Abstract
Linear porphyrin arrays self-assembled by either hydrogen bonding or metal ion coordination self-organize into lipid bilayer membranes. The length of the transmembrane assemblies is determined both by the thermodynamics of the intermolecular interactions in the supermolecule and by the dimension and physical chemical properties of the bilayer. Thus, the size of the porphyrin assembly can self-adjust to the thickness of the bilayer. An aqueous electron acceptor is placed on one side of the membrane and an electron donor is placed on the opposite side. When illuminated with white light, substantial photocurrents are observed. Only the assembled structures give rise to the photocurrent, as no current is observed from any of the component molecules. The fabrication of this photogated molecular electronic conductor from simple molecular components exploits several levels of self-assembly and self-organization.
There has been a rapid development in the synthesis and characterization of both inorganic and organic nanoscaled materials where much of the synthesis exploits various self-assembly processes and much of the characterization relies on scanning-probe microscopies (1–6). The excellent work on the self-assembly of a large variety of supramolecular systems (7–9) that have potential photonic, magnetic, catalytic, and analytical properties has been well matched by the development of physical methods to characterize these systems on the nanometer scale (1). The potential usefulness and functionality of these materials and systems has largely rested on proof of principle experiments, such as those using scanning-probe tips to construct and evaluate the device. In general, however, the methods to incorporate these nanoscaled self-assembled materials or objects into devices on a production (economically feasible) scale have significantly lagged behind. It has been recognized, albeit a burgeoning area of materials research, that these self-assembled systems will have to subsequently, or concomitantly, self-organize into hierarchical structures that may also form the interconnects to the macroscopic world (1, 3, 9). For many of these devices based on organic or hybrid inorganic-organic compounds, in analogy to biological systems, the primary, secondary, tertiary, and quaternary levels of structural organization will need to be controlled. The present work utilizes self-assembled porphyrin arrays as the functional components of self-organizing photogated conductors.
The primary structure in this case is the molecule—and we have exquisite control and understanding of how to synthesize, characterize, and predict molecular properties. The secondary structure is how these molecules self-assemble into larger, often times discrete, structures on the scale of less than a few nanometers. Again, this is a mature field tracing its modern origins to the work of Pedersen, Cram, and Lehn over 25 years ago (2, 8). The tertiary structure, then, is how the self-assembled supermolecules self-organize into entities that are many tens to hundreds of nanometers in at least one direction (1, 3, 9). Lastly, the quaternary structure involves the architecture of the self-organized systems in the actual device or material (1, 3, 9).
Reported here is the design of porphyrin molecules (primary structure) that self-assemble into supramolecular entities (secondary structure), which are then self-organized into a liquid crystalline matrix (tertiary structure), and form a functioning device (quaternary structure). Specifically, lipid bilayers are used as a means to self-organize the self-assembled porphyrin arrays in terms of both size and orientation. The ≈1 mm across and ≈8-nm-thick disk-shaped device is connected to the macroscopic world by using standard laboratory electrodes and a solution of 0.1 M KCl. The objective is to show that self-assembled systems can indeed be incorporated into hierarchical systems and function as devices, and also that connecting to these devices does not have to entail nanoscaled electrodes a priori. This work combines our studies on synthetic supramolecular chemistry and on the potential of using self-organizing systems such as bilayers to construct devices.
Experimental Procedures
The experimental design for the photoconductance measurements is described elsewhere (10–16) but is summarized here. Lipid bilayers are formed from a solution containing diphytanoylphosphatidyl choline (a methyl branched saturated lipid), dodecane (10% vol/vol), and the components of the self-assembled porphyrin arrays (≈3 mM) all in chloroform. They are formed across a 1-mm hole in a 0.2-mm-thick Teflon partition dividing two 3.5-ml compartments containing 0.1 M KCl in a 10 mM (pH 7.1) phosphate buffer (for cell design, see supporting information, which is published on the PNAS web site, www.pnas.org). The ≈8-nm width is calculated from the membrane capacitance (17). Anthraquinonesulfate (0.5–1 mM) and 1–4 mM Fe(II) complex are placed on opposite sides of the membrane. The solutions on both sides of the membrane are gently stirred by using magnetic stirring bars. Because the saturated calomel electrodes on each side of the partition are nonpolarizing, they can be used to monitor conduction for tens of minutes. A homemade operational amplifier (capable of clamping the voltage when desired) measures the current. A glass slide covers an aperture to allow light to impinge directly on the bilayer. The entire system is enclosed in a copper Faraday cage resting on a vibration-damping table (both homemade). The porphyrin synthesis and characterization of both linear and square arrays are also described elsewhere (18–21).
Design Features and Principles
The incorporation of porphyrins into bilayer lipid membranes (BLM) to study interfacial electron transfer reactions to aqueous quinones eventually led to the formation of a molecular electronic device that functioned as a photogated transistor (10–12). This self-organizing device used the interfacial photo-induced electron transfer reaction to form a lattice of porphyrin cations on each side of the BLM and an applied voltage to gate a current of 102- to 104-fold more lipophilic ions across the BLM than formed by the photochemical event. A Grotthus-type mechanism was proposed to be the basis of conduction across the bilayer, wherein the initial charge transfer led to the spontaneous formation of an ion chain of alternating lipophilic porphyrin cations and lipophilic anions that spans the bilayer (11, 12). Later devices (13, 14) used other charge carriers such as C60 and protonophores, which led to a device that pumped protons across the membrane (15, 16). The substantial changes in the electrostatics of the bilayer itself were also proposed to play a significant role in the conduction mechanism (22). An example of an alternative approach by Gust and coworkers (23) uses multifunctional molecules wherein the electron donor, transporter, and acceptor are covalently linked. When some of these triads are incorporated into vesicle membranes, along with protonophores and the ATP synthase proteins, the photosynthetic generation of ATP is observed (23). The present work bridges the gap between the above two systems in many respects. The self-assembly of the electron donor-linker acceptor is much more efficient than the former electrostatic assembly, because the functional (conducting) molecules are preassembled before the device is turned on. Self-assembly also overcomes the difficult organic synthesis (and low yields) of the latter triad molecular approach (24). The systems described below are composed of small molecules that can be produced in large quantities, and because self-assembly and self-organization are exploited, the large-scale production of devices based on these concepts are economically feasible.
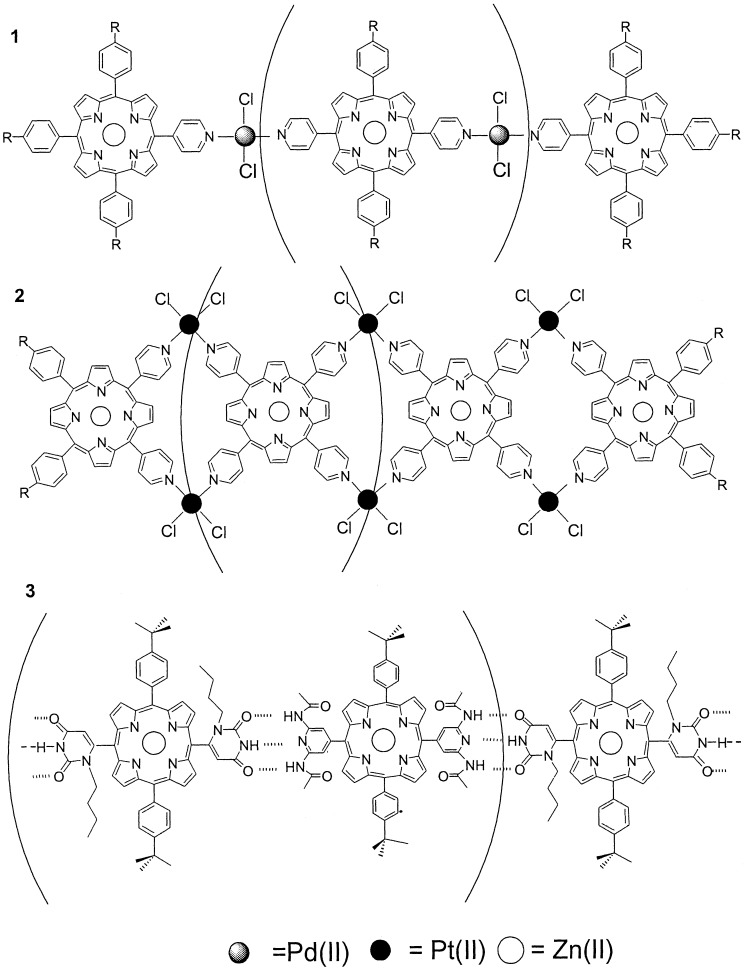
In general, the self-assembly of discrete porphyrin arrays can be accomplished by hydrogen bonding (18, 19, 25, 26) or metal ion coordination (20, 21, 25, 26) in organic solvents. Three linear tapes and one system (18) that may be linear, irregular, or circular are used in the photogated conducting devices described below. Closed-shell Zn(II) metalloporphyrins are used to eliminate oxidative and reductive degradation of the system and to make the electron transfer reaction more efficient. In principle, as observed for other systems, the use of the much more labile Mg(II) porphyrins should increase the electron transfer efficiency by increasing the singlet excited-state lifetime (27). It is well understood that the length of linear self-assembled systems is dictated by the thermodynamics of the intermolecular interactions (2, 7, 8, 19), but in this case the length is also governed by the width of the bilayer, vide infra. The combination of a set of two porphyrins in a 2:2 ratio (one bearing two pyridyl groups at 180° and one bearing a single pyridyl group) with three trans palladium units forms a population of linear systems with substantial rotational and conformational flexibility (Fig. 1, assembly 1) (21). The rotational and conformational flexibility is substantially less in a similar system based on pyridyl moieties coordinating to a cis platinum complex (Fig. 1, assembly 2) (21). In addition to the geometry of labile coordination sites, the metal ion linker should not have any low-lying metal-centered excited (d,d) states that may quench the electron or energy transfer.
Figure 1.
The three types of self-assembled linear porphyrinic tapes used in this study, where the average number of porphyrins needed to span the bilayer is 4. The parentheses indicate that length of the self-assembled photoconductor adjusts to the thickness of the bilayer.
Because the mode of self-assembly can have a profound influence on the photonic properties of chromophore arrays, in the above cases because of the heavy atom effect, self-assembled arrays mediated by H-bonds were also studied. H-bond-assembled arrays are also more relevant to electron/energy transfer processes in biological systems such as photosynthetic antenna complexes (28). Thus, 180° topology of complementary hydrogen-bonding groups, each on a different porphyrin, results in the self-assembly of linear porphyrin polymers in toluene. In the present case, diacetamidopyridyl groups are complementary to uracil groups (Fig. 1, assembly 3) (19). A second H-bond-assembled array is formed from dibutlybarbituric acid and triaminotriazine appended with two zinc tetraphenylporphyrin (ZnTPP) moieties. Although this last H-bond system forms a rosette from three equivalents each of the barbituric acid and the triazine derivatives (Fig. 2, assembly 4) in organic solvents (18), it may form other open structures in the bilayer.
Figure 2.
Three bis-porphyrin triaminotriazine and three dibutylbarbituric acid molecules self-assemble by complementary hydrogen bonds to form a rosette, assembly 4, in toluene and other organic solvents, but may also exist in open structures inside the BLM for reasons discussed in the text.
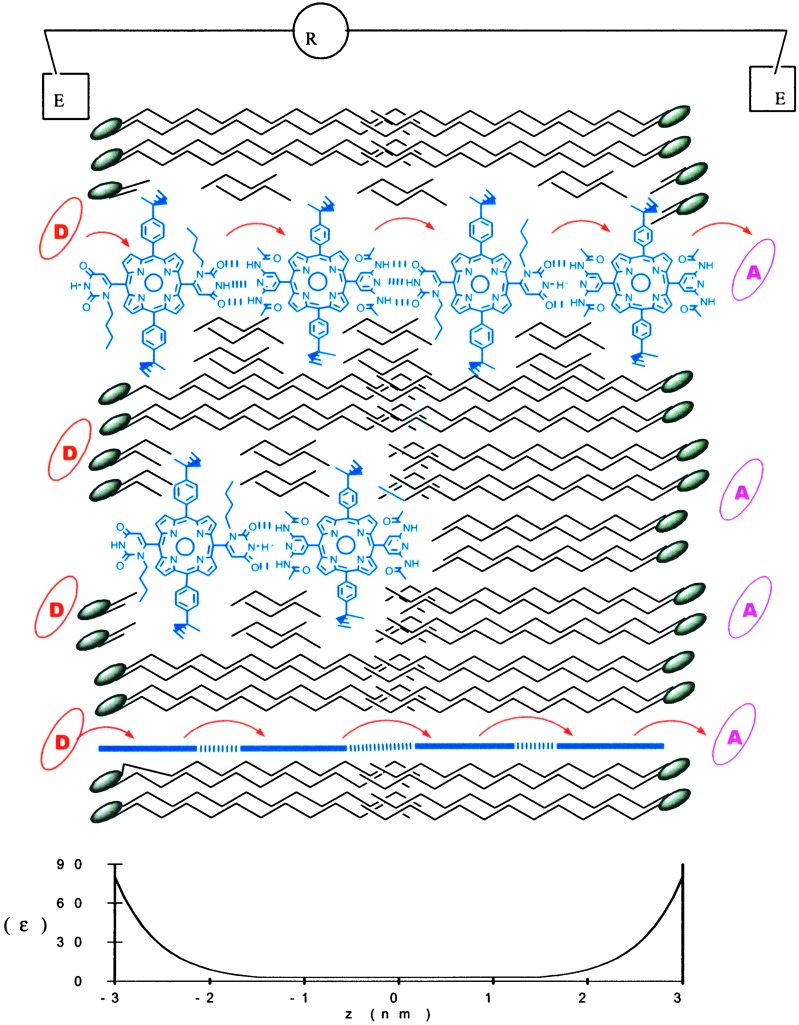
Linear tapes self-assembled by a combination of two porphyrin components form a statistical mixture of linear polymers weighted by the thermodynamics of the intermolecular bonds (19). However, the additional constraints of the bilayer also limit the length of the self-assembled tapes. The dielectric constant of lipid bilayers exponentially decreases from the water interface via the head group to the hydrocarbon center, going from ɛ≈80 to ɛ≈2 over about 2 nm on each side of the bilayer, which leaves a ≈4-nm hydrocarbon center (Fig. 3 Lower) (22). As such, the core is essentially a low dielectric organic solvent. Structures self-assembled by means of H-bonding will be favored at the BLM core. They will form to a lesser extent in the polar ester region, and will not form at all in the water-containing zwitterionic head group or aqueous surroundings (Fig. 3), because water effectively competes for the H-bonding sites on the porphyrins. Similar considerations apply to the pyridyl moieties and to the labile Pt and Pd coordination sites where water, phosphate, and chloride ions compete. With the caveat that the length of the assemblies is dynamic, vide supra, 3–4 porphyrins subunits in 1–4 are sufficient to span the BLM. Thus, one of the unique features of this mode of self-organization is that the self-assembling systems tailor their length to the thickness of the bilayer or device. The hydrocarbon chains and exponentially varying dielectric of the bilayer also serve as a means to orient the self-assembled porphyrin conductors perpendicular to the BLM–water interface and parallel to the hydrocarbon chains.
Figure 3.
Schematic of one of four self-assembling porphyrin systems self-organized into bilayers to form a functional device. The orientation of the porphyrin tape (top and bottom arrays) can be in any direction such that the porphyrin planes are perpendicular to the bilayer-water interface. It is difficult to quantify the yield of membrane-spanning porphyrin arrays, so it is reasonable to expect some monomers-to-trimers to be present in the system (middle). The electron donor, D, is K4Fe(CN)6 and the acceptor, A, is anthraquinonesulfate (AQS). The bottom graph illustrates the exponentially varying dielectric constant.
The bilayer containing the self-assembling porphyrin tapes resides in a 1-mm hole in a Teflon partition that separates two compartments containing buffered salt solutions (see Experimental Procedures). Another feature of this self-organizing device is that the electron donor [K4Fe(CN)6] and electron acceptor (anthraquinonesulfate) molecules are chosen such that they partition at the bilayer–water interface and are added to opposite sides of the membrane. Lastly, connection to the macroscopic world is mediated by the conduction of salt water.
Any system constructed from monolayers or bilayers is dynamic. The thermal (translational) and vibrational fluctuations of the bilayer will have significant effects on the equilibria of the self-assembled systems. Counteracting these is the higher viscosity and the general orientation of the hydrocarbon chains. Thus, the thermodynamics of self-assembly and the supramolecular dynamics of these conducting tapes in the bilayer may be different from what is found in toluene and other organic solvents. The stability of these 1 mm in diameter membranes is limited to a few hours to at most a day. However, forming the bilayers on millipore filters increases their stability to several days, and formation on nanopore filters may dramatically increase their lifetime, as observed in other systems (1, 29).
Results and Discussion
Significantly, when the self-assembled photoconductors shown in Fig. 1 are self-organized into the lipid bilayer membrane, a large photocurrent (Table 1) is observed upon illumination of the membrane system (Fig. 3) with ≈100 W of white light and with no applied potential. The electrochemical potential between the donor and the acceptor (≈0.5 V) is the driving force for the photocurrent. The magnitude of the photocurrents depends on light intensity and a variety of other factors such as electron transfer quantum yield, dynamics/stability of the system, and electronic coupling between the porphyrins, vide infra. Control experiments using the component molecules result in no photocurrent and little or no mobility in the dark under an applied potential. Thus, when 0.5–1 mM of any of the individual porphyrins of the self-assembled conductors are mixed into the bilayer-forming solution, there is virtually no observable trans membrane photocurrent. For comparison, under the same conditions Mg-octaethylporphyrin and Zn-tetraphenylporphyrin exhibit currents of ≈0.5 nA (which is expected because they are more lipophilic). As expected, there is very little current because of the porphyrins crossing the membrane under an applied potential clamped at ±40 mV with no light (<0.1 nA), whereas both organic-soluble metal ion species lead to a small observable dark current (<1.5 nA) (supporting information).
Table 1.
Photocurrents
| Species | Dark current, nA (± 40 mV applied) | Photo current, nA (no applied V) | Assembly current Porphyrin current |
|---|---|---|---|
| MgOEP | <0.5 | ∼1 | |
| ZnTPP | <0.5 | 0.5 | |
| ZnTPyP | <0.1 | 0.2 | |
| Zn5-PyP | <0.1 | 0.5 | |
| Zn5,15-PyP | <0.1 | 0.4 | |
| Zn5,10-PyP | <0.1 | 0.3 | |
| Pt(Bn)2Cl2 | 1.2 | <0.1 | |
| Pd(Bn)2Cl2 | 1.6 | <0.1 | |
| Zn5,15-UrP | <0.1 | <0.2 | |
| Zn5,15-APyP | <0.1 | <0.2 | |
| DiP-triazine | <0.1 | <0.1 | |
| DiBuBarb | <0.1 | 0.3 | |
| Assembly 1 | <0.1 | 85 (±5) | 170 (Zn5-PyP) |
| Assembly 2 | <0.1 | 65 (±5) | 163 (Zn5,15-PyP) |
| Assembly 3 | <0.1 | 85 (±5) | 425 (Zn5,15-UrP) |
| Assembly 4 | <0.1 | 45 (±5) | 150 (DiBuBarb) |
Zn5-PyP, 5-(4-pyridyl)-10,15,20-Tris(4-tert-butylphenyl)porphyrinato zinc; Zn5,10-PyP, 5,10-bis(4-pyridyl)-15,20-bis(4-tert-butylphenyl)porphyrinato zinc; Zn5,15-PyP, 5,15-bis(4-pyridyl)-10,20-bis(4-tert-butylphenyl) porphyrinato zinc; ZnTPyP, 5,10,15,20-tetrakis(4-pyridyl)porphyrinato zinc; Zn5,15-APyP, 5,15-bis(3,5-diacetamido-4-pyridyl)-10,20-bis(4-tert- butylphenyl)porphyrinato zinc; Zn5,15-UrP, 5,15-bis(1′-butyl-6′-uracyl)-10,20-bis(4-tert-butylphenyl)porphyrinato zinc; Bn, benzonitrile; DiP-triazine, N,N-bis(tetraphenylporphyrinatozinc)triaminotriazine; DiBuBarb, dibutylbarbituric acid; ZnTPP, zinc tetraphenylporphyrin; MgOEP, magnesium octaethylporphyrin.
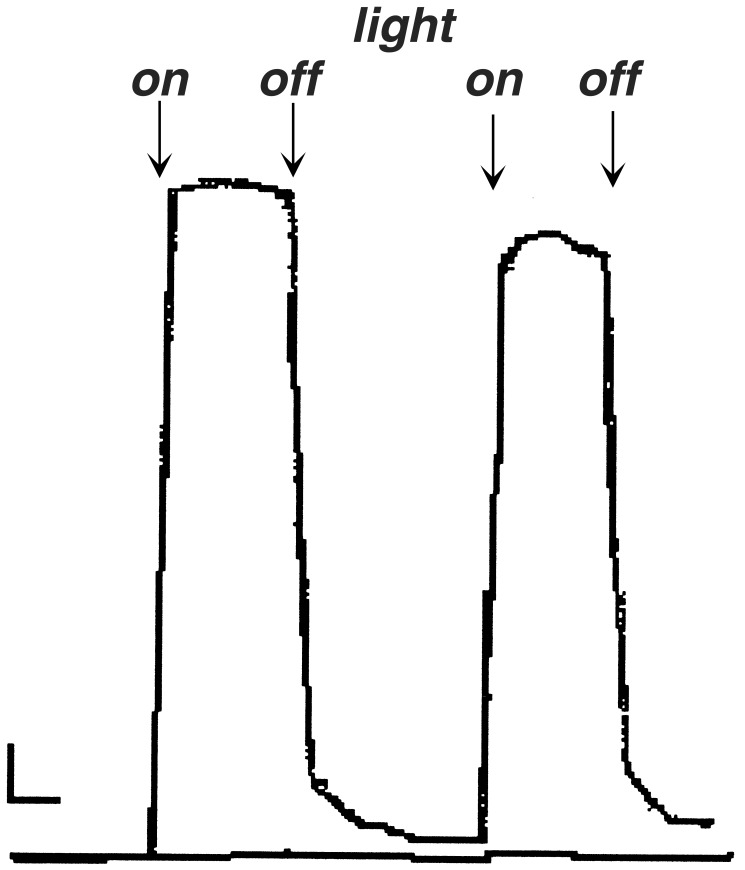
As seen in Fig. 4, there is a substantial increase in the transmembrane current because of the presence of the self-assembled “tetramer” (assembly 3) after illumination of the system, whereas the bottom trace shows the conductance due to only the 5,15-bis(1′-butyl-6′-uracyl)-10,20-bis(4-tert-butylphenyl)porphyrinato zinc (Zn5,15-UrP) under the same conditions. Note that the time constant for the amplifier is 0.1 s. Three observations are noteworthy. (i) The current is nonlinear at constant illumination, (ii) the system takes about a minute to completely return to zero after the light is turned off, and (iii) the current observed for subsequent light cycles is not as great as the initial on cycle. These can be explained by several factors and are part of our continuing efforts to improve the system. The variable steady-state current is readily explained as follows. Initially, a certain quantity of the donor and acceptor molecules is associated with the membrane device, and under constant illumination these are depleted such that fresh donor and acceptor molecules must diffuse to the bilayer–water interface. Eventually, the solutions bathing the membrane are depleted in these redox species. Another factor is that the system is dynamic and there may be reorganization of the self-assembled conductors, the donor and acceptor molecules, and the membrane itself. These latter effects (diffusion and dynamics) also may contribute to the long relaxation back to zero. These factors are also consistent with the observation that when the device is on for shorter periods of time the return to zero is proportionally shorter. The building up of charge on the membrane interface may be a contributing factor as well (22), but studies on other systems indicate these are small effects (17). The relaxation times are greater than simple diffusion of the redox species, therefore they may also reflect surface-binding energetics (17). Increasing the salt concentration, from 0.1 M to 0.3 M, thereby decreasing the Debey length, decreases the relaxation time by <20%. To estimate the number of porphyrins in the functional (i.e., conducting) tapes, current vs. concentration studies were done (11, 17, 22), where a ≈3.8th power concentration dependence was observed for assembly 3. This result indicates that on average there are likely four porphyrins in the conducting species mediated by H-bonds. Similar studies on the Pt-assembled tape, 2, also indicate four porphyrins participate in the conduction process.
Figure 4.
Strip chart recording of the photocurrent of assembly 3 self-organized into the bilayer. The horizontal scale bar represents 1 min, and the vertical scale bar represents 6.9 nA. The lower trace is the photocurrent of Zn5,15-UrP only. (See supporting information for photocurrents for all assemblies.)
The above discussion implies that devices containing the tapes assembled by both Pd and Pt, which are known to be more robust and to have greater electronic communication between subunits (19–21, 25, 26), should exhibit a more constant current, and that is what is observed. The relaxation time is only moderately shorter, which further supports membrane charging and diffusion as the dominant reasons for the long relaxation times. The interfacial charge transfer reaction is known to occur on tens of ps time scales (13, 14). The charge-transfer reactions in similar metalloporphyrin assemblies (both H-bond- and metal-mediated) in organic solvents are on the same scale (30). Thus, it is reasonable to expect that the assemblies studied here have generally similar electron-transfer rates and are consistent with other transmembrane currents (10–16). Because the time constant for the amplifier is 0.01–0.1 s for these experiments, the time constant to turn on the device is as of yet unknown.
Conclusions
Whereas previous studies focused on the synthetic supramolecular chemistry of porphyrinic materials, the present one begins to explore the photonic functionality of these self-assembled systems and to develop methodologies to self-organize them into devices. The experiments describe here are a direct measure of the photoconductivity of the self-organized devices, as such there is little ambiguity in interpretation of the data. These studies also demonstrate that nanometer-scale electrodes are not a priori necessary to connect to nanometer-scaled devices. The themes and design principles successfully demonstrated by these photogated conductivity experiments may well be generally applicable to a variety of systems.
Supplementary Material
Acknowledgments
This work is dedicated to Prof. David Mauzerall on the occasion of his becoming emeritus at The Rockefeller University. Drs. K. Sun and S. Niu are thanked for their assistance. Financial support from The Rockefeller University, National Science Foundation Grant CHE-0135509, the U.S.–Israel Binational Science Foundation 1999082, and Hunter College is gratefully acknowledged.
Abbreviation
- BLM
bilayer lipid membrane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Alivisatos A P, Barbara P F, Castleman A W, Chang J, Dixon D A, Klein M L, McLendon G L, Miller J S, Ratner M A, Rossky P J, et al. Adv Mater. 1998;10:1297–1336. [Google Scholar]
- 2.Lehn J-M. Pure Appl Chem. 1994;66:1961–1966. [Google Scholar]
- 3.Aviram A, Ratner M, editors. Molecular Electronics Science and Technology. New York: N.Y. Acad. Sci.; 1998. [Google Scholar]
- 4.Stang P J, Olenyuk B. Acc Chem Res. 1997;30:502–518. [Google Scholar]
- 5.Philp D, Stoddart J F. Angew Chem Int Ed Engl. 1996;35:1154–1196. [Google Scholar]
- 6.Daganni R. Chem Eng News. 1998;76:35–44. [Google Scholar]
- 7.Steed J W, Atwood J L. Supramolecular Chemistry. New York: Wiley; 2000. [Google Scholar]
- 8.Lehn J-M. Supramolecular Chemistry: Concepts and Perspectives. New York: VCH; 1995. [Google Scholar]
- 9.Kurth D G, Lehmann P, Schütte M. Proc Natl Acad Sci USA. 2000;97:5704–5707. doi: 10.1073/pnas.97.11.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drain C M, Mauzerall D. Bioelectrochem Bioenerg. 1990;24:263–266. [Google Scholar]
- 11.Drain C M, Mauzerall D. Biophys J. 1992;63:1556–1563. doi: 10.1016/S0006-3495(92)81739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drain C M, Christensen B, Mauzerall D. Proc Natl Acad Sci USA. 1989;86:6959–6962. doi: 10.1073/pnas.86.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu S, Mauzerall D. J Am Chem Soc. 1996;118:5791–5795. [Google Scholar]
- 14.Hwang K C, Mauzerall D. Nature (London) 1993;361:138–140. doi: 10.1038/361138a0. [DOI] [PubMed] [Google Scholar]
- 15.Sun K, Mauzerall D. Proc Natl Acad Sci USA. 1996;93:10758–10762. doi: 10.1073/pnas.93.20.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun K, Mauzerall D. Biophys J. 1996;71:295–308. doi: 10.1016/S0006-3495(96)79225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tien H T, Salamon Z, Kutnik J, Krysinski P, Kotowski J, Ledermann D, Janas T. J Mol Elec. 1988;4:S1–S30. [Google Scholar]
- 18. Drain, C. M., Russell, K. C. & Lehn, J.-M. (1996) J. Chem. Soc. Chem. Commun., 337–338.
- 19.Shi X, Barkigia K M, Fajer J, Drain C M. J Org Chem. 2001;66:6513–6522. doi: 10.1021/jo010108c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drain, C. M. & Lehn, J.-M. (1994) J. Chem. Soc. Chem. Commun., 2313–2315.
- 21.Drain C M, Nifiatis F, Vasenko A, Batteas J D. Angew Chem Int Ed Engl. 1998;37:2344–2347. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2344::AID-ANIE2344>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Mauzerall D, Drain C M. Biophys J. 1992;63:1544–1555. doi: 10.1016/S0006-3495(92)81738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg-Yfrach G, Liddell P A, Hung S-C, Moore A L, Gust D, Moore T A. Nature (London) 1997;385:239–241. [Google Scholar]
- 24.Lindsey J S, Gust D, Moore T A, Moore A L, Weghorn S J, Johnson T E, Lin S, Liddell P A, Kuciauskas D. J Am Chem Soc. 1999;121:8604–8614. [Google Scholar]
- 25.Chambron J-C, Heitz V, Sauvage J-P. In: The Porphyrin Handbook. Kadish K M, Smith K M, Guilard R, editors. Vol. 6. New York: Academic; 2000. pp. 1–42. [Google Scholar]
- 26.Chou J-H, Kosal M E, Nalwa H S, Rakow N A, Suslick K. In: The Porphyrin Handbook. Kadish K M, Smith K M, Guilard R, editors. Vol. 6. New York: Academic; 2000. pp. 43–131. [Google Scholar]
- 27.Li F, Gentemann S, Kalsbeck W A, Seth J, Lindsey J S, Holten D, Bocian D F. J Mater Chem. 1997;7:1245–1262. [Google Scholar]
- 28.Balaban T S, Tamiaki H, Holzwarth A R, Schaffner K. J Phys Chem B. 1997;101:3424–3431. [Google Scholar]
- 29. Yu, J. S., Kim, J. Y., Lee, S., Mbindyo, J. K. N., Martin, B. R. & Mallouk, T. E. (2000) J. Chem. Soc. Chem. Commun., 2445–2446.
- 30.Sessler J L, Wang B, Harriman A. J Am Chem Soc. 1995;117:704–714. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.