Abstract
Background
To elucidate the correlation between immunoglobulin G (IgG) N-glycosylation patterns (IGPs) and diabetes complications, including diabetic nephropathy (DN), diabetic retinopathy (DR) and diabetic maculopathy (DM). We performed a two-sample Mendelian randomization (MR) study.
Methods
We obtained genome-wide association studies (GWAS) summary statistics related to 77 IGPs and diabetic complications from the MRC Human Genetics Unit and the FinnGen consortium. In our two-sample MR, genetic variants associated with IGPs were used to estimate correlations. The inverse-variance weighted (IVW) method was used as the primary analysis. Various complementary methods, including MR-Egger, weighted median (WM), and weighted mode were implemented to detect the causal relationship. Sensitivity analyses including MR-PRESSO, MR-Egger, Cochran’s Q and leave-one-out methods were used to validate the robustness of the results.
Results
After MR-PRESSO outlier correction and a stringent False Discovery Rate (FDR) correction, a significant causal association was identified: higher levels of IGP48 were robustly associated with an increased risk of diabetic maculopathy (DM) (OR: 1.21, 95% CI: 1.09–1.35, P = 0.001, FDR = 0.004). Additionally, several other nominal associations (P < 0.05) were observed that did not withstand FDR correction but suggest areas for future investigation. These included protective associations with DM for IGP18 (OR: 0.86, P = 0.033) and IGP35 (OR: 0.90, P = 0.015), and risk associations for IGP23 (OR: 1.19, P = 0.028) and IGP28 (OR: 1.17, P = 0.038). For diabetic retinopathy (DR), a nominal protective association was found for IGP35 (OR: 0.94, P = 0.026) and a risk association for IGP48 (OR: 1.07, P = 0.042). Sensitivity analyses detected no significant horizontal pleiotropy, and the results were stable.
Conclusion
Our study provides robust genetic evidence identifying IGP48 as a causal risk factor for DM. Other nominal associations with DM and DR require further validation. IGP48 emerges as a promising biomarker and therapeutic target for DM, warranting further research into its underlying mechanisms and clinical utility.
Trial registration
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01858-7.
Keywords: Mendelian randomization, Instrumental variables, Diabetic complications, IgG N-glycosylation, Diabetic retinopathy
Background
Diabetes mellitus is a complex and multifactorial metabolic disease characterized by insulin resistance and insufficient insulin secretion. Globally, the number of people with diabetes mellitus has quadrupled in the last three decades and diabetes mellitus is the ninth leading cause of death in humans [1]. The global prevalence of diabetes among people aged 20–79 years is 10.5% as of 2021 and is estimated to rise to 12.2% by 2045 [2]. Studies have shown that diabetes mellitus and diabetes complications (including cardiovascular, renal, peripheral vascular, ophthalmic, hepatic, or neurological disease) place a greater burden on patients [3]. Understanding the role of genetics, lifestyle, and environment in diabetes and diabetes complications is critical for prevention and the development of targeted therapies.
N-glycans show great promise as biomarkers for different diseases, especially those involving inflammatory changes [4]. IgG, the prevalent isotype of antibodies, plays a key role in humoral immunity against pathogens because it mediates the immune response to viral infections and participates in systemic antiviral immunity and inflammatory responses [5]. IgG mediates the immune response through the interaction of its fragment crystallizable (Fc)-Fc γ receptor (FcγR) and Fc complement 1q [6]. The functional diversity of IgG is mediated by heterogeneous glycosylation of the Fc structural domain Asn297, which carries complex N-glycans [7]. Studies have shown that immunoglobulin G (IgG) N-glycosylation patterns (IGPs) are potential biomarker for people with diabetes and diabetes complications [8]. Specifically, in the context of diabetic complications, a compelling rationale for targeting IgG N-glycosylation arises from its potential to modulate critical pathogenic pathways; for instance, altered IgG glycoforms may promote pro-inflammatory responses and endothelial dysfunction, thereby contributing to the vascular damage characteristic of diabetic retinopathy and nephropathy, highlighting its significance as a potential therapeutic target [9, 10]. A cross-sectional study showed that there was a significant difference in urinary IgG between the control and diabetic nephropathy (DN) groups, and that it was significantly elevated with the progression of DN [11]. In addition, Zhang et al. concluded that glomerular IgG deposition was significantly associated with the progression of DN and that IgG1 deposition tended to predominate in patients [8]. Another clinical study showed that IGP32 and IGP54 were significantly associated with diabetic retinopathy (DR) [12]. Moreover, urinary IgG levels were significantly higher in DM patients compared to control [13]. The results of a single-center retrospective study suggest that in patients with diabetic nephropathy, deposition of IgG subclasses in multiple locations is associated with shorter duration of nephropathy and more severe tubulointerstitial injury [14]. However, the results of a prospective study showed that there was no statistically significant difference between the four IgG subtypes (IgG1-4) in the vitreous humor of patients with type 2 diabetes (T2D) compared to controls [15]. Notably, these observational studies may be limited by sample size and potential confounders. And, it is unclear whether patients with diabetic complications benefit from control interventions for IgG glycome composition, especially in the general population. Therefore, it is of interest to assess the potential of IGPs to serve as a target for preventive interventions for diabetic complications and to investigate its etiologic role in diabetic complications.
Mendelian randomization (MR) is a novel method based on Mendel’s first and second laws of genetic inheritance: the law of segregation and the law of independent assortment [16]. Most MR studies use genetic variants significantly associated with exposure as instrumental variable (IV) to assess the association between genetically predicted exposure and outcome [17]. MR, which uses genetic variation as an instrumental variable to mimic the design of a randomized controlled trial (RCT) [18], is effective in reducing confounders and reverse causality bias in traditional observational studies because genotypes are randomly assigned at birth and are independent of disease status [17]. In exploring the association of IgG N-glycosylation patterns with diabetic complications, MR may provide more reliable causal evidence and help identify potential drug targets (e.g., specific glycosyltransferases). In addition, MR results may provide preferred intervention directions for subsequent RCT design, optimizing the efficiency and cost-effectiveness of clinical trials. For example, Sun et al. found a causal relationship between human IgG N-Glycosylation and aging [19]. And Hong et al. used MR to discover genetic determinants of neuroticism and potentially druggable gene targets [20].
However, the relationship between IGPs and diabetes complications, including DN, diabetic retinopathy (DR) and diabetic maculopathy (DM), using the MR method has not yet been investigated. In this study, we applied an MR analysis to investigate the causal relationship between IGPs and three diabetes complications.
Methods
Study design
The MR analysis followed the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines [21]. Our MR analysis was conducted based on three IV assumptions [22]: (a) it does not affect the outcome; (b) it is not associated with the outcomes due to confounding pathways; (c) it is associated with the exposure. A study frame diagram was provided in Fig. 1 to depict the design of our study. Due to the fact that the GWAS data included in our study were publicly available, no additional ethical approvals were required.
Fig. 1.
Overview of Mendelian randomization
Data source
In order to investigate the causal relationship between IGPs and diabetes complications, the two-sample MR method was used in this study. 77 IGP served as exposure factors, whereas diabetes complications, including DN, DR, and DM, served as outcome factors.
The genome-wide association studies (GWAS) summary dataset for DN (5,579 case and 59,084 control), DR (14,142 case and 82,287 control), and DM (4,603 case and 82,287 control) were obtained from the FinnGen R12 database of European ancestry. GWAS of 77 IGPs (8,090 samples) were obtained from MRC Human Genetics Unit of European ancestry (https://datashare.ed.ac.uk/handle/10283/3238) [23]. The detailed information on the GWAS summary data of the exposures and outcomes were provided in Table 1.
Table 1.
The GWAS data for exposure and outcomes
| Trait | GWAS ID | Population | SNPs | |
|---|---|---|---|---|
| case/control | Decent | |||
| DN | FinnGen_R12_DM_NEPHROPATHY_EXMORE | 3,283/181,704 | European | 16,380,336 |
| DR | FinnGen_R12_DM_RETINOPATHY | 14,584/202,082 | European | 16,380,459 |
| DM | FinnGen_R12_DM_MACULOPATHY | 1,811/211,566 | European | 16,380,450 |
| IgG N-glycosylation level | https://datashare.ed.ac.uk/handle/10283/3238 | NA | European | NA |
Diabetic nephropathy (DN); Diabetic retinopathy (DR); Diabetic maculopathy (DM); Immunoglobulin G (IgG)
IVs selection
Single nucleotide polymorphisms (SNPs) robustly associated with each Immunoglobulin G N-glycosylation pattern (IGP) were selected as instrumental variables (IVs). Primarily, SNPs reaching genome-wide significance (P < 5 × 10− 8) for association with specific IGPs (IGP2, IGP5, IGP7 to 11, IGP13 to 15, IGP17, IGP22, IGP24, IGP26, IGP28 to 31, IGP34 to 40, IGP42, IGP45 to 47, IGP49, IGP51 to 55, and IGP58 to 77) were chosen. For IGPs where an insufficient number of IVs were identified at this strict threshold (IGP1, IGP3 to 4, IGP12, IGP16, IGP18 to 21, IGP23, IGP25, IGP27, IGP32 to 33, IGP41, IGP43 to 44, IGP48, IGP50, and IGP56 to 57), the P-value threshold was relaxed to P < 5 × 10− 6 to ensure adequate instrument strength and number [24, 25]. Then, we screeded SNPs with a minimum minor allele frequency (MAF) > 0.01 [26]. Linkage disequilibrium (LD) between SNPs was then removed using the following criteria: R2 < 0.001 and window size = 10,000kb [27]. The proportion of variance in each IGP explained by each individual SNP (R²) was calculated using the formula [28]:
 |
If the selected IVs were not present in the resulting summary data, we searched for SNPs with high LD (R2 > 0.8) to the IVs as proxy SNPs to replace the existing IVs. The strength of the IV was estimated by calculating the F-value for each SNP in the IV to exclude possible weak instrument bias between the IV and exposure factors as follows [29]:
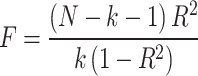 |
Where R2 represents the proportion of exposure variance explained by the SNP in the IV (R2 is the proportion of exposure variance explained by SNPs. N represents the sample size of the exposure factor GWAS data). The requirement for the F-value was > 10 [30].
MR analysis
Inverse-variance weighted (IVW) was used as the primary method to assess the causal association between IGPs and diabetes complications by calculating the odds ratio (OR) and 95% confidence interval (CI) [31]. To ensure the robustness of our findings, we also utilized three additional MR methods: MR-Egger, weighted median (WM), and weighted mode [32]; The MR-Egger method takes into the presence of interception and provides accurate estimates of causal effects in the presence of pleiotropic bias; if pleiotropic effects were not reversed (P-pleiotropy < 0.05), the IVW estimate had a significant bias [31, 33]; the WM method assumes that half of the IVs were valid, analyzing the causal association between exposure and outcome [34]. All analyses in this study were linked together using the Two Sample MR package, version R 4.0.5. MR results were visualized using scatter plots and forest plots. Given the multiple associations tested, P-values from the primary IVW analysis were corrected using the False Discovery Rate (FDR) method, with an FDR-adjusted P < 0.05 deemed statistically significant.
Sensitivity analysis
A sensitivity analysis was performed to detect potential heterogeneity and horizontal pleiotropy in MR studies. Cochran’s Q test was used to assess heterogeneity between IVs, with P > 0.05 indicating low heterogeneity, meaning that the IV scores were randomly distributed between IVs and had little effect on the IVW results [35]. Given the possible influence of genetic variability on the estimation of association effects, this study used the MR-Egger regression method to investigate the presence of horizontal pleiotropy [36, 37]. If the MR-Egger regression intercept is close to zero or not statistically significant, this indicates the absence of pleiotropy; to account for multiple comparisons, the P-values for the MR-Egger intercept were also FDR-corrected. In addition, the MR-PRESSO method was used to identify potential outliers (i.e., SNPs with P < 0.05) and to reassess causal associations after their removal to correct for horizontal pleiotropy [37]. Funnel plots and leave-one-out plots were used to assess the stability and consistency of the results.
Results
IVs selection
Instrumental variables (IVs) were selected for each IGP, ensuring all instruments were sufficiently strong (F-statistic > 10) to mitigate weak instrument bias. Detailed information on the selected IVs is provided in the Supplementary Material (Table S1 and Table S2).
Initial analyses, sensitivity checks, and outlier correction
Initially, we conducted a two-sample MR analysis to investigate the causal associations between 77 Immunoglobulin G N-glycosylation patterns (IGPs) and three diabetic complications: DN, DR, and DM. The complete set of initial MR estimates for all 77 IGPs is presented in Table S3. Sensitivity analyses of these initial results, including heterogeneity tests (Cochran’s Q) and pleiotropy assessments (MR-Egger intercept), are detailed in Table S4. Significant heterogeneity was observed for several associations.
To address the potential influence of outliers and refine the causal estimates, we employed the MR-PRESSO test. This method identified potential outlier SNPs across various IGP-diabetic complication associations, which were subsequently removed from the respective analyses. Full details of the identified outliers and the MR-PRESSO test results are provided in Table S5.
Causal associations after outlier correction
Following the removal of outliers, the primary IVW MR analysis, with a stringent False Discovery Rate (FDR) correction for multiple testing, revealed one robustly significant causal association (Table 2; Fig. 2). Higher genetically predicted levels of IGP48 were significantly associated with an increased risk of diabetic maculopathy (DM) (OR: 1.21, 95% CI: 1.09–1.35, P = 0.001, FDR = 0.004).
Table 2.
Mendelian randomization associations between IgG N-glycosylation traits (IGPs) and diabetes complications after MR-PRESSO outlier correction (Positive results)
| Exposure | Outcome | N.SNP | Methods | OR_CI | P-value | FDR |
|---|---|---|---|---|---|---|
| IGP18 | Diabetic maculopathy | 11 | Inverse variance weighted | 0.86 (0.75–0.99) | 0.033 | 0.03346 |
| IGP23 | Diabetic maculopathy | 5 | Inverse variance weighted | 1.19 (1.02–1.39) | 0.028 | 0.915 |
| IGP28 | Diabetic maculopathy | 6 | Inverse variance weighted | 1.17 (1.01–1.35) | 0.038 | 0.037767 |
| IGP35 | Diabetic maculopathy | 7 | Inverse variance weighted | 0.90 (0.83–0.98) | 0.015 | 0.915 |
| IGP48 | Diabetic maculopathy | 13 | Inverse variance weighted | 1.21 (1.09–1.35) | 0.001 | 0.000502 |
| IGP35 | Diabetic retinopathy | 7 | Inverse variance weighted | 0.94 (0.90–0.99) | 0.026 | 0.915 |
| IGP48 | Diabetic retinopathy | 12 | Inverse variance weighted | 1.07 (1.00–1.15) | 0.042 | 0.042194 |
Fig. 2.
The IVW and forest plot between IGPs and diabetes complications (positive results)
Additionally, our analysis identified several nominal associations (IVW P < 0.05) that did not withstand FDR correction but suggest areas for future investigation (Table 2). For DM, these included a protective effect for higher levels of IGP18 (OR: 0.86, 95% CI: 0.75–0.99, P = 0.033) and IGP35 (OR: 0.90, 95% CI: 0.83–0.98, P = 0.015), and an increased risk with higher levels of IGP23 (OR: 1.19, 95% CI: 1.02–1.39, P = 0.028) and IGP28 (OR: 1.17, 95% CI: 1.01–1.35, P = 0.038). For diabetic retinopathy (DR), a nominally protective association was found for IGP35 (OR: 0.94, 95% CI: 0.90–0.99, P = 0.026), while a nominal risk association was observed for IGP48 (OR: 1.07, 95% CI: 1.00–1.15, P = 0.042). No significant causal associations were identified for diabetic nephropathy (DN).
Supplementary sensitivity analyses confirmed the robustness of these findings. For the identified positive associations, there was no evidence of significant heterogeneity (Cochran’s Q test) or directional pleiotropy (MR-Egger intercept test) after outlier correction (Table 3). A comparison of the MR estimates before and after the MR-PRESSO outlier correction demonstrated the stability of these associations, with no significant distortion observed (Table 4). Furthermore, the MR-PRESSO global test indicated no significant remaining outliers for these reported associations (Supplementary Table S8). The detailed results for all outlier-corrected MR methods (IVW, MR-Egger, Weighted Median, Weighted Mode) and further sensitivity analyses (heterogeneity and pleiotropy tests) are presented in Supplementary Table S6 and Supplementary Table S7, respectively. The specific definitions of these IGPs, along with their associated candidate genes, are detailed in Supplementary Table S9.
Table 3.
Assessment of heterogeneity and horizontal Pleiotropy for associations between IGPs and diabetes complications after outlier correction (Positive results)
| Exposure | Outcome | Heterogeneity | Pleiotropy | ||
|---|---|---|---|---|---|
| Q statistic (IVW) | P value | MR-Egger Intercept | P value | ||
| IGP18 | Diabetic maculopathy | 6.50041199 | 0.771616306 | 0.000707963 | 0.972331073 |
| IGP23 | Diabetic maculopathy | 4.584854 | 0.332604 | 0.018933 | 0.602826 |
| IGP28 | Diabetic maculopathy | 1.999835892 | 0.849167743 | 0.020097563 | 0.676856075 |
| IGP35 | Diabetic maculopathy | 4.709818 | 0.581533 | -0.01948 | 0.28238 |
| IGP48 | Diabetic maculopathy | 10.29302844 | 0.590270309 | -0.019491902 | 0.34071981 |
| IGP35 | Diabetic retinopathy | 3.305817 | 0.7696 | -0.00321 | 0.754314 |
| IGP48 | Diabetic retinopathy | 8.249926299 | 0.690756277 | -0.003931636 | 0.750310558 |
Table 4.
Comparison of associations before and after MR-PRESSO outlier correction (Positive results)
| Exposure | Outcome | Raw | Outlier corrected | Global p | outliers | Distortion p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR_CI | P | OR_CI | P | |||||||
| IGP18 | Diabetic maculopathy | 0.86 (0.77–0.96) | 0.02 | NA ( NA - NA ) | NA | 0.811 | NA | NA | ||
| IGP23 | Diabetic maculopathy | 1.19 (1.02–1.39) | 0.09 | NA ( NA - NA ) | NA | 0.471 | NA | NA | ||
| IGP28 | Diabetic maculopathy | 1.17 (1.06–1.28) | 0.02 | NA ( NA - NA ) | NA | 0.855 | NA | NA | ||
| IGP35 | Diabetic maculopathy | 0.90 (0.84–0.97) | 0.03 | NA ( NA - NA ) | NA | 0.563 | NA | NA | ||
| IGP48 | Diabetic maculopathy | 1.21 (1.10–1.34) | 0 | NA ( NA - NA ) | NA | 0.641 | NA | NA | ||
| IGP35 | Diabetic retinopathy | 0.94 (0.91–0.98) | 0.02 | NA ( NA - NA ) | NA | 0.844 | NA | NA | ||
| IGP48 | Diabetic retinopathy | 1.07 (1.01–1.14) | 0.04 | NA ( NA - NA ) | NA | 0.708 | NA | NA | ||
Discussion
To our knowledge, this study represents the first two-sample Mendelian randomization analysis to systematically investigate the causal relationships between a comprehensive panel of 77 IgG N-glycosylation patterns (IGPs) and major diabetic complications. After applying a stringent False Discovery Rate (FDR) correction and removing outliers via MR-PRESSO, our analysis identified one robustly significant causal association: higher genetically predicted levels of IGP48 were significantly associated with an increased risk of diabetic maculopathy (DM). While several other nominal associations with DM and diabetic retinopathy (DR) were observed, they did not withstand multiple testing correction and thus should be considered as preliminary signals requiring further investigation. The robustness of our primary finding is supported by multiple sensitivity analyses, providing strong evidence for this novel link.
These findings highlight that specific structural features of IgG glycans may differentially contribute to the pathogenesis of diabetic complications. The identified IGPs represent distinct glycosylated forms within the N-linked oligosaccharide chains on the IgG molecule [8], with their specific characteristics detailed in Supplementary Table S9. Our robustly significant finding, IGP48, is defined as the percentage of G1 [3]F glycan in total neutral IgG glycans, a structure often associated with a pro-inflammatory profile. In addition to this primary finding, the IVW analysis provided further, albeit preliminary, insights. For instance, the protective association for IGP18 corresponds to the percentage of G2FNS1 glycan, while the risk-associated IGP23 and IGP28 correspond to the percentage of G2FNS2 glycan and monosialylated fucosylated structures, respectively. Moreover, the protective association for IGP35 with DR represents a ratio of complex sialylated structures with a bisecting GlcNAc. It is well-established that such glycosylation patterns significantly influence IgG’s effector functions, including its Fc receptor binding affinity and overall immunomodulatory capabilities [38], and previous observational studies have indeed indicated associations between various aspects of IgG N-glycosylation and diabetic complications [9], providing a plausible framework for their role.
The causal links identified likely involve complex molecular mechanisms, potentially modulated by the candidate genes associated with these IGPs (detailed in Supplementary Table S9). The regulation of immune metabolism appears central. Our most robust finding centers on IGP48 (candidate gene: TMEM121), whose pro-inflammatory potential might be a key driver of its association with increased DM risk. It could exacerbate retinal neurovascular unit damage by upregulating inflammatory factors like TNF-α and IL-6, a mechanism consistent with findings on other glycan structures [39].The nominal associations, while requiring cautious interpretation, may point to similar or complementary pathways. For instance, the risk-associated IGP23 (candidate genes including RUNX3, TXLNB, IKZF1) could also promote a pro-inflammatory state. Conversely, the nominally protective glycoforms like IGP18 (candidate genes including TXLNB, IKZF1, RUNX1) and IGP35 (candidate gene: ELL2) could mitigate disease progression by reducing vascular endothelial cell activation and inhibiting inflammatory signaling pathways such as MAPK-AP-1. Secondly, vascular endothelial dysfunction, particularly the disruption of the blood-retinal barrier in DR [40], could be exacerbated by abnormal IgG glycosylation. The risk conferred by IGP48 and potentially the nominally-associated IGP23 might involve altered nitric oxide (NO) production or activity; for example, IGP23 could further inhibit NO synthesis by promoting ADMA expression, building upon the known effect of ADMA in hyperglycemic states [41]. Thirdly, neurodegenerative changes contributing to DM-related retinal neurosensory dysfunction [42], might be influenced by IgG glycosylation’s impact on neuronal survival via modulation of glial cell function or oxidative stress. The risk association of IGP23, for instance, could involve accelerated neuronal apoptosis through enhanced glial cell activation. The candidate genes identified, such as MGAT3 for IGP28, offer valuable targets for future research to dissect the precise genetic and enzymatic pathways dictating these critical IgG glycan structures and their pathological roles in diabetic complications.
Retrospective studies have shown that the relative abundance of IGPs correlates with DN [10]. However, the present MR results did not reveal a causal relationship between IGPs and DN. We believe that DN is a complex disease whose onset and progression may involve the interaction of multiple genes, the environment, and lifestyle; and that IGPs may be only one of the many factors that are not sufficient to cause DN on their own [43], but may have a significant effect in combination with other factors. In terms of sample size and statistical methods, MR studies may be limited by sample size, resulting in insufficient statistical validity to detect weak causality [44]; meanwhile, observational studies may be confounded by confounding factors and reverse causality, leading to biased results [45]. In addition, IGPs modifications may change with disease progression and treatment [46]. Therefore, multiple measurements at different time points are needed to more accurately assess its relationship with DN.
Our study has several strengths, including the use of a two-sample MR design with large-scale GWAS data and robust IVs (F-statistics > 10), which minimizes reverse causation and confounding. However, certain limitations must be acknowledged. First, our study primarily included participants of European ancestry, which may limit the generalizability of our findings to other populations. Future research should focus on validating these associations in more diverse cohorts. Second, while we employed multiple sensitivity analyses to assess horizontal pleiotropy, we acknowledge that more advanced methods such as multivariate MR (MVMR) or CAUSE were not utilized. MVMR could have helped disentangle the effects of highly correlated IGPs, while CAUSE could have further distinguished causality from shared genetic confounding. The absence of these analyses represents a methodological limitation, and their future application would provide a more refined understanding of the observed associations. Third, while applying a stringent FDR correction was crucial for identifying robust causal associations, this approach may be overly conservative for hypothesis generation in a mechanism-focused study like ours. The strict correction likely led to the dismissal of potentially true, albeit weaker, causal links that did not meet the stringent statistical threshold. Therefore, the nominal associations reported should be interpreted as valuable preliminary evidence that warrants further exploration in dedicated mechanistic and validation studies, rather than being definitively dismissed. Despite these limitations, our findings provide a strong foundation for future research. Future work combining more sophisticated statistical models with clinical cohort validation is essential to improve the accuracy and biological explanatory power of these causal inferences, ultimately aiding in the development of new diagnostic and therapeutic strategies for diabetic complications.
Conclusion
In conclusion, our two-sample Mendelian randomization study provides robust genetic evidence for a causal relationship between higher levels of the IgG N-glycosylation trait IGP48 and an increased risk of diabetic maculopathy. While other nominal associations were identified, they require further validation. These findings highlight IGP48 as a promising biomarker for risk stratification and a potential therapeutic target in diabetic complications, emphasizing the importance of specific glycan structures in the disease process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Figure S1. The Forest plots between IGPs and diabetes complications (Positive results). (A)-(E): The Forest plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Forest plots of IGP35 and IGP48 on DR.
Supplementary Material 2: Figure S2. The Scatter plots between IGPs and diabetes complications (Positive results). (A)-(E): The Scatter plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Scatter plots of IGP35 and IGP48 on DR.
Supplementary Material 3: Figure S3. The Funnel plots between IGPs and diabetes complications (Positive results). (A)-(E): The Funnel plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Funnel plots of IGP35 and IGP48 on DR.
Supplementary Material 4: Figure S4. The Lol plots between IGPs and diabetes complications (Positive results). (A)-(E): The Lol plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Lol plots of IGP35 and IGP48 on DR.
Acknowledgements
Not applicable.
Abbreviations
- IgG
Immunoglobulin G
- IGPs
IgG N-glycosylation patterns
- DN
Diabetic nephropathy
- DR
Diabetic retinopathy
- DM
Diabetic maculopathy
- MR
Mendelian randomization
- GWAS
Genome-wide association studies
- IVW
Inverse-variance weighted
- WM
Weighted median
- T2D
Type 2 diabetes
- IV
Instrumental variable
- RCT
Randomized controlled trial
- SNPs
Single nucleotide polymorphisms
- LD
Linkage disequilibrium
Author contributions
This article has only one author, and all contributions are attributed to Xiao-Huang Zhang alone.
Funding
This study was supported by the Henan Natural Science Foundation (grant number: 242300421502).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. 2022;65:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plavša B, Szavits-Nossan J, Blivajs A, Rapčan B, Radovani B, Šesto I et al. The N-Glycosylation of total plasma proteins and IgG in atrial fibrillation. Biomolecules. 2023;13. [DOI] [PMC free article] [PubMed]
- 5.Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-Engineering for modulated effector Functions-Improving antibodies for Cancer treatment. Antibodies (Basel). 2020;9. [DOI] [PMC free article] [PubMed]
- 7.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 2017;114:3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Zhang J, Zhang R, Wang Y, Liang Y, Yang Z, et al. Implications of Immunoglobulin G deposit in glomeruli in Chinese patients with diabetic nephropathy. J Diabetes. 2020;12:521–31. [DOI] [PubMed] [Google Scholar]
- 9.Memarian E, Heijmans R, Slieker RC, Sierra A, Gornik O, Beulens JWJ, et al. IgG N-glycans are associated with prevalent and incident complications of type 2 diabetes. Diabetes Metab Res Rev. 2023;39:e3685. [DOI] [PubMed] [Google Scholar]
- 10.Bermingham ML, Colombo M, McGurnaghan SJ, Blackbourn LAK, Vučković F, Pučić Baković M, et al. N-Glycan profile and kidney disease in type 1 diabetes. Diabetes Care. 2018;41:79–87. [DOI] [PubMed] [Google Scholar]
- 11.Abdou AE, Anani HAA, Ibrahim HF, Ebrahem EE, Seliem N, Youssef EMI, et al. Urinary igg, serum CX3CL1 and miRNA-152-3p: as predictors of nephropathy in Egyptian type 2 diabetic patients. Tissue Barriers. 2022;10:1994823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Pan H, Liu D, Zhou D, Tao L, Zhang J, et al. Variation of IgG N-linked glycosylation profile in diabetic retinopathy. J Diabetes. 2021;13:672–80. [DOI] [PubMed] [Google Scholar]
- 13.Ohara N, Hanyu O, Hirayama S, Nakagawa O, Aizawa Y, Ito S, et al. Hypertension increases urinary excretion of Immunoglobulin G, ceruloplasmin and transferrin in normoalbuminuric patients with type 2 diabetes mellitus. J Hypertens. 2014;32:432–8. [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Wan F, Zhu Q, Ye T, Jiang X, Yang H. IgG subclass deposition in diabetic nephropathy. Eur J Med Res. 2022;27:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Hu Y, Wu Q, Zeng Y, Xiao Y, Zeng X, et al. Qualitative and quantitative analysis of B-Cell-Produced antibodies in vitreous humor of type 2 diabetic patients with diabetic retinopathy. J Diabetes Res. 2020;2020:4631290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. 2022;12. [DOI] [PMC free article] [PubMed]
- 17.Guo JZ, Xiao Q, Gao S, Li XQ, Wu QJ, Gong TT. Review of Mendelian randomization studies on ovarian Cancer. Front Oncol. 2021;11:681396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W, Jian X, Zhang J, Meng X, Wang H, Zheng D et al. The causality between human Immunoglobulin G (IgG) N-Glycosylation and aging: A Mendelian randomization study. Molecules. 2024;29. [DOI] [PMC free article] [PubMed]
- 20.Hong Y, Wang Y, Shu W. Deciphering the genetic underpinnings of neuroticism: A Mendelian randomization study of druggable gene targets. J Affect Disord. 2025;370:147–58. [DOI] [PubMed] [Google Scholar]
- 21.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology. 2006;17:260–7. [DOI] [PubMed] [Google Scholar]
- 23.Klarić L, Tsepilov YA, Stanton CM, Mangino M, Sikka TT, Esko T, et al. Glycosylation of Immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci Adv. 2020;6:eaax0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Zhang Y, Li S, Tao Y, Gao R, Xu W, et al. The association between genetically predicted systemic inflammatory regulators and polycystic ovary syndrome: A Mendelian randomization study. Front Endocrinol (Lausanne). 2021;12:731569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Tang M, Gao X, Tian S, Liu W. Mendelian randomization analyses explore the relationship between cathepsins and lung cancer. Commun Biol. 2023;6:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Song M, Kim MS, Natarajan P, Do R, Myung W, et al. An atlas of associations between 14 micronutrients and 22 cancer outcomes: Mendelian randomization analyses. BMC Med. 2023;21:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 genomes project: data management and community access. Nat Methods. 2012;9:459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. 2020;11:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mi Y, Zhu Q, Chen Y, Zheng X, Wan M, Li Y. Impact of physical activity frequency, duration, and intensity on senile cataract risk: A Mendelian randomization study. Transl Vis Sci Technol. 2024;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50:1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess S, Foley CN, Allara E, Staley JR, Howson JMM. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat Commun. 2020;11:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulinskaya E, Dollinger MB. An accurate test for homogeneity of odds ratios based on cochran’s Q-statistic. BMC Med Res Methodol. 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal Pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.do Rosário MMT, Noleto GR, de Oliveira Petkowicz CL. Degalactosylation of xyloglucans modify their pro-inflammatory properties on murine peritoneal macrophages. Int J Biol Macromol. 2017;105:533–40. [DOI] [PubMed] [Google Scholar]
- 40.Antonetti DA, Silva PS, Stitt AW. Current Understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieczór AM, Wieczór R, Kulwas A, Rość D. Asymmetric dimethylarginine and angiogenesis: biological significance. Int Angiol. 2018;37:431–6. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Raman R, Kulothungan V, Sharma T. Association of systemic and ocular risk factors with neurosensory retinal detachment in diabetic macular edema: a case-control study. BMC Ophthalmol. 2014;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11. [DOI] [PMC free article] [PubMed]
- 45.Thiese MS. Observational and interventional study design types; an overview. Biochem Med (Zagreb). 2014;24:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudman N, Kifer D, Kaur S, Simunović V, Cvetko A, Pociot F, et al. Children at onset of type 1 diabetes show altered N-glycosylation of plasma proteins and IgG. Diabetologia. 2022;65:1315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1. The Forest plots between IGPs and diabetes complications (Positive results). (A)-(E): The Forest plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Forest plots of IGP35 and IGP48 on DR.
Supplementary Material 2: Figure S2. The Scatter plots between IGPs and diabetes complications (Positive results). (A)-(E): The Scatter plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Scatter plots of IGP35 and IGP48 on DR.
Supplementary Material 3: Figure S3. The Funnel plots between IGPs and diabetes complications (Positive results). (A)-(E): The Funnel plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Funnel plots of IGP35 and IGP48 on DR.
Supplementary Material 4: Figure S4. The Lol plots between IGPs and diabetes complications (Positive results). (A)-(E): The Lol plots of IGP18, IGP23, IGP28, IGP35 and IGP48 on DM; (F)-(G): The Lol plots of IGP35 and IGP48 on DR.
Data Availability Statement
All data generated or analysed during this study are included in this published article.




