Abstract
We discuss a nanoengineering approach for supramolecular chemistry and self assembly. The collective properties and biofunctionalities of molecular ensembles depend not only on individual molecular building blocks but also on organization at the molecular or nanoscopic level. Complementary to “bottom-up” approaches, which construct supramolecular ensembles by the design and synthesis of functionalized small molecular units or large molecular motifs, nanofabrication explores whether individual units, such as small molecular ligands, or large molecules, such as proteins, can be positioned with nanometer precision. The separation and local environment can be engineered to control subsequent intermolecular interactions. Feature sizes as small as 2 × 4 nm2 (32 alkanethiol molecules) are produced. Proteins may be aligned along a 10-nm-wide line or within two-dimensional islands of desired geometry. These high-resolution engineering and imaging studies provide new and molecular-level insight into supramolecular chemistry and self-assembly processes in bioscience that are otherwise unobtainable, e.g., the influence of size, separation, orientation, and local environment of reaction sites. This nanofabrication methodology also offers a new strategy in construction of two- and three-dimensional supramolecular structures for cell, virus, and bacterial adhesion, as well as biomaterial and biodevice engineering.
Supramolecular chemistry has become an area of intense research (1–4), partly inspired by biological ensembles in nature, such as collagen and enzymes or protein assemblies in general. In nature, the collective properties and biofunctionalities of these ensembles depend not only on the individual molecular units but also (perhaps even more importantly) on the organization at the molecular or nanoscopic level (1, 2, 4, 5). Such organization dependence can be attributed to polyvalent interactions in biological systems (6). “Bottom-up” approaches have been taken to mimic nature and have resulted in creative synthesis of small molecular units (7, 8) and large molecular motifs (9). These molecular building blocks contain the desired charge, polarization, or chemical functionalities that will affect intermolecular interactions such as van der Waals forces, hydrogen bonding, polar attractions, and/or hydrophobic interactions (7–9). These interactions dictate the subsequent assembly into supramolecular structures (4, 9–11). Complementary to these synthetic approaches, we explore whether individual units such as small molecular ligands or large molecules such as proteins can be positioned with nanometer precision by using nanoengineering methodologies. The separation and local environment can be engineered to influence subsequent intermolecular interactions.
Micrometer-sized patterns of biomolecules can be produced by using relatively well known microfabrication technologies. Without using templates, DNA micropatterns may be directly produced by using photolithography (12–14). Micropatterns of proteins have also been created by first making patterned surfaces as templates, followed by selective adsorption of proteins (15). More precise positioning of biomolecules requires new fabrication strategies. Scanning probe microscopy (SPM), such as scanning tunneling microscopy (STM) (16) and atomic force microscopy (AFM) (17), are best known for their ability to visualize surfaces of materials with the highest spatial resolution (18–21). Taking advantage of the sharpness of the tips and strong and localized tip–surface interactions, SPM has also been used to manipulate atoms on metal surfaces and to fabricate nanopatterns of metal and semiconductor surfaces (22–28). Various approaches to controlling the local interactions between the tip and surface molecules have also been reported. These methods include AFM-based lithography such as tip-catalyzed surface reactions (29), dip-pen nanolithography (DPN) (30), tip-directed formation of metal-oxide devices (31, 32), and STM-based lithography such as tip-assisted electrochemical etching and field-induced desorption (33, 34). Domains of collagen and collagen-like molecules with dimensions of 30–50 nm were produced by using an AFM-based method, DPN (35). We focus on control of the tip–molecule interaction to more precisely position molecular units ranging from small molecular ligands to proteins. As discussed below, nanoislands of only 32 alkanethiol molecules are produced on metal surfaces. Individual proteins may be aligned on a 10 × 150 nm2 line. This level of precise control provides a new mechanism for studying supramolecular chemistry and for constructing complex structures, as one can preposition the molecular units to dictate the subsequent assembly or reactions.
Materials and Methods
Preparation of Self-Assembled Monolayers (SAM).
All alkanethiol compounds were purchased from Aldrich, with purity greater than 95%. Compounds of 3-mercapto-1-propanal and 11-mercapto-1-undecanal were synthesized by oxidation with pyridium dichromate (36). Solutions of thiols in ethanol or 2butanol were prepared by using 5- to 20-min sonication to accelerate dissolution.
Two types of gold thin films were used: high vacuum deposited films and ultraflat gold films. Gold (Alfa Aesar, 99.999%) was deposited in a high vacuum evaporator (Denton Vacuum, Model DV502-A, Moorestown, NJ) at ≈10−6 torr (1 torr = 133 Pa) onto freshly cleaved mica substrates (Mica, New York; clear ruby muscovite). To enhance the formation of terraced Au(111) domains, the mica was preheated to 325°C before deposition by using quartz lamps mounted behind the substrate. Typical evaporation rates were 3 Å/s, and the thickness of the films was 150 nm. The Au films were annealed at 325°C under vacuum for approximately 15 min after deposition. This procedure produced samples with flat Au(111) terraces as large as 300 × 300 nm2 in dimension according to our AFM images. Ultraflat gold, 150 nm in thickness, was prepared according to the method developed by Hegner et al. (37) and Wagner et al. (38). The resulting gold surfaces have a mean roughness of 2–5 Å according to our AFM measurements. Thiol SAMs, were prepared by immersing the freshly prepared gold films into the corresponding thiol solutions (0.1–1.0 mM) for at least 18 h.
Preparation of Protein Solutions.
Bovine serum albumin (BSA) (fraction V, which is essentially fatty acid free), lysozyme (LYZ, from hen egg, 95% purity), rabbit IgG (purity 95%), and mouse anti-rabbit IgG were purchased from Sigma and used immediately as received. The proteins were diluted to the desired concentrations of 10 μg/ml in Hepes buffer solutions before AFM experiments.
AFM Imaging and Fabrication.
The AFM used for this study incorporates a home-constructed deflection-type scanner controlled by commercial electronics and software (RHK Technology, Troy, MI). The instrument allows simultaneous acquisition of multiple images such as topography, frictional force, and elasticity. The scanner may be operated under ambient laboratory conditions, in vacuum, or in solution (39). The Si3N4 cantilevers were either sharpened microlevers from ThermoMicroscopes (Sunnyvale, CA), with a force constant of 0.1 N/m, or standard microlevers from Digital Instruments (Santa Barbara, CA) with a force constant of 0.38 N/m. Images were acquired under contact mode with a typical load of 0.15 nN (measured from force–distance curves). The fabrication force was determined for individual systems by using procedures described previously (40).
Results
High-Resolution Imaging and Fabrication.
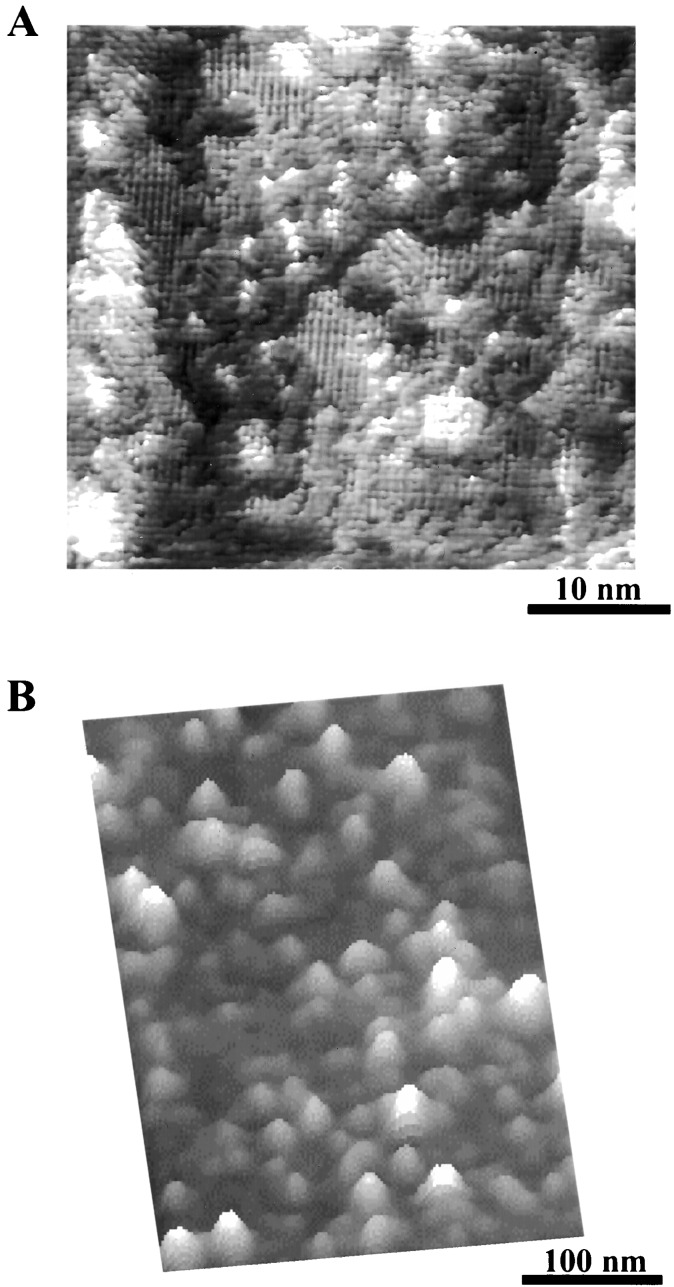
Although scanning tunneling microscopy is known for its capability of atomic and molecular resolution (41–46), researchers have not been optimistic about the resolution of AFM, especially when imaging assemblies of organic molecules or biological or polymeric motifs because of the relatively large tip size and the “soft-and-sticky” nature of the sample. Through technical advances in the design and construction of scanning heads and the development of new imaging modes for organic and biosystems, molecular resolution images are obtained for SAMs by using AFM (47, 48), despite the structural complexity of the molecules. Fig. 1A is a high-resolution image of an alkane–thiol SAM on gold formed during nanografting (48, 49). The structural features are visualized in striking detail: (i) ordered structure within domains with a lattice constant of 0.5 nm and (ii) molecular level defects such as domain boundaries and single atomic steps. For immobilized proteins such as LYZ on a planar surface, individual molecules are visible from the AFM topograph in Fig. 1B, from which their heights are measured. The orientation of the proteins can be determined from the height measurements (36).
Figure 1.
High-resolution AFM topographs of organic thin films and proteins. (A) A 40 × 40 nm2 scan of CH3(CH2)17S/Au(111) SAM imaged under 2-butanol. This image is taken within a nanofabricated region, thus contains newly formed Au(111) single atomic islands. Ordered domains are clearly visible. Bright areas are 0.24 nm above the surrounding because of single atomic Au(111) steps. Domain boundaries appear darker in contrast than the ordered domains. (B) A 300 × 400 nm2 scan of LYZ molecules immobilized on a H(CO)(CH2)10S/Au(111) SAM.
The fact that molecules can be resolved by using AFM indicates that the tip–molecule interactions are localized to molecular dimensions, especially in the case of SAMs. Therefore, in principle, by enhancing the local interactions, molecules may be moved, and chemical bonds may be selectively broken. The detailed methodology in controlling these local interactions is the key to positioning molecules precisely. The methods used in this report are nanografting (49, 50) and nanopen reader and writer (NPRW) (51). The surface structure of SAM resists is first characterized under a very low force or load. Fabrication locations are then selected, normally in flat regions, e.g., Au(111) plateau areas. Then nanopatterns are engineered under high forces. In nanografting, the SAM and the AFM cantilevers are immersed in a solution containing another thiol with protein adhesive terminal groups. As the AFM tip plows through the matrix, the thiol molecules in the solution adsorb on the newly exposed gold surface. Protein adhesive thiols are positioned following the scanning trajectory of the tip. In NPRW, the tip is precoated with the desired molecules by soaking the tip in the desired solution, then drying it in nitrogen. These molecules are transferred under high force to the exposed substrate (51). Nanostructures of thiol molecules serve as templates for attaching proteins. Selectivity of protein adsorption can be achieved by using the variation in protein affinity toward different SAMs (52–58).
Precise Positioning of Small Molecules and Proteins on Surfaces.
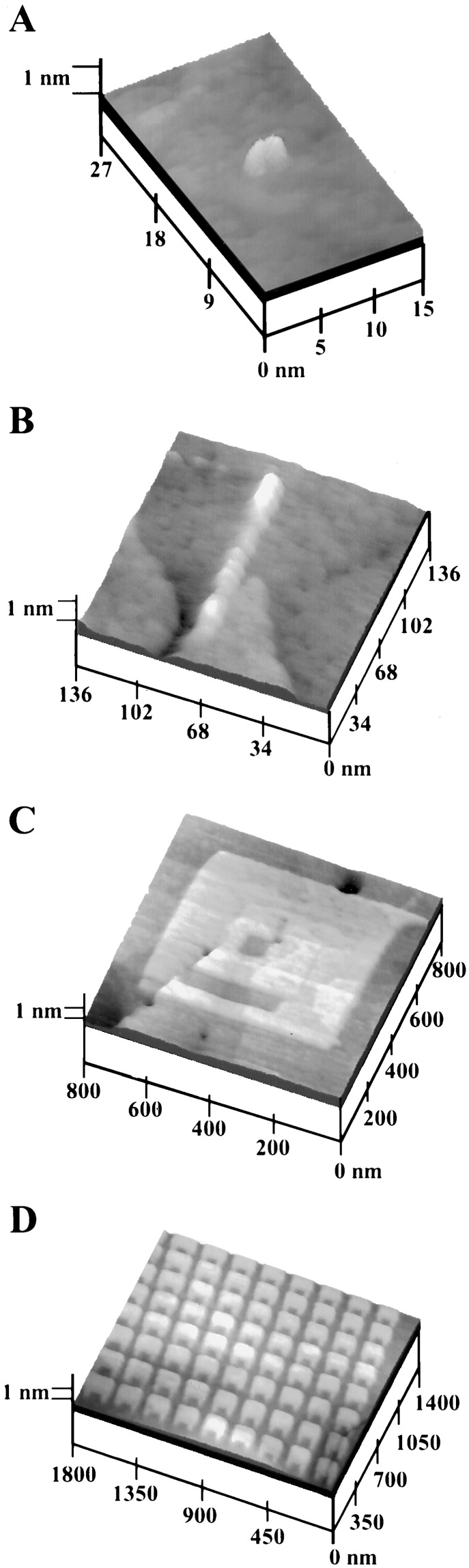
Compared with other fabrication techniques, nanografting and NPRW have the highest precision and resolution (40, 51). An edge resolution of 1 nm is routinely obtained. The smallest feature fabricated is 2 × 4 nm2 (shown in Fig. 2A), consisting of 32 thiol molecules (49). One-dimensional features, such as 10-nm-wide lines (Fig. 2B) or two-dimensional nanoislands with various shapes (40, 49), can also be produced. More importantly for positioning biomolecular building blocks, bioadhesive ligands such as biotin, -COOH, and -CHO can be positioned with nanometer precision on surfaces (49). In the AFM image shown Fig. 2C, an HS(CH2)17CH3 pattern (600 × 600 nm2) was first grafted within an HS(CH2)9CH3 matrix, resulting in a nanosquare of positive contrast. Then, two nanostructures of aldehyde-terminated thiols, a 70 × 70 nm2 square and a 70 × 300 nm2 rectangle, were produced within the HS(CH2)17CH3 nanosquare. The example shown in Fig. 2C demonstrates the capability of nanografting for producing nanostructures with various heights and functionalities. For research in supramolecular chemistry and self assembly, making arrays of nanostructures is essential. Fig. 2D is a proof-of-concept experiment, in which a 9 × 8 array of nanostructures is created. Each element shaped similar to a “space invader” icon has a 150 × 115 nm2 head attached to two small “legs” of 40 × 45 nm2. These nanostructures consist of octadecanethiol, 0.8 nm taller than the decanethiol matrix. The examples in Fig. 2 demonstrate the precision of AFM-based lithography in positioning small molecules, as well as flexibility in producing various nanostructures and arrays. The nanostructures of these ligands exhibit little lateral diffusion, as they are organized into closely packed structures and are surrounded by matrix SAMs (40).
Figure 2.
Nanopatterns produced and imaged within a CH3(CH2)9S/Au(111) monolayer. (A) Thirty-two CH3(CH2)17SH molecules are grafted, forming a 2 × 4 nm2 “dot.” The “dot” is 8.8 Å higher than the surrounding monolayer, indicating that thiols are closely packed. (B) A 10 × 100 nm2 line of CF3(CF2)11(CH2)2SH molecules produced using NPRW. (C) Nanostructures with multiple components may be produced. Two small patterns of aldehyde terminated SAMs are grafted in a 600 × 600 nm2 square of CH3(CH2)17SH area. (D) Fabrication of arrays of nanostructures. Each element consists of CH3(CH2)17SH molecules. This array was engineered within 4 min.
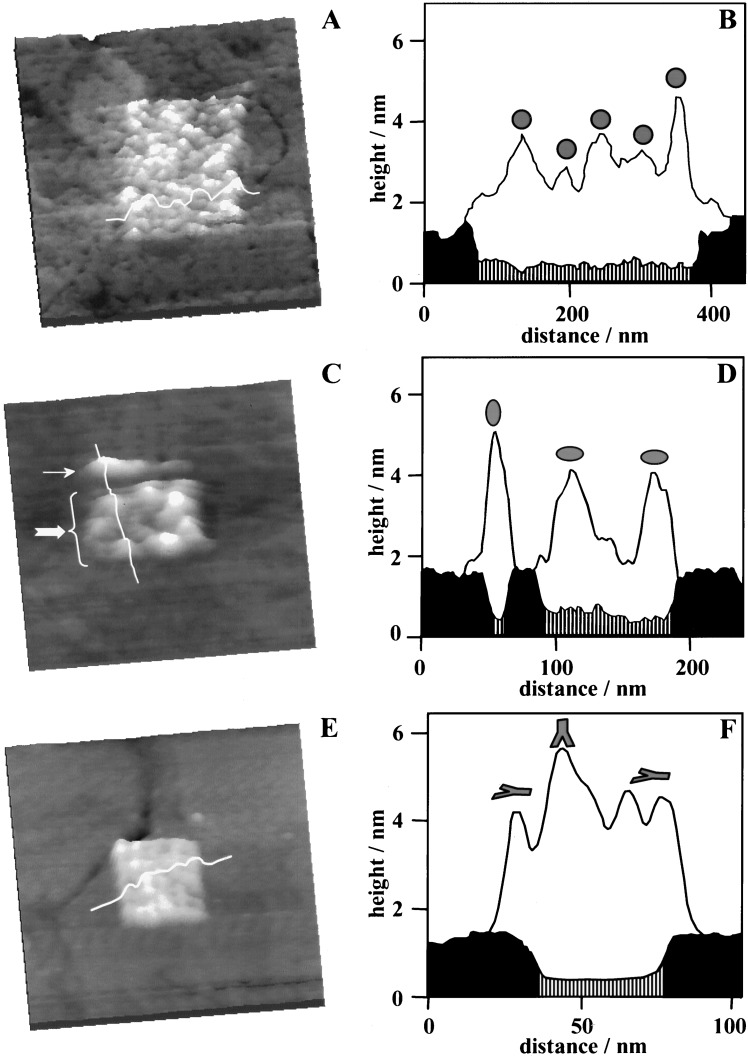
The precision in organizing large molecular units depends on the quality of template nanostructures and the selectivity in pattern transfer reactions. With templating nanostructures precisely engineered, proteins can be selectively attached to the bioadhesive areas. As shown in Fig. 3A, a 300 × 350 nm2 pattern of a small protein, BSA was successfully produced. The SAM-based template was first produced within a hexanethiol matrix using mercapto-propanal and washed thoroughly with deionized water. Next, the medium was replaced with a 20 mM Hepes buffer solution (pH 6.5) containing 10 μg/ml of BSA. After about 3 min of immersion, a near monolayer of BSA was observed exclusively on the aldehyde-terminated area. BSA molecules may adsorb individually or as aggregates. The heights measured for individual BSA are shown in the cursor plot (Fig. 3B) and are consistent with the known dimensions of its active conformation: approximately spherical in shape with a diameter of 4 nm (59).
Figure 3.
Positioning of proteins on surfaces. (A) BSA molecules are assembled onto a 200 × 250 nm2 pattern of H(CO)(CH2)2S/Au(111) SAM. (B) Cursor profile as indicated in A. (C) Three LYZ molecules are aligned along a 10 × 150 nm2 line (indicated by the small arrow). Eight LYZ molecules are confined within a 100 × 150 nm2 rectangle of HO(CO)(CH2)2S/Au SAM (large arrow). (D) Cursor profiles as indicated in C but taken as each step of nanofabrication. Black and shaded areas represent the matrix and patterned SAM regions, respectively, whereas the clear region corresponds to adsorbed protein molecules. (E) IgG molecules are immobilized onto a 40 × 40 nm2 nanopattern of H(CO)(CH2)2SH. Little adsorption is observed on the surrounding CH3(CH2)9S/Au(111) matrix. (F) Cursor profile as indicated in E. In the cursor plots, the origin is the gold.
A less symmetric protein molecule, LYZ, can also be positioned, following a similar procedure. The template in this case contains two nanopatterns of mercapto-propanoic acid in a decanethiol SAM: a narrow line (10 × 150 nm2) above a rectangle (100 × 150 nm2). The two patterns are separated by 30 ± 5 nm. The surface was rinsed thoroughly to completely remove any residual thiols, first with deionized water, then with 20 mM Hepes buffer (pH 7.0). After rinsing, a 10 μg/ml solution of LYZ was injected. Within 3 min, proteins adsorbed exclusively onto the two patterned areas, as shown in Fig. 3C. The high selectivity observed at pH 7 is mostly because of electrostatic interactions between the LYZ molecules and the carboxylate-terminated nanopatterns. Because the isoelectric point of LYZ is 11.1 (60), LYZ exhibits a net positive charge at pH 7. The pKa value of mercapto-propanoic acid SAM is 8 (61), thus at neutral pH, approximately 10% of the nanopatterned area has a net negative charge. Consequently, the selectivity of LYZ adsorption is mediated by electrostatic attraction. Under these conditions, little adsorption was observed at methyl-terminated areas within the time frame of the entire experiment (4 h). Further, the boundary between the two nanopatterns remained clearly visible.
Individual LYZ particles can be resolved in the AFM image of Fig. 3C. Three LYZ molecules are positioned along the 10 × 150 nm2 nanoline, whereas eight protein particles are confined within the 100 × 150 nm2 nanorectangle. The corresponding cursor profiles in Fig. 3D reveal that the immobilized protein molecules exhibit two different heights: 4.3 ± 0.2 nm and 3.0 ± 0.2 nm. It is known that physical interactions are not specific, therefore various orientations with respect to the surface are observed for the adsorbed proteins. Because LYZ molecules are ellipsoidal with the approximate dimensions 4.5 × 3.0 × 3.0 nm3 from x-ray crystallographic studies (62), the observed heights correspond to side-on and end-on orientations of LYZ, respectively.
Large proteins such as IgG can also be assembled within nanometer confinement. In Fig. 3E, rabbit IgG molecules are immobilized within a 40 × 40 nm2 square. IgG molecules within the nanopattern are near close-packed and cover the entire nanopattern. At pH 6.5, the aldehyde groups react with primary amines (e.g., in lysine residues) in IgG, forming covalent imine bonds (63, 64). The hydrophobic adsorption of IgG on methyl-terminated areas may be removed by washing with 1% Tween 20 surfactant solution, because the attachment is relatively weak. The cursor profile shown in Fig. 3F reveals heights ranging from 4 to 6 nm for immobilized IgG molecules. The observed heights result from several possible orientations that the Y-shaped IgG (65) can adopt, as the IgG has multiple lysine residues.
Reactivity of the Immobilized Proteins with Nanostructures.
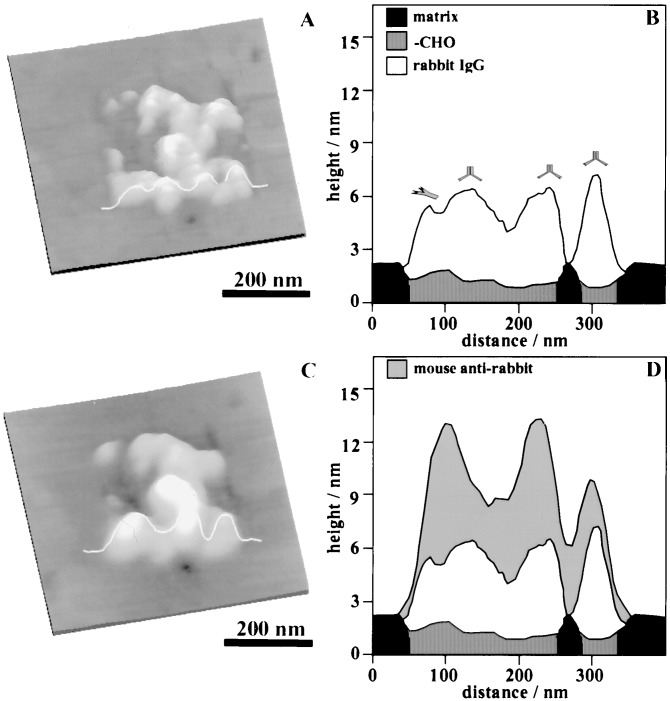
The reactivity of each molecular unit is important for subsequent construction of supramolecular assemblies. In the case of protein nanostructures, the activity may be tested by the corresponding biochemical reactions. Fig. 4 illustrates how the bioactivity of a nanopattern of rabbit IgG was tested by reaction toward a specific antibody, mouse anti-rabbit IgG. In Fig. 4A, rabbit IgG molecules are positioned within a 300 × 300 nm2 frame. The ligands under the IgG are 11-mercapto-1-undecanal grafted within an octadecanethiol SAM. The weakly bound IgG molecules on the methyl-terminated areas were removed by washing with 1% Tween 20 surfactant solution. Within 5 min of injecting 10 μg/ml of mouse anti-rabbit IgG, recognition (or antibody-antigen binding) occurred, resulting in adsorption of the secondary IgG onto the nanosquare. By comparing the topographic images before and after secondary IgG injection, a height increase was observed, which indicates the binding of mouse anti-rabbit IgG. The height measurements are illustrated from the cursor profiles in Figs. 4 B and D. In Fig. 4B, the proteins appear 3.0–4.4 nm taller than the surrounding matrix (or 3.8–5.2 nm above the nanopattern). Analysis of the entire pattern indicates that IgG molecules exhibit heights ranging from 3.8 to 7.0 nm. The variation in height is consistent with the expectation that each rabbit IgG has 57 lysine residues containing amines (PDB ID code 1IGT). Thus immobilized IgG may adopt various configurations after reacting with aldehyde. Immersion of the IgG nanopatterns in a solution of mouse anti-rabbit IgG resulted in a further increase in height, ranging from 2.8 to 7.5 nm, indicating the attachment of secondary antibodies. Such a wide height range is expected because the rabbit IgG molecules within the nanopatterns can have various orientations on surfaces. The end of the Fc fragment of each rabbit IgG serves as the binding site for the mouse anti-rabbit IgG. The ends of the Fab fragment of the Y-shaped mouse anti-rabbit IgG bind specifically to the rabbit IgG. Various orientations of the rabbit IgG lead to different configurations of the antibody–antigen-binding complexes. In contrast, injection of nonspecific antibodies did not result in any observable height increase. The spatial precision and specificity achieved by using this nanoengineering method provides an opportunity for three-dimensional construction of bioassemblies.
Figure 4.
Specific antibody–antigen recognition process for proteins immobilized on nanopatterns. (A) Rabbit IgG are immobilized on a 300 × 300 nm2 H(CO)(CH2)10SH nanosquare. (C) The same area after the introduction of mouse anti-rabbit IgG. Cursor profiles in B and D are taken from the same location and at each step of nanofabrication. Black and shaded areas represent the matrix and patterned SAM regions, respectively. The clear region corresponds to adsorbed rabbit IgG, whereas the gray area represents the secondary IgG molecules. An increase in height (compare cursor B and D) is observed, which suggests that specific binding of antibody to antigen occurs.
Discussion
A nanoengineering approach is used to position molecules on surfaces. The molecules range from small alkanethiols to larger biosystems such as proteins or DNA (M. Liu, N.A.A., J. C. Garno, C. Chow, and G.-Y.L., unpublished work; ref. 66). The nanofabrication method may be used as a generic approach to position other molecules such as enzymes, collagens, fibronectin, or synthetic motifs.
The smallest features produced include a 2 × 4 nm2 dot (32 thiol molecules), and a 10 × 150 nm2 line (containing three proteins). These high-resolution engineering and imaging studies reveal nanoscopic and molecular level information for supramolecular chemistry and self-assembly processes in bioscience that are otherwise unobtainable, e.g., the influence of size, separation, orientation, and the local environment of reaction sites. Work is in progress to explore the possible improvement in nanofabrication methods to reach single molecule precision and in protein immobilization chemistry to control both the lateral and perpendicular orientations of molecules.
In terms of technique development, nanolithography is complementary to the “bottom-up” material synthesis approach, in providing a means to fabricate ultra-small electronic components, sensing elements, and scaffolds for biomaterial engineering. One concern with scanning probe lithography is the serial nature of the process and low throughput. Two approaches are in progress to address these issues: automating the nanofabrication (66–70) and/or using parallel probes (70–73). Nanostructures of SAMs are sufficiently stable, at least 40 h without observable desorption in pure solvent and 8 h without observable exchange in thiol solutions (50). Protein nanopatterns can sustain washing with buffer and surfactant solutions, as well as mechanical forces (64). Detachment or denaturation was not observed within the maximum duration of our experiments, i.e., 48 h. The observed stability makes nanolithography a promising technique for applications. Two specific areas of applications include: (i) construction of two- or three-dimensional supramolecular structures with well positioned binding sites for cell, virus, and bacterial adhesion, and for biomaterial engineering; and (ii) construction of inorganic and biomolecular hybrid systems in which functional building blocks are positioned with molecular precision.
Acknowledgments
We appreciate many helpful technical and scientific discussions with Jayne Garno, Kapila Wadu-Mesthrige, Sylvain Cruchon-Dupeyrat, and Song Xu. We thank all four reviewers for their careful reading of the manuscript, as well as their constructive and insightful comments. N.A. thanks the National Science Foundation Integrative Graduate Education and Research Traineeship program for a graduate research fellowship. This work is also supported by The National Science Foundation (Grant CHE-9733400), The Whitaker Foundation (Biomedical Engineering Grant), and the University of California at Davis.
Abbreviations
- AFM
atomic force microscopy
- NPRW
nanopen reader and writer
- SAM
self-assembled monolayer
- LYZ
lysozyme
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Niemeyer C M. Angew Chem Int Ed Engl. 2001;40:4128–4158. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Greig L M, Philp D. Chem Soc Rev. 2001;30:287–302. [Google Scholar]
- 3.Nguyen S T, Douglas L G, Hupp J T, Zhang X. Proc Natl Acad Sci USA. 2001;98:11849–11850. doi: 10.1073/pnas.201373898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Shen J C. Adv Mater. 1999;11:1139–1143. [Google Scholar]
- 5.Dinolfo P H, Hupp J T. Chem Mat. 2001;13:3113–3125. [Google Scholar]
- 6.Mammen M, Choi S K, Whitesides G M. Angew Chem Int Ed Engl. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Atwood J L, Lehn J M, Gokel G W, Vogtle F, Osa T, Szejtli J, Murakami Y, Suslick K S, MacNicol D D, Toda F. Comprehensive Supramolecular Chemistry. New York: Pergamon; 1996. [Google Scholar]
- 8.Lehn J M. Supramolecular Chemistry: Concepts and Perspectives: A Personal Account. New York: VCH; 1995. [Google Scholar]
- 9.Leininger S, Olenyuk B, Stang P J. Chem Rev. 2000;100:853–907. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]
- 10.Miller S A, Ding J H, Gin D L. Curr Opin Colloid Int. 1999;4:338–347. [Google Scholar]
- 11.Miller S A, Kim E, Gray D H, Gin D L. Angew Chem Int Ed Engl. 1999;38:3022–3026. doi: 10.1002/(sici)1521-3773(19991018)38:20<3021::aid-anie3021>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Fodor S P A, Leighton Read J, Pirrung M C, Stryer L, Lu A T, Solas D. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 13.Tarlov M J, Burgess D R F, Gillen G. J Am Chem Soc. 1993;115:5305–5306. [Google Scholar]
- 14.Huang J Y, Dahlgren D A, Hemminger J C. Langmuir. 1994;10:626–628. [Google Scholar]
- 15.Lopez G P, Biebuyuck H, Kumar A, Whitesides G. J Am Chem Soc. 1993;115:10774–10781. [Google Scholar]
- 16.Binnig G, Rohrer H, Gerber C, Weibel E. Phys Rev Lett. 1982;49:57–61. [Google Scholar]
- 17.Binning G, Quate C F, Gerber C. Phys Rev Lett. 1986;56:930–936. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 18.Chen C J. Introduction to Scanning Tunneling Microscopy. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 19.Sarid D. Scanning Force Microscopy, with Applications to Electric, Magnetic and Atomic Forces. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 20.Frommer J. Angew Chem Int Ed Engl. 1992;31:1298–1328. [Google Scholar]
- 21.Hamers R J. J Phys Chem B. 1996;100:13103–13120. [Google Scholar]
- 22.Eigler D M, Schweizer E K. Nature (London) 1990;244:524–526. [Google Scholar]
- 23.Zeppenfeld P, Lutz C P, Eigler D M. Ultramicroscopy. 1992;42–44:128–133. [Google Scholar]
- 24.Crommie M F, Lutz C P, Eigler D M. Science. 1993;262:218–220. doi: 10.1126/science.262.5131.218. [DOI] [PubMed] [Google Scholar]
- 25.Jung T A, Schlittler R R, Gimzewski J K, Tang H, Joachim C. Science. 1996;271:181–184. [Google Scholar]
- 26.Avouris P. Acc Chem Res. 1995;28:95–102. [Google Scholar]
- 27.Lyo I, Avouris P. Science. 1991;253:173–176. doi: 10.1126/science.253.5016.173. [DOI] [PubMed] [Google Scholar]
- 28.Stipe B C, Bezaei M A, Ho W, Gao S, Persson M, Lundqvist B I. Phys Rev Lett. 1997;78:4410–4413. [Google Scholar]
- 29.Muller W T, Klein D L, Lee T, Clarke J, Mceuen P L, Schultz P G. Science. 1995;268:272–273. doi: 10.1126/science.268.5208.272. [DOI] [PubMed] [Google Scholar]
- 30.Piner R D, Zhu J, Xu F, Hong S H, Mirkin C A. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 31.Snow E S, Campbell P M. Science. 1995;270:1639–1641. [Google Scholar]
- 32.Dagata J A, Inoue T, Itoh J, Matsumoto K, Yokoyama H. J Appl Phys. 1998;84:6891–6900. [Google Scholar]
- 33.Ross C B, Sun L, Crooks R M. Langmuir. 1993;9:632–636. [Google Scholar]
- 34.Schoer J K, Zamborini F P, Crooks R M. J Phys Chem. 1996;100:11086–11091. [Google Scholar]
- 35.Wilson D L, Martin R, Hong S, Cronin-Golomb M, Mirkin C A, Kaplan D L. Proc Natl Acad Sci USA. 2001;98:13660–13664. doi: 10.1073/pnas.241323198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadu-Mesthrige K, Xu S, Amro N A, Liu G-Y. Langmuir. 1999;15:8580–8583. [Google Scholar]
- 37.Hegner M, Wagner P, Semenza G. Surf Sci. 1993;291:39–46. [Google Scholar]
- 38.Wagner P, Hegner M, Guntherodt H J, Semenza G. Langmuir. 1995;11:3867–3875. [Google Scholar]
- 39.Kolbe W F, Ogletree D F, Salmeron M B. Ultramicroscopy. 1992;42:1113–1117. doi: 10.1016/0304-3991(92)90411-c. [DOI] [PubMed] [Google Scholar]
- 40.Liu G-Y, Xu S, Qian Y. Acc Chem Res. 2000;33:457–466. doi: 10.1021/ar980081s. [DOI] [PubMed] [Google Scholar]
- 41.Poirier G E, Pylant E D, White J M. J Chem Phys. 1996;105:2089–2092. [Google Scholar]
- 42.Poirier G E, Tarlov M J. Langmuir. 1994;10:2853–2856. [Google Scholar]
- 43.Mcdermott C A, Mcdermott M T, Green J B, Porter M D. J Phys Chem. 1995;99:13257–13267. [Google Scholar]
- 44.Delamarche E, Michel B, Biebuyck H A, Gerber C. Adv Mater. 1996;8:719–729. [Google Scholar]
- 45.Weiss P S, Abrams M J, Cygan M T, Ferris J H, Kamna M M, Krom K R, Stranick S J, Youngquist M G Y. Anal Chim Acta. 1995;307:355–363. [Google Scholar]
- 46.Kang J, Rowntree P A. Langmuir. 1996;12:2813–2819. [Google Scholar]
- 47.Butt H J, Seifert K, Bamberg E. J Phys Chem. 1993;97:7316–7320. [Google Scholar]
- 48.Xu S, Laibinis P E, Liu G-Y. J Am Chem Soc. 1998;120:9356–9361. [Google Scholar]
- 49.Xu S, Miller S, Laibinis P E, Liu G-Y. Langmuir. 1999;15:7244–7251. [Google Scholar]
- 50.Xu S, Liu G Y. Langmuir. 1997;13:127–129. [Google Scholar]
- 51.Amro N A, Xu S, Liu G-Y. Langmuir. 2000;16:3006–3009. [Google Scholar]
- 52.Norde W, Giesbers M, Pingsheng H. Colloids Surf B. 1995;5:255–263. [Google Scholar]
- 53.Buijs J, Britt D W, Hlady V. Langmuir. 1998;14:335–341. doi: 10.1021/la970669s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner P, Hegner M, Kernen P, Zaugg F, Semenza G. Biophys J. 1996;70:2052–2066. doi: 10.1016/S0006-3495(96)79810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel N, Davies M C, Hartshorne M, Heaton R J, Roberts C J, Tendler S J B, Williams P M. Langmuir. 1997;13:6485–6490. [Google Scholar]
- 56.Wadu-Mesthrige K, Amro N A, Liu G-Y. Scanning. 2000;22:380–388. doi: 10.1002/sca.4950220607. [DOI] [PubMed] [Google Scholar]
- 57.Browning-Kelly M E, Wadu-Mesthrige K, Hari V, Liu G Y. Langmuir. 1997;13:343–350. [Google Scholar]
- 58.Lee G U, Kidwell D A, Colton R J. Langmuir. 1994;10:354–357. [Google Scholar]
- 59.Rosenoer V M, Oratz M, Rothschild M A. Albumin Function and Uses. New York: Pergamon; 1977. [Google Scholar]
- 60.Imoto T, Johnson L N, North A C T, Philips D C, Rupley J A. Vertebrate Lysozymes. New York: Academic; 1972. [Google Scholar]
- 61.Hu K, Bard A J. Langmuir. 1997;13:5114–5119. [Google Scholar]
- 62.Blake C F, Koenig D F, Mair G A, North A C T, Phillips D C, Sarma V R. Nature (London) 1965;206:757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- 63.Baker A, Zidek L, Wiesler D, Chmelik J, Pagel M, Novotny M V. Chem Res Toxicol. 1998;11:730–740. doi: 10.1021/tx970167e. [DOI] [PubMed] [Google Scholar]
- 64.Wadu-Mesthrige K, Amro N A, Garno J C, Xu S, Liu G-Y. Biophys J. 2001;80:1891–1899. doi: 10.1016/s0006-3495(01)76158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverton E W, Navia M A, Davies D R. Proc Natl Acad Sci USA. 1977;74:5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz P V. Langmuir. 2001;17:5971–5977. [Google Scholar]
- 67.Cruchon-Dupeyrat S, Porthun S, Liu G Y. Appl Surf Sci. 2001;176:636–642. [Google Scholar]
- 68.Baur C, Gazen B C, Koel B, Ramachandran T R, Requicha A A G, Zini L. J Vacuum Sci Technol B. 1997;15:1577–1580. [Google Scholar]
- 69.Ramachandran T R, Baur C, Bugacov A, Madhukar A, Koel B E, Requicha A, Gazen C. Nanotechnology. 1998;9:237–245. [Google Scholar]
- 70.Hang S H, Mirkin C A. Science. 2000;288:1808–1811. doi: 10.1126/science.288.5472.1808. [DOI] [PubMed] [Google Scholar]
- 71.Hong S H, Zhu J, Mirkin C A. Science. 1999;286:523–525. doi: 10.1126/science.286.5439.523. [DOI] [PubMed] [Google Scholar]
- 72.Binnig G, Rohrer H. IBM J Res Dev. 2000;44:279–293. [Google Scholar]
- 73.Despont M, Brugger J, Drechsler U, Durig U, Haberle W, Lutwyche M, Rothuizen H, Stutz R, Widmer R, Binnig G, et al. Sensors Actuators A. 2000;80:100–107. [Google Scholar]