Abstract
Background
Controlled clinical trials investigating ongoing questions about extracorporeal membrane oxygenation (ECMO) for patients with the acute respiratory distress syndrome (ARDS), including what the optimal mechanical ventilation (MV) tidal volume (TV) strategies are and whether ECMO potentiates injurious host responses, are difficult. We therefore conducted a systematic literature search and review to characterize studies investigating ECMO in adult animal lung injury models and to determine whether they inform these questions.
Methods
A systematic literature search with relevant search terms was conducted of four data bases through 2/2/24.
Results
Forty-five studies met inclusion criteria, and most parameters examined were represented similarly in studies with (n = 24) or without (n = 21) severe ARDS PaO2/FiO2s levels (≤ 100 mmHg or > 100 mmHg). Overall, while only 11 studies were published from 1971 to 2005, 5, 8, and 11 were published in subsequent 5-year periods up to 2020 and then 10 through 2/2/24 (Figure 1). Most studies investigated pig or sheep models (n = 32), but since 2016, six studies employed rat models. Eighteen studies administered lung lavage alone or with another lung injury challenge (17 with PaO2/FiO2s ≤ 100) and 9 used oleic acid. Although seven studies administered lipopolysaccharide, very different from clinical ARDS only one used a bacterial and none a viral challenge. Thirty-two studies employed V-V ECMO. The most frequent duration of ECMO investigated was 24 h in 16 studies but only 2 studies investigated longer periods (48 and 96 h). Differences in study questions, methodologies and outcome measures precluded formal meta-analysis. However, overall in studies that compared mechanical ventilation alone (MV) to ECMO groups or that compared differing ECMO groups: in 5 studies ECMO supported tidal volume reductions that approached apneic levels in 2; all but 1 of 10 studies indicated that ECMO with or without TV reductions either did not increase or reduced lung injury measures; 2 studies did while 4 did not find that ECMO aggravated molecular or cellular markers of inflammation; and only 2 studies examined host thrombotic responses with ECMO.
Conclusion
Animal models to date have addressed important questions facing ECMO use for ARDS, but ones more closely simulating ARDS in patients appear warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40635-025-00781-5.
Keywords: ECMO, ARDS, Lung injury, Animal, Preclinical, Systematic review
Background
Extracorporeal membrane oxygenation (ECMO) is increasingly employed in adults with progressive but potentially reversible acute respiratory distress syndrome (ARDS) [1–3]. Veno-venous ECMO (V-V ECMO), the most common form, as well as veno-arterial (V-AECMO) and arterial-venous ECMO (A-V ECMO), provide oxygenation and ventilatory support and may allow reductions in the potentially injurious higher tidal volumes and airway pressures related to mechanical ventilation alone [4, 5]. ECMO is most frequently applied for infection-related ARDS, and was used extensively during the SARS-CoV-2 pandemic for periods ranging from several days to weeks in COVID-19 patients [6–9]. Although its clinical application is increasing, questions remain about ECMO use [10–12]. While ECMO can support gas exchange, the best practices for mechanical ventilation (MV) such as tidal volume management are unclear [13–15]. There are also concerns that complications related to ECMO instrumentation, such as stimulated host immune and thrombotic responses, might aggravate underlying lung injury [10, 16–19]. Research is ongoing directed at these and other questions.
Animal models of ARDS and acute lung injury (ALI) contributed to the development of ECMO support for respiratory failure and continue to be used to understand and improve ECMO application [20, 21]. Preclinical ECMO research for ALI is evolving. For example, while a prior systematic review included only large animal models, several recent studies have investigated ECMO with ALI in rat models [20–22].
Therefore, to inform preclinical ECMO research going forward, we conducted a more current systematic review of studies investigating ECMO in adult animal models of ARDS and ALI. Because ECMO is typically administered to patients diagnosed with severe ARDS, retrieved studies were grouped for review based on whether the model of lung injury employed produced PaO2/FiO2 levels ≤ 100 or > 100. We first examined characteristics of the overall group of retrieved studies and categorized studies based on the design and types of comparisons made within studies. We then reviewed the questions asked, measures conducted, and conclusions made from each study. Our primary goal was to examine the research presently available from preclinical studies addressing with controlled study designs, the questions noted above regarding management of mechanical ventilation with ECMO and ECMO’s effects on lung injury and host inflammatory or thrombotic responses.
Methods
This systematic review was prepared using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement on guidance for literature review and data extraction [Supplemental File-A (SupFile-A)]. It was registered with the International Prospective Register of Systematic Reviews on December 20, 2022 (PROSPERO-2022-CRD42023773983).
Literature search and study inclusion
Using search terms and strategies listed in SupFile-B, three authors (M.A., P.Q.E., P.T.P.) identified relevant studies published in the following databases from inception through February 2, 2024: PubMed, EMBASE, Web of Science and Biosis. These terms were modified from a previously published search of Pubmed and EMBASE [21]. Recovered reports were reviewed for additional references. Published studies that investigated either a veno-venous (V-V), veno-arterial (V-A) or arterio-venous (A-V) ECMO system established percutaneously in adult animals administered a challenge that would produce direct or indirect lung injury, were selected for further review. Non-English publications were excluded.
Data extraction and organization of studies
Two investigators (M.A., P.Q.E) independently extracted data from reports using tables with formats comparable to those presented in this report. These data included: country and year of publication; species, strain, age and weight of animals; type, dose, route, timing and targeted level of the lung injury challenge or the level of hypoxemia resulting from the challenge; ECMO system, mode, duration, equipment, settings, ventilatory support, and anticoagulation employed; type and method of mechanical ventilatory support provided; and type of anesthetic agents and hemodynamic support used. Also extracted were the experimental groups compared, questions addressed, measures obtained, and a brief summary of the authors’ conclusions.
Studies were organized into two groups based on whether the level of lung injury targeted or produced resulted in severe ARDS PaO2/FiO2 levels (≤ 100 mmHg) or not (> 100 mmHg). Studies were further categorized based on the groups that were investigated and compared including: a mechanical ventilation alone (MV) versus a single ECMO group; an MV versus two different ECMO groups; two or more ECMO groups, all with lung injury; a single group with serial measures; or two ECMO groups, one with and one without lung injury.
To identify those studies employing concurrent controlled designs, either ECMO vs. MV groups or two of more ECMO groups, that investigated questions described in the introduction, the following criteria were employed. Studies investigating MV strategies for ECMO compared the effects of differing TVs, airway pressures (e.g., positive end expiratory pressures) or other MV maneuvers in MV vs ECMO groups or ECMO vs ECMO groups. Studies investigating the effects of ECMO on lung injury compared histologic lung injury measures (e.g., lung injury scores), wet-to-dry lung weight, or lung water measures in MV vs. ECMO groups. Studies investigating the effects of ECMO on host inflammatory or thrombotic responses compared inflammatory cytokines, other molecular markers of inflammation or inflammatory cell populations or measures of coagulation and thrombosis in MV vs. ECMO groups.
Data synthesis and analysis
Relevant study information was summarized and presented in figures and tables where appropriate. Based on differences in the models and methods employed and endpoints investigated across studies, meta-analysis was not included in this systematic review.
Results
The literature search retrieved 3221 reports. After exclusion of 1394 duplicates and 1689 others based on review of titles and abstract, 138 reports underwent full paper review, of which 45 reports were found to meet inclusion criteria [15–17, 22–63] (Supplemental [21]) Figure 1 (Sup Fig. S1).
Fig. 1.
Flow diagram for the literature search
Study characteristics
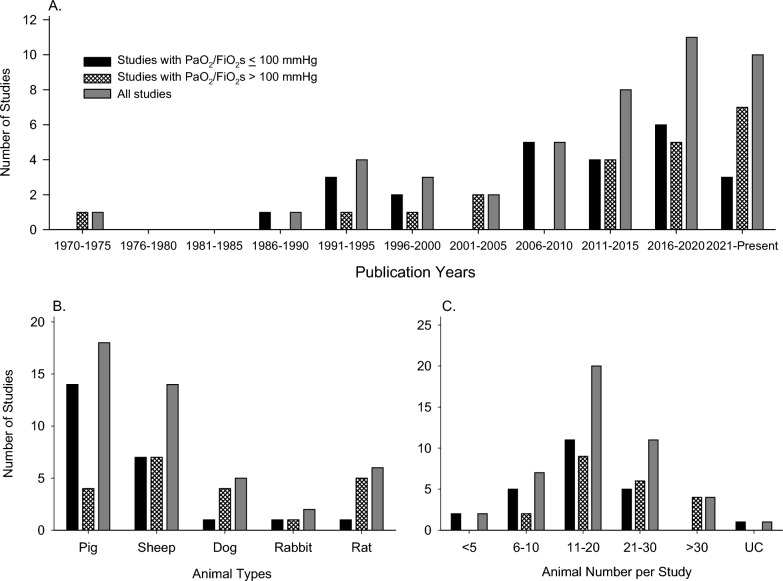
Study characteristics are summarized in Table 1A and B for studies grouped based on PaO2/FiO2 levels targeted or reported and the groups compared as described in the methods. The overall number of included reports steadily increased over time, with 11 published from 1971 to 2005, but then 5, 8, and 11 published in each subsequent 5-year period up to 2020 and 10 through 2/2/24 (Figure 1). Whether studies targeted or reported PaO2/FiO2 levels ≤ 100 mmHg (n = 24 studies) vs. > 100 mmHg (n = 21 studies) was variable over the different time periods. While most studies investigated either pig or sheep large animal models (n = 18 and 14, respectively), since 2016, 6 studies employed rat models including one with PaO2/FiO2 levels ≤ 100 mmHg. Most studies (n = 31) included between 11 and 30 animals. No study examining PaO2/FiO2 levels ≤ 100 mmHg employed > 30 animals.
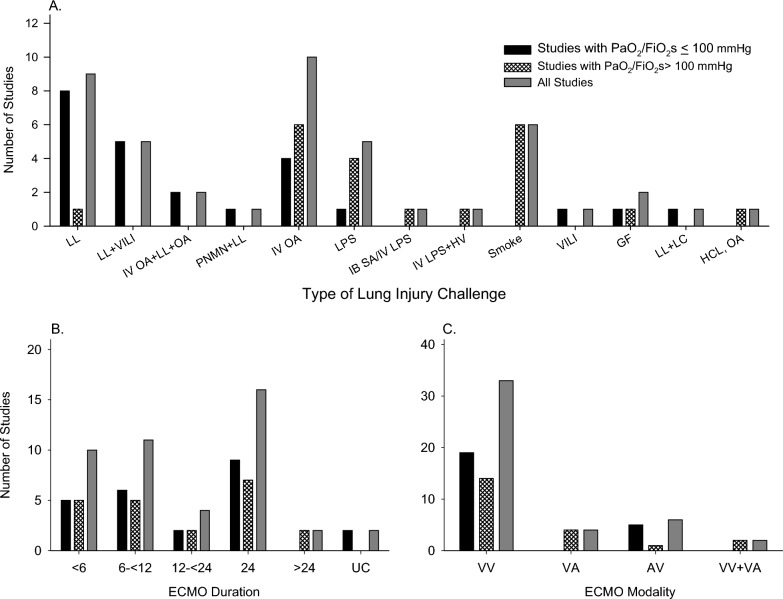
Over all studies, lung lavage alone and intravenous oleic acid alone were the most common types of lung injury challenges employed (9 and 11, respectively) but lung lavage was also used in combination with other challenges in 9 studies (Table 1, Fig. 2). While seven studies employed a lipopolysaccharide (LPS) lung injury challenge, only one employed a bacterial challenge (intrabronchial S. aureus) and this was in combination with LPS. No study used a virus challenge. While 16 of the studies with PaO2/FiO2s ≤ 100 mmHg employed lung lavage alone or combined with another challenge, only one study with PaO2/FiO2s > 100 mmHg used this challenge type. About equal numbers of studies grouped by PaO2/FiO2 level employed oleic acid but the only study with a bacteria challenge, most with LPS challenges, and all with smoke challenges targeted or resulted in PaO2/FiO2s > 100 mmHg (see Fig. 3).
Table 1.
A and B Summary of study characteristics
| Author | Publication | ECMO | Lung injury type |
Animal | |||
|---|---|---|---|---|---|---|---|
| Year | Country | Mode | Time (h) | Type | Total number |
||
| A. Studies with PaO2/FiO2 ≤ 100 | |||||||
| Studies that compared an MV alone group with an ECMO group | |||||||
| Plotz | 1993 | Netherlands | V-V | 7 | LL | Rabbit | 12 |
| German | 1996 | Austria, US | V-V | 6 | IV OA | Sheep | 30 |
| Iglesias | 2008 | Spain | A-V | 12 | PNMN-LL | Pig | 15 |
| Araos | 2016 | Chile | V-V | 24 | LL + VILI | Pig | 18 |
| Huang | 2022 | China | V-V | 4 | IV OA | Rat | UC |
| Studies that compared an MV alone group to two different ECMO groups | |||||||
| Yanos | 1990 | US | V-V | 6 | IV OA | Dog | 18 |
| Johannes | 2014 | Austria, Germany | A-V | 24 | LL | Sheep | 24 |
| Pilarczyk | 2015 | Germany | V-V | 8 | LL | Pig | 14 |
| Studies that compared differing ECMO groups all with lung injury | |||||||
| Hirschl | 1995 | US | V-V | 4 | LL + IV OA | Sheep | 12 |
| Hirschl | 1996 | US | V-V | 4 | LL + IV OA | Sheep | 10 |
| Kopp | 2010 | Canada, Germany | V-V | 24 | LL | Pig | 24 |
| Kopp | 2012 | Canada, Germany | V-V | 24 | LL | Pig | 24 |
| Araos | 2019 | Chile | V-V | 24 | LL + VILI | Pig | 24 |
| Dubo | 2020 | Chile | V-V | 24 | LL + VILI | Pig | 12 |
| Millar | 2020 | Australia, UK, US | V-V | 24 | IB LPS | Sheep | 14 |
| Qaqish | 2020 | Canada | V-V | 6 | GF | Pig | 9 |
| Araos | 2021 | Chile | V-V | 24 | LL + VILI | Pig | 20 |
| Studies with a single group and serial measures | |||||||
| Booke | 1995 | Germany | V-V | UC | VILI | Sheep | 7 |
| Brederlau | 2006 | Germany | A-V | 2.7 | LL | Pig | 15 |
| Zick | 2006 | Germany | A-V | UC | LL | Pigs | 7 |
| Muellenbach | 2009 | Germany | A-V | 8 | LL | Pig | 8 |
| Langer | 2014 | Italy, US | V-V | 12/16 | IV OA | Sheep | 11 |
| Mendes | 2022 | Brazil | V-V | 5 | LL + LC | Pig | 5 |
| Andresen | 2018 | Chile, China | V-V | 24 | LL + VILI | Pig | 5 |
| B Studies with studies with PaO2/FiO2 > 100 | |||||||
| Studies that compared an MV alone group with an ECMO group | |||||||
| Zwischenberger | 1993 | US | V-A/V-V | 96 | Smoke | Sheep | 29 |
| Hayes | 2015 | Australia | V-V | 2/24 | Smoke | Sheep | 43 |
| MacDonald | 2015 | Australia | V-V | 24 | Smoke | Sheep | 40 |
| Du | 2016 | China | V-V | 24 | IP LPS | Rat | 40 |
| Passmore | 2016 | Australia | V-V | 24 | Smoke | Sheep | 27 |
| Passmore | 2017 | Australia | V-V | 24 | Smoke | Sheep | 27 |
| Stenlo | 2021 | Sweden, US | V-A | 7 | LPS | Pig | 21 |
| Kayumov | 2022 | Australia, Korea, SG | V-A | 3 | LPS | Rat | 80 |
| Lim | 2020 | South Korea | V-V | 12 | IB SA/IV LPS | Pig | 8 |
| Brusatori* | 2023 | Italy, Germany, UK, US | V-V | 24 | HCL, OA | Pig | 24 |
| Studies that compared an MV alone group to two different ECMO groups | |||||||
| Zhang | 2021 | China | V-V | 9 | IV OA | Dog | 30 |
| Studies that compared differing ECMO groups all with lung injury | |||||||
| Trittenwein | 1999 | Austria, US | V-V/ V-A | 1 | IV LPS + HV | Rabbit | 18 |
| Kim | 2004 | South Korea | V-A | 2 | IV OA | Dog | 16 |
| Prat | 2015 | France, Italy, US | V-V | 10 | IV OA | Sheep | 9 |
| 2021 | China | V-A | 24 | IT/IV LPS | Rat | 15 | |
| Zhang | 2022 | China | V-V | 2 | IV OA | Rat | 15 |
| Study with differing ECMO groups with lung injury and also a single group with serial measures | |||||||
| LeFrack† | 1973 | US | V-V | 8 | GF | Dog | 14 |
| Studies with a single group and serial measures | |||||||
| Li | 2021 | China | V-V | 3 | IV OA | Rat | 12 |
| Ju | 2018 | China | A-V | 48 | IV OA | Dog | 12 |
| Studies that compared ECMO groups with and without lung injury | |||||||
| Dembinski | 2003 | Germany | V-V | 6 | LL | Pig | 12 |
| Shekar | 2015 | Australia | V-V | 12 | Smoke | Sheep | 20 |
A-V, arterio-venous; ECLA, extracorporeal lung assist; GF, gastric fluid; gps, groups; HCL, hydrochloric acid; HV, hypoventilation; IB, intrabronchial; IP, intraperitoneal; IT, intratracheal; IV, intravenous; LA, linoleic acid; LC, lung collapse; LL, lung lavage; OA, oleic acid; PNMN, pneumonectomy; Pub., publication; SG, Singapore; UK, United Kingdom; US, United States; V-V, veno-venous; SA, S. aureus; UC, unclear; VILI, ventilator induced lung injury
*Two experiments with different lung injury challenge types
†Two experiments each addressing a different comparison and questions
Fig. 2.
This figure shows the number of studies with PaO2/FiO2s ≤ 100 mmHg or PaO2/FiO2s > 100 mmHg and overall examining extracorporeal membrane oxygenation (ECMO) in adult animal lung injury models included in this systematic review that; were published during five year periods beginning in 1970 (A); employed either pig, sheep, dog, rabbit or rat models (B); and employed either ≤ 5, 6 to 10, 11 to 20, 21 to 30, > 30 or an unclear number of animals (C)
Fig. 3.
This figure shows the number of studies with PaO2/FiO2s ≤ 100 mmHg or PaO2/FiO2s > 100 mmHg and overall examining extracorporeal membrane oxygenation (ECMO) in adult animal acute lung injury models and included in this systematic review that; employed the different types of lung injury challenges reported on (A); investigated either veno-venous (V-V), veno-arterial (V-A), arterial-venous (AV), or both V-V and V-A ECMO systems; and investigated ECMO over < 6 h, 6 to < 12 h, 12 to < 24 h, 24 h, > 24 h or for an unclear period (C)
The ECMO modalities investigated included V-V, V-A or A-V ECMO in 32, 8, and 3 studies, respectively, and 2 examined both V-V and V-A systems. V-V ECMO was employed about equally in studies that targeted or achieved PaO2/FiO2 levels ≤ vs. > 100 mmHg. Over all studies, the most frequent duration of ECMO investigated was 24 h in 16 reports. These durations were also about equal comparing studies with PaO2/FiO2 levels ≤ vs. > 100 mmHg. However, two reports, both with PaO2/FiO2s > 100 mmHg, studied periods longer than 24 h (48 and 96 h) (see Fig. 3).
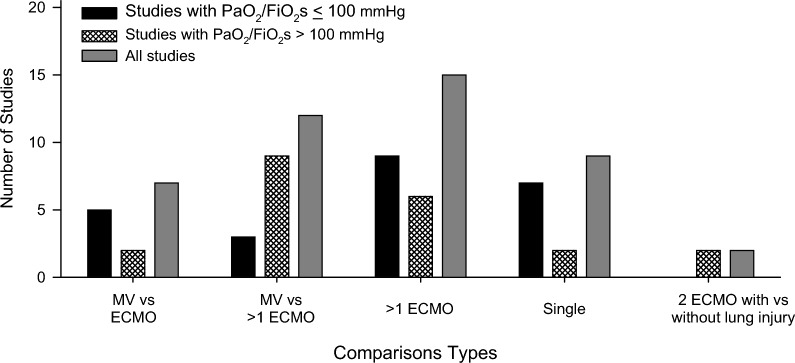
Studies made the following comparisons: 15 studies, 7 with PaO2/FiO2s ≤ 100, compared an MV alone group to an ECMO group; 4 studies, 3 with PaO2/FiO2s ≤ 100, compared an MV alone group to two ECMO groups; 14 studies, 9 with PaO2/FiO2s ≤ 100, compared 2 or more ECMO groups; 9 studies, 7 with PaO2/FiO2s ≤ 100, included single groups with serial measures; and 2 studies with PaO2/FiO2s > 100, compared 2 ECMO groups, 1 group with and 1 without lung injury (see Fig. 4). One study with PaO2/FiO2s > 100, included two experiments, one with a single group and serial measures and one with three ECMO groups [42]. Another study with PaO2/FiO2s > 100, also included two experiments, one employing a hydrochloric acid challenge and one using oleic acid [28]. Of note, seven of the studies comparing an MV group to an ECMO group with lung injury, all with PaO2/FiO2s > 100 mmHg, also included MV and ECMO groups that had not received lung injury challenges [16, 17, 30, 45, 49, 62, 63].
Fig. 4.
This figure shows the number of studies with PaO2/FiO2s ≤ 100 mmHg or PaO2/FiO2s > 100 mmHg and overall examining extracorporeal membrane oxygenation (ECMO) in adult animal lung injury models and included in this systematic review the numbers of studies that compared: a group with mechanical ventilation alone (MV) to a single group with ECMO (MV vs ECMO); an MV group alone to two ECMO groups (MV vs > 1 ECMO); more than one ECMO group (> 1 ECMO); a single group with serial measures (Single); and two ECMO groups, one group with and one group without lung injury (2 ECMO with vs without lung injury)
Additional details regarding lung injury challenges, anesthesia and hemodynamic support, and the ECMO employed in reviewed reports are provided in SupTables 1, 2, and 3. Of note, studies employing smoke challenges typically targeted carboxyhemoglobin and not oxygenation levels (SupTable 2). Because six rat models examining ECMO with lung injury were either not included in a prior review of ECMO in animal lung injury models (one study) or have been published since that review and because this type of small animal model may be of use by a wider number of investigator groups, we highlight methods in these studies in rats in Table 2.
Table 2.
Summary of the lung injury, anesthesia and ECMO methodologies in studies employing rat models
| Author | Year | Total N | Mode | Config | Time (h) | Lung injury type | Pump | Oxygenator | Blood Flow (mL/min) | Sweep Gas | Membrane Gas O₂% | Anesthesia (Ind.) | Anesthesia (Maint.) | ET | Comparisons |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Du | 2016 | 40 | V-V | NA | 24 | IP LPS | NR | NR | NR | NR | NR | NR | Iso | NR | MV vs ECMO |
| Li | 2021 | 12 | V-V | RJV–RJV | 3 | IV OA | PreFluid Peristaltic | Xijing | 80–90 | 80–100 | 0.90, Titrate to PaO₂ | Sev | – | – | Single gp/serial measures |
| 2021 | 15 | V-A | RIJ–RCA | 24 | IT/IV LPS | Kewei | Kewei | 150 | 200 | 1 | Sumianxin, Zoletil | Sumianxin, Zoletil | ET | Differing ECMO gps | |
| Huang | 2022 | UC | V-V | RJV–RJV | 4 | IV OA | PreFluid Peristaltic | Xijing | 80–90 | 80–100 | 0.90, Titrate to PaO₂ | Sevo | Sevo | ET | MV vs ECMO |
| Kayumov | 2022 | 80 | V-A | REJV–LFA | 3 | LPS | Watson-Marlow Pumps | Micro-1 | 50 | NR | NR | Ket, Xylazine | Isoflurane | ET | MV vs ECMO |
| Zhang | 2022 | 15 | V-V | RJV–RJV | 2 | IV OA | PreFluid Peristaltic | Xijing | 80–90 | 80–100 | 0.9 | Pen | Pro or Sevo | ET | Differing ECMO gps |
ALI, acute lung injury; ET, endotracheal tube; IP, intraperitoneal; IV, intravenous; IT, intratracheal; LPS lipopolysaccharide; OA, oleic acid; NR, not reported; PFP, PreFluid peristaltic pump; RCA, right carotid artery; REJV, right external jugular vein; RIJ, right internal jugular vein; RJV, right jugular vein; VA, veno-arterial; VV, veno-venous; Xijing, Kewei, Micro-1, commercial oxygenators; Iso, isoflurane; Sev, sevoflurane; Pen, pentobarbital; Pro, propofol; Ket, ketamine
Study questions, measures and conclusions
Study questions, measures and conclusions are briefly summarized in Tables 3 and 4 for studies targeting or producing PaO2/FiO2 levels ≤ 100 mmHg and > 100 mmHg, respectively. In each table, studies are organized based on the types of comparisons made (see methods). Both groups of studies asked and reported a range of questions and conclusions, respectively. We were most interested in determining whether studies with concurrent controlled study designs had examined questions noted in the introduction regarding the management of MV with ECMO, and the effects of ECMO on lung injury and host inflammatory and thrombotic responses.
Table 3.
Summary of study questions, measures and conclusions for studies with lung injury targeting or resulting in PaO2/FiO2s ≤ 100mmHg
| Author (y) | Question | Measures* | Conclusions† |
|---|---|---|---|
| Studies that compared an MV alone group with an ECMO group‡ | |||
| Plotz (1993) | Compared surfactant with positive pressure-controlled ventilation vs. with V-V ECMO with sighs | Weaning success, GasEx§, lung mechanics§, ventilator efficiency | Large volume surfactant instillation was possible with V-V ECMO and sighs |
| German (1996) | Compare effects of MV with NO vs with V-V ECMO on Hemos, GasEx and lung lymph drainage during | Hemos§, GasEx, lung lymph flow and protein concentration | NO reduced PVR and increased oxygenation and lung lymph flow; ECMO improved oxygenation more and removed CO2 |
| Iglesias (2008) | Compare conventional MV with 10-12mL/kg or 6mL/kg vs A-V ECMO with near-static ventilation | Weaning success, Hemos, GasEx, lung mechanics, LIS, lung IL-6, IL-8, TNFα, surfactant | A-V ECMO and near-static ventilation improved outcomes versus conventional MV |
| Araos (2016) | Compare the effects of V-V ECMO to MV alone | Survival, Hemos, GasEx, lung mechanics, LIS, W/D | ECMO rescued an MV refractory lung injury model and will allow testing of other V-V ECMO interventions |
| Huang (2022) | Investigate if V-V ECMO is protective and if Hippo/YAP signaling contributes to that protection | Oxygenation, LIS, W/D, AT2 cells, BAL and lung tissue IL-6, IL-8 and TNFα, Hippo/YAP signaling markers | V-V ECMO stimulated Hippo/YAP signaling aided recovery of injured alveolar epithelium |
| Studies that compared an MV alone group to two different ECMO groups | |||
| Yanos (1990) | Investigate whether V-V ECMO supported hypopnea would reduce lung edema | Hemos, GasEx, lung mechanics, W/D, lung H2O | V-V ECMO supported hypopnea decreased Paw and PAP and increased PaO2 but didn’t alter lung water and increased venous admixture |
| Johannes (2014) | Compare effects of reducing TV to 3mL/kg or apneic ventilation with A-V ECMO vs. MV with 6mL/kg alone | Hemos, GasEx, lung mechanics, regional LIS | A-V ECMO allowed TV decreases to 0mL and reduced upper lung hyperinflation but increased lower lung inflammation |
| Pilarczyk (2015) | Compare conventional V-V ECMO to a miniaturized oxygenator with rotary pump | Hemos, GasEx, CBC, LFTs, free Hb, pump characteristics | Miniaturized system supported Hemos and GasEx but increased hemolysis |
| Studies that compared differing ECMO groups | |||
| Hirschl (1995) | Investigate if liquid vs gas ventilation improves lung function during support with V-V ECMO | Hemos, GasEx, lung mechanics, histology | Liquid ventilation improved GasEx, lung compliance, and lung injury and inflammation |
| Hirschl (1996) | Investigate if total followed by partial liquid ventilation vs. gas ventilation improves lung function supported with V-V ECMO | Hemos, GasEx, lung mechanics, histology | Total followed by partial liquid ventilation improved GasEx, lung injury and inflammation but only total liquid ventilation improved compliance |
| Kopp (2009) | Investigate if a low resistance oxygenator without blood pump or an oxygenator with a miniaturized pump improves hemocompatibility vs. conventional V-V ECMO | Coagulation studies (ACT, PTT, PT, TAT, Fibrinogen, PLTs), thromboelastography, CBC, serum IL-8 and TNF, EM of filters | Both systems were hemocompatible but absence of a blood pump did not increase this |
| Kopp (2012) | Compare Hemos and GasEx with miniaturized V-V ECMO vs. A-V ECMO system | Hemos, GasEx and pump characteristics | Both systems facilitated LPV but A-V ECMO limited oxygenation and CO2 exchange and increased cardiac work |
| Araos (2019) | Compare nonprotective (10mL/kg), protective (6mL/kg) or near-apneic MV with V-V ECMO | Hemos, GasEx, lung mechanics, LIS, W/D, myofibroblast protein markers | ECMO supported near-apneic MV and reduced lung injury on histology and myofibroblast marker expression |
| Dubo (2020) | Investigate if low spontaneous breathing efforts worsens lung injury vs. controlled near-apneic ventilation during V-V ECMO | Hemos, GasEx, lung mechanics, vasopressors, fluid balance, lactate, LIS, W/D, lung cytokines | Low intensity spontaneous breathing with high RRs and low TVs did not worsen lung injury versus near-apneic controlled ventilation |
| Millar (2020) | Investigate safety and efficacy of mesenchymal stromal cell (MSC) administration with V-V ECMO | Hemos, GasEx, lung mechanics, LIS, W/D, BAL protein, ECMO characteristics | MSC diminished oxygenator function and did not improve oxygenation but reduced lung injury and inflammation on histology |
| Qaqish (2020) | Establish lung injury with V-V ECMO model with and without surfactant | Hemos, GasEx, lung mechanics, LIS, W/D, plasma and BAL inflammatory cytokines, BAL bile acid | A lung injury with V-V ECMO model developed and surfactant was tolerated but did not alter function |
| Araos (2021) | Compare PEEP 0, 10 and 20 levels during near-apneic ventilation with V-V ECMO | Hemos, GasEx, lung mechanics, LIS, W/D | V-V ECMO with PEEP = 10 limited lung injury, benefited GasEx, and did not worsen Hemos |
| Studies with a single group and serial measures | |||
| Booke (1995) | Investigate if percutaneous V-V ECMO can provide lung support | Hemo, GasEx | V-V ECMO maintained gas exchange even in paralyzed animals after ALI |
| Brederlau (2006) | Investigate A-V ECLA’s contribution to gas exchange with different gas flows | Hemo, GasEx | A-V ECLA removed CO2 but oxygenation was reduced during severe hypoxia by increased shunt fraction |
| Zick (2006) |
Test oxygenation with a pumpless interventional lung assist device (ILA) |
GasEx, oxygenator blood flow | ILA significantly increased oxygenation but the effect was small |
| Muellenbach (2009) | Investigate TV reductions with A-V ECMO and an open lung approach | Hemos, GasEx, lung mechanics | A-V ECMO and an open lung approach provided CO2 removal with TVs of 0-2mL/kg and maintained oxygenation increases |
| Langer (2014) | Investigate V-V ECMO during spontaneous ventilation with 6 different gas flows, before and then after lung injury | GasEx, lung mechanics, lung CT scans, plasma IL-1b, TNF, IL-8, IL-10 | V-V ECMO can control spontaneous ventilation in healthy sheep and ones with ARDS |
| Andresen (2018) | Investigate meropenem PKs and a rapid response PK biosensor during V-V ECMO supported | Meropenem PK measures | Biosensor provided reliable meropenem PK data which ECMO did not alter |
| Mendes (2022) | Investigate V-V ECMO effects on lung perfusion and Hemos during one-sided lung ventilation and lung collapse and lavage | Hemo, GasEx, lactate, Hb | ECMO decreased PAP and may have increased shunt, but did not alter lung perfusion distribution with varying V/Q mismatches |
ACT, activated thromboplastin time; ALI, acute lung injury; AT2, alveolar type 2 cells; AV, arterio-venous; BAL, bronchoalveolar lavage; BUN, blood urea nitrogen; CBC, complete blood count; CO2, carbon dioxide; CT, computerized tomography; ECLA, extracorporeal lung assist; ECMO, extracorporeal membrane oxygenations; EM, electron micrography; FiO2, fractional inspired oxygen concentration; GasEx, gas exchange; GP, group; H2O, water; Hb, hemoglobin; Hemos, hemodynamics; IL, interleukin; LFTs, liver function tests; LIS, lung injury score on histology; LPV, lung protective ventilation; MV, mechanical ventilation; NO, inhaled nitric oxide; O2, oxygen; PaO2, arterial oxygen pressure; PAP, pulmonary artery pressure; Paw, airways pressure; PEEP, positive end expiratory pressure; PK, pharmacokinetics; PLT -platelet; PTT, partial thromboplastin time; PT, prothrombin time; PVR, pulmonary vascular resistance; Rx, treatment; TAT, thrombin–antithrombin complexes; TNF, tumor necrosis factor; TV, tidal volume; V-A, veno-arterial; V/Q, ventilation/perfusion; V-V, veno-venous; W/D, wet to dry lung ratio;
*Listed measures may not include all those reported in a study
‡†Summarized from reports’ findings or conclusions
‡ALI is employed whether a study designated the model as an ALI or acute respiratory distress syndrome one
§depending on the study hemodynamics included systemic and/or pulmonary vascular measures, gas exchange included measures of oxygenation and/or carbon dioxide removal and lung mechanics included static lung compliance and/or airway pressures
Table 4.
Summary of study questions, measures and conclusions for studies with lung injury targeting or resulting in PaO2/FiO2s > 100mmHg
| Author (y) | Question | Measures* | Conclusions† |
|---|---|---|---|
| Studies that compared an MV alone group to an ECMO group‡ | |||
| Zwischenberger (1993) | Investigate effects of S-ALI treated with V-A/V-V ECMO on blood inflammatory mediators and GasEx | Hemos§, GasEx§, CBC, plasma thromboxane B2, conjugated dienes, lung histology, W/D | ECMO exacerbated the inflammatory response to S-ALI and may have impaired GasEx |
| Hayes (2015) | Investigate V-V ECMO and hyperoxia effects on PLT dysfunction | Hemos, GasEx, ADP and collagen-induced PLT aggregation, Ca2+ | ECMO did not alter PLT activity, but hyperoxia with ECMO may have desensitized ADP-dependent PLT activation |
| MacDonald (2015) | Investigate S-ALI, V-V ECMO and RBC Rx effects on oxidative stress and plasma selenium levels | Hemos, GasEx, LFTs, COHb%, plasma TBARS, GTX and selenium activity | S-ALI increased TBARS; Adding ECMO to S-ALI reduced selenium more than S-ALI and ECMO alone |
| Du (2016) | Investigate V-V ECMO effects during LPS induced lung injury | Hemos, GasEx, histology, W/D, lung stress kinases | ECMO aided oxygenation and cardiac function |
| Passmore (2016) | Investigate S-ALI and ECMO effects alone and together on lung and blood inflammatory cells, cytokines and tissue remodeling | GasEx, lung mechanics§, fluid balance, lung inflammatory cells, LIS, W/D, BAL protein and cells, plasma IL-1b, IL-6 and IL-8, HCT, lung MMP2 and 9 | Addition of ECMO augments the inflammatory response in a host with pre-existing lung injury |
| Passmore (2017) | Investigate S-ALI and ECMO effects alone and together on parameters of hemostasis | CBC, coagulation and PLT function tests, pump flow | ECMO stimulated collagen-induced PLT aggregation and reduced factor VIII, vWF and fibrinogen levels, changes augmented with ECMO and lung injury together |
| Stenlo (2021) | Investigate whether a particle flow rate test (PFR) tracks lung injury during V-AECMO | Hemos, GasEx, lung mechanics, Hb, plasma and BAL IL-6, LIS, W/D, proteomics, PFR | A PFR test tracked lung injury |
| Kayumov (2022) | Investigated cardiac left ventricular performance and oxygen consumption with ECMO in LPS challenged rats | Left ventricular performance measures | ECMO in LPS challenged rats resulted in higher myocardial oxygen consumption |
| Lim (2020) | Compared ultralow 3mL/kg TVs with V-V ECMO to conventional MV with 15mL/kg TVs | Hemos, GasEx, BAL cytokines, lung ultrasound, LIS, W/D | V-V ECMO with low TVs was possible and protected against VILI |
| Brusatori (2023) | Compare V-V ECMO, ECCO2R and MV alone on Hemo, GasEx, lung mechanics with HCL or OA induced lung injury | Hemo, GasEx, lung mechanics, oxygen consumption | Compared to MV, ECMO had better effects on Hemo, GasEx and oxygen consumption than ECCO2R |
| Studies that compared an MV alone group to two different ECMO groups | |||
| Zhang (2021) | Investigate whether CRRT reduces inflammatory markers during lung injury and V-V ECMO |
Hemos, GasEx, Hb, plasma IL-6, TNFa (MV + ALI vs ECMO + ALI vs ECMO + Rx + ALI) |
V-V ECMO and CRRT removed inflammatory mediators and improved oxygenation and hypercapnia |
| Studies that compared differing ECMO groups | |||
| LeFrack (1973)¶ | Establish a V-V ECMO supported gastric fluid lung injury model and compare Travenol, Peirce GE, Lande-Ed oxygenators |
Hemos, GasEx, lung mechanics (Single gp/serial measures) |
Travenol oxygenator delivered most O2 based on membrane surface area, Pierce GE had highest O2 transfer irrespective of area |
| Trittenwein (1999) | Compare if an oxygenator delivering FiO2 = 1.0 with V-V vs V-AECMO would increase lipid peroxidation and lung injury after LPS priming and hypoxia | Hemos, GasEx, lung tissue and plasma MDA levels | After hypoxia, ECMO with FiO2 = 1.0 in LPS challenged animals increased oxidative lipid damage, an effect dependent on PaO2 with V-V ECMO |
| Kim (2004) | Investigate whether a pressure relieving compliance chamber (PRCC) reduces RBC injury in a pulsatile V-AECMO system | Hemos, GasEx, plasma free Hb, CBC, LFTs, BUN | PRCC with a pulsatile pump decreased RBC trauma but not as well as a non-pulsatile pump alone; the pulsatile pump improved oxygenation |
| Prat (2015) | Compare one initial low dose of heparin vs a standard regimen, both with miniaturized V-V ECMO | CBC, coagulation factors, thromboelastography, PLT aggregometry and P-selectin, TAT, plasmin/antiplasmin complexes | Single low dose heparin bolus maintained anticoagulation for 10h |
| Xing (2021) | Investigate YTHDF1 KOmac effects on immunity during LPS challenge and V-AECMO | GasEx, inflammatory markers, flow cytometry, western blot, RNA methylation, brain histopathology | In V-AECMO supported septic rats, YTHDF1 KOmacs benefitted immune paralysis and brain injury |
| Zhang (2022) | Compare the effects of sevoflurane vs. propofol anesthesia with V-V ECMO on oxygenation and inflammatory injury | Hemos, GasEx; TNFa and IL-1b in BAL, serum and lung tissue; BAL protein, lung myeloperoxidase, LIS, W/D | Compared to propofol, sevoflurane with lung injury and ECMO improved oxygenation and reduced inflammatory markers |
| Studies with a single group and serial measures | |||
| Li (2021) | Establish a rat model of V-V ECMO and lung injury | Hemos, GasEx, HCT, Hb, lytes, LIS, W/D, BAL protein | A V-V ECMO rat model of lung injury was established |
| Ju (2018) | Investigate correct by-pass flow for A-V ECLA and A-V ECLA’s effects on GasEx and inflammatory cytokines | Hemos, GasEx, serum cytokines, lactate and urine output | A-V ECLA provided GasEx without excessive inflammation or tissue hypoperfusion |
| Studies that compared ECMO groups with and without lung injury | |||
| Dembinski (2003) | Investigate the safety and efficacy of V-V ECMO with heparin anticoagulation during normal and injured lung function | Hemos, GasEx, ECMO pump dynamics, CBC, fibrinogen, macroscopic examination of the pump and oxygenator for visible clots | During lung injury, V-V ECMO with low dose heparin provided stable Hemos, adequate GasEx, and no major blood damage or external clot formation |
| Shekar (2015) | Investigated whether several antibiotic properties predict antibiotic behavior with V-V ECMO | Hemos, GasEx, PK data for 8 different antibiotics | With ECMO, lipophilic antibiotics increased Vss and decreased CL while protein bound antibiotics decreased Vss and CL |
ADP -adenosine diphosphate; ALI, acute lung injury; AV, arterio-venous; BAL, bronchoalveolar lavage; BUN, blood urea nitrogen; CBC, complete blood count; Cl, clearance; CRRT, continuous renal replacement therapy; ECCO2R, extracorporeal CO2 removal; ECLA, extracorporeal lung assist; ECMO, extracorporeal membrane oxygenations; FiO2, fractional inspired oxygen concentration; GasEx, gas exchange; GP, group; GTX, glutathione peroxidase; Hb, hemoglobin; HCL, hydrochloric acid; HCT, hematocrit; Hemos, hemodynamics; IL, interleukin; KOmac, knock out macs; LFTs, liver function tests; LIS, lung injury score on histology; LPS, lipopolysaccharide; Lytes, serum electrolytes; MDA, malondialdehyde; MMP, matrix metalloproteinase; MV, mechanical ventilation; O2, oxygen; OA, oleic acid; PaO2, arterial oxygen pressure; PK, pharmacokinetics; PLT -platelet; RBC, red blood cell; S-ALI, smoke induced ALI; TAT, thrombin–antithrombin complexes; TBAR, thiobarbituric; TNF, tumor necrosis factor; TV, tidal volume; V-A, veno-arterial; VILI, ventilator induced lung injury; Vss, steady state distribution volume; VV, veno-venous; vWF, von Willebrand factor; W/D, wet to dry lung ratio;
*Listed measures may not include all those reported in a study
†Summarized from reports’ findings or conclusions
‡ALI is employed whether a study designated the model as an ALI or acute respiratory distress syndrome one
§Depending on the study hemodynamics included systemic and/or pulmonary vascular measures, gas exchange included measures of oxygenation and/or carbon dioxide removal and lung mechanics included static lung compliance and/or airway pressures
¶This study included two sets of experiments, one with a single group and serial measures and another with differing ECMO groups all with ALI
In concurrent controlled studies comparing ECMO to MV groups, three with PaO2/FiO2s ≤ 100 mmHg examined the effects of lowering TVs with ECMO [35, 36, 58], while one study with PaO2/FiO2s > 100 mmHg study investigated this question [44]. ECMO supported TV reductions in these studies, although one reported increased lower lung inflammation with the maneuver [36]. In two studies by one group comparing different ECMO groups and with PaO2/FiO2s ≤ 100, ECMO supported reductions in TV in one [15] while one concluded that an intermediate PEEP level of 10 cmH2O would be optimal with ECMO [25]. Also, in studies comparing different ECMO groups with PaO2/FiO2s ≤ 100 mmHg, one reported large volume surfactant instillation was possible with ECMO [51] and two determined liquid vs. gas ventilation improved ECMO application, but these were both very short-term studies (7 h and 3 h, respectively) [33, 34].
Whether it was a primary question of study or not, four studies with PaO2/FiO2s ≤ 100 mmHg reported the effects of ECMO compared to MV alone on lung injury scores and/or lung wet/dry weight ratios or lung water measures [22, 24, 35, 58], while six studies with PaO2/FiO2s > 100 mmHg provided such data [17, 28, 30, 44, 55, 62] (Table 3). ECMO was either associated with no significant effects or with reductions in these measures in all but one study which reported increases in W/D after 96 h of ECMO [62]. One study with PaO2/FiO2s ≤ 100 mmHg which compared differing ECMO groups, reported that decreasing TV levels with ECMO produced progressive decreases in global LISs but not W/Ds [15]. Another study by this group which also compared differing ECMO groups but with differing PEEP levels, reported that PEEPs of 10 cmH2O reduced global LISs and PEEPs of 10 and 20 cmH2O reduced W/Ds [25]. In two studies comparing ECMO and MV groups, survival appeared improved in the ECMO vs. MV group [24, 28].
In studies comparing ECMO and MV groups, two with PaO2/FiO2s ≤ 100 [22, 35] and five with PaO2/FiO2s > 100 mmHg [17, 30, 44, 55, 59, 62] provided BAL, lung and/or plasma IL-1b, IL-6, IL-8 and/or TNFa levels or other molecular or cellular markers of inflammation. In six of these studies providing an overall assessment of ECMO’s effects, in four ECMO appeared to have beneficial ones [22, 35, 44, 45] and in two it was potentially harmful [17, 62]. Only two studies comparing ECMO and MV groups and both with PaO2/FiO2s > 100 mmHg, reported on the possible effects of ECMO on parameters of coagulation and thrombosis [16, 49]. In one, increased oxygen levels with ECMO may have desensitized ADP associated platelet activation [16] and in the other ECMO with smoke associated lung injury may have augmented platelet activation and reduced factor VIII, Von Willebrand factor and fibrinogen levels [49].
Discussion
This systematic review of studies investigating ECMO in adult animal lung injury models included 45 reports, which was 28 more than the last systematic review in this area of research we are aware of [21]. While the increased number of overall studies in the present review may relate in part to our use of four rather than two databases, the pace of preclinical research examining ECMO for ALI is increasing. For example, different from the prior review which presented no rodent studies, the present one included six rat models, five of which were published in 2021 or later. Consistent with clinical ECMO use, most studies employed V-V ECMO. However, very different from what’s encountered clinically, only one study employed ECMO in an infectious disease model of pneumonia which was bacterial. Despite ECMO’s application in both the earlier H1N1 influenza and frequent use in the recent SARS-CoV-2 pandemics, no animal model investigated ECMO with a viral challenge. Also, very different from clinical use, only two studies employed ECMO for more than 24 h. Most studies either compared MV with one or more than one ECMO group (n = 19) or compared differing ECMO groups (n = 15) and these comparison types were similar between studies with PaO2/FiO2s ≤ 100 vs > 100 mmHg.
A notable difference between studies with models that targeted or produced PaO2/FiO2s ≤ 100 mmHg vs. > 100 mmHg was the type of lung injury challenge administered. Most of the former employed lung lavage alone or in combination with an additional challenge whereas most of the latter employed smoke or LPS challenges. About equal numbers of the two study types employed oleic acid challenges. These differences may reflect the potency of the different challenges. However, studies with smoke challenge typically targeted carboxyhemoglobin levels, which may not have produced the same level of injury and hypoxemia as either lung lavage or oleic acid. Also, LPS challenge sufficient to produce PaO2/FiO2s ≤ 100 mmHg may result in unacceptable hemodynamic instability, especially when used in combination with anesthesia.
While differences among study designs, models and endpoints prevented meta-analysis, limited numbers of controlled animal studies reviewed here did address questions facing the clinical application of ECMO. Several investigated and supported reductions in tidal volumes used with MV during ECMO and one examined how PEEP might be managed with ECMO [25]. Except for one 96 h study, ECMO did not appear to aggravate histologic and lung water measures and may, with or without TV reductions, have reduced injury in some studies [17, 23, 26, 30, 32, 37, 46, 57, 60, 64]. Similarly, markers of host inflammatory responses were not increased with ECMO in most but not all studies examining these [17, 23, 37, 46, 47, 64]. There were only two controlled studies examining how ECMO might alter potentially associated with thrombotic responses [29, 52].
Despite differing methodologies, the studies reviewed here all addressed questions important for the application of ECMO in the setting of lung injury. However, the limited duration of ECMO administration in these animal studies and the absence of infectious disease as a source of lung injury, limits their interpretation in the context of clinical ECMO use for ARDS. Longer periods of ECMO exposure in combination with either a bacterial or viral source of infection associated lung injury might provide further insights into the questions we examined in studies. Such studies would best combine strategies designed to limit potential injury related to accompanying MV, one goal of ECMO use clinically. Longer term models and the use of infectious challenges appear possible. Notably, one of the studies comparing ECMO and MV groups analyzed here, supported sheep for 96 h, a period approaching ones encountered in patients [62]. Also, a 96 h model of sedated and mechanically ventilated canines infected with S. aureus pneumonia has been employed extensively in the study of the treatment and support of sepsis [64–66]. However, such large animal models requiring continuous intensive care unit type support are resource intensive and not possible for most laboratories. But given the increasing clinical use of ECMO for ARDS and difficulty of conducting controlled ECMO clinical studies examining key questions like the optimal ventilatory management of ECMO patients and its net effects on lung injury measures, it may be appropriate for governmental health agencies to fund such research in longer term large animal models.
Our review does indicate that ECMO is being increasingly investigated in rat ALI models which are potentially less resource intensive than large animal ones [22, 30, 30, 43, 57, 63]. While the sensitivity of these rodent models to the depressive hemodynamic effects of sedation and analgesia may preclude longer term study, these models do provide the tools to examine ongoing questions such as the effect of ECMO systems on host inflammatory and thrombotic responses [67–69]. Rodent studies would be facilitated by the wider range of reagents available for biologic study in these species. However, among the studies cited here, there is considerable methodological heterogeneity in how models were constructed, including variation in circuit configuration (V–V vs. V–A), cannulation strategies, oxygenator design, flow parameters, and anesthetic regimens. These differences could shape important physiologic and immunologic responses, complicating cross-study interpretation and limiting reproducibility. Anesthetics employed such as isoflurane, ketamine/xylazine, or sevoflurane differ in their hemodynamic effects and influence on immune responses. Likewise, the most common lung injury models—lipopolysaccharide and oleic acid—activate differing inflammatory pathways and differ in both severity and relevance to human disease. Despite infection being the most frequent clinical trigger for ECMO, none of the included rat studies employed a live bacterial challenge. Future ECMO studies in rats should incorporate Gram-positive or Gram-negative bacterial models to better reflect clinical conditions and explore how pathogen-driven inflammation interacts with the extracorporeal circuit. Such bacterial pneumonia models have been widely used to investigate other aspects of inflammatory lung injury [70, 71]. The rat is also susceptible to strains of beta-coronavirus that can be studied in biosafety level-2 laboratories [72]. Since SARS-CoV-2 continues to cause clinical infection and has the potential to mutate into a more resistant form, studies of ECMO in rat beta-coronavirus models should be considered. The small size of the rat model allows study with larger numbers of groups and samples and would facilitate multifactorial study designs comparing the individual and combined effects of pulmonary infection and ECMO.
Several limitations of this study include the following. Non-English reports were not reviewed, but even without those, the study included 45 studies overall. None of the findings from studies comparing ECMO and MV groups or differing ECMO groups could be blinded ones. This is a similar problem that clinical trials contend with. This review did not include studies conducted in neonatal and infant lung injury models. Finally, as noted, the differences in the models employed, limited numbers of studies addressing questions now facing clinical application of ECMO for ARDS, and the lack of standardized reporting among studies precluded meta-analysis.
In conclusion, animal lung injury models made an important contribution to the development of ECMO to support patients with ARDS. Animal models will continue to be important for the development and refinement of ECMO system performance. Data from existing animal studies provide important insights into ongoing questions about the degree to which ECMO can support reductions in TVs and airways pressures and about the host’s inflammatory and thrombotic responses to ECMO. However, among animal studies going forward, ones addressing these questions in models more closely simulating common sources of lung injury and the durations of ECMO encountered clinically appear warranted.
Supplementary Information
Acknowledgements
The NIH had no role in the design of the study or the collection, analysis, and interpretation of the data. The opinions expressed in this manuscript reflect those of the authors and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or of the United States Government.
Take-home message: This systematic review provides insights into how animal models might be designed to inform questions presently facing the clinical use of ECMO for ARDS.
Author contributions
PTP and MA conceived and designed the study with contributions from JS and PQE. NT conducted the initial search and contributed to the manuscript. PTP, MA and PQE reviewed search results and MA, MJ, YL and PQE extracted data. PTP, JS, MA, and PQE wrote and edited the manuscript. All authors reviewed and approved the final version of this manuscript for submission.
Funding
Intra-mural funding from the National Institutes of Health (NIH) supported this work.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brodie D, Slutsky AS, Combes A (2019) Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA 322(6):557–568 [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C et al (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975 [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Peek GJ, Hajage D, Hardy P, Abrams D, Schmidt M et al (2020) ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 46(11):2048–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E (2019) Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 7(2):163–172 [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369(22):2126–2136 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D et al (2020) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med 8(11):1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G et al (2021) Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal life support organization registry. Lancet Lond Engl 398(10307):1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS et al (2021) Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care Lond Engl 25(1):211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Extracorporeal Life Support Organization. Registry dashboard of ECMO-supported COVID-19 patient data. https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx. Accessed 30 Nov 2021.
- 10.Munshi L, Brodie D, Fan E (2022) Extracorporeal support for acute respiratory distress syndrome in adults. NEJM Evid. 1(10):EVIDra2200128 [DOI] [PubMed] [Google Scholar]
- 11.MacLaren G, Fisher D, Brodie D (2022) Treating the most critically ill patients with COVID-19: the evolving role of extracorporeal membrane oxygenation. JAMA 327(1):31–32 [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S et al (2014) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 190(5):488–496 [DOI] [PubMed] [Google Scholar]
- 13.Assouline B, Combes A, Schmidt M (2023) Setting and monitoring of mechanical ventilation during venovenous ECMO. Crit Care 27(1):95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Minnen O, Jolink FEJ, van den Bergh WM, Droogh JM, Oude Lansink-Hartgring A, Dutch ECLS Study Group (2024) International survey on mechanical ventilation during extracorporeal membrane oxygenation. ASAIO J Am Soc Artif Intern Organs. 70(4):300–304 [Google Scholar]
- 15.Araos J, Alegria L, Soto D, Garcia P, Garcia A, Dubo S et al (2018) How much airway pressure to apply while resting the lungs in severe ARDS supported with ECMO? Intensive Care Med Exp 6:807 [Google Scholar]
- 16.Hayes RA, Foley S, Shekar K, Diab S, Dunster KR, McDonald C et al (2015) Ovine platelet function is unaffected by extracorporeal membrane oxygenation within the first 24 h. Blood Coagul Fibrinolysis 26(7):816–822 [DOI] [PubMed] [Google Scholar]
- 17.Passmore MR, Fung YL, Simonova G, Foley SR, Dunster KR, Diab SD et al (2016) Inflammation and lung injury in an ovine model of extracorporeal membrane oxygenation support. Am J Physiol Lung Cell Mol Physiol 311(6):L1202-l1212 [DOI] [PubMed] [Google Scholar]
- 18.Patroniti N, Bonatti G, Senussi T, Robba C (2018) Mechanical ventilation and respiratory monitoring during extracorporeal membrane oxygenation for respiratory support. Ann Transl Med 6(19):386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF (2016) The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care Lond Engl 20(1):387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayumov M, Habimana R, Kim D, Obiweluozor FO, Jeong IS, Cho HJ (2023) Extracorporeal circulation models in small animals: beyond the limits of preclinical research. Acute Crit Care 38(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar JE, Bartnikowski N, von Bahr V, Malfertheiner MV, Obonyo NG, Belliato M et al (2019) Extracorporeal membrane oxygenation (ECMO) and the acute respiratory distress syndrome (ARDS): a systematic review of pre-clinical models. Intensive Care Med Exp 7(1):18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Zhang R, Zhai K, Li J, Yao M, Wei S et al (2022) Venovenous extracorporeal membrane oxygenation promotes alveolar epithelial recovery by activating Hippo/YAP signaling after lung injury. J Heart Lung Transpl 41(10):1391–1400 [DOI] [PubMed] [Google Scholar]
- 23.Andresen M, Araos J, Wong KY, Leung YC, So LY, Wong WT et al (2018) Evaluation of meropenem pharmacokinetics in an experimental acute respiratory distress syndrome (ARDS) model during extracorporeal membrane oxygenation (ECMO) by using a PenP beta-lactamase biosensor. Sensors 18(5):1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araos J, Alegria L, Garcia P, Damiani F, Tapia P, Soto D et al (2016) Extracorporeal membrane oxygenation improves survival in a novel 24-hour pig model of severe acute respiratory distress syndrome. Am J Transl Res 8(6):2826–2837 [PMC free article] [PubMed] [Google Scholar]
- 25.Araos J, Alegria L, Garcia A, Cruces P, Soto D, Erranz B et al (2021) Effect of positive end-expiratory pressure on lung injury and haemodynamics during experimental acute respiratory distress syndrome treated with extracorporeal membrane oxygenation and near-apnoeic ventilation. Br J Anaesth 127(5):807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booke M, Packheiser J, Witt T, Rohe G, Kappius P, Soeparwata R et al (1995) Veno-venous ECMO allows complete gas exchange in ovine ARDS. Appl Cardiopulm Pathophysiol 5(3):161–167 [Google Scholar]
- 27.Brederlau J, Muellenbach R, Kredel M, Schwemmer U, Anetseder M, Greim C et al (2006) The contribution of arterio-venous extracorporeal lung assist to gas exchange in a porcine model of lavage-induced acute lung injury. Perfusion 21(5):277–284 [DOI] [PubMed] [Google Scholar]
- 28.Brusatori S, Zinnato C, Busana M, Romitti F, Gattarello S, Palumbo MM et al (2023) High-versus low-flow extracorporeal respiratory support in experimental hypoxemic acute lung injury. Am J Respir Crit Care Med 207(9):1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dembinski R, Kopp R, Henzler D, Hochhausen N, Oslender N, Max M et al (2003) Extracorporeal gas exchange with the DeltaStream rotary blood pump in experimental lung injury. Artif Organs 27(6):530–536 [DOI] [PubMed] [Google Scholar]
- 30.Du QR, Shen Y, Yu J, Huang SP, Pan SM (2016) Extracorporeal membrane oxygenation (EMCO) is an optimal method to cure the pneumonia caused by endotoxin in mice. Int J Clin Exp Pathol 9(10):10796–10802 [Google Scholar]
- 31.Dubo S, Oviedo V, Garcia A, Alegria L, Garcia P, Valenzuela ED et al (2020) Low spontaneous breathing effort during extracorporeal membrane oxygenation in a porcine model of severe acute respiratory distress syndrome. Anesthesiology 133(5):1106–1117 [DOI] [PubMed] [Google Scholar]
- 32.Germann P, Balassa A, Roeder G, Kaider A, Schlag G, Zimpfer M et al (1997) Effects of inhaled nitric oxide and extracorporeal membrane oxygenation on pulmonary hemodynamics and lymph flow in oleic acid lung injury in sheep. Crit Care Med 25(11):1881–1887 [DOI] [PubMed] [Google Scholar]
- 33.Hirschl RB, Parent A, Tooley R, McCracken M, Johnson K, Shaffer TH et al (1995) Liquid ventilation improves pulmonary-function, gas-exchange, and lung injury in a model of respiratory-failure. Ann Surg 221(1):79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirschl RB, Tooley R, Parent A, Johnson K, Bartlett RH (1996) Evaluation of gas exchange, pulmonary compliance, and lung injury during total and partial liquid ventilation in the acute respiratory distress syndrome. Crit Care Med 24(6):1001–1008 [DOI] [PubMed] [Google Scholar]
- 35.Iglesias M, Jungebluth P, Petit C, Matute MP, Rovira I, Martínez E et al (2008) Extracorporeal lung membrane provides better lung protection than conventional treatment for severe postpneumonectomy noncardiogenic acute respiratory distress syndrome. J Thorac Cardiovasc Surg 135(6):1362–1371 [DOI] [PubMed] [Google Scholar]
- 36.Johannes A, Kredel M, Zollhoefer B, Schlegel N, Von Kirschbaum C, Brederlau J et al (2014) Influence of apneic oxygenation and minimal tidal volumes on ventilator-associated lung injury. Minerva Anestesiol 80(5):526–536 [PubMed] [Google Scholar]
- 37.Ju ZH, Ma JH, Wang C, Yu J, Qiao YR, Hei FL (2018) Effects of pumpless extracorporeal lung assist on hemodynamics, gas exchange and inflammatory cascade response during experimental lung injury. Exp Ther Med 15(2):1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TS, Sun K, Lee KB, Lee HW, Baek KJ, Park SY et al (2004) Application of a pressure-relieving air compliance chamber in a single-pulsatile extracorporeal life support system: an experimental study. Artif Organs 28(12):1106–1109 [DOI] [PubMed] [Google Scholar]
- 39.Kopp R, Bensberg R, Henzler D, Niewels A, Randerath S, Rossaint R et al (2010) Hemocompatibility of a miniaturized extracorporeal membrane oxygenation and a pumpless interventional lung assist in experimental lung injury. Artif Organs 34(1):13–21 [DOI] [PubMed] [Google Scholar]
- 40.Kopp R, Bensberg R, Wardeh M, Rossaint R, Kuhlen R, Henzler D (2012) Pumpless arterio-venous extracorporeal lung assist compared with veno-venous extracorporeal membrane oxygenation during experimental lung injury. Br J Anaesth 108(5):745–753 [DOI] [PubMed] [Google Scholar]
- 41.Langer T, Vecchi V, Belenkiy SM, Cannon JW, Chung KK, Cancio LC et al (2014) Extracorporeal gas exchange and spontaneous breathing for the treatment of acute respiratory distress syndrome: an alternative to mechanical ventilation? Crit Care Med 42(3):E211–E220 [DOI] [PubMed] [Google Scholar]
- 42.Lefrak EA, Stevens PM, Nicotra MB (1973) An experimental model for evaluating extracorporeal membrane oxygenator support in acute respiratory failure. Am Surg 39(1):20–30 [PubMed] [Google Scholar]
- 43.Li YN, Huang J, Zhang RZ, Wang SX, Cheng XD, Zhang PB et al (2023) Establishment of a venovenous extracorporeal membrane oxygenation in a rat model of acute respiratory distress syndrome. Perfusion 38:85 [DOI] [PubMed] [Google Scholar]
- 44.Lim SY, Cho YJ, Kim DJ, Kim JS, Jheon S, Chung JH et al (2020) Effects of ultralow-tidal-volume ventilation under veno-venous extracorporeal membrane oxygenation in a porcine model with ventilator-induced lung injury. Membranes 10(12):379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald CI, Fung YL, Shekar K, Diab SD, Dunster KR, Passmore MR et al (2015) The impact of acute lung injury, ECMO and transfusion on oxidative stress and plasma selenium levels in an ovine model. J Trace Elem Med Biol 30:4–10 [DOI] [PubMed] [Google Scholar]
- 46.Mendes PV, Park M, de Azevedo LCP, Morais CCA, Amato MBP, Costa ELV (2022) Lung perfusion during veno-venous extracorporeal membrane oxygenation in a model of hypoxemic respiratory failure. Intensive Care Med Exp 10(1):15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Millar JE, Bartnikowski N, Passmore MR, Obonyo NG, Malfertheiner MV, von Bahr V et al (2020) Combined mesenchymal stromal cell therapy and extracorporeal membrane oxygenation in acute respiratory distress syndrome. A randomized controlled trial in sheep. Am J Respir Crit Care Med 202(3):383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muellenbach RM, Kredel M, Kuestermann J, Klingelhoefer M, Schuster F, Wunder C et al (2009) Combining “open-lung” ventilation and arteriovenous extracorporeal lung assist: Influence of different tidal volumes on gas exchange in experimental lung failure. Med Sci Monit 15(8):213–220 [PubMed] [Google Scholar]
- 49.Passmore MR, Fung YL, Simonova G, Foley SR, Diab SD, Dunster KR et al (2017) Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit Care 21(1):191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilarczyk K, Heckmann J, Lyskawa K, Strauß A, Haake N, Wiese I et al (2016) Comparison of a new miniaturized extracorporeal membrane oxygenation system with integrated rotary blood pump to a standard system in a porcine model of acute lung injury. Artif Organs 40(7):645–658 [DOI] [PubMed] [Google Scholar]
- 51.Plotz FB, Mook PH, Heikamp A, Brus F, Okken A, Oetomo SB et al (1993) Large volume instillation of surfactant during extracorporeal life support improves lung function in lung lavaged rabbits. Asaio J 39(3):M470–M474 [DOI] [PubMed] [Google Scholar]
- 52.Prat NJ, Meyer AD, Langer T, Montgomery RK, Parida BK, Batchinsky AI et al (2015) Low-dose heparin anticoagulation during extracorporeal life support for acute respiratory distress syndrome in conscious sheep. Shock 44(6):560–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qaqish R, Watanabe Y, Galasso M, Summers C, Ali AA, Takahashi M et al (2020) Veno-venous ECMO as a platform to evaluate lung lavage and surfactant replacement therapy in an animal model of severe ARDS. Intensive Care Med Exp 8(1):63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL et al (2015) Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care 19:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stenlo M, Silva IAN, Hyllén S, Bölükbas DA, Niroomand A, Grins E et al (2021) Monitoring lung injury with particle flow rate in LPS- and COVID-19-induced ARDS. Physiol Rep 9(13):e14802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trittenwein G, Rotta AT, Gunnarsson B, Steinhorn DM (1999) Lipid peroxidation during initiation of extracorporeal membrane oxygenation after hypoxia in endotoxemic rabbits. Perfusion 14(1):49–57 [DOI] [PubMed] [Google Scholar]
- 57.Xing Y, Cheng DL, Shi CS, Shen ZQ (2021) The protective role of YTHDF1-knock down macrophages on the immune paralysis of severe sepsis rats with ECMO. Microvasc Res 137:104178 [DOI] [PubMed] [Google Scholar]
- 58.Yanos J, Presberg K, Crawford G, Meller J, Wood LD, Sznajder JI (1990) The effect of hypopnea on low-pressure pulmonary edema. Am Rev Respir Dis 142(2):316–320 [DOI] [PubMed] [Google Scholar]
- 59.Zhang RZ, Zhai KR, Huang J, Wei SL, Yang JB, Zhang YC et al (2024) Sevoflurane alleviates lung injury and inflammatory response compared with propofol in a rat model of VV ECMO. Perfusion. 10.1177/02676591221131217 [DOI] [PubMed] [Google Scholar]
- 60.Zhang K, Ma M, Pan H, Chen Y, Hou D, Yin H (2021) Effect of CRRT combined with low-flow ECMO on canines with ARDS and hypercapnia. J Artif Organs 24(3):336–342 [DOI] [PubMed] [Google Scholar]
- 61.Zick G, Frerichs I, Schädler D, Schmitz G, Pulletz S, Cavus E et al (2006) Oxygenation effect of interventional lung assist in a lavage model of acute lung injury: a prospective experimental study. Crit Care 10(2):R56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zwischenberger JB, Cox CS, Minifee PK, Traber DL, Traber LD, Flynn JT et al (1993) Pathophysiology of ovine smoke-inhalation injury treated with extracorporeal membrane-oxygenation. Chest 103(5):1582–1586 [DOI] [PubMed] [Google Scholar]
- 63.Kayumov M, Kim D, Raman S, MacLaren G, Jeong IS, Cho HJ (2022) Combined effects of sepsis and extracorporeal membrane oxygenation on left ventricular performance in a murine model. Sci Rep 12(1):22181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon SB, Minneci PC, Deans KJ, Feng J, Eichacker PQ, Banks SM et al (2009) Effects of intra-aortic balloon counterpulsation in a model of septic shock. Crit Care Med 37(1):7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweeney DA, Cui X, Solomon SB, Vitberg DA, Migone TS, Scher D et al (2010) Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis 202(12):1885–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minneci PC, Deans KJ, Hansen B, Parent C, Romines C, Gonzales DA et al (2007) A canine model of septic shock: balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol 293(4):H2487-2500 [DOI] [PubMed] [Google Scholar]
- 67.Karzai W, von Specht BU, Parent C, Haberstroh J, Wollersen K, Natanson C et al (1999) G-CSF during Escherichiacoli versus Staphylococcusaureus pneumonia in rats has fundamentally different and opposite effects. Am J Respir Crit Care Med 159(5 Pt 1):1377–1382 [DOI] [PubMed] [Google Scholar]
- 68.Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y et al (2004) Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol 286(4):R699-709 [DOI] [PubMed] [Google Scholar]
- 69.Qiu P, Li Y, Ding Y, Weng J, Banks SM, Kern S et al (2011) The individual survival benefits of tumor necrosis factor soluble receptor and fluid administration are not additive in a rat sepsis model. Intensive Care Med 37(10):1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haley M, Parent C, Cui X, Kalil A, Fitz Y, Correa-Araujo R et al (2005) Neutrophil inhibition with L-selectin-directed MAb improves or worsens survival dependent on the route but not severity of infection in a rat sepsis model. J Appl Physiol Bethesda Md. 98(6):2155–2162 [DOI] [PubMed] [Google Scholar]
- 71.Solomon SB, Cui X, Gerstenberger E, Danner RL, Fitz Y, Banks SM et al (2006) Effective dosing of lipid A analogue E5564 in rats depends on the timing of treatment and the route of Escherichiacoli infection. J Infect Dis 193(5):634–644 [DOI] [PubMed] [Google Scholar]
- 72.Singh A, Singh RS, Sarma P, Batra G, Joshi R, Kaur H et al (2020) A comprehensive review of animal models for coronaviruses: SARS-CoV-2, SARS-CoV, and MERS-CoV. Virol Sin 35(3):290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.