Abstract
The genomes of hyperthermophilic Archaea encode dozens of methylation guide, C/D box small RNAs that guide 2′-O-methylation of ribose to specific sites in rRNA and various tRNAs. The genes encoding the Sulfolobus homologues of eukaryotic proteins that are known to be present in C/D box small nucleolar ribonucleoprotein (snoRNP) complexes were cloned, and the proteins (aFIB, aNOP56, and aL7a) were expressed and purified. The purified proteins along with an in vitro transcript of the Sulfolobus sR1 small RNA were reconstituted in vitro, into an RNP complex. The order of assembly of the three proteins onto the RNA was aL7a, aNOP56, and aFIB. The complex was active in targeting S-adenosyl methionine (SAM)-dependent, site-specific 2′-O-methylation of ribose to a short fragment of ribosomal RNA (rRNA) that was complementary to the D box guide region of the sR1 small RNA. The presence of aFIB was essential for methylation; mutant proteins having amino acid replacements in the SAM-binding motif of aFIB were able to assemble into an RNP complex, but the resulting complexes were defective in methylation activity. These experiments define the minimal number of components and the conditions required to achieve in vitro RNA guide-directed 2′-O-methylation of ribose in a target RNA.
The eukaryotic nucleolus is a highly specialized organelle where rRNA is transcribed, processed, folded, and assembled along with ribosomal proteins into small and large ribosomal subunits (1–5). During this process, up to a hundred or more nucleotide modifications are introduced into the ribosomal RNA (rRNA) by two distinct families of small nucleolar ribonucleoprotein (snoRNP) complexes. The snoRNAs in these RNP complexes contain short antisense guide elements that are used to target modifications to specific locations within the rRNAs. One guide family, the C/D box snoRNPs, targets site-specific 2′-O-methylation of ribose (6–9), and the other guide family, the H/ACA snoRNPs, targets site-specific conversion of uridine to pseudouridine (10).
The C/D box snoRNAs are characterized by a bipartite structure with conserved C box (RUGAUGA) and D box (CUGA) motifs near their respective 5′ and 3′ ends and related C′ (UGAUGA) and D′ (CUGA) motifs near the center of the molecule. The antisense elements are located upstream of the D or D′ motifs and are generally 10 or more nucleotides (nt) in length. Methylation is directed to the rRNA nucleotide that participates in a Watson–Crick base pair five nucleotides upstream from the start of the D or D′ box; this is the N plus five rule (10–12). Although the general mechanism used by these RNP complexes in mediating modification has been deduced from in vivo biochemical and genetic observations, isolation and characterization of the structure and the in vitro activity of these guide complexes have not been described.
The human C/D box snoRNAs associate with several essential proteins, including fibrillarin, NOP56, and NOP58 (paralogous proteins derived from a gene duplication event), and a 15.5-kDa protein (8, 12–14). The corresponding proteins in Saccharomyces cerevisiae are designated Nop1p, Nop56p, Nop58p, and Snu13p. Proteins of the fibrillarin family contain a conserved S-adenosyl methionine (SAM)-binding motif (15, 16). Replacement of two amino acids in this motif (A157V and P219S) in the S. cerevisiae Nop1p protein resulted in temperature sensitivity and a dramatic reduction in methylation of nascent rRNA transcripts under restrictive conditions (17). The 15.5-kDa protein is a component of both the C/D box snoRNPs and the U4/U6⋅U5 tri small nuclear RNP (snRNP), and belongs to a larger family of related RNA-binding proteins that include human L7a and S12 and yeast L30 ribosomal proteins (14, 18, 19). These proteins bind to a common RNA structural motif consisting of an internal purine-rich asymmetric loop with an unusual fold. The fold is generally characterized by two tandem G⋅A base pairs, a high degree of purine stacking, and a single base rotated out of the RNA loop that inserts into a pocket of the protein (20). The motif has been described either as the “kink-turn,” because of the sharp bend present in the phosphodiester backbone of the RNA axis, or as the “GA motif,” based on the presence of the highly conserved GA dinucleotide in the asymmetric internal loop (21, 22). The motif is a highly conserved feature of the 5′ stem loop of U4 snRNA, is generated by the C and D box (or C′ and D′) sequences of C/D box snoRNAs, and is present in other RNAs from both eukaryotes and prokaryotes (21, 22).
Archaea are prokaryotic organisms, distinct from Bacteria and believed to be related to the earliest eukaryotes (23). By employing biochemical and computational methods, the presence of up to 50 or more distinct C/D box small RNAs (sRNAs) was demonstrated in several species of hyperthermophilic Archaea (24–26). The archaeal sRNAs appear to guide methylation not only to rRNA during ribosome biogenesis, but also to 21 different positions within various tRNAs. The genome of the archaeon Sulfolobus solfataricus encodes three proteins, designated aFIB, aNOP56, and aL7a, that are the homologues of the human nucleolar proteins fibrillarin, NOP56/58, and 15.5-kDa. The Sulfolobus aL7a protein has been annotated as a ribosomal protein although it exhibits greater sequence similarity to the human 15.5-kDa protein than to either of the paralogous human L7a or S12 ribosomal proteins.
Archaeal sRNAs exhibit a bipartite structure with well-defined C and D box sequences near their respective 5′ and 3′ ends and related C′ and D′ sequences near the center of the molecules. These can be modeled into structural motifs that are similar to those recognized by the human 15.5-kDa protein (20). Here we show that the aL7a protein binds directly to a Sulfolobus C/D box sRNA, and, together with aNOP56 and aFIB, forms an RNP complex that is active in vitro in site-directed methylation of a fragment of rRNA.
Materials and Methods
Expression and Purification of Recombinant Wild-Type and Mutant Proteins.
The genes encoding the S. solfataricus proteins aL7a (also called HS6), aFIB, and aNOP56 (GenBank accession nos. S75397, AAK41216, and AAK41215) were amplified by PCR and cloned between the NcoI and BamHI sites of pET3d (aL7a, aFIB) or NcoI and EcoRI sites of pET28a (aNOP56). The aL7a gene was amplified with primers AO66 (forward: 5′-AGAATTCCCATGGACGCGATGTCAAAAGCTAG-3′) and AO67 (reverse: 5′-TTAGGATCCTTAACTTGAAGTTTTACCTTTAATC-3′); the aFIB gene was amplified with primers AO70 (forward: 5′- AAAGATCTCCATGGCTGAAGTAATTACCGTAAAAC-3′) and AO71 (reverse: 5′-TTAGGATCCCTACCCTTTATATTTGCTAAGAAC-3′); and the aNOP56 gene was amplified with primers SZ102 (forward: 5′-CCGATATCCATGGTGAAAATATACCTAATTGA-3′) and SZ103 (reverse: 5′-CCGAATTCTCACTTTCTTTTACCTCTTCTCT-3′). The NcoI, BamHI, and EcoRI sites are underlined; as a consequence of NcoI cloning, the three respective proteins contain the following amino acid replacements at the cloning site: N2D, S2A, and M2V. Site-specific mutations in the S. solfataricus aFIB gene were generated by multistep PCR mutagenesis using primers AO70, AO71, HE70 (forward: 5′- GGTCTTATATTTAGGTGTTGCTTCTGGAACTACAATAAG-3′) and HE71 (reverse: 5′-CTTATTGTAGTTCCAGAAGCAACACCTAAATATAAGACC-3′) to generate the A85V mutation and AO70, AO71, HE83 (forward: 5′-GGAGGCCTAATATCTTTGTACTATTGGCTGATGCAAG-3′) and HE84 (reverse: 5′-CTTGCATCAGCCAATAGTACAAAGATATTAGGCCTCC-3′) to generate the P129V mutation. The altered nucleotides creating the desired mutations are underlined. The recombinant proteins were expressed in Escheria. coli BL21(DE3) (aNOP56), E. coli BL21 (DE3)pLysS (aFIB), or E. coli BL21 (DE3)pLysE (aL7a), and initially separated from the majority of host E. coli proteins by thermoprecipitation. Details of the purification will be presented elsewhere.
In Vitro Transcription of Guide and Target RNAs.
A clone (pPD1260) containing the S. acidocaldarius sR1 cDNA inserted between the EcoRI and BamHI sites of plasmid pGEM 3Zf(+) was linearized with BamHI and used as template in a T7 RNA polymerase transcription reaction to generate sR1 sRNA (24). Uniformly labeled sR1 transcript was generated by reducing the appropriate NTP concentration to 13 μM and including 10 μCi (1 Ci = 37 GBq) of the corresponding [α-32P]-NTP in the reaction. End-labeled sR1 was generated by using 5′-32P-labeled cytidine-5′,3′-bisphosphate ([32P]pCp), and T4 RNA ligase (27).
To generate target RNA, a 112-bpfragment of rDNA spanning the predicted site of methylation (position U52) was amplified by PCR from S. acidocaldarius genomic DNA using primers AO63.1 (5′-GTAATACGACTCACTATAGGGATAAGCCATGGGAG-3′; the T7 promoter region is underlined), and AO65 (5′-TATTTAGGTGACACTATAGGTTAGCCACGTGTTACTCAGCC-3′; the SP6 promoter region is underlined). The product was cleaved with SmaI (at position 69 in rDNA); the resulting 51-bp fragment was purified by gel electrophoresis and used as a template in a T7 transcription reaction. The sequence of the generated target RNA product is 5′-GGGAUAAGCCA[U]GGGAGUCUUACACUCCC-3′; the expected site for methylation is bracketed.
The T7 templates used to transcribe mutant guide RNA were generated by PCR using plasmid pPD1260 as template and the following primers containing nucleotide substitutions (in brackets) at the desired positions: T7 (5′-TAATACGACTCACTATAGGG-3′; the T7 promoter is underlined), and HE45 (5′-GTTATCAGACCA[A]GGGAGTTAAG-3′). The T7 template used to transcribe mutant target RNA was generated from a partially single-stranded DNA template, by annealing the T7 promoter primer to the oligonucleotide OSZ117 (5′-GGGAGTGTAAGACTCCC[T]TGGCTTATCCCTATAGTGAGTCGTATTA-3′; the T7 promoter is underlined). The complement of the expected site for methylation is bracketed.
Where required, PCR products were sequenced to ensure that they contained the expected substitutions or alterations.
Gel Mobility Retardation Assays.
A constant amount of uniformly labeled S. acidocaldarius sR1 RNA (0.2 pmol) was either mock treated or mixed with serial dilutions of recombinant aL7a protein (0.25, 0.5, 1, 2, 4, or 8 pmol) in binding buffer A (25 mM phosphate buffer, pH 7/100 mM NaCl/1 mM MgCl2) in a 10 μl reaction. After 10-min incubation at 70°C, 1 μl of the loading buffer (1% bromophenol blue/10% glycerol) was added and 5 μl of the mixture was loaded on a nondenaturing 10% polyacrylamide gel containing 25 mM phosphate buffer, pH 7. The gel was run in 50 mM phosphate buffer, pH 7, at 150 V, for 2 h at room temperature. The distribution of free (sR1) and retarded RNA (sR1–aL7a; complex I) was visualized by autoradiography. The nonspecific RNA-binding activity of aL7a was tested by using in vitro transcribed S. acidocaldarius RNase P RNA, tRNAGly, or RNA transcribed from the polylinker region of pGEM3Zf(+). Competition assays contained aL7a protein (1 pmol), radiolabeled sR1 (0.2 pmol), and competitor RNA (0.02 to 20 pmol of nonradioactive sR1 or RNase P RNA). The same procedure as described above was used to assemble higher-order complexes consisting of sR1–aL7a–aNOP56 (complex II) and sR1–aL7a–aNOP56–aFIB (complex III), except that the amount of each of the included proteins was 1 pmol. These larger complexes were routinely resolved on a nondenaturing 6% polyacrylamide gel prepared as described above.
Immunoprecipitation of Complexes Containing aFIB Mutants.
The sR1 transcript (1 pmol), 3′ end-labeled with [32P]pCp, was incubated with various combinations of aL7a, aNOP56, and either wild-type aFIB or one of the aFIB mutants (20 pmol of each protein) at 70°C for 15 min in a 100-μl reaction volume. Rabbit anti-S. solfataricus aFIB serum (20 μl) was incubated for 2 h at room temperature with 100 μl of settled protein A-Sepharose suspension in the binding buffer A. Using the same buffer throughout, excess unbound antibodies were removed by three 10-min washes. The complexes resulting from different RNA–protein combinations were then added to the preformed protein A-anti-aFIB beads, and incubation was continued for 30 min at room temperature. Unbound material was removed by three 10-min washes. The RNA present in the bound material was recovered after phenol/chloroform extraction, run on a denaturing 6% polyacrylamide gel, and visualized by autoradiography.
In Vitro Methylation Assay.
Equimolar amounts (720 pmol) of sR1 guide RNA and target RNA were mixed (final volume of 30 μl, in 25 mM phosphate buffer, pH 7/100 mM NaCl), denatured by incubating for 1 min at 95°C, and renatured by cooling rapidly to 55°C. The RNAs were added at 0°C to 90 μl containing aFIB, aNOP56, aL7a (24 pmol of each), and [methyl-3H]SAM (360 pmol, 3.9 Ci/mmol, Amersham Pharmacia) in the binding buffer A. Aliquots (20 μl) were removed and transferred to 70°C; after 10, 20 30, 60, or 120 min the individual 20-μl samples were removed and precipitated at 0°C for 10 min with 5% trichloroacetic acid. The precipitates were collected on 0.2-μm nitrocellulose filters (Sartorius), and dried, and radioactivity was measured by scintillation counting. The predenaturation and annealing of the guide and target RNAs, although routinely performed, are not required for the reconstitution of methylase activity; all of the components are able to fully reassemble into an active complex at the reaction temperature of 70°C (data not shown).
TLC Analysis of Modified Nucleotides.
Guide and target RNAs were mixed in a 20 μl reaction in the standard methylation assay (except that [methyl-3H]SAM specific activity used was 72.15 Ci/mmol; 60 pmol per assay) and incubated at 70°C for 1 h. The methylated RNA target was digested with 0.01 unit of P1 nuclease (Roche Diagnostics) for 12 h at 37°C and products were mixed with 5 nmol each of pAm, pCm, pGm, pUm, and pUmG standards (Dharmacon Research, Lafayette CO) before the two-dimensional TLC separation (28). TLC analysis was carried out on thin-layer cellulose plates (10 cm × 10 cm, Merck) by using the following chromatographic system: first dimension, isobutyric acid/concentrated NH4OH/H2O (66:0.5:33.5; vol/vol/vol); second dimension, 0.1 M sodium phosphate, pH 6.8/ammonium sulfate/n-propyl alcohol (100:60:2; vol/wt/vol). Unlabeled standards were detected by UV shading. Radioactivity was detected by 3H-imaging, and the spots corresponding to the UV-detectable standards were excised from the plate and subjected to scintillation counting.
Results and Discussion
Binding of aL7a to Archaeal sR1 Methylation Guide sRNA.
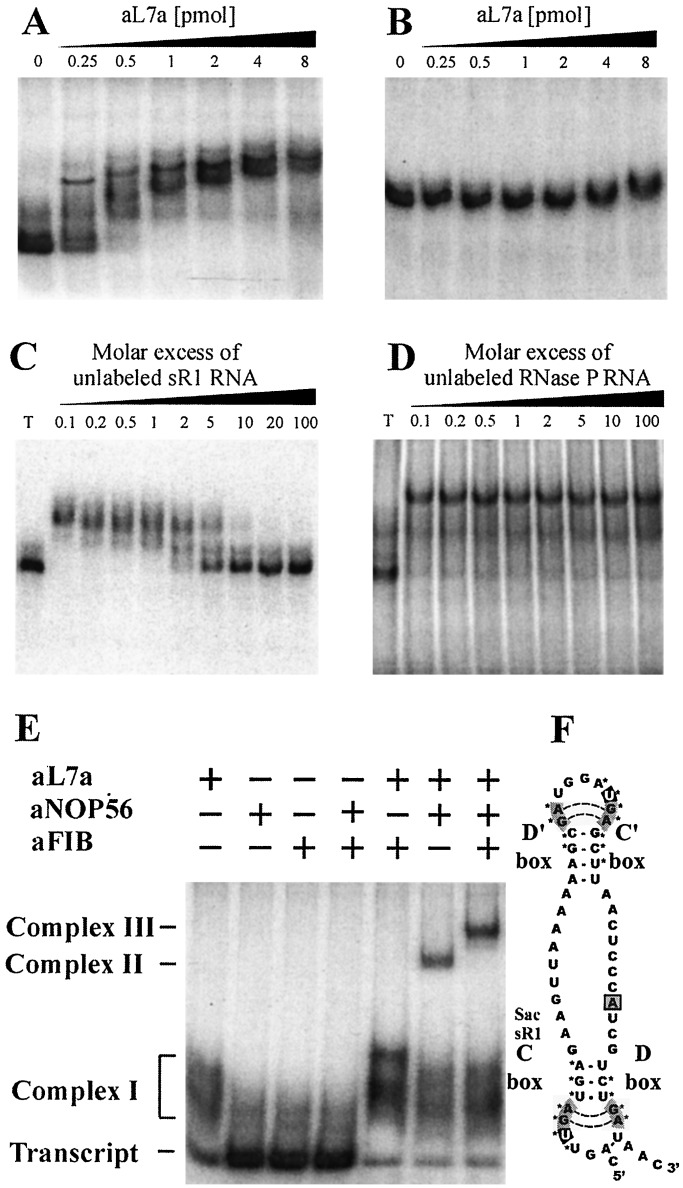
The human 15.5-kDa protein has been shown to interact directly with the 5′ stem loop of U4 snRNA and a similar structural motif generated by the interaction between the C and D box sequences in U3, U8, and U14 snoRNAs (18). Archaeal sRNAs exhibit a bipartite structure with well-defined box C and D sequences near their respective 5′ and 3′ ends and related C′ and D′ sequences near the center of the molecules. Although some archaeal sRNAs lack the terminal hairpin that is found in most eukaryotic C/D box snoRNAs, their box sequences can be modeled into structural motifs that are similar to the binding motifs of the human 15.5-kDa protein in U4 snRNA and U3, U8, and U14 snoRNAs (Fig. 1F). A gel electrophoresis retardation assay was used to investigate whether recombinant S. solfataricus aL7a would bind to in vitro transcribed sR1 sRNA (Fig. 1A). Increasing amounts of protein caused a dramatic shift in the mobility of the sR1 sRNA. At higher protein concentrations, complexes with distinct electrophoretic mobilities were observed, suggesting that there are multiple conformational isomers of the sR1–aL7a complex. The presence of box C-like and D-like motifs appeared to be required for the binding of aL7a to the RNA. For example, the mobility of the RNA component of S. acidocaldarius RNase P, which lacks these sequence motifs, was not altered by the presence of aL7a protein, and RNase P RNA failed to disrupt the sR1–aL7a interaction (Fig. 1 B, C–D). Similarly, the aL7a protein failed to bind in vitro transcripts containing tRNAGly, a fragment of 16S rRNA, or the polylinker region of pGEM7 (data not shown).
Figure 1.
In vitro assembly of archaeal sR1 sRNA into an RNP complex. In vitro transcribed RNAs were uniformly labeled with [α-32P]ATP and used in gel mobility retardation assays to monitor interaction with recombinant aL7a protein. S. acidocaldarius C/D box sR1 sRNA (0.2 pmol) (A), or RNase P RNA (1 pmol) (B), was mixed with increasing amounts of recombinant aL7a protein (0 to 8 pmol per assay) at 0°C, transferred to 70°C for 10 min, separated on a nondenaturing 10% polyacrylamide gel, and visualized by autoradiography. The competition assays contained radiolabeled sR1 RNA (0.2 pmol), aL7a protein (1 pmol), and nonradiolabeled competitor sR1 (C), or RNase P (D) RNAs (0.02 to 20 pmol). T, transcript. To detect higher-order complexes, uniformly labeled sR1 transcript (0.2 pmol) was mixed with one or more of the proteins (1 pmol of each protein per reaction) at 0°C, transferred to 70°C for 10 min, separated on a nondenaturing 6% polyacrylamide gel, and visualized by autoradiography (E). The positions of free transcript, complex I (sR1 sRNA–aL7a), complex II (sR1 sRNA–aL7a–aNOP56), and complex III (sR1 sRNA–aL7a–aNOP56–aFIB) are indicated. A secondary structural model of sR1s RNA is depicted (F). The aL7a protein is predicted to bind to the loops generated by the C/D or C′/D′ motifs (indicated by *). The base predicted to rotate out of the loop and insert into the pocket of the protein is the first U residue in the C or C′ box sequence and is highlighted in black (20).
Higher-Order RNP Complexes Containing aL7a, aNOP56, and aFIB.
The observation that both eukaryotic and archaeal box C/D methylation guide RNAs can be coimmunoprecipitated with antibodies against NOP56/58 or fibrillarin implies that these proteins are components of the methylation guide RNP complex (11, 12, 24, 29). A gel electrophoresis retardation assay was used to determine whether the archaeal proteins (aNOP56 and aFIB) can bind directly to sR1 sRNA or be assembled into C/D box RNPs. In contrast to aL7a protein, neither aFIB nor aNOP56, nor the two proteins together, were able to retard the mobility of the sR1 RNA (Fig. 1E). Because the aL7a protein binds sR1, we decided to investigate whether it could serve to nucleate the binding of aNOP56 and aFIB to the complex. When aNOP56 was added to the sR1–aL7a complex I, a clear supershift in mobility was observed; this new species was designated complex II. In contrast, when aFIB was added to the sR1–aL7a complex I, no discernible shift was detected. In a final experiment, aFIB was added to the preformed sR1–aL7a–NOP56 complex II. This resulted in an even greater retardation in mobility, indicative of aFIB binding to create the even larger complex III. These experiments suggest that the aL7a protein is the primary box C/D sRNA-binding protein; when bound to the sRNA it appears to nucleate the step-wise addition of first aNOP56, then aFIB, to the complex. The optimum temperature for assembly of these complexes was 70°C; this is slightly below the 75–80°C optimum growth temperature for Sulfolobus species. Once assembled the complexes were stable for several hours at room temperature.
Methylation Activity of in Vitro Reconstituted C/D Box RNPs.
The crystal structure of the Methanococcus jannaschii fibrillarin protein has been determined (17). The carboxyl-terminal region contains a motif that is structurally related to the SAM binding sites in several methyltransferase enzymes and appears to be responsible for the methylation activity (14, 16, 17). We next asked whether our in vitro complex, when assembled in the presence of a suitable target RNA, has methyltransferase activity when SAM is provided as substrate. The target RNA (29 nt) was designed to contain the region of Sulfolobus 16S rRNA that is recognized in vivo by the D box guide of the sR1 sRNA. Methylation occurs at position U52 in the rRNA (24, 25). In the presence of target RNA and SAM radiolabeled at the methyl position, radioactivity was rapidly incorporated into acid-insoluble material (Fig. 2A). The methylation depended both on the addition of a suitable complementary target RNA (data not shown) and on the presence of the aFIB protein in the complex. In control reactions where only RNAs and either single proteins or combinations of only two proteins were added, radioactive methyl incorporation into acid-insoluble material was not detected (data not shown). The amount of product formed after 60 min (about 8 pmol per reaction) was approximately two times the molar amount of aFIB present (4 pmol per reaction). This implies that, under this reaction condition, each molecule of aFIB was, on average, able to participate in the methylation of two target RNAs. The plateau reached in the reaction appears to be a consequence of the degradation of the guide and target RNAs and the SAM substrate at the high temperature (70°C) required for catalysis, rather than a consequence of inactivation of the three proteins. Supplementation of the reaction after 45 min with additional guide and target RNAs (120 pmol of each) and SAM (60 pmol) resulted in a 50–100% increase in the production of methylated target, whereas supplementation with 4 pmol of each of the three proteins had no effect (data not shown). The mechanism for enzymatic turnover is currently unclear. Either multiple target RNAs are able to associate and dissociate from a single preassembled RNP complex, or the RNP complexes are disassembled after each methylation and reassembled before the next round of methylation. The supplementation experiments suggest that some reassembly occurs.
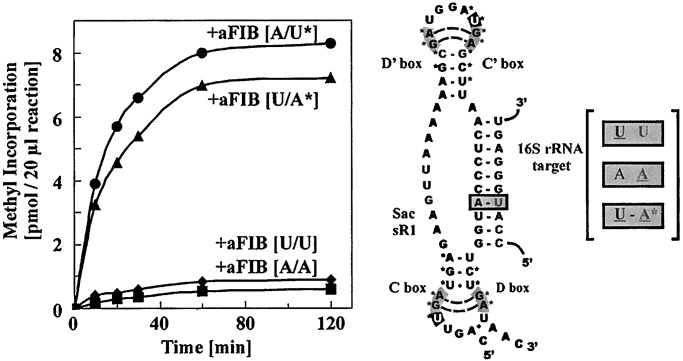
Figure 2.
RNP guide-dependent methyl incorporation into a complementary target RNA. RNP complex was assembled by renaturing in vitro transcribed sR1 sRNA and target RNA (5′-GGGAUAAGCCA[U]GGGAGUCUUACACUCCC-3′; the expected site for methylation is bracketed) and mixing with aFIB, aNOP56, aL7a, and [methyl-3H]SAM at 0°C. The mixture (120 μl containing 720 pmol of sR1 guide RNA, 720 pmol of target RNA, 360 pmol of radioactive SAM, and 24 pmol of each of the three recombinant proteins) was divided into six 20-μl reactions and transferred to 70°C. At time intervals, single 20-μl reactions were removed and precipitated at 0°C with 5% trichloroacetic acid. The precipitates were collected on nitrocellulose filters, and dried, and radioactivity was determined by scintillation counting. The kinetics of methyl incorporation are shown on the left (A) and the predicted secondary structure of the guide and target RNAs are shown on the right (B). The wild type (wt) and the two mutant aFIB proteins (containing A85V or P129V replacements) were tested for their ability to assemble into RNP particles by using 3′-end-labeled sR1 sRNA transcript (1 pmol) and coimmunoprecipitation with antibodies against aFIB. The coprecipitated RNAs were displayed on a denaturing 6% polyacrylamide gel and detected by autoradiography (C). The ability of the A85V mutant to assemble into complex III (D) was examined as described in the legend to Fig. 1.
An S. cerevisiae mutant strain that contains replacement of two highly conserved amino acids in the putative SAM-binding motif in the Nop1p fibrillarin protein (A175V and P219S) exhibits temperature sensitivity and a dramatic reduction in methylation of nascent rRNA transcripts under restrictive conditions (17). By site-directed mutagenesis, single amino acid replacements (A85V or P129V) were introduced at the corresponding positions in the Sulfolobus aFIB protein. The two mutant proteins were expressed, purified, and mixed with one or both of the aNOP56 or aL7a proteins and sR1 sRNA (3′ end-labeled with [32P]pCp) in an assembly assay (Fig. 2C). The mixtures were immunoprecipitated with antibodies against aFIB, and the presence of sR1 sRNA in the precipitate was detected by gel electrophoresis. In the absence of aFIB protein, little or no sR1 sRNA was detected in the precipitate. In contrast, both aFIB wild-type and mutant proteins were able to assemble with comparable efficiencies, into sR1-containing RNP complexes in the presence of aL7a and aNOP56 (Fig. 2C). Moreover, the A85V mutant protein was shown to be fully competent for assembly into complex III (Fig. 2D). When tested in the methylation reaction, the complex containing the A85V replacement was inactive, whereas the complex containing the P129V replacement was partially active (Fig. 2A). These results suggest (i) that the two aFIB mutants retain structural integrity and are able to assemble into RNP complexes and (ii) that the putative SAM-binding motif in the aFIB protein is essential for methylation of target RNA. This result provides further evidence that fibrillarin acts as the methyltransferase in RNA guide-directed methylation.
Importance of the Watson–Crick Base Pairing at the Site of Methylation.
In vivo studies in eukaryotes have indicated that a Watson–Crick base pair between the guide and target is required at the site of methylation, five nucleotides upstream from the start of the D or D′ box (7). An A to U mutation was introduced into the sR1 D box guide at this position and a U to A mutation was introduced at the position equivalent to U52 in the rRNA target. Radioactive methyl incorporation was reduced to background in assays using either the mutant sR1 guide and a wild-type target or a wild-type sR1 guide and the mutant target (Fig. 3). In contrast, when the mutant guide and the mutant target were used together, base pairing at the predicted site of methylation was regenerated because of the compensatory nature of the mutations, and methyl incorporation was restored to near the wild-type level.
Figure 3.
Effects of nucleotide substitution in the methylation guide and target RNA sequences. Nucleotide substitutions were introduced into the sR1 D box guide (A to U at the N plus five position relative to the start of the D box) and the rRNA target (U to A at position corresponding to U52 in 16S rRNA). Methylation assays, as described in the legend to Fig. 2, were carried out by using (i) wild-type guide and wild-type target (●), (ii) mutant guide and wild-type target (♦), (iii) wild-type guide and mutant target (■), or (iv) compensatory mutant guide and mutant target (▴). The base pair at the methylation site for each assay is illustrated on the right and the incorporation kinetics for the four reactions is illustrated on the left.
Specificity of the Methylation Reaction.
Based on the N plus five rule, the site of methyl modification within the target RNA is predicted to occur at the nucleotide position corresponding to U52 in 16S rRNA (1, 2). This residue within the target RNA is part of a unique UG dinucleotide (Fig. 4A). To show that methylation is specific to the U52 position, target RNA was incubated in the presence of [methyl-3H]SAM in the standard methylation assay and then subjected to digestion with nuclease P1 under conditions where non methylated nucleotides are completely hydrolyzed and methylated nucleotides are only partially hydrolyzed. The hydrolysis products were mixed with 2′-O-methylated ribose standards, pAm, pCm, pGm, pUm, and pUmG, and separated by TLC (28) (Fig. 4B). The positions of individual standards were observed by UV shading and superimposed on the 3H image plate. The 3H-radioactivity was observed only in pUm and pUmG and was quantified by scintillation counting of the individual UV-detectable spots (pUm, 1,129 cpm; pUmG, 5,955 cpm; pAm, 23 cpm; pCm, 37 cpm; and pGm, 28 cpm). Omission of any of the RNA or protein components from the reaction mixture results in background levels of incorporation of radioactivity into acid-insoluble material (see Fig. 2). These experiments provide strong evidence that the in vitro reconstituted archaeal C/D box RNP accurately directs methylation to the nucleotide in the target that forms a Watson–Crick base pair, five nucleotides upstream from the start to the D box. This nucleotide corresponds to position U52 in 16S rRNA.
Figure 4.

Thin-layer chromatographic separation of the hydrolysis products of the target RNA. The standard 29-nt long target RNA contains a single unique UG dinucleotide. The U residue in this dinucleotide is the expected site of methylation and corresponds to position U52 in 16S rRNA (A). Guide and target RNAs were mixed in a 20-μl reaction in the standard methylation assay using [methyl-3H]SAM and incubated at 70°C for 1 h. The RNA was extracted, digested with 0.01 unit of P1 nuclease (Roche Diagnostics) for 12 h at 37°C, and products were mixed with 5 nmol each of pAm, pCm, pGm, pUm, and pUmG standards before the two-dimensional TLC separation (28). Unlabeled standards were detected by UV shading and radioactivity was detected by 3H-imaging (B). The spots corresponding to the UV-detectable standards were excised from the plate and subjected to scintillation counting (see text). Omission of any of the RNA or protein components from the reaction mixture results in background levels of incorporation of radioactivity into acid-insoluble material (see Fig. 2).
Perspectives.
Both eukaryotic and archaeal organisms possess small C/D box RNAs with complementary guide regions that are used to direct methylation to specific nucleotide positions in rRNA during ribosome assembly. The guide RNA–rRNA interaction and the associated methylation may have more than one function. The interaction may be used as a means to channel localized folding or mediate structural rearrangement within the rRNA during the assembly process. In addition, the deposition of the methyl group on the 2′-O position of ribose may provide rigidity and structural stability to the rRNA within the assembled ribosome. Although the predicted positions of most methylations are not highly conserved, virtually all are confined to the structurally important core regions of small and large subunit rRNAs (25).
Our attempts to reconstitute an RNP complex that is active in vitro in guide-directed methylation have resulted in a remarkable observation: the core catalytic complex from Archaea is much simpler than anticipated. It requires only an sRNA and three proteins, the core RNA-binding aL7a protein, the aNOP56 protein of largely unknown function, and the aFIB protein which appears to possess the methyltransferase activity. It is perhaps surprising that an RNA helicase (30) has yet to be found in our partially purified in vivo RNP complexes, nor is it required for in vitro methylation in our reconstituted system. The absence of the helicase requirement may be related to the high temperature of the in vitro reaction (70°C), or to the small size of the RNA target used in the assay. In Sulfolobus cell extracts, C/D box RNPs are large heterogeneous complexes, which sediment between 10 S and 50 S in a sucrose or glycerol density gradient (24). The larger sedimentation values may reflect the in vivo association of the core RNP complexes with additional proteins or with precursors of small and large ribosomal subunits.
The number of easily identifiable C/D box sRNAs in particular archaeal species is correlated to the optimum growth temperature. Genera such as Pyrococcus, Pyrobaculum, and Sulfolobus contain dozens of sRNAs with guides that are complementary to both rRNAs and tRNAs, whereas mesophilic archaeal genera, such as Halobacterium, contain few if any sRNAs (24, 25). The optimum growth temperature for Sulfolobus is between 75° and 80°C and is reflected in the high temperature requirement for the assembly and methylation reactions described here. A temperature of at least 60°C is required to reconstitute the RNP complex, whereas efficient in vitro methylation occurs in an even narrower range around 70°C. This likely reflects a combination of the temperature optimum of the proteins, the structural stability of the guide–target duplex, and the chemical stability of the RNAs and SAM substrate.
In addition to rRNA, a large number of other cellular transcripts, including tRNAs, snRNAs, and mRNAs, transit through the nucleolus during their maturation, en route to their final cellular destination. At least some of these RNAs are substrates for guide–directed methylation (31–36). The modifications, in addition to their influence on local RNA secondary structure (37), can play important roles in potentiating RNA/RNA and RNA/protein interactions (38–40). For example, methyl modification has been implicated as a possible switch for controlling A/I editing and splice site selection in brain-specific pre-mRNAs (41, 42). The experiments presented here demonstrate aFIB, aNOP56, and aL7a are sufficient for in vitro methylation. Our ability to reconstitute the archaeal methylation machinery from recombinant components opens the way for more detailed studies on the structure, mechanism, and diverse biological functions of these complex RNP machines.
Acknowledgments
We are grateful to Nick Watkins for sharing with us information on the properties of the human 15.5-kDa protein before publication and for suggesting a secondary structure for the archaeal sR1 sRNA. This work was supported by a grant from the Canadian Institute for Health Research to P.P.D. P.P.D. is an associate of the Canadian Institute for Advanced Research, program in Evolutionary Biology.
Abbreviations
- RNP
ribonucleoprotein
- snoRNA and snoRNP
small nucleolar RNA and RNP
- snRNA and snRNP
small nuclear RNA and RNP
- SAM
S-adenosyl-methionine
References
- 1.Maxwell E S, Fournier M J. Annu Rev Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- 2.Lafontaine D L J, Tollervey D. In: Modification and Editing of RNA. Grosjean H, Benne R, editors. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 281–288. [Google Scholar]
- 3.Bachellerie J-P, Cavaille J, Qu L-H. In: The Ribosome: Structure, Function, Antibiotics, and Cellular Interactions. Garrett R A, Douthwaite S R, Liljas A, Matheson A T, Moore P B, Noller H F, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 199–203. [Google Scholar]
- 4.Venema J, Tollervey D. Annu Rev Genet. 1999;33:261–311. doi: 10.1146/annurev.genet.33.1.261. [DOI] [PubMed] [Google Scholar]
- 5.Filipowicz W, Pelczar P, Pogacic V, Dragon F. Acta Biochim Pol. 1999;46:377–389. [PubMed] [Google Scholar]
- 6.Weinstein L B, Steitz J A. Curr Opin Cell Biol. 1999;11:378–384. doi: 10.1016/S0955-0674(99)80053-2. [DOI] [PubMed] [Google Scholar]
- 7.Cavaille J, Nicoloso M, Bachellerie J-P. Nature. 1996;383:732–735. doi: 10.1038/383732a0. [DOI] [PubMed] [Google Scholar]
- 8.Kiss-Laszlo Z, Henry Y, Bachellerie J-P, Caizergues-Ferrer M, Kiss T. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- 9.Nicoloso M, Qu L-H, Michot B, Bachellerie J-P. J Mol Biol. 1996;260:178–195. doi: 10.1006/jmbi.1996.0391. [DOI] [PubMed] [Google Scholar]
- 10.Ni J, Tien A L, Fournier M J. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 11.Wu P, Brockenbrough J S, Metcalfe A C, Chen S, Aris J P. J Biol Chem. 1998;273:16453–16463. doi: 10.1074/jbc.273.26.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schimmang T, Tollervey D, Kern H, Frank R, Hurt E C. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier T, Berges T, Tollervey D, Hurt E C. Mol Cell Biol. 1997;17:7088–7098. doi: 10.1128/mcb.17.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins N J, Segault V, Charpentier B, Nottrott S, Fabrizio P, Bachi A, Wilm M, Rosbash M, Branlant C, Luhrmann R. Cell. 2000;103:457–466. doi: 10.1016/s0092-8674(00)00137-9. [DOI] [PubMed] [Google Scholar]
- 15.Koonin E V, Tatusov R L, Rudd K E. Proc Natl Acad Sci USA. 1995;92:11921–11925. doi: 10.1073/pnas.92.25.11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Boisvert D, Kim K K, Kim R, Kim S H. EMBO J. 2000;19:317–323. doi: 10.1093/emboj/19.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tollervey D, Lehtonen H, Jansen R, Kern H, Hurt E C. Cell. 1993;72:443–457. doi: 10.1016/0092-8674(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 18.Nottrott S, Hartmuth K, Fabrizio P, Urlaub H, Vidovic I, Ficner R, Luhrmann R. EMBO J. 1999;18:6119–6133. doi: 10.1093/emboj/18.21.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao H, White S A, Williamson J R. Nat Struct Biol. 1999;6:1139–1147. doi: 10.1038/70081. [DOI] [PubMed] [Google Scholar]
- 20.Vidovic I, Nottrott S, Hartmuth K, Luhrmann R, Ficner R. Mol Cell. 2000;6:1331–1342. doi: 10.1016/s1097-2765(00)00131-3. [DOI] [PubMed] [Google Scholar]
- 21.Klein D J, Schmeing T M, Moore P B, Steitz T A. EMBO J. 2001;20:4214–4221. doi: 10.1093/emboj/20.15.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler W C, Grundy F J, Murphy B A, Henkin T M. RNA. 2001;7:1165–1172. doi: 10.1017/s1355838201002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woese C R, Kandler O, Wheelis M L. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omer A D, Lowe T M, Russell A G, Ebhardt H, Eddy S R, Dennis P P. Science. 2000;288:517–522. doi: 10.1126/science.288.5465.517. [DOI] [PubMed] [Google Scholar]
- 25.Dennis P P, Omer A, Lowe T. Mol Microbiol. 2001;40:509–519. doi: 10.1046/j.1365-2958.2001.02381.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaspin C, Cavaille J, Erauso G, Bachellerie J-P. J Mol Biol. 2000;297:895–906. doi: 10.1006/jmbi.2000.3593. [DOI] [PubMed] [Google Scholar]
- 27.England T E, Uhlenbeck O C. Nature (London) 1978;275:560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- 28.Keith G. Biochimie. 1995;77:142–144. doi: 10.1016/0300-9084(96)88118-1. [DOI] [PubMed] [Google Scholar]
- 29.Lafontaine D L J, Tollervey D. RNA. 1999;5:455–467. doi: 10.1017/s135583829998192x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugeron M C, Kressler D, Linder P. RNA. 2001;7:1317–1334. doi: 10.1017/s1355838201010640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jady B E, Kiss T. EMBO J. 2001;20:541–551. doi: 10.1093/emboj/20.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganot P, Jady B E, Bortolin M L, Darzacq X, Kiss T. Mol Cell Biol. 1999;19:6906–6917. doi: 10.1128/mcb.19.10.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tycowski K T, You Z H, Graham P J, Steitz J A. Mol Cell. 1998;2:629–638. doi: 10.1016/s1097-2765(00)80161-6. [DOI] [PubMed] [Google Scholar]
- 34.Bond V C, Wold B. Mol Cell Biol. 1993;13:3221–3230. doi: 10.1128/mcb.13.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson M R, Pederson T. Proc Natl Acad Sci USA. 1998;95:7981–7986. doi: 10.1073/pnas.95.14.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertrand E, Houser-Scott F, Kendall A, Singer R H, Engelke D R. Genes Dev. 1998;12:2463–2468. doi: 10.1101/gad.12.16.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis R. In: Modification and Editing of RNA. Grosjean H, Benne R, editors. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 85–102. [Google Scholar]
- 38.Baidya N, Uhlenbeck O C. Biochemistry. 1995;34:12363–12368. doi: 10.1021/bi00038a033. [DOI] [PubMed] [Google Scholar]
- 39.Chowrira B M, Berzal-Herranz A, Keller C F, Burke J M. J Biol Chem. 1993;268:19458–19462. [PubMed] [Google Scholar]
- 40.Abramovitz D L, Friedman R A, Pyle A M. Science. 1996;271:1410–1413. doi: 10.1126/science.271.5254.1410. [DOI] [PubMed] [Google Scholar]
- 41.Cavaille J, Buiting K, Kiefmann M, Lalande M, Brannan C I, Horsthemke B, Bachellerie J-P, Brosius J, Huttenhofer A. Proc Natl Acad Sci USA. 2000;97:14311–14316. doi: 10.1073/pnas.250426397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipowicz W. Proc Natl Acad Sci USA. 2000;97:14035–14037. doi: 10.1073/pnas.97.26.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]