Abstract
Background
Compared with paroxysmal atrial fibrillation, persistent atrial fibrillation (AF) is more harmful and difficult to treat, and the efficacy of different catheter ablation for both also varies greatly. So this study aimed to systematically evaluate the efficacy and safety of cryoballoon (CB) and radiofrequency (RF) ablation in the treatment of persistent AF.
Methods
We searched the PubMed, Embase, and Cochrane library databases for studies comparing the efficacy and safety between CB and RF ablation for persistent AF. All included studies met our inclusion criteria.
Results
A total of 11 studies, 2551 patients were enrolled in this study, including 1256 patients in CB group and 1295 patients in RF group. Meta-analysis results showed that the freedom from atrial tachyarrhythmia (ATA) recurrence was similar between the CB and RF groups (OR 1.00, 95% CI 0.85 to 1.18, I2 16%). The results of repeated ablation events in the two groups were similar (OR 0.85, 95% CI 0.64 to 1.12, I245%), while the operative time in the CB group was shorter than that in the RF group (mean reduction 45.27 min, 95% CI 61.34 to 29.20 min, I2 95%). There was no significant difference in fluoroscopy time between the two groups (mean difference 2.12 min, 95% CI 7.83 to 12.07 min, I2 99%). The incidence of total complications was similar between the two groups (OR 1.08, 95% CI 0.74 to 1.58, I2 0%), but phrenic nerve palsy (PNP) was more likely to occur in the CB group (OR 4.84, 95% CI 1.84 to 12.71, I2 0%). The incidence of pericardial tamponade was not statistically different between the two groups (OR 0.72, 95% CI 00.32 to 1.58, I2 0%).
Conclusions
CB can be used as an alternative therapy to RF for persistent AF, both of which have considerable efficacy and safety. CB can significantly reduce the operation time with the probability of high PNP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-025-02911-x.
Keywords: Persistent atrial fibrillation, Cryoballoon ablation, Radiofrequency ablation, Meta-analysis
Introduction
Catheter ablation has emerged as a well-established therapeutic intervention for atrial fibrillation (AF), with widespread clinical adoption. It achieves arrhythmia control through three principal mechanisms: elimination of AF triggers, electrophysiological substrate modification, and modulation of the autonomic nervous system [1]. Pulmonary vein isolation (PVI) serves as the cornerstone strategy in catheter ablation for both paroxysmal and persistent AF. In recent years, besides traditional radiofrequency (RF) ablation therapy, an updated technique-cryoballoon ablation (CB) is widely used in the treatment of AF, especially paroxysmal AF [2]. The 2024 ESC Guidelines [3], which provide a Class I recommendation for PVI in paroxysmal AF and Class IIa recommendation for endoscopic and hybrid ablation procedures in symptomatic persistent AF refractory to antiarrhythmic drug (AAD) therapy. However, until now there have been a few articles to compare the efficacy of CB and RF ablation on persistent AF. This meta-analysis aims to compare the safety and efficacy of CB and RF ablation for the treatment of persistent AF.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement was used for the conduct of this meta-analysis [4].
Data sources and inclusion criteria
Three databases, PubMed, Embase, and Cochrane library databases, were searched during the study and conducted from the data of 2003 to March 2024. The medical terms used in the search were ((((((("Radiofrequency Ablation"[Mesh]) OR (Ablation, Radiofrequency[Title/Abstract])) OR (Radio-Frequency Ablation[Title/Abstract])) OR (Ablation, Radio-Frequency[Title/Abstract])) OR (Radio Frequency Ablation[Title/Abstract])) OR (Ablation, Radio Frequency[Title/Abstract])) AND (persistent atrial fibrillation)) AND ((cryoballoon ablation) OR (cryoablation)).The inclusion criteria for this study, established a priori, were as follows: (1) The patient had a medical history of persAF; (2) The population enrolled must be ablation-naïve; (3) Studies regarding the safety and efficacy of CB and RF for persistent AF; (4) Follow-up time was greater than one year. Additionally, Manual search was performed by screening the references cited in this manuscript (backward citation tracking).
Outcomes
In this study, clinical efficacy and safety were selected as outcome indicators. Clinical efficacy was the primary end and assessed by the recurrence of AF/atrial tachycardia (AT) after the blanking period (BP). According to the 2024 European Heart Rhythm Association (EHRA) consensus, the blanking period has been redefined as 8 weeks following catheter ablation for atrial fibrillation [5]. However, all 11 included studies published prior to 2024 employed a BP definition of 3 months post-procedure [6]. Secondary outcomes included total procedural duration, fluoroscopy duration, and safety outcomes (total complications, phrenic nerve palsy (PNP), pericardial effusion, or tamponade).
Data collection and quality assessment
The selected articles in this meta-analysis were read and screened independently by inclusion and exclusion criteria. The above process was extracted independently by two calibrated examiners, and any disagreements were resolved through open discussion. The following study characteristics were retrieved: the first author of included studies, time of publication, sample size, ablation method, gender, age, time of follow-up, adverse events, interventions and outcome indicators. For all included studies, observational studies and randomized controlled trials used Newcastle–Ottawa Scale and RevMan 5.4 software for quality evaluation, respectively.
Statistical analysis
The included studies were tested for heterogeneity to ensure the reliability of the meta-analysis. The I2 was used to access the heterogeneity of the test results [7]. I2 > 50% and P < 0.1 represents significant heterogeneity among studies, and random-effects model was used for combining. If I2 < 50% and P > 0.1, the heterogeneity among the test results was considered small, and a fixed-effects model was used for combining. Sources of heterogeneity were sensitivity analyzed by case-by-case literature exclusion or subgroup analysis. The studied indicators were collected and collated, and the meta-analysis was performed using Review Manager5.4 software. The dichotomous variables were described by odds ratio (OR), and the continuous variables were described by mean difference (MD). Each effect size was estimated with 95% confidence intervals (95% CI), and P < 0.05 was set for statistical significance.
Result
Study selection
By searching three databases, a total of 1262 studies were selected for the first time(PubMed: 1,067; Embase: 120; Cochrane: 75), 70 duplicate literatures were eliminated, and 152 reviews, meta-analysis, case reports, conference abstracts, and letters were eliminated through the classification function of the database. After reading the title and abstract, 22 studies were confirmed for full reading. Eleven studies were excluded due to unavailability of original data, duplicate publication, and being non-original research. Finally, 11 studies were included in meta-analysis, including 3 randomized controlled trial (RCT) and 8 observational studies. The process of literature screening results is shown in Fig. 1 [8–18]. Notably, the studies by Guler et al. [19] and Kosmidou et al. [20] were not included because no separate comparison of CB and RF was made. The study by Gallagher et al. [21] was not included because the ablation protocol used in this study was different from other studies, and the purpose of the study was irrelevant to us. Hoffman et al.’s [22] study was not accepted because it did not extract useful data.
Fig. 1.
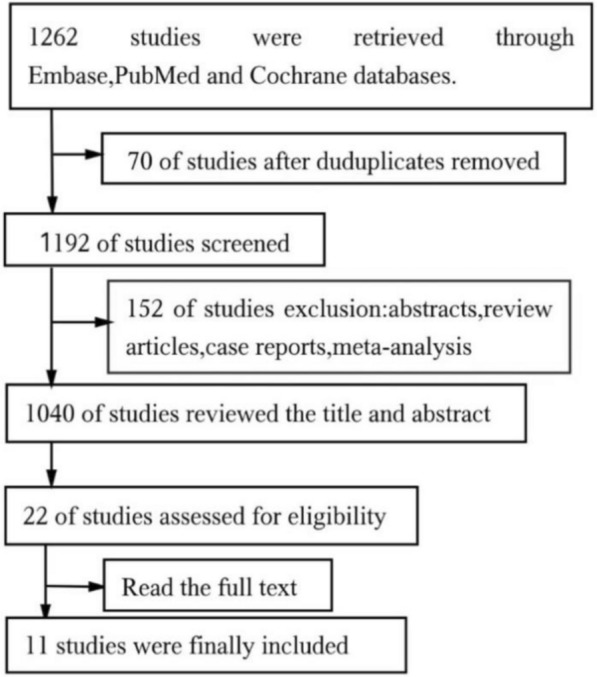
PRISMA Flow Diagram
Characteristics and quality of studies
11 relevant studies were included in this meta-analysis, including a total of 2551 patients, 1256 patients in CB group and 1295 patients in RF group. The basic characteristics of the included studies are shown in Table 1. Baseline patient characteristics are shown in Table 2. For CB, most studies used second-generation balloons as the ablation protocol. For RF, most studies used the contact force (CF)—sensing catheters. In particular, Boveda et al.'s study used first-generation balloons and non-CF catheters [9]. The average follow-up time ranged from 12 to 48 months. Most studies monitor recurrent use Holter ECG for 1 to 7 days, Mörtsell et al. [12] and Yokokawa et al. [11] use Cardiac Implantable Electronic Device (CIED) and Implantable Loop Recorder (ILR) to monitor the recurrence of ATA, and Baimbetov et al. [17] use the Reveal XT Implantable loop recorder (Medtronic, Minnesota) to monitor relapse. After the CB group was isolated in the pulmonary veins, most studies were allowed to tinker with different additional ablation techniques. At subsequent follow-up, most studies also performed repeated ablation in patients with recurrent ATA. The quality of all studies on observational was evaluated using Newcastle–Ottawa Quality Assessments, and the scores of all studies were 7–9 points, indicating a low risk of bias and a high quality of the selected studies. The RCTs were assessed by the Cochrane risk-of-bias tool,which had some unclear risk of bias. The details about the author’s judgments of the risk of bias assessment are outlined in (supplementary material: Table S1, Fig. S1, Fig. S2).
Table 1.
Characteristics of studies included in this meta-analysis
RF Radiofrequency, CB Cryoballoon, LAD Left arital diameter, LVEF left ventricular ejection fraction, CB-Adv Arctic front advance™, Gen Generation, CF Contact fore, CTI cavotricuspid isthmus, CFAE complex fractionated atrial electrograms, MI mitral isthmus, RL roof line, RFA radiofrequency ablation, PVI pulmonary vein isolation, BOX isolation of the posterior left atrium
Table 2.
Baseline patient characteristics
| Study | Ablation Type | Age | Male(%) | Duration of AF (months) | BMI | Hyper-tension(%) | LVEF(%) | LAD(mm) |
|---|---|---|---|---|---|---|---|---|
| Akkaya,2018 |
CB RF |
62 (54, 69) 61 (56, 68) |
69.4 64.9 |
39.6 (7.2, 74.4) 30 (8.4, 106.8) |
28.3 (26.0, 32.3) 28.4 (25.5, 31.9) |
73.0 76.6 |
62 (57, 62) 61 (56, 62) |
44 (41/48) 45 (41, 49) |
| Bisignani,2021 |
CB RF |
55.3 ± 8.6 58.16 ± 9 |
55 61.7 |
26.9 ± 21.9 29.8 ± 26.2 |
NR NR |
NR NR |
57.5 ± 6.5 56.6 ± 7.9 |
44 ± 9 46.2 ± 6.9 |
| Boveda,2016 |
CB RF |
59.9 ± 11.6 59.8 ± 9.9 |
90 89 |
49.2 ± 48 42 ± 49.2 |
28.1 ± 3.5 29.8 ± 5.4 |
19.0 19.0 |
48% with LVEF > 50% 49% with LVEF > 50% |
NR NR |
| Baimbetov,2022 |
CB RF |
61.3 ± 10.2 61.6 ± 6.5 |
62 58 |
19 ± 17 22 ± 15 |
NR NR |
NR NR |
59 ± 5 54 ± 6 |
41 ± 5 39 ± 7 |
| Cicont,2015 |
CB RF |
62.4 ± 9.8 62.4 ± 9.5 |
72 76 |
32.7 ± 37.6 26.7 ± 23.7 |
27.5 ± 3.4 28.7 ± 4.0 |
52.0 68.0 |
57.5 ± 3.7 56.3 ± 4.1 |
46.0 ± 7.2 47.2 ± 6.2 |
| Kobori,2022 |
CB RF |
70.0 ± 9.6 70.7 ± 9.1 |
78.3 69.8 |
5 (4–7) 6 (4–7) |
NR NR |
54.9 60.4 |
58.9 ± 7.9 56.7 ± 9.6 |
41.4 ± 5.2 42.9 ± 5.9 |
| Mörtsell,2019 |
CB RF |
59.9 ± 10.5 59.2 ± 10.3 |
66.1 69.1 |
NR NR |
28.1 ± 4.1 28.5 ± 4.6 |
51.7 56.6 |
NR NR |
NR NR |
| Mililis,2023 |
CB RF |
60.22 ± 9.87 62.74 ± 9.09 |
83.3% 77.3% |
67.56 ± 45.36 72.84 ± 77.88 |
30.07 ± 5.28 33.07 ± 3.11 |
NR NR |
55.15 ± 7.10 55.70 ± 6.16 |
44.89 ± 4.79 43.55 ± 4.74 |
| Natale,2021 |
CB RF |
65.3 ± 10 64.7 ± 9.6 |
69.6 74.5 |
NR NR |
NR NR |
13.2 13.5 |
NR NR |
NR NR |
| Shi,2022 |
CB RF |
62.4 ± 8.4 64 ± 8.7 |
86.5 71.4 |
8 (1–14) 8 (0–12) |
29.6 ± 4.7 28.8 ± 4.5 |
55.8 58.0 |
56.0 ± 7.2 56.8 ± 8.1 |
46 ± 6 44 ± 7 |
| Yokokawa,2018 |
CB RF |
64 ± 10 64 ± 8 |
71 79 |
27 ± 21 30 ± 28 |
31 ± 5 32 ± 5 |
63.0 69.0 |
54 ± 12 53 ± 14 |
46 ± 5 46 ± 6 |
The results of meta-analysis
Freedom from recurrent ATA
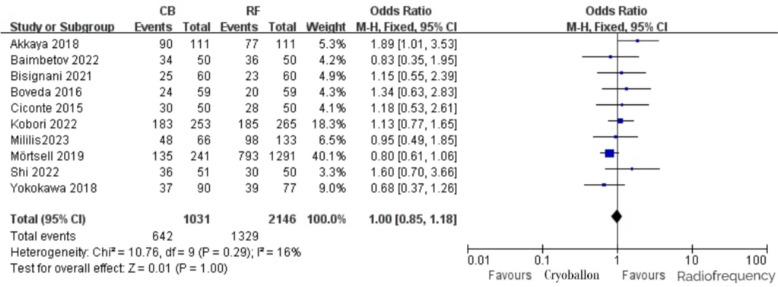
Among the included studies, 10 studies reported the occurrence of freedom from ATA events in patients, and the incidence of freedom from ATA events in the CB group and the RF group was 62.3% and 61.9%, respectively. After heterogeneity test: P = 0.29, I2 = 16%, it was considered that the heterogeneity among the studies was minimal. A fixed-effect model analysis demonstrated no statistically significant difference between the two groups in outcomes for patients without ATA, with an OR of 1.00 (95% CI [0.85,1.18], P = 1.00; Fig. 2).
Fig. 2.
Freedom from recurrent ATA with RF vs. CB ablation
Repeat ablation
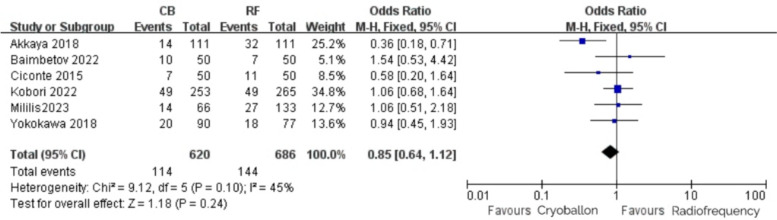
Among the included studies, 6 studies reported repeated ablation events in patients, and the incidence of repeated ablation events in the CB group and the RF group were 18.4% and 21.0%, respectively. After heterogeneity test: P = 0.10, I2 = 45%, it was considered that the heterogeneity among studies was low. The fixed-effect model was used, and the results were as follows: OR = 0.85, 95% CI [0.64,1.12], P = 0.24 (Fig. 3), indicating that there was no statistical difference in repeated ablation of patients between the two groups.
Fig. 3.
Repeat ablation with RF vs. CB ablation
Total procedural time and fluoroscopy time
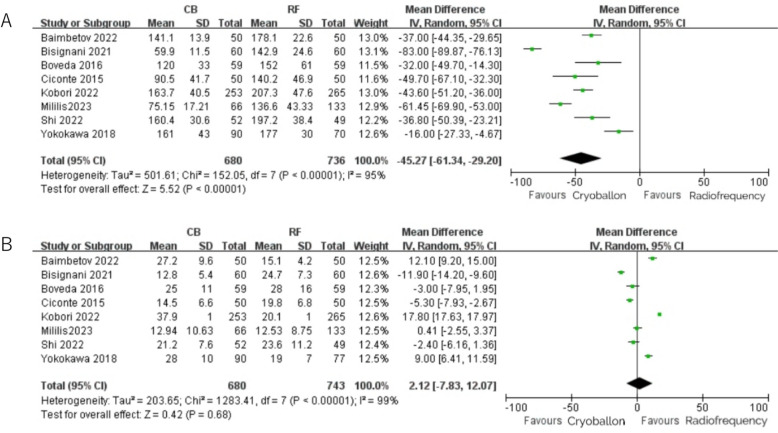
Total procedural time and fluoroscopy time were reported in 8 studies. The total procedural time in the CB group was shorter than that in the RF group, with an average shortening of 45.27 min (95% CI 61.34 to -29.20) (Fig. 4A), and there was a significant statistical difference between the two groups. There was non-significantly shorter fluoroscopy time with CB compared with RF (MD = 2.12, 95%CI [− 7.83, 12.07], P = 0.68) (Fig. 4B).
Fig. 4.
A Total procedural time with RF vs. CB ablation; B: Fluoroscopy time with RF vs. CB ablation
Complications
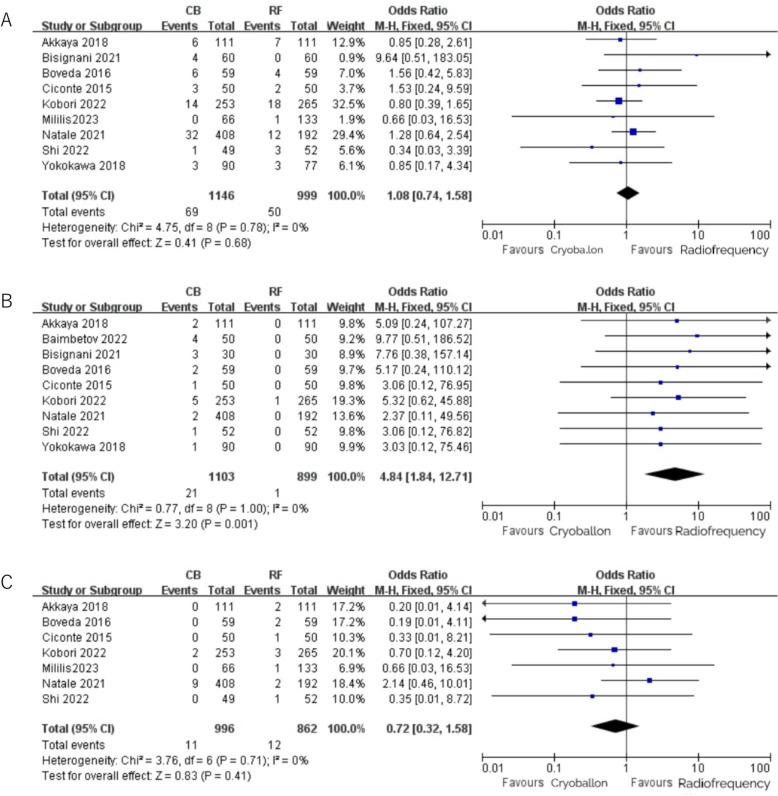
In our study, nine studies reported the occurrence of total complications in patients, and there was no statistical difference in the occurrence of complications between the CB group and the RF group (CB 0.6% vs. RF 0.5%, OR = 1.08, 95%CI [0.74,1.58], P = 0.68) (Fig. 5A). PNP and cardiac tamponade were reported as stand-alone complication. In our meta-analysis, nine studies provided detailed data on the incidence of PNP following CB and RF ablation. All studies consistently reported a higher incidence of PNP in the CB group compared to the RF group, which aligns with the conclusions drawn from our pooled analysis (CB 1.9% vs. RF 0.1%, OR = 4.84, 95% CI [1.84,12.71], P = 0.001) (Fig. 5B). Cardiac tamponade was reported in seven studies, and the results were as follows: OR = 0.72, 95% CI [0.32,1.58], P = 0.41 (Fig. 5C), indicating that there was no statistical difference in the occurrence of pericardial tamponade events between the CB group and the RF group.
Fig. 5.
A Total complications with RF vs. CB ablation; B: PNP with RF vs. CB ablation; C: Cardiac tamponade with RF vs. CB ablation
Subgroup analysis
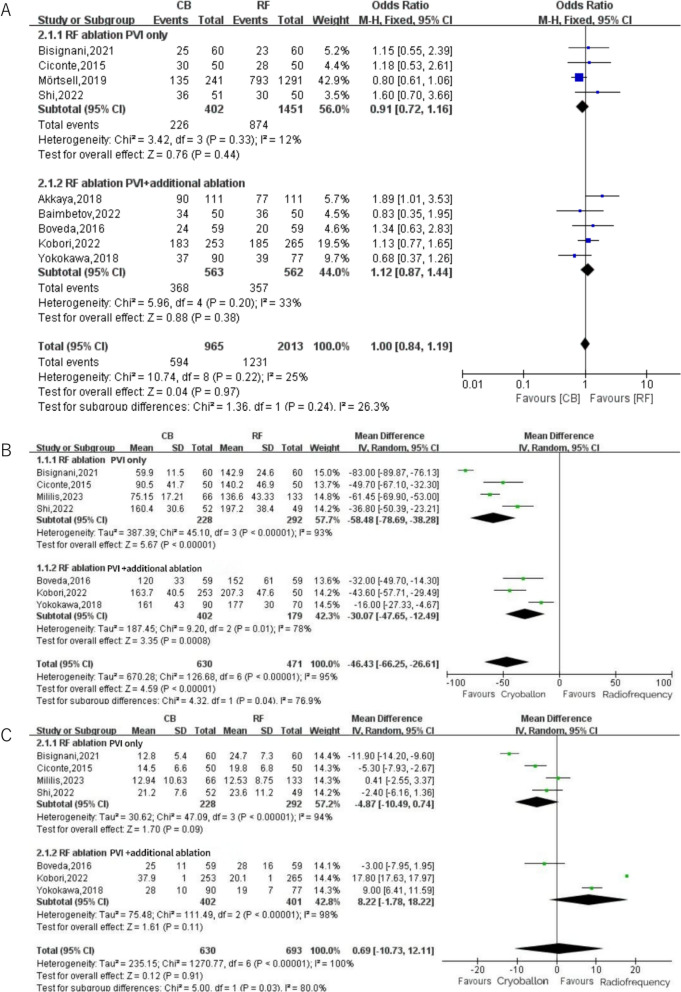
In our study, subgroup analysis comparing freedom from ATA recurrence between RF ablation with PVI alone and RF ablation combined with extra-PVI lesions (Fig. 6A) revealed no significant difference between the two groups (P = 0.97). The subgroup analysis demonstrated statistically significant differences in procedural duration (P = 0.04) and fluoroscopy time (P = 0.03). Notably, in studies limited to PVI-only RF ablation, cryoballoon (CB) technology was associated with reductions of 58.48 min in procedural time and 4.87 min in fluoroscopy time (Fig. 6B, C).
Fig. 6.
A Subgroup analysis for freedom from ATA by trial RF ablation strategy (PVI only or additional ablation permitted) B: Subgroup analysis for procedure time by trial RF ablation strategy (PVI only or additional ablation permitted) C: Subgroup analysis for fluoroscopy time by trial RF ablation strategy (PVI only or additional ablation permitted)
Variations in post-ablation monitoring modalities (e.g., Holter monitoring, ILRs, or CIEDs) may introduce heterogeneity in primary outcome ascertainment and thereby influence study conclusions. To further investigate this, we performed a prespecified subgroup analysis stratifying studies by monitoring sensitivity into high-sensitivity and low-sensitivity cohorts. The analysis revealed no difference in freedom from recurrent ATA by subgroup (P = 0.31), which contradicts findings from benchmark trials (e.g., CIRCA-DOSE). Detailed data and the corresponding forest plots are presented in Supplementary Fig. S3.
Sensitivity analysis
In the statistical analysis of the above 6 indicators, significant heterogeneity was found in the total procedural time and fluoroscopy time. Sensitivity analysis was conducted for these two indicators respectively. In terms of total procedural time, after excluding the study of Bisignani et al. [14], which had a significant outlier, the duration of surgery in the CB group was still shorter than that in the RF group, which was the same as the previous results. Kobori et al.'s study was also regarded as a significant outlier in perspective time, and there was still no significant difference between the results of the two groups after removal and re-analysis.
Discussion
Atrial fibrillation is the most common persistent tachyarrhythmia, and PVI has long been shown to be the cornerstone of catheter ablation for atrial fibrillation [23]. In recent years, CB has become an alternative to conventional RF catheter ablation technology. Technically, compared to RF, CB has the advantages of simplified surgical procedures and a shorter learning curve [24]. In the study of STOP Persistent AF, 54.8% of patients who used CB for catheter ablation maintained sinus rhythm, so CB also had a higher success rate compared with RF [25]. Over the past 15 years, several studies have evaluated the efficacy of CB and RF in the treatment of persistent atrial fibrillation, and both have shown comparable efficacy. Therefore, CB’s application in persistent AF has been widely adopted. For patients undergoing PVI surgery, arrhythmia recurrence constitutes the most frequent cause of rehospitalization, accounting for 40–60% of readmissions within 1 year according to large-scale registries [26, 27], so effectively reducing postoperative recurrence and complications is crucial to accelerate the rehabilitation process. However, although the efficacy of the two techniques is comparable, some people still relapse after PVI surgery, so we need to know the effectiveness and safety of both in patients with persistent atrial fibrillation.
Main findings
In the form of meta-analysis, the following conclusions were drawn by comparing the efficacy of CB and RF in the treatment of persistent atrial fibrillation: (1) Primary outcome: Freedom from recurrent ATA rates were similar between the two groups; (2) Secondary outcomes: There were no significant differences in repeat ablation, fluoroscopy time, total complications, and pericardial tamponade. Total procedural time of CB group was significantly shorter than that of RF group. PNP events occurred less in RF group. Our conclusions are consistent with the results of two previous meta-analyses by Liu et al. [28]and Kim et al. [29].
Efficacy
There was no difference in ATA-free survival rate between the two groups of patients. This result has been confirmed by a large number of data. ATA recurrence during the post-ablation blanking BP is termed early recurrence and is not considered indicative of procedural failure, as these episodes generally resolve spontaneously and typically do not warrant repeat ablation [5]. Conversely, recurrence after the 12-month postoperative period is classified as late recurrence, primarily attributed to progressive atrial fibrosis, sustained inflammatory responses, and pathological autonomic nervous system remodeling [30]. According to the 2024 ESC guidelines, catheter ablation is recommended as the first-line treatment for patients with paroxysmal atrial fibrillation, advocating PVI alone, and for patients with symptomatic persistent AF refractory to antiarrhythmic drug (AAD) therapy, minimally invasive thoracoscopic ablation or hybrid ablation procedures should be considered to prevent AF-related symptoms, recurrence, and disease progression (Class of Recommendation IIa, Level of Evidence A) [3]. However, for persistent atrial fibrillation, the superiority of ablation over pharmacological therapy remains unclear, and the optimal ablation strategy has yet to be definitively established. In addition, some studies such as Shinsuke Miyazaki et al. [31] performed additional ablation of the left roof line in addition to CB for PVI. Patients with persistent AF who underwent complete roof line modification demonstrated a significantly higher rate of freedom from ATA recurrence compared to those without comprehensive roof ablation (94.4% vs. 75.0%, p = 0.0049). Although more extensive ablation strategies beyond PVI have been advocated clinically for persistent AF, no conclusive evidence has established that additional extra-PV ablation approaches further improve procedural success rates [14]. This conclusion aligns with the results derived from our subgroup analysis. Future studies should prioritize investigating the most efficacious ablation strategy for persistent AF, particularly through rigorous comparison of lesion sets and verification of substrate-specific targets. Among the included articles, only the study by Akkaya et al. was based on patients with persistent atrial fibrillation and left atrial enlargement. The study still found that among patients with single pulmonary vein isolation without roof lines (RLs), the midterm survival rate without AF/ LA tachycardia (LAT) was comparable between the CB group and the RF group. However, for patients who received additional RLs, the survival rate without AF/LAT was lower in the RF group than in the CB group. Therefore, RL creation during CB can be a viable alternative to RF for patients with persistent atrial fibrillation and left atrial enlargement.
Procedure and fluoroscopy time
In terms of safety, it can be observed that the total procedural time of CB is significantly shorter than that of RF. Among the 8 literatures that included the study of operative time, significant heterogeneity can be observed, which may be caused by differences in countries, patients' basic conditions, surgeons' proficiency, and implemented intervention measures. However, this does not affect the results we obtained, and the final results of all studies are consistent with ours. In terms of fluoroscopy time, there is no significant difference between the two groups. However, there is also large heterogeneity among the related studies. The sources of heterogeneity may be related to national differences, different patient ethnic groups, anatomical variations, surgeons' proficiency, and different intervention measures. This phenomenon may be attributed to several factors: (1) The proficiency of the operator; (2) preferential use of RF ablation in cases with anatomically complex substrates requiring extended fluoroscopic verification; and (3) heterogeneity in 3D electroanatomical mapping system utilization (e.g., Carto3 vs. EnSite Precision), which differentially impacts fluoroscopy reduction potential.
safety
Regarding complications in this meta-analysis, only PNP shows a statistical difference between the CB group and the RF group, with a higher incidence in the CB group. This result is consistent with that of paroxysmal AF. PNP is the most commonly observed complication of CB ablation, with an incidence of approximately 6% (ranging from 3 to 11%) in clinical surgeries [32–34], mainly related to its anatomical structure. The right phrenic nerve is short in distance from the right inferior pulmonary vein and the right superior pulmonary vein, especially the shortest distance from the right superior pulmonary vein is about 2.1 mm [35]. Therefore, the phrenic nerve is more likely to be damaged during CB ablation in the right superior pulmonary vein. In the early STOP AF study [36], the incidence of PNP in CB ablation was approximately 11.2%. Fortunately, most patients' symptoms were temporary, and only about 1.5% of patients still had persistent PNP symptoms after one year. For this common complication, surgeons will conduct certain computed tomographic (CT) examinations before the operation to evaluate the anatomical structure of the left atrium and pulmonary veins. During the operation, the pacing electrode is placed in the superior vena cava above the cryoballoon for pacing, or the pacing electrode is placed in the right subclavian vein for stable pacing. At the same time, the surgeon manually presses under the xiphoid process to feel the diaphragmatic pulsation. If the occurrence of PNP is observed during the operation, the operation should be stopped immediately to allow the tissue to rewarm as soon as possible to prevent further aggravation of the damage. Moreover, compared with paroxysmal atrial fibrillation, the incidence of PNP in persistent atrial fibrillation is higher. The reason may be that in addition to pulmonary veins, a larger-scale ablation may be required for persistent atrial fibrillation. Currently, most of the cryoballoons commonly used in our clinical practice are second-generation CB. Among the literatures we included, only the studies by Boveda et al. and Hoffman et al. used the first-generation CB. The study by Furrnkranz et al. [37] concluded that compared with the first-generation balloon, the second-generation CB significantly improves the success rate of pulmonary vein isolation, reduces the operative time and fluoroscopy time, and some subsequent prospective studies have confirmed this. However, the second-generation CB increases the incidence of PNP while enhancing the cryo efficacy. Therefore, more stringent monitoring of diaphragmatic movement is required during the operation.
Limitations
Our study has several limitations. Significant heterogeneity was observed in key outcomes, particularly procedural and fluoroscopy times. Potential sources of heterogeneity include operator experience, variations in surgical interventions, ablation scope, and whether additional ablation strategies beyond PVI were performed. Subgroup analysis of the efficacy of RF ablation strategies revealed no statistically significant differences. However, these findings require cautious interpretation due to uneven covariate distribution and limited sample size in each subgroup. The predominance of observational studies in our analysis introduces inherent methodological constraints. Certain studies employed non-randomized allocation of ablation modalities, as evidenced by the exclusion of CB ablation in patients presenting with severe left atrial wall thinning or pulmonary venous anatomical anomalies [16]. Procedural variability was further compounded by operator decisions to perform additional ablation targets (LA roof, posterior wall, mitral isthmus, or complex fractionated atrial electrograms) based on individual intraoperative findings, leading to baseline characteristic disparities between groups that increase susceptibility to selection bias and indication confounding. Follow-up duration discrepancies (12–24 months) across studies and inconsistent use of implantable monitoring devices for asymptomatic atrial fibrillation detection introduced outcome definition variability. In the subsequent follow-up, only a few studies used implantable devices for monitoring, which led to missed detections of asymptomatic atrial fibrillation recurrence and variations in outcome definitions. Current evidence from most studies confirms substantial differences in arrhythmia detection rates among various post-ablation monitoring strategies, with implantable cardiac monitors (ICMs) established as the gold standard [38]. The CRYSTAL-AF trial by Healey et al. [39] demonstrated that implantable cardiac monitors (ILRs/CIEDs) achieved an 8- to tenfold higher detection rate of occult atrial fibrillation compared to conventional monitoring strategies (29.3% vs. 3.0% over 12 months, HR = 8.8, 95% CI 4.2–18.4, P < 0.001). In subgroup analyses stratified by different post-ablation monitoring modalities, our findings demonstrate significant discrepancies compared to prior studies. This discrepancy may stem from limited sample sizes within subgroups (high-sensitivity: n = 3 studies; low-sensitivity: n = 5 studies), resulting in insufficient statistical power to detect true heterogeneity. We acknowledge this as a methodological limitation and advocate for standardized monitoring protocols in future studies to mitigate surveillance bias. These critical confounding factors were not quantitatively analyzed in our current study and require further validation through prospective investigations with standardized protocols.
Conclusions
CB can be used as an alternative therapy to RF for persAF, both of which have considerable efficacy and safety. No significant differences were observed between CB and RF ablation in terms of freedom from ATA recurrence or fluoroscopy time. However, CB can significantly reduce total procedural time with the probability of high PNP.
Supplementary Information
Additional file 1. Table S1 Risk-of-Bias Assessment Tool for Observational Studies. Fig. S1 Risk-of-Bias Summary of RCT studies. Fig. S2 Risk-of-Bias Chart of RCT studies. Fig. S3 Subgroup analysis for freedom from recurrent ATA by trial post-ablation monitoring strategies (high-sensitivity and low-sensitivity cohorts)
Acknowledgements
We would like to thank all the authors of the 11 articles included in this Meta-analysis.
Author contributions
Conceptualization, CTZ; writing—original draft preparation,GYM; writing—review and editing, MHL; supervision, CTZ; data analysis, GYM & MHL. The authors revised the manuscript for intellectual content. All authors have read and approved the final manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
There are no ethical issues involved in this meta-analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tian-Zhi Cai, Email: caitianzhi1978@126.com.
Hong-Lan Ma, Email: mahlmed@163.com.
References
- 1.Benali K, et al. Impact of catheter ablation of atrial fibrillation on disease progression. JACC Clin Electrophysiol. 2025;11(2):421–35. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G, et al. Catheter ablation of atrial fibrillation: current status, techniques, outcomes and challenges. Kardiol Pol. 2018;76(12):1680–6. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder IC, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314–414. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzeis S, et al. 2024 European heart rhythm association/heart rhythm society/Asia pacific heart rhythm society/Latin American heart rhythm society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2024;26(4):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calkins H, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Heart Rhythm. 2017;14(10):e445–94. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 8.Ciconte G, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17(4):559–65. [DOI] [PubMed] [Google Scholar]
- 9.Boveda S, et al. Outcomes after cryoballoon or radiofrequency ablation for persistent atrial fibrillation: a multicentric propensity-score matched study. J Interv Card Electrophysiol. 2016;47(2):133–42. [DOI] [PubMed] [Google Scholar]
- 10.Akkaya E, et al. Ice or fire? Comparison of second-generation cryoballoon ablation and radiofrequency ablation in patients with symptomatic persistent atrial fibrillation and an enlarged left atrium. J Cardiovasc Electrophysiol. 2018;29(3):375–84. [DOI] [PubMed] [Google Scholar]
- 11.Yokokawa M, et al. Cryoballoon antral pulmonary vein isolation vs contact force-sensing radiofrequency catheter ablation for pulmonary vein and posterior left atrial isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2018;15(12):1835–41. [DOI] [PubMed] [Google Scholar]
- 12.Mörtsell D, et al. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace. 2019;21(4):581–9. [DOI] [PubMed] [Google Scholar]
- 13.Natale A, et al. Real-world safety of catheter ablation for atrial fibrillation with contact force or cryoballoon ablation. J Interv Card Electrophysiol. 2021;60(3):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisignani A, et al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity-matched score comparison between different strategies. J Interv Card Electrophysiol. 2022;64(1):9–16. [DOI] [PubMed] [Google Scholar]
- 15.Shi LB, et al. Cryoballoon vs. radiofrequency catheter ablation: insights from NOrwegian randomized study of PERSistent Atrial Fibrillation (NO-PERSAF study). Europace. 2022;24(2):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori A, et al. Comparison of cryoballoon and contact force-sensing radiofrequency ablation for persistent atrial fibrillation in clinical practice. Circ J. 2022;86(2):290–8. [DOI] [PubMed] [Google Scholar]
- 17.Baimbetov AK, et al. Comparative effectiveness and safety of cryoablation versus radiofrequency ablation treatments for persistent atrial fibrillation. Am J Cardiol. 2022;184:22–30. [DOI] [PubMed] [Google Scholar]
- 18.Mililis P, et al. Radiofrequency versus cryoballoon catheter ablation in patients with persistent atrial fibrillation: a randomized trial. J Cardiovasc Electrophysiol. 2023;34(7):1523–8. [DOI] [PubMed] [Google Scholar]
- 19.Guler TE, et al. Combined cryoballoon and radiofrequency ablation versus radiofrequency ablation alone for long-standing persistent atrial fibrillation. Am J Med Sci. 2017;354(6):586–96. [DOI] [PubMed] [Google Scholar]
- 20.Kosmidou I, et al. Comparing safety and efficacy of irrigated radiofrequency catheter ablation versus combined cryoballoon and catheter ablation for persistent atrial fibrillation. J Atr Fibrillation. 2013;6(3):924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher MM, et al. Multi-catheter cryotherapy compared with radiofrequency ablation in long-standing persistent atrial fibrillation: a randomized clinical trial. Europace. 2021;23(3):370–9. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann E, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21(9):1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, et al. Chinese guidelines for the diagnosis and management of atrial fibrillation. Pacing Clin Electrophysiol. 2024;47(6):714–70. [DOI] [PubMed] [Google Scholar]
- 24.Velagic V, et al. Learning curve using the second-generation cryoballoon ablation. J Cardiovasc Med (Hagerstown). 2017;18(7):518–27. [DOI] [PubMed] [Google Scholar]
- 25.Su WW, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP persistent AF trial. Heart Rhythm. 2020;17(11):1841–7. [DOI] [PubMed] [Google Scholar]
- 26.Samuel M, et al. Association of atrial fibrillation burden with health-related quality of life after atrial fibrillation ablation: substudy of the Cryoballoon vs contact-force atrial fibrillation ablation (CIRCA-DOSE) randomized clinical trial. JAMA Cardiol. 2021;6(11):1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noseworthy PA, et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the american heart association. Circulation. 2019;140(25):e944–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XH, et al. Cryoballoon versus radiofrequency ablation for persistent atrial fibrillation: a systematic review and meta-analysis. Kardiol Pol. 2020;78(1):20–9. [DOI] [PubMed] [Google Scholar]
- 29.Kim JA, Chelu MG. Comparison of cryoballoon and radiofrequency ablation for persistent atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2023;66(3):585–95. [DOI] [PubMed] [Google Scholar]
- 30.El-Harasis MA, et al. Recurrence after atrial fibrillation ablation and investigational biomarkers of cardiac remodeling. J Am Heart Assoc. 2024;13(6): e031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki S, et al. Cryoballoon left atrial roof ablation for persistent atrial fibrillation-Analysis with high-resolution mapping system. Pacing Clin Electrophysiol. 2022;45(5):589–97. [DOI] [PubMed] [Google Scholar]
- 32.Andrade JG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8(9):1444–51. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi F, et al. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011;8(6):885–91. [DOI] [PubMed] [Google Scholar]
- 34.Sarabanda AV, et al. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol. 2005;46(10):1902–12. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Quintana D, et al. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16(3):309–13. [DOI] [PubMed] [Google Scholar]
- 36.Packer DL, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–23. [DOI] [PubMed] [Google Scholar]
- 37.Furnkranz A, et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol. 2013;24(5):492–7. [DOI] [PubMed] [Google Scholar]
- 38.Aguilar M, et al. Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: implications for practice and clinical trial design. Circulation. 2022;145(1):21–30. [DOI] [PubMed] [Google Scholar]
- 39.Sanna T, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1 Risk-of-Bias Assessment Tool for Observational Studies. Fig. S1 Risk-of-Bias Summary of RCT studies. Fig. S2 Risk-of-Bias Chart of RCT studies. Fig. S3 Subgroup analysis for freedom from recurrent ATA by trial post-ablation monitoring strategies (high-sensitivity and low-sensitivity cohorts)
Data Availability Statement
No datasets were generated or analysed during the current study.