Abstract
Traditional drug delivery systems are constrained by limitations such as low drug-loading efficiency, immunogenicity, and functional simplicity, rendering them inadequate to address the demands of complex pathological scenarios. Microalgae have emerged as a promising alternative system, possessing not only health benefits derived from their bioactive compounds but also inherent properties that naturally align with delivery system requirements. These advantageous characteristics include exceptional biocompatibility, easily modifiable surface properties, and photosynthetic oxygen-generating capacity. Therefore, using microalgae to develop functional natural delivery systems that combine the delivery of therapeutic substances with the regulation of body health is promising. This review provides a detailed exposition of microalgae-based delivery strategies, including the characteristics, advantages, and types of both microalgae and their derived delivery systems. Furthermore, it pays particular attention to their delivery applications in various biomedical fields, as well as the clinical progress, research gaps, and future trends. Overall, microalgae have great potential for the development of natural delivery systems that promote organismal health and enhance biological functionality. In the future, the integration of emerging biosensing and nanotechnologies with microalgae platforms holds great promise for advancing next-generation therapeutic delivery systems with intelligent responsiveness, precise targeting, and personalized treatment capabilities.
Keywords: Delivery system, Nanotechnology, Hydrogels, Microrobots, Biomedical applications
Graphical abstract
Highlights
-
•
Microalgae offer natural bioactive carriers for drug delivery and health modulation.
-
•
Microalgae-based systems enable targeted, controlled, and responsive drug release.
-
•
Various delivery forms include microcapsules, nanocarriers, and hybrid hydrogels.
-
•
Microalgae enhance PDT, SDT, wound healing, and multimodal medical imaging.
-
•
Research trends highlight growing interest in biomedical microalgae applications.
1. Introduction
Modern precision medicine has set forth numerous requirements for drug delivery systems, including the biocompatibility of the carriers, controllability of drug loading, and intelligent targeting capabilities [[1], [2], [3]]. Traditional synthetic materials, despite their excellent mechanical properties, are often limited by high immunogenicity and complex metabolic pathways [[4], [5], [6]]. Moreover, their relatively singular functionality makes them inadequate for complex pathological scenarios [7,8]. As reported by Nature Medicine and Nature Biomedical Engineering, “The media almost daily reports on the progress of new drugs, but the methods of drug delivery are rarely highlighted [9].” However, “Drug delivery technologies have enabled the development of many pharmaceutical products [10].” This highlights the critical yet often overlooked role of drug delivery systems in modern medicine. Meanwhile, modern disease treatment is transitioning from monotherapy to a tripartite model that integrates “treatment, repair, and prevention,” imposing a dual mission on delivery systems: to precisely deliver bioactive substances while also modulating the disease microenvironment [[11], [12], [13]]. This has prompted researchers to turn their attention to natural biological carriers with evolutionarily optimized characteristics. Given that, the development of functionalized natural carriers that can both deliver therapeutic agents and regulate systemic health has become a strategic high ground in the field of biomedical materials.
Microalgae are among the most efficient photosynthetic microorganisms in nature. Despite their simple structures, they are capable of highly efficient metabolism, synthesis, and accumulation of a wide range of valuable nutrients [14]. These high-value bioactive compounds mainly include proteins, lipids, carbohydrates, pigments, and vitamins, which endow microalgae and their derivatives with potent anti-inflammatory and immune-regulatory properties, as well as other potential health benefits [[14], [15], [16], [17]]. However, the potential of microalgae extends beyond their ability to enhance health. In addition to being rich in these functional components, and more significantly, as ancient photosynthetic organisms, they offer a natural blueprint for the development of novel delivery systems due to their unique hierarchical structures and inherent properties. For instance, the nanoporous siliceous cell walls of diatoms exhibit tunable and readily modifiable surface chemistry [18,19]. Chlamydomonas reinhardtii can move rapidly and is guided by visible light after sensing it (a phenomenon known as phototaxis) [20,21]. The three-dimensional helical structure and intrinsic fluorescence of Spirulina have been proven to enable efficient drug loading, while also allowing non-invasive in vivo monitoring [22,23]. Therefore, microalgae, which possess health benefits and natural delivery attributes, hold promise to meet the multifaceted demands of precision medicine for advanced intelligent delivery architectures.
By conducting a statistical analysis of publications related to microalgae in the Web of Science database and selecting the most highly cited papers each year from 2015 to 2024 for further analysis, the general evolution of research hotspots in the field of microalgae can be clearly illustrated (Fig. 1). The statistical results indicate that over the past decade, the field of microalgae research has shown a trend of diversification, with the most highly cited papers in different years focusing on distinct research hotspots. For example, early studies (from 2015 to 2020) tended to focus on the fundamental biological characteristics of microalgae and their potential applications in environmental remediation. However, as research has deepened and technology has advanced, the research hotspots in recent years (from 2022 to 2024) have gradually shifted towards the exploration of microalgae in cutting-edge fields such as materials science and biomedical applications, with a particular focus on drug delivery. This shift in research hotspots also fully reflects that the potential of using microalgae and their derived resources for delivery is increasingly gaining attention.
Fig. 1.
Evolution of hotspots in microalgae related research in recent ten years. (Note: According to the retrieval rules of the core database of Web of Science, taking “microalgae∗” as the topic, the most frequently cited research publications related to microalgae in that year were selected for analysis. The color block represents research areas. Retrieval date: February 28, 2025.)
Fig. 2 presents a bibliometric analysis that illustrates the research landscape and developmental trends of microalgae-based biomedical applications over the past decade. Panel A maps the top publication journals and their corresponding categories in Web of Science. Notably, leading journals such as Chemical Engineering Journal, ACS Sustainable Chemistry & Engineering, and Advanced Functional Materials highlight the strong presence of microalgae-related work in engineering and materials, while another cluster of journals, such as Food Chemistry, Nutrients, and Journal of Agricultural and Food Chemistry, reflects growing interest in food science, nutrition, and therapeutic bioactivity. This interdisciplinary distribution underscores microalgae's diverse applicability and growing traction across both engineering technology and life science domains. Panel B shows a year-by-year increase in the number of publications from 2015 to 2024, rising from fewer than 500 articles in 2015 to over 1700 articles in 2024. This consistent growth trend demonstrates sustained scholarly interest and expanding research momentum in the field of microalgae-based biomedical technologies. Panel C displays a keyword co-occurrence network, highlighting the strong associations between “microalgae” and “drug delivery,” “nanoparticles,” and “hydrogel.” These linkages reflect the growing research focus on microalgae-based multifunctional delivery systems. It also indicates the trend of integration of microalgae in engineered nanomaterials and smart delivery systems. Overall, the network confirms drug delivery as a central and evolving application of microalgae in biomedical research.
Fig. 2.
Overview of publications on topics related to this review according to the Web of Science database. (A) Sankey diagrams of the top five publications corresponding to the categories related to the retrieval topic; (B) Trends in the number of publications related to this review topic; (C) Keyword co-occurrence network map of related topics based on VOSviewer analysis. (Note: According to the retrieval rules, taking microalga∗ AND (health∗ OR benefit∗ OR function∗ OR deliver∗ OR transmit∗ OR biomedic∗ as the topic. Retrieval date range is from 1 January 2015–1 January 2025.).
Based on all the above backgrounds, a review of this topic is conducive to systematically summarizing the research progress of microalgae in health benefits and delivery strategies, facilitating the integration of different disciplines, and driving the development of microalgae-based advanced intelligent delivery systems with clinical application value for precision medicine. Currently, several reviews have summarized the progress of microalgae in biomedical applications, primarily focusing on microalgae encapsulation technology [24], the role of microalgae in tissue repair [25], the use of microalgal biomass as drug carriers [26], and their applications in tumor therapy [27]. Although the above reviews provide valuable insights into the biomedical applications of microalgae, they still leave some knowledge gaps in the comprehensive understanding and optimization of microalgal delivery strategies.
To address the growing interest and evolving research landscape of microalgae-derived delivery systems, this review aims to provide a comprehensive and in-depth analysis of their development and biomedical potential. We first summarize the intrinsic characteristics and engineering advantages that make microalgae suitable for therapeutic delivery, including biocompatibility, low toxicity, biodegradability, targeting ability, and oxygen-generating capacity. We then categorize the main types of microalgae-based delivery systems, such as microcapsules, nanocarriers, composite hydrogels, and microrobots, and elaborate on their respective preparation methods and functional properties. In addition, we highlight their diverse applications in drug delivery, oxygen supply, medical imaging, and registered clinical trials. Finally, we discuss current challenges and outline future perspectives, with an emphasis on interdisciplinary integration and clinical transformation. This review is intended to serve as a systematic reference and conceptual framework for researchers working in biomedicine, materials science, and nanotechnology.
2. Characteristics or advantages of microalgae-based delivery system
Microalgae and their active ingredients, characterized by diversity and functionality, show great potential for applications in the fields of medicine, nutrition and health. In addition, microalgae are gaining attention for their ability to act as a unique delivery vehicle. Microalgae are not only a source of functional ingredients, but their cellular structure and biological properties also provide them an irreplaceable advantage in delivery systems. This dual identity makes microalgae exhibit distinct characteristics from traditional carriers in the delivery of drugs and functional substances. Therefore, exploring the strategies related to microalgae delivery will provide a more comprehensive understanding and support for their application value. Fig. 3 shows the characteristics or advantages of microalgae-based delivery systems.
Fig. 3.
Characteristics or advantages of microalgae-based delivery system. (A) Biocompatibility: Microalgae demonstrate excellent biocompatibility with cells and tissues and do not induce significant immune or toxic effects. (B) Low toxicity: The composition of microalgae is inherently safe, containing natural components including proteins, lipids (ALA, DHA, EPA), carbohydrates, minerals (K, Fe, Se, Ca, Zn, Mg), and vitamins, which contribute to their minimal toxicity profile. (C) Degradability: Microalgae can be degraded through multiple pathways including lysosomal degradation of macrophages and metabolism of gut microbiota. (D) Targeting capabilities: Microalgae can passively participate in physiological and immune responses through innate cell recognition mechanisms, such as macrophage phagocytosis, and can be artificially modified with targeting ligands for active targeting to specific cells or tissues. (E) Large specific surface area (SSA): Three-dimensional modeling reveals the high specific surface area of microalgae, which enables high drug loading efficiency and increased contact area for enhanced therapeutic efficacy. (F) High oxygen-generating efficiency: Microalgal photosynthetic capability allows for efficient oxygen production, which can improve the oxygenation of hypoxic tissues and enhance therapeutic outcomes in oxygen-depleted environments.
2.1. Biocompatibility
One of the core advantages of microalgae as a component of delivery systems lies in their excellent biocompatibility (Fig. 3A). Microalgae can achieve stable in vivo interactions with the host organism without inducing significant immune or toxic reactions, which is mainly attributed to their low immunogenicity [28]. From a molecular mechanism perspective, microalgal cells are rich in polysaccharides, proteins, and lipids, which have high affinity for surface receptors on human cells, thereby effectively avoiding excessive immune recognition and rejection of exogenous substances by the body [9,29,30]. Based on this characteristic, algal-mediated nanoparticles (AMNPs) can avoid triggering inflammatory responses or immune clearance during in vivo delivery, making them ideal carriers for the administration of drugs, bioactive macromolecules, and gene-editing components [31]. On the other hand, the abundant amino and carboxyl groups on the surface of microalgae can load drugs through non-covalent interactions such as electrostatic adsorption, effectively avoiding the immunogenicity issues that may arise from chemical modifications [25]. In vitro co-culture experiments further confirmed the biocompatibility of microalgae: when co-cultured with endothelial cells, both maintained stable morphology and viability, and microalgae exhibited similarities to red blood cells in size and rheological properties [32]. Animal experimental results also supported the good biocompatibility of microalgae. Short-term systemic perfusion experiments showed that microalgae could achieve uniform distribution in the vascular system, and even high-dose systemic injection did not induce rejection reactions in live mice [32]. In summary, microalgae can not only be safely applied for in vivo delivery but also significantly reduce adverse reactions caused by immunogenicity during the delivery process, thereby enhancing the overall therapeutic effect to some extent.
2.2. Low toxicity
Most microalgae themselves, as natural organisms, have low toxicity, especially after proper processing and modification. Microalgae cells have shown a good safety profile in a wide range of organisms, and their low toxicity is mainly due to the structure of the microalgae cell wall and the minimal stimulation of the host immune system by these natural constituents (Fig. 3B) [33]. A recent study has explored the potential of microalgae as coatings for surgical sutures and specifically tested their toxicity and antimicrobial properties. These microalgae not only showed promising antimicrobial activity against Staphylococcus aureus, but also exhibited no toxic effects on L929 mouse fibroblasts. Their findings have important implications for the development of novel surgical materials that can reduce the incidence of surgical infections while maintaining tissue integrity and safety [34]. In addition, Taroncher et al. [35] conducted a comprehensive assessment of the cytotoxicity of several microalgae on HepG2 cells and showed that no significant cytotoxicity was observed after exposure to these microalgae. Evaluating the toxic effects of nano/micro-materials is an important step in their biomedical applications [36]. Therefore, we summarized Table 1, taking several representative microalgae species as examples. It is worth mentioning that when microalgae form a composite system with other novel materials, it is necessary to consider whether the shading effect and agglomeration effect of materials on microalgae may induce toxicity to some extent [37].
Table 1.
Toxicity/safety evaluation results of representative microalgae-based delivery systems.
| Study topic | Microalgae species/system | Model | Dose/Exposure | Assay | Results | Reference |
|---|---|---|---|---|---|---|
| Microalgal biohybrid microrobots in IBD therapy | Hp@CS-PNAs@PAA | In vivo biodistribution and toxicity: BALB/c mice | Oral administration of Hp@CS-PNAs@PAA (Hp: 5 mg/mL; PNAs: 4.5 mg/mL) | Fluorescence imaging and histological analysis (H&E staining) | No detectable damage to major organs (heart, liver, spleen, lungs, kidneys) after treatment with Hp@CS-PNAs@PAA. | [41] |
| Microalgal biomass for oral intestinal disease treatment | SP@Curcumin | Cytotoxicity: intestinal epithelial cells (IEC-6) | Incubated with different concentrations of SP, curcumin, and SP@Curcumin for 24 h | MTT assay | SP@Curcumin exhibited significantly lower cytotoxicity compared to free curcumin. For example, at a concentration of 50 μg/ml SP, SP@Curcumin showed cell viability of about 80 %, while free curcumin showed cell viability of less than 60 %. | [30] |
| Biosafety: Healthy female Balb/c mice (6 weeks old) | Intragastric administration for 30 days | Blood routine and blood biochemistry parameters, H&E staining of major organs (heart, liver, spleen, kidney, lung) | No significant differences in blood parameters or histological damage observed compared to control group. | |||
| pH-responsive microalgal hydrogels for oral insulin delivery | CV@INS@ALG | Cytotoxicity: IEC-6 | Various concentrations (6.25−200 μg/mL) | MTT assay | Cell viability: >90 % at all tested concentrations (6.25−200 μg/mL). | [95] |
| Long-term in vivo biosafety: C57BL/6J mice | Daily oral administration for 30 days (CV = 366.5 mg/kg, insulin = 20 IU/kg) | Histology and blood biochemistry tests | No obvious pathological changes or abnormal inflammation in gastrointestinal tract and other major organs. | |||
| Biodistribution and degradation: BALB/c nude mice | Single oral administration (CV = 366.5 mg/kg, insulin = 20 IU/kg) | Fluorescence imaging, TEM/SEM of gastrointestinal contents | Fluorescence signal detected in upper abdomen at 0.5 h, spread to lower abdomen by 2 h, and almost undetectable at 8 h. | |||
| Microalgal cross-scale delivery for GBM treatment | Diatom microrobots (DMs) | Cytotoxicity: human brain astroblastoma cells (U87 cells) | Incubated with concentrations ranging from 0.01 to 1.5 mg/mL of DMs | CCK-8 assay | negligible cytotoxicity at concentrations up to 1.5 mg/mL (cell viability >50 %). | [111] |
| Euglena hydrogel system for gout | Eug-Col@Fucar | Biosafety evaluation: male KM mice (4 weeks) | Daily oral administration for 15 days: Eug (4 mg/mL), Col (100 μg/mL), Fucar (10 mg/mL/7.5 mg/mL), Eug-Col@Fucar (4 mg/mL, 100 μg/mL, 10 mg/mL/7.5 mg/mL) | Blood routine analysis, blood chemistry analysis, histological evaluation (H&E staining) of major organs | No significant differences in blood routine and biochemical parameters between treatment groups and control group. No detectable damage or histopathological lesions in heart, spleen, lungs, or kidneys. | [145] |
| Side effect assessment: male KM mice (4 weeks) | 17 days of treatment: Control (PBS 10 mg/kg), Model (PBS 10 mg/kg), Eug (4 mg/mL), Col (100 μg/mL), Fucar (10 mg/mL/7.5 mg/mL), Eug-Col@Fucar (4 mg/mL, 100 μg/mL, 10 mg/mL/7.5 mg/mL) | Organ weight ratio to body weight, histological analysis (H&E staining) of stomach and liver, measurement of colon length, histological analysis (H&E staining) of colon | Eug-Col@Fucar group: Normal organ indices, restored colon length (10.675 ± 0.998 cm), mitigated gastrointestinal and hepatic damage. | |||
| Light-driven microalgae micromotor for PDT | Drug-loaded Chlamydomonas reinhardtii micromotor (R-motor) | In vivo biosafety: Balb/c mice | Single intravenous injection of R-motor (4 × 109 kg−1 of Chlamydomonas reinhardtii) | Body weight monitoring, survival rate, hematology tests (PLT, AST, ALT, HGB, WBC, Neu, UREA, UA), histological analysis (H&E staining) | No significant weight loss was observed in treated mice; Only one mortality was recorded in both the algae-alone group and the control group over the 10-day treatment period; No significant deviations in blood indices compared to the untreated tumor-bearing group; No discernible damage was observed in major organs | [29] |
Note: ALG, sodium alginate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CV, Chlorella vulgaris; CS, chitosan; Col, colchicine; Eug, Euglena; Fucar, fucoidan-carrageenan complex; GBM, glioblastoma; H&E, hematoxylin and eosin; HGB, hemoglobin; Hp, Haematococcus pluvialis; INS, insulin; IU, international unit; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Neu, neutrophils; PDT, photodynamic therapy; PAA, poly(acrylic acid); PNAs, platinum nanoparticle assemblies; PLT, platelet count; SP, Spirulina platensis; UA, uric acid; UREA, urea; WBC, white blood cell count.
2.3. Degradability
The biodegradable properties of microalgae ensure their safety and sustainability in the physiological environment. Microalgae derivatives are gradually decomposed in vivo through various biodegradation processes, ultimately transforming into non-toxic products, which makes microalgae delivery systems safer and more controllable during drug delivery [38]. For instance, biohybrid microalgae-robots evade immune recognition through red blood cell membrane camouflage and achieve targeted drug release in the lungs. Following drug release, residual microalgae fragments are phagocytosed by alveolar macrophages and decomposed by lysosomal acidic hydrolases into amino acids and sugars, ultimately leading to complete clearance of the microalgae microrobots (Fig. 3C) [39]. Similarly, microalgae-derived nanoalgosomes can be internalized by mammalian cells through endocytosis, where their lipid bilayers are degraded by intracellular esterases, leading to the release of contents (such as drugs or nucleic acids) into the cytoplasm [40].
In addition, the degradation pathway varies depending on the administration route. SEM observations of Haematococcus pluvialis oral drug delivery systems reveal detailed in vivo degradation processes [41]. Results demonstrate that these carriers maintain structural integrity in the stomach and duodenum but begin to deteriorate in the jejunum and ileum, ultimately undergoing complete decomposition in the colon. This degradation pattern is closely related to gut microbiota decomposition and utilization, as studies have shown that microalgae-based delivery carriers can significantly influence intestinal microbial community structure and improve microbial abundance [22]. The metabolic behavior of microalgae has been further characterized by comparing the fluorescence intensity of chlorophyll in urine samples with time-dependent biodistribution. The results reveal consistency between metabolic behavior and time-dependent distribution in the kidney-urinary system, indicating that microalgae can ultimately be degraded and eliminated from the body through renal clearance [42].
The degradability of microalgae delivery systems not only reduces the cumulative toxicity of the drug delivery system itself but also prevents bioaccumulation issues that may arise from long-term use. The biosafety testing results presented in Table 1 indirectly demonstrate the biological safety of degradation products, with no observed organ accumulation or toxic reactions. With the development of microalgae biomaterials, microalgae-based bioplastic systems have been extensively studied, further promoting the clinical application prospects of microalgae-based delivery systems [43]. In summary, these findings collectively establish that microalgae-based delivery systems possess excellent in vivo degradability through multiple complementary pathways, ensuring both therapeutic efficacy and biological safety.
2.4. Surface and structural features supporting high drug loading
The size and morphology of microalgae not only characterize their appearance, but also determine their access to light and nutrients, as well as their drug loading efficiency [44]. Microalgae have a large specific surface area (SSA), which allows them to provide more binding sites for adsorption or loading of drug molecules, thereby enhancing delivery efficiency (Fig. 3E) [26,45]. The surface of microalgae can be functionally modified or genetically engineered to adaptively load multiple drug molecules. The larger SSA makes this process easier to carry out and allows microalgae to be further modified to enhance their drug delivery capabilities [46]. In addition, a larger SSA can increase the chance of drug contact with target cells, contributing to rapid drug release. This is particularly important for the treatment of acute diseases or in situations where rapid drug action is required, as microalgae can immediately release delivered drugs upon exposure to specific stimuli (e.g., pH, temperature, or enzymes) [26,47]. A recent large-scale morphometric study by Borics et al. calculated the surface area and biovolume of over 2000 freshwater microalgae taxa using realistic 3D models derived from a combination of light microscopy images, identification book illustrations, and direct microscopic observations [44]. Although absolute SSA values were not directly reported in the article, they provide a large number of quantitative parameters such as microalgae equivalent sphere diameter, microalgae species length/width ratio, 3D visualization ratio and their deduction process in the supplementary material. Their analysis revealed substantial variability in surface-area-to-volume (SA/V) ratios across taxonomic groups, reflecting distinct morphological adaptations. These SA/V differences are known to affect nutrient absorption and light utilization, and may also have implications for drug loading capacity in potential biomedical applications, given the role of surface area in material exchange processes. Notably, the high surface area of microalgae, as inferred from their high SA/V ratios, may provide a significant advantage in these applications due to their enhanced capacity for material exchange and interaction with the surrounding environment. The large SSA of microalgae also facilitates intracellular delivery of drugs via endocytosis. During cellular uptake of microalgae, more drugs are able to enter the cell together with them, thereby enhancing the intracellular therapeutic effect of the drug. This mechanism is particularly important in the treatment of diseases that require intracellular action, including cancer [45,48,49].
In addition to SSA, the unique surface properties and structural characteristics of microalgal-derived delivery systems are associated with their drug-loading performance. The surface of microalgal cells is rich in negatively charged functional groups, which can efficiently load positively charged small-molecule drugs through electrostatic adsorption. For example, the negatively charged surface of Spirulina platensis can adsorb curcumin through electrostatic interactions, with a drug-loading efficiency exceeding 80 % [30]. Moreover, the water channels and connecting pores (14–16 nm) in the microalgal cell membrane allow small-molecule drugs (e.g., fluorescein isothiocyanate) to diffuse into the cells under osmotic pressure, thereby further increasing the drug-loading capacity [42]. The morphological characteristics of microalgae further enhance their drug-loading ability. The silica shell of diatoms has a three-dimensional hierarchical porous structure with a large SSA, providing numerous adsorption sites for drugs and realizing sustained release through physical adsorption or chemical bonding. For instance, the slow release of mesalamine and prednisone from diatom carriers has been reported [36]. The helical structure of Spirulina platensis significantly increases its surface area, enabling it to adsorb more drug molecules. Additionally, this geometric feature prolongs the retention time of drugs in the intestinal villi, thereby enhancing bioavailability [30]. Furthermore, the flagellar structure of Chlamydomonas reinhardtii is prone to covalently bond drugs (e.g., vancomycin) [50].
2.5. Multifunctionality
2.5.1. High oxygen-generating efficiency
Microalgae have a high photosynthetic efficiency, with oxygen production rates of up to 10 mg/L min [51]. This efficient photosynthesis makes microalgae an important source of oxygen generation in the microenvironment, especially in localized hypoxic environments (Fig. 3F). From the perspective of molecular mechanisms, the Photosystem II (PSII) and Photosystem I (PSI) within microalgal cells work in concert to drive the photolysis of water molecules by capturing photon energy, thereby generating oxygen, protons, and electrons. The high efficiency of this process is attributed to the precisely regulated photosynthetic electron transport chain system within microalgal cells, which can maintain a stable rate of oxygen production under light conditions. Additionally, microalgae possess a CO2 concentrating mechanism (CCM), enabling them to achieve high photosynthetic rates even in environments with low CO2 concentrations [52,53]. Moreover, microalgal cells are equipped with a unique non-photochemical quenching (NPQ) protective mechanism that safeguards the photosynthetic system from damage under high light conditions, thus maintaining continuous and efficient oxygen production [54]. These self-protective mechanisms ensure that microalgae can maintain stable oxygen generation capabilities under variable environmental conditions, providing a reliable foundation for oxygen supply when used as a carrier for delivery systems.
2.5.2. Targeting
Microalgae can be engineered as targeted carriers to deliver drugs precisely to diseased sites [55]. This targeting capability can be achieved through multiple mechanisms that can be categorized into three main strategies: intrinsic motility, passive targeting, and ligand modification (Fig. 3D). The inherent motility of certain microalgae species enables active navigation toward target sites, facilitating controlled drug delivery through their natural swimming behavior and chemotactic responses [56]. Section 3.4 of this paper provides a detailed description, and thus it will not be reiterated here. Passive targeting mechanisms rely on the natural recognition and uptake processes within biological systems. For instance, microalgae can be engulfed by immune cells, such as macrophages, thereby engaging in physiological and immune responses within the human body. Through this mechanism, microalgae can transport drugs to specific tissues or organs [57]. Additionally, passive targeting can be enhanced through external modulation strategies. For example, coating microalgae with a cell membrane can significantly improve their circulation time and targeting ability in vivo. This approach helps microalgae evade immune system clearance, increasing drug accumulation at the target site [58,59]. Active targeting can be achieved through surface functionalization with specific targeting molecules. Furthermore, the surface of microalgae can be modified with specific targeting molecules, such as antibodies, peptides, or small molecules, to enhance their binding affinity to diseased cells or tissues [60]. These surface modifications enable precise recognition and binding to target receptors or biomarkers expressed on diseased cells.
These integrated strategies ensure that the drug exerts its therapeutic effects only at the desired location, minimizing distribution to normal tissues and thus improving therapeutic efficacy while reducing side effects.
2.5.3. Fluorescence characteristics
The chlorophyll abundant in microalgae possesses fluorescent properties: upon the absorption of light energy by chlorophyll molecules, electrons transition from the ground state to the excited state, and subsequently return to the ground state by emitting photons to form fluorescence. This fluorescence process originates primarily from chlorophyll a, which plays a central role in both photosystem I and II, making it a key photoreactive component in microalgal cells [61]. During this process, non-radiative decay such as internal conversion or vibrational relaxation leads to energy loss, resulting in emitted photons of longer wavelength [62]. Specifically, chlorophyll a exhibits strong absorption in the blue (430–450 nm) and red (640–660 nm) regions of the spectrum and emits characteristic red fluorescence centered around 680–685 nm, which is easily detectable using confocal or fluorescence microscopy [63]. Due to the partial dissipation of energy in the form of heat, the wavelength of fluorescence is longer than that of the absorbed light, typically in the red light region. Although chlorophyll a exhibits two principal absorption peaks in the blue (430–450 nm) and red (640–660 nm) regions, with the latter commonly used for fluorescence excitation due to better tissue penetration and spectral compatibility. Upon stimulation, it emits characteristic red fluorescence, making it an intrinsic optical marker for real-time tracking of microalgae in biomedical applications [25,64,65]. These optical properties of microalgae are widely employed for real-time tracking in biological systems, without the need for additional fluorescent labeling, thus offering a non-invasive and extra label-free imaging advantage.
3. Types of microalgae-derived delivery system
It is widely recognized that drug delivery systems play a crucial role in achieving the efficacy of new drugs. As May [9] points out, simply designing a new drug is not sufficient to ensure its efficacy, it is also critical that the drug effectively reaches the target site. The realization of this goal relies on the application of a variety of delivery vehicles, including nanoparticles, microneedles, erythrocytes, and microalgae. These delivery vehicles can play a key role in improving drug targeting, controlling the rate of drug release, and minimizing side effects, thereby greatly enhancing the therapeutic effect of drugs.
Innovations in drug delivery systems have not only facilitated therapeutic efficacy, but also significantly improved patient compliance and safety, especially in areas such as chronic disease and oncology treatment, which can reduce adverse effects and improve the acceptability of treatment through precise drug delivery [24,26]. Therefore, drug delivery, as an important complementary area of drug development, is gradually becoming a core component of modern pharmaceutical research. The synergy between a drug and its delivery vehicle is the key to effective treatment, ensuring that the drug is able to maximize its effectiveness at the right time and place.
Given the above background, microalgae-derived delivery systems have attracted increasing attention. Section 2 has summarized the characteristics or advantages of microalgae-based delivery systems, which have fully demonstrated their great potential in precision drug delivery. Therefore, an in-depth discussion on the types of microalgae-derived delivery systems and their applications is not only of great academic significance, but also offers new perspectives and possibilities for the advancement of the drug delivery field.
3.1. Microcapsules
Microencapsulation technology aims to encapsulate solid, liquid, or gaseous substances in a cover layer or shell with a diameter of 1–1000 μm, thereby achieving the protection of the encapsulated material, controlling its release, improving its stability, and optimizing its application effect. Currently, microencapsulation technology is widely used in many fields such as drug delivery, food processing, agriculture, and environmental protection [66,67]. Fully utilizing the functional properties and advantages of microalgae and combining them with encapsulation technology is a valuable research direction for application. Overall, researchers have developed several microalgae-based microencapsulation techniques (as core or wall materials), including emulsion polymerization (oil-water emulsification), spray drying, ionic gelation, and freeze drying (Fig. 4A). In addition to microalgae biomass itself, which can be used as a wall material for microcapsules to encapsulate and deliver substances, microalgae-derived extracellular polymers [67], frustule [68], and extract powder [69] have also made some progress in application. Table 2 summarizes the preparation techniques, delivery substances, and application scenarios of microencapsulated microalgae-derived delivery systems in detail.
Fig. 4.
Representative preparation processes or characterization of microalgae-derived microcapsules and nanocarriers delivery systems. (A) Preparation of microalgae-derived microcapsules. (a) Preparation of microalgae-derived microcapsules by oil-water emulsification method, cited from Ref. [24], Copyright © Elsevier. (b) Preparation of microalgae-derived microcapsules by freeze drying method, cited from Ref. [138], Copyright © Elsevier. (c) Preparation of microalgae-derived microcapsules by spray drying method, cited from Ref. [67], Copyright © Elsevier. (d) Preparation of microalgae-derived microcapsules by ionic gelation method, cited from Ref. [124], Copyright © Elsevier. (B) Preparation of microalgae-derived nanocarriers. (a) Types of natural nanocarriers, cited from Ref. [71], Copyright © Royal Society of Chemistry. (b) Characterization of surface functionalized diatoms suitable for different application scenarios, cited from Ref. [139], Copyright © Elsevier. (c) Preparation of microalgae-derived protein nanocarriers, cited from Ref. [75], Copyright © Elsevier. (d) Characterization of microalgae-derived lipids nanocarrier application, cited from Ref. [80], Copyright © Elsevier. (e) Preparation of microalgae-derived extracellular vesicles (nanoalgosomes) nanocarriers, cited from Ref. [140], Copyright © Frontiers Media. (f) Preparation of microalgae-derived metal nanocarriers, cited from Ref. [85], Copyright © Royal Society of Chemistry.
Table 2.
Preparation techniques, delivery substances and applications of different types of microalgae-derived delivery systems.
| Type | Microalgae resources carrier | Substances delivered or loaded | Preparation technique | Application | Reference |
|---|---|---|---|---|---|
| Microcapsule | Cyanothece sp. CCY 0110 (Derived extracellular polymeric substance) | Vitamin B12 | Spray-drying | Controlled release in food products. | [67] |
| Synechococcus elongates 7942 (Biomass) | O2 | Ionic gelation | Constructing hyperoxia microenvironment to induce ferroptosis. | [124] | |
| Chlorella vulgaris (Biomass) | Curcumin | Physical adsorption and encapsulation | Stabilize pharmacological activities of curcumin. | [66] | |
| Coscinodiscus concinnus (Derived frustule) | Streptomycin | Evaporated with ultra-desiccator | Applications for oral drug delivery in vitro. | [68] | |
| Aulacoseira sp. (Derived frustule) | Indomethacin | Surface modification | Delivery of water-insoluble drugs. | [146] | |
| Aulacoseira sp. (Derived frustule) | Levofloxacin | Surface modification | Stimulus-responsive antibiotic drug delivery. | [147] | |
| Arthrospira platensis (Biomass) | Astaxanthin | Not mentioned | Prevention of cognitive impairment in rats. | [148] | |
| Spirulina (Extract powder) | Ellagic acid | Freeze-drying after compounding | Controlled release in simulated gastrointestinal tract. | [69] | |
| Chlorella vulgaris and Spirulina platensis (Biomass) | Protein of microalgae itself | Freeze-drying after compounding | Baby biscuits's nutritional fortifier. | [149] | |
| Spirulina maxima UTEX LB2342 (Biomass) | Antioxidants of microalgae itself | Freeze-drying after compounding | Alleviate cadmium-induced testicular toxicity in rats. | [138] | |
| Spirulina sp. LEB-18 (Biomass) | Bioactives of microalgae itself | Spray-drying | Improve the physical and chemical properties of chocolate milk. | [150] | |
| Nanocarrier | Chlorella pyrenoidosa (Derived protein) | Probiotic | Microfluidic electrospinning | Improve the survival rate of Lactobacillus plantarum in gastrointestinal environment. | [151] |
| Spirulina platensis (Derived protein) | Curcumin | Freeze-drying after compounding | Improve the antioxidant capacity and stability of curcumin in vivo. | [75] | |
| Nannochloropsis sp. (Derived Lipids) | Thymol | Self-assembly and ultrasonic homogenization | As an antibacterial agent for plant disease control. | [79] | |
| Nannochloropsis oceanica (Derived Lipids) | Curcumin and α-tocopherol | Ultrasonic treatment after cyclic freezing and thawing | Protective carrier of antioxidants. | [80] | |
| Diatom (Derived diatomite) | Ibuprofen | Ultrasonic homogenization followed by mixing and impurity removal | Ibuprofen drug carrier with high load, sustained-release and low cost. | [130] | |
| Diatom (Derived diatomite) | Galunisertib | Stirring and crosslinking after mixing | Improve drug efficacy and reduce drug toxicity and side effects. | [19] | |
| Diatom (Derived diatomite) | Doxorubicin HCl and Paclitaxel | Self-assembled | Dual drug delivery to treat relapsing tumors. | [139] | |
| Diatom (Derived diatomite) | Rhenium(I) tricarbonyl anticancer complexes | Dry with argon after mixing | Targeted, sustained-release and synergistic chemotherapy drug delivery. | [152] | |
| Thraustochytrium sp. (Derived oil vesicles) | Lutein and curcumin | Ultrasonic dispersion after forced formation in water phase | Super-long stable drug and nutritional supplement carrier. | [82] | |
| Coelastrella aeroterrestrica BA_Chlo4 (Biomass) | Hexagonal Ag nanoparticles | Freeze-drying after compounding | Anticancer, antibacterial and antioxidant for medical uses. | [86] | |
| Spirulina platensis (Derived extracts) | Fe3O4/Ag nanoparticles | Drying after mixing | Inhibition of breast cancer cells (in vitro). | [87] | |
| Chlorella pyrenoidosa FWAC 7066 (Derived exopolysaccharides) | Ag nanoparticles | Incubate after mixing | Antibacterial agents in pharmaceutical and biomedical areas. | [88] | |
| Hydrogel | Chlamydomonas reinhardtii (Biomass) | Ciprofloxacin and O2 | Chemical crosslinking method | Promote wound healing. | [99] |
| Spirulina (Derived protein) | Catechol@chitosan | Chemical crosslinking method | Repair full-thickness skin injury. | [100] | |
| Synechocystis sp. PAK 13 (Biomass) | Urea | Physical crosslinking method | Water conservation and improvement of soil nutrient utilization rate. | [103] | |
| Porphyridium sp., Dixoniella grisea and Porphyridium aerugineum (Biomass) | Zn2+ | Physical crosslinking method | Antimicrobial wound dressings. | [104] | |
| Spirulina platensis (Biomass) | Berberine | Chemical crosslinking method | Accelerate wound healing and reduce diabetic inflammation infected with MRSA. | [97] | |
| Spirulina (Biomass) | 5-Fluorouracil | Chemical crosslinking method | Anti-cancer drug delivery system with varying drug loading and release according to pH. | [105] | |
| Microcystis aeruginosa (Biomass) | Microcystins | Chemical crosslinking method | As an anti-tumor therapeutic by augmenting tumor immunogenicity and immune responses | [98] | |
| Spirulina platensis (Biomass) | Rhein | Physical crosslinking method | Treating inflammatory bowel disease and its related anxiety and depression. | [90] | |
| Chlorella vulgaris (Biomass) | Insulin | Chemical crosslinking method | PH-responsive oral insulin delivery system with hypoglycemic and insulin sensitizing effects. | [95] | |
| Chlorella vulgaris (Biomass) | Berberine | Chemical crosslinking method | Natural oral hydrogel system for synergistic treatment of lead poisoning-related diseases. | [101] | |
| Schizochytrium sp. (Derived cell wall) | Bovine serum albumin | Chemical crosslinking method | Hydrogel vaccine system applied to mucosal pathway. | [102] | |
| Microrobot | Chlamydomonas reinhardtii (Biomass) | Vancomycin and ciprofloxacin | Surface modification | Biohybrid microswimmers against bacterial infections. | [141] |
| Chlamydomonas reinhardtii (Biomass) | 5- aminolevulinic acid and O2 | Surface modification | Used for photodynamic therapy of breast cancer. | [29] | |
| Chlamydomonas reinhardtii (Biomass) | Doxorubicin | Noncovalent self-assembly | High-yield microrobots that deliver drugs to tumor cells on demand. | [20] | |
| Thalassiosira weissflogii (Biomass) | Temozolomide and tetrakis (4-sulfonatophenyl) porphine | Vacuum loading | Combined chemotherapy and photodynamic therapy for glioblastoma. | [111] | |
| Spirulina (Biomass) | Doxorubicin | Solution-gel method | Minimally invasive delivery of drug release triggered by pH and near infrared. | [113] | |
| Spirulina platensis (Biomass) | Magnetite nanoparticles | Vacuum annealing after mixed freeze-drying | Multifunctional microrobots suitable for photothermal antibacterial therapy, metabolic detoxification and gastrointestinal targeted delivery. | [110] | |
| Chlorella pyrenoidosa (Biomass) | Fe3O4 nanoparticles | Electrostatic adherence | Magnetic-driven microrobots for precise photothermal muscle contraction. | [114] | |
| Chlamydomonas reinhardtii (Biomass) | Doxorubicin | Surface modification | Local active delivery of drug-loaded nanoparticles to inhibit lung metastasis of cancer. | [39] | |
| Chlamydomonas reinhardtii (Biomass) | Ciprofloxacin | Surface modification | Treatment of acute bacterial pneumonia by delivering antibiotics in vivo with the potential of reducing drug resistance | [142] | |
| Chlamydomonas reinhardtii (Biomass) | O2 | Surface modification | Accelerating the healing of diabetic wounds by producing oxygen and combining inflammatory chemokines. | [21] | |
| C. Pitschmannii (Biomass) | Fluorescent dye | Self-assembly | Drug delivery in harsh acidic environment. | [143] | |
| Micromonas pusilla CCMP 1545 (Biomass) | Vancomycin | Surface modification | Non-invasive drug delivery for pneumonia. | [153] | |
| Others (Conventional simple carrier) | Spirulina platensis (Biomass) | Probiotics | Electrostatic self-assembly | Regulate intestinal homeostasis and relieve intestinal inflammation. | [115] |
| Spirulina platensis (Biomass) | Doxorubicin | Electrostatic adsorption | High lung-accumulating and PH-responsive drug sustained release. | [42] | |
| Spirulina platensis (Biomass) | Astaxanthin nanoparticles | Centrifuge collection after mixing | Relieve radiation damage. | [116] | |
| Diatom (Derived silica microparticles) | Prednisone and mesalamine | Immersion method | Sustained release of drugs and enhanced drug permeability. | [36] | |
| Spirulina platensis (Biomass) | Curcumin | Freeze-drying after compounding | Treatment of intestinal diseases by oral administration | [30] | |
| Spirulina platensis (Biomass) | Selenium Nanoparticles | Electrostatic adsorption | Relieve inflammatory bowel disease. | [117] | |
| Spirulina platensis (Biomass) | Amifostine | Freeze-drying after compounding | Protect the intestine from damage caused by radiotherapy. | [22] |
3.2. Nanocarriers
Nanotechnology has a wide range of applications in nutrition, medicine, environment, materials, and other natural sciences. Different forms of nanostructured carriers (mainly including natural nanoparticles, nanoliposomes, extracellular vesicles and nanopolymers) have been successfully applied in drug delivery and diagnostic imaging [[70], [71], [72]].
Diatoms are the most widely used microalgae-based nanocarriers (Table 2). The cell wall of diatoms consists of silica and has a fine mesh structure with nanoscale pores. Therefore, through surface modification or doping with other nanomaterials, the surface of diatoms can be further functionalized to suit different delivery scenarios (Fig. 4B) [73,74]. The diatomite, diatomaceous earth, and frustule used in the current study are essentially closely related and are just different forms of diatom siliceous cell walls.
Besides, protein-based nanocarriers have become a hot research target for encapsulated carriers due to their excellent biocompatibility, biodegradability, and loading capacity (Table 2). The economy and sustainability of microalgae-derived plant proteins compared with animal proteins make them more in line with the trend of green chemistry.The protein content of Spirulina platensis is as high as 460–630 g/kg, which is higher than that of soybean (400 g/kg) and wheat (100–160 g/kg) [75]. Therefore, it is regarded as a new sustainable natural protein source. Many experiments have demonstrated the great potential of Pickering emulsions [76], double cross-linked hydrogels [77], and self-assembled nanoparticles [75] based on Spirulina platensis proteins as packaging carriers of delivery systems.
Lipid nanocarriers are a type of carrier for multiple therapeutic agents that have attracted much attention in the pharmaceutical industry, especially playing an important role in the field of drug and vaccine delivery (Table 2). Lipid nanocarriers include liposomes, solid lipid nanoparticles, nanostructured lipid carriers, and cationic lipid-nucleic acid complexes [78]. Microalgal lipid-based nanocarriers are usually prepared by techniques such as self-assembly, solvent evaporation, and ultrasonic emulsification (Fig. 4B). Many microalgae species under the Nannochloropsis genus (e.g., N. oceanica, N. limnetica, and N. gaditana) have a high lipid yield (accounting for 30–70 % of biomass), and thus occupy an important place in the current development of naturally sourced lipid nanocarriers [79,80].
In addition to lipids of microalgae, microalgae-derived extracellular vesicles as nanocarriers have also been an important research direction in nanobiotechnology in recent years (Table 2). Extracellular vesicles secreted by cells are biological membrane-encapsulated nanoparticles, which have evolved into natural vehicles for transferring endogenous and exogenous bioactive molecules between cells and within or outside organisms through natural evolution [81]. Nanosized small extracellular vesicles isolated from microalgae are commonly referred to as “nanoalgosomes” by researchers. The most studied nanoalgosomes are those derived from the marine photosynthetic microalgae Tetraselmis chuii. These nanoalgosomes have been proven to be one of the most promising microalgae-derived nanocarriers due to their cross kingdom communication capability and biocompatibility, especially their unique osteotropic properties [40,81,82]. Therefore, these nanoalgosomes carriers are very suitable for further exploration as an inspiring clinical therapeutic tool.
Microalgae and their derivatives are also potential natural sources for the green synthesis of nanoparticles (Table 2). Unlike conventional processing methods, the green biosynthesis of nanoparticles by self-assembly is environmentally friendly and highly biocompatible. In particular, some bacteria, fungi, yeasts, and microalgae have been shown to serve as precursors for biosynthesis or conversion into nanoparticles [83]. Specifically, biomolecules in microalgae have the ability to reduce metal ions, and thus are often used as reducing agents and encapsulation carriers for nanoparticle synthesis [84]. Chlorella, Spirulina, Dunaliella, and Scenedesmus are the main microalgae genera used to assist the green synthesis of metal/non-metal nanoparticles, such as gold, silver, copper and selenium [[85], [86], [87], [88]]. In addition to being carriers for drug delivery, microalgae-derived metal nanoparticles themselves can also be regarded as carriers of metal ions in some form, and thus have multiple roles in biomedicine. For example, microalgae-derived metal nanoparticles cause the death of these microorganisms by interacting with bacterial cell membranes, fungal cell walls, and viral surfaces [85]. These nanoparticles also exhibit potential anti-tumor activity by modulating oxidative stress, causing damage to cellular proteins, which leads to cellular dysfunction [89]. In addition, microalgae-derived nanoparticles have the potential to modulate oxidative stress-related diseases, such as cardiovascular disease and diabetes by scavenging free radicals [85,86]. Therefore, the prospects of microalgae-derived nanoparticles in biomedicine deserves further development.
3.3. Composite hydrogels
Hydrogels are a class of hydrophilic polymeric materials with a three-dimensional network structure that can absorb water, swell, and form a gel [90]. Due to their good biocompatibility, tunability, and high loading capacity, hydrogels are widely used in drug delivery systems [[91], [92], [93], [94]]. Hydrogels are not only able to encapsulate drugs and control their release, but also respond to changes in different environmental conditions (e.g., pH, temperature) to achieve targeted drug release [90,95,96]. Combining microalgae with hydrogels to form microalgae composite hydrogels can confer additional functionalities to hydrogels, such as enhanced biocompatibility, biodegradability, antioxidant properties, and immunomodulatory functions (Table 2) [97,98].
Similar to the preparation methods of conventional hydrogels, the preparation of microalgae composite hydrogels is mainly carried out by chemical cross-linking and physical cross-linking (Fig. 5). In the chemical cross-linking method, microalgae and their derived components are combined with cross-linking agents (e.g., chemical cross-linking agents, cross-linking initiators) to form a stable three-dimensional mesh structure through chemical reactions (formation of covalent bonds) [[99], [100], [101], [102]]. In physical cross-linking methods, microalgae and their derived components can be bound to the hydrogel matrix through hydrogen bonding, Van der Waals forces, or electrostatic interactions to form a looser composite network structure [90,103,104].
Fig. 5.
Representative preparation processes of microalgae-derived composite hydrogels. (A) Preparation of microalgae-derived composite hydrogel by hydrogen bonding and electrostatic interaction, cited from Ref. [103], Copyright © Springer Nature. (B) Preparation of microalgae-derived composite hydrogel by self-assembly, cited from Ref. [90], Copyright © John Wiley & Sons. (C) Preparation of microalgae-derived composite hydrogel by crosslinking reaction and coordination of metal ions, cited from Ref. [100], Copyright © MDPI. (D) Preparation of microalgae-derived hydrogel by crosslinking reaction and polymerization, cited from Ref. [98], Copyright © John Wiley & Sons. (E) Preparation of microalgae-derived hydrogel by forming ionic bonds, cited from Ref. [95], Copyright © American Chemical Society.
It is important to critically state that many of the studies do not directly account for the functions that microalgae and their derivatives play in the preparation of hydrogel delivery systems beyond adjuvant therapy. However, we believe that these microalgae do play a key role in the composite hydrogel system derived from them in regulating the loading and delivery capacity of the system. For example, by regulating the amount of Spirulina biomass and the environmental pH in the genipin cross-linked chitosan hydrogel, the water absorption capacity and drug release characteristics of the composite hydrogel can be strongly affected [105].
In general, on the one hand, these microalgae and their derivatives can be directly used as raw materials to synthesize a hydrogel, through which functional substances can be subsequently loaded and delivered. On the other hand, these microalgae and their derivatives can be combined with functional substances to form a complex first, which is then loaded into the hydrogel component through physical and chemical cross-linking to form a composite hydrogel. This composite hydrogel can then be designed to perform a variety of functions as a bioactive hydrogel delivery system. With the development of precision medicine and personalized therapy, the advantages of microalgae composite hydrogels as drug delivery carriers will become increasingly prominent. First, microalgae composite hydrogels have important applications in local drug delivery, antitumor drugs, anti-inflammatory drugs, and vaccine delivery [90,98,102,105]. In addition, the biodegradability and good biocompatibility of microalgae composite hydrogels make them safer for clinical applications [90,101,102]. With the further development of processed microalgae and the development of bioengineering technology, the preparation process and application areas of microalgae composite hydrogels will continue to expand. In the future, they are expected to become an ideal drug delivery carrier and bring new breakthroughs in the treatment of diseases.
3.4. Microrobots
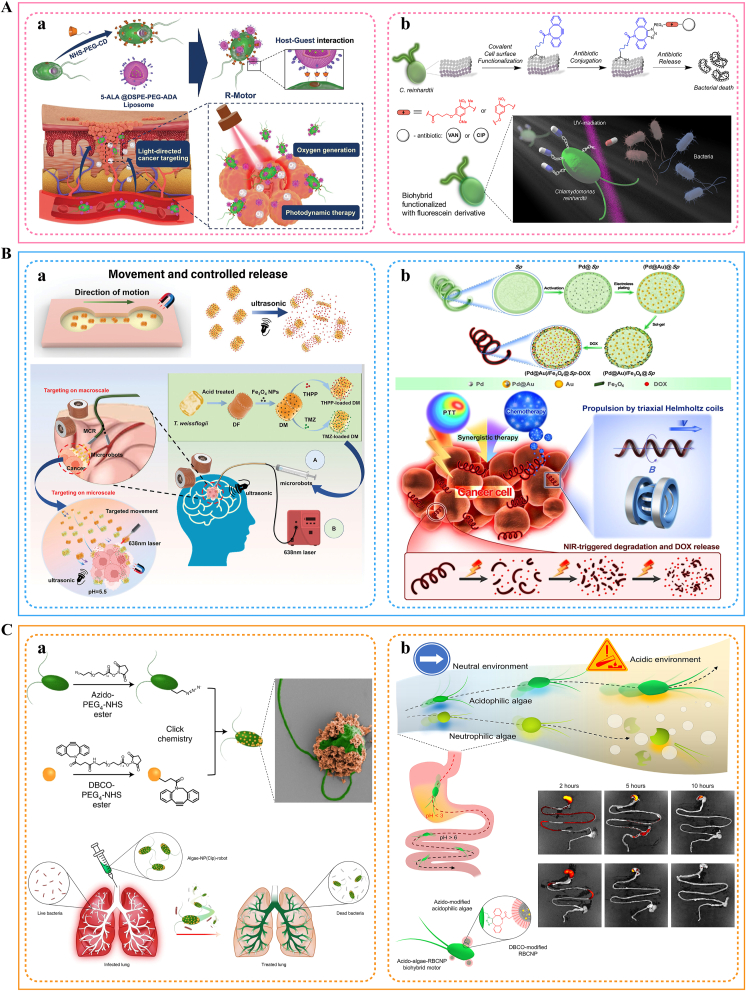
The traditional delivery system still faces challenges in overcoming biological barriers, precise control, and real-time response. To cope with this situation, researchers have specially developed microrobots for the delivery system. Microrobots, alternatively referred to as micro- or nano-scale machines, rotors, motors, or swimmers, are autonomous micro/nanostructures that can operate at the micrometer scale. They possess the ability to perform specific tasks that are requested, either outside or within cells [106]. The characteristics or advantages of microalgae-based delivery systems (Section 2) make them an ideal “bio-machine” fusion mode for intelligent delivery. In particular, some special category of microalgae, such as green algae and Spirulina (e.g., Chlamydomonas reinhardtii, Spirulina platensis), with their responsiveness and self-driven ability under stimuli such as light and magnetic field, offer great potential for the development of microalgae-derived microrobots for delivery systems [65]. Table 2 summarizes in detail the applications of these microrobots derived from engineered microalgae in delivery.
Fig. 6 depicts the basic knowledge of microalgae movement mechanisms. Engineered microalgae-derived microrobots can usually be classified into two main categories based on their driving mechanisms: light-driven microrobots and magnetically-driven microrobots. Light-driven microrobots can be categorized into ordinary light-driven and precision optical tweezer-driven microrobots. The freshwater green alga Chlamydomonas reinhardtii (CR) is used as an example to briefly explain these driving principles. The common light-driven pattern uses the phototropism (behavior in response to light) of microalgae as the driving force to make the microrobot in directional motion through stimulation from an external light source. CR senses light through subcellular organelles called “eyespot”. When the CR flagellum pulsates at its intrinsic frequency of 60–70 Hz, its eyespot is able to receive light signals from different directions. Subsequent triggering of intracellular Ca2+ flow generates a photocurrent that disrupts the balance of motion between the flagella. If the motion path of the CR is parallel to the direction of the light source, the eyespot will continue to perceive the light signal and approach the light source; otherwise, the flagella will redirect the motion of the microalgae [64,107]. As for precision optical tweezers actuation, is a technology that utilizes a three-dimensional potential well formed by the mechanical effect of momentum transfer between light and matter to capture and manipulate particles. Specifically, this technique realizes non-contact manipulation by “clamping” tiny objects through the forces (mainly scattering and gradient forces) generated when the light beam interacts with the object. Currently, the controlled movement of CR in various biological media has been achieved using optical forces [64,65,107,108]. In summary, optical tweezers delivery focuses on using the mechanical properties of light to manipulate and deliver substances, while ordinary light-driven delivery focuses on using the chemical or physical effects of light to control movement.
Fig. 6.
The basics of microalgae-derived microrobots. (A) Intrinsic motion properties, surface functionalization, and applications of microalgae-derived microrobots. (B) Schematic diagram of multi-modal visualization of microalgae movement mechanism. (a) The process of flagellar movement in an algal micromotor that is in motion. (b) The various flagellar configurations of the green alga Chlamydomonas, depicted in distinct colors throughout a single cycle of beating, with tracking done at intervals of 2 ms. (c) The fluid dynamics created by the movement of algae. (d) The propulsion of spiral cyanobacteria (Spirulina) that have been coated with magnetic nanoparticles and are driven by magnetic forces. (e) The motion of a hierarchical diatom (Achnanthes) decorated with catalyst, triggered by chemical reactions. (f) A diagram (i) and a real-time photograph (ii) demonstrating the phototactic behavior of algal motors. The scale bar represents 1 cm. Adapted from Ref. [64], Copyright © John Wiley & Sons.
Magnetically driven microrobots rely on external magnetic fields to control microalgae [109]. These structurally appropriate microalgae, such as diatoms, Spirulina, and Chlorella, readily respond to external magnetic fields through surface modifications. By combining these microalgae with magnetic materials such as Ni, Co, Fe, Fe2O3, and Fe3O4, the direction and speed of motion of these biohybrid microalgae robots can be precisely controlled [65,110]. The magnetically driven system is particularly effective in high-penetration scenarios for targeted delivery and localization in deep tissues [111,112]. The development of the delivery system, drug-carrying efficiency, targeted propulsion performance, and the effectiveness of combined photodynamic therapy (PDT) for this type of micro-robot have been validated in a wide range of clinical application scenarios [110,113,114].
Microalgae-based microrobots have been shown to have longer tissue retention time, controlled drug release characteristics, and contact-free teleguidance performance compared to conventional drug delivery systems, thus contributing to more precise, smarter, and easily adjustable targeted delivery (Fig. 7). Applications in this area will be introduced later.
Fig. 7.
Representative schematic diagram of preparation and targeting processes of microalgae-derived microrobots delivery systems. (A) Light-driven microrobots. (a) Binding of adamantane-modified liposomes (containing photosensitizers 5-Aminolevulinic acid) to cyclodextrin-modified Chlamydomonas reinhardtii surfaces via host-guest interactions, then targeted accumulation in the tumor site by light drive, cited from Ref. [29], Copyright © John Wiley & Sons. (b) Covalently attaching antibiotics with photo-cleavable linker to Chlamydomonas reinhardtii biomixed micro-swimmer, and then effectively delivered to the bacterial infection site by light drive, cited from Ref. [141], Copyright © Elsevier. (B) Magnetic-driven microrobots. (a) Cross-scale drug delivery system of diatom microrobots based on a magnetic continuum robot to realize the combination of PDT and chemotherapy, cited from Ref. [111], Copyright © John Wiley & Sons. (b) Schematic diagram of propulsion, near infrared trigger and synergistic chemophototherapy targeting cancer cells of magnetic micro-robot loaded with doxorubicin, cited from Ref. [113], Copyright © American Chemical Society. (C) Other types. (a) Schematic diagram of preparation and in vivo treatment of microalgae-drug-loaded polymer nanoparticles hybrid microrobots, cited from Ref. [142], Copyright © Springer Nature. (b) Schematic diagram of preparation and gastrointestinal delivery route of acidophilic microalgae microrobots, cited from Ref. [143], Copyright © American Association for the Advancement of Science.
3.5. Others
Last but not least, there are some conventional delivery systems based on microalgae. They usually make direct use of the structural features of microalgae and do not require additional modification, functionalization, or complex preparation steps for efficient loading and delivery of functional substances. According to our summary, Spirulina platensis is the preferred carrier for conventional microalgae-derived delivery systems. Researchers have developed a number of convenient and effective delivery strategies by taking full advantage of its standard spiral structure [115], negatively charged surface [42,116], stimulated fluorescence of chlorophyll [30], and acid environment tolerance [22,117]. In a word, these delivery systems have no complicated design and thus show great prospects for future transformation.
4. Applications of microalgae-derived delivery system
4.1. Oxygen supply
Oxygen is the basis for cellular metabolism, energy production, and tissue repair in living organisms. Particularly in hypoxic or low-oxygen environments, the availability of oxygen is critical. The hypoxic state is common in pathological conditions such as tumors, trauma, and stroke, and this state leads to cellular dysfunction, which in turn aggravates disease progression. Tumor cells are often in a hypoxic state due to rapid growth and inadequate vascular oxygen supply, which not only promotes tumor aggressiveness but also induces resistance to treatments such as radiation and chemotherapy [118,119]. Similarly, stroke and trauma are often accompanied by localized hypoxia, which affects neural and tissue recovery. Therefore, an effective oxygen delivery system can significantly improve cell and tissue function in these hypoxic environments [25,120]. Previously developed methods of oxygen delivery have certain limitations. For example, hemoglobin oxygen carriers, while capable of delivering oxygen, have been hampered from widespread regulatory approval due to their high mortality rate and overall incidence of myocardial infarction [121]. Artificial erythrocytes, while better able to mimic natural erythrocytes in delivering oxygen to tissues throughout the body, still face high costs and production complexity [122]. Although nanoparticle-based oxygen delivery systems have better stability, their toxicological controversies still limit clinical applications [123]. Section 2 details the high oxygen production efficiency and other advantages of the natural microalgae delivery system, revealing the great potential of this system as an ideal oxygen delivery vehicle. Several representative applications of microalgae-based oxygen delivery systems in medicine will be presented next.
To improve the efficacy of melanoma treatment, Jiang et al. [124] designed a miniature oxygen factory, microalgae photosynthesis microcapsules (MPMCs), which utilizes the oxygen-producing capacity of microalgae to enhance the effectiveness of radiation therapy. The system drives the MPMCs through near-infrared laser irradiation to continuously supply oxygen to the tumor microenvironment, which is essential for increasing the sensitivity of melanoma cells to radiation therapy. The results show that MPMCs not only increase oxygen levels in the hypoxic tumor microenvironment through their photosynthetic activity, but also enhance the effectiveness of X-ray therapy by triggering lipid peroxidation and ferroptosis in melanoma cells (Fig. 8A). Integrating of oxygen-producing microalgae into light-driven therapeutic microrobots is a novel idea to enhance PDT. Wang et al. [29] designed a micromotor system loaded with a photosensitizer (5-aminolevulinic acid) using green alga CR, which not only exhibited good biocompatibility and phototropism, but also targeted and accumulated in tumor area to actively produce oxygen (Fig. 8B). Theoretically, this oxygen-producing micromotor system solves an important challenge in PDT, namely, the scarcity of oxygen seriously limits the generation efficiency of reactive oxygen species (ROS) such as singlet oxygen. The results also show that the micromotor system based on microalgae can significantly increase the local drug concentration in the tumor site to synergistically enhance the overall PDT response, ultimately resulting in the complete regression of the tumor in the preclinical animal model of breast cancer. In order to avoid the problem of light penetration depth, Chang et al. [125] developed an oxygen delivery system consisting of CaAl2O4:Eu,Nd persistent luminescent materials and cyanobacteria. As an internal light source independent of long-term external illumination, it can cooperate with cyanobacteria to continuously supply oxygen to the tumor microenvironment, thereby enhancing PDT effect (Fig. 8C). This process overcomes the challenges related to light penetration and minimizes the risk of biotoxicity caused by long-term light radiation. Microalgae-based oxygen delivery systems can also assist and enhance sonodynamic therapy (SDT) for hypoxic tumors. Lu et al. [126] prepared a kind of bio-mixed acoustic sensitizer that combined subminiature bimetallic oxide Mn1.4WOx nano acoustic sensitizer with cyanobacteria. The biological hybrid system can not only alleviate the tumor hypoxia microenvironment under light exposure, but also significantly improve the ROS level activated by ultrasonic irradiation. It is worth noting that the persistent oxygen production of cyanobacteria can even inhibit the gene expression of hypoxia-inducible factor 1α, thereby further enhancing the efficiency of SDT (Fig. 8D).
Fig. 8.
Applications of oxygen delivery system based on microalgae in biomedicine. (A) Continuous oxygen production by MPMCs for synergistic tumour therapy with X-rays by inducing ferroptosis. Examination of oxygen levels in tumors at different (a) times and (b) depths. (c) Confocal images of hypoxia status in tumour sections. (d) Representative images of immunohistochemical staining of tumour sections. (e) Assessment of GPX4 expression in tumour tissue. (f) General schematic diagram. Adapted from Ref. [124], Copyright © Elsevier. (B) Effect of light-guided microalgae microrobot for enhancing photodynamic therapy in tumor-bearing mouse model. (a) The fluorescent trajectory of the microalgae microrobot moving in stereospace. (b) Analysis of oxygen production efficiency of microalgae microrobot under different light sources. (c) Image of mouse tumor at the end of treatment. (d) Weight statistics of different groups of mice during treatment. (e) Survival statistics during treatment. (f) H&E stained slice images of heart, liver, spleen, lung and kidney of mice treated in different groups. Adapted from Ref. [29], Copyright © John Wiley & Sons. (C) Photodynamic therapy platform based on microalgae without external light source activation. (a) Preparation of exogenous irradiation-free photosynthetic microalgae-based photodynamic therapy platform.(b) Enhancement mechanism: continuous output of O2 (relieving tumor hypoxia) and singlet oxygen (tumor treatment). (c) In vitro therapeutic effect: Confocal laser scanning microscope images of 4T1 cancer cells after different treatments. (d) In vivo therapeutic effect: Individual tumor-growth curve of mice after various treatments. Adapted from Ref. [125], Copyright © Elsevier. (D) Enhanced sonodynamic nanotherapy through oxygen production by cyanobacteria. (a) Overall schematic diagram of treatment course. (b) Cell viabilities of Cos7 cells and 4T1 cancer cells treated with biohybrid sonosensitizers. (c) Cell viabilities of 4T1 cancer cells after different treatments under either hypoxic or normoxic conditions. (d) Flow cytometry analysis of 4T1 cancer cells as stained. (e) Weights of dissected tumors for each group after different treatments. (f) ROS and HIF-1α fluorescence images of tumor sections for each group after different treatments. Adapted from Ref. [126], Copyright © John Wiley & Sons.
In addition, the potential of microalgae-based oxygen delivery systems to promote wound healing has also been explored. In the field of wound care, microalgae can also deliver drugs while providing dissolved oxygen to wounds, helping to alleviate acute and chronic tissue hypoxia, thereby promoting cell repair and regeneration [25,127,128]. Since this topic has been introduced in Section 2 and Tables 2 and it will not be reiterated in this section.
4.2. Drug delivery
Microalgae-derived drug delivery systems can be broadly categorized into two types: one is based on simple preparation, and the other is based on surface modification.
The simple preparation system can make full use of the inherent properties of microalgae for drug loading and therapeutic applications. Liu et al. [129] recently developed a novel microalgae-based drug delivery microspheres Eug/Lut@HAMA, in which the microalgae (euglena) loaded with luteolin was encapsulated in methacrylated hyaluronic acid microspheres (HAMA). The system can effectively reduce uric acid level and protect kidney by using the adsorption ability of microalgae to uric acid and the inhibition of luteolin to xanthine oxidase. The microspheres are adhesive, which can prolong the drug retention time in the intestine and achieve sustained release of euglena and luteolin. This innovative approach offers a safe and efficient alternative for the treatment of hyperuricemia and its associated renal complications (Fig. 9A). Microalgae-derived drug delivery systems offer new strategies to improve the efficacy and safety of osteoarthritis (OA) therapy. Liang et al. [23] developed a dual drug delivery system using Spirulina platensis as a carrier for metformin (Met). The system can be responsive to the weakly acidic microenvironment of the joints to achieve controlled release of Met and subsequent release of phycocyanin. This dual release mechanism not only prolongs the retention time of Met, but also provides a synergistic therapeutic effect of anti-oxidation and anti-inflammation from phycocyanin. The system has demonstrated significant improvements in OA animal models and has the potential to restore cartilage metabolic balance. As a natural and biodegradable carrier, microalgae further enhance the safety of the long-term use of the delivery system (Fig. 9B). Zhang et al. [22] constructed an amifostine delivery system (SP@AMF) based on Spirulina platensis. This system can significantly improve the survival rate of IEC-6 cells after radiation, effectively prevent early and delayed radiation damage to small intestine in mice, and maintain the healthy state of gut microbiota, demonstrating good radiation protection effect. Zhong et al. [30] also utilized Spirulina platensis to encapsulate curcumin (SP@Cur). This system demonstrated promising antitumor effects in vitro. Subsequently, its ability to inhibit tumor growth and improve survival rates in a colorectal cancer mouse model further confirmed its potential for clinical applications in gastrointestinal diseases. Ibuprofen, as one of the most commonly used analgesic and anti-inflammatory drugs, has limitations such as rapid degradation and low bioavailability. To enhance its delivery and release performance, a dual encapsulation approach using diatomite and chitosan was developed [130]. This composite system allows for the control of ibuprofen release rate by adjusting the chitosan-to-diatomite ratio, potentially meeting the therapeutic needs of various chronic diseases.
Fig. 9.
Applications of drug delivery system based on microalgae. (A) Schematic diagram of microalgae-based drug delivery microspheres used to treat hyperuricemia with renal injury. (a) Preparation process of Eug/Lut@HAMA. (b) Mechanism of protective effect on acute hyperuricemia with renal injury. Adapted from Ref. [129], Copyright © Elsevier. (B) Schematic diagram of dual drug delivery system based on microalgae (SP@Met). (a) Preparation and release mechanism of SP@Met. (b) Mechanism of effectively alleviating disease progression in OA mice. Adapted from Ref. [23], Copyright © John Wiley & Sons. (C) Gastrointestinal tract drug delivery using functionalized microalgae motors embedded in a degradable capsule. (a) Schematic diagram of manufacturing process of the functionalized microalgae. (b)–(c) Schematic diagram of functionalized microalgae capsule with double coating layers for oral drug delivery applications. (d) Bright-field (top) and fluorescent (bottom) images of functionalized capsule, scale bar, 2 mm. Adapted from Ref. [131], Copyright © American Association for the Advancement of Science. (D) Au-SP@CF biological hybrid system based on microalgae can realize photothermal treatment of breast cancer. (a) Preparation process of Au-TSP. (b) The process of modifying Au-TSP to generate targeted Au-SP@CF biological mixture. (c) Schematic diagram of the application of Au-SP@CF in tumor treatment. Adapted from Ref. [144], Copyright © American Chemical Society.
To develop a smart microalgae-based drug delivery system with higher drug loading efficiency and enhanced functionality, researchers have employed various chemical and physical modification techniques to functionalize the surface of microalgae cells. To enhance the efficiency of oral drug delivery to the gastrointestinal tract, Zhang et al. [131] loaded fluorescent dye and erythrocyte membrane-encapsulated nanoparticles of the chemotherapeutic drug doxorubicin (DOX) onto Chlorella vulgaris via chemical conjugation. To protect the naturally motile Chlorella from damage caused by gastric acid, they further designed a capsule with an inner hydrophobic coating and an outer pH-responsive enteric polymer coating. The inner coating is designed to maintain the viability of the microalgae, while the outer coating dissolves in the intestine, subsequently releasing the functionalized microalgae motors. These microalgae motors are uniformly distributed and retained for extended periods in the gastrointestinal environment, and they can achieve higher drug concentrations in the small intestinal tissue (Fig. 9C). Hosseini et al. engineered a multifunctional biohybrid system based on Spirulina platensis and Au nanoparticles (AuNPs). Specifically, the surface of the microalgae was initially functionalized with thioglycolic acid to enhance the loading efficiency of AuNPs (called Au-TSP). To augment the targeting capability of this biohybrid, folic acid was employed as a targeting agent, and L-cysteine was utilized as a linker to achieve targeted modification of Au-TSP (Au-SP@CF). In vitro assessments revealed that under near-infrared laser irradiation, Au-SP@CF demonstrated significant photothermal performance against triple-negative breast cancer cells. Furthermore, this system exhibited good in vivo biocompatibility and a high tumor suppression effect in tumor-bearing mice (Fig. 9D). Vona et al. [132] achieved high loading and controlled release of the anti-inflammatory drug naproxen by introducing n-octyl chains onto the surface of diatoms. They utilized a 3D skin tissue model to evaluate the transdermal permeation of the drug and its effects on skin cells. The results indicated that the system can achieve gradual drug release and good skin adhesion, without significantly impacting on the morphology and viability of skin cells. Studer et al. [133] devised a thiol-sensitive biocomposite microswimmer based on Chlamydomonas reinhardtii for antibiotic delivery. Initially, the microalgal surface was functionalized through dibenzocyclooctyne NHS-activated anchors. Subsequently, vancomycin conjugates were covalently linked to the microalgal surface via disulfide bonds. The microswimmer itself does not exhibit antibiotic activity, only becomes active under thiol-mediated conditions. This feature is expected to limit the development of antibiotic resistance. In addition to delivering conventional drugs, microalgae-derived biohybrid microrobots can also be considered as a novel type of therapeutic agent for disease treatment. Li et al. [134] encapsulated macrophage membranes onto poly(lactic-co-glycolic acid) nanoparticle cores using an ultrasonic method to form M@NPs. Subsequently, click chemistry was employed to conjugate M@NPs with Chlamydomonas reinhardtii, thereby constructing the microrobots. This system integrates the motility characteristics of Chlamydomonas reinhardtii with the cytokine-binding capability of macrophage membranes, offering a promising strategy for actively neutralizing pro-inflammatory cytokines in a biotherapeutic manner.
4.3. Medical imaging
Medical imaging is an indispensable component of modern medical diagnosis, extensively used for early disease detection, disease progression tracking, and therapeutic efficacy evaluation. Leveraging thier fluorescence characteristics, microalgae can achieve non-invasive in vivo tracking without the need for additional fluorescent labeling. As previously mentioned, the microalgae-based delivery system possesses multiple biological advantages, the most significant of which is its ability to achieve targeted delivery by modulating its surface properties. Consequently, this system is capable of enhancing the signal intensity at the lesion site and conducting imaging precisely at the lesion location. Moreover, by loading different types of imaging probes, microalgae can facilitate multimodal imaging to compensate for the information deficiencies among various imaging techniques [135].
Wang et al. [135] developed a multifunctional biohybrid, magnetism-driven microrobot based on microalgae, named “Volbot”. Volbot integrates live volvox with chitosan and polydopamine (PDA)-functionalized magnetic nanoparticles loaded with the photosensitizer Chlorin e6 (Ce6-Chitosan-PDA@Fe3O4). This system leverages the auto-fluorescence of microalgae and their responsiveness to external stimuli, which not only facilitates the in vivo localization of Volbot but also provides real-time feedback on its distribution and activity. Moreover, the photoacoustic imaging capability of the Volbot system enhances imaging contrast and resolution, enabling more accurate assessment of the size, shape, and depth of tumors. Thanks to the integration of microalgae and magnetic nanoparticles, Volbot also exhibits robust compatibility with magnetic resonance imaging (MRI). The Volbot system not only offers a non-invasive means of monitoring the therapeutic process but also provides a platform for the concurrent PDT and evaluation of its efficacy (Fig. 10A). Zhong et al. [42] investigated a novel approach of employing Spirulina platensis for targeted drug delivery and imaging-guided therapy against breast cancer lung metastasis. Through surface electrostatic adsorption, the microalgae can simply and efficiently load doxorubicin to form SP@DOX. The drug release of this system is significantly enhanced at pH 5.5, rendering SP@DOX a promising alternative strategy for targeted drug release within tumor tissues, given the acidic nature of the tumor microenvironment. Additionally, the inherent chlorophyll of SP@DOX and the intrinsic fluorescence of doxorubicin enable non-invasive in vivo tracking and real-time monitoring without the need for additional fluorescent labeling. This dual-modal fluorescence imaging characteristic allows for simultaneous visualization under both SP and DOX channels, providing a comprehensive display of drug distribution and therapeutic efficacy. The passive lung-targeting capability of the SP@DOX system, combined with its sustained drug release and monitorable properties, makes it a promising platform for the treatment of breast cancer lung metastasis, capable of enhancing therapeutic outcomes while minimizing systemic toxicity (Fig. 10B). Zhong et al. [136] also employed Spirulina platensis as a biological template to encapsulate superparamagnetic iron oxide nanoparticles on its surface, thereby fabricating photosynthetic biomimetic nanorobots (PBNs) that can be externally magnetically driven. Owing to their inherent chlorophyll, these nanorobots possess both fluorescence and photoacoustic imaging capabilities, which facilitate precise localization and monitoring of the targeting situation. Specifically, fluorescence imaging can track the distribution and accumulation of the nanorobots within the body, while photoacoustic imaging provides deeper tissue penetration and higher resolution, which is conducive to accurately assessing the oxygenation status and therapeutic efficacy of tumors (Fig. 10C). This dual-imaging modality holds promise as a powerful tool in the field of cancer theranostics, as it offers a more comprehensive and dynamic view of the disease treatment process.
Fig. 10.
Applications of medical imaging based on microalgae delivery system. (A) Volbot microalgae microrobot for multi-mode accurate imaging and treatment. (a) Schematic diagram of preparation of Volbot. (b) Fluorescent imaging picture of Volbot. (c) Microscopic pictures of volvox and its flagella, scale bar, 10 μm. (d) Schematic diagram of Volbot used in photodynamic/photothermal combined treatment of cancer and accurate imaging. (e) Scanning electron microscope picture of volvox, scale bar, 10 μm. (f) Image of Volbot flow, scale bar, 100 μm. (g) Images from magnetic resonance imaging (MRI), photothermal therapy (PT), photoacoustic therapy (PA), and whole-body fluorescence (FL) following intratumoral Volbot injection. Adapted from Ref. [135], Copyright © John Wiley & Sons. (B) Microalgae-derived systems for targeted drug delivery and dual-modal fluorescence imaging-guided therapy for lung metastasis of breast cancer. (a) Inherent properties of Spirulina platensis. (b) Schematic diagram showing how SP@DOX-mediated lung targeted drug administration and fluorescence imaging-guided treatment prevent breast cancer from spreading to the lung. (c) Time-dependent in vivo fluorescence pictures of nude mice receiving an intravenous injection of SP@DOX. Average metastatic nodules (d) and H&E staining (e) of lung tissue in nude mice model of breast cancer with lung metastasis at 15 days, scale bar, 200 μm. Adapted from Ref. [42], Copyright © John Wiley & Sons. (C) Photosynthetic microalgal hybrid nano-swimmer system for imaging-guided enhanced radiation-photodynamic therapy of tumors. (a) Schematic diagram of preparation of magnetic microrobot based on Spirulina platensis and tumor treatment mechanism. In vivo photoacoustic imaging (b) and magnetic resonance imaging (c) of 4T1 tumor-bearing mice before and after intravenous injection of engineered microalgae robots. Adapted from Ref. [136], Copyright © John Wiley & Sons.
The microalgae-based delivery system, in addition to leveraging its inherent fluorescence properties to assist in medical imaging, can also function as a biosensor to enhance the fluorescence imaging effect. For instance, by synergistically utilizing the two-dimensional periodic porous nanostructure and photonic crystal characteristics of diatoms, it is possible to achieve femtogram-level detection of mouse Immunoglobulin G, with a detection precision that is 10–100 times higher than that of conventional surface-enhanced Raman scattering immunoassays [137].
4.4. Registered clinical trials
The implementation of 13C labeling of microalgae ([13C]-microalage) facilitates researchers in tracking the distribution and metabolic processes of the labeled substances or specific materials containing the labels within organisms, thereby assessing and monitoring their in vivo behaviors. In many clinical trials retrieved (Table 3), Spirulina has been employed as a marker for gastric emptying tests to evaluate the gastric emptying rates. For instance, in NCT01248221, researchers utilized [13C]-Spirulina to compare the stomach emptying breath tests and scintigraphy in healthy subjects and those with dyspepsia. Additionally, the purpose of NCT05223881 is to ascertain whether an investigational device known as the [13C]-Spirulina gastric emptying breath test (GEBT) can accurately diagnose gastroparesis (delayed emptying of the stomach) in patients with cystic fibrosis. In order to assess the impact of the gastroparesis candidate drug PCS12852 on gastric emptying rate, the [13C]-Spirulina GEBT was similarly employed as an evaluation tool (NCT05270460). Furthermore, a trial (NCT00680277) was conducted wherein orally administered intrinsically labeled Spirulina was tracked to monitor the enrichment of its β-carotene and its cleavage product, vitamin A, thereby evaluating the bioavailability and bioconversion rate of β-carotene from Spirulina to vitamin A. Additionally, given that at present there is no standard method for the evaluation of in vivo cuff leak and aspiration, and considering the high cost of radioactive labeled markers, Berra et al. (NCT02301845) designed a suspension of diatom shells as a marker for cuff leak and microaspiration in mechanically ventilated, critically ill patients. This intervention allows for the assessment of leak and aspiration by detecting the presence of diatom shells in the tracheal secretions below the cuff.
Table 3.
Clinical trials related to microalgae-derived delivery system (Source: https://clinicaltrials.gov/, retrieval date: February 28, 2025).
| Clinical trial ID | Official title | Interventional model | Intervention/Treatment | Outcome measure related to the role of microalgae | Measure description | Trial purpose |
|---|---|---|---|---|---|---|
| NCT02301845 | Validation of a marker for aspiration and endotracheal tube cuff leak. | Single group assignment | Instillation of suspended diatom shells - 10 mg q12h. | Cuff leak and microaspiration. | The investigators will collect samples of tracheal secretions below the cuff every 4 h after the first pharyngeal administration of diatom shells and after 12 h from the other 5 scheduled administrations of diatom shells. Samples will be centrifuged and the sediment observed under microscopy for detection of shells in tracheal secretions below the endotracheal tube cuff. Their presence will be a sign of cuff leak and aspiration. The ratio between administered diatom shells and collected diatom shells will be used to give an estimate of leakage. | Aimed at validating diatom shells as a marker for cuff leak and microaspiration in mechanically ventilated, critically ill patients. |
| NCT00680277 | Vitamin A value of Spirulina carotenoids in humans | Single group assignment | Dietary Supplement: Spirulina β-carotene. | The enrichment of spirulina β-carotene and its cleavage product vitamin A were tracked after taken the oral dose of intrinsically labeled Spirulina. | Not mentioned. | To determine the vitamin A value (equivalence) of Spirulina. |
| NCT06016933 | Effect of locally delivered Spirulina gel on treatment of stage II, grade B periodontitis | Parallel assignment | Oral hygiene instructions and SRP of all teeth, followed by insertion of the spirulina gel. | Clinical attachment level. | Clinical attachment level was measured at baseline, 1 and 3 months after gel application. | To assess the clinical and immunological effect of spirulina in treatment of stage II periodontitis. |
| Probing pocket depth. | Probing pocket depth was measured at baseline, 1 and 3 months after gel application. | |||||
| Interleukin 6. | Enzyme linked immune-assay for the quantitative measurement of interleukin 6 concentration in gingival cervicular fluid at baseline, 1 and 3 months after gel application. | |||||
| NCT01248221 | [13C]-Spirulina Platensis GEBT - dual-label validation study | Parallel assignment | [13C]-Spirulina platensis and 99mTc sulfur colloid. | Gastric emptying half time measured by [13C]-Spirulina GEBT. | Gastric emptying half time is the time for half of the ingested solids to leave the stomach. This value was measured by the [13C]-Spirulina GEBT. | To compare stomach emptying using [13C]-Spirulina platensis breath test and scintigraphy in healthy subjects and subjects with dyspepsia. |
| NCT04635306 | Determination of the effect of [13C]-Spirulina nitrogen content on in vivo [13C]-Spirulina GEBT results | Single group assignment | Approved GEBT test meal. | Difference in vivo [13C]-Spirulina GEBT response. | Determining the difference between measure gastric emptying rate (kPCD) results produced from the low %N [13C]-Spirulina GEBT test meal and the FDA-approved test meal. | To determine whether there is a difference in the human in vivo response to [13C]-Spirulina meals manufactured using [13C]-Spirulina containing different levels of protein (as measured by %nitrogen). |
| NCT06699654 | Comparative study between Spirulina gel and Spirulina nanoparticles gel for treatment of stage II grade B periodontitis: randomized control clinical study with radiological and biochemical assessment | Parallel assignment | Spirulina gel. | Reduction in PPD. | The mean reduction in probing pocket depth in millimeters at periodontal sites treated with Spirulina gel and Spirulina nanoparticles gel. | To investigate the effectiveness of Spirulina gel and Spirulina nanoparticles gel in treating stage II grade B periodontitis. |
| NCT04772261 | [13C]-Spirulina breath test ([13C]-GEBT) lot-to-lot and biological variability study | Parallel assignment | Diagnostic test: [13C]-Spirulina GEBT. | Biological Variability. | Participant kPCD values at each measured timepoint from the same [13C]-Spirulina GEBT test meal will be plotted to compare against each other. | To collect information regarding lot-to-lot variability in [13C]-Spirulina test meal lets and within-subject biological variability. |
| NCT06004596 | Establishing a pediatric reference range for the [13C]-Spirulina GEBT | Single group assignment | Diagnostic Test: [13C]-Spirulina GEBT. | kPCD at 15/30/45/60/90/120/150/180/210/240 min. | 13CO2 excretion rate calculated at 15/30/45/60/90/120/150/180/210/240 min after meal completion. | To define the normal response to the [13C]-Spirulina GEBT in children, so that researchers can use this test to help diagnose children that are suspected of having a condition called gastroparesis, which means that food doesn't empty from their stomach normally. |
| NCT05223881 | [13C]-Spirulina Platensis GEBT for diagnosis of gastroparesis in patients with cystic fibrosis | Parallel assignment | Device: [13C]-Spirulina Platensis GEBT. | Rate of gastric emptying. | The kPCD value (13CO2 excretion rate) at any time t, is proportional to the rate of gastric emptying. Increasing kPCD values represent increasing rates of gastric emptying. | To determine if an investigational device called the [13C]-Spirulina GEBT, can accurately diagnose gastroparesis (delayed emptying of the stomach) in patients with cystic fibrosis. |
| NCT02755064 | Relationship between gastric emptying and glycemic variability in type 1 diabetes mellitus | Parallel assignment | Carbon-13 Spirulina breath test. | Relationship between gastric emptying and glycemia. | Gastric emptying was measured by [13C]-Spirulina GEBT. Glycemia was measured with continuous glucose monitoring. | To learn if the rate at which food empties from the stomach affects blood sugar values. |
| NCT05055336 | Longitudinal evaluation of gastric emptying and accommodation in children with dyspepsia: Toward individualized care in functional abdominal pain disorders | Parallel assignment | Gastric emptying breath test. | Gastric emptying rate. | As measured by the Spirulina breath test. | To further study the relationship between gastroparesis (a condition in which the stomach cannot empty itself of food in a normal fashion) and functional dyspepsia (frequent symptoms of indigestion that have no obvious cause). |
| NCT05270460 | A phase 2A, placebo-controlled, randomized, dose response study of the safety, pharmacokinetics and efficacy of PCS12852 on gastric emptying rate assessed by [13C]-Spirulina GEBT in patients with moderate to severe gastroparesis | Parallel assignment | Drug: PCS12852. | Change in gastric emptying rate from baseline as determined by the AUC of the gastric emptying rate. | Change from baseline in gastric emptying rate was determined by the AUC by GEBT at Day 28 after administration of PCS12852 or placebo. | To compare the effect of 2 different dosage regimens of PCS12852 on gastric emptying time to placebo in both idiopathic gastroparesis and diabetic gastroparesis patients. |
| NCT05426551 | Validation of the dual-isotope method for measuring ileal protein digestibility | Parallel assignment | [13C]-Spirulina as the reference protein. | Indirect amino acid digestibility. | Indirect ileal amino acid digestibility (%) = AAi ileal digestibility Spirulina or free AA mix (%) × Ratio plasma AAi/Ratio meal AAi | To assess the IAA digestibility of intrinsically 2H-hen's egg in healthy volunteers using both indirect and direct methods. |
Note: AUC, area under the curve; GEBT, gastric emptying breath test; IAA, indispensable amino acid; kPCD, kinetic percent of carbon dioxide; PPD, probing pocket depth; q12h, every 12 h; SPR, scaling and root planing.
In the aforementioned clinical trials, microalgae primarily functioned as diagnostic tools for the delivery of labeled substances, thereby providing valuable instruments for clinical diagnosis. Moreover, in the clinical trials NCT06016933 and NCT06699654, the therapeutic effects of locally delivered Spirulina gel on Stage II Grade B periodontitis were investigated. And the clinical and immunological efficacies of Spirulina gel and Spirulina nanoparticle gel were assessed by measuring clinical attachment levels, probing depths, and interleukin-6 concentrations in gingival crevicular fluid. All these clinical trials have proved the practical application value of the microalgae delivery system in modern medicine.
5. Current challenges
Despite the advantages of microalgae-derived delivery systems, there are still some challenges in their practical application scenarios (Fig. 11), such as follows.
-
(1)
Although microalgae possess favorable biocompatibility, the complex impact of their derived or engineered delivery systems on the immune regulation of the body remains an issue that cannot be overlooked. This is because there are certain differences between the microalgae-derived delivery systems and the original microalgal biomass in terms of structure, composition, and biological functions. For instance, when microalgae are used as drug delivery carriers, after certain processing and modification, their surface properties and the binding modes with the loaded drugs, potentially influencing their distribution, absorption, and interactions with immune cells in the body, thereby affecting the immune response. Moreover, orally administered microalgae-derived delivery systems may indirectly affect the host's immune response by modulating the composition and function of the gut microbiota.
-
(2)
The targeting mechanisms of microalgae-derived delivery systems largely rely on the specific binding of surface ligands to receptors. However, in complex physiological environments, the heterogeneity and dynamic changes in receptor expression, as well as the interference of non-specific binding, can affect the targeting precision. Although surface modification and other techniques can enhance targeting specificity, the in vivo stability and functional maintenance of modified microalgae remain incompletely understood.
-
(3)
The mechanisms by which microalgae-based delivery systems achieve rapid and synchronous drug release upon reaching the target site, as well as stable release under varying physiological conditions, remain a current research focus. Currently, the triggers for drug release largely depend on external stimuli or microenvironmental changes. However, the penetration and uniformity of these stimuli are difficult to ensure, which may lead to unstable efficiency and outcomes of drug release.
-
(4)
The growth of microalgae is influenced by multiple factors, including light, temperature, and nutrient availability. Variations in these factors can lead to differences in the physiological state and functional component content of microalgae across different batches. Furthermore, quality fluctuations during the preparation process of the delivery systems may impose limitations on the consistency of quality in scaled-up production.
-
(5)
Although microalgae-based delivery systems have demonstrated promising applications in laboratory research, several barriers exist in the transition from preclinical studies to clinical applications. These include the complexity of the human physiological environment, individual differences, long-term safety, and the therapeutic efficacy assessment. More clinical trial data are required to substantiate their safety and effectiveness. Furthermore, the lag in regulatory policies and standards results in a lack of clear guidance and specifications for algae-derived products in areas such as product registration, approval processes, and quality control. This, to some extent, restricts their rapid entry into the market and clinical use.
Fig. 11.
Current challenges and future trends in the application of microalgae-derived delivery system.
6. Future trends
With the continuous development of microalgae and their derived delivery systems in the biomedical field, future research will focus on overcoming current challenges and promoting their translation into clinical applications. First, to address the complex impact on the immune system, researchers should place greater emphasis on the precise regulation of microalgae-derived delivery systems. By further optimizing their surface properties and modification strategies, researchers can explore more efficient immune evasion or immune tolerance mechanisms to minimize potential immune effects. Additionally, in terms of targeted delivery, as understanding of receptor expression dynamics and targeting mechanisms deepens, future research should fully integrate nanotechnology and biomarkers screening to enhance the precision and stability of delivery systems, reduce non-specific binding interference, and thereby improve therapeutic outcomes.
Regarding drug release, with in-depth research on microenvironmental stimulus-responsive mechanisms, future delivery systems will place greater emphasis on achieving controlled, timed, and synchronized drug release, and on developing new stimulus-responsive materials to meet the challenges posed by different physiological conditions. As for the safety issue, it is expected to be comprehensively evaluated by in situ validation combined with predictive approaches (such as fluorescent probe technology and optical tweezers technology combined with computer simulation). Finally, despite obstacles in the transition from preclinical research to clinical applications, with the continuous accumulation of clinical trials and the improvement of regulatory policies, the clinical application prospects of microalgae-derived delivery systems will become increasingly clear, and related products will enter the market under strict quality control and safety assessment frameworks. Therefore, microalgae-based delivery systems will demonstrate broader application potential in the future, especially in the context of the medical development trend characterized by the integration of biochemistry, pharmacology, and materials science.
7. Conclusion
Microalgae are not only rich in bioactive substances but also possess inherent properties naturally suitable for delivery systems. This positions microalgae-based platforms as promising vehicles for developing naturally functionalized delivery systems. This review provides a detailed exposition of microalgae-based delivery strategies, including the characteristics, advantages, and types of both microalgae and their derived delivery systems. The unique combination of biocompatibility, low toxicity, targeting capabilities, large specific surface area, and high oxygen-generating efficiency enables microalgae's adaptable application across diverse delivery platforms, including but not limited to microcapsules, nanocarriers, composite hydrogels, and microrobots. Such versatility in delivery formats expands the application scenarios of microalgae-based systems. Beyond efficient drug delivery for therapeutic interventions, these systems demonstrate multifaceted biomedical potential through mechanisms that accelerate wound healing and synergistically enhance PDT and SDT. Furthermore, by loading various imaging probes, microalgae enable multimodal imaging capabilities that bridge the informational gaps between different medical imaging modalities. Despite these promising prospects, challenges remain in translating microalgae-based delivery systems from preclinical research to clinical applications. As successful advancements continue to accumulate in precision delivery, targeting mechanisms, and clinical implementation, microalgae and their derivative systems are poised to unlock unprecedented possibilities in biomedical engineering. Future developments should focus on overcoming current limitations while harnessing their multifunctional potential for next-generation therapeutic strategies.
CRediT authorship contribution statement
Peiyi Wang: Writing – review & editing, Writing – original draft, Visualization, Investigation, Data curation, Conceptualization. Changhong Liu: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. Lei Zheng: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (U23A2081, U23A20265), the Key R&D Program of Anhui Province (2023n06020052, 2023n06020010), and the Fundamental Research Funds for the Central Universities Special Funding Project (JZ2024HGTG0286).
Contributor Information
Changhong Liu, Email: changhong22@hfut.edu.cn.
Lei Zheng, Email: lzheng@hfut.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Harimoto T., Jung W.-H., Mooney D.J. Delivering living medicines with biomaterials. Nat. Rev. Mater. 2025 doi: 10.1038/s41578-024-00766-y. [DOI] [Google Scholar]
- 2.Shahiwala A. Advancing drug delivery research: sustainable strategies for innovation and translation. Drug Delivery and Translational Research. 2025 doi: 10.1007/s13346-024-01767-8. [DOI] [PubMed] [Google Scholar]
- 3.Oyejide A.J., Adekunle A.A., Abodunrin O.D., Atoyebi E.O. Artificial intelligence, computational tools and robotics for drug discovery, development, and delivery. Intelligent Pharmacy. 2025 doi: 10.1016/j.ipha.2025.01.001. [DOI] [Google Scholar]
- 4.Kapnick S.M., Martin C.A., Jewell C.M. Engineering metabolism to modulate immunity. Adv. Drug Deliv. Rev. 2024;204 doi: 10.1016/j.addr.2023.115122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam J., Kim A., Kim K., Moon J.H., Baig J., Phoo M., Moon J.J., Son S. Engineered polysaccharides for controlling innate and adaptive immune responses. Nature Reviews Bioengineering. 2024;2(9):733–751. doi: 10.1038/s44222-024-00193-2. [DOI] [Google Scholar]
- 6.Griffin K.V., Saunders M.N., Lyssiotis C.A., Shea L.D. Engineering immunity using metabolically active polymeric nanoparticles. Trends Biotechnol. 2024 doi: 10.1016/j.tibtech.2024.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N., Liu J., Zhao S., Li Y., Wei Q. Skin-inspired bimodal pressure sensor for static and dynamic intelligent interactive perception system. Chem. Eng. J. 2024;502 doi: 10.1016/j.cej.2024.158012. [DOI] [Google Scholar]
- 8.Cetin F.N., Mignon A., Van Vlierberghe S., Kolouchova K. Polymer- and lipid-based nanostructures serving wound healing applications: a review. Adv. Healthcare Mater. 2025;14(1) doi: 10.1002/adhm.202402699. [DOI] [PubMed] [Google Scholar]
- 9.May M. Why drug delivery is the key to new medicines. Nat. Med. 2022;28(6):1100–1102. doi: 10.1038/s41591-022-01826-y. [DOI] [PubMed] [Google Scholar]
- 10.Vargason A.M., Anselmo A.C., Mitragotri S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021;5(9):951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Wang Y.-N., Luo Y.-G., Yang H., Ren J., Li X. Advances in synthetic biology-based drug delivery systems for disease treatment. Chin. Chem. Lett. 2024;35(11) doi: 10.1016/j.cclet.2024.109576. [DOI] [Google Scholar]
- 12.Huang L., Huang X.-H., Yang X., Hu J.-Q., Zhu Y.-Z., Yan P.-Y., Xie Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024;201 doi: 10.1016/j.phrs.2024.107100. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q., Liu Q., Wang Y., Chen J., Schmid O., Rehberg M., Yang L. Bridging smart nanosystems with clinically relevant models and advanced imaging for precision drug delivery. Adv. Sci. 2024;11(14) doi: 10.1002/advs.202308659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Tang T., Shi Q., Zhou Z., Fan J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022;126:99–112. doi: 10.1016/j.tifs.2022.06.016. [DOI] [Google Scholar]
- 15.Gohara-Beirigo A.K., Matsudo M.C., Cezare-Gomes E.A., Carvalho J.C.M.d., Danesi E.D.G. Microalgae trends toward functional staple food incorporation: sustainable alternative for human health improvement. Trends Food Sci. Technol. 2022;125:185–199. doi: 10.1016/j.tifs.2022.04.030. [DOI] [Google Scholar]
- 16.Yang S., Wang Y., Wang J., Cheng K., Liu J., He Y., Zhang Y., Mou H., Sun H. Microalgal protein for sustainable and nutritious foods: a joint analysis of environmental impacts, health benefits and consumer's acceptance. Trends Food Sci. Technol. 2024;143 doi: 10.1016/j.tifs.2023.104278. [DOI] [Google Scholar]
- 17.De Bhowmick G., Guieysse B., Everett D.W., Reis M.G., Thum C. Novel source of microalgal lipids for infant formula. Trends Food Sci. Technol. 2023;135:1–13. doi: 10.1016/j.tifs.2023.03.012. [DOI] [Google Scholar]
- 18.Min K.H., Kim D.H., Youn S., Pack S.P. Biomimetic diatom biosilica and its potential for biomedical applications and prospects: a review. Int. J. Mol. Sci. 2024;25(4):2023. doi: 10.3390/ijms25042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Managò S., Tramontano C., Delle Cave D., Chianese G., Zito G., De Stefano L., Terracciano M., Lonardo E., De Luca A.C., Rea I. SERS quantification of galunisertib delivery in colorectal cancer cells by plasmonic-assisted diatomite nanoparticles. Small. 2021;17(34) doi: 10.1002/smll.202101711. [DOI] [PubMed] [Google Scholar]
- 20.Akolpoglu M.B., Dogan N.O., Bozuyuk U., Ceylan H., Kizilel S., Sitti M. High-Yield production of biohybrid microalgae for On-Demand cargo delivery. Adv. Sci. 2020;7(16) doi: 10.1002/advs.202001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H., Kim B., Jeong S.H., Kim T.Y., Kim D.-P., Oh Y.-K., Hahn S.K. Microalgae-based biohybrid microrobot for accelerated diabetic wound healing. Small. 2023;19(1) doi: 10.1002/smll.202204617. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D., Zhong D., Ouyang J., He J., Qi Y., Chen W., Zhang X., Tao W., Zhou M. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat. Commun. 2022;13(1):1413. doi: 10.1038/s41467-022-28744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang F., Zhao C., Zheng Y., Zhong D., Zhou M. Microalgae-based dual drug delivery system with enhanced articular cavity retention for osteoarthritis treatment. Adv. Funct. Mater. 2024;34(30) doi: 10.1002/adfm.202401055. [DOI] [Google Scholar]
- 24.da Silva A.F., Moreira A.F., Miguel S.P., Coutinho P. Recent advances in microalgae encapsulation techniques for biomedical applications. Adv. Colloid Interface Sci. 2024;333 doi: 10.1016/j.cis.2024.103297. [DOI] [PubMed] [Google Scholar]
- 25.Liu S., Shi L., Luo H., Chen K., Song M., Wu Y., Liu F., Li M., Gao J., Wu Y. Processed microalgae: green gold for tissue regeneration and repair. Theranostics. 2024;14(13):5235–5261. doi: 10.7150/thno.99181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H., Lang Y., Wang S., Zhou M. Microalgae-based drug delivery systems in biomedical applications. Eng. Regen. 2024;5(3):361–374. doi: 10.1016/j.engreg.2024.01.002. [DOI] [Google Scholar]
- 27.Wang R., Wang Z., Zhang M., Zhong D., Zhou M. Application of photosensitive microalgae in targeted tumor therapy. Adv. Drug Deliv. Rev. 2025;219 doi: 10.1016/j.addr.2025.115519. [DOI] [PubMed] [Google Scholar]
- 28.Jia J., Liu J., Shi W., Yao F., Wu C., Liu X., Na J., Jin Z., Xu C., Zhang Q., Zhao Y., Liao Y. Microalgae-loaded biocompatible alginate microspheres for tissue repair. Int. J. Biol. Macromol. 2024;271 doi: 10.1016/j.ijbiomac.2024.132534. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Yang Z., Li S., Kwong C.H.T., Zhang D., Wei J., Gao C., Zhang Q.-w., Wang R. Light-directed microalgae micromotor with supramolecular backpacks for photodynamic therapy. Adv. Funct. Mater. 2024;(n/a) doi: 10.1002/adfm.202411070. n/a. [DOI] [Google Scholar]
- 30.Zhong D., Zhang D., Chen W., He J., Ren C., Zhang X., Kong N., Tao W., Zhou M. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci. Adv. 2021;7(48) doi: 10.1126/sciadv.abi9265. eabi9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng S., Xie X., Liu J., Li A., Wang Q., Guo D., Li S., Li Y., Wang Z., Guo T., Zhou J., Tang D.Y.Y., Show P.L. A potential paradigm in CRISPR/cas systems delivery: at the crossroad of microalgal gene editing and algal-mediated nanoparticles. J. Nanobiotechnol. 2023;21(1):370. doi: 10.1186/s12951-023-02139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrenfeld C., Veloso-Giménez V., Corrales-Orovio R., Rebolledo R., Boric M.P., Egaña J.T. Microalgae share key features with human erythrocytes and can safely circulate through the vascular system in mice. Appl. Microbiol. Biotechnol. 2023;107(14):4621–4633. doi: 10.1007/s00253-023-12588-z. [DOI] [PubMed] [Google Scholar]
- 33.Raj T., Morya R., Chandrasekhar K., Kumar D., Soam S., Kumar R., Patel A.K., Kim S.-H. Microalgae biomass deconstruction using green solvents: challenges and future opportunities. Bioresour. Technol. 2023;369 doi: 10.1016/j.biortech.2022.128429. [DOI] [PubMed] [Google Scholar]
- 34.Tokgöz M., Yarkent Ç., Köse A., Oncel S.S. The potential of microalgal sources as coating materials: a case study for the development of biocompatible surgical sutures. Lett. Appl. Microbiol. 2023;76(8) doi: 10.1093/lambio/ovad086. [DOI] [PubMed] [Google Scholar]
- 35.Taroncher M., Rodríguez-Carrasco Y., Barba F.J., Ruiz M.J. Evaluation of cytotoxicity, analysis of metals and cumulative risk assessment in microalgae. Toxicol. Mech. Methods. 2023;33(5):388–400. doi: 10.1080/15376516.2022.2152514. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Shahbazi M.-A., Mäkilä E.M., da Silva T.H., Reis R.L., Salonen J.J., Hirvonen J.T., Santos H.A. Diatom silica microparticles for sustained release and permeation enhancement following oral delivery of prednisone and mesalamine. Biomaterials. 2013;34(36):9210–9219. doi: 10.1016/j.biomaterials.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Lau Z.L., Low S.S., Ezeigwe E.R., Chew K.W., Chai W.S., Bhatnagar A., Yap Y.J., Show P.L. A review on the diverse interactions between microalgae and nanomaterials: growth variation, photosynthetic performance and toxicity. Bioresour. Technol. 2022;351 doi: 10.1016/j.biortech.2022.127048. [DOI] [PubMed] [Google Scholar]
- 38.Pedrioli G., Paganetti P. Hijacking endocytosis and autophagy in extracellular vesicle communication: where the inside meets the outside. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.595515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F., Guo Z., Li Z., Luan H., Yu Y., Zhu A.T., Ding S., Gao W., Fang R.H., Zhang L., Wang J. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci. Adv. 2024;10(24):eadn6157. doi: 10.1126/sciadv.adn6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamo G., Fierli D., Romancino D.P., Picciotto S., Barone M.E., Aranyos A., Božič D., Morsbach S., Raccosta S., Stanly C., Paganini C., Gai M., Cusimano A., Martorana V., Noto R., Carrotta R., Librizzi F., Randazzo L., Parkes R., Capasso Palmiero U., Rao E., Paterna A., Santonicola P., Iglič A., Corcuera L., Kisslinger A., Schiavi E.D., Liguori G.L., Landfester K., Kralj-Iglič V., Arosio P., Pocsfalvi G., Touzet N., Manno M., Bongiovanni A. Nanoalgosomes: introducing extracellular vesicles produced by microalgae. J. Extracell. Vesicles. 2021;10(6) doi: 10.1002/jev2.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J., Ye J., Li R., Li R., Liu X., Han J., Yang Y., Ran N., Yuan M., Zhang Z., Chong W., Ji X. Nanozyme-functionalized microalgal biohybrid microrobots in inflammatory bowel disease treatment. Biomaterials. 2025;319 doi: 10.1016/j.biomaterials.2025.123231. [DOI] [PubMed] [Google Scholar]
- 42.Zhong D., Zhang D., Xie T., Zhou M. Biodegradable microalgae-based carriers for targeted delivery and imaging-guided therapy toward lung metastasis of breast cancer. Small. 2020;16(20) doi: 10.1002/smll.202000819. [DOI] [PubMed] [Google Scholar]
- 43.Roy Chong J.W., Tan X., Khoo K.S., Ng H.S., Jonglertjunya W., Yew G.Y., Show P.L. Microalgae-based bioplastics: future solution towards mitigation of plastic wastes. Environ. Res. 2022;206 doi: 10.1016/j.envres.2021.112620. [DOI] [PubMed] [Google Scholar]
- 44.Borics G., Lerf V., T-Krasznai E., Stanković I., Pickó L., Béres V., Várbíró G. Biovolume and surface area calculations for microalgae, using realistic 3D models. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2021.145538. [DOI] [PubMed] [Google Scholar]
- 45.Benedetti M., Vecchi V., Barera S., Dall'Osto L. Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb. Cell Fact. 2018;17(1):173. doi: 10.1186/s12934-018-1019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiji M.G., Ninan M.A., Thomas V.P., Thomas B.T. Edible microalgae: potential candidate for developing edible vaccines. Vegetos. 2024;37(3):788–793. doi: 10.1007/s42535-023-00636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L., Weathers P.J., Xiong X.-R., Liu C.-Z. Microalgal bioreactors: challenges and opportunities. Eng. Life Sci. 2009;9(3):178–189. doi: 10.1002/elsc.200800111. [DOI] [Google Scholar]
- 48.Usher P.K., Ross A.B., Camargo-Valero M.A., Tomlin A.S., Gale W.F. An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels. 2014;5(3):331–349. doi: 10.1080/17597269.2014.913925. [DOI] [Google Scholar]
- 49.Dolganyuk V., Belova D., Babich O., Prosekov A., Ivanova S., Katserov D., Patyukov N., Sukhikh S. Microalgae: a promising source of valuable bioproducts. Biomolecules. 2020;10(8):1153. doi: 10.3390/biom10081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shchelik I.S., Sieber S., Gademann K. Green Algae as a drug delivery system for the controlled release of antibiotics. Chem. Eur J. 2020;26(70):16644–16648. doi: 10.1002/chem.202003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Najm Y., Jeong S., Leiknes T. Nutrient utilization and oxygen production by Chlorella vulgaris in a hybrid membrane bioreactor and algal membrane photobioreactor system. Bioresour. Technol. 2017;237:64–71. doi: 10.1016/j.biortech.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 52.Hu J., Meng W., Su Y., Qian C., Fu W. Emerging technologies for advancing microalgal photosynthesis and metabolism toward sustainable production. Front. Mar. Sci. 2023;10 doi: 10.3389/fmars.2023.1260709. [DOI] [Google Scholar]
- 53.Rozenberg J.M., Sorokin B.A., Mukhambetova A.N., Emelianova A.A., Kuzmin V.V., Belogurova-Ovchinnikova O.Y., Kuzmin D.V. Recent advances and fundamentals of microalgae cultivation technology. Biotechnol. J. 2024;19(3) doi: 10.1002/biot.202300725. [DOI] [PubMed] [Google Scholar]
- 54.Cazzaniga S., Perozeni F., Baier T., Ballottari M. Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnology for Biofuels and Bioproducts. 2022;15(1):77. doi: 10.1186/s13068-022-02173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong X., Wu W., Pan P., Zhang X.-Z. Engineered living materials for advanced diseases therapy. Adv. Mater. 2023;(n/a) doi: 10.1002/adma.202304963. [DOI] [PubMed] [Google Scholar]
- 56.Liu S., Yang H., Ho M.Y., Xing B. Recent advances of material-decorated photosynthetic microorganisms and their aspects in biomedical applications. Adv. Opt. Mater. 2023;11(11) doi: 10.1002/adom.202203038. [DOI] [Google Scholar]
- 57.Dehghani J., Movafeghi A., Mathieu-Rivet E., Mati-Baouche N., Calbo S., Lerouge P., Bardor M. Microalgae as an efficient vehicle for the production and targeted delivery of therapeutic glycoproteins against SARS-CoV-2 variants. Mar. Drugs. 2022;20(11):657. doi: 10.3390/md20110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y., Luo J., Chen X., Liu W., Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 2019;11(1):100. doi: 10.1007/s40820-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao C., Kwong C.H.T., Wang Q., Kam H., Xie B., Lee S.M.-Y., Chen G., Wang R. Conjugation of macrophage-mimetic microalgae and liposome for antitumor sonodynamic immunotherapy via hypoxia alleviation and autophagy inhibition. ACS Nano. 2023;17(4):4034–4049. doi: 10.1021/acsnano.3c00041. [DOI] [PubMed] [Google Scholar]
- 60.Kerschgens I.P., Gademann K. Antibiotic algae by chemical surface engineering. Chembiochem : a European journal of chemical biology. 2018;19(5):439–443. doi: 10.1002/cbic.201700553. [DOI] [PubMed] [Google Scholar]
- 61.Krause G.H., Weis E. vol. 42. 1991. pp. 313–349. (Chlorophyll Fluorescence and Photosynthesis: the Basics). 1991. [DOI] [Google Scholar]
- 62.Lichtman J.W., Conchello J.-A. Fluorescence microscopy. Nat. Methods. 2005;2(12):910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 63.Campbell D., Hurry V., Clarke Adrian K., Gustafsson P., Öquist G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998;62(3):667–683. doi: 10.1128/mmbr.62.3.667-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang F., Li Z., Chen C., Luan H., Fang R.H., Zhang L., Wang J. Biohybrid microalgae robots: design, fabrication, materials, and applications. Adv. Mater. 2024;36(3) doi: 10.1002/adma.202303714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou M., Yin Y., Zhao J., Zhou M., Bai Y., Zhang P. Applications of microalga-powered microrobots in targeted drug delivery. Biomater. Sci. 2023;11(23):7512–7530. doi: 10.1039/D3BM01095C. [DOI] [PubMed] [Google Scholar]
- 66.Jafari Y., Sabahi H., Rahaie M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016;211:700–706. doi: 10.1016/j.foodchem.2016.05.115. [DOI] [PubMed] [Google Scholar]
- 67.Estevinho B.N., Mota R., Leite J.P., Tamagnini P., Gales L., Rocha F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019;343:644–651. doi: 10.1016/j.powtec.2018.11.079. [DOI] [Google Scholar]
- 68.Gnanamoorthy P., Anandhan S., Prabu V.A. Natural nanoporous silica frustules from marine diatom as a biocarrier for drug delivery. J. Porous Mater. 2014;21(5):789–796. doi: 10.1007/s10934-014-9827-2. [DOI] [Google Scholar]
- 69.Yağmur N., Şahin S. Encapsulation of ellagic acid from pomegranate peels in microalgae optimized by response surface methodology and an investigation of its controlled released under simulated gastrointestinal studies. J. Food Sci. 2020;85(4):998–1006. doi: 10.1111/1750-3841.15085. [DOI] [PubMed] [Google Scholar]
- 70.Chamundeeswari M., Jeslin J., Verma M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019;17(2):849–865. doi: 10.1007/s10311-018-00841-1. [DOI] [Google Scholar]
- 71.Huang L., Luo S., Tong S., Lv Z., Wu J. The development of nanocarriers for natural products. WIREs Nanomedicine and Nanobiotechnology. 2024;16(3) doi: 10.1002/wnan.1967. [DOI] [PubMed] [Google Scholar]
- 72.Zhou J., Zhou L., Chen Z.-y., Sun J., Guo X.-w., Wang H.-r., Zhang X.-y., Liu Z.-r., Liu J., Zhang K., Zhang X. Remineralization and bacterial inhibition of early enamel caries surfaces by carboxymethyl chitosan lysozyme nanogels loaded with antibacterial drugs. J. Dent. 2025;152 doi: 10.1016/j.jdent.2024.105489. [DOI] [PubMed] [Google Scholar]
- 73.Delasoie J., Zobi F. Natural diatom biosilica as microshuttles in drug delivery systems. Pharmaceutics. 2019;11(10):537. doi: 10.3390/pharmaceutics11100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tiwari A., Melchor-Martínez E.M., Saxena A., Kapoor N., Singh K.J., Saldarriaga-Hernández S., Parra-Saldívar R., Iqbal H.M.N. Therapeutic attributes and applied aspects of biological macromolecules (polypeptides, fucoxanthin, sterols, fatty acids, polysaccharides, and polyphenols) from diatoms — a review. Int. J. Biol. Macromol. 2021;171:398–413. doi: 10.1016/j.ijbiomac.2020.12.219. [DOI] [PubMed] [Google Scholar]
- 75.Xu T., Yan L., Liu C., Zheng L. Novel functional carriers based on Spirulina protein for the delivery of curcumin with improved stability and antioxidant efficiency. Food Chem. 2025;464 doi: 10.1016/j.foodchem.2024.141729. [DOI] [PubMed] [Google Scholar]
- 76.Liu R., Li Y., Zhou C., Tan M. Pickering emulsions stabilized with a spirulina protein–chitosan complex for astaxanthin delivery. Food Funct. 2023;14(9):4254–4266. doi: 10.1039/D3FO00092C. [DOI] [PubMed] [Google Scholar]
- 77.Arjeh E., Rostami H., Pirsa S., Fathi M. Synthesis and characterization of novel Spirulina protein isolate (SPI)-based hydrogels through dual-crosslinking with genipin/Zn2+ Food Res. Int. 2022;162 doi: 10.1016/j.foodres.2022.112107. [DOI] [PubMed] [Google Scholar]
- 78.Tenchov R., Bird R., Curtze A.E., Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 79.Menicucci F., Michelozzi M., Raio A., Tredici M., Cencetti G., Clemente I., Ristori S. Thymol-loaded lipid nanovectors from the marine microalga Nannochloropsis sp. as potential antibacterial agents. Biocatal. Agric. Biotechnol. 2021;32 doi: 10.1016/j.bcab.2021.101962. [DOI] [Google Scholar]
- 80.Clemente I., Bonechi C., Rodolfi L., Bacia-Verloop M., Rossi C., Ristori S. Lipids from algal biomass provide new (nonlamellar) nanovectors with high carrier potentiality for natural antioxidants. Eur. J. Pharm. Biopharm. 2021;158:410–416. doi: 10.1016/j.ejpb.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Adamo G., Santonicola P., Picciotto S., Gargano P., Nicosia A., Longo V., Aloi N., Romancino D.P., Paterna A., Rao E., Raccosta S., Noto R., Salamone M., Deidda I., Costa S., Di Sano C., Zampi G., Morsbach S., Landfester K., Colombo P., Wei M., Bergese P., Touzet N., Manno M., Di Schiavi E., Bongiovanni A. Extracellular vesicles from the microalga Tetraselmis chuii are biocompatible and exhibit unique bone tropism along with antioxidant and anti-inflammatory properties. Commun. Biol. 2024;7(1):941. doi: 10.1038/s42003-024-06612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Y.-C., Arief T., Mondal S., Pen Y., Chang C.-H. Fabrication of microalgae oil vesicles for drug delivery applications. J. Oleo Sci. 2024;73(4):583–591. doi: 10.5650/jos.ess23213. [DOI] [PubMed] [Google Scholar]
- 83.Bin-Meferij M.M., Hamida R.S. Biofabrication and antitumor activity of silver nanoparticles utilizing novel nostoc sp. Bahar M. International journal of nanomedicine. 2019;14:9019–9029. doi: 10.2147/ijn.S230457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arévalo-Gallegos A., Garcia-Perez J.S., Carrillo-Nieves D., Ramirez-Mendoza R.A., Iqbal H.M., Parra-Saldívar R. Botryococcus braunii as a bioreactor for the production of nanoparticles with antimicrobial potentialities. International journal of nanomedicine. 2018;13:5591–5604. doi: 10.2147/ijn.S174205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arteaga-Castrejón A.A., Agarwal V., Khandual S. Microalgae as a potential natural source for the green synthesis of nanoparticles. Chem. Commun. 2024;60(29):3874–3890. doi: 10.1039/D3CC05767D. [DOI] [PubMed] [Google Scholar]
- 86.Hamida R.S., Ali M.A., Almohawes Z.N., Alahdal H., Momenah M.A., Bin-Meferij M.M. Green synthesis of hexagonal silver nanoparticles using a novel microalgae Coelastrella aeroterrestrica strain BA_Chlo4 and resulting anticancer, antibacterial, and antioxidant activities. Pharmaceutics. 2022;14(10):2002. doi: 10.3390/pharmaceutics14102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salehzadeh A., Naeemi A.S., Khaknezhad L., Moradi-Shoeili Z., Shandiz S.A.S. Fe3 O4/Ag nanocomposite biosynthesised using Spirulina platensis extract and its enhanced anticancer efficiency. IET Nanobiotechnol. 2019;13(7):766–770. doi: 10.1049/iet-nbt.2018.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navarro Gallón S.M., Alpaslan E., Wang M., Larese-Casanova P., Londoño M.E., Atehortúa L., Pavón J.J., Webster T.J. Characterization and study of the antibacterial mechanisms of silver nanoparticles prepared with microalgal exopolysaccharides. Mater. Sci. Eng. C. 2019;99:685–695. doi: 10.1016/j.msec.2019.01.134. [DOI] [PubMed] [Google Scholar]
- 89.Sidorowicz A., Fais G., Casula M., Borselli M., Giannaccare G., Locci A.M., Lai N., Orrù R., Cao G., Concas A. Nanoparticles from microalgae and their biomedical applications. Mar. Drugs. 2023;21(6):352. doi: 10.3390/md21060352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong D., Jin K., Wang R., Chen B., Zhang J., Ren C., Chen X., Lu J., Zhou M. Microalgae-Based hydrogel for inflammatory bowel disease and its associated anxiety and depression. Adv. Mater. 2024;36(24) doi: 10.1002/adma.202312275. [DOI] [PubMed] [Google Scholar]
- 91.Shi T., Lu H., Zhu J., Zhou X., He C., Li F., Yang G. Naturally derived dual dynamic crosslinked multifunctional hydrogel for diabetic wound healing. Compos. B Eng. 2023;257 doi: 10.1016/j.compositesb.2023.110687. [DOI] [Google Scholar]
- 92.Liu Y., Wang Y., Fu Y., Wang N., Liu Q., Zhao S., Yang H.Y., Liu C. Fabrication of temperature and pH dual-sensitive semi-interpenetrating network hydrogel with enhanced adhesion and antibacterial properties. Polymer. 2025;326 doi: 10.1016/j.polymer.2025.128343. [DOI] [Google Scholar]
- 93.He W., Wang Y., Li X., Ji Y., Yuan J., Yang W., Yan S., Yan J. Sealing the Pandora's vase of pancreatic fistula through entrapping the digestive enzymes within a dextrorotary (D)-peptide hydrogel. Nat. Commun. 2024;15(1):7235. doi: 10.1038/s41467-024-51734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y., Wang Y., Fu Y., Wang N.N., Zhan S.Q., Sheng L., Yang H.Y., Liu C. Healable and transparent ionic conductive hydrogels based on PNATF as multiple-signal sensors. ACS Appl. Polym. Mater. 2025;7(4):2529–2540. doi: 10.1021/acsapm.4c03794. [DOI] [Google Scholar]
- 95.Ren C., Zhong D., Qi Y., Liu C., Liu X., Chen S., Yan S., Zhou M. Bioinspired pH-Responsive microalgal hydrogels for oral insulin delivery with both hypoglycemic and insulin sensitizing effects. ACS Nano. 2023;17(14):14161–14175. doi: 10.1021/acsnano.3c04897. [DOI] [PubMed] [Google Scholar]
- 96.Cheng S., Yang J., Song J., Cao X., Zhou B., Yang L., Li C., Wang Y. A motion-responsive injectable lubricative hydrogel for efficient Achilles tendon adhesion prevention. Mater. Today Bio. 2025;30 doi: 10.1016/j.mtbio.2025.101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu H., Zhong D., Li W., Lin X., He J., Sun Y., Wu Y., Shi M., Chen X., Xu F., Zhou M. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022;42 doi: 10.1016/j.nantod.2021.101368. [DOI] [Google Scholar]
- 98.Meng X., Liu Z., Yang Y., Li J., Ran Z., Zhu Y., Fu J., He Y., Hao Y. Engineered Microcystis aerugiosa hydrogel as an anti-tumor therapeutic by augmenting Tumor immunogenicity and immune responses. Adv. Funct. Mater. 2024;34(7) doi: 10.1002/adfm.202305915. [DOI] [Google Scholar]
- 99.Xing X., Liu C., Zheng L. Preparation of photo-crosslinked microalgae-carboxymethyl chitosan composite hydrogels for enhanced wound healing. Carbohydr. Polym. 2025;348 doi: 10.1016/j.carbpol.2024.122803. [DOI] [PubMed] [Google Scholar]
- 100.Mao Y., Sun Y., Yang C. Compound microalgae-type biofunctional hydrogel for wound repair during full-thickness skin injuries. Polymers. 2024;16(5):692. doi: 10.3390/polym16050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C., Ye Q., Hua S., Huang H., Zhong D., Liang F., Zhou M. Microalgae-based natural oral hydrogel system for synergistic treatment of lead poisoning-related diseases. Nano Today. 2023;53 doi: 10.1016/j.nantod.2023.102034. [DOI] [Google Scholar]
- 102.García-Silva I., Olvera-Sosa M., Ortega-Berlanga B., Ruíz-Rodríguez V., Palestino G., Rosales-Mendoza S. Synthesis and characterization of innovative microgels based on Polyacrylic acid and microalgae cell wall and their potential as antigen delivery vehicles. Pharmaceutics. 2023;15(1):133. doi: 10.3390/pharmaceutics15010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sarhan N., Arafa E.G., Elgiddawy N., Elsayed K.N.M., Mohamed F. Urea intercalated encapsulated microalgae composite hydrogels for slow-release fertilizers. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-58875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Netanel Liberman G., Ochbaum G., Bitton R., Arad S. Antimicrobial hydrogels composed of chitosan and sulfated polysaccharides of red microalgae. Polymer. 2021;215 doi: 10.1016/j.polymer.2020.123353. [DOI] [Google Scholar]
- 105.Aydınoğlu D., Ünal M. Evaluation of the influence of spirulina microalgae on the drug delivery characteristics of genipin cross-linked chitosan hydrogels. International Journal of Polymeric Materials and Polymeric Biomaterials. 2019;68(17):1020–1033. doi: 10.1080/00914037.2018.1525545. [DOI] [Google Scholar]
- 106.Chen W., Zhou H., Zhang B., Cao Q., Wang B., Ma X. Recent progress of Micro/Nanorobots for cell delivery and manipulation. Adv. Funct. Mater. 2022;32(18) doi: 10.1002/adfm.202110625. [DOI] [Google Scholar]
- 107.Li Z., Liu T., Sun X., Zhou Q., Yan X. Natural algae-inspired microrobots for emerging biomedical applications and beyond. Cell Rep. Phys. Sci. 2024;5(6) doi: 10.1016/j.xcrp.2024.101979. [DOI] [Google Scholar]
- 108.Pan T., Shi Y., Zhao N., Xiong J., Xiao Y., Xin H., Li B. Bio-Micromotor tweezers for noninvasive bio-cargo delivery and precise therapy. Adv. Funct. Mater. 2022;32(16) doi: 10.1002/adfm.202111038. [DOI] [Google Scholar]
- 109.X. Liang, Z. Chen, Y. Deng, D. Liu, X. Liu, Q. Huang, T. Arai, Field-controlled microrobots fabricated by photopolymerization, Cyborg and Bionic Systems. 4 9, 10.34133/cbsystems.0009. [DOI] [PMC free article] [PubMed]
- 110.Zheng C., Li Z., Xu T., Chen L., Fang F., Wang D., Dai P., Wang Q., Wu X., Yan X. Spirulina-templated porous hollow carbon@magnetite core-shell microswimmers. Appl. Mater. Today. 2021;22 doi: 10.1016/j.apmt.2021.100962. [DOI] [Google Scholar]
- 111.Li M., Wu J., Li N., Zhou J., Cheng W., Wu A., Liu L., Jiao N. Cross-scale drug delivery of diatom microrobots based on A magnetic continuum robot for combined chemical and photodynamic therapy of glioblastoma. Adv. Funct. Mater. 2024;34(37) doi: 10.1002/adfm.202402333. [DOI] [Google Scholar]
- 112.Zhang Z., He R., Han B., Ren S., Fan J., Wang H., Zhang Y.-L., Ma Z.-C. Magnetically switchable adhesive millirobots for universal manipulation in both air and water. Adv. Mater. 2025;(n/a) doi: 10.1002/adma.202420045. n/a. [DOI] [PubMed] [Google Scholar]
- 113.Wang X., Cai J., Sun L., Zhang S., Gong D., Li X., Yue S., Feng L., Zhang D. Facile fabrication of magnetic microrobots based on Spirulina templates for targeted delivery and synergistic chemo-photothermal therapy. ACS Appl. Mater. Interfaces. 2019;11(5):4745–4756. doi: 10.1021/acsami.8b15586. [DOI] [PubMed] [Google Scholar]
- 114.Liu L., Wu J., Chen B., Gao J., Li T., Ye Y., Tian H., Wang S., Wang F., Jiang J., Ou J., Tong F., Peng F., Tu Y. Magnetically actuated biohybrid microswimmers for precise photothermal muscle contraction. ACS Nano. 2022;16(4):6515–6526. doi: 10.1021/acsnano.2c00833. [DOI] [PubMed] [Google Scholar]
- 115.Han Z.-Y., Zhang C., An J.-X., Qiao J.-Y., Zhang X.-Z. Microalgal biomass-assisted delivery of probiotics for modulation of gut homeostasis and alleviation of intestinal inflammation. Nano Today. 2024;54 doi: 10.1016/j.nantod.2023.102093. [DOI] [Google Scholar]
- 116.Zhang D., He J., Cui J., Wang R., Tang Z., Yu H., Zhou M. Oral Microalgae-Nano integrated system against radiation-induced injury. ACS Nano. 2023;17(11):10560–10576. doi: 10.1021/acsnano.3c01502. [DOI] [PubMed] [Google Scholar]
- 117.Chen T., Chi X., Li Y., Li Y., Zhao R., Chen L., Wu D., Hu J.-N. Orally deliverable Microalgal-based carrier with selenium nanozymes for alleviation of inflammatory bowel disease. ACS Appl. Mater. Interfaces. 2024;16(38):50212–50228. doi: 10.1021/acsami.4c08020. [DOI] [PubMed] [Google Scholar]
- 118.Bertout J.A., Patel S.A., Simon M.C. The impact of O2 availability on human cancer. Nat. Rev. Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.West J.B. Physiological effects of chronic hypoxia. N. Engl. J. Med. 2017;376(20):1965–1971. doi: 10.1056/NEJMra1612008. [DOI] [PubMed] [Google Scholar]
- 120.Zheng W., Yao S.-Y., Hu H., Chen X., Qian Z., Liu W., Zhu Y., Mao Z., Guo D.-S., Gao C. Hypoxia-responsive calixarene-grafted self-assembled peptide hydrogel for inflammation suppression in ischemic stroke. Nano Today. 2024;54 doi: 10.1016/j.nantod.2023.102064. [DOI] [Google Scholar]
- 121.Estep T.N. Issues in the development of hemoglobin based oxygen carriers. Semin. Hematol. 2019;56(4):257–261. doi: 10.1053/j.seminhematol.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 122.Swi Chang T.M. In: Principles of Tissue Engineering. fourth ed. Lanza R., Langer R., Vacanti J., editors. Academic Press; Boston: 2014. Chapter 49 - red blood cell substitutes; pp. 1059–1073. [Google Scholar]
- 123.Sharma S., Parveen R., Chatterji B.P. Toxicology of nanoparticles in drug delivery. Current Pathobiology Reports. 2021;9(4):133–144. doi: 10.1007/s40139-021-00227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang J., Wang W., Zheng H., Chen X., Liu X., Xie Q., Cai X., Zhang Z., Li R. Nano-enabled photosynthesis in tumours to activate lipid peroxidation for overcoming cancer resistances. Biomaterials. 2022;285 doi: 10.1016/j.biomaterials.2022.121561. [DOI] [PubMed] [Google Scholar]
- 125.Chang M., Feng W., Ding L., Zhang H., Dong C., Chen Y., Shi J. Persistent luminescence phosphor as in-vivo light source for tumoral cyanobacterial photosynthetic oxygenation and photodynamic therapy. Bioact. Mater. 2022;10:131–144. doi: 10.1016/j.bioactmat.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu S., Feng W., Dong C., Song X., Gao X., Guo J., Chen Y., Hu Z. Photosynthetic oxygenation-augmented sonodynamic nanotherapy of hypoxic tumors. Adv. Healthcare Mater. 2022;11(3) doi: 10.1002/adhm.202102135. [DOI] [PubMed] [Google Scholar]
- 127.Y. Qiao, F. Yang, T. Xie, Z. Du, D. Zhong, Y. Qi, Y. Li, W. Li, Z. Lu, J. Rao, Y. Sun, M. Zhou, Engineered algae: a novel oxygen-generating system for effective treatment of hypoxic cancer, Sci. Adv. 6 (21) eaba5996, 10.1126/sciadv.aba5996. [DOI] [PMC free article] [PubMed]
- 128.Cui H., Su Y., Wei W., Xu F., Gao J., Zhang W. How microalgae is effective in oxygen deficiency aggravated diseases? A comprehensive review of literature. Int. J. Nanomed. 2022;17(null):3101–3122. doi: 10.2147/IJN.S368763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu X., Dong J., Cui J., Zheng Y., Hu H., Wang R., Wang K., Zhong D., Huang H., Zheng Y., Zhou M. Microalgae-based drug delivery microspheres for treatment of hyperuricemia with renal injury. Nano Today. 2025;61 doi: 10.1016/j.nantod.2024.102607. [DOI] [Google Scholar]
- 130.Ibrahim S.M., Bin Jumah M.N., Othman S.I., Alruhaimi R.S., Al-Khalawi N., Salama Y.F., Allam A.A., Abukhadra M.R. Synthesis of Chitosan/Diatomite composite as an advanced delivery system for ibuprofen drug; equilibrium studies and the release profile. ACS Omega. 2021;6(20):13406–13416. doi: 10.1021/acsomega.1c01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang F., Li Z., Duan Y., Abbas A., Mundaca-Uribe R., Yin L., Luan H., Gao W., Fang R.H., Zhang L., Wang J. Gastrointestinal tract drug delivery using algae motors embedded in a degradable capsule. Sci. Robot. 2022;7(70) doi: 10.1126/scirobotics.abo4160. eabo4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vona D., Flemma A., Piccapane F., Cotugno P., Cicco S.R., Armenise V., Vicente-Garcia C., Giangregorio M.M., Procino G., Ragni R. Drug delivery through epidermal tissue cells by functionalized Biosilica from diatom Microalgae. Mar. Drugs. 2023;21(8):438. doi: 10.3390/md21080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Studer T., Morina D., Shchelik I.S., Gademann K. Biohybrid microswimmers for antibiotic drug delivery based on a thiol-sensitive release platform. Chem. Eur J. 2023;29(26) doi: 10.1002/chem.202203913. [DOI] [PubMed] [Google Scholar]
- 134.Li Z., Duan Y., Zhang F., Luan H., Shen W.-T., Yu Y., Xian N., Guo Z., Zhang E., Yin L., Fang R.H., Gao W., Zhang L., Wang J. Biohybrid microrobots regulate colonic cytokines and the epithelium barrier in inflammatory bowel disease. Sci. Robot. 2024;9(91) doi: 10.1126/scirobotics.adl2007. [DOI] [PubMed] [Google Scholar]
- 135.Wang J., Soto F., Liu S., Yin Q., Purcell E., Zeng Y., Hsu E.-C., Akin D., Sinclair B., Stoyanova T., Demirci U. Volbots: Volvox Microalgae-Based robots for Multimode precision imaging and therapy. Adv. Funct. Mater. 2022;32(50) doi: 10.1002/adfm.202201800. [DOI] [Google Scholar]
- 136.Zhong D., Li W., Qi Y., He J., Zhou M. Photosynthetic biohybrid nanoswimmers System to alleviate tumor hypoxia for FL/PA/MR imaging-guided enhanced radio-photodynamic synergetic therapy. Adv. Funct. Mater. 2020;30(17) doi: 10.1002/adfm.201910395. [DOI] [Google Scholar]
- 137.Squire K., Kong X., LeDuff P., Rorrer G.L., Wang A.X. Photonic crystal enhanced fluorescence immunoassay on diatom biosilica. J. Biophot. 2018;11(10) doi: 10.1002/jbio.201800009. [DOI] [PubMed] [Google Scholar]
- 138.Mostafa Mohammed D., El-Messery T.M., Baranenko D.A., Hashim M.A., Tyutkov N., Marrez D.A., Elmessery W.M., El-Said M.M. Effect of Spirulina maxima microcapsules to mitigate testicular toxicity induced by cadmium in rats: optimization of in vitro release behavior in the milk beverage. J. Funct.Foods. 2024;112 doi: 10.1016/j.jff.2023.105938. [DOI] [Google Scholar]
- 139.Kabir A., Nazeer N., Bissessur R., Ahmed M. Diatoms embedded, self-assembled carriers for dual delivery of chemotherapeutics in cancer cell lines. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118887. [DOI] [PubMed] [Google Scholar]
- 140.Picciotto S., Santonicola P., Paterna A., Rao E., Raccosta S., Romancino D.P., Noto R., Touzet N., Manno M., Di Schiavi E., Bongiovanni A., Adamo G. Extracellular vesicles from microalgae: uptake studies in human cells and Caenorhabditis elegans. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.830189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shchelik I.S., Molino J.V.D., Gademann K. Biohybrid microswimmers against bacterial infections. Acta Biomater. 2021;136:99–110. doi: 10.1016/j.actbio.2021.09.048. [DOI] [PubMed] [Google Scholar]
- 142.Zhang F., Zhuang J., Li Z., Gong H., de Ávila B.E.-F., Duan Y., Zhang Q., Zhou J., Yin L., Karshalev E., Gao W., Nizet V., Fang R.H., Zhang L., Wang J. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 2022;21(11):1324–1332. doi: 10.1038/s41563-022-01360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.F. Zhang, Z. Li, Y. Duan, H. Luan, L. Yin, Z. Guo, C. Chen, M. Xu, W. Gao, R.H. Fang, L. Zhang, J. Wang, Extremophile-based biohybrid micromotors for biomedical operations in harsh acidic environments, Sci. Adv. 8 (51) eade6455, 10.1126/sciadv.ade6455. [DOI] [PMC free article] [PubMed]
- 144.Hosseini M., Ahmadi Z., Kefayat A., Molaabasi F., Ebrahimpour A., Naderi Khojasteh far Y., Khoobi M. Multifunctional gold helix phototheranostic biohybrid that enables targeted image-guided photothermal therapy in breast cancer. ACS Appl. Mater. Interfaces. 2022;14(33):37447–37465. doi: 10.1021/acsami.2c10028. [DOI] [PubMed] [Google Scholar]
- 145.Liu X., Dong J., Wu Z., Cui J., Zheng Y., Zhou M. Microalgae-based hydrogel drug delivery system for treatment of gouty arthritis with alleviated colchicine side effects. Bioact. Mater. 2025;52:17–35. doi: 10.1016/j.bioactmat.2025.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aw M.S., Bariana M., Yu Y., Addai-Mensah J., Losic D. Surface-functionalized diatom microcapsules for drug delivery of water-insoluble drugs. J. Biomater. Appl. 2013;28(2):163–174. doi: 10.1177/0885328212441846. [DOI] [PubMed] [Google Scholar]
- 147.Vasani R.B., Losic D., Cavallaro A., Voelcker N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B. 2015;3(21):4325–4329. doi: 10.1039/C5TB00648A. [DOI] [PubMed] [Google Scholar]
- 148.Martin M., Pusceddu M.M., Teichenné J., Negra T., Connolly A., Escoté X., Torrell Galceran H., Cereto Massagué A., Samarra Mestre I., del Pino Rius A., Romero-Gimenez J., Egea C., Alcaide-Hidalgo J.M., del Bas J.M. Preventive treatment with Astaxanthin microencapsulated with Spirulina powder, administered in a dose range equivalent to human consumption, prevents LPS-Induced cognitive impairment in rats. Nutrients. 2023;15(13):2854. doi: 10.3390/nu15132854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mayasari E., Raya I., Dwyana Z., Anshar A.M., Arif A.R. Modification of microencapsulate protein crude extract formula from Chlorella vulgaris and Spirulina platensis for baby biscuit. J. Phys. Conf. 2019;1341(3) doi: 10.1088/1742-6596/1341/3/032046. [DOI] [Google Scholar]
- 150.Batista de Oliveira T.T., Miranda dos Reis I., Bastos de Souza M., da Silva Bispo E., Fonseca Maciel L., Druzian J.I., Lordelo Guimarães Tavares P.P., de Oliveira Cerqueira A., dos Santos Boa Morte E., Abreu Glória M.B., Lima Deus V., Radomille de Santana L.R. Microencapsulation of Spirulina sp. LEB-18 and its incorporation in chocolate milk: properties and functional potential. LWT. 2021;148 doi: 10.1016/j.lwt.2021.111674. [DOI] [Google Scholar]
- 151.Wang K., Chen E., Lin X., Tian X., Wang L., Huang K., Skirtach A.G., Tan M., Su W. Core-shell nanofibers based on microalgae proteins/alginate complexes for enhancing survivability of probiotics. Int. J. Biol. Macromol. 2024;271 doi: 10.1016/j.ijbiomac.2024.132461. [DOI] [PubMed] [Google Scholar]
- 152.Delasoie J., Schiel P., Vojnovic S., Nikodinovic-Runic J., Zobi F. Photoactivatable surface-functionalized diatom microalgae for colorectal cancer targeted delivery and enhanced cytotoxicity of anticancer complexes. Pharmaceutics. 2020;12(5):480. doi: 10.3390/pharmaceutics12050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Z., Guo Z., Zhang F., Sun L., Luan H., Fang Z., Dedrick J.L., Zhang Y., Tang C., Zhu A., Yu Y., Ding S., Wang D., Chang A.-Y., Yin L., Russell L.M., Gao W., Fang R.H., Zhang L., Wang J. Inhalable biohybrid microrobots: a non-invasive approach for lung treatment. Nat. Commun. 2025;16(1):666. doi: 10.1038/s41467-025-56032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.