altered cytotoxicity T cell profile suggesting that activated CTLs expressing granzymes are recruited to the joint, the target site of chronic inflammation in AS.5 We next sought to compare expression levels of granzyme B and perforin in CD127+ versus CD127- IR+ CTLs in AS SFMCs and matching PBMCs. We had expected that the CD127-fraction of IR-expressing CTLs would downregulate their cytotoxic capabilities owing to their putative exhausted phenotype. Surprisingly, CD127- PD-1+ TIGIT+ CTLs in SF expressed significantly higher levels of Granzyme B and perforin compared to their CD127+ IR-expressing counterparts (Figures 5A and 5B). Mass cytometric analyses revealed an overall reduction in granzyme B+ and perforin+ CTLs in SFMCs, compared to PBMCs (Figures 5A and 5B). CD38, a marker robustly induced during inflammation is also expressed at a higher level in CD127- PD-1+ TIGIT+ CTLs, compared to the CD127+ counterparts in SFMCs and PBMCs (Figure 5C). Taken together, our mass cytometric analyses indicate that SF CD127- IR+ CTLs retain their cytotoxic capacities and may perpetuate ongoing joint inflammation in patients with AS.
Figure 5.
Synovial fluid CD127- PD-1+ TIGIT+ CTLs retain some capacity to produce cytolytic molecules and retain the capacity to produce inflammatory cytokines upon stimulation
(A and B) Mass cytometric analyses to quantify median expression levels of intracellular granzyme B (A) and perforin (B) peripheral blood (PB) CTLs and synovial fluid (SF) CTLs from 8 pairs of active patients with AS. ∗p < 0.05 calculated with Friedman repeated measures tests with Bonferroni correction.
(C) Mass cytometric analyses to quantify median expression levels of cell surface CD38 in peripheral blood (PB) CTLs and synovial fluid (SF) CTLs from 8 pairs of patients with active AS. ∗p < 0.05 calculated with Friedman repeated measures tests with Bonferroni correction.
(D) Cytokine release assay; Gating strategy to measure intracellular IFNγ and TNFα. CD127+ and CD127- PD-1+ TIGIT+ CTLs were FACS-sorted from paired SFMC and PBMC samples, then stimulated with PMA and ionomycin for 4 h in the presence of Brefeldin A. Intracellular IFNγ and TNFα were measured by flow cytometry.
(E and F) Graphs display proportions of CD8+ T cells expressing intracellular IFNγ (E) and TNFα (F) ∗p < 0.05; calculated by Friedman repeated measures test with Bonferroni correction.
CD127- IR expressing cytotoxic cells retain their capacity to producing inflammatory cytokines
To further resolve whether CD127- IR+ CTLs in AS SF are truly exhausted, we tested the capacity of these cells to produce the pro-inflammatory cytokines IFNγ and TNFα. We FACS- sorted CD127+ and CD127- PD-1+ TIGIT+ CTLs from paired SFMCs and PBMCs, then stimulated CTLs with PMA and ionomycin for 4 h in the presence of Brefeldin A to measure the intracellular expression of IFNγ and TNFα by flow cytometry (Figure 5D). As expected, CD127+ “memory” IR + CTLs from SFMCs and PBMCs produced a significant amount of IFNγ and TNFα upon stimulation, whereas cytokine production was drastically reduced from the corresponding CD127- IR+ CTLs in the blood (Figures 5E and 5F). Consistent with the expression of granzyme B and perforin, SF CD127- IR+ CTLs retain the capacity to produce IFNγ and TNFα upon stimulation (Figures 5E and 5F) These findings further suggest that SF CD127- CTLs may not be truly exhausted during chronic joint inflammation despite expressing classical immune checkpoint markers- PD-1, TIGIT, and LAG-3.
TOX, a regulator of cytotoxic cell exhaustion is downregulated in SF CD127- IR expressing cytotoxic cells
The transcription factor TOX (thymocyte selection associated high-mobility group box protein) is a master regulator of T cell exhaustion, especially in the context of chronic viral infections and cancer.19,20 Robust expression of TOX results in the transcriptional and epigenetic commitment of exhausted T cells by activating a distinctive exhausted transcriptional program.21 To confirm that SF CD127- PD-1+ TIGIT+ CTLs in patients with AS with active disease resist CTL exhaustion, we measured TOX expression in CD127-and CD127+ PD-1+ CTLs in paired SFMCs and PBMCs by intracellular flow cytometry (Figure 6A). TOX expression was significantly reduced specifically in SF CD127- PD-1+ CTLs compared to matched CTLs from the peripheral blood (Figure 6B), suggesting that the pathological process of resisting CTL exhaustion occurs in the local inflammatory milieu of the joint. TOX expression in CD127+ PD-1+ CTLs from SF was slightly reduced, but not statistically significant in comparison to CD127+ PD-1+ CTLs from the blood (Figure 6B). Our single cell transcriptomic data corroborated these findings. TOX expression was not uniformly absent in all CTL clusters identified in AS SF. For instance, TOX is expressed in clusters 7, 9, and 10, these clusters represent a small fraction of terminally differentiated and canonically exhausted CTLs (Figure 6C). However, TOX transcript expression was undetectable specifically in CTLs expressing PDCD1, TIGIT, and LAG3 (cluster 2) (Figure 6C). This cluster of CTLs comprises a large majority of CTLs in AS SF (Figure 3A). Taken together, these results lend support to the notion that a subset of highly activated CTLs expressing IRs seems to resist CTL exhaustion, which may be fundamental to sustain chronic inflammation, a hallmark of AS.
Figure 6.
Downregulation of TOX expression in CD127- PD-1+ CTLs in SFMCs from patients with ankylosing spondylitis
(A) Representative flow cytometric analyses of blood or synovial fluid (SF) PD-1+ CD127+ and CD127- CTL for TOX transcription factor expression. CD127+ and CD127- CTLs were gated from live CD3+ CD8+ PD-1+ cells. Fluorescence minus one (FMO) control were used to define CD127+, CD127-and PD-1+ CD8+ T cells.
(B) TOX expression, measured by median fluorescence intensities (MFI), in CD127- (left) and CD127+ (right) PD-1+ CTLs in paired blood and SF from 6 patients. ∗p < 0.05, calculated by two-sided Wilcoxon-signed ranked test; ns-not significant.
(C) UMAP clustering (top) of CTLs captured by sequencing mature CD45RO + CTLs from PBMCs of healthy control (n = 4) and patients with AS (n = 4); SFMCs of patients with AS (n = 4) and PD-1+ TIGIT+ CD127- CTL sorted from SFMCs of patients with AS (n = 3). Dot plot (bottom) shows the relative, scaled expression of the indicated genes (TIGIT, PDCD1, CTLA4, LAG3, HAVCR2, TOX, and TOX2; x axis) for each Seurat cluster (y axis).
Discussion
Perpetual, chronic inflammation is one of the hallmarks of AS and accounts for both the symptoms of the disease and the progressive, irreversible damage to the spine. A central role for CTLs in AS inflammation is supported by our recent discovery of a novel subset of integrin-expressing mature synovial CTLs4 and identification of clonally expanded TRBV9-CDR3β-TRBJ2.3 CTLs in patients with HLA-B27 with AS.11,22,23,24 These discoveries reinvigorated the long-standing arthritogenic peptide theory to explain the immunopathogenesis of AS and implicate a strong antigen-driven immune response in AS. Antigen-driven expansion of CTLs in inflammation and infection conditions is normally truncated by T cell exhaustion, which is considered a host homeostatic mechanism that normally restrains autoreactive CTLs. Here, using single cell immunophenotyping analyses, we identified a subset of clonally expanded synovial antigen-experienced CTLs expressing classical coinhibitory receptors PD-1, TIGIT, and LAG-3 that retained some key cytotoxic effector functions, such as the ability to produce granzymes, perforin, and to release proinflammatory cytokines upon further stimulation.
Further analysis of this CTL subset by scRNA-seq suggested that these CTLs may not be completely exhausted due to a lack of expression of other exhaustion-related gene transcripts. Our data suggest that AS synovial CTLs exhibit an activated effector phenotype that differs from canonical T cell exhaustion commonly characterized in chronic viral infection and cancer. This resistance to canonical exhaustion lends support to the notion that autoinflammation forms the pathological basis of AS, in which host homeostatic mechanisms fail to curb persistent inflammation. Furthermore, our in-silico trajectory analyses suggest that CD127- PD-1+ TIGIT+ LAG-3+ CTLs may bifurcate to differentiate into either activated effector or exhausted CTLs. Future studies should explore and define the molecular signals present in the AS inflammatory microenvironment that drive the differentiation of these CD127- PD-1+ TIGIT+ LAG-3+ CTLs into potent, activated effector CTLs.
Intriguingly, from our scRNA-seq DEG analysis, CD74 was found to be highly upregulated in PD-1+ TIGIT+ CD127- SF CTLs. The overexpression of the intracellular domain of CD74 in PD-1+ TIGIT+ CD127- SF CTLs was observed in our flow cytometry experiments. CD74, which encodes for the MHCII invariant chain, plays an important role in regulating the MHCII antigen presentation pathway. Additionally, the intracellular domain (ICD) of CD74 also cooperates with CD44 and CXCR2 to initiate multiple pro-inflammatory pathways upon binding of macrophage inhibitory factor (MIF). Multiple studies in recent years demonstrated that MIF induces inflammation and predicts spinal ankylosis in patients with AS and in the SKG murine model of spondyloarthritis.12,25 To our knowledge, the role of CD74 signaling in contributing to CTL dysregulation in AS remains undefined. Our profiling studies suggest that CD74 could represent a promising target to reverse CTL dysregulation in AS. Humanized anti-CD74 monoclonal antibodies (milatuzumab) are currently being studied as a treatment for systemic lupus erythematosus (SLE) to control underlying overactivated immune responses responsible of autoinflammation.26
Chronic antigenic exposure and TCR stimulation are key prerequisites for CTLs to develop T cell exhaustion. Both prerequisites are present within the AS inflammatory milieu. First observed in settings of chronic viral infections and later in response to tumors, immune exhaustion is an evolutionarily conserved adaptation to chronic antigen stimulation that is critical to limit overactivated immune activation stemming from autoreactive T cells. On one hand, the blockade of inhibitory receptors using monoclonal antibodies partially rescues the CTL exhaustion phenotype to permit adequate CTL responses, which is therapeutically relevant in cancer treatment. On the other hand, inducing CTL exhaustion to tame autoreactive T cells could be a therapeutic strategy to treat autoimmunity and autoinflammatory conditions such as AS. The existence of CTL exhaustion and its contribution to disease outcomes in autoimmunity and autoinflammatory settings is an active research forefront.
In our study, we addressed whether chronic inflammation in AS is a direct consequence of CTL failing to undergo immune exhaustion. High dimensional immune profiling of CTLs from patients with AS revealed considerably high heterogeneity amongst memory CTLs in the peripheral blood and synovial fluid. Of particular interest were the expression patterns of commonly expressed inhibitory receptors, cytolytic markers, and modulators and hallmarks of the exhaustion program, such as TOX and TCF1. It is well known that terminally exhausted CTLs have high expression levels of TOX and low expression of TCF-1.7 Our high-dimensional immune profiling experiments revealed that AS CTLs highly express some canonical inhibitory receptors (PD-1, TIGIT, and LAG-3) which is likely a consequence of prolonged immune activation within the AS inflammatory milieu rather than a consequence of T cell exhaustion as PD-1+ TIGIT+ LAG-3+ CD127- CTLs from patients with AS failed to express TOX at a protein level. TOX transcript expression was detectable in some synovial mature CTL subsets, but not in the PD-1+ TIGIT+ LAG-3+ CD127- subset. AS synovial CTLs that express PD-1 and TIGIT also comprise a subpopulation that is CD127-positive and TOX-negative that also expresses some levels of TCF-1. We speculate that these cells retain T cell effector function, maintain proliferation capacity and polyfunctionality. Our observation of synovial CTLs that seem to resist exhaustion and retain polyfunctionality may not be unique to AS. Recently, in a mouse model of type 1 diabetes, it was found that intra-islet CTLs exhibit a phenotype that differs from canonical T cell exhaustion despite expressing inhibitory receptors, reminiscent of the similar phenotypes found in our samples of AS synovial CTLs.27 In both diseases, further research is warranted to assess the mechanisms by which these IR+ CTLs contribute to autoinflammation, as well as molecular mechanisms that could be used therapeutically to induce exhaustion for these autoinflammatory CTLs.
Historically, studies in AS have shown a limited pathogenic role for CTLs despite the disease being highly linked to HLA-B27 and the enrichment of CD8+ T cell-related genes in genome-wide association studies. In recent years, emerging immune profiling studies have revealed unique CTL phenotypes expressed in patients with AS and support the notion that there is a general broad dysregulation of CTLs in patients with AS. Our previous study showed that GZM and PRF1 gene expression were reduced in patients with AS compared to age-matched healthy controls.5 Flow cytometry experiments of peripheral CTLs confirmed that the frequencies of perforin and granzyme - expressing lymphocytes were significantly reduced in AS compared to healthy controls. Consistent with these observations, our mass cytometry data showed a significant reduction in the frequencies of PhenoGraph clusters that express perforin and granzyme. In addition, the negative correlation of the frequency of a PhenoGraph cluster that is highly enriched for the expression of cytolytic molecules (PRF+ GZMB+ GZMA+ GNLY+) with BASDAI scores is suggestive of an inverse relationship between CTL cytolytic function and disease activity. We hypothesize that the reduction of perforin-expressing CTLs in the peripheral blood may contribute to CTL overactivation through “frustrated killing,” which results in the release of IFNγ and other cytolytic molecules, further augmenting AS autoinflammation. It is worth pointing out that there may seem to be a paradox that if activated CTLs are resistant to exhaustion, these cells should be secreting higher levels of perforin and granzyme. We do not know yet whether the inexhaustion and the perforin deficiency hypotheses are mechanistically linked. It is plausible that both pathological processes are concurrently happening in AS. Our CyTOF and scRNAseq data suggest that resistance to exhaustion may only happen within the inflammatory SF milieu, while the loss of perforin expressing CTLs occurs in the peripheral blood. The retention of perforin expression in AS synovial CTLs supports the hypothesis of key cytotoxic cell activity within the inflamed joint. A future study that investigates the relationship between CTL inexhaustion and perforin dysregulation in AS is certainly warranted.
Our discovery of an enhanced CD38 expression of AS peripheral effector memory CD8+ T cells provides further support for CTL dysregulation in AS and raises the possibility of using CD38 as a potential biomarker to evaluate AS disease progression. CD38 has been clearly linked to inflammation and infection.28 CD38 expression is under the transcriptional control of type I or II interferon, as well as TNFα/NF-κB stimulation.29,30 Therefore, it is not surprising that its expression on a broad range of immune cells is robustly induced during acute and chronic inflammation. For instance, in rheumatoid arthritis (RA), high CD38 expression on peripheral CD3+ and synovial tissue CD3+ cells was reported. The expression of CD38 was also significantly correlated with levels of rheumatoid factor (RF) among patients with RF + RA.31 Expanded CD38hiCD8+ T cells were also reported in patients with SLE that are susceptible to frequent infections.32 Mechanistically, highly levels of CD38 in peripherally expanded CTLs resulted in decrease in CTL cytotoxicity through acetylated EZH2.33 This dysregulation of cytotoxicity found in patients with SLE is reminiscent of the dysregulated cytotoxicity of peripheral CTLs found in our cohort of patients with AS.5
More recently, a population of clonally expanded CD38hi CD127-activated effector CTLs was identified in synovial fluid of patients with cancer with inflammatory arthritis induced after treatment with checkpoint inhibitors (ICI-A). This subset of CTLs that is highly enriched for effector functions accumulates uniquely in ICI-A joints compared to RA and PsA. This population of CD38hi CTLs bears a strong resemblance to the IR-expressing CTLs identified in our study. First, extensive clonal expansion was observed in both populations of CTLs. Secondly, transcriptomic analysis of both populations revealed a significant dependence on the type I interferon response pathway, with common significantly upregulated IFN inducible genes such as ISG15 and MX1. And lastly, both populations of CTLs play a key role in sustaining autoinflammation in the joints of ICI-A and AS. Although we did not directly compare the transcriptomics profiles of CD38hi CTLs identified in patients with ICI-A, we could not exclude the possibility that the expansion of synovial IR+ CD127- CTLs identified in our cohort of patients is unique to AS. Additionally, in a separate cohort of juvenile idiopathic arthritis (JIA), it was also found that PD-1 expressing effector CTLs in synovial fluids of patients with JIA also retained the capacity for clonally expansion.34 These CTLs were enriched for tissue-resident memory (Trm) transcriptional signatures with no signs of exhaustion.34 Analogous to these studies, a subset of TIM-3+ CX3CR1- CTLs with hallmarks of terminal exhaustion yet retaining cytotoxic polyfunctionality, was recently discovered in the tumor microenvironment of patients with multiple myeloma and lymphoma.35 These CTLs are transcriptionally distinct from dysfunctional exhausted T cells found in chronic viral infections.35 In consideration of other similar CTLs described in the current literature, resistance of CTL exhaustion in the synovial inflammatory milieu in AS represents a deviation of host homeostatic response to excess and chronic T cell stimulation. Whereas in cancer, resistance to T cell exhaustion is beneficial to eliminate tumor cells, in autoinflammatory conditions, such as in AS, resisting exhaustion may perpetuate and amplify undesired CTL effector functions.
In summary, amidst a resurgent interest in evaluating the role of CTLs in contributing to AS inflammation, we provide single cell transcriptomic and proteomic data showing that CTLs from patients with AS are broadly dysregulated with regard to their ability to undergo T cell exhaustion. Our analyses support the concept of clonally expanded, pathogenic effector CTLs that play a significant role in perpetuating chronic joint inflammation. More importantly, we propose a mechanism- resistance to T cell exhaustion, by which effector CTLs utilize to perpetually sustain chronic inflammation in AS. Currently, there is a major unmet need for effective treatments which can prevent progressive damage to the spine for patients with AS. We envision that those interventions based on intercepting chronic inflammation provide the best chance of achieving these goals. Our approach of profiling CTLs to gain a deeper understanding of CTL dysregulation in AS lays the groundwork for the future development of novel therapeutics targeting AS inflammation.
Limitations of the study
Our study has several limitations. Our observations in peripheral blood and synovial fluid were derived from a small group cohort of patients; a larger cohort of patients will be needed to assess the magnitude of pathological contributions of IR-expressing CTLs and their associations with clinical outcomes. Additionally, our analysis lacked the inclusion of synovial tissue derived from the axial skeleton. We used synovial fluid as a surrogate sample to evaluate CTL phenotypes from an inflamed environment. We did not compare other forms of inflammatory joint disease, limiting our ability to assess whether CTL inexhaustion is a pathological process uniquely characterized in AS.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the corresponding author, Dr. Robert D. Inman (Robert.inman@uhn.ca).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
scRNA-seq data can be accessed via GEO using the accession code: GSE288581.
-
•
This article does not report original code.
-
•
Additional information needed to reanalyze the data reported in this article and permitted by UHN REB 20–5026 is available from the lead contact upon request.
Acknowledgments
We thank Mr. Edwin Speck from Princess Margaret Flow Cytometry (https://pmflow.ca), University Health Network, for their assistance in FACS cell sorting. We also thank the technical staff from the SickKids Center for Advanced Single Cell Analysis (CASCA), Toronto, Ontario (https://lab.research.sickkids.ca/casca/) and the Princess Margaret Genomics Center (http://pmgenomics.ca) for their assistance in acquiring samples for mass cytometry experiments and single cell sequencing experiments. The researchers are supported by a Strategic Operating Grant from Arthritis Society Canada (19-0518) and a Project Grant from Canadian Institutes of Health Research (399454). M.T. was supported by a training postdoctoral fellowship award from Arthritis Society Canada. The graphical abstract was created with BioRender.com.
Author contributions
M.T. and R.D.I. conceptualized and designed the study. M.T. and Z.Q. designed and analyzed the single cell RNA sequencing and mass cytometry experiments. M.T., Z.Q., and M.L. performed all other experiments. M.T. wrote the first draft of the article. R.D.I. supervised the study. All authors edited and approved the article.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 145Nd-anti-CD4 (Clone RPA-T4) | Standard BioTools (Formerly Fluidigm) | Cat# 3145001B; RRID: AB_3661845 |
| 151Eu-anti-CD14 (Clone M5E2) | Standard BioTools (Formerly Fluidigm) | Cat# 3151009B; RRID: AB_2810244 |
| 152Sm-anti-TCRγδ (Clone 11F2) | Standard BioTools (Formerly Fluidigm) | Cat# 3162008B; RRID: AB_2687643 |
| 165Ho-anti-CD16 (Clone 3G8) | Standard BioTools (Formerly Fluidigm) | Cat# 3145008B; RRID: AB_2888924 |
| 176Yb-anti-CD56 (Clone HCD56) | Standard BioTools (Formerly Fluidigm) | Cat# 3176009B; RRID: AB_2811096 |
| 89Y-anti-CD45 (Clone HI30) | Standard BioTools (Formerly Fluidigm) | Cat# 3089003B; RRID: AB_2938863 |
| 146Nd-anti-CD8a (Clone RPA-T8) | Standard BioTools (Formerly Fluidigm) | Cat# 3146001B; RRID: AB_3661846 |
| 147Sm-anti-CD3 (Clone UCHT1) | Purified antibody from BioLegend | Cat# 300402; RRID: AB_314056 |
| 169Tm-anti-CD19 (Clone HIB19) | Standard BioTools (Formerly Fluidigm) | Cat# 3169001B; RRID: AB_2893034 |
| 174Yb-anti-HLA-DR (Clone L243) | Standard BioTools (Formerly Fluidigm) | Cat# 3174001B; RRID: AB_2665397 |
| 149Sm-anti-CD45RO (Clone UCHL1) | Purified antibody from BioLegend | Cat# 304202; RRID: AB_314418 |
| 160Gd-anti-CD28 (Clone CD28.2) | Standard BioTools (Formerly Fluidigm) | Cat# 3160003B; RRID: AB_2868400 |
| 162Dy-anti-CD27 (Clone L128) | Standard BioTools (Formerly Fluidigm) | Cat# 3162009B; RRID: AB_2756422 |
| 144Nd-anti-CD69 (Clone FN50) | Standard BioTools (Formerly Fluidigm) | Cat# 3144018B; RRID: AB_2687849 |
| 171Yb-anti-CD38 (Clone HIT2) | Purified antibody from BioLegend | Cat# 303502; RRID: AB_314354 |
| 156Gd-anti-CD95 (Clone DX2) | Purified antibody from BioLegend | Cat# 305602; RRID: AB_314540 |
| 163Dy-anti-CD49a (Clone TS2/7) | Standard BioTools (Formerly Fluidigm) | Cat# 3163015B; RRID: AB_2893061 |
| 161Dy-anti-KLRG1 (Clone SA231A2) | Purified antibody from BioLegend | Cat# 367702; RRID: AB_2632738 |
| 164Dy-anti-CD62L (Clone DREG56) | Purified antibody from BioLegend | Cat# 304802; RRID: AB_314462 |
| 175Lu-anti-CD103 (Clone Ber-ACT8) | Purified antibody from BioLegend | Cat# 350202; RRID: AB_10639864 |
| 172Yb-anti-CX3CR1 (Clone 2A9-1) | Standard BioTools (Formerly Fluidigm) | Cat# 3172917B |
| 167Er-anti-CCR7 (Clone G043H7) | Purified antibody from BioLegend | Cat# 353202; RRID: AB_10945157 |
| 143Nd-anti-CD127 (Clone A019D5) | Standard BioTools (Formerly Fluidigm) | Cat# 3143012B; RRID: AB_2810240 |
| 141Pr-anti-CD45RA (Clone HI100) | Purified antibody from BioLegend | Cat# 304102; RRID: AB_314406 |
| 150Nd-anti-LAG-3 (Clone 11C3C65) | Standard BioTools (Formerly Fluidigm) | Cat# 3150030B; RRID: AB_3661851 |
| 159Tb-anti-PD-L1 (Clone 29.EA2A3) | Purified antibody from BioLegend | Cat# 329702; RRID: AB_940372 |
| 153Eu-anti-TIGIT (Clone MBSA43) | Purified antibody from ThermoFisher Scientific (Life Technologies) | Cat# 16-9500-92; RRID: AB_10718831 |
| 155Gd-anti-PD-1 (Clone EH12.2H7) | Purified antibody from BioLegend | Cat# 329902; RRID: AB_940488 |
| 166Er-anti-Perforin (Clone dG9) | Purified antibody from BioLegend | Cat# 3081022; RRID: AB_314700 |
| 173Yb-anti-Granzyme B (Clone GB11) | Standard BioTools (Formerly Fluidigm) | Cat# 3173006B; RRID: AB_2811095 |
| 158Gd-anti-Granzyme A (Clone CB9) | Purified antibody from BioLegend | Cat# 507202; RRID: AB_315468 |
| 168Er-anti-Granzyme K (Clone GM26E7) | Purified antibody from BioLegend | Cat# 370502; RRID: AB_2566673 |
| 142Nd-anti-Granulysin (Clone DH2) | Purified antibody from BioLegend | Cat# 348007; RRID: AB_2563601 |
| FITC anti-human CD4 (Clone RPA-T4) | BioLegend | Cat# 300506; RRID: AB_314074 |
| FITC anti-human CD14 (Clone M5E2) | BioLegend | Cat# 301804; RRID: AB_314186 |
| FITC anti-human CD16 (Clone 3G8) | BioLegend | Cat# 302006; RRID: AB_314206 |
| FITC anti-human CD20 (Clone 2H7) | BioLegend | Cat# 302304; RRID: AB_314252 |
| FITC anti-human CD11c (Clone Bu15) | BioLegend | Cat# 337214; RRID: AB_2129792 |
| FITC anti-human CD19 (Clone HIB19) | BioLegend | Cat# 302206; RRID: AB_314236 |
| FITC anti-human TCRγδ (Clone B1) | BioLegend | Cat# 331208; RRID: AB_1575108 |
| APC anti-human CD3 (Clone UCHT1) | BioLegend | Cat# 300412; RRID: AB_314066 |
| PE-Cyanine 7 anti-human CD8 (Clone SK1) | BioLegend | Cat# 344712; RRID: AB_2044008 |
| APC/Cyanine 7 anti-human CD45RO (Clone UCHL1) | BioLegend | Cat# 304228; RRID: AB_10895897 |
| APC/Cyanine 7 anti-human PD-1 (Clone EH12.2H7) | BioLegend | Cat# 329922; RRID: AB_10933429 |
| APC anti-human PD-1 (Clone E12.2H7) | BioLegend | Cat# 329908; RRID: AB_940475 |
| Brilliant Violet 605 anti-human TIGIT (Clone A15153G) | BioLegend | Cat# 372712; RRID: AB_2632927 |
| Brilliant Violet 421 anti-human CD127 (Clone A019D5) | BioLegend | Cat# 351310; RRID: AB_10960140 |
| PE anti-human CD74 (Clone Pn.1) | BioLegend | Cat# 357604; RRID: AB_2561939 |
| PE anti-human TOX (Clone TXRX10) | ThermoFisher Scientific (Life Technologies) | Cat# 12-6503-80; RRID: AB_10853657 |
| Brilliant Violet 421 anti-human TCF1 (Clone S33-966) | BD Biosciences | Cat# 566692; RRID: AB_2869822 |
| PE anti-human IFN-γ (Clone 4S.B3) | BioLegend | Cat# 502509; RRID: AB_315234 |
| PE anti-human TNF-α (Clone MAb11) | BioLegend | Cat# 502909; RRID: AB_315261 |
| Biological samples | ||
| Healthy adult peripheral blood | UHN Spondylitis Program | UHN REB 08-0126 |
| AS patient peripheral blood and synovial fluid | UHN Spondylitis Program | UHN REB 20-5026 |
| Chemicals, peptides, and recombinant proteins | ||
| Cell-ID Cisplatin-194Pt | Standard BioTools (Formerly Fluidigm) | Cat# 201194 |
| Cell-ID Intercalator-Ir- 125μM | Standard BioTools (Formerly Fluidigm) | Cat# 201192A |
| Cytofix Fixation Buffer | BD Biosciences | Cat# 554655 |
| Intracellular Staining Permeabilization Wash Buffer (10X) | BioLegend | Cat# 421002 |
| Fixable Viabillity Stain 780 | BD Biosciences | Cat# 565388; RRID: AB_2869673 |
| Human TruStain FcX | BioLegend | Cat# 422302; RRID: AB_2818986 |
| Cell Activation Cocktail | BioLegend | Cat# 423301 |
| Brefeldin A | BD Biosciences | Cat# 555029; RRID: AB_2869014 |
| Ficoll-Paque | GE Healthcare/Cytiva | Cat# 17144003 |
| Critical commercial assays | ||
| Cell-ID 20-Plex Pd Barcoding Kit | Standard BioTools (Formerly Fluidigm) | Cat# 201060 |
| Foxp3/ Transcription Factor Staining Buffer set | eBioscience/ThermoFischer Scientific | Cat# 00-5523-00 |
| Chromium Next GEM Single Cell 5' Reagent Kit v2 | 10X Genomics | Cat# PN-1000263 |
| Chromium Single Cell V(D)J Amplification Kit | 10X Genomics | Cat# PN-1000253 |
| Deposited data | ||
| Single cell RNA/TCR-seq data | https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi | Dataset can be accessed using GEO accession code GSE288581 |
| Software and algorithms | ||
| PRISM | GraphPad Software, Inc | Version 10 |
| FlowJo | FlowJo/BD | Version 10.5.3 |
| R | The R project for Statistical Computing | Version 4.4.0 |
| R Studio | Posit | Version 2024.04.0 |
| Seurat | Seurat package in R | Version 5.1.0 |
| Platypus | Platypus package in R | Version 4.4.0; https://github.com/alexyermanos/Platypus |
| Cell Ranger Single Cell Software Suite | 10X Genomics | Version 5.0.1 |
| Monocle3 | Monocle3 package in R | Version 1.3.7; https://cole-trapnell-lab.github.io/monocle3/ |
| Cytobank | Cytobank, Inc (Beckman Coulter) | https://premium.cytobank.org/ |
| PhenoGraph | Levine et al.13 | RRID: SCR_016919 |
| diffcytR | Weber et al.36 | https://www.bioconductor.org/packages/release/bioc/html/diffcyt.html |
| UMAP | uwot pckage in R | Version 0.2.2 |
| Other | ||
| Human reference genomic NCBI build 38, GRCh28 | Genome Reference Consortium | https://www.ncbi.nlm.nih.gov/grc/human |
Experimental model and study participant details
Patient cohorts
Patients with axSpA and healthy control (HC) subjects were recruited from the Spondylitis rheumatology clinic at Toronto Western Hospital, University Health Network/UHN (Toronto, Ontario, Canada) according to an approved study per University Health Network Review Ethics Board polices (CAPCR ID 20–5026). Study protocols were approved by the Research Ethics Board of the University Health Network in accordance with the Helsinki Declaration and good clinical practice guidelines (UHN REB 20–5026). All patients and controls provided written, informed UHN Research Ethics Board- approved consent prior to sample collection and study participation.
We established a biobank of peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) from axSpA patients in our longitudinal observation cohort. All axSpA patients fulfilled the modified New York (mNY) criteria for ankylosing spondylitis.37 Patients with active disease were defined using a surrogate ASDAS-CRP score of greater than 2.1. A surrogate ASDAS-CRP score was calculated for each patient using BASDAI and CRP values recorded on the day of clinic assessment as described by Ortolan et al.38 Joint aspiration was performed when clinically indicated. Active joint effusion in axSpA patients reflects active joint inflammation. HC subjects were age- and sex-matched. HCs who were recruited had no history of clinical autoimmune disease or recent infections or vaccinations using a self-reported questionnaire. The biological sex and age of the study subjects are reported in Tables S1 and S2.
Method details
Preparation of mononuclear cells
For PBMC and SFMC isolation, venous blood and aspirated synovial fluid were collected in heparinized tubes (BD) prior to cell isolation with Ficoll-Paque plus (GE Healthcare) density centrifugation. All mononuclear cells used in this study were cryopreserved with 55% fetal bovine serum (FBS) and 10% DMSO in RPMI 1640 and stored in liquid nitrogen for subsequent use. For experiments involving FACS sorting, thawed PBMC and SFMC were rested overnight in complete media (RPMI 1640 with L-glutamine + 10% heat inactivated FBS + penicillin/streptomycin + 50 μM mercaptoethanol) before use.
Mass cytometry time-of-flight (CyTOF)
PBMCs and SFMCs stored in liquid nitrogen were thawed and used immediately for mass cytometry staining. A summary of cohort demographics for healthy control subjects and AS patients is provided in Table S1. Target cells were blocked with anti-CD16/CD32 Fc receptor blocking solution (BioLegend) to minimize nonspecific background staining. Cells were stained with a customized 33-marker antibody panel (Table S3). In the final panel, markers were broadly categorized into the following: major immune lineage, CD8 T cell activation, immune checkpoints, and intracellular cytolytic CTL functional markers. All antibodies were validated by conventional flow cytometry for expression on fresh vs thawed PBMC or SFMC. Where indicated, antibodies were purchased pre-conjugated from Standard BioTools (Formerly Fluidigm) or custom conjugated with metal tags using MaxPar Ready monoclonal antibodies from Biolegend of Thermofisher, according to the manufacturer’s protocol. Antibodies were titrated to determine optimal concentration for full panel staining, and MMO (metal-minus-one) were used as gating controls when required. After staining with primary metal-conjugated antibodies for cell surface markers, cells were barcoded using the Cell-ID™ 20-Plex Pd Barcoding Kit (Standard BioTools) according to manufacturer’s protocol, then pooled for intracellular staining using metal tagged antibodies. Live cells were identified using 1μM of monoisotopic cisplatin platinum-194 (Standard BioTools). DNA intercalation solution was added for overnight (0.077μM) to distinguish cells from debris. All matched PBMC and SFMC samples were thawed and stained on the same day to minimize batch effects. Samples were acquired on the CyTOF2 instrument (Standard BioTools) at the Peter Gilgan Center for Research and Learning (PGCRL), Hospital for Sick Children, Toronto, Canada. EQ Four Element Calibration Beads (Standard BioTools) were used to normalize signal intensity over time using the CyTOF software version 6.7.
CyTOF data analysis
FCS files were manually debarcoded and analyzed using Cytobank6.2 (Beckman Coulter),R (v.4.4.0), and RStudio (v2024.04.0). Heatmaps were generated in R using the ‘blue-red’, ‘viridis’, and the ‘gplots’ package; median signal intensity values were used to generated heatmaps. Data were pre-transformed by ‘cytofAsinh’ in R package ‘flowCore’ using a co-factor of 5 or by Arcsinh transformation (with co-factor= 5) in the software ‘Cytobank’. After Arcsinh transformation, CD3+ CD8+ cells were manually gated on R using the ‘ggplots2’ package or on the software ‘Cytobank’. R package ‘uwot’ was used to obtain UMAP projections. We defined the number of local neighbours= 30 with a minimum Euclidean distance of 0.3. PhenoGraph clustering analyses were performed with R package ‘RPhenograph’ (data transformation = cytofAsinh, seeds = 42, k = 30 for analysis in Figures 1 and 2). Unpaired or paired differential abundance (i.e. fold change) values were calculated using negative binomial generalized linear regression modelling edgeR tests, through the ‘edgeRdiffcyt’ R package. An adjusted p value < 0.05 using the Benjamin-Hochberg method was considered statistically significant for the comparisons made. For graphical visualizations, the following R packages were used: ‘ggplots2’, ‘stats’, ‘gplots’, ‘pheatmap’ and ‘ComplexHeatmap’. For some of the analysis mentioned above, we relied on published techniques.39,40
Library preparation for single cell transcriptomics studies and scRNAseq
CD45RO+ CD8+ CD3+ cells were FACS sorted from thawed PBMCs and SFMCs of healthy controls and AS subjects. Cell sorting was performed on the BD Aria III at the Toronto Western Krembil Discovery Tower (KDT)-UHN Flow Cytometry Facility, Toronto, Canada. Gating strategy for FACS sorting is depicted in Figure S4A. A summary of cohort demographics for healthy control subjects and AS patients is provided in Table S3. Freshly sorted, live single cell suspensions with viability of greater than 80% were prepared for subsequent library generation at the Princess Margaret Genomics Center, Toronto, Canada. Cell concentration and viability was assessed via Trypan Blue staining, using a haemocytometer. Gel beads-in-emulsion generation, barcoding, cDNA amplification, 5’ gene expression library construction, V(D)J amplification from cDNA and V(D)J library construction were performed with Chromium Next GEM Single Cell 5’ Reagent Kits v2 (10x Genomics) in accordance with manufacturer’s instructions. 2,000 cells were targeted for capture from each sample, sequenced to a depth of 50,000 reads for both GEX and TCR analyses. Both 5’ gene expression and V(D)J enriched libraries were quantified and evaluated via bioanalyzer high sensitivity chip (Agilent) prior to sequencing. The final libraries were sequenced on a NovaSeq 6000 S4 flow cell (Illumina) with an R1-26, i7-10, i5-10, R2-90 configuration. 31,184 single cell RNA-seq profiles across all groups were generated for downstream gene expression analyses.
Quality control of scRNAseq data
Raw scRNAseq data were preprocessed using the Cell Ranger Single Cell Software Suite (V.5.0.1) provided by 10x Genomics for demultiplexing cellular barcodes, read alignment and generation of the gene-cell matrix under the GRCh38 human reference genome. Detailed quality control metrics were generated and evaluated by Seurat R package (V.4.5.0), RStudio (v2024.04.0). Genes detected in less than 3 cells and cells in which detected transcripts were fewer than 200 or more than 8000 genes, or >70% UMIs derived from mitochondrial genes or log10 gene count/log10 UMI count >0.80 were filtered out and excluded from the subsequent analysis.
Single cell transcriptomics analysis
The R package ‘Platypus’ (v.4.4.0) was used to process and analyse sequenced data in this study. The package is publicly available at github.com/alexyermanos/Platypus. For single cell gene expression (GEX) analysis, the output directory from cellranger count was supplied as input to the function automate_GEX, that analyzes and integrates transcription data using functions from the R package Seurat (v3.1.1), RStudio (v.2024.04.0). GEX libraries were integrated using the SCTransform function from Seurat. During data pre-processing, cells containing more than 20% mitochondrial genes were removed. Transcriptional cluster and clonotype information were integrated using the VDJ_GEX_integrate function in Platypus under default parameters. For the 31,184 cells in the healthy control and AS data set, we identified 12 distinct CD8+ clusters (Figure 3E), we categorized CD8+ clusters based on canonical gene expression (e.g. CCR7, TCF7, IL7R, LEF for early differentitated memory CTL cluster; Figures 3, S5, and S6) For trajectory analysis, the R packages ‘Platypus’ and Monocle3 were used to generated slingshot and Monocle3 trajectory inferences in Figures 4A and 4B, respectively. Monocle3 package is publicly available at github.com/cole-trapnell-lab/monocle3. Root nodes to construct developmental trajectories and subsequent pseudo-time analyses were chosen based on a priori CTL cluster determination of early differentiated memory cells expressing key transcripts such as TCF7, CCR7, and IL7R).
VDJ analysis
28,405 scTCR-seq profiles were generated for downstream T cell receptor clonal analyses, with a mean of 84.18% (SEM ± 1.44) of cells with productive V-J gene pairing. For clonotype analysis, clonotyping information was extracted directly from the output directory of cellranger using the function analyze_VDJ in the R package ‘Platypus’. The function VDJ_clonotype was used to quantify the number of unique clones in each sample, with clone.strategy set to either ‘cdr3a.a’, ‘hvj.lvj’, ‘hvj.lvj.cdr3length.cdr3homology’, or ‘hvj.lvj.cdr3lengths’. In addition, the R package ‘scRepertoire’ was used to calculate diversity indices of TCR profiles and relative abundance of ‘hyperexpanded’, ‘large’, ‘medium’, ‘small’, and ‘rare’ clonotypes in Figure 4.
Flow cytometry and intracellular transcription factor staining
Thawed PBMC and SFMC single-cell suspensions were first stained with a Live/Dead fixable cell stain (FVS780, BD) according to the recommendations of the manufacturer. Cells were blocked with FcX (Biolegend) prior to staining with cell surface antibodies. For experiments in which TCF-1, TOX, IFN-γ, and TNF-α were stained, cells were fixed with 4% paraformaldehyde buffer and permeabilized with intracellular staining buffer (BioLegend). Antibodies used are listed in Supplementary Table (X). Data were acquired on FACSCanto II (BD) at the Toronto Western Krembil Discovery Tower (KDT)-UHN Flow Cytometry Facility, Toronto, Canada, and analyzed with FlowJo software.
CTL cell sorting
Paired SFMC and PBMC from 5 AS patients were thawed and prepared from frozen samples for cell staining with fluorescent conjugated antibodies. Prepared single cell suspensions were stained with the following anti-human antibodies (CD14- FITC, CD4- FITC, CD11c-FITC, CD20-FITC, CD16- FITC, CD3- APC, CD8- PE-Cy7, CD45RO- APC-Cy7, PD-1- PE, TIGIT- BV605, CD127- BV421) and sorted on the BD Aria III at the Toronto Western Krembil Discovery Tower (KDT)-UHN Flow Cytometry Facility, Toronto, Canada, with gating strategy illustrated in Figure S2D. Between 10,000-50,000 cells per sample were collected in RPMI supplemented with 50% heat-inactivated FCS. CD45RO+ CD127+ PD-1+ TIGIT+ CD3+ CD8+ and CD45RO+ CD127- PD-1+ TIGIT+ CD3+ CD8+ populations were sorted for cytokine release assay.
Cytokine release assay
Sorted CD45RO+ CD127+ PD-1+ TIGIT+ CTLs and CD45RO+ CD127- PD-1+ TIGIT+ CTLs were stimulated for a total of 4 hours with Cell Activation Cocktail (Biolegend) containing PMA and ionomycin according to manufacturer’s protocol; GolgiPlug protein transport inhibitor containing Brefeldin A (BD Biosciences) was added for the last 3.5 hours of culture. Afterwards, cells were incubated with surface antibodies (CD3-APC; CD8- PE-Cy7; Lineage- FITC) and then fixed, permeabilized, and intracellularly stained with anti-human IFN-γ-PE, and TNF-α-PE.
Quantification and statistical analysis
All statistical analyses were performed using GraphPad Prism software. Data were tested for normality before selection of statistical tests. Values are presented as mean ± SEM. Two-tailed statistical tests were used, with specific tests indicated in the figure legends. p values less than 0.05 were considered statistically significant.
Published: June 6, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.112821.
Supplemental information
References
- 1.Inman R.D., Sieper J. Oxford University Press; 2016. Axial Spondyloarthritis. [Google Scholar]
- 2.Mauro D., Thomas R., Guggino G., Lories R., Brown M.A., Ciccia F. Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat. Rev. Rheumatol. 2021;17:387–404. doi: 10.1038/s41584-021-00625-y. [DOI] [PubMed] [Google Scholar]
- 3.Bowness P. Hla-B27. Annu. Rev. Immunol. 2015;33:29–48. doi: 10.1146/annurev-immunol-032414-112110. [DOI] [PubMed] [Google Scholar]
- 4.Qaiyum Z., Gracey E., Yao Y., Inman R.D. Integrin and transcriptomic profiles identify a distinctive synovial CD8+ T cell subpopulation in spondyloarthritis. Ann. Rheum. Dis. 2019;78:1566–1575. doi: 10.1136/annrheumdis-2019-215349. [DOI] [PubMed] [Google Scholar]
- 5.Gracey E., Yao Y., Qaiyum Z., Lim M., Tang M., Inman R.D. Altered Cytotoxicity Profile of CD8+ T Cells in Ankylosing Spondylitis. Arthritis Rheumatol. 2020;72:428–434. doi: 10.1002/art.41129. [DOI] [PubMed] [Google Scholar]
- 6.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E., Lynn R.C., Philip M., Rao A., Restifo N.P., et al. Defining 'T cell exhaustion'. Nat. Rev. Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osuch S., Metzner K.J., Caraballo Cortes K. Reversal of T Cell Exhaustion in Chronic HCV Infection. Viruses. 2020;12:799. doi: 10.3390/v12080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collier J.L., Weiss S.A., Pauken K.E., Sen D.R., Sharpe A.H. Not-so-opposite ends of the spectrum: CD8(+) T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021;22:809–819. doi: 10.1038/s41590-021-00949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinney E.F., Lee J.C., Jayne D.R.W., Lyons P.A., Smith K.G.C. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Garner L.I., Zvyagin I.V., Paley M.A., Komech E.A., Jude K.M., Zhao X., Fernandes R.A., Hassman L.M., Paley G.L., et al. Autoimmunity-associated T cell receptors recognize HLA-B∗27-bound peptides. Nature. 2022;612:771–777. doi: 10.1038/s41586-022-05501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura A., Zeng F., Nakamura S., Reid K.T., Gracey E., Lim M., Leng L., Jo S., Park Y.S., Kusuda M., et al. Macrophage migration inhibitory factor drives pathology in a mouse model of spondyloarthritis and is associated with human disease. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abg1210. [DOI] [PubMed] [Google Scholar]
- 13.Levine J.H., Simonds E.F., Bendall S.C., Davis K.L., Amir E.a.D., Tadmor M.D., Litvin O., Fienberg H.G., Jager A., Zunder E.R., et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieland D., Kemming J., Schuch A., Emmerich F., Knolle P., Neumann-Haefelin C., Held W., Zehn D., Hofmann M., Thimme R. TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat. Commun. 2017;8 doi: 10.1038/ncomms15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gounari F., Khazaie K. TCF-1: a maverick in T cell development and function. Nat. Immunol. 2022;23:671–678. doi: 10.1038/s41590-022-01194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson A.H., Zhang F., Dunlap G., Gomez-Rivas E., Watts G.F.M., Faust H.J., Rupani K.V., Mears J.R., Meednu N., Wang R., et al. Granzyme K(+) CD8 T cells form a core population in inflamed human tissue. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abo0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang R., Rangachari M., Kuchroo V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019;42 doi: 10.1016/j.smim.2019.101302. [DOI] [PubMed] [Google Scholar]
- 18.Wolfl M., Kuball J., Ho W.Y., Nguyen H., Manley T.J., Bleakley M., Greenberg P.D. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfei F., Kanev K., Hofmann M., Wu M., Ghoneim H.E., Roelli P., Utzschneider D.T., von Hoesslin M., Cullen J.G., Fan Y., et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 20.Scott A.C., Dündar F., Zumbo P., Chandran S.S., Klebanoff C.A., Shakiba M., Trivedi P., Menocal L., Appleby H., Camara S., et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan O., Giles J.R., McDonald S., Manne S., Ngiow S.F., Patel K.P., Werner M.T., Huang A.C., Alexander K.A., Wu J.E., et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faham M., Carlton V., Moorhead M., Zheng J., Klinger M., Pepin F., Asbury T., Vignali M., Emerson R.O., Robins H.S., et al. Discovery of T Cell Receptor beta Motifs Specific to HLA-B27-Positive Ankylosing Spondylitis by Deep Repertoire Sequence Analysis. Arthritis Rheumatol. 2017;69:774–784. doi: 10.1002/art.40028. [DOI] [PubMed] [Google Scholar]
- 23.Komech E.A., Koltakova A.D., Barinova A.A., Minervina A.A., Salnikova M.A., Shmidt E.I., Korotaeva T.V., Loginova E.Y., Erdes S.F., Bogdanova E.A., et al. TCR repertoire profiling revealed antigen-driven CD8+ T cell clonal groups shared in synovial fluid of patients with spondyloarthritis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.973243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komech E.A., Pogorelyy M.V., Egorov E.S., Britanova O.V., Rebrikov D.V., Bochkova A.G., Shmidt E.I., Shostak N.A., Shugay M., Lukyanov S., et al. CD8+ T cells with characteristic T cell receptor beta motif are detected in blood and expanded in synovial fluid of ankylosing spondylitis patients. Rheumatology. 2018;57:1097–1104. doi: 10.1093/rheumatology/kex517. [DOI] [PubMed] [Google Scholar]
- 25.Ranganathan V., Ciccia F., Zeng F., Sari I., Guggino G., Muralitharan J., Gracey E., Haroon N. Macrophage Migration Inhibitory Factor Induces Inflammation and Predicts Spinal Progression in Ankylosing Spondylitis. Arthritis Rheumatol. 2017;69:1796–1806. doi: 10.1002/art.40175. [DOI] [PubMed] [Google Scholar]
- 26.Wallace D.J., Figueras F., Wegener W.A., Goldenberg D.M. Experience with milatuzumab, an anti-CD74 antibody against immunomodulatory macrophage migration inhibitory factor (MIF) receptor, for systemic lupus erythematosus (SLE) Ann. Rheum. Dis. 2021;80:954–955. doi: 10.1136/annrheumdis-2020-219803. [DOI] [PubMed] [Google Scholar]
- 27.Grebinoski S., Zhang Q., Cillo A.R., Manne S., Xiao H., Brunazzi E.A., Tabib T., Cardello C., Lian C.G., Murphy G.F., et al. Autoreactive CD8(+) T cells are restrained by an exhaustion-like program that is maintained by LAG3. Nat. Immunol. 2022;23:868–877. doi: 10.1038/s41590-022-01210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piedra-Quintero Z.L., Wilson Z., Nava P., Guerau-de-Arellano M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.597959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauvois B., Durant L., Laboureau J., Barthélémy E., Rouillard D., Boulla G., Deterre P. Upregulation of CD38 gene expression in leukemic B cells by interferon types I and II. J. Interferon Cytokine Res. 1999;19:1059–1066. doi: 10.1089/107999099313299. [DOI] [PubMed] [Google Scholar]
- 30.Schiavoni I., Scagnolari C., Horenstein A.L., Leone P., Pierangeli A., Malavasi F., Ausiello C.M., Fedele G. CD38 modulates respiratory syncytial virus-driven proinflammatory processes in human monocyte-derived dendritic cells. Immunology. 2018;154:122–131. doi: 10.1111/imm.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X., Yue L., Liu W., Wang Y., Wang L., Xu B., Wang Y., Pan J., Yan X. CD38 and E2F transcription factor 2 have uniquely increased expression in rheumatoid arthritis synovial tissues. Clin. Exp. Immunol. 2014;176:222–231. doi: 10.1111/cei.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio J., Humbel M., Mulki L., Katsuyama E., Krishfield S., O'Connell J., Tsokos G.C., Kyttaris V. Expanded CD8(+)CD38(+) T Cell Population in Patients With Systemic Lupus Erythematosus Is Linked to Increased Infection Rates: A Prospective Study. ACR Open Rheumatol. 2024;6:801–806. doi: 10.1002/acr2.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsuyama E., Suarez-Fueyo A., Bradley S.J., Mizui M., Marin A.V., Mulki L., Krishfield S., Malavasi F., Yoon J., Sui S.J.H., et al. The CD38/NAD/SIRTUIN1/EZH2 Axis Mitigates Cytotoxic CD8 T Cell Function and Identifies Patients with SLE Prone to Infections. Cell Rep. 2020;30:112–123.e4. doi: 10.1016/j.celrep.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrelli A., Mijnheer G., Hoytema van Konijnenburg D.P., van der Wal M.M., Giovannone B., Mocholi E., Vazirpanah N., Broen J.C., Hijnen D., Oldenburg B., et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Investig. 2018;128:4669–4681. doi: 10.1172/JCI96107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minnie S.A., Waltner O.G., Zhang P., Takahashi S., Nemychenkov N.S., Ensbey K.S., Schmidt C.R., Legg S.R.W., Comstock M., Boiko J.R., et al. TIM-3(+) CD8 T cells with a terminally exhausted phenotype retain functional capacity in hematological malignancies. Sci. Immunol. 2024;9 doi: 10.1126/sciimmunol.adg1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber L.M., Nowicka M., Soneson C., Robinson M.D. diffcyt: Differential discovery in high-dimensional cytometry via high-resolution clustering. Commun. Biol. 2019;2:183. doi: 10.1038/s42003-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Linden S., Valkenburg H.A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 38.Ortolan A., Ramiro S., van Gaalen F., Kvien T.K., Landewe R.B.M., Machado P.M., Ruyssen-Witrand A., van Tubergen A., Bastiaenen C., van der Heijde D. Development and validation of an alternative ankylosing spondylitis disease activity score when patient global assessment is unavailable. Rheumatology. 2021;60:638–648. doi: 10.1093/rheumatology/keaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen L.R., Leipold M.D., Pedersen C.B., Maecker H.T. The anatomy of single cell mass cytometry data. Cytometry A. 2019;95:156–172. doi: 10.1002/cyto.a.23621. [DOI] [PubMed] [Google Scholar]
- 40.Olsen L.R., Pedersen C.B., Leipold M.D., Maecker H.T. Getting the Most from Your High-Dimensional Cytometry Data. Immunity. 2019;50:535–536. doi: 10.1016/j.immuni.2019.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
scRNA-seq data can be accessed via GEO using the accession code: GSE288581.
-
•
This article does not report original code.
-
•
Additional information needed to reanalyze the data reported in this article and permitted by UHN REB 20–5026 is available from the lead contact upon request.