Abstract
The internal ribosome entry sites (IRES),
IRES and IRES
and IRES , precede
the coat protein (CP) and movement protein (MP) genes of
crucifer-infecting tobamovirus (crTMV), respectively. In the present
work, we analyzed the activity of these elements in transgenic plants
and other organisms. Comparison of the relative activities of the crTMV
IRES elements and the IRES from an animal virus—encephalomyocarditis
virus—in plant, yeast, and HeLa cells identified the 148-nt
IRES
, precede
the coat protein (CP) and movement protein (MP) genes of
crucifer-infecting tobamovirus (crTMV), respectively. In the present
work, we analyzed the activity of these elements in transgenic plants
and other organisms. Comparison of the relative activities of the crTMV
IRES elements and the IRES from an animal virus—encephalomyocarditis
virus—in plant, yeast, and HeLa cells identified the 148-nt
IRES as the strongest element that also
displayed IRES activity across all kingdoms. Deletion analysis
suggested that the polypurine (A)-rich sequences (PARSs) contained in
IRES
as the strongest element that also
displayed IRES activity across all kingdoms. Deletion analysis
suggested that the polypurine (A)-rich sequences (PARSs) contained in
IRES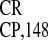 are responsible for these features. On the
basis of those findings, we designed artificial PARS-containing
elements and showed that they, too, promote internal translation from
dicistronic transcripts in vitro, in tobacco protoplasts
and in HeLa cells. The maximum IRES activity was obtained from multiple
copies of either (A)4G(A)2(G)2 or
G(A)2–5 as contained in IRES
are responsible for these features. On the
basis of those findings, we designed artificial PARS-containing
elements and showed that they, too, promote internal translation from
dicistronic transcripts in vitro, in tobacco protoplasts
and in HeLa cells. The maximum IRES activity was obtained from multiple
copies of either (A)4G(A)2(G)2 or
G(A)2–5 as contained in IRES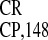 .
Remarkably, even homopolymeric poly(A) was moderately active, whereas a
poly(G) homopolymer was not active. Furthermore, a database search for
existing PARS sequences in 5′-untranslated regions (5′UTR) of genes in
tobacco genome allowed the easy identification of a number of IRES
candidates, in particular in the 5′UTR of the gene encoding
Nicotiana tabacum heat-shock factor 1 (NtHSF1).
Consistent with our prediction, the 5′UTR of NtHSF1 turned out to be an
IRES element active in vitro, in plant protoplasts and
HeLa cells. We predict that PARS elements, when found in other mRNAs,
will show a similar activity.
.
Remarkably, even homopolymeric poly(A) was moderately active, whereas a
poly(G) homopolymer was not active. Furthermore, a database search for
existing PARS sequences in 5′-untranslated regions (5′UTR) of genes in
tobacco genome allowed the easy identification of a number of IRES
candidates, in particular in the 5′UTR of the gene encoding
Nicotiana tabacum heat-shock factor 1 (NtHSF1).
Consistent with our prediction, the 5′UTR of NtHSF1 turned out to be an
IRES element active in vitro, in plant protoplasts and
HeLa cells. We predict that PARS elements, when found in other mRNAs,
will show a similar activity.
Translation of most eukaryotic mRNAs occurs by traditional cap-dependent ribosome scanning (1–5). However, the initiation of translation of a variety of viral and cellular mRNAs takes place by an alternative mechanism of internal ribosome entry mediated by internal ribosome entry sites (IRESs). IRESs of about 350–450 nt have been identified and most extensively characterized in the 5′-untranslated regions (5′UTRs) of RNA of viruses belonging to the Picornaviridae and Flaviviridae families (6–8), whereas IRESs of about 200 nt were found on the RNAs of insect RNA viruses (9–10). The IRES elements of different origin differ largely in structural organization, sequence, length and functional requirements. It is generally believed that there are kingdom-specific limitations of viral IRES activity; thus none of the animal virus IRES elements seem to be active in yeast cells (7). Contrary to this concept, Urwin et al. (11) reported that the encephalomyocarditis virus (EMCV) IRES (IRESEMCV) was also moderately active in plant cells.
IRES elements have also been found in the 5′UTRs of several animal mRNAs. Importantly, IRES-dependent translation has been reported for cellular mRNAs when their cap-dependent translation is impaired (e.g., under conditions of viral infection, heat shock, apoptosis, and at the G2/M phase of the cell cycle) (12–14).
It is obvious that mRNAs of those plant viruses that are naturally uncapped (e.g., members of the Potyviridae, Comoviridae, and Luteoviridae families) must be translated by a cap-independent process (15–18). Indeed, two distinct regulatory elements revealed within the 5′UTR of tobacco etch potyvirus were capable of mediating internal translation from dicistronic constructs (19).
In accordance with the ribosome-scanning mechanism, only the
5′-proximal gene of tobamovirus genomic RNA can be directly translated
by ribosomes, whereas the other genes are expressed from two separate
3′-coterminal subgenomic RNAs (sgRNAs). The dicistronic
I2 sgRNA is translated to produce the movement
protein (MP), whereas the 3′-proximal coat protein (CP) gene is silent.
The CP gene is expressed from a small monocistronic sgRNA (for review,
see ref. 20). Recently, a new tobamovirus [crucifer-infecting
tobamovirus (crTMV)] capable of systemically infecting members of the
Brassicaceae family has been isolated and characterized
(21). We reported that the 148-nt region upstream of the CP gene of
crTMV RNA contains an IRES (IRES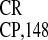 ), promoting
cap-independent and internal translation of the CP gene and different
reporter genes from dicistronic constructs (22, 23). Recently, the
ability of IRES
), promoting
cap-independent and internal translation of the CP gene and different
reporter genes from dicistronic constructs (22, 23). Recently, the
ability of IRES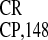 to promote internal translation
was confirmed in a potato virus X vector-based system (24). The
capacity of crTMV IRES
to promote internal translation
was confirmed in a potato virus X vector-based system (24). The
capacity of crTMV IRES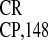 to mediate internal
translation distinguishes this tobamovirus from the well-known type
member of the genus, TMV U1; the equivalent 148-nt sequence from TMV U1
RNA (U1
to mediate internal
translation distinguishes this tobamovirus from the well-known type
member of the genus, TMV U1; the equivalent 148-nt sequence from TMV U1
RNA (U1 ) was incapable of mediating internal
translation (22). Recently, it has been shown that the 228- and 75-nt
regions upstream of the MP gene of crTMV RNA,
IRES
) was incapable of mediating internal
translation (22). Recently, it has been shown that the 228- and 75-nt
regions upstream of the MP gene of crTMV RNA,
IRES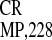 and its 5′-truncated variant
IRES
and its 5′-truncated variant
IRES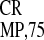 , are also active (23).
, are also active (23).
In this study, the activities of IRES ,
IRES
,
IRES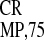 , and the well-characterized mammalian
IRESEMCV were compared in a dicistronic
translation assay in plant, yeast, and HeLa cells. It was found that
IRES
, and the well-characterized mammalian
IRESEMCV were compared in a dicistronic
translation assay in plant, yeast, and HeLa cells. It was found that
IRES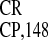 exhibited a high capacity to mediate
translation of the 3′-proximal β-glucuronidase (GUS) gene
located on a dicistronic transcript in all of the types of cells
tested. The sequence elements responsible for this cross-kingdom
activity were identified. The results allowed us to artificially design
novel IRES elements and to identify plant-derived IRES elements in
plant genes that all demonstrate cross-kingdom activity.
exhibited a high capacity to mediate
translation of the 3′-proximal β-glucuronidase (GUS) gene
located on a dicistronic transcript in all of the types of cells
tested. The sequence elements responsible for this cross-kingdom
activity were identified. The results allowed us to artificially design
novel IRES elements and to identify plant-derived IRES elements in
plant genes that all demonstrate cross-kingdom activity.
Materials and Methods
Plasmid Constructs.
Dicistronic plasmids contained crTMV CP or green fluorescent protein (GFP) gene as the first cistron and the GUS gene as the second, separated by various intercistronic sequences (ICS). T7 and 35S promoter-based constructs of CP-ICS-GUS and hairpin (H)-CP-ICS-GUS series were described previously (22, 23). The analogue procedure (precisely described in supporting information on the PNAS web site, www.pnas.org) was used to construct T7-H-GFP-ICS-GUS and 35S-GFP-ICS-GUS plasmids. Artificial ICS were obtained from pairs of complementary oligonucleotides. Nicotiana tabacum heat-shock factor 1 (NtHSF-1) mRNA (European Molecular Biology Laboratory accession no. AB014483) untranslated leader was obtained by PCR from N. tabacum total genomic DNA. Corresponding 35S-based cassettes were transferred into pBIN19 vector for plant transformation. To perform experiments in yeast the CP-ICS-GUS, fragments of T7-based plasmids were inserted into pYeDP1/8–2 yeast expression vector.
In vitro transcription and translation were performed according to manufacturer protocols for the RiboMax kit, wheat germ extract, and rabbit reticulocyte lysate (all from Promega). Transcripts were purified by 2M LiCl precipitation. The mRNA concentration in translation reactions was 0.5 μM in all cases.
Generation and Characterization of Transgenic Tobacco Plants.
Plasmid constructs were transformed into Agrobacterium tumefaciens strain AGL1 (25) by using standard procedures (26). Transgenic R0 plants were obtained from discs of N. tabacum var. Samsun and characterized by Northern and Western analyses, which were performed according to membrane manufacturer protocols (Amersham Pharmacia)—Hybond N+ and polyvinylidene difluoride membrane, correspondingly.
Protoplast Preparation and Transfection.
Protoplasts were isolated from N. tabacum (cv. W38) leaves and electroporated with pFF19-based dicistronic DNA constructs “CP-ICS-GUS” and “GFP-ICS-GUS” as described earlier (23).
Determination of GUS Activity.
GUS activity was determined according to ref. 27 and measured in relative light units. GUS activity was normalized with the protein concentration estimated by using a Bio-Rad protein assay kit. For each experiment, background GUS activity associated with nontransfected protoplasts was subtracted. The mean values (with SE bars) for three to ten independent experiments are given.
Yeast Cell Transformation and Analysis.
The yeast strain 2805 was transformed according to ref. 28. Transformants were selected on minus-histidine medium. Bicistronic mRNA transcription was induced by galactose. Total protein was extracted from yeast spheroplasts by three cycles of freezing in liquid nitrogen and rapid warming up to 42°C in the presence of 0.1% sarkosyl/0.1% Triton X-100. The supernatant, clarified by centrifugation, was collected, and the total protein content was determined (29).
Transfection of HeLa Cells by Modified Vaccinia Virus Encoding T7 RNA Polymerase and T7 Promoter-Based GUS-Expressing Plasmids “H-GFP-ICS-GUS.”
HeLa cell monolayers were grown on 3.5-cm-diameter Petri dishes in Dulbecco's modified MEM supplemented with 10% heat-inactivated FCS and 100 units/ml of streptomycin and penicillin. Virus stocks of modified vaccinia virus Ankara, expressing bacteriophage T7 RNA polymerase, were prepared as described (30). HeLa cell dishes that were 80–90% confluent were infected with virus at 30–40 plaque-forming units/cell. After 45-min absorption, the cells were washed and transfected by using Opti-MEM (Life Technologies, Gaithersburg, MD), plasmid DNA, and Lipofectin (GIBCO/BRL). A transfection mixture of 2 μg of DNA in 5 μl of Lipofectin was used for each 3.5-cm plate; six plates were used in each experiment for each construct. Cells were incubated at 37°C for 6 h. After incubation, the medium was removed, cells were washed twice with PBS and lysed directly on the plate in 250 μl of lysis buffer (100 mM potassium phosphate, pH 7.8/0.2% Triton X-100/0.5 mM DTT) for 10 min. The lysate was collected, clarified by centrifugation at 2,000 × g for 10 min, and stored at −70°C.
Results
crTMV IRES-Mediated Expression of the 3′-Proximal GUS Gene in Transgenic Plants.
crTMV RNA contains two IRES elements capable of promoting
internal translation of the 3′-proximal genes from dicistronic
constructs even when translation of the first gene was blocked by a
5′-terminal H structure (22, 23). These results were obtained in
cell-free translation systems [rabbit reticulocyte lysates (RRL) wheat
germ extracts (WGE)] and in electroporated protoplasts. To exclude
possible discrepancies between the functional activities of
crTMV-derived IRESs in vitro and in planta, we
compared the relative efficiencies of different crTMV IRESs in
transgenic tobacco plants. To this end, a series of
R0 tobacco plants were generated, transgenic for
dicistronic constructs containing a 5′-proximal crTMV CP gene separated
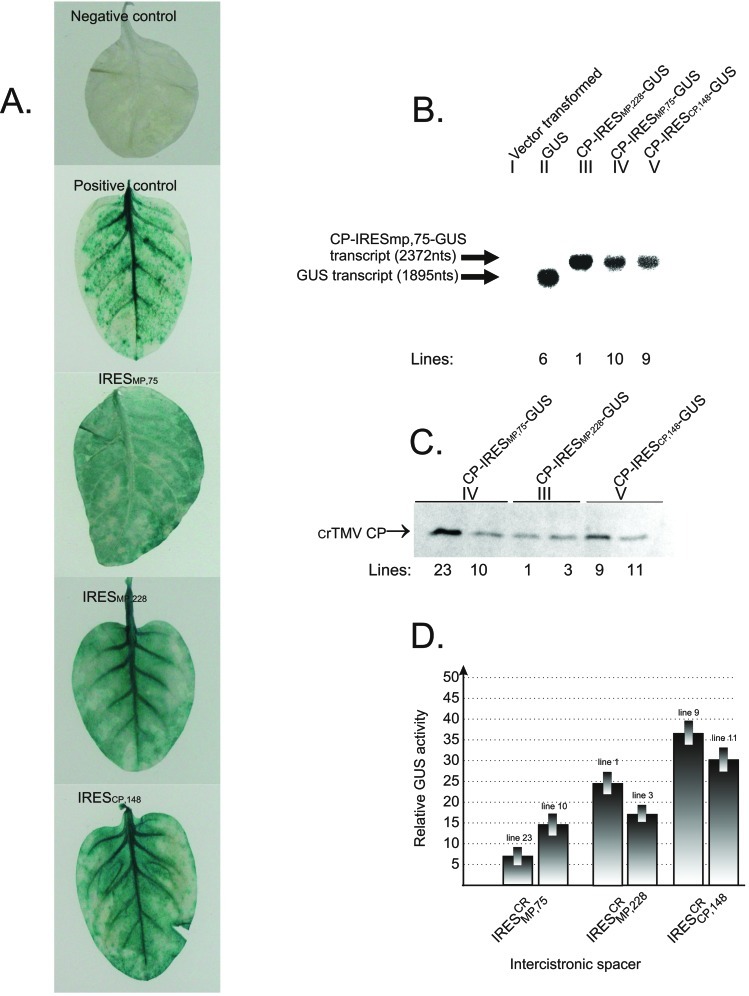
from the second gene (GUS) by one of the IRES elements. Fig.
1A shows that GUS activity
could be readily detected by histochemical methods in plants transgenic
for dicistronic IRESCR-carrying constructs. The
integrity of dicistronic and monocistronic transcripts produced in
these transgenic plants was proven by Northern blotting. Average RNA
samples extracted from the leaves of plants transformed with
monocistronic (II in Fig. 1B) or dicistronic (III–V in Fig.
1B) transgenes were used for analysis. It can be seen from
Fig. 1B that dicistronic and monocistronic constructs
yielded transcripts of the predicted size; no visible bands
corresponding to monocistronic products of dicistronic transcript
degradation could be detected. In addition, these results provided
evidence that the IRES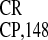 sequence functions as an
IRES and not as a transcriptional promoter. The results of Western blot
analysis with antibodies to crTMV CP indicated that the 5′-proximal CP
gene was expressed in transgenic plants, although the expression level
varied from plant to plant (Fig. 1C). In Fig. 1D,
the relative GUS activity mediated by crTMV IRESs was normalized
relative to the amount of CP produced by the 5′-proximal gene of
dicistronic CP-IRES-GUS mRNAs in individual transgenic lines (Fig. 1
C and D). In other words, GUS activity was
normalized with respect to the amount of dicistronic transcript
produced by individual lines.
sequence functions as an
IRES and not as a transcriptional promoter. The results of Western blot
analysis with antibodies to crTMV CP indicated that the 5′-proximal CP
gene was expressed in transgenic plants, although the expression level
varied from plant to plant (Fig. 1C). In Fig. 1D,
the relative GUS activity mediated by crTMV IRESs was normalized
relative to the amount of CP produced by the 5′-proximal gene of
dicistronic CP-IRES-GUS mRNAs in individual transgenic lines (Fig. 1
C and D). In other words, GUS activity was
normalized with respect to the amount of dicistronic transcript
produced by individual lines.
Figure 1.
IRES-mediated GUS gene expression in tobacco plants transgenic for
dicistronic CP-IRES-GUS constructs. Five series of transgenic plants
differing in IRES sequences were generated: (I) Negative control:
vector-transformed plants; (II) positive control: plants transgenic for
monocistronic GUS gene; (III–V) IRES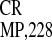 ,
IRES
,
IRES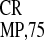 , and IRES
, and IRES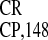 elements,
respectively, were used as intercistronic spacers. (A)
Histochemical detection of GUS activity. (B) Northern
blot of total RNA isolated from transgenic tobacco leaves probed with a
GUS gene DNA probe. Positions of synthetic monocistronic (GUS) and
dicistronic (CP-IRES
elements,
respectively, were used as intercistronic spacers. (A)
Histochemical detection of GUS activity. (B) Northern
blot of total RNA isolated from transgenic tobacco leaves probed with a
GUS gene DNA probe. Positions of synthetic monocistronic (GUS) and
dicistronic (CP-IRES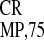 -GUS) RNA transcripts are
marked by arrows. (C) Western blot analyses of the crTMV
CP in transgenic tobacco leaves. The arrow indicates the position of
crTMV CP. Arabic numerals (B, C) denote
the number of the transgenic plant line used. Roman numerals denote
transgenic plants transformed with different constructs indicated
above. (D) IRESCR-mediated GUS activity
expressed in two different transgenic lines (denoted by Arabic
numerals). The relative GUS activity was normalized to the CP content
measured by densitometry of the CP bands presented in
C.
-GUS) RNA transcripts are
marked by arrows. (C) Western blot analyses of the crTMV
CP in transgenic tobacco leaves. The arrow indicates the position of
crTMV CP. Arabic numerals (B, C) denote
the number of the transgenic plant line used. Roman numerals denote
transgenic plants transformed with different constructs indicated
above. (D) IRESCR-mediated GUS activity
expressed in two different transgenic lines (denoted by Arabic
numerals). The relative GUS activity was normalized to the CP content
measured by densitometry of the CP bands presented in
C.
The relative efficiency of GUS gene expression by monocistronic
transgene and by the 3′-proximal GUS gene of dicistronic transgene was
also examined. Comparison of GUS activities in samples taken from
plants transgenic for dicistronic (CP-IRES-GUS) and monocistronic (GUS)
constructs (Fig. 1, Roman numerals III–V and II, respectively) showed
that the average levels of IRES-mediated GUS expression (in relative
light units) reached 21% (IRES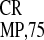 and
IRES
and
IRES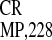 ) and 31% (IRES
) and 31% (IRES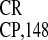 )
of monocistronic GUS expression.
)
of monocistronic GUS expression.
Cross-Kingdom Conservation of IRES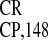 Activity: Comparative Efficiencies of IRESEMCV and crTMV
IRESs in Tobacco Protoplasts, HeLa, and Yeast Cells.
Activity: Comparative Efficiencies of IRESEMCV and crTMV
IRESs in Tobacco Protoplasts, HeLa, and Yeast Cells.
Our earlier data showed that the crTMV IRES elements were active both
in plant cell-derived (WGE) and animal cell-derived (RRL) cell-free
systems (22, 23). In a series of experiments, the relative efficiencies
of GUS gene expression mediated by IRESEMCV,
IRES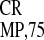 and IRES
and IRES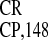 were
compared. The activity of IRESEMCV was negligible
in WGE, whereas in RRL, the level of
IRESEMCV-directed GUS gene activity was more than
two times higher than IRES
were
compared. The activity of IRESEMCV was negligible
in WGE, whereas in RRL, the level of
IRESEMCV-directed GUS gene activity was more than
two times higher than IRES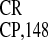 , the most active
crTMV IRES element (Fig. 2
D
and E).
, the most active
crTMV IRES element (Fig. 2
D
and E).
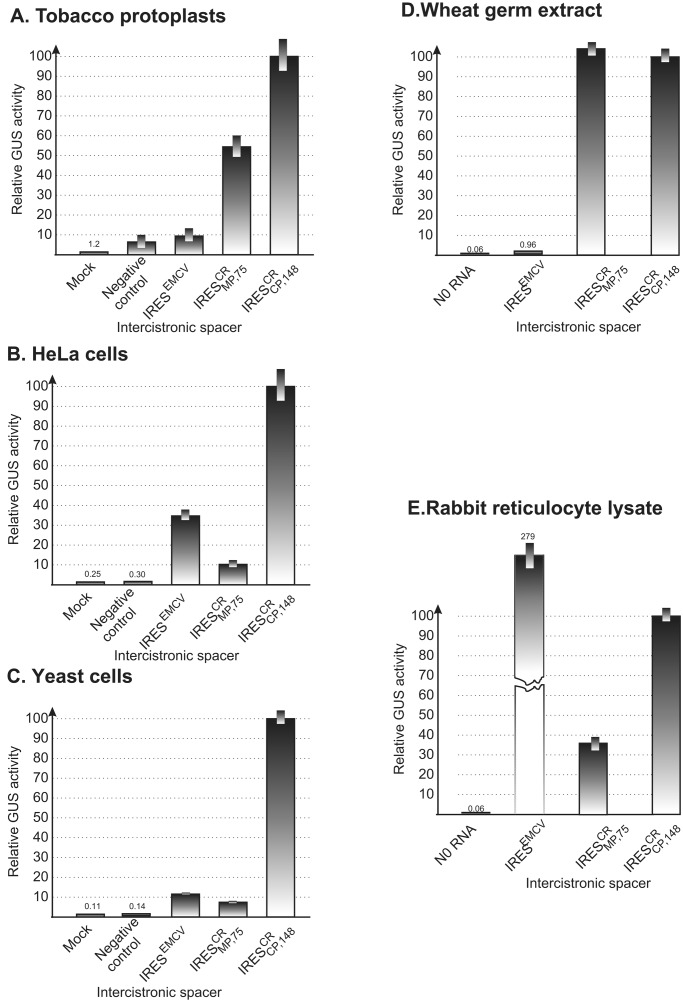
Figure 2.
Cross-kingdom conservation of IRES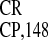 activity. Expression of the 3′-proximal GUS gene from dicistronic
CP-IRES-GUS constructs in tobacco protoplasts (A), HeLa
(B), yeast (C) cells and cell-free
translation systems WGE (D), and RRL (E).
The 72-nt synthetic GC-rich polylinker-derived (PL72)
spacer (23) (A, B) and the 148-nt region
upstream from start codon of the CP gene of TMV U1
(U1
activity. Expression of the 3′-proximal GUS gene from dicistronic
CP-IRES-GUS constructs in tobacco protoplasts (A), HeLa
(B), yeast (C) cells and cell-free
translation systems WGE (D), and RRL (E).
The 72-nt synthetic GC-rich polylinker-derived (PL72)
spacer (23) (A, B) and the 148-nt region
upstream from start codon of the CP gene of TMV U1
(U1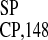 , ref. 22) (C) were used as
negative controls. GUS gene expression in HeLa (B) and
yeast (C) cells transfected with animal cell or yeast
cell promoter-based dicistronic constructs H-GFP-IRES-GUS and
CP-IRES-GUS, respectively.
, ref. 22) (C) were used as
negative controls. GUS gene expression in HeLa (B) and
yeast (C) cells transfected with animal cell or yeast
cell promoter-based dicistronic constructs H-GFP-IRES-GUS and
CP-IRES-GUS, respectively.
We then compared the relative activities of the EMCV and two
crTMV IRESs (IRES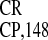 and
IRES
and
IRES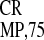 ) in different types of cell cultures,
including tobacco protoplasts, HeLa cells, and yeast cells. Appropriate
promoter-based plasmids were constructed to transcribe a dicistronic
RNA including the IRES sequences in question intercistronically
upstream of the GUS gene. In all cases tested, GUS expression was
negligible from negative control constructs (Fig. 2
A–C). In tobacco protoplasts (Fig.
2A), the relative activity of the crTMV RNA-derived
IRESs was markedly higher than that of IRESEMCV,
which is consistent with the negligible activity of
IRESEMCV in WGE (Fig. 2D).
Furthermore, the relative efficiency of IRESEMCV
was considerably higher in HeLa cells than in nonanimal (tobacco
protoplasts and yeast) cells, and the activity of
IRES
) in different types of cell cultures,
including tobacco protoplasts, HeLa cells, and yeast cells. Appropriate
promoter-based plasmids were constructed to transcribe a dicistronic
RNA including the IRES sequences in question intercistronically
upstream of the GUS gene. In all cases tested, GUS expression was
negligible from negative control constructs (Fig. 2
A–C). In tobacco protoplasts (Fig.
2A), the relative activity of the crTMV RNA-derived
IRESs was markedly higher than that of IRESEMCV,
which is consistent with the negligible activity of
IRESEMCV in WGE (Fig. 2D).
Furthermore, the relative efficiency of IRESEMCV
was considerably higher in HeLa cells than in nonanimal (tobacco
protoplasts and yeast) cells, and the activity of
IRES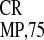 was high in plant protoplasts but
dramatically reduced in the nonplant cells. The most unexpected result
was that the activity of the 148-nt IRES
was high in plant protoplasts but
dramatically reduced in the nonplant cells. The most unexpected result
was that the activity of the 148-nt IRES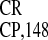 was
invariably the highest in all of the cells tested. Remarkably, it was
even higher than that of IRESEMCV in HeLa cells
(Fig. 2B). This observation provides additional
evidence that the requirements for IRES-mediated translation may differ
in vitro and in vivo. These results demonstrate
an unusual cross-kingdom conservation of the crTMV
IRES
was
invariably the highest in all of the cells tested. Remarkably, it was
even higher than that of IRESEMCV in HeLa cells
(Fig. 2B). This observation provides additional
evidence that the requirements for IRES-mediated translation may differ
in vitro and in vivo. These results demonstrate
an unusual cross-kingdom conservation of the crTMV
IRES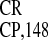 activity. It was therefore reasonable to
assume that some specific features of this sequence are responsible for
its functional universality.
activity. It was therefore reasonable to
assume that some specific features of this sequence are responsible for
its functional universality.
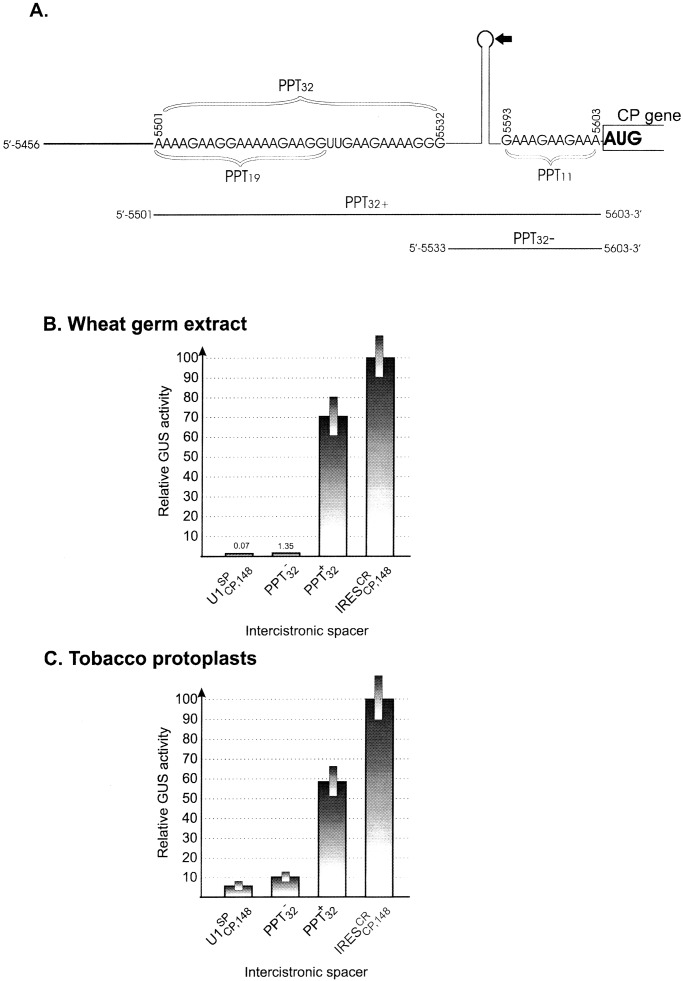
Deletion Analysis.
The structural organization of IRES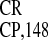 is
relatively simple (see figure 6 in ref. 22). It can be folded into a
secondary structure containing the 32-nt polypurine tract (PPT),
PPT32, upstream of a potentially stable hairpin,
and the 11-nt PPT11, just downstream of this
stem–loop structure (22). In addition, the short
5′-5562-AGAAGUA-5568–3′ motif (PPT7) is located
downstream of PPT32 (22). Earlier studies had
shown that neither the 5′ nor the 3′ half of
IRES
is
relatively simple (see figure 6 in ref. 22). It can be folded into a
secondary structure containing the 32-nt polypurine tract (PPT),
PPT32, upstream of a potentially stable hairpin,
and the 11-nt PPT11, just downstream of this
stem–loop structure (22). In addition, the short
5′-5562-AGAAGUA-5568–3′ motif (PPT7) is located
downstream of PPT32 (22). Earlier studies had
shown that neither the 5′ nor the 3′ half of
IRES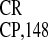 , comprising nucleotides 5456–5568 and
5569–5603, was active as single sequences (22). Here we analyzed
whether less drastic deletions of IRES
, comprising nucleotides 5456–5568 and
5569–5603, was active as single sequences (22). Here we analyzed
whether less drastic deletions of IRES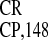 retain
activity. In fact, sequence 5501–5603 (PPT
retain
activity. In fact, sequence 5501–5603 (PPT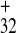 ), which
includes all three of the PPTs, retained about 70% of intact
IRES
), which
includes all three of the PPTs, retained about 70% of intact
IRES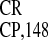 activity, whereas sequence 5533–5603
(PPT
activity, whereas sequence 5533–5603
(PPT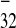 ), lacking PPT32, was
inactive in WGE and extremely low in plant protoplasts (Fig.
3
B and C,
respectively). Sequence 5501–5592, lacking
PPT11, lost about 30% of activity in WGE (data
not shown). In summary, these results indicate that
PPT32 and PPT11 are the
essential elements of IRES
), lacking PPT32, was
inactive in WGE and extremely low in plant protoplasts (Fig.
3
B and C,
respectively). Sequence 5501–5592, lacking
PPT11, lost about 30% of activity in WGE (data
not shown). In summary, these results indicate that
PPT32 and PPT11 are the
essential elements of IRES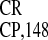 .
.
Figure 3.
IRES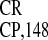 deletion analysis. (A)
Simplified schematic representation of the IRES
deletion analysis. (A)
Simplified schematic representation of the IRES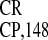 structure (see ref. 22 for details) and its deletion mutants. Letters
indicate the sequences of the 32-nt polypurine tract PPT32
(which includes the 19-nt element PPT19) located upstream
of the hairpin–loop structure and the 11-nt tract (PPT11)
just upstream of the CP gene, respectively. Arabic numerals indicate
the nucleotide positions in full-length crTMV genomic RNA (21). The
arrow points to the position resulting in formation of two deletion
mutants (Δ5′IREScp and Δ3′IREScp) described in ref. 22. The lines
indicated by PPT
structure (see ref. 22 for details) and its deletion mutants. Letters
indicate the sequences of the 32-nt polypurine tract PPT32
(which includes the 19-nt element PPT19) located upstream
of the hairpin–loop structure and the 11-nt tract (PPT11)
just upstream of the CP gene, respectively. Arabic numerals indicate
the nucleotide positions in full-length crTMV genomic RNA (21). The
arrow points to the position resulting in formation of two deletion
mutants (Δ5′IREScp and Δ3′IREScp) described in ref. 22. The lines
indicated by PPT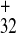 and PPT
and PPT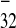 correspond to the respective IRES
correspond to the respective IRES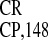 deletion
mutants used in the present study. GUS gene expression by internal
translation from dicistronic constructs in WGE (B) and
tobacco protoplasts (C) under control of the intact
IRES
deletion
mutants used in the present study. GUS gene expression by internal
translation from dicistronic constructs in WGE (B) and
tobacco protoplasts (C) under control of the intact
IRES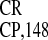 and its deletion mutants
(PPT
and its deletion mutants
(PPT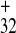 and PPT
and PPT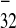 ).
UI
).
UI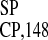 sequence (22) was taken as a negative
control.
sequence (22) was taken as a negative
control.
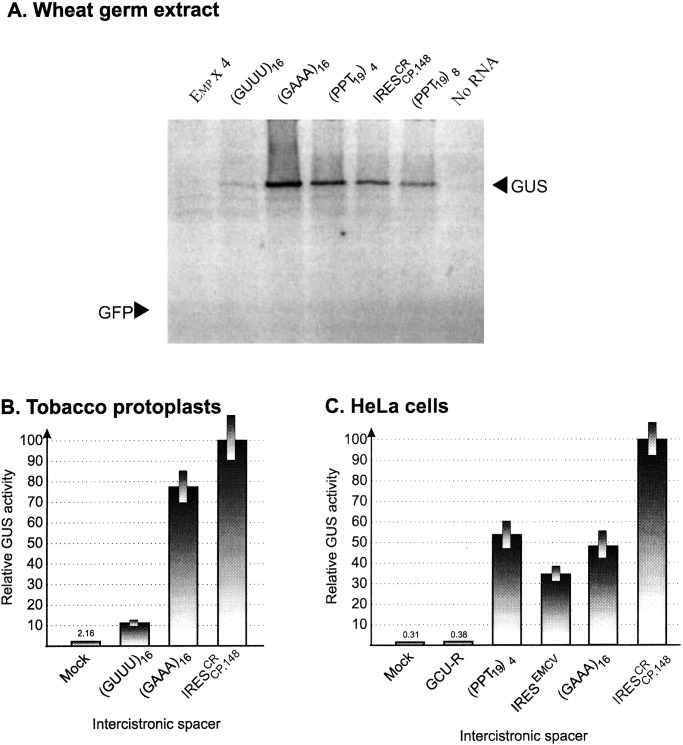
Artificial Polypurine (A)-Rich Sequence (PARS) Elements Exhibit IRES Activity in Vitro and in Plant and Animal Cells.
PPT32 contains the 19-nt sequence
AAAAGAAGGAAAAAGAAGG (PPT19) representing a direct
tandem repeat of the AAAAGAAGG
[(A4)G(A2)G2]
element in combination with the 11-nt sequence GAAGAAAAGGG. A similar
motif (GAAAGAAGAAA) is present in PPT11 (Fig.
3A). Therefore, all three PARSs can be seen as multiple
copies of a G(A)2–5 module. To test whether
these modules are in fact the important elements of
IRES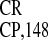 , artificial PARSs were created and used as
intercistronic spacers in dicistronic H-GFP-ICS-GUS constructs. The
IRES activity of these artificial sequences was examined in WGE,
tobacco protoplasts, and HeLa cells (Fig.
4).
(PPT19)4 and
(PPT19)8 were in fact able
to promote internal translation in WGE (Fig. 4A). It
is noteworthy that (GAAA)16 also directed
translation of the downstream GUS gene efficiently, despite the fact
that translation of the first gene (GFP) was invariably blocked by the
hairpin structure H. The results of in vitro translation are
generally consistent with those experiments made in tobacco protoplasts
(Fig. 4B) and HeLa cells (Fig. 4C). The IRES
activity of the sequence (GAAA)16 was notably
high in both tobacco protoplasts and human HeLa cells. Comparable
levels of activity in HeLa cells were exhibited by the spacers
(GAAA)16 and
(PPT19)4 (Fig.
4C). Significantly, these levels approached (or even
exceeded) the level of GUS gene expression promoted by
IRESEMCV in HeLa cells (Fig. 4C). On
the other hand, the levels of GUS gene expression mediated by
(GUUU)16 were very low in vitro (Fig.
4A) and in tobacco protoplasts (Fig. 4B),
and the 68-nt GCU-rich (GCU-R) sequence did not exhibit IRES activity
in HeLa cells (Fig. 4C).
, artificial PARSs were created and used as
intercistronic spacers in dicistronic H-GFP-ICS-GUS constructs. The
IRES activity of these artificial sequences was examined in WGE,
tobacco protoplasts, and HeLa cells (Fig.
4).
(PPT19)4 and
(PPT19)8 were in fact able
to promote internal translation in WGE (Fig. 4A). It
is noteworthy that (GAAA)16 also directed
translation of the downstream GUS gene efficiently, despite the fact
that translation of the first gene (GFP) was invariably blocked by the
hairpin structure H. The results of in vitro translation are
generally consistent with those experiments made in tobacco protoplasts
(Fig. 4B) and HeLa cells (Fig. 4C). The IRES
activity of the sequence (GAAA)16 was notably
high in both tobacco protoplasts and human HeLa cells. Comparable
levels of activity in HeLa cells were exhibited by the spacers
(GAAA)16 and
(PPT19)4 (Fig.
4C). Significantly, these levels approached (or even
exceeded) the level of GUS gene expression promoted by
IRESEMCV in HeLa cells (Fig. 4C). On
the other hand, the levels of GUS gene expression mediated by
(GUUU)16 were very low in vitro (Fig.
4A) and in tobacco protoplasts (Fig. 4B),
and the 68-nt GCU-rich (GCU-R) sequence did not exhibit IRES activity
in HeLa cells (Fig. 4C).
Figure 4.
Comparative dicistronic analysis of IRES activities of multiple
G(A)3 modules and natural IRESs
(IRES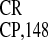 and IRESEMCV) in WGE
(A), tobacco protoplasts (B), and HeLa
cells (C). Artificial sequences tested:
(i) (PPT19)4 and
(PPT19)8 representing the tandem repeats of
four (76-nt) and eight (152-nt) copies of the 19-nt AAAAGAAGGAAAAAGAAGG
sequence derived from PPT32 (see Fig. 3), respectively;
(ii) the 64-nt (GAAA)16 sequence consisting
of 16 G(A)3 elements; (iii) control U-rich
sequence (GUUU)16; (iv) the control Emp
× 4 sequence consisting of four copies of the U-rich
CGUUUGCUUUUUGUAGUA element derived from another crTMV IRES
(IRES
and IRESEMCV) in WGE
(A), tobacco protoplasts (B), and HeLa
cells (C). Artificial sequences tested:
(i) (PPT19)4 and
(PPT19)8 representing the tandem repeats of
four (76-nt) and eight (152-nt) copies of the 19-nt AAAAGAAGGAAAAAGAAGG
sequence derived from PPT32 (see Fig. 3), respectively;
(ii) the 64-nt (GAAA)16 sequence consisting
of 16 G(A)3 elements; (iii) control U-rich
sequence (GUUU)16; (iv) the control Emp
× 4 sequence consisting of four copies of the U-rich
CGUUUGCUUUUUGUAGUA element derived from another crTMV IRES
(IRES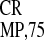 ) and (v) the GCU-rich
sequence (GCU-R) containing four copies of CGCGGGCG blocks linked via
the 7-nt sequence UUUGUUU used as an additional negative control.
(A) Analysis of proteins directed in WGE by dicistronic
H-GFP-ICS-GUS T7 transcripts containing artificial sequences as the
intercistronic spacer. Arrows indicate the position of GUS and GFP.
(B and C) GUS gene expression in tobacco
protoplasts (B) and HeLa (C) cells
transfected with dicistronic GFP-IRES-GUS constructs containing
different IRES sequences. “Mock” indicates that DNA-free solution
was used for transfection.
) and (v) the GCU-rich
sequence (GCU-R) containing four copies of CGCGGGCG blocks linked via
the 7-nt sequence UUUGUUU used as an additional negative control.
(A) Analysis of proteins directed in WGE by dicistronic
H-GFP-ICS-GUS T7 transcripts containing artificial sequences as the
intercistronic spacer. Arrows indicate the position of GUS and GFP.
(B and C) GUS gene expression in tobacco
protoplasts (B) and HeLa (C) cells
transfected with dicistronic GFP-IRES-GUS constructs containing
different IRES sequences. “Mock” indicates that DNA-free solution
was used for transfection.
To estimate the impact of each type of purine on the IRES
activity of PARSs, the homopolymers poly(A)60 and
poly(G)60 were inserted into the bicistronic
transcripts H-GFP-GUS and tested (Table
1). Poly(G)60
exhibited no IRES activity in vitro, whereas
poly(A)60 promoted GUS gene expression even more
efficiently than IRES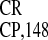 . To show that the GUS
gene is not being translated from degraded
H-GFP-poly(A)60-GUS RNA, the
32P-labeled dicistronic transcripts were
incubated in RRL. No significant changes in electrophoretic mobility or
integrity of transcripts were observed after 60-min incubation (data
not shown). Remarkably, the IRES activity of the
poly(A)60 sequence was drastically reduced in
HeLa cells, suggesting that a combination of A and G nucleotides is
required for IRES activity in vivo (Table 1). Taken
together, these data suggest that multiple PARS modules are responsible
for conservation of cross-kingdom activity of
IRES
. To show that the GUS
gene is not being translated from degraded
H-GFP-poly(A)60-GUS RNA, the
32P-labeled dicistronic transcripts were
incubated in RRL. No significant changes in electrophoretic mobility or
integrity of transcripts were observed after 60-min incubation (data
not shown). Remarkably, the IRES activity of the
poly(A)60 sequence was drastically reduced in
HeLa cells, suggesting that a combination of A and G nucleotides is
required for IRES activity in vivo (Table 1). Taken
together, these data suggest that multiple PARS modules are responsible
for conservation of cross-kingdom activity of
IRES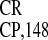 .
.
Table 1.
Dicistronic analysis of IRES activity of poly(A) and poly(G) sequences used as an intercistronic spacers
| Spacer | Activity in RRL assay (%) | Activity in HeLa cells (%) |
|---|---|---|
| Poly(A)60 | 150 | 10 |
| Poly(G)60 | 1 | 1 |
IRES
|
100 | 100 |
| No RNA (mock) | 0.3–0.8 | 0.5–1.0 |
The H-GFP-spacer-GUS constructs were used in dicistronic assays.
Relative GUS activity was expressed as in ref. 23;
IRES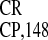 activity was taken as 100%. The mean
values of three independent experiments are given.
activity was taken as 100%. The mean
values of three independent experiments are given.
IRES Activity of a PARS-Containing 5′UTR Derived from Tobacco Heat-Shock Factor mRNA.
It is reasonable to expect that IRES-mediated translation is
typical for mRNAs with long and highly structured 5′UTRs. The 5′UTR
sequences of a number of plant genes encoding heat-shock protein (HSP)
mRNAs were examined for the presence of purine-rich tracts by using the
European Molecular Biology Laboratory cDNA nucleotide database. Some of
the sequences analyzed contained PARSs of different sizes (accession
nos. AB014483, AB017273, AF005993, and AF035460). For example, two long
polypurine tracks were revealed in the 453-nt 5′UTR of NtHSF-1 mRNA
(accession no. AB014483), 5′-74-AAAGAAGAGAGAAAACUGAAAAGGCAGAAAA-105–3′
and 5′-420-AGAGAAACAGAGAAAUACAGGGGAAAAACAAGGGAUG-456–3′),
suggesting that the 5′-leader of NtHSF-1 mRNA exhibits IRES activity.
To test this hypothesis, the 453-nt 5′UTR of NtHSF-1 (5′UTR NtHSF) was
isolated from tobacco genomic DNA and used as an intercistronic spacer
in dicistronic analysis of IRES activity. GFP and GUS expression was
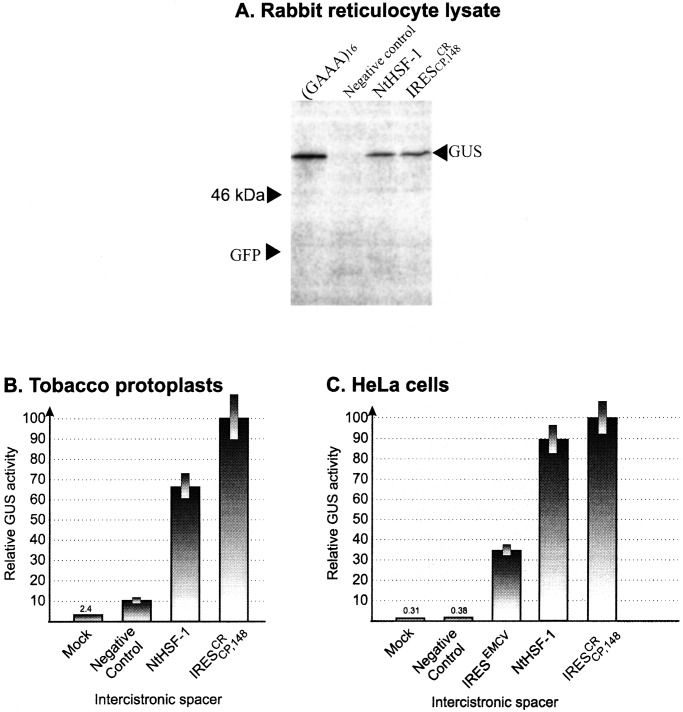
analyzed from an H-GFP-5′UTR-NtHSF-GUS construct in RRL (Fig.
5A), as well as in transfected
tobacco protoplasts (Fig. 5B) and HeLa cells (Fig.
5C). The presence of H at the 5′-terminal position abolished
GFP gene expression initiated by ribosome scanning. However, GUS was
expressed by internal initiation in all three systems (Fig. 5) and also
in WGE (not shown). The activities were comparable to those mediated by
IRES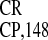 , which was used as a positive control. The
68-nt GCU-rich (GCU-R) sequence and (GUUU)16 were
used as negative controls. Consequently, the 5′UTR of NtHSF-1 mRNA is
an IRES and it exhibits cross-kingdom conservation of internal ribosome
entry activity. In both of these otherwise unrelated elements,
IRES
, which was used as a positive control. The
68-nt GCU-rich (GCU-R) sequence and (GUUU)16 were
used as negative controls. Consequently, the 5′UTR of NtHSF-1 mRNA is
an IRES and it exhibits cross-kingdom conservation of internal ribosome
entry activity. In both of these otherwise unrelated elements,
IRES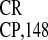 and IRESNtHSF-1,
multiple PARS elements are apparently responsible for the activity
across the kingdoms analyzed.
and IRESNtHSF-1,
multiple PARS elements are apparently responsible for the activity
across the kingdoms analyzed.
Figure 5.
Dicistronic analysis of IRES activity of the 5′-UTR of NtHSF-1 mRNA (5′UTR NtHSF) in RRL (A), tobacco protoplasts (B), and HeLa cells (C). Tested H-GFP-ICS-GUS RNA transcripts contained as intercistronic spacers the 453-nt 5′UTR of NtHSF-1 mRNA (5′UTR NtHSF) and other synthetic sequences indicated in the legend to Fig. 4.
Our preliminary results indicate that 5′UTR regions from two other mRNAs of this type, i.e., those encoding the tobacco poly(A)-binding protein and 48-kDa MAP kinase, also promote internal translation in a similar way (see below).
Discussion
IRESs of different origins differ greatly in sequence, length, secondary structure organization, and functional requirements (4). Significant variability was revealed in sets of translation initiation factors and/or noncanonical transacting factors required for the activity of different IRES elements (8, 31–34). It was reasonable to presume that the activity of IRESs in heterologous cell types will be limited because of kingdom-specific differences in cap-independent translation mechanisms. Therefore, it was not unexpected that animal virus (picornaviruses, hepatitis C virus) IRESs were inactive in yeast cells (35–37), despite the fact that IRES-mediated translation of cellular mRNAs has been reported in yeast (38). On the other hand, Urwin et al. (11) found that IRESEMCV was active both in animal and, moderately, in plant cells. Taken together, the problem of kingdom-specific differences in IRES activity have so far remained ill-defined.
In the first series of experiments presented here, we showed that
IRES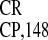 and IRES
and IRES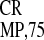 are
functionally active in plants transgenic for dicistronic constructs. In
addition, these results provided evidence that the
IRES
are
functionally active in plants transgenic for dicistronic constructs. In
addition, these results provided evidence that the
IRES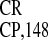 sequence functions in vivo as
an IRES, rather than a transcriptional promoter. Next, the activity of
crTMV IRESs (IRES
sequence functions in vivo as
an IRES, rather than a transcriptional promoter. Next, the activity of
crTMV IRESs (IRES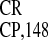 and
IRES
and
IRES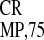 ) was compared with that of
IRESEMCV in plant, animal, and yeast cells.
Surprisingly, comparison of the relative activities of these IRESs
showed that IRES
) was compared with that of
IRESEMCV in plant, animal, and yeast cells.
Surprisingly, comparison of the relative activities of these IRESs
showed that IRES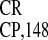 invariably exhibited a unique
cross-kingdom conservation of maximum IRES activity in organisms as
diverse as plants, animal cells and yeast (Fig. 2). The relative
efficiency of IRESEMCV was considerably higher in
HeLa cells than in nonanimal (tobacco and yeast) cells; however, the
relative activity of IRES
invariably exhibited a unique
cross-kingdom conservation of maximum IRES activity in organisms as
diverse as plants, animal cells and yeast (Fig. 2). The relative
efficiency of IRESEMCV was considerably higher in
HeLa cells than in nonanimal (tobacco and yeast) cells; however, the
relative activity of IRES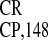 was invariably the
greatest in all cell types tested (Fig. 2
A–C).
was invariably the
greatest in all cell types tested (Fig. 2
A–C).
The IRES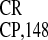 sequence can be folded into a
simple secondary structure containing two PARSs: the 32-nt PARS
(PPT32) upstream of a stable hairpin loop and the
11-nt PARS (PPT11) upstream of the CP gene AUG
codon. In addition the short (PPT)7 also contains
a PARS motif. Both the PPT32 and
PPT11 sequences could be represented as multiple
copies of a G(A)2–5 module. Deletion analysis
suggested that PARSs might be responsible for the activity of
IRES
sequence can be folded into a
simple secondary structure containing two PARSs: the 32-nt PARS
(PPT32) upstream of a stable hairpin loop and the
11-nt PARS (PPT11) upstream of the CP gene AUG
codon. In addition the short (PPT)7 also contains
a PARS motif. Both the PPT32 and
PPT11 sequences could be represented as multiple
copies of a G(A)2–5 module. Deletion analysis
suggested that PARSs might be responsible for the activity of
IRES . The capacity of artificial sequences to
promote internal translation from dicistronic H-GFP-IRES-GUS constructs
was tested in vitro (WGE, Fig. 4A) and
in vivo (tobacco protoplasts, Fig. 4B; HeLa
cells, Fig. 4C). In agreement with our proposal, the maximum
IRES activity was exhibited by PARS elements, in particular by
(GAAA)16. Remarkably, the activities of
IRES
. The capacity of artificial sequences to
promote internal translation from dicistronic H-GFP-IRES-GUS constructs
was tested in vitro (WGE, Fig. 4A) and
in vivo (tobacco protoplasts, Fig. 4B; HeLa
cells, Fig. 4C). In agreement with our proposal, the maximum
IRES activity was exhibited by PARS elements, in particular by
(GAAA)16. Remarkably, the activities of
IRES -derived
(PPT19)4 and of
(GAAA)16 appeared to be even somewhat higher than
that of IRESEMCV. In contrast, the GCU-rich and
(GUUU)16 spacers had almost no effect on the
in vivo expression of the second gene. Taken together, our
results suggest that PARS elements are archetypal IRES elements
responsible for cross-kingdom conservation of IRES activity. To further
explore the impact of each type of purine in IRES activity of PARSs,
the homopolymers poly(A) and poly(G) were used in dicistronic analysis.
Table 1 shows that in RRL, the IRES activity of
poly(A)60 was very high, whereas
poly(G)60 was not active.
However, in HeLa cells, the IRES activity of
poly(A)60 was considerably lower than that of
IRES
-derived
(PPT19)4 and of
(GAAA)16 appeared to be even somewhat higher than
that of IRESEMCV. In contrast, the GCU-rich and
(GUUU)16 spacers had almost no effect on the
in vivo expression of the second gene. Taken together, our
results suggest that PARS elements are archetypal IRES elements
responsible for cross-kingdom conservation of IRES activity. To further
explore the impact of each type of purine in IRES activity of PARSs,
the homopolymers poly(A) and poly(G) were used in dicistronic analysis.
Table 1 shows that in RRL, the IRES activity of
poly(A)60 was very high, whereas
poly(G)60 was not active.
However, in HeLa cells, the IRES activity of
poly(A)60 was considerably lower than that of
IRES (Table 1) or the heteropolymeric
artificial IRESs listed above (data not shown). These data indicate
that a certain optimal ratio and sequence arrangement of A and G
residues is required in order for a PARS to exhibit IRES activity.
(Table 1) or the heteropolymeric
artificial IRESs listed above (data not shown). These data indicate
that a certain optimal ratio and sequence arrangement of A and G
residues is required in order for a PARS to exhibit IRES activity.
As outlined above, IRESs of different origins differ significantly in their translational requirements. In particular, the IRES of hepatitis virus C (HCV) is distinct from the EMCV- and poliovirus-like groups of IRESs. In particular, IRESs of the HCV type bind 40S ribosomal subunits in the absence of initiation factors (8, 33). This phenomenon could be because of: (i) specific interaction of the IRES with ribosomal protein(s) (33, 39) and/or (ii) base pairing between the IRES and the 18S rRNA. The possibility that complementarity between short modules in eukaryotic mRNAs to 18S rRNA might play a role in IRES–ribosome interaction has been discussed (e.g., see refs. 38, 40–42).
The mechanism of possible interaction of the 40S ribosome with PARS elements is obscure. Apparently, the requirement for internal initiation of translation in a plant cell may differ from requirements in animal and yeast cells. Presumably, such IRES elements can overcome kingdom-specific barriers to translation of the second gene because of their unique capability to exploit only those translation initiation factors and noncanonical transacting proteins that are able to express their function universally in different types of cell. It is possible that the ribosome per se, as the most conserved element of the eukaryotic translation apparatus, is responsible for cross-kingdom IRES activity.
It is believed that IRES-mediated translation of cellular mRNAs is activated by physiological stimuli, which play a regulatory role in switching from traditional cap-dependent to IRES-dependent mechanisms (reviewed in refs. 7, 12, and 14). In particular, HSP mRNAs could be regarded as possible candidates for dual cap-dependent and IRES-mediated translation. We suggested that PARSs naturally occurring in long 5′UTRs of plant mRNAs (i) confer IRES activity and (ii) confer this activity across kingdoms. Two long (32- and 34-nt) and several short PARS elements with multiple (G)1–4(A)2–5 modules were identified in the 453-nt 5′UTR of N. tabacum heat-shock factor 1 mRNA. When tested in dicistronic constructs (GFP-5′UTR-NtHSF1-GUS), this sequence functioned as an IRES in WGE, RRL, and in tobacco protoplasts and human HeLa cells. These data further support the idea that PARSs are involved in cross-kingdom conservation of IRES activity.
To the best of our knowledge, no IRES elements of plant origin have been described to date.
Analysis of European Molecular Biology Laboratory databases showed that the 5′UTRs of numerous cellular mRNAs contain PARSs that could be regarded as putative plant IRESs. Our preliminary results indicate that two additional mRNAs of this type, i.e., those encoding the tobacco poly(A)-binding protein (43) and 48-kDa mitogen-activated protein kinase (44), also promote internal translation. The approach could thus be used to identify IRES elements in eukaryotic genomes.
Supplementary Material
Acknowledgments
We thank Dr. Helen Rothnie for correcting this paper and R. Karapetian for technical assistance in yeast work. This work was funded in part by the Russian Foundation for Basic Research (RFBR) Grant 9904-49119, International Association for the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union (INTAS) Grant 99-00720, RFBR-Deutsche Forschungsgemeinschaft Grant 9904-04000, and Federation of European Biochemical Societies short-term fellowship grant (M.S.).
Abbreviations
- IRES
internal ribosome entry site
- crTMV
crucifer-infecting tobamovirus
- MP
movement protein
- CP
coat protein
- EMCV
encephalomyocarditis virus
- ICS
intercistronic sequences
- RRL
rabbit reticulocyte lysates
- WGE
wheat germ extracts
- PPT
polypurine tract
- PARS
polypurine A-rich sequences
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- H
hairpin
- NtHSF
N. tabacum heat-shock factor
References
- 1.Pain V M. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 2.Kozak M. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 3.Dever T E. Trends Biol Sci. 1999;24:398–403. doi: 10.1016/s0968-0004(99)01457-7. [DOI] [PubMed] [Google Scholar]
- 4.Jackson R J. In: Translation Control of Gene Expression. Sonenberg N, Hershey J W B, Mathews W B, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 2000. pp. 127–184. [Google Scholar]
- 5.Gale M, Tan S-L, Katze M G. Microbiol Mol Biol Rev. 2000;64:239–280. doi: 10.1128/mmbr.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belsham G J, Sonenberg N. Trends Microbiol. 2000;8:330–335. doi: 10.1016/s0966-842x(00)01788-1. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Salas E, Ramos R, Lefuente E, Lopez de Quinto S. J Gen Virol. 2001;82:973–984. doi: 10.1099/0022-1317-82-5-973. [DOI] [PubMed] [Google Scholar]
- 8.Pestova T V, Kolupaeva V G, Lomakin I B, Pilipenko E V, Shatsky I N, Agol V I, Hellen C U T. Proc Natl Acad Sci USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki J, Nakashima N. Proc Natl Acad Sci USA. 2000;97:1512–1515. doi: 10.1073/pnas.010426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson J E, Powell M, Hoover S E, Sarnow P. Mol Cell Biol. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urwin P, Yi L, Martin H, Atkinson H, Gilmartin P M. Plant J. 2000;24:583–589. doi: 10.1046/j.1365-313x.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 12.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk R G. Nat Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- 13.Werner R. Int Union Biochem Mol Biol (IUBMB) Life. 2000;50:173–176. doi: 10.1080/152165400300001480. [DOI] [PubMed] [Google Scholar]
- 14.Sachs A B. Cell. 2000;101:243–245. doi: 10.1016/s0092-8674(00)80834-x. [DOI] [PubMed] [Google Scholar]
- 15.Carrington J C, Freed D D. J Virol. 1990;64:1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacke E D, Prufer F, Salamini F, Rohde W. J Gen Virol. 1990;71:2265–2272. doi: 10.1099/0022-1317-71-10-2265. [DOI] [PubMed] [Google Scholar]
- 17.Thomas A A M, Ter Haar E, Wellink J, Voorma H O. J Virol. 1991;65:2953–2959. doi: 10.1128/jvi.65.6.2953-2959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallie D R, Tanguay R, Leathers V. Gene. 1995;165:233–238. doi: 10.1016/0378-1119(95)00521-7. [DOI] [PubMed] [Google Scholar]
- 19.Niepel M, Gallie D R. J Virol. 1999;73:9080–9088. doi: 10.1128/jvi.73.11.9080-9088.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palukaitis P, Zaitlin M. In: Plant Virus. Van Regenmortel M H V, Fraenkel-Conrat M, editors. Vol. 2. New York: Plenum; 1986. pp. 105–131. [Google Scholar]
- 21.Dorokhov Yu L, Ivanov P A, Novikov V K, Agranovsky A A, Morozov S Yu, Efimov V A, Casper R, Atabekov J G. FEBS Lett. 1994;350:5–8. doi: 10.1016/0014-5793(94)00721-7. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov P A, Karpova O V, Skulachev M V, Tomashevskaya O L, Rodionova N P, Dorokhov Yu L, Atabekov J G. Virology. 1997;232:32–43. doi: 10.1006/viro.1997.8525. [DOI] [PubMed] [Google Scholar]
- 23.Skulachev M V, Ivanov P A, Karpova O V, Korpela T, Rodionova N P, Dorokhov Yu L, Atabekov J G. Virology. 1999;263:139–154. doi: 10.1006/viro.1999.9928. [DOI] [PubMed] [Google Scholar]
- 24.Toth R L, Chapman S, Carr F, Santa Cruz S. FEBS Lett. 2001;489:215–219. doi: 10.1016/s0014-5793(01)02091-9. [DOI] [PubMed] [Google Scholar]
- 25.Lazo G R, Stein P A, Ludwig R A. Biotechnology. 1991;9:963–967. doi: 10.1038/nbt1091-963. [DOI] [PubMed] [Google Scholar]
- 26.Horsh R B, Fry J E, Hoffman N L, Eichholtz D, Rogers S G, Fraley R T. Science. 1985;227:1229–1231. [Google Scholar]
- 27.Jefferson R A. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- 28.Hill J, Donald K A, Griffiths D E, Donald G. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt L S, Moss B, Rozenblatt S. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- 31.Kolupaeva V G, Hellen C U T, Shatsky I N. RNA. 1996;2:1199–1212. [PMC free article] [PubMed] [Google Scholar]
- 32.Kaminski A, Jackson R J. RNA. 1998;4:626–638. doi: 10.1017/s1355838298971898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pestova T V, Shatsky I N, Fletcher S P, Jackson R J, Hellen C U T. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilipenko E V, Pestova T V, Kolupaeva V G, Khitrina E V, Poperechnaya A N, Agol V I, Hellen C U T. Genes Dev. 2000;14:2028–2045. [PMC free article] [PubMed] [Google Scholar]
- 35.Coward P, Dasgupta A. J Virol. 1992;66:286–295. doi: 10.1128/jvi.66.1.286-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evstafieva A G, Beletsky A V, Borovjagin A V, Bogdanov A A. FEBS Lett. 1993;335:273–276. doi: 10.1016/0014-5793(93)80745-g. [DOI] [PubMed] [Google Scholar]
- 37.Das S, Ott M, Yamane A, Venkatesan A, Gupta S, Dasgupta A. Front Biosci. 1998;3:D1241–D1252. doi: 10.2741/a359. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Edelman G M, Mauro W. Proc Natl Acad Sci USA. 2001;98:1531–1536. doi: 10.1073/pnas.98.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushi S, Okada M, Stahl J, Kageyama T, Hoshino F B, Katayama K. J Biol Chem. 2001;276:20824–20826. doi: 10.1074/jbc.C100206200. [DOI] [PubMed] [Google Scholar]
- 40.Pestova T V, Hellen C U T, Wimmer E. J Virol. 1991;65:6194–6204. doi: 10.1128/jvi.65.11.6194-6204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauro V P, Edelman G M. Proc Natl Acad Sci USA. 1997;94:422–497. doi: 10.1073/pnas.94.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu M C-Y, Tranque P, Edelman G M, Mauro V P. Proc Natl Acad Sci USA. 1999;96:1339–1344. doi: 10.1073/pnas.96.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le H, Gallie D R. Plant Sci. 2000;152:101–114. [Google Scholar]
- 44.Zhang S, Klessig D F. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.