Abstract
Tumor-infiltrating lymphocyte (TIL) therapy represents an emerging and promising approach in the field of cancer immunotherapy, harnessing the body’s own immune cells to target and eliminate cancer cells. This review provides an in-depth analysis of TIL therapy, focusing on its mechanisms, clinical applications, challenges, and future prospects. TILs, particularly CD8 + T cells, are isolated from a patient’s tumor, expanded ex vivo, and reinfused to promote anti-tumor immunity. The success of TIL therapy has been demonstrated in various cancers, particularly melanoma, where it has led to durable responses and, in some cases, complete remission. However, significant challenges remain, including the heterogeneity of TIL populations, difficulties in large-scale expansion, and immune-related adverse events. We also explore current strategies aimed at overcoming these limitations, including genetic modification of TILs, combinatory approaches with checkpoint inhibitors, and optimization of tumor infiltration protocols. With ongoing research and technological advancements, TIL therapy holds substantial promise as a cornerstone of personalized cancer treatment. This review synthesizes the current landscape of TIL therapy, providing insights into its clinical efficacy, potential for broader application, and the future of cancer immunotherapy.
Keywords: Tumor, Immunotherapy, Melanoma, Lymphocytes, TIL therapy, Antigens
Introduction
Tumor-infiltrating lymphocyte (TIL) therapy has emerged as a promising and innovative approach in the field of cancer immunotherapy, harnessing the body’s own immune cells to combat malignancies. TILs are a population of immune cells that naturally migrate to and accumulate within tumors, where they attempt to mount an immune response against cancer cells. These lymphocytes, primarily consisting of CD8 + cytotoxic T cells, are capable of recognizing and destroying tumor cells that express specific antigens. However, in many cancers, the tumor microenvironment suppresses or evades immune responses, limiting the effectiveness of these naturally occurring TILs. TIL therapy involves isolating these lymphocytes directly from a patient's tumor, expanding them ex vivo, and then reinfusing them into the patient in larger quantities to enhance anti-tumor immune responses. This adoptive cell transfer approach has shown substantial success, particularly in melanoma, where clinical trials have demonstrated durable responses, and in some cases, complete remissions. TIL therapy is distinct from other forms of immunotherapy, such as monoclonal antibodies or checkpoint inhibitors, by utilizing the patient’s own immune cells rather than synthetic agents or drugs to stimulate immunity. Despite the promising results, TIL therapy faces several challenges, including the technical difficulties of isolating and expanding sufficient numbers of TILs, overcoming the immunosuppressive tumor microenvironment, and managing potential immune-related adverse events. Furthermore, the heterogeneity of TIL populations and the varied responses across different tumor types necessitate ongoing research to optimize this approach [1].
General overview of TIL therapy
Tumor-infiltrating lymphocyte (TIL) therapy is a modern cancer treatment used to combat notoriously hard-to-treat solid tumors. Like other adoptive cell therapies, TIL harnesses the body’s immune system to combat malignancies by using T cells, a type of white blood cell integral to immune response. Except in TIL therapy, these T cells are directly extracted from tumors.
Having survived the tumor microenvironment, these lymphocytes are already primed to recognize and attack cancer cells, offering a personalized approach to cancer immunotherapy. Unlike traditional treatments that often have a broader target, TIL therapy is specifically tailored to each patient’s unique tumor, increasing the potential for effective treatment outcomes. This innovative method reflects a significant shift in oncology, emphasizing the role of individualized treatment strategies in combating various forms of cancer.
TIL therapy process
Tumor-infiltrating lymphocyte therapy is a multi-step process that mobilizes the body’s immune system to fight cancer. These are the major steps in creating an intricately tailored treatment for each patient:
Extraction of TILs: The process begins by surgically removing a piece of the patient’s tumor, from which TILs are extracted. These lymphocytes are already fighting the cancer but are typically outnumbered within the tumor environment.
Expansion and activation: Once isolated, these TILs are taken to a laboratory where they are cultured and stimulated to proliferate. This step involves the use of cytokines, such as interleukin-2 (IL-2), to activate and expand the TILs, significantly increasing their numbers and enhancing their ability to fight tumor cells.
Reinfusion into patients: After sufficient expansion, these activated TILs are infused back into the patient. Prior to the reinfusion, patients typically undergo a lymphodepletion regimen, including chemotherapy, to reduce the number of other immune cells in the body. This step enhances the effectiveness of the infused TILs.
Post-infusion monitoring: Following the reinfusion, the patient’s response to the cell therapy is closely monitored. The expanded TILs, now in large numbers, navigate through the body to locate and destroy cancer cells.
Supportive care: Patients often receive additional treatments, such as IL-2-based immunotherapy medications, to support the TILs’ activity.
This therapy has shown promise in treating various types of cancers, particularly those where other treatments have failed. Its effectiveness lies in the personalized approach, using the patient’s immune cells to target the tumor. However, the process is complex and requires facilities and expertize found only at specialized cancer centers.
Clinical results and efficiency of tumor-infiltrating lymphocyte therapy
Tumor-infiltrating lymphocyte (TIL) therapy is an emerging form of adoptive cell therapy (ACT) that leverages a patient's own immune system to fight cancer. TILs are immune cells, predominantly T cells, that are extracted from a patient's tumor, expanded in the laboratory, and reinfused into the patient. This approach aims to boost the immune system's ability to recognize and destroy cancer cells.
Clinical results
TIL therapy has shown promising results, particularly in advanced cancers that are resistant to conventional therapies. Here are highlights of clinical findings across key cancer types:
Melanoma
Historical development of TIL therapy:
1970s: Discovery of Tumor-Infiltrating Lymphocytes
Early Observations: Researchers observed lymphocytes infiltrating tumor tissues in cancer patients, suggesting an immune response against tumors.
Tumor Immunity Hypothesis: This period marked the beginning of understanding how the immune system interacts with tumors.
1980s: Initial Development of TIL Therapy
Adoptive Cell Therapy (ACT) Concept: The groundwork for ACT was laid by Dr. Steven Rosenberg and colleagues at the National Cancer Institute (NCI), who pioneered the concept of using immune cells to fight cancer.
First TIL Isolation (1986): Rosenberg's team successfully isolated TILs from melanoma tumors, expanded them ex vivo, and reinfused them into patients.
Early Successes: Initial clinical trials demonstrated objective tumor regression in melanoma patients, proving the concepts.
1990s: Refinement and Expansion
Improved Culture Techniques: Researchers optimized methods to expand TILs in the laboratory using interleukin-2 (IL-2), a cytokine that promotes T-cell proliferation.
High-Dose IL-2: Administering IL-2 alongside TILs improved their survival and activity in patients.
Challenges: Despite promising results, the therapy faced hurdles, including inconsistent responses and severe toxicity from IL-2.
2000s: Advancements in TIL Therapy
Lymphodepletion: Introduction of preconditioning regimens (e.g., chemotherapy or radiation) to deplete the patient's lymphocytes before TIL infusion improved engraftment and response rates.
Durable Responses: Long-term follow-up studies confirmed durable complete responses in subsets of melanoma patients, sparking renewed interest in TIL therapy.
Expansion Beyond Melanoma: Research extended TIL therapy to other cancers, such as cervical cancer, renal cell carcinoma, and non-small cell lung cancer (NSCLC).
2010s: Modernization and Broader Applications
Checkpoint Inhibitors Integration: Combining TIL therapy with immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1) enhanced efficacy, addressing tumor-induced immune suppression.
Clinical Trials: Multicenter trials demonstrated significant responses in previously untreatable cancers, such as metastatic melanoma and cervical cancer.
Commercial Development: Companies began developing TIL products for regulatory approval, such as Iovance Biotherapeutics' lifileucel for melanoma and cervical cancers.
2020s: Technological Innovations and Scaling
Gene Editing: Advanced tools like CRISPR enabled genetic modifications to enhance TIL function, such as improving specificity or resistance to exhaustion.
Neoantigen Selection: Techniques to select TILs targeting tumor-specific neoantigens improved precision and efficacy.
Commercialization: TIL therapy moved closer to routine clinical use, with regulatory filings and approvals for lifileucel and other TIL-based treatments.
Automated Manufacturing: Efforts to streamline and automate the TIL production process aimed to reduce costs and improve accessibility.
- Cervical Cancer
- The FDA granted TIL therapy (lifileucel) a breakthrough designation based on promising Phase II trial results in patients with recurrent, metastatic cervical cancer.
- ORRs of ~ 30–35% have been reported in this hard-to-treat population.
- Non-Small Cell Lung Cancer (NSCLC)
- Preliminary studies show promise, with ORRs in the range of 20–30%, though further trials are ongoing.
- Other Solid Tumors
- Head and Neck Cancers, Breast Cancer, and Colorectal Cancer: Trials have demonstrated varying degrees of efficacy, typically influenced by tumor mutational burden (TMB) and the immunogenicity of the tumor.
Efficiency factors
TIL therapy's success depends on multiple factors:
Tumor Mutational Burden (TMB): Tumors with higher mutational loads provide more neoantigens, which enhance T cell recognition.
Patient Selection: Immunogenic tumors and patients with less immunosuppressive tumor microenvironments respond better.
Preconditioning: Lymphodepleting regimens before TIL infusion improve outcomes by creating space for TIL expansion and reducing regulatory T cells.
Expansion Protocols: Laboratory methods to expand TILs and select highly active clones influence the therapy's effectiveness.
Historical TIL therapy versus modern TIL therapy
Historical TIL therapy, which began to gain traction in the 1980s and 1990s, marked the beginning of efforts to harness the body’s immune system to target and fight cancer. The therapy was based on the observation that tumors contain immune cells, specifically T-cells, which infiltrate the tumor tissue. Researchers realized that by isolating these T-cells, expanding them in the laboratory, and reintroducing them into the patient, they could potentially enhance the body’s immune response against the tumor. However, early trials faced numerous obstacles, including the difficulty in extracting sufficient quantities of T-cells, the lengthy process of growing them in the laboratory, and the inability to guarantee that the expanded cells would have the desired tumor-killing properties.
In addition to technical challenges, historical TIL therapy faced significant limitations in the effectiveness of the immune cells that were extracted. Not all tumors contained T-cells capable of recognizing and destroying cancerous cells, which led to inconsistent outcomes. Another obstacle was the tumor microenvironment, which often suppresses immune cell activity. Tumors can create an immunosuppressive environment through various mechanisms, such as the secretion of inhibitory molecules, which hinder the effectiveness of T-cells. As a result, the immune cells harvested and reinfused into patients often faced a hostile environment that diminished their therapeutic potential.
The introduction of chemotherapy as a conditioning regimen in early TIL therapy was intended to suppress the patient’s immune system to improve the effectiveness of the infused T-cells. However, this approach came with significant drawbacks. Chemotherapy’s broad suppression of the immune system left patients vulnerable to infections and other complications, and it could also damage healthy tissues. The need for such intensive chemotherapy was a major concern for both patients and researchers, and it limited the applicability of the therapy, particularly for older or more vulnerable patients.
Modern TIL therapy has advanced considerably due to a better understanding of the immune system, cancer biology, and cell culture techniques. One of the most significant developments is the ability to identify and isolate T-cells that are more likely to target cancer cells effectively. Today’s TIL therapy utilizes advanced techniques to genetically modify or select for T-cells that can better recognize tumor antigens. This refinement in cell selection has led to much higher success rates, with patients showing better clinical responses. Moreover, modern TIL therapy is often combined with other immunotherapies, such as checkpoint inhibitors like anti-PD-1 and anti-CTLA-4, which help to block the mechanisms by which tumors suppress immune activity.
The combination of TIL therapy with checkpoint inhibitors represents one of the key advancements in modern cancer treatment. These inhibitors work by blocking proteins that tumors use to evade immune detection, such as PD-1 or CTLA-4, thereby enhancing the activity of T-cells. When used together, checkpoint inhibitors and TILs create a synergistic effect, leading to a stronger and more sustained immune response against the tumor. Furthermore, advances in the manufacturing process have enabled the rapid expansion of T-cells, reducing the time patients must wait for treatment and improving overall efficiency. Additionally, modern TIL therapy is becoming more personalized, with treatments being tailored to individual patients based on their tumor profile and immune system characteristics. This personalized approach has resulted in better outcomes and reduced side effects compared to earlier approaches.
While modern TIL therapy is still an evolving field, it has proven to be a highly promising treatment, especially for cancers like melanoma, ovarian cancer, and other solid tumors. Modern advancements have not only improved the effectiveness of TIL therapy but also minimized the side effects that were prevalent in historical approaches. Patients now experience less intense immune suppression, and some newer protocols have even reduced or eliminated the need for chemotherapy altogether. These improvements, combined with faster and more efficient TIL expansion, make modern TIL therapy one of the most innovative approaches in cancer immunotherapy today, offering hope for patients who were previously facing limited treatment options.
Lifileucel: the first commercial TIL product
Lifileucel, marketed as Amtagvi, is an autologous tumor-infiltrating lymphocyte (TIL) therapy approved by the U.S. Food and Drug Administration (FDA) in February 2024 for adults with unresectable or metastatic melanoma previously treated with a PD-1 blocking antibody, and if BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor. In the first-line treatment setting for advanced melanoma, the combination of lifileucel with PD-1 inhibitors is under active investigation. The IOV-COM-202 phase 2 trial (NCT03645928) evaluated lifileucel combined with pembrolizumab in patients with advanced melanoma who had not received prior immune checkpoint inhibitor therapy. This study reported an overall response rate (ORR) of 67%, indicating promising efficacy for this combination in the frontline settings. Building upon these findings, the TILVANCE-301 phase 3 trial (NCT05727904) has been initiated to further assess the efficacy and safety of lifileucel combined with pembrolizumab as a first-line treatment for advanced melanoma. This randomized, open-label study aims to enroll approximately 670 participants, comparing the combination therapy against pembrolizumab monotherapy. Primary endpoints include objective response rate and progression-free survival, with secondary endpoints evaluating overall survival, complete response rate, duration of response, event-free survival, and adverse events. Notably, the trial design allows participants in the pembrolizumab monotherapy group to cross over to receive lifileucel monotherapy upon confirmed disease progression.
Recent advancements in tumor-infiltrating lymphocyte (TIL) therapy have focused on enhancing its efficacy by genetically modifying TILs to lack the programmed cell death protein 1 (PD-1). PD-1 is an inhibitory receptor that, when engaged by its ligand PD-L1, can suppress T-cell activity, allowing tumors to evade immune surveillance. By knocking out PD-1 in TILs, researchers aim to prevent this inhibitory signaling, thereby enhancing the anti-tumor activity of these cells. A notable development in this area is the use of CRISPR-Cas9 gene-editing technology to create PD-1-deficient TILs. In preclinical studies, TILs with PD-1 knocked out demonstrated a significant reduction in PD-1 expression—up to 87.53%—after expansion. These modified TILs exhibited enhanced anti-tumor efficacy, as evidenced by their superior ability to delay tumor growth in models of PD-L1-expressing human melanoma. Building upon these promising preclinical results, clinical trials have been initiated to evaluate the safety and efficacy of PD-1 knockout TILs in patients with advanced cancers. For instance, Iovance Biotherapeutics is conducting a Phase 1/2 study (IOV-GM1-201) to evaluate IOV-4001, a genetically modified autologous PD-1 knockout TIL product, in patients with unresectable or metastatic melanoma or stage III or IV non-small-cell lung cancer. These efforts represent a significant step forward in the development of more effective adoptive cell therapy strategies for melanoma and potentially other cancers. By genetically enhancing TILs to resist tumor-induced immunosuppression, therapies like PD-1 knockout TILs hold promise for improving patient outcomes in the future.
Advancements in adoptive cell therapy (ACT) for non-small cell lung cancer (NSCLC) have focused on enhancing the efficacy of tumor-infiltrating lymphocytes (TILs) by genetically modifying them to lack the programmed cell death protein 1 (PD-1). PD-1 is an inhibitory receptor that, when engaged by its ligand PD-L1, can suppress T-cell activity, allowing tumors to evade immune surveillance. By knocking out PD-1 in TILs, researchers aim to prevent this inhibitory signaling, thereby enhancing the anti-tumor activity of these cells. A notable development in this area is the use of CRISPR-Cas9 gene-editing technology to create PD-1-deficient TILs. In preclinical studies, TILs with PD-1 knocked out demonstrated a significant reduction in PD-1 expression—up to 87.53%—after expansion. These modified TILs exhibited enhanced anti-tumor efficacy, as evidenced by their superior ability to delay tumor growth in models of PD-L1-expressing human melanoma. Building upon these promising preclinical results, clinical trials have been initiated to evaluate the safety and efficacy of PD-1 knockout TILs in patients with advanced cancers, including NSCLC. For instance, a Phase I trial is investigating the use of PD-1-deficient engineered T cells with CRISPR/Cas9 in patients with advanced NSCLC. These efforts represent a significant step forward in the development of more effective ACT strategies for NSCLC. By genetically enhancing TILs to resist tumor-induced immunosuppression, therapies like PD-1 knockout TILs hold promise for improving patient outcomes in the future.
The application of tumor-infiltrating lymphocyte (TIL) therapies in non-small cell lung cancer (NSCLC) is an area of significant interest, particularly with advancements such as genetically modified TILs (e.g., PD-1 knockout TILs). Here's how these approaches are being explored in NSCLC:
- Genetic Modification of TILs for NSCLC:
-
oSimilar to melanoma, NSCLC tumors also express PD-L1, which engages PD-1 receptors on T-cells, limiting their ability to mount an effective anti-tumor response. To overcome this, researchers have been genetically modifying TILs to knock out PD-1, thereby preventing this inhibitory signaling and boosting their anti-tumor activity.
-
oPreclinical Findings: In preclinical models, PD-1 knockout TILs demonstrated enhanced anti-tumor efficacy, significantly delaying tumor progression in NSCLC models, particularly when PD-L1 was expressed on the tumors. These TILs had a more potent cytotoxic effect compared to unmodified TILs.
-
oCRISPR-Cas9 Technology: CRISPR is being employed to edit TILs, creating genetically modified cells with PD-1 knockouts. These TILs have been shown to have improved tumor-fighting capabilities, with the potential to overcome the immune evasion mechanisms of tumors in NSCLC.
-
o
- Clinical Trials in NSCLC:
-
oPhase 1/2 Trials: Clinical trials are underway to evaluate PD-1 knockout TILs in patients with advanced NSCLC. One such trial by Iovance Biotherapeutics, which investigates the combination of genetically modified TILs and immune checkpoint inhibitors, is exploring their potential in advanced NSCLC patients.
-
oTIL-Based Therapies: Clinical studies are also exploring the use of expanded TILs (without PD-1 knockouts) as an adjunctive or alternative approach for NSCLC treatment, especially for patients with advanced or metastatic disease. These studies are investigating TILs' efficacy when used alongside therapies like PD-1 inhibitors (e.g., pembrolizumab).
-
oCombination with PD-1/PD-L1 Inhibitors: The potential synergy between PD-1 knockout TILs and PD-1 inhibitors is being studied, combining both strategies to enhance the immune response against NSCLC tumors. These trials are assessing improved response rates and durable outcomes in patients.
-
o
- Recent Research and Developments:
-
oSome studies have found that the combination of engineered TILs (such as PD-1 knockout or cytokine-modified) and immune checkpoint inhibitors like pembrolizumab or nivolumab can result in enhanced and prolonged responses, even in patients with heavily pretreated or refractory NSCLC.
-
oResearchers are also exploring how TIL therapies might be combined with other emerging immunotherapies or targeted therapies for NSCLC.
-
o
These developments suggest a promising future for TIL-based immunotherapies in NSCLC, offering a potential path forward in overcoming the challenges of immune evasion in this hard-to-treat cancer.
T-cell biology and tumor microenvironment
In adoptive cell therapy, also referred to as TIL therapy, the primary cells targeted and expanded are tumor-infiltrating lymphocytes. Immunotherapy now targets these T-cells because of.
their strong ability to eradicate tumors though various T-cell types, including cytotoxic T cells, helper T.8 cells, and regulatory T cells, also contribute to T-cell-mediated responses in the tumor microenvironment (TME), TIL is crucial for anti-tumor immunity [2]. B cells, innate lymphoid cells, CD8 + T cells, and CD4 + T cells are among the lymphoid components that must be present, activated, and co-stimulated for an anti-tumor immune response to be effective [3]. The immune system's anti-tumor effects, including the distinct cell types of CD8 + effector T cells, are explained by tumor antigen-specific cytotoxic CD8 + TILs that express T-cell receptors [4]. Better clinical outcomes are linked to TIL presence, but the kind and function of TILs as well as the TME localization of various TILs are important determinants of final tumor progression or control. TME components such as tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), regulatory T-cells (Tregs), and immunosuppressive molecules and metabolites will prevent TILs from killing tumor cells [5].
Tumor-infiltrating immune cells and their associations with immunotherapies
Immune cells, especially T cells, can be used to eradicate tumor cells, as shown by the efficacy of cancer immunotherapy treatments like ACT and ICI therapies. However, only a small percentage of cancer patients benefit from them despite their long-term therapeutic success [6]. Immune infiltrates, a significant part of the TME, have been shown to influence immunotherapy responses and tumor development. Determining the processes of immunotherapies, establishing predictive biomarkers, and discovering new therapeutic targets thus need a deeper comprehension of both innate and adaptive immune cells in the TME [7].
T lymphocytes.
Tumor immunology has turned its attention to T cells because of their powerful potential to eradicate tumors. TCR interaction with short peptides of tumor antigens presented by major histocompatibility complex (MHC) molecules or human leukocyte antigen initiates T cell activity [8]. Genetic rearrangements involving several random recombinations of TCR gene segments produce TCRs, which can result in a variety of TCR repertoires that give T cells their uniqueness and specificity. Different T cell types, including as cytotoxic T cells, T helper (TH) cells, and regulatory T cells (Tregs), are engaged in T-cell-mediated immune responses inside the tumor context, and TILs are essential for efficient anti-tumor immunity [9].
The primary effector cells are CTLs, which work with cytotoxic substances like perforin and granzymes. One hundred Even while research has demonstrated a favorable correlation between TILs, namely CTLs, and patient survival across a variety of cancers, CTLs that infiltrate tumor sites frequently fall short of controlling tumor development because of fatigue or malfunction shaped by the immunosuppressive TME. T-cell fatigue was first identified in mouse models of chronic lymphocytic choriomeningitis virus infection and has since been shown to be common in human tumors. It is typified by the overexpression of PD-1 and other inhibitory molecules. Thommen et al., for example, examined the characteristics of three groups of intratumoral CD8 + TILs from patients with non-small cell lung cancer (NSCLC) with varying degrees of PD-1 expression. They discovered that TILs with high PD-1 expression were depleted but still indicative of how NSCLC patients might react to anti-PD-1 therapy. Together with the remarkable clinical effectiveness of ICIs, our results emphasize how crucial it is to treat cancer by addressing T-cell malfunction. TH cells and Tregs are components of CD4 T cells. By assisting CD8 effector T cells or by functioning as cytotoxic T cells to eradicate tumor cells directly, TH cells support anti-tumor immunity by directly impairing T-cell function through immunosuppressive soluble factors and indirectly preventing T-cell activation through CTLA-4-mediated inhibition of APC costimulatory signals, Tregs, on the other hand, which are essential for preserving homeostasis, orchestrate anti-tumor immunity. Anti-CTLA-4 antibodies may also cause Treg depletion, despite the fact that inhibiting negative signaling strengthens T-cell priming. This highlights the intricate ways in which ICIs support anti-tumor immunity [10].
B cells
B cells are humoral immune cells that are part of the adaptive immune system's humoral immunity. B cells can develop into memory B cells or plasma cells in response to tumor or infected cells. The latter can release immunoglobulins (Igs), commonly referred to as antibodies, which bind and neutralize target antigens. Notably, antigens interact with the B-cell receptor (BCR), a membrane-bound form of Ig (mIg) that gives B cells antigen specificity, to activate B cells. Because the Ig gene segments are randomly rearranged, each B-cell has a distinct BCR that is obtained from a very varied pool of the BCR repertoire. The BCR repertoire has a variety of antigen specificities, and the chosen BCR may undergo further modification upon encountering an antigen [11].
B cells are essential for humoral immunity because they produce antibodies, but they also support cellular immunity by acting as APCs to boost T-cell-mediated immunity and by regulating immunological responses via regulatory B cells or cytokines. Furthermore, in locations of chronic inflammation and tumors, B cells aid in the maintenance of secondary lymphoid organ architecture and promote the development of tertiary lymphoid structures (TLSs), which are highly ordered structures made up of aggregation of immune cells like T cells, B cells, and follicular DCs. Long-term immunity is aided by TLSs, which are especially crucial for the recruitment and local activation of B cells and T cells [12].
NK cells
NK cells are the quintessential innate lymphoid cells that enhance the MHC-restricted tumor lysis carried out by cytotoxic T cells by performing cytotoxic actions without MHC specificity. NK cells use cytolytic granules to directly destroy tumor cells and collaborate with other immune cells by producing chemokines and proinflammatory cytokines. Crucially, activating and inhibitory receptors produced on the surface of NK cells work together to induce NK cell activation. In particular, activating receptors detect the signs of cellular stress linked to viral infection or carcinogenesis when virus-infected cells or tumor cells lose MHC class I expression, which results in NK activation and effector function. Inhibitory receptors, on the other hand, interact with MHC class I molecules expressed on normal cells and help NK cells develop self-tolerance [13].
Adoptive transfer of autologous NK cells, which involves transferring ex vivo activated and expanded NK cells into patients, is one of the NK-based immunotherapies that have been investigated; CAR-NK cell treatments, in which modified NK cells expressing CARs against a particular tumor antigen are transfused; cytokine treatments, which increase NK cell function by infusing certain cytokines; and mAb-based treatments, which relate to the administration of antibodies to NK cells in order to disrupt their inhibitory receptors.
Similar to ICIs, which block T cell inhibitory pathways, blocking NK cell inhibitory receptors shows promise as well. A number of NK cell inhibitory receptors have been investigated for their potential as therapeutics and for use in clinical settings. The primary inhibitory receptors on human NK cells are the killer immunoglobulin receptor (KIR) family and the CD94/NKG2A heterodimer Antibodies that target KIRs, either by themselves or in conjunction with other therapeutic drugs, can increase the anti-tumor activity of NK cells. Furthermore, monalizumab new anti-NKG2A antibody, is presently undergoing clinical trials to assess its anticancer potential. Antibodies that target NKG2A also shown efficacy in inducing NK cell responses. Crucially, activating receptors might be used in addition to inhibitory receptors, for example, by delivering cytokines to increase either by introducing antibodies that coat target cells to induce NK cytotoxicity or by expressing them [14].
Andrade et al. created antibodies to stop human cancer cells from losing cell surface MICA and MICB, two stress-induced molecules that are detected by activating NKG2D receptors on NK cells. They discovered that these antibodies inhibited tumor growth by enhancing anti-tumor immunity that is primarily mediated by NK cells. This is a promising example of such strategies. All things considered, using NK cells for medicinal purposes is a viable choice that merits more research.
Myeloid cells
Granulocytes and mononuclear phagocytes are two examples of the many cell types that make up myeloid lineage cells, which have been demonstrated to be essential for tumor immunity. The most prevalent granulocyte subtype, neutrophils, are known to have a role in innate defence against bacterial and fungal infections, although their functions in tumor immunity are still up for debate. While Ponzetta et al. discovered that neutrophils were crucial for the polarization of a subset of unconventional T cells with an innate-like phenotype, which benefited anti-tumor immunity, Szczerba et al. demonstrated that neutrophils escorted circulating tumor cells (CTCs) within the bloodstream and facilitated the metastatic potential of CTCs. Furthermore, fridlender et al. found that neutrophils in the TME exhibited varying stages of activation, with the N1 phenotype adopting an anti-tumorigenic role and the N2 phenotype is acting in a protumorigenic manner. The functional variety of tumor-associated neutrophils (TANs) is shown by these data taken together [15].
Clinical trials have demonstrated strong responses to adoptive cell therapy, which uses autologous, ex vivo-expanded tumor-infiltrating lymphocytes (TILs) to treat solid tumors. Lifileucel is currently the first TIL cell therapy product to get FDA approval in the United States because to its promising effectiveness, manageable safety profile, and improvements in a single production process. To guarantee that this modality is successfully incorporated into clinical care, treatment management and delivery practice guidelines are required. The TIL Working Group, which is made up of globally renowned hematologists and oncologists with expertize in TIL cell therapy, produced clinical and toxicity management recommendations for the TIL cell treatment regimen. These guidelines relate to patient care and operational elements suggestions for patient care based on expert consensus. In the context of possible standard-of-care TIL use, expert consensus recommendations for patient management are discussed, including screening tests, patient eligibility, and clinical and toxicity management with TIL cell therapy, including tumor tissue procurement surgery, non-myeloablative lymphodepletion, TIL infusion, and IL-2 administration. These suggestions offer helpful pointers for the best clinical care throughout the TIL cell treatment regimen's administration and for identifying and managing toxicities later on. The multidisciplinary teams of doctors, nurses, and other stakeholders that provide care for these patients are the main emphasis of these recommendations [16].
Current clinical state of TIL therapy in melanoma
TIL treatment has worked when tried on the unusual and difficult-to-treat uveal melanoma, first indicated in 2017 [17]. The complete response (CR) and partial response (PR) among patients followed up receiving TIL and HD-IL-2 was 4.5% and 31.8%, respectively [18]. A notable step was Iovance’s implementation of a successful phase II trial of TIL product lifileucel (LN-144) in 2021 that was done on patients with advanced melanoma after their disease progressed on ICI [19]. Mean amounts of TILs received by patients was 2.73 × 10^10, with 80% disease control rate (DCR), 36% objective response rate (ORR), 3% complete response (CR), and 33% partial response (PR) [20]. It is encouraging to note that TIL therapy is an option poised to treat patients with advanced melanoma disease after ‘progression following immunotherapy’ [21]. Other authors have reported more objective clinical response rate among patients with advanced melanoma to be 50% with two complete response and three partial response patients [22]. Currently, TIL therapy is being evaluated as 2nd line therapy but melanoma remains the most evaluated tumor type in several clinical trials [23].
Successful application of TIL therapy in other solid tumors
Besides melanoma, TIL therapy has shown impressive clinical benefits for patients with cervical cancer and has shown prelimin efficacy in colorectal cancer cholangiocarcinoma, non-small cell lung cancer as well as breast cancer [24]. HPV viruses have forever been established as the cause of virtually all cervical cancers. A clinical study was conducted to verify if ACT with HPV-TILs could be used to reverse the course of metastatic cervical cancer, and nine eligible cervical patients were recruited. The HPV-TILs were started and selected based on their reactivity against HPV E6 and E7. A single load of cold TIL-HVP was given to the treated subjects before them receiving a high amount of IL-2. Three patients had an objective tumor response, out of the five that received injection of two Tanya had total responses over one year, while the other two had partial responses [25]. Rosenberg et al. used this methodology to demonstrate the utilizing of whole-exome sequencing to further screen tumor-specific antigens through a personalized immunogenomic approach. Moreover, the team also carried out a phase II clinical trials on the effectiveness of TIL therapy in patients with metastatic disease associated with human HPV [26]. Though modest, these results lend support to the premise that TIL-ACT can mediate tumor regression and that there are great prospects in using TIL-ACT in the treatment of metastatic human HPV-associated cancers [27].
The TIL product of LN-145 worked well in patients suffering from advanced cervical cancer in a phase two clinical trial, surprisingly. The mean number of TILs administered was 2.8 × 1010; the overall response rate was 44% with one complete response, nine partial responses, and two unconfirmed partial responses [28]. In this context, the Food and Drug Administration (FDA) has given LN-145 the status of a breakthrough treatment. In the end, this landmark study has proved both the safety and effectiveness of this approach in patients with cervical cancer and will guarantee the further progress of adoptive cell therapy in these types of malignancies [29].
In 2014, Tran et al. were able to effectively expand neoantigen-specific TILs from a cholangiocarcinoma patient with metastasis. The patient experienced rampant improvement in liver and lung metastasis after having an IL-2 infusion along with TIL infusion containing substantial amounts of CD4 + neoantigen-specific T cells [30].
A 50 year old woman suffering from metastatic colorectal cancer was enrolled in a phase two clinical trial (NCT01174121) and was administered with a single infusion of TILs (1.48 × 1011 TILs which were CD8 + T cells capable of recognizing the KRASG12D mutant). In this patient, all metastatic lesions showed regression and they were able to achieve partial response for nine months [31].
In 2017, Lee et al. effectively confined and increased TILs in vitro from patients with breast cancer which appeared their response to autologous tumor cells. Zacharakis et al. administrated TIL treatment to patients with hormone receptor-positive metastatic breast cancer in 2018. In brief, the understanding experienced total and tough tumor relapse after accepting a combination treatment of TIL treatment and anti-PD-1 monoclonal antibodies. It is worth noticing that they screened out the TILs that were basically CD4 + T cells (62.5%), which seem recognize the mutant of four proteins (SLC3A2, KIAA0368, CADPS2, and CTSB) [32].
In 2020, it was detailed that the combination of PD-1 inhibitor and TIL treatment appeared preparatory adequacy within the treatment of NSCLC. In this stage one trial (NCT03215810), patients with metastatic NSCLC who advanced after nivolumab treatment were treated with TILs. Patients gotten the implantation of TILs and IL-2, taken after by nivolumab to increase the perseverance of the TILs. Two of 13 evaluable patients accomplished strong total reactions. The energizing comes about of this clinical trial offer trust for patients who are advancing after anti-PD-1 treatment, and show that TIL treatment combined with a PD-1 inhibitor may be a promising choice for patients with metastatic NSCLC. As of now, a clinical trial utilizing ACT with TIL for the treatment of NSCLC is enlisting patients (NCT04614103), and a few trials of TIL combined with PD-1 inhibitor for the treatment of NSCLC have been propelled (NCT03903887, NCT03645928) [33].
An outline of the general procedures for TIL cell therapy
The procedure starts when the main oncologist discusses therapy alternatives and does a preliminary assessment to determine whether TIL is appropriate [34]. It would be good to refer potentially eligible patients to an authorized treatment center (ATC) if they choose to pursue TIL cell therapy. Centers accredited to provide TIL cell therapy as part of a clinical study or standard of treatment are known as TIL ATCs [35]. In order to qualify as an ATC, the centers must have care team members who have completed a TIL treatment training program and/or treatment teams that have treated patients with TIL cell therapy [36]. After a patient has been When a patient is thought to be a good candidate for TIL cell treatment, the necessary supplies for tumor tissue collection operations are acquired, and the TIL Good Manufacturing Practice (GMP) manufacturer—both in commercial and academic settings—is informed [37]. Together with the treating physician, the surgeon determines the best lesion or lesions and surgical strategy. Currently, TIL creation requires a tumor that is at least 1.5 cm in diameter (1.5–4 cm) [38]. The surgical team resects and prosects (trims and fragments) the tumor tissue under sterile conditions during a tumor tissue procurement surgery, frequently in conjunction with pathology (with the utmost care to ensure that any material used for pathology review is kept separate from the tissue used for TIL manufacturing) [39]. The tumor tissue is prepared fresh on the day of the tumor tissue procurement procedure and kept between 2 °C and 8 °C until the courier arrives for transport pick-up [40]. It is then transported for TIL manufacture in sterile medium containing hypothermosol, amphotericin B, and gentamicin41. In a commercial scenario, fresh tumor tissue is delivered to a centralized GMP facility to commence TIL, whereas in an academic or institutional setting, the TIL product is often created on site in a nearby certified GMP facility production [41]. Following manufacturing, the cryopreserved TIL infusion product is returned to the ATC and administered while being monitored by the treatment team [42].
Patients undergo a non-myeloablative lymphodepleting regimen (usually consisting of fludarabine and cyclophosphamide) for 5–7 days before to TIL infusion [43]. Depending on the treatment team's preference, this regimen may be given inpatient or outpatient [44]. Current TIL products involve either freshly manufactured TIL or cryopreserved TIL that has been thawed before infusion. After non-myeloablative lymphodepletion is finished, a brief course of high-dose bolus IL-2 is administered every 8–12 h for 2–5 days to support the growth and activity of the infused TIL [45]. Up to six doses of IL-2 were frequently given in research looking into lifileucel TIL cell treatment, while other studies looking into TIL cell therapy have utilized up to fifteen doses of IL-2 [46]. Hospitalization is now necessary for monitoring and supportive treatment during high-dose IL-2 administration and TIL infusion [47]. About 14 days following TIL infusion, patients are released when the hospital care team determines it is acceptable, following adequate hematological recovery and relief from any IL-2 toxicity [48]. After being discharged, some patients will need transfusion assistance for packed red blood cells and/or platelets, as well as hydration, thus careful monitoring and care coordination are required [49]. Patients are advised to stay near the treatment facility (within 30 to 50 miles or less than an hour) for a certain amount of time with a designated carer, just like with other cellular treatments. An acceptable milestone is thirty days following TIL injection, however the temporal frame may vary in length according on patient fitness, comorbidities, and treatment-related toxicities as well as institutional requirements [50].
Preparation of TIL
Although TILs exist generally within the tumor microenvironment, this does not cruel TILs can be effectively disconnected and increased from tumor tissues, nor does it cruel tumor cells can be recognized and disposed of by separated TILs [24]. The capacity of TILs to slaughter tumor cells will be inhibited by different variables within the tumor microenvironment, such as the presence of immunosuppressive subsets counting administrative T cells (Tregs), myeloid-derived silencer cells (MDSCs), and tumor-associated macrophages, as well as the increment in immunosuppressive atoms and metabolites [25]. Moreover, the number of TILs within the tumor microenvironment is little and troublesome to increase [26]. In this manner, it is basic to optimize the planning prepare of TILs [27]. In common, the era of TILs can be isolated into two steps: the pre-rapid development strategy (pre-REP), which is the beginning development organize of TILs from tumor tissue, and the large-scale development of the 14-day REP [28].
Unselected/young TILs
Early studies focused on pre-selecting tumor-reactive cultures that detected interferon-gamma (IFN-γ) for further outgrowth. However, this method limited the clinical application of TIL therapy, adding time to TIL expansion and making it difficult to obtain tumor cells or materials [29]. To simplify the generation process and improve TIL culture properties, a modified TIL production protocol was tested using "young" TILs, which have no pre-selection step for tumor reactivity based on IFN-γ. The clinical response rate of protocols using "young" TILs is not low, indicating rapid improvement in their status [30]. "Young" TILs have longer telomeres and higher levels of costimulatory molecules CD27 and CD28 compared to selected TILs, making them conducive to the proliferation, survival, and persistence of TILs in vivo [31]. TIL selection strategies involve screening effector T cells with anti-tumor activity, and various isolation strategies have been proposed and developed for this program [32]. This summary summarizes known strategies for isolating tumor-reactive T cells [33].
TIL selection strategies
The selection of TILs from the tumor microenvironment is crucial for the development of anti-tumor effector T cells. Isolation strategies have been proposed for this program, and this summary aims to summarize known methods for isolating tumor-reactive T cells [51].
Studies have shown that CD103 + TILs indicate strong prognostic significance in ovarian and oral cancer. In 2017, Ganesan et al. demonstrated that CD103 and CD8 are important phenotypes in the tissue-resident memory of T cells in lung cancers. Furthermore, Simoni et al. reported that CD39 could be a marker for recognizing tumor-reactive CD8 + T cells. In 2018, Doohen et al. proved that CD103 + , CD39 + , and CD8 TILs are unique tumor-reactive cells in the tumor microenvironment, which can be isolated from the tumor digests by immunomagenetic beads and fluorescence-activated cell sorting (FACS) [52]. The co-culture experiment of TILs and tumor cells showed that CD103 + , CD39 + , and CD8 TILs exhibited enhanced tumor reactivity. In 2020, Kortekaas and his colleagues highlighted that CD39 + T cells have specific tumor reactivity, and have further proven the importance of CD39 in identifying and isolating tumor-reactive T cells [53]. However, CD39 was also highly expressed on exhausted CD8 TILs with low proliferation potential, and in order to improve the proliferation potential of tumor-specific CD8 TILs a new sorting strategy with negative CD39 was defined [54].
PD-1/CD279 may be better described as a marker for tumor-reactive T cells to identify tumor cells, even if it has been described as an immunosuppressive checkpoint that is expressed on activated T cells and taken over by tumors to evade immune surveillance and create immunological tolerance [55]. Using immunomagnetic beads and the FACS approach, Inozume et al. pre-selected CD8 + PD-1 + T cells, which demonstrated higher tumor reactivity than CD8 + PD-1 − or non-selected TILs. This idea is directly supported by Fernandez-Poma et al.’s findings that the expression of PD-1 on CD8 + TILs precisely identified the tumor-reactive cells and assessed the anticancer activity of CD8 + PD-1 + TILs in vivo [56].
PD-1/CD279 may be better described as a marker for tumor-reactive T cells to identify tumor cells, even if it has been described as an immunosuppressive checkpoint that is expressed on activated T cells and taken over by tumors to evade immune surveillance and create immunological tolerance. Using immunomagnetic beads and the FACS approach, Inozume et al. pre-selected CD8 + PD-1 + T cells, which demonstrated higher tumor reactivity than CD8 + PD-1 − or non-selected TILs. This idea is directly supported by Fernandez-Poma et al.’s findings that the expression of PD-1 on CD8 + TILs precisely identified the tumor-reactive cells and assessed the anticancer activity of CD8 + PD-1 + TILs in vivo [57]. Nevertheless, because CD137 is a costimulatory molecule, prolonged stimulation causes it to become over-differentiated and impairs its capacity for proliferation. In order to identify TCRs and enrich tumor-reactive T cells, Parkhurst et al. suggested a method that screens up-regulates CD137 following antigen-specific stimulation [58]. It is then added to less-differentiated PBLs for reinfusion treatment. However, a long preparatory period is unjustified for the majority of patients with advanced metastatic disease. A streamlined and effective protocol was suggested by Seliktar-Ofir et al. In short, the standard rapid amplification procedures were performed after the CD137 positive TIL was immediately extracted from the co-culture system of TILs and tumor cells. Steps of TCR sequencing were skipped [59].
Selection by Neoantigens
For TIL treatment, it is essential to identify specific tumor antigens, especially neoantigens. To do this, Rosenberg and his colleagues employed whole-exome sequencing (WES) technology in combination with human leukocyte antigen (HLA) class I to identify and candidate the mutant proteins produced in tumor cells that vary from healthy cells. These modified epitopes are delivered to MHC-matched antigen-presenting cells (APC) once they have been synthesized [60]. The ability of the TILs to recognize was evaluated by ELISPOT testing of IFN-γ after they were co-cultivated with the original TIL. Before being cultivated in vitro, the identified neoantigen-specific CD8 + T cells can be further purified using FACS based on active markers such as CD137 [61]. The ability to recognize and detect neoantigen-specific TILs has increased with the development of the tandem minigene (TMG) library. Because TIL skips the process of discovering and synthesizing a large number of peptides, it has been further enhanced. Neo antigen-specific effector TILs have been effectively administered to patients with ovarian, breast, colorectal, melanoma, and cholangiocarcinoma cancers using these methods [62]. However, the majority of advanced patients should not use this procedure since it requires complicated equipment and takes a long time to prepare the TIL for reinfusion. Furthermore, prolonged antigen stimulation and amplification will also reduce TIL’s capacity to proliferate [63]. A possible remedy may be offered by Deniger and associates’ recent translocation of the TP53 “hot spot” mutation-reactive T cell receptor to peripheral blood T cells [64].
Pre-rapid expansion procedure
Tumor tissue can be obtained through surgery and processed quickly for the initial growth of tumor-reactive immune cells (TILs). These TILs can be cut into fragments or single-cell suspensions and cultured in a complete medium with IL-2 [65]. The tumor location may affect the production of TILs, as it may be contaminated with microbes or contain ineffective T cells. A modified TIL production protocol has been tested to simplify the generation process and improve the properties of TIL cultures [66]. "Young" TILs, which have longer telomeres and higher levels of costimulatory molecules CD27 and CD28, are conducive to the proliferation, survival, and persistence of TILs in vivo. Isolation strategies for isolating tumor-reactive T cells have been proposed and developed for this program [67].
Rapid expansion procedure
As soon as a sufficient amount of the original TIL is collected, it is either cryopreserved or used straight away for further REP, which typically takes around 14 days according to standard methodology. IL-2, anti-CD3 antibody (which is added just at the beginning of REP), and irradiation feeder cells activate and further increase TILs to treatment levels during the REP [68]. Allogeneic or autologous peripheral blood mononuclear cells, which can activate and release growth factors to encourage TIL proliferation, are the source of the irradiation feeder cells [69]. TILs are typically cultivated in a culture flask during the REP, and the cultures are subsequently expanded by moving them to gas-permeable bags. Jin et al. employed gas-permeable flasks to increase the TIL’s development rates for the initial growth and REP [70].
Role of lymphodepletion
An essential step in TIL treatment is a non-myeloablative (NMA) lymphodepletion regimen that includes chemotherapy or total body irradiation (TBI) prior to TIL injection [71]. The use of the lymphodepletion regimen is based on a mouse model where lymphodepletion increases TIL efficiency [72]. The elimination of Tregs, an increase in host homeostatic cytokines (such as IL-7 and IL-15), and a decrease in endogenous lymphocytes, which compete for these trophic cytokines, are some of the possible ways that the lymphodepletion regimen may increase the effect of TILs [73]. Additionally, lymphodepletion can increase antigen-presenting cell (APC) activation, which is crucial for controlling adoptively transplanted T lymphocytes [74].
Rosenberg et al. (1994) demonstrated that the ORR was 31% for patients with metastatic melanoma treated with lymphodepletion and 35% for those treated without. Likewise, in 2002, Dudley and associates showed that adoptive transfer of TILs might improve ORR in patients with metastatic melanoma following lymphodepletion with fludarabine (Flu) 25 mg/m2/day for 5 days and cyclophosphamide (Cy) 60 mg/kg/day for 2 days [75]. According to certain clinical research, the response rate may be increased by including TBI in the NMA chemotherapy regimen. However, a randomized experiment showed no discernible impact. The regimen of Cy (30 mg/kg for two days) and Flu (25 mg/m2 for five days) is a superior option, according to the recent research that compiled several NMA regimens [76]. Notably, the majority of patients have suffered from hematological side effects such neutropenia, lymphopenia, and coagulopathy brought on by the NMA lymphodepletion regimen. The majority of non-hematological problems, such as nausea, headaches, diarrhea, lack of appetite, neutropenic fever, and hyperbilirubinemia, may be controlled with normal supportive care [77]. It's interesting to note that Santos et al. created a regimen that substituted oncolytic adenoviruses for NMA lymphodepleting in order to circumvent the extreme toxicity of the former, and this approach proved to be quite effective [78].
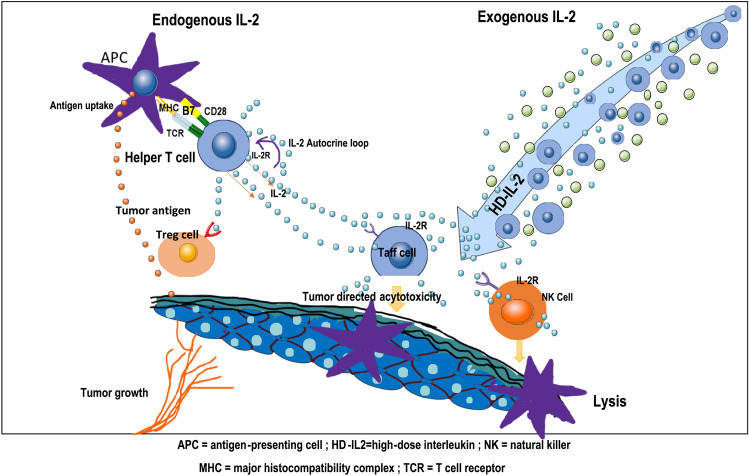
Role of interleukin-2
An essential cytokine, IL-2 promotes the survival and proliferation of effector T cells. When IL-2 was added to cultured lymphocytes, Rosenberg and his colleagues discovered that it encouraged the lymphocytes to lyse autologous tumor cells. Furthermore, they discovered that combining IL-2 with a TIL infusion might enhance its therapeutic impact. After that, Rosenberg used TIL therapy in conjunction with IL-2 to treat melanoma patients. In 1988, he published the first report on the effective use of TIL-ACT to treat melanoma.
Nevertheless, it has been observed that IL-2 is a necessary cytokine for the growth and operation of Tregs, which would compete with CD8 + T cells for IL-2 and inhibit the CD8 + T cells' response. IL-2 has been shown by Liu et al. to control tumor-reactive CD8 + T cell fatigue [79]. In short, excessive IL-2 causes CD8 + T lymphocytes to malfunction by upregulating their inhibitory receptors and decreasing their cytokine and effector molecule output. While IL-7, IL-15, and IL-21 can result in poorly differentiated T cells and increased proliferation. T cell fatigue and terminal differentiation may result from IL-2 [80]. The amounts of cytokines increases when IL-15 and IL-21 are introduced to the culture medium rather than only IL-2. The number of TILs cultivated from lung and colorectal tumors may rise if the cytokines IL-15 and IL-21 are added to the culture media as opposed to using IL-2 alone. Nevertheless, IL-2 is primarily used in the present clinical studies to sustain and grow TILs; more clinical trials are required to ascertain if these cytokines may take the position of IL-2 [81].
The ideal IL-2 infusion dosage has not yet been established. For decades, individuals with metastatic melanoma have been treated with a regimen of TILs and IL-2 since Rosenberg and his colleagues published their results [82]. When administered following TIL infusion, the HD-IL-2 (≥ 720,000 IU/kg) regimen has been shown to continually maintain the development and activity of infused TILs. Nevertheless, HD-IL-2 is linked to serious side effects, such as capillary leakage syndrome, which manifests as edema, hypotension, oliguria, and even hypovolemic shock. Thus, it is worthwhile to look at LD IL-2 (< 720,000 IU/kg) in combination with a TIL infusion. There is currently insufficient data to conclude that LD IL-2 regimens can provide a long-lasting response on par with HD-IL-2 regimens. These findings are encouraging, nevertheless, and need immediate attention as well as more clinical trial research. Hsu et al. have developed an IL-2 prodrug to address the issues of short half-life and high toxicity in vivo [83].
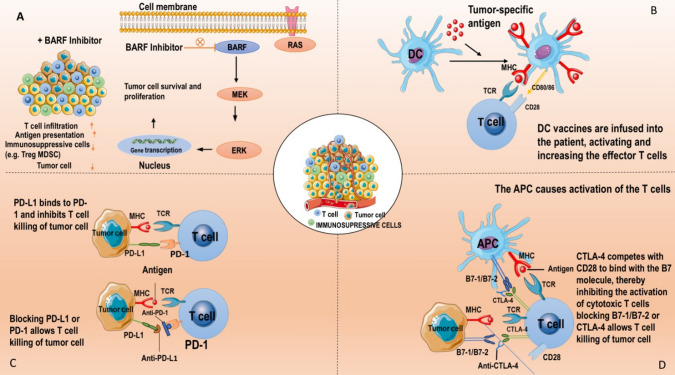
In several recent trials, the combination of TIL treatment and anti-PD-1/PD-L1 antibody therapy has produced encouraging first outcomes. T cell surface expression of immune checkpoint receptors (e.g., CTLA-4 and PD-1/PD-L1) is the immune system's self-defence strategy [84]. Effector T cells' CTLA-4 and PD-1 molecules are increased in cancer patients, and they bind to antigen-presenting cells' or tumor cells' B7-1/B7-2 and PD-L1 molecules, respectively. Anti-CTLA-4 and anti-PD-1 antibodies can prevent this, which leads to reduced T cell function. Furthermore, research has shown that CD8 + T cells will undergo apoptosis, or adopt an aberrant differentiation stage with high expression of inhibitory receptors and almost no responsiveness to particular tumor antigens, following prolonged exposure to tumor antigens [85]. Furthermore, these findings were confirmed by the in vivo treatment model. These processes provide TILs and ICIs a theoretical foundation. Therefore, the ICIs should be administered prior to the removal of tumor tissues and during the early proliferation of TILs in order to acquire enhanced tumor-reactive TILs. ICIs were also given following TIL infusion to increase the tumoricidal effect of infused TILs. Clinical trials are now being conducted to determine the effectiveness of TILs in conjunction with anti-PD-1 therapy as a first-line treatment. Until early findings are published, it is not possible to assess the additive impact of TILs and anti-PD-1. According to recent research, dendritic cells (DC) and tumor cells both exhibit high amounts of PD-L1, which can reduce the activation of anti-tumor activities and suppress T cells [86]. This will give TILs and PD-L1 inhibitors a theoretical foundation for treating cancer patients with elevated PD-L1 expression.
BRAF inhibitor
Cell development and differentiation are significantly influenced by the BRAF gene. BRAF mutations alter the ERK/MAPK signal cascade in some malignancies, which leads to an increase in cell proliferation [86]. About 50% of cutaneous melanomas have been shown to possess the BRAF mutation, which is the most prevalent mutation that results in overactivation of the MAPK pathway. immunological-escape mechanisms, which render them "dull" in immunity and enable them to elude T-cell immunological responses, can be induced by the activating BRAF mutation (mostly V600E) [87]. According to studies, vemurafenib, a BRAF inhibitor, can improve the presentation of melanoma antigens, decrease immunosuppressive cells, enable lymphocyte infiltration, and lessen associated immunosuppressive signals. When used to treat BRAFV600E mutant melanoma, the ORR of the BRAF inhibitor vemurafenib can reach 50%, increasing both the overall and progression-free survival rate. However, BRAF inhibitors often have a brief clinical response, and patients have fewer options for other therapy as the cancer worsens [88]. BRAF/MEK inhibitors have been shown by Peiffer et al. to stimulate the growth of T lymphocytes specific to melanoma in melanoma patients. In a recent clinical trial, seven out of eleven patients with metastatic melanoma who were treated with a combination of vemurafenib, HD-IL-2, and TILs had an objective clinical response; two of these patients had a full response. Notably, the DC vaccination can activate and enhance TILs and elicit an immunological response and clinical studies are now assessing its combination with TIL treatment. Additionally, the combination of oncolytic virus and TIL treatment is being investigated. By generating cytokines that enhance TIL’s anti-tumor impact, the virus can counteract tumor immunosuppression. Five out of thirteen patients in a clinical study using TIL therapy and adenovirus to treat metastatic melanoma experienced objective responses, with three of them achieving full responses [89].
| Combination strategy with TIL’s | Structure | Function | Type of tumor |
|---|---|---|---|
| Chemotherapy |
Cisplastin Methotrexate + Cisplatin + doxorubicin |
Increase the CR rate from 30 to 70% with RFS of 15 months Significantly increased DFS and OS compared to monotherapy with no additional adverse effects |
Ovarian Osteosarcoma |
| Immune checkpoint inhibitors [ICI] |
Anti-PD1\TIL Therapy Anti-PD1\TIL therapy Anti-CTLA4- TIL Therapy Anti-PD1 and Anti-CTLA4-TIL therapy |
Improved prognosis and enhanced survival time Reduced adverse effects and enhanced safety Improved anti-tumor immune response and increased survival time Manageable cytotoxicity and sizable tumor regression |
Cervical Metastatic osteosarcoma Metastatic melanoma Heavy and neck cholangiocarcinoma |
| Oncolytic virus |
Adenoma Virus TIL therapy Herpes simplex virus(HSV-1) Pox virus Adeno virus Reo virus Herpes simplex virus |
Desired TILs delivery system and increasing its cytotoxicity Increased T cell activation Enhance TIL s selectivity by TME altering Choosing the best virus to increase the performance of TIL therapy |
Pancreatic cell line Oral cancer Colon Solid tumors |
| Cancer factor |
Mutant peptide Mutant intracellular protein Whole tumor lysate of Dc vaccine Matured Dc vaccine in presence of IL −12 and Toll like receptor agonists |
Increasing survival rate Durable tumor regression Increase viability and safety of treatment Allogenic T cell activation co related with IL-12 |
Melanoma Metastatic Melanoma Metastatic Melanoma Melanoma |
Best practices and guidelines for the clinical care of patients undergoing TIL cell therapy
Identification and selection of patients
Timely and effective planning and operational execution are essential given the difficulties in controlling advanced illness and the several procedures involved in TIL cell treatment. A multidisciplinary team comprising the surgeon, medical oncologist, and frequently a cellular therapy or hematopoietic stem cell transplant specialist works together to identify patients for TIL cell treatment. A multidisciplinary tumor board should be the setting for case discussions. Patients who are currently being considered for TIL cell therapy will have advanced disease that has progressed on previous lines of treatment, such as an antiprogrammed cell death protein 1 (anti-PD-1)–containing regimen. However, they must be healthy enough to endure surgical procedures, non-myeloablative lymphodepletion (NMA-LD), cell infusion, and IL-2, as well as to be able to wait for TIL manufacturing and infusion scheduling, which may take anywhere from 22 to 60 days. For TIL to be manufactured, patients must have at least one tumor lesion that can be removed, and in clinical studies, an extra site of detectable disease has been needed to gauge response. It is uncertain if it is effective when the illness has been fully removed [90].
TIL therapy-related short- and long-term toxicity
Since TIL therapy side effects have mostly been linked to chemotherapy or IL2, almost all patients receiving treatment experience Common Terminology Criteria for Adverse Events (CTCAE) grade 3–4 treatment-related adverse events (TRAE). While neutropenia puts patients at high risk of infection, conditioning chemotherapy with cyclophosphamide (60 mg/kg) and fludarabine phosphate (25 mg/m2) causes reversible pancytopenia, requiring repeated transfusions of red blood cells (RBC) and thrombocytes in the majority of patients. In a group of patients with and without melanoma undergoing TIL treatment and low-dose IL2, Kverneland and colleagues assessed the immune system's recovery following lymphodepleting chemotherapy. When granulocyte colony-stimulating factor (G-CSF) was given in this trial, the median duration of neutropenia was six days [91].
TIL-related biomarkers
The effectiveness of TIL therapy may be influenced by a number of factors pertaining to patient conditioning and the manufacturing process before cell product injection.
TIL expansion seems to be insensitive to the location of the removed lesion. Patients with various metastasectomy locations, including brain metastases, have shown objective responses [92]. 48Anatomic sites that permit minimally invasive procedures and reduce complications (such as lymph nodes or subcutaneous/soft tissue nodules) are preferred because myelotoxic CT will be needed a few weeks after surgery. On the other hand, sites that have a higher risk of bacterial contamination (such as skin, bowel lesions, preirradiated, or ulcerated tumors) should be avoided at all costs [93].Due to bystander non-tumor-reactive antibodies, procurement from secondary lymphoid organs (such as the gut or spleen) may provide challenges. Lymphocytes have the ability to grow within the culture in a preferred manner.49As opposed to necrotic, hemorrhagic, or adipose regions, which have a negative correlation with TIL growth, lymph nodes or peripheral tumor sites near blood or lymphatic arteries are recommended. The effectiveness of TIL therapy may be influenced by a number of factors pertaining to patient conditioning and the manufacturing process before cell product injection [94]
TIL expansion seems to be insensitive to the location of the removed lesion. Patients with various metastasectomy locations, including brain metastases, have shown objective responses. Anatomic sites that provide minimally invasive treatments and reduce problems (such as lymph nodes or subcutaneous/soft tissue nodules) are preferred since myelotoxic CT will be needed a few weeks following surgery, but those with a Avoid areas with a higher risk of bacterial contamination, such as skin, intestinal lesions, preirradiated, or ulcerated tumors. [95].Because of the possibility of bystander non-tumor-reactive lymphocytes preferentially growing within the culture, procurement from secondary lymphoid organs (such as the intestine or spleen) may present challenges [96]. As opposed to necrotic, hemorrhagic, or adipose regions, which have a negative correlation with TIL proliferation, lymph nodes or peripheral tumor sites nearer blood or lymphatic vessels are recommended [97]. A brief ex vivo cell culture period is a reliable indicator of TIL growth and TIL-ACT responsiveness. Longer culture times have been shown to impair TIL function since the early investigations.51 52 The median TIL age at REP beginning was considerably lower among responders (13.2 days vs 19.7 days; p < 0.001) and < 20 days in all of them in a study of 31 MM patients with 15 objective responses (ORR 48.4%)0.47 Besser et al.46 found that 20 MM patients had similar outcomes. Patients with objective responses and persistent tumor-reactive T cell clonotypes have been reported to have longer telomeres in the infused TIL, which are inversely linked with culture time53.7.According to Shen et al., TIL was unable to cause telomerase activity in vivo, seeing a sharp decrease in telomere length a few days following injection. For "older" TIL with shorter baseline telomeres, this might result in quick replicative senescence, indicating that "young" TIL is those that can endure and mediate anti-tumor effects [98]. The idea that cell senescence is a harmful indicator is supported by the observation that "young" TIL with an earlier differentiation state appears to have greater levels of expression of the costimulatory markers CD27 and CD28. For TIL-ACT, adequate conditioning is typically regarded as a prerequisite. By inducing antigenic presentation, eliminating immunosuppressive CD4 + regulatory T cells (Treg) and natural killer (NK) cells, and lowering TIL competition for stimulating cytokines, lymphodepletion improves the anti-tumor response of infused T cells [99]. Patients who experience more severe lymphodepletion have been found to have higher serum levels of lymphocyte homeostatic cytokines (IL-7 and IL-15) and proinflammatory cytokines (IL-2, IL-6, IFN-γ, TNF-α, and IL-1α)58 60. The tumor-reactive CD8 + TIL ratio and treatment effectiveness have been linked to the degree of lymphodepletion in various studies. Despite their usefulness as predictive biomarkers, these findings suggest that elevated serum levels of these cytokines may serve as a stand-in for sufficient pretreatment conditioning [100].
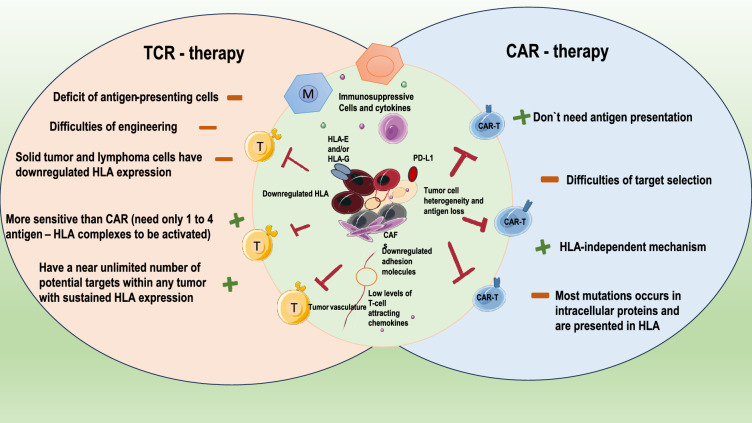
The drawbacks of TIL therapy and prospects for the future
Patients with metastatic melanoma and other solid tumors have benefited from TIL treatment. TIL treatment provides distinct benefits over other ACTs (such CAR-T and TCR-T). TILs are more successful in responding to tumor heterogeneity because they are made up of T cells with various TCR clones, which can act against tumor-specific neo antigens in addition to directly acting against common self-antigens [101]. TILs are simpler to find in the tumor tissue after transfusion because they often have a high number of effector memory T cells and express chemokine receptors after being triggered by the tumor antigen in vivo. Furthermore, TILs are produced directly from patients without gene alteration, indicating TIL treatment does, of course, have certain drawbacks. First off, effector T cells with anti-tumor activity need to be present in the tumor to produce long-lasting anti-tumor responses, but this isn't always the case for solid tumors. The NK cells, on the other hand, are more feasible to produce. A number of inhibitory elements in the cancer microenvironment can readily impact NK cells, which are often suppressed in cancer patients [102]. Tumor-infiltrating γδT cells are another possible translational anti-tumor cell type. They are independent of tumor-associated antigens and typically carry out their anti-tumor action by secreting interferon (IFN)γ and tumor necrotic factor (TNF). Numerous techniques, such as topical injection, bispecific compounds, and combination therapy, have been used to address the homing issues of γδT cells that the technique has little toxicity [103].
Second, even though TIL selection techniques have advanced significantly, the broad use of TIL therapy in a range of cancers remains difficult because of the difficulty in effectively identifying and isolating neoantigen-specific lymphocytes and the barrier of the immunosuppressive tumor microenvironment. T cell receptor-engineered T cell (TCR-T) treatment, in contrast to TIL therapy, alters endogenous T cell surface TCRs to identify tumor-specific neoantigens. TCR-T therapy, a possible cellular immunotherapy for the treatment of different malignancies, is therefore unrestricted by the target cells' surface antigen expression. Importantly, individuals with TP53 mutations may benefit from a novel therapy thanks to the TCRs that react to these mutations, which were discovered by Deniger et al. may provide patients who express the matching HLA types and carry these mutations a novel therapy. Furthermore, CAR technology is a viable method for altering TILs to recognize tumor-associated antigens or neoantigens and improve the therapeutic efficacy of TILs, as CAR-T cells are not necessary in APC presentation to operate against antigen-positive cancer cells. Through the transduction of a CAR to operate against the common tumor antigen Her2, a recent work by Mills et al. created dual-specific TILs (anti-Her2 CAR-TILs); further research shown that anti-Her2 CAR-TILs could function against Her-2 positive tumors both in vitro and in vivo. However, it is challenging to construct universal CARs-TILs since tumor cells often exhibit a variety of gene alterations that will produce a wide range of neoantigens cytometry [104].
In the meantime, the immunosuppressive tumor microenvironment may cause invading cytotoxic T cells to become exhausted, which lowers the cancer cells' capacity to be eliminated. Therefore, it is necessary to investigate novel particular fatigue indicators. The properties of heterogeneous TILs may be better understood with the development of new technologies, such as single-cell level analytic technologies; novel T cell markers or TIL subsets may be discovered with therapeutic potential or function as targets against solid tumors. The zinc-finger transcription factor Gata-3 was found to be a regulator of CD8 + TIL dysfunction after Singer et al. used single-cell RNA sequencing to distinguish between the activation and dysfunction gene modules in defective CD8 + TILs. Wagner et al. discovered high-grade ER + breast tumors infiltrated using single-cell mass Furthermore, the injected TIL has a brief in vivo survival period. TIL modification technology has been investigated to enhance the survival and tumor-homing capacity of TILs following reinfusion into patients. Interestingly, the primary sites that allow lymphocyte entrance into tumors are the tumor-associated high endothelial venules (TA-HEVs), according to research from the Jean-Philippe Girard research group at the University of Toulouse in France. The effectiveness of ICI can be enhanced by increasing the density and maturity of TA-HEV endothelial cells (TA-HECs), which can encourage tumor-specific CD8 + T cell infiltration. Additionally, the effectiveness of ICI in melanoma patients is directly linked to TA-HEVs, which might be a strong predictor of clinical therapy and a possible target of combined cell therapy. Although TIL therapy offers certain special benefits in the treatment of solid tumors, there are still a number of obstacles to overcome. The primary barrier to TIL treatment remains the tumor immunosuppressive microenvironment. Additionally, the isolation and growth of efficient tumor-reactive T cells still require significant improvement, and other combination treatments still require investigations [105].
Differentiating TIL treatment characteristics in solid tumors and the latest developments in clinical trials
The following characteristics of solid tumors might represent significant obstacles to the development of successful adoptive cellular therapy. The considerable heterogeneity of solid tumors makes it difficult to identify a perfect target for every tumor cell, in contrast to hematological malignancies with lineage malignancy. When a single tumor antigen is targeted, more aggressive clones typically return or the antigen is lost. Furthermore, even after adoptively transferring a high number of T cells, a significant portion of solid tumors are difficult to infiltrate. Furthermore, a number of immune-suppressive mechanisms make it challenging for T cells to perform their full function in the tumor microenvironment (TME), including but not limited to restricted to the existence of immune regulatory subsets including Tregs, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), the downregulation of costimulatory molecules, and the overexpression of immune inhibitory chemicals, cytokines, and metabolites. When it comes to treating solid tumors, TIL could have some unique benefits. First off, compared to other adoptive cellular therapies like chimeric antigen receptor T (CAR-T) and TCR-T cell therapy, TIL may be more effective at addressing tumor heterogeneity because it is made up of T cells with multiple T cell receptor (TCR) clones that can recognize a variety of tumor antigens. Accordingly, TIL has proven to be more clinically effective than CAR-T in solid tumors with a high mutation load, such melanoma. Second, effect memory T (Tem) cells, which express chemokine receptors such CCR5 and CXCR3 on their surface, likely to make up the majority of TIL after being triggered by tumor antigens. TIL can readily combine with their tumor-specific TCRs to home to tumors and other anti—genetically different tissues after being introduced into patients. Finally, because TCRs of TIL are negatively selected during the early development of T cell immunity, off-target damage has seldom been seen in TIL treatment. Conversely, if the affinity-enhanced TCR in TCR-T products or the tailored tumor-targeting single-chain variable fragments (scFv) in CAR-T exhibit cross-reactivity with antigens on normal tissues, they may be hazardous [106].
Development of next-generation TIL therapy
Next-generation TIL products are being actively researched to increase the in vivo survival and function of classic TIL treatment while also reducing toxicities linked to high-dose IL-2. Next-generation TIL is TIL that has been genetically altered to either knock off (KO) the target gene using tools like CRISPR or TALEN, or overexpress a gene of interest by viral transduction. However, there can be some significant obstacles in the way of the creation of next-generation TIL. Because TIL has a different cellular makeup and development rate than PBMC, gene editing in TIL can be technically challenging. TIL from metastatic melanoma may be transduced by retrovirus using a technique reported by Forget et al., with transduction efficiencies ranging from around 31% to 58% and effective expansion to clinically meaningful quantities; nonetheless, individuals' transduction and expansion rates varied greatly. Furthermore, choosing the right genes to target in TIL is crucial yet difficult. Up until now, the majority of next-generation TIL clinical trials have concentrated on modifying TIL to overexpress cytokines like IL-2 and IL-12. IL-2 insertion into TIL demonstrated improved survival in vitro following IL-2 withdrawal, but there was no increase in persistence in vivo and therapeutic effectiveness in a phase I/II study [24]. Compared to ~ 50% ORR with non-modified TIL and high-dose IL-2, just 2/12 (17%) ORR was seen in individuals who received IL-2 transduced TIL. In metastatic melanoma, a different phase I trial employing TIL transduced with IL-12 expressed under the control of a nuclear factor of activated T cells (NFAT)-inducible promoter demonstrated positive clinical efficacy—patients treated with 0.3 − 3e9 NFAT-IL-12 engineered TILs achieved a 63% ORR, which is 10–100 times lower than the cell number used. However, there was also notable toxicity and an unexpectedly high amount of IL-12 and IFN-γ in the serum. However, preclinical testing is also being done to determine the viability and usefulness of genetically going T cell negative regulators in TIL, such as PD-1 and CISH. It is currently unknown how to logically choose or pinpoint the target genes that will provide the most advantages for TIL treatment. Using CRISPR technologies for screening, such as CRISPR KO, interference (CRISPRi), or activation (CRISPRa), may be a potential method to find new targets for TIL in this context. Furthermore, before entering the clinic, genetically engineered TIL must undergo a thorough safety and function assessment. Animal models continue to be used in addition to in vitro characterization [107].
Current advancements in TIL therapy
Mice with micrometastasis from different tumor types were used in the initial research employing adoptive transfer of TILs expanded in interleukin-2 (IL2). According to these studies, TILs were 50 to their potency is 100 times greater than that of lymphokine-activated killer cells. In this study, mice with adenocarcinoma that received cyclophosphamide, TIL, and IL2 showed a 100% cure rate for advanced hepatic metastases and a 50% cure rate for advanced pulmonary metastases. Rosenberg and colleagues conducted the first in-human series of Phase I/II clinical trials employing a TIL therapy treatment at the NIH in the late 1980s and early 1990s. In these trials, patients received autologous TILs in addition to high-dose IL2 administration and lymphodepleting conditioning [108]. Clinical trials are presently being conducted to evaluate the effectiveness of TIL therapy in treating solid tumor indications, despite the fact that the FDA has not yet approved any TIL therapies.
According to one retrospective analysis report, TIL therapy was used to treat 21 patients with histologically confirmed metastatic cutaneous melanoma who had no other options for standard-of-care treatment between 2011 and 2019. The study's findings included an overall response rate of 67%, a complete response rate of 19%, an 86% disease control rate, and a median overall survival of 21.3 months. Overall, -month overall median survival. Five patients in total experienced long-lasting, continuous responses longer than 30 months after infusion, and those who experienced a full response stayed alive and free from illness. The reliance on high doses of IL2, which can result in toxicities for patients, is one of the drawbacks of TIL therapy. 18 One pilot study examined the infusion of the TIL product followed by low-dose IL2 in order to address this problem, and it found that even at low IL2 dosages, comprehensive and long-lasting responses were obtained. TIL therapy's beneficial effects on melanoma have prompted numerous centers around the world to carry out research to either replicate of maximize this procedure. PD-1 blockade or an anti-PD-1 combination therapy is currently the first line of treatment for patients with advanced melanoma [109]. Clinical trials are currently being conducted on the use of TIL in conjunction with anti-PD-1, in addition to the.
additional clinical studies that are currently assessing the best ways to treat TIL production. Between 2011 and 2020, there were TIL therapy trials (Fig. 1A), involving different types of TIL products. Of these, 54% were open, 17% were completed, and the remaining ones were closed or terminated; more than 50% of the trials were carried out in the USA, and 15% were in China. TIL therapy may eventually become the standard of care for late-stage solid tumors, particularly advanced melanoma, after showing encouraging results in Phase I/II clinical trials. Refractory, relapsed, and Patients with metastatic cancers have limited options if their disease worsens or does not respond to immune checkpoint inhibitors, and metastatic cancers represent a significant unmet medical need. TIL treatment does have certain drawbacks though [110].
Fig. 1.
Tumor Infiltration Lymphocyte (TIL) Therapy
Although effector T-cells with anti-tumor activity must be present in the tumor to produce strong anti-tumor responses, this is not always the case in solid.
tumors. The immunosuppressive nature of the tumor microenvironment can wear down cytotoxic T cells that have infiltrated, reducing the capacity of cancer cells to be eradicated. Furthermore, IL2 is commonly used to stimulate TIL activity and growth, but it can also cause systemic toxicity and encourage suppressive Tregs in the TME. To optimize this treatment for solid tumors, ACT engineering or combination therapy with additional agents that enable ACT to overcome these obstacles will be required. The effectiveness of TIL-based treatments is limited, but there are ways to improve this procedure [111].
The capacity to increase TILs ex vivo offers a chance to alter and improve the.
infusion product using techniques such as genetic engineering, T cell metabolism modification, and enrichment of tumor-reactive TILs. Given that these reactions will take place gradually and activate several immune compartments, it is widely accepted that TIL therapy will work best when used in conjunction with other immunomodulatory treatments.
T cells, B lymphocytes, macrophages, natural killer cells, and dendritic cells—collectively referred to as TILs—are among the many different types of cells that make up the tumor stroma. Polyclonal, primarily tumor-specific CD8 + and CD4 + T cells make up the majority of this cell mixture. These cells can bind to multiple TAAs, overcoming tumor heterogeneity and antigen escape. These cells lose their capacity to attack the tumor because of the immunosuppressive TME, but they become reactivated to target the neoplastic cells when they are removed from the tumor and grown ex vivo. Rosenberg and associates were the first to show that autologous TIL therapy was a useful anticancer treatment for patients with metastatic melanoma. They expanded TILs in vitro after removing them from the patient's own tumor. Figure 2 illustrates the in vitro T cell expansion procedure. The patients who had lost lymph were given the cryopreserved cells again. In the intensive care unit, the patients subsequently received multiple doses of weight-based IL-2 immune modulation. Eleven out of the twenty patients treated in this trial experienced tumor regression, which was greater than the objective response rates attained with IL-2 or lymphokine-activated killer cells given alone. Early TIL trials in lung cancer produced poor results, despite the high burden of clonal mutations and the high number of tumors neo antigens in NSCLC that could predict a positive response to immunotherapy, likely because of the development of neoantigen-specific T cells that guide anti-tumor immunity. None of the patients in Kradin et al.' s initial clinical trial of TILs in lung cancer patients saw a reduction in total tumor burden of more than 50%. In a different study with NSCLC patients, low response rates were also observed. In their post-operative testing of TILs in stage II and III non-small cell lung cancer, Ratto et al. discovered that 18 of 113 cultures failed to grow. The remaining individuals were assigned at random to be treated normally as opposed to TILs. Patients who received TILs had a higher three-year survival rate. Stage IIIB patients benefited, but stage II patients did not, indicating the potential viability of TILs in NSCLC (Figs. 3, 4 and 5).
Fig. 2.
T-Cell biology and tumor microenvironment
Fig. 3.
Over view of predictive biomarkers for response to ICIs
Fig. 4.
Dual mechanism of action of interleukin—2
Fig. 5.
The combination of TIL therapy and other therapies. The following four methods of combined therapy are described in the form of figures A a BRAF inhibitor can reduce immunosuppressive signals and facilitate tumor infiltration of lymphocytes; B DCs are stimulated by signals and loaded with tumor-specific antigens on MHC to activate the antigen-specific T-cells; C PD-L1 or PD-1 blockers may prevent the interaction of PD-1 and PDL-1 between TILs and tumor cells; and D blocking CTLA-4, which competes with CD28 to bind the B7 molecule, allows T cell killing of tumor cell
This type of treatment has the potential to be a breakthrough for a deadly disease with few second-line therapy options due to improved understanding of TME and tumor biology, including the immunological composition of tumors, immune checkpoints, and immune exhaustion. Although the exact lymphocyte subtype responsible for TIL therapy's efficacy is still unknown, it seems to be connected to neoantigen reactive T cells in the TME. It has been shown that FOXP3 + T cell infiltration results in a lower overall survival (OS) in patients with non-small cell lung cancer (NSCLC), whereas higher levels of CD8 + , CD4 + , and CD3 + T cells in the tumor stroma are linked to better OS. (Fig. 3). Furthermore, the interaction between CD4 + T cells and CD8 + T cells that have infiltrated tumors may be more significant in slowing the development of NSCLC than their isolated presence alone, since CD4 + T cells contribute by releasing cytokines like IL-2 (Fig. 4), which stimulates the growth and multiplication of CD8 + T cells. However, the presence of T regulatory cells has been linked to tumor growth and metastasis and promotes the spread of immunosuppressive TME. Avi and colleagues investigated the viability of TIL therapy in patients with non-small cell lung cancer (NSCLC) using their enhanced knowledge of lung tumor immunobiology and technological advancements. Five NSCLC patients had multiple TIL cultures that they were able to successfully isolate and grow.
This preclinical evaluation's findings demonstrated that TILs are feasible in a population with unmet needs Creelan et al. evaluated the safety and effectiveness of TILS on patients with pretreated non-small cell lung cancer in a recent phase I single-arm clinical trial. Patients with EGFR or ALK translocations who had advanced on at least one prior approved TKI were included in this trial. Individuals who had received immunotherapy before the clinical trial and those with active brain metastases were not included. Patients who had never used PD-(L)1 blockade were chosen in order to lower the percentage of T cells that have reached terminal differentiation in culture. Peripheral blood lymphocytes are being genetically modified for autologous cellular therapy protocols in an attempt to increase the effectiveness of TILs. In the majority of these genetic engineering techniques, T cells' programmed cell death protein 1 (PD-1, PDCD1) is eliminated, preventing it from interacting with antigen-presenting and tumor cells' programmed death ligand 1 (PD-L1). In addition to improving TIL effectiveness, PD-1/PD-L1 axis manipulation (Fig. 5) may also prevent immunotherapy side effects caused by T cells impacted at non-tumor sites. Zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR-Cas9 are frequently employed gene-editing methods. However, this strategy did not produce an effective anti-tumor response or result in prolonged TIL survival in vivo in melanoma patients. Previous in vitro studies had demonstrated that genetically engineering TILs to express IL-2 could prolong their survival after IL-2 withdrawal while maintaining their tumor specificity and function. Additional strategies to improve TIL effectiveness have included genetically modifying T cells to withstand exogenous TGF-β signaling, which is known to suppress tumor-specific cellular immunity and introducing the CXCR2 gene into tumor-specific T cells to improve their tumor-specific migration, localization, and ultimately anti-tumor response [112].
TIL production begins with the enzymatic digestion and/or fragmentation of newly obtained tumor tissue to isolate TILs. These TILs are then cultured as single-cell suspensions or as tiny tumor fragments in culture medium containing high levels of interleukin-2 (HD-IL-2). Following an initial TIL outgrowth that is accompanied by the removal of malignant cells, a rapid expansion protocol employing polyclonal T-cell stimulation with soluble anti-CD3 antibodies and irradiated allogeneic feeder cells in the presence of HD-IL-2 allows the TILs to grow to extremely high cell numbers. Approximately 4–6 weeks are needed for this manufacturing process, which produces 5 × 109–2 × 1011 cells, the majority of which are CD3 + T cells. Patients receive lymphodepleting chemotherapy, which attempts to eliminate endogenous cytokines, before receiving an intravenous infusion of the expanded TILs. In order to create physical space for the survival and growth of the transferred TILs, cytokine sinks consume the homeostatic cytokines IL-7 and IL-15. Although little is currently known about these effects, it is likely that the chemotherapy also affects the myeloid compartment. Lastly, intravenous boluses of IL-2 are given to patients after TIL infusion to promote the infused cells' continued growth. Recent research has demonstrated that, despite the robustness of this manufacturing process, the final TIL infusion products still exhibit a considerable degree of heterogeneity with respect to antigen specificities and T-cell differentiation stages. Three significant findings have been noted: Our group and others have demonstrated that a significant portion of TILs, referred to as "bystander" TILs, recognize antigens unrelated to cancer, while only a small percentage of tumor-resident TILs in human cancers are able to recognize autologous tumor cells. TIL’s express high or elevated levels of inhibitory molecules, such as PD-1, LAG-3, and TIGIT, and exhibit characteristics of tissue residency and prior antigenic stimulation. They can also exist in various states of T cell dysfunction. As an alternative, they are members of the so-called progenitor dysfunctional T cells, which are capable of self-renewal. According to spatial transcriptomics and proteomics, tumor-reactive TILs may be found near other immune and tumor cells or in clusters within tertiary lymphoid structures. Designing innovative production protocols that aim for TIL products enriched for tumor reactivity and optimal functional states—which we believe are necessary to overcome some of the current limitations of TIL therapy—will be made easier with this knowledge [113]. We must first gain a better understanding of which antigens may cause anti-tumor reactivity in order to increase the number of tumor-reactive cells and thereby increase TIL efficacy. There are various subclasses into which tumor-associated antigens (TAA) can be separated. TAA is separated into shared and private antigens according to a commonly used classification [114]. Antigens from cell-lineage specific proteins, cancer/testis gene products, and overexpressed self-antigens are found in the first group. Neoantigens are antigens that fall into the latter category. Tumor-specific mutations produce mutant epitopes, or neoantigens. Neoantigens are not native to the immune system, unlike the majority of tumor-associated shared antigens. As a result, T cells that are specific for these antigens have not been subjected to negative thymic selection, which increases the possibility of discovering high affinity TCRs against these antigens [115]. Neoantigens are appealing targets for T-cell therapy because of their potential for high affinity T cells against these antigens and their lack of expression on healthy cells. Unknown and possibly undervalued is the role that T cells that target common antigens play in the clinical response to TIL therapy. Selection for neoantigen-specific T cells may be used to enhance the tumor-reactive potential of TIL therapy, as evidenced by findings that the frequency of neoantigen-specific T cells in TIL products is linked to an improved response following TIL therapy. One of the challenges with this approach is that translation and processing are currently not taken into consideration when identifying appropriate neoantigens using algorithms that predict binding to the patient's HLA molecules [116]. Less than 2% of the predicted neoantigens in tumors elicit T-cell responses, according to a recent study by Parkhurst et al. This suggests that T-cell responses against these predicted neoantigens need to be validated, but it may also offer important insight into the range of T-cell repertoires against these antigens. Based on phenotypic markers, novel techniques have recently been developed to directly identify tumor-reactive T cells in the tumor microenvironment (TME). According to several studies, CD8 + TILs that express PD-1, CD39, and CD103 are more reactive to tumors, particularly those that target neoantigens. To increase a TIL product's tumor-reactive potential, it might be enough to select for tumor-resident T cells that have these characteristics [117].
Molecular and cellular mechanisms underlying the function of TILs
TCRs, which are heterodimers made up of an α and β chain, have a very broad repertoire that enables T cells to identify various major histocompatibility complex I-presenting tumor antigens. To guarantee signal transduction and propel the antigen-specific immune response against the cancer cell, TCRs combine with CD3ϵ/γ/δ/ζ subunits after interacting with tumor antigens [118]. The TCR and CD3 complex plays an essential role in the activation of TILs and their anti-tumor effects by transducing extracellular signals to intracellular effectors. To enhance their ability to kill tumor cells, activated TILs release a variety of cytokines. Activated TILs, for instance, increase the expression of CD107a on the cell surface, which is linked to degranulation and cytotoxicity. Additionally, the release of IFN-γ, a crucial effector of TIL's anti-tumor activity, is increased by the cellular signals. Crucially, by upregulating apoptosis-related proteins like caspase-3, Bak, and Fas, IFN-γ can cause apoptosis in both Fas-high and Fas-low cancer cells. The anti-tumor function of TILs is further enhanced by activated TIL’s increased production of TNF-α, perforin, and granzyme [119]. TNF-α up-regulates death receptors 4 and 5 to trigger cancer-cell apoptosis via p53-dependent pathways. Remarkably, TNF-α can also increase cells' susceptibility to cell death caused by the Fas ligand (FasL). TILs release the chemicals perforin and granzyme, which have both been demonstrated to have anticancer effects in a variety of cancer types [120]. Granzyme B and perforin cause cancer-cell apoptosis and stop cancer-cell foci from forming. TILs release the chemicals perforin and granzyme, which have both been demonstrated to have anticancer effects in a variety of cancer types. Granzyme B and perforin cause cancer-cell apoptosis and stop cancer-cell foci from forming [121]. Granzyme B, mechanistically, causes reactive oxygen species to build up, which leads to dysregulated mitochondrial function and cytochrome c release, ultimately accelerating the apoptosis of cancer cells. The expression of death ligands, such as FasL and TNF-related apoptosis-inducing ligand (TRAIL), on the surface of TILs is up regulated when TCRs and tumor antigens interact [122]. Cancer-cell apoptosis can be induced by TRAIL and FasL, which can identify the TRAIL receptor and Fas on cancer cells, respectively. The expression of death ligands, such as FasL and TNF-related apoptosis-inducing ligand (TRAIL), on the surface of TILs is up regulated when TCRs and tumor antigens interact [123]. Cancer-cell apoptosis can be triggered by TRAIL and FasL, which can identify the TRAIL receptor and Fas on cancer cells, respectively. The caspase family-dependent apoptosis signaling, including caspase-8, −10, and −3, is activated when TRAIL binds to its receptor. Through the Bid-Bax/Bak pathway, activated caspase-8 causes permeabilization of the outer membrane of the mitochondria, causing mitochondrial dysfunction and the release of cytochrome C, which speeds up the apoptosis of cancer cells [124]. Additionally, FasL binds Fas to trigger apoptosis in cancer cells via the signaling pathways of MAPK, NF-κB, and caspase-8. Effective cancer treatment is still a very difficult task to complete globally. At the moment, conventional treatment approaches are ineffective in curing cancer, which leads to significant financial losses. In order to precisely target and eradicate tumor cells, autologous TILs are gathered, grown, and then reinfused into patients as part of TIL therapy, an advanced immunotherapy technique for the treatment of cancer [125]. TIL therapy is currently very effective in treating cancerous tumors like colorectal cancer and melanoma. Given that TILs have a remarkably diverse TCR repertoire, can target tumors, and have strong cytotoxic effects, adoptive TIL therapy is a promising approach to treating cancer. The safety and effectiveness of this strategy have been repeatedly shown in numerous clinical trials. The acquisition of a sufficient number and quality of immune cells, the improvement of cell preparation procedures, and the resolution of issues with drug resistance and immune evasion are some of the difficulties and problems that TIL therapy also faces [126]. These important issues need to be resolved immediately. Furthermore, because immune cells may unintentionally damage healthy tissue while attacking tumor cells, immune toxicity resulting from targeting normal tissues is a serious concern from a clinical standpoint. From a production perspective, cell therapy's lengthy manufacturing cycle, high costs, and difficult pricing structure make it difficult for patients to access the treatment and create commercial barriers for this new treatment. Therefore, it is essential to further improve TIL’s anti-tumor properties in a number of ways, such as through combination therapies (Fig. 5), gene editing, and the enrichment of functional TILs [127]. One of the most effective methods for altering TIL properties and enhancing their capacity to kill tumors is gene editing, which opens the door for TIL commercialization and future clinical uses. One very promising method of treating cancer is TIL infusion. The TIL market for tumor treatment will expand more quickly if unmet clinical needs are addressed and novel TIL-based treatments are created. One promising treatment option for cancer is TIL therapy [128]. TILs can be isolated from tumors, screened using surface markers, or obtained by altering the tumor neoantigen reactivity, rapidly expanding, and then infusing the patient again following NMA lymphodepletion. These are the primary steps in TIL therapy. TILs are primarily isolated using mechanical techniques or enzymatic treatment; amplification is primarily accomplished by co-culturing TILs with elevated IL-2 concentrations; and lymphodepletion is typically accomplished by Cy and Flu. A specific quantity of IL-2 must typically be given when reinfusing TILs into the patient [129]. To improve the activity and isolation efficiency of TILs, new isolation techniques have been developed, building on established practices. Techniques for gene modification are used to TIL recruitment and effectiveness against tumors. While novel techniques in lymphodepletion and infusion are intended to lessen side effects, new stimuli are also being tested to sustain TIL activity during amplification and avoid senescence. Combination therapy and genetic modification give us the chance to improve TIL’s chemotaxis, survival, and anti-tumor potential. The entire therapeutic process, from the laboratory to commercialization, must be put forth for TIL therapy to be successful [130].
The next-generation of TILs
As the field of TIL therapy rapidly advances, technological advancements are being made to increase the anti-tumor efficacy of TIL products. The future evolution of TILs is based on the principles of effective immune activation. First, TCR recognition of intratumor clonal neoantigen heterogeneity determines the adaptive immune response to cancer. In TIL therapies like lifileucel and ITIL-168, it has been shown that an unrestricted TCR repertoire in TIL products opposes tumor heterogeneity and produces clinical responses (ORRs 36%–67%; refs. 12). Neoantigen-specific TILs have also been demonstrated to maintain their activity for nearly three years after TIL injection. Clinical studies of particular neoantigens are needed to develop TIL and TCR [131]
Conclusion
T-cell transfer therapy (TIL therapy) has emerged as a promising and innovative treatment for cancer, particularly for patients with melanoma and other solid tumors. This therapy involves isolating tumor-infiltrating lymphocytes (TILs) from a patient's own tumor, expanding them in the laboratory to increase their number, and then infusing them back into the patient to attack and destroy cancer cells. The clinical evidence supports the potential of TIL therapy in providing durable responses, particularly for patients with advanced cancers who have not responded well to conventional treatments like chemotherapy or immunotherapy. Studies have demonstrated significant tumor regression and long-term survival in some patients, showing that TIL therapy can lead to meaningful clinical benefits. However, challenges such as the complexity and cost of the procedure, as well as the potential for side effects, must be addressed. While TIL therapy is not yet widely available as a standard treatment, ongoing research, technological advancements, and clinical trials are rapidly improving the technique's efficacy and accessibility. With continued optimization of protocols, personalized treatments, and expanded indications, TIL therapy has the potential to become a cornerstone of cancer immunotherapy, offering hope to patients with difficult-to-treat cancers.
Author contributions
Writing original draft, conception and design: Royyala Sai Abhistika. Data collection, writing review and editing: Inapanuri Bhanu. Drafting the editorial article: Akshaya Durgam. Figures and illustrations: Adeeb Unnisa and Huda khan. Writing conceptualization, design and drafting, methodology, supervision: Rahaman Shaik.
Funding
The authors did not receive any financial support or funding for this research.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ding Z-Y, Zou X-L, Wei Y-Q. Cancer microenvironment and cancer vaccine. Cancer Microenviron. 2012;5(3):333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buhrmann C, Kraehe P, Lueders C, Shayan P, Goel A, Shakibaei M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT. PLoS ONE. 2014;9(9): e107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiavoni G, Gabriele L, Mattei F. The tumor microenvironment: a pitch for multiple players. Front Oncol. 2013;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32(1–2):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–47. [DOI] [PubMed] [Google Scholar]
- 6.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podlaha O, Riester M, De S, Michor F. Evolution of the cancer genome. Trends Genet. 2012;28:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 10.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seager RJ, Hajal C, Spill F, Kamm RD, Zaman MH. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg Sci Phys Oncol. 2017;3: 034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demaria O, et al. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. [DOI] [PubMed] [Google Scholar]
- 14.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74. [DOI] [PubMed] [Google Scholar]
- 15.Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderton GK, Bordon Y. Tumour immunotherapy-leukocytes take up the fight. Nat Rev Immunol. 2012;12:237. [DOI] [PubMed] [Google Scholar]
- 17.Dummer R , Flaherty K , Robert C , Arance AM , G JWd , Garbe C , et al . Five-year overall survival (OS) in COLUMBUS: a randomized phase 3 trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients (pts) with BRAF V600-mutant melanoma. J Clin Oncol 39:15s, 2021: (suppl. abstr. 9507) [DOI] [PMC free article] [PubMed]
- 18.Ugurel S, Röhmel J, Ascierto PA, Becker JC, Flaherty KT, Grob JJ, et al. Survival of patients with advanced metastatic melanoma: the impact of MAP kinase pathway inhibition and immune checkpoint inhibition-Update 2019. Eur J Cancer. 2020;130:126–38. [DOI] [PubMed] [Google Scholar]
- 19.Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384:2229–40. [DOI] [PubMed] [Google Scholar]
- 20.Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long-term survival results on metastatic melanoma treatment. J Immunother Cancer. 2020;8: e000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss SA, Wolchok JD, Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25:5191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Watkins R, Vilgelm AE. Cell therapy with TILs: training and taming T cells to fight cancer. Front Immunol. 2021;12: 690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94:S3–9. [DOI] [PubMed] [Google Scholar]
- 25.Spiess PJ, Yang JC, Rosenberg SA. In vivo antitumor activity of tumor-infiltrating lymphocytes expanded in recombinant interleukin-2. J Natl Cancer Inst. 1987;79:1067. [PubMed] [Google Scholar]
- 26.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–2131. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. [DOI] [PubMed] [Google Scholar]
- 28.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: Intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–800. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zacharakis N, Chinnasamy H, Black M, Xu H, Lu Y-C, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24:724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:1543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review [DOI] [PubMed]
- 35.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancerJ. Clin Oncol. 2010;28(1):105–13. [DOI] [PubMed] [Google Scholar]
- 36.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without Carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Lee JJ, Song IH, Park IA, Kang J, Yu JH, et al. Prognostic and predictive value of nanostring-based immune-related gene signatures in a neoadjuvant setting of triple-negative breast cancer: relationship to tumor-infiltrating lymphocytes [DOI] [PubMed]
- 38.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK activation is associated with reduced tumor-Infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors Clin. Cancer Res. 2016;22(6):1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group Ann. Oncol. 2015;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial Ann. Oncol. 2014;25(8):1544–50. [DOI] [PubMed] [Google Scholar]
- 42.Pruneri G, Lazzeroni M, Bagnardi V, Tiburzio GB, Rotmensz N, DeCensi A, et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast [DOI] [PubMed]
- 43.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer [DOI] [PubMed]
- 44.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. [DOI] [PubMed]
- 45.Asano, Kashiwagi S, Goto W, Takada M, Takashima T, Morisaki T, et al. Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes (TILs) and residual cancer burden (RCB). [DOI] [PMC free article] [PubMed]
- 46.Garcia-Martinez E, Gil GL, Benito AC, Gonzalez-Billalabeitia E, Conesa MA, Garcia T, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. [DOI] [PMC free article] [PubMed]
- 47.Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. [DOI] [PMC free article] [PubMed]
- 48.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. [DOI] [PubMed]
- 49.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy N. Engl J Med. 2017;376(22):2147–59. [DOI] [PubMed] [Google Scholar]
- 50.Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–64. [DOI] [PubMed] [Google Scholar]
- 51.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, Landin AM, Mullinax JE, Saller JJ, Saltos AN, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deniger DC, Pasetto A, Robbins PF, Gartner JJ, Prickett TD, Paria BC, Malekzadeh P, Jia L, Yossef R, Langhan MM, et al. T-cell Responses to TP53 “Hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res. 2018;24:5562–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dafni U, Michielin O, Martin Lluesma S, Tsourti Z, Polydoropoulou V, Karlis D, Besser MJ, Haanen J, Svane I-M, Ohashi PS, Kammula US, Orcurto A, Zimmermann S, Trueb L, Klebanoff CA, Lotze MT, Kandalaft LE, Coukos G. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. 2019;30(12):1902–13. 10.1093/annonc/mdz398. [DOI] [PubMed] [Google Scholar]
- 54.Chandran SS, Somerville RPT, Yang JC, Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott D, Paria BC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017;18:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, Thomas SS, Domingo-Musibay E, Pavlick AC, Whitman ED, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol. 2021;39:2656–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Berg JH, Heemskerk B, van Rooij N, Gomez-Eerland R, Michels S, van Zon M, de Boer R, Bakker NAM, Jorritsma-Smit A, van Buuren MM, et al. Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer. 2020;8: e00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevanović S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong MLM, Somerville RPT, Klebanoff CA, Kammula US, Sherry RM, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:1486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, Slomovitz BM, Walther A, Thomas SS, Chesney JA, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;37(Suppl. 15):2538. [Google Scholar]
- 59.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. [DOI] [PubMed] [Google Scholar]
- 60.Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017. 10.1126/science.aak9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ, Kim Y-A, Sim CK, Heo S-H, Song IH, Park HS, Park SY, Bang WS, Park IA, Lee M, et al. Expansion of tumor-infiltrating lymphocytes and their potential for application as adoptive cell transfer therapy in human breast cancer. Oncotarget. 2017;8:113345–211335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang C-H, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003. 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen LT, Yen PH, Nie J, Liadis N, Ghazarian D, Al-Habeeb A, Easson A, Leong W, Lipa J, McCready D, et al. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs). PLoS ONE. 2010;5: e13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poch M, Hall M, Joerger A, Kodumudi K, Beatty M, Innamarato PP, Bunch BL, Fishman MN, Zhang J, Sexton WJ, et al. Expansion of tumor infiltrating lymphocytes (TIL) from bladder cancer. Oncoimmunology. 2018;7: e1476816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wickström S, Lövgren T. Expansion of tumor-infiltrating lymphocytes from melanoma tumors. Methods Mol Biol. 2019. 10.1007/978-1-4939-8979-9_7. [DOI] [PubMed] [Google Scholar]
- 68.Wu R, Forget M-A, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–55. [DOI] [PubMed] [Google Scholar]
- 70.Aebersold P, Hyatt C, Johnson S, Hines K, Korcak L, Sanders M, Lotze M, Topalian S, Yang J, Rosenberg SA. Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. J Natl Cancer Inst. 1991. 10.1093/jnci/83.13.932. [DOI] [PubMed] [Google Scholar]
- 71.Aebersold P, Hyatt C, Johnson S, Hines K, Korcak L, Sanders M, Lotze M, Topalian S, Yang J, Rosenberg SA. Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. J Natl Cancer Inst. 1991;83:932–93. [DOI] [PubMed] [Google Scholar]
- 72.Schwartzentruber DJ, Hom SS, Dadmarz R, White DE, Yannelli JR, Steinberg SM, Rosenberg SA, Topalian SL. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;12:1475–83. [DOI] [PubMed] [Google Scholar]
- 73.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donia M, Junker N, Ellebaek E, Andersen MH, Straten PT, Svane IM. Characterization and comparison of ‘standard’ and ‘young’ tumour-infiltrating lymphocytes for adoptive cell therapy at a Danish translational research institution. Scand J Immunol. 2012;75:157–216. [DOI] [PubMed] [Google Scholar]
- 75.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16:6122–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Itzhaki O, Hovav E, Ziporen Y, Levy D, Kubi A, Zikich D, Hershkovitz L, Treves AJ, Shalmon B, Zippel D, et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–20. [DOI] [PubMed] [Google Scholar]
- 77.Foppen MG, Donia M, Svane IM, Haanen JB. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol. 2015;9:1918–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015;3:926–35. [DOI] [PubMed] [Google Scholar]
- 81.Xiao Y, Li H, Mao L, Yang QC, Fu LQ, Wu CC, Liu B, Sun ZJ. CD103+ T and dendritic cells indicate a favorable prognosis in oral cancer. J Dent Res. 2019;98:1480–7. [DOI] [PubMed] [Google Scholar]
- 82.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. Bystander CD8 T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–9. [DOI] [PubMed] [Google Scholar]
- 84.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kortekaas KE, Santegoets SJ, Sturm G, Ehsan I, van Egmond SL, Finotello F, Trajanoski Z, Welters MJP, van Poelgeest MIE, van der Burg SH. CD39 identifies the CD4 tumor-specific T-cell population in human cancer. Cancer Immunol Res. 2020;8:1311–21. [DOI] [PubMed] [Google Scholar]
- 86.Martinez-Usatorre A, Carmona SJ, Godfroid C, Yacoub Maroun C, Labiano S, Romero P. Enhanced phenotype definition for precision isolation of precursor exhausted tumor-infiltrating CD8 T cells. Front Immunol. 2020;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. [DOI] [PubMed] [Google Scholar]
- 90.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Investig. 2014;124:2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inozume T, Hanada K-I, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheño U, Elizalde E, Alignani D, Zubeldia N, Otano I, et al. Expansion of tumor-infiltrating CD8(+) T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy. Cancer Res. 2017;77:3672–84. [DOI] [PubMed] [Google Scholar]
- 93.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye Q, Song D-G, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014. 10.1158/1078-0432.CCR-13-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakellariou-Thompson D, Forget M-A, Creasy C, Bernard V, Zhao L, Kim YU, Hurd MW, Uraoka N, Parra ER, Kang Y, et al. 4–1BB agonist focuses CD8 tumor-infiltrating T-Cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res. 2017;23:7263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seliktar-Ofir S, Merhavi-Shoham E, Itzhaki O, Yunger S, Markel G, Schachter J, Besser MJ. Selection of shared and neoantigen-reactive T cells for adoptive cell therapy based on CD137 separation. Front Immunol. 2017;8:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prickett TD, Crystal JS, Cohen CJ, Pasetto A, Parkhurst MR, Gartner JJ, Yao X, Wang R, Gros A, Li YF, et al. Durable complete response from metastatic melanoma after transfer of autologous T cells recognizing 10 mutated tumor antigens. Cancer Immunol Res. 2016;4:669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, Weber J, Hwu P, Pilon-Thomas S, Radvanyi L. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21:611–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tavera RJ, Forget MA, Kim YU, Sakellariou-Thompson D, Creasy CA, Bhatta A, Fulbright OJ, Ramachandran R, Thorsen ST, Flores E, et al. Utilizing T-cell activation signals 1, 2, and 3 for tumor-infiltrating lymphocytes (TIL) expansion: the advantage over the sole use of Interleukin-2 in cutaneous and uveal melanoma. J Immunother. 2018;41:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parkhurst M, Gros A, Pasetto A, Prickett T, Crystal JS, Robbins P, Rosenberg SA. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin Cancer Res. 2017;23:2491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robbins PF, Lu Y-C, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME, Stroncek DF, Rosenberg SA. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J Immunother. 2012;35:283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan C-C, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salem ML, Cole DJ. Dendritic cell recovery post-lymphodepletion: a potential mechanism for anti-cancer adoptive T cell therapy and vaccination. Cancer Immunol Immunother. 2010;59:341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. [DOI] [PubMed] [Google Scholar]
- 107.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:2389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nissani A, Lev-Ari S, Meirson T, Jacoby E, Asher N, Ben-Betzalel G, Itzhaki O, Shapira-Frommer R, Schachter J, Markel G, et al. Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy. J Immunother Cancer. 2021;9: e001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santos JM, Cervera-Carrascon V, Havunen R, Zafar S, Siurala M, Sorsa S, Anttila M, Kanerva A, Hemminki A. adenovirus coding for interleukin-2 and tumor necrosis factor alpha replaces lymphodepleting chemotherapy in adoptive T cell therapy. Mol Ther. 2018;26:2243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng LE, Ohlén C, Nelson BH, Greenberg PD. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proc Natl Acad Sci USA. 2002;99:3001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41(Pt 1):4420–5. [PubMed] [Google Scholar]
- 115.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–5. [DOI] [PubMed] [Google Scholar]
- 117.Kalia V, Sarkar S. Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing act. Front Immunol. 2018;9:2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, Fang Y, Deng J, Gao Y, Liang X, et al. IL-2 regulates tumor-reactive CD8 T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021. 10.1038/s41590-020-00850-9. [DOI] [PubMed] [Google Scholar]
- 119.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meng Q, Liu Z, Rangelova E, Poiret T, Ambati A, Rane L, Xie S, Verbeke C, Dodoo E, Del Chiaro M, et al. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J Immunother. 2016;39:81–9. [DOI] [PubMed] [Google Scholar]
- 121.Liu Z, Meng Q, Bartek J, Poiret T, Persson O, Rane L, Rangelova E, Illies C, Peredo IH, Luo X, et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology. 2017;6: e1252894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frank LSA, Lotze M, Ritthipichai K, Mosychuk C. The T-cell growth factor cocktail IL-2/IL-15/IL-21 enhances expansion and effector function of tumor-infiltrating T cells in a novel process developed by iovance. In Proceedings of the SITC Annual Meeting, National Harbor, MD, USA, 8 November 2012
- 123.Marabondo S, Kaufman HL. High-dose interleukin-2 (IL-2) for the treatment of melanoma: safety considerations and future directions. Expert Opin Drug Saf. 2017;16:1347–57. [DOI] [PubMed] [Google Scholar]
- 124.Acquavella N, Kluger H, Rhee J, Farber L, Tara H, Ariyan S, Narayan D, Kelly W, Sznol M. Toxicity and activity of a twice daily high-dose bolus interleukin 2 regimen in patients with metastatic melanoma and metastatic renal cell cancer. J Immunother. 2008;31:569–76. [DOI] [PubMed] [Google Scholar]
- 125.Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met Ö, Hölmich LR, Andersen RS, Hadrup SR, Andersen MH, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ullenhag GJ, Sadeghi AM, Carlsson B, Ahlström H, Mosavi F, Wagenius G, Tötterman TH. Adoptive T-cell therapy for malignant melanoma patients with TILs obtained by ultrasound-guided needle biopsy. Cancer Immunol Immunother. 2012;61:725–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ, Hölmich LR, Hendel HW, Met Ö, Andersen MH, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res. 2016;22:3734–45. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen LT, Saibil SD, Sotov V, Le MX, Khoja L, Ghazarian D, Bonilla L, Majeed H, Hogg D, Joshua AM, et al. Phase II clinical trial of adoptive cell therapy for patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and low-dose interleukin-2. Cancer Immunol Immunother. 2019;68:773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsu EJ, Cao X, Moon B, Bae J, Sun Z, Liu Z, Fu Y-X. A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat Commun. 2021;12:2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang C, Li M, Wei R, Wu J. Adoptive transfer of TILs plus anti-PD1 therapy: an alternative combination therapy for treating metastatic osteosarcoma. J Bone Oncol. 2020;25: 100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Investig. 2015;125:3335–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.