Summary
Metabolic dysfunction-associated steatotic liver disease (MASLD) affects more than a quarter of the adult population worldwide. MASLD can progress to metabolic dysfunction-associated steatohepatitis (MASH), which is associated with increased risk of progression to liver fibrosis, cirrhosis and hepatocellular carcinoma, as well as cardiovascular complications. The pathogenesis of MASLD is complex and initiated by altered metabolic signalling circuits between the adipose tissue, muscle, gut and liver. Liver fibrosis is largely driven by the crosstalk of steatotic hepatocytes with macrophages and hepatic stellate cells and constitutes the primary determinant of outcomes in MASLD. Therefore, fibrosis regression is a key therapeutic goal for MASH therapies. Here, we review therapeutic strategies that directly or indirectly reduce liver fibrosis and discuss novel therapeutic concepts. Among these, the targeting of hepatocytes and metabolism have yielded fibrosis reduction in clinical trials and led to the first FDA-approved therapy for MASH. However, these therapies reduce fibrosis only in a subset of patients and have not yet shown benefits beyond the F2-F3 fibrosis stage. Direct antifibrotics and macrophage-based therapies may be more suitable for advanced stages of MASH, but are still in the developmental stage. The arsenal of therapies for MASLD is rapidly expanding and includes macrophage transplantation, hepatocyte-specific oligonucleotides, as well as CAR T cell-based therapies. Integrating these novel therapeutic concepts into stage-specific and/or combination therapies targeting divergent pathogenic mechanisms and cell types is the focus of ongoing research, which may lead to fibrosis reduction in a higher percentage of patients with MASH.
Keywords: Non-alcoholic fatty liver disease, NAFLD, non-alcoholic steatohepatitis, NASH, HSC, Kupffer cells, inflammation, cirrhosis, hepatocellular carcinoma, outcomes, portal hypertension, pharmacologic
Graphical abstract
Keypoints.
-
•
Liver fibrosis is the key determinant of outcomes in patients with metabolic dysfunction-associated steatotic liver disease (MASLD).
-
•
In MASLD, liver fibrosis is driven by crosstalk between different cell types, including hepatocytes, macrophages, immune cells and hepatic stellate cells (HSCs).
-
•
To date, the only strategies shown to reduce fibrosis in MASLD have been indirect, i.e. targeting hepatocytes and metabolism.
-
•
The success of hepatocyte- and metabolism-targeting drugs has mostly been validated in patients with MASLD and F2-F3 fibrosis.
-
•
Direct antifibrotic drugs, targeting HSCs, extracellular matrix production or degradation, would be desirable for MASLD patients with advanced fibrosis or cirrhosis. However, so far, direct antifibrotics have not achieved a strong reduction of fibrosis in clinical trials and/or their development has been terminated.
-
•
A wide range of direct antifibrotics remain under investigation, including cell therapies.
-
•
Drugs targeting macrophages, for example to increase restorative macrophages, may reduce fibrosis or promote fibrosis resolution, and are currently under investigation.
-
•
Understanding different cell states and cell-cell communication in MASLD will likely lead to new antifibrotic and regenerative therapies.
Introduction
Metabolic dysfunction-associated liver disease (MASLD) is closely associated with obesity and type 2 diabetes mellitus.1,2 With nearly 2 billion adults worldwide and 75% of adult Americans being overweight or obese,3 the rates of MASLD have dramatically increased, affecting about 30% of adults worldwide.4,5 Alarmingly, the rates of obesity and MASLD among adolescents have also increased steadily,3,6,7 leading to an earlier onset and longer duration of MASLD, which is likely to increase the risk of developing MASLD-associated complications in the lifetime of affected individuals. MASLD can progress to a more aggressive form, termed metabolic dysfunction-associated steatohepatitis (MASH), which is characterised by inflammation and elevated risk for disease progression toward fibrosis, cirrhosis and the development of hepatocellular carcinoma (HCC).1,2
Liver fibrosis represents the primary determinant of mortality in patients with MASLD, and is associated with increased liver-related events, including the development of HCC, as well as cardiovascular outcomes.[8], [9], [10], [11], [12] The accurate and sensitive identification of patients with MASH and liver fibrosis remains challenging, as liver biopsy is rarely performed nowadays. Clinically, liver fibrosis is routinely assessed via non-invasive tests, including imaging approaches that include vibration-controlled transient elastography (e.g. FibroScan), magnetic resonance elastography, corrected T1 weighted imaging, or serologic marker-based scores, such as the Fibrosis-4 index and enhanced liver fibrosis (ELF) test.[13], [14], [15], [16] However, many non-invasive tests perform best in detecting advanced fibrosis stages (>F3).14,15,17 Although pathologic scoring of fibrosis by stepwise scoring systems has been the basis for assessing severity and changes in fibrosis,18 it is increasingly clear that digital methodologies are far more accurate and quantitative, not only in assessing fibrosis, but also other structural and cellular features of disease (reviewed in Refs.19,20). While their advantages are incontrovertible, they have not yet been approved by regulatory agencies as alternative endpoints for clinical trials.
Since recent therapeutic concepts embrace earlier treatment, more refined strategies have been developed to reliably quantify fibrosis at earlier stages by combining imaging and serologic tests such as FibroScan plus aspartate aminotransferase (FAST) score, the MRI plus AST (MAST) score and the MRE plus FIB-4 (MEFIB) index.14 Newer serological tests (e.g. NIS2+) aim at identifying “at-risk MASH” based on steatohepatitis activity and/or relevant fibrosis.21 For further details on current tests and future developments, we refer to recent reviews on this topic.13,14,22,23
MASLD is a systemic disease involving crosstalk between the liver, adipose tissue, muscle and gut.1,24 Whereas the development of steatosis in early MASLD stages is driven by systemic alterations of lipid and glucose metabolism in multiple organs, the progression to MASH fibrosis and MASH cirrhosis is, in large part, a consequence of the crosstalk among different cell types within the liver.25,26 Key players in this intrahepatic crosstalk include hepatocytes, hepatic stellate cells (HSCs), liver sinusoidal endothelial cells (LSECs) and specific subsets of immune cells.27 While the dynamics of this cellular crosstalk have not been fully unravelled, these interactions are often bi- or multidirectional, involving multiple cell types that closely interact and form cellular modules rather than single cell types that act as isolated disease drivers.25,28 For example, immune cell recruitment and subsequent inflammation appear to be a consequence of metabolic hepatocyte stress and injury, but inflammatory cells may also drive hepatocyte steatosis and injury.29,30 Likewise, HSC activation is a consequence of hepatocyte injury but the loss of hepatoprotective factors in activated HSCs may also contribute to increased hepatocyte injury.31,32 The healthy liver contains negative feedback loops that preserve homeostasis,31,32 but these are replaced by feed-forward loops in the injured liver, amplifying steatosis, injury and fibrogenesis.[33], [34], [35], [36] In addition to MASLD-promoting diets and lifestyle, there are also a wide range of genetic factors that influence its development and progression.1,2,37,38 Thus, treatment options for liver fibrosis span a wide range of cellular targets and interventions. Because of the multicellular signalling circuits in MASH, targeting one cell type in the liver may impact many other cell types.39
Herein, we review current concepts for antifibrotic therapies in MASLD. While HSCs represent the primary fibrogenic cell type of the liver, there are currently no approved direct antifibrotic treatments for MASLD. However, targeting hepatocyte metabolism has proven to be an effective approach for MASLD and may indirectly improve liver fibrosis (Fig. 1). Moreover, macrophages not only play a key role in activating HSCs but also contribute to the resolution of liver fibrosis, and are currently being investigated as fibrosis-resolving therapies in clinical trials (Fig. 1).26,[40], [41], [42],43 We review the underlying pathophysiology and key players, stage-specific therapies and the combination of direct and indirect antifibrotic therapies. Furthermore, we highlight emerging therapeutic concepts, including hepatocyte-directed, RNA-based therapies, as well as those that harness the restorative properties of macrophage and HSC subpopulations to restore liver architecture and function.
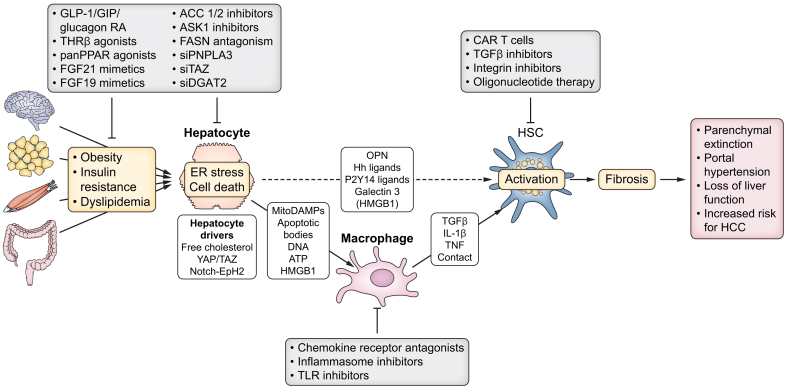
Fig. 1.
Therapeutic interruption of the cellular crosstalk that promotes liver fibrosis in MASLD.
Obesity, insulin resistance and dyslipidaemia increase hepatocyte steatosis, ER stress and cell death as well as activation of the YAP/TAZ pathway and NOTCH pathways. TAZ- and NOTCH-driven secretion of hedgehog ligands and OPN, as well as secretion of galectin 3 and DAMPs like P2Y14 ligand UDP-glucose may directly promote the activation of HSCs. Apoptotic bodies, mitochondrial DAMPs and other DAMPs, such as DNA and HMGB1, activate macrophages, which in turn secrete TGFβ, IL-1β and TNF to promote HSC activation and survival in MASH. Together, this may result in progressive liver fibrosis with parenchymal extinction and loss of liver function as well as the development of portal hypertension and increased risk for the development of HCC. Several therapies that target metabolism and hepatocytes, including GLP-1/GIP/glucagon RA, THRβ agonists, pan-PPAR agonists, FGF21 mimetics, as well as a large number of drugs still under investigation, may improve hepatocyte steatosis, stress, cell death and mediators that promote HSC and macrophage activation and, thereby, reverse liver fibrosis. Targeting macrophages (e.g. via chemokine receptor antagonism, inflammasome inhibitors and TLR inhibitors) and HSCs (e.g. via CAR T cells, TGFβ inhibitors, integrin inhibitors or oligonucleotide therapy) has not yet been proven to reverse liver fibrosis in patients with MASLD but remains promising. CAR, chimeric antigen receptor; DAMPs, damage-associated molecular patterns; ER, endoplasmic reticulum; HCC, hepatocellular carcinoma; Hh, hedgehog; HMGB1, high molecular group box 1; OPN, osteopontin; TGFβ, transforming growth factor β; TLR, Toll-like receptor; TNF, tumour necrosis factor.
HSC cell states and functions in MASLD
HSCs are the primary fibrogenic cell type in the liver and, hence, one of the key targets for direct antifibrotic therapies. However, the few clinical trials testing direct antifibrotic therapies targeting HSCs and/or fibrogenesis have not been successful, suggesting that more refined strategies may be required. Recent studies suggest distinct HSC states differentially impact homeostasis, liver function, fibrosis and disease progression.39 Understanding these distinct functions and cell states will be important for the development of novel antifibrotic therapies, with the focus being on targeting pathogenic HSC states and mediators linked to fibrosis and inflammation, while restoring HSC states and mediators associated with homeostasis, hepatoprotection and fibrosis resolution (Fig. 2) (see “Direct antifibrotic therapies targeting HSCs in MASH – Emerging strategies”).
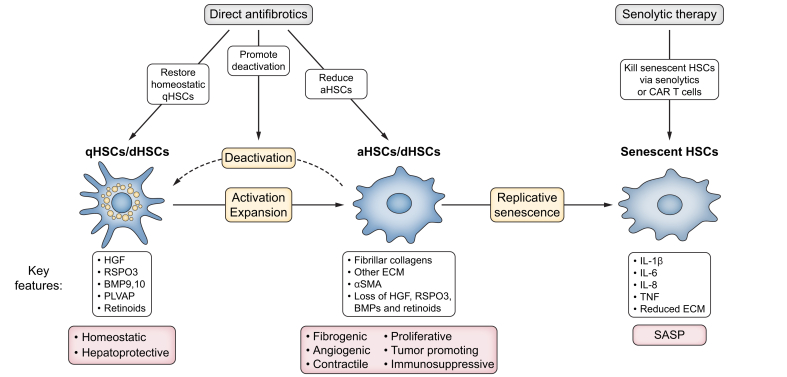
Fig. 2.
Targeting distinct HSC states in MASLD.
In MASLD, HSCs exist in four main states: quiescent, activated, deactivated, and senescent. qHSCs are characterised by their homeostatic and hepatoprotective properties, expressing mediators such as HGF, RSPO3 and BMPs. Following MASH-induced liver injury, HSCs activate and proliferate and acquire fibrogenic, angiogenic, contractile, immunosuppressive and tumour-promoting properties through the expression of fibrillar collagens, non-collagenous ECM, αSMA and the loss of hepatoprotective mediators, HGF, RSPO3 and BMPs. With improved MASLD, HSCs may deactivate (dHSCs) and return to a near-quiescent state. In progressive MASLD, HSCs may undergo senescence, characterised by the "senescence-associated secretory phenotype" (SASP), with increased IL-1β, IL-6, IL-8, and TNF expression as well as lower ECM expression. HSC-directed therapeutic strategies in MASLD include the restoration of a healthy HSC balance by reducing pathogenic aHSCs and increasing protective qHSCs/dHSCs; as well as by eliminating sHSCs. aHSCs, activated HSCs; BMP, bone morphogenetic protein; dHSCs, deactivated HSCs; ECM, extracellular matrix; HGF, hepatocyte growth factor; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; qHSCs, quiescent HSCs; PLVAP, plasmalemma vesicle-associated protein; RSPO3, R-spondin 3; SASP, senescence-associated secretory phenotype; sHSCs, senescent HSCs.
Quiescent HSCs promote liver homeostasis
In the healthy liver, HSCs maintain a quiescent and non-proliferative phenotype. Quiescent HSCs (qHSCs) are the main reservoir for vitamin A, which store 50-80% of the body’s total vitamin A within cytoplasmic lipid droplets in the form of retinyl esters.44 Retinyl ester storage in HSCs is mediated by lecithin retinol acyltransferase, which is highly enriched in HSCs.44 In addition to maintaining systemic levels of retinoids, lecithin retinol acyltransferase has a role in promoting liver regeneration after 70% partial hepatectomy.45 Beyond the storage of vitamin A, increasing evidence suggests that qHSCs are also responsible for maintaining key aspects of liver homeostasis, including the metabolic functions of hepatocytes.32,39,[46], [47], [48] Their position within the space of Disse and long cellular projections allow HSCs to maintain close contact with liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs) and hepatocytes. Wake and colleagues proposed that individual HSCs can contact up to twenty hepatocytes and several LSECs in a multicellular unit termed a “stellon”.49 This concept is further supported by single-cell RNA-seq analyses, indicating that HSCs are among the most interactive of cell types in the liver.50 Along this line, qHSCs are enriched in several cytokines and growth factors through which they can maintain crosstalk with hepatocytes, KCs and LSECs.28,31,50,51 Key qHSC-enriched mediators include hepatocyte growth factor (HGF), R-spondin 3 (RSPO3), neurotrophin-3 (NTF3) and bone morphogenetic protein 9 and 10 (BMP9, BMP10)32,46,48 (Fig. 2). A central function of qHSCs, mediated by RSPO3, is the regulation of liver zonation.32 HSCs show a pericentral to periportal gradient of RSPO3 expression, which is required for the activation of WNT/β-catenin signalling in pericentral to mid-lobular hepatocytes, and contributes to the zonal expression of characteristic WNT-regulated genes such as Cyp2e1 and Cyp1a2.32,52 Moreover, HSC-expressed RSPO3 is required for efficient liver regeneration, consistent with the key role of the WNT/β-catenin pathway in hepatocyte proliferation and liver regeneration.32 BMP9 and BMP10 are additional growth factors that are enriched in qHSCs and exert critical functions in liver homeostasis.46 HSC-derived BMP9 and BMP10, which often act in tandem as they are the only known ligands for Alk1,53 provide signals that maintain endothelial cell and KC identity.46 Furthermore, via their effects on LSECs, they also affect liver zonation and regulate iron metabolism.46
HGF is a growth factor enriched in qHSCs that does not have an established role in liver homeostasis, apart from protecting hepatocytes from injury in the healthy liver. Although HGF is also a complete mitogen for hepatocytes, its deletion in HSCs does not affect liver regeneration, as LSECs also express HGF.31 NTF3 is also enriched in qHSCs and may drive liver regeneration, as shown by the mitogenic effects of recombinant NTF3 in vitro and increased hepatocyte proliferation following NTF3 overexpression in vivo.48,54 In summary, qHSCs express several growth factors that maintain liver homeostasis and zonation, protect the liver from injury and promote regeneration.39 These beneficial functions of qHSCs are progressively lost as MASLD progresses, as detailed in the following section.
Activated HSCs promote fibrogenesis and lose homeostatic functions
Activated HSCs are the primary collagen-producing cells of the liver in a wide range of diseases, including MASLD.[55], [56], [57] In response to liver injury, qHSCs undergo a well-characterised activation process, and transdifferentiate into extracellular matrix-producing, contractile myofibroblasts.58 Activated HSCs display profound morphologic and transcriptomic alterations, including the loss of their characteristic lipid droplets, a more myofibroblastic spindle-like shape, and the acquisition of proliferative, migrative, chemotactic and contractile capabilities.59 The differentiation from qHSCs to activated HSCs is mediated by a wide range of signalling molecules, including transforming growth factor-β (TGFβ), the most potent activator of HSCs, as well as platelet-derived growth factor (PDGF) and connective tissue growth factor (CTGF or CCN2), which drive the expansion of HSCs and their migration. Additional mediators promoting HSC activation include angiotensin, leptin, interleukin (IL)-1β, -17, -20, C–C motif chemokine ligand (CCL)-2, -3, -5, damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns, osteopontin and hedgehog ligands.58 The profibrogenic effects of these molecules are mediated by downstream pathways, including the Smad, Hippo-YAP-TAZ, Erk and MAP kinase pathways.58 Collectively, these divergent features of HSCs reflect a level of cellular heterogeneity that has only become apparent with the emergence of single-cell analytic techniques.60
The production of extracellular matrix (ECM) is considered the primary disease-driving feature of activated HSCs, promoting the formation of fibrotic septa, and disrupting the normal liver’s architecture and biomechanical properties.61,62 Conceptually, the amount of fibrosis present in the liver reflects the balance between pro-fibrogenic and fibrinolytic mechanisms.61 To date, however, this concept has not been formally validated, but the general assumption is that fibrosis accumulation results from excess profibrogenic activity relative to fibrinolysis.
The architectural changes in advanced fibrosis are associated with parenchymal extinction and severe clinical sequelae including the loss of liver function, the development of portal hypertension and an increased risk of liver cancer.[60], [63], [64] A set of core genes (e.g. GAS7, SPON1, SERPINE1, LTBP2, KLF9, EFEMP1) drive the fibrogenic activation of HSC subclusters, as evidenced from patient biopsies, HSC cultures and rodent models.65 Activated HSCs produce a wide range of ECM molecules, including type I collagen, the most abundant ECM component of the fibrotic liver,[66], [67], [68] other fibrillar and non-fibrillar collagens, along with glycoproteins that include hyaluronan, tenascin, decorin, fibronectin, periostin, lumican and laminins.[69], [70], [71] In the early stages of MASH fibrosis, an increase of pericellular fibrosis leads to the characteristic perisinusoidal fibrosis, especially in the pericentral zone (zone 3). In later stages, fibrotic septa and bridging fibrosis may develop.61 In advanced fibrosis, collagen becomes crosslinked, making it stiffer and more resistant to degradation. This process is initiated by enzymes that include lysyl oxidases,72 among others.73 In addition to the replacement of functional parenchyma by ECM, the increased stiffness also impairs the function of hepatocytes. Stiffness leads to a loss of hepatocyte function and dedifferentiation, e.g. via the downregulation of transcription factor HNF4α (hepatocyte nuclear factor 4α) and/or upregulation of the YAP/TAZ pathway.[74], [75], [76] Moreover, many ECM components signal via specific receptors such as integrins and discoidin domain receptors in resident and non-resident liver cells, affecting a wide range of responses such as proliferation and regeneration, differentiation and inflammation.69,77,78
A second characteristic feature of activated HSCs is their increased contractility. αSMA (α-smooth muscle actin) is strongly upregulated during HSC activation and contributes to HSCs’ regulation of vascular tone,63 along with protocadherin 7.79 At the same time, HSCs may also promote angiogenesis via the secretion of VEGF (vascular endothelial growth factor) and angiopoietin-1.80,81 Together with the increased stiffness of the fibrotic liver, angiogenesis and higher HSC contractility promote the development of portal hypertension, a characteristic feature of advanced liver disease, and may contribute to many of its complications, including decreased liver function, variceal bleeding and ascites formation.82
Finally, activated HSCs engage in a bidirectional crosstalk with discrete immune cell populations. While immune cells such as macrophages, T cells and B cells contribute to HSC activation in MASH,30,83 HSCs may regulate hepatic inflammation and immunity by controlling immune cell recruitment and activity in MASH.84 The secretion of chemokines, including MCP-1 (also known as CCL2), IL-8, RANTES SDF-1/CXCL-12, and the expression of adhesion molecules ICAM-1 and VCAM-1 promote the infiltration of lymphocytes and monocyte-derived macrophages.[85], [86], [87], [88], [89] HSCs can modulate immune responses by acting as MHC class II-expressing antigen-presenting cells and by simulating T cells via CD86 expression.[90], [91], [92] However, HSCs have limited antigen-presenting capabilities in vivo and likely contribute to the tolerogenic environment of the liver through expression of ICAM-1, IDO, PD-L1, retinoic acid and TGFβ, affecting cytotoxic and regulatory T cells, B cells and myeloid-derived suppressor cells.[93], [94], [95], [96], [97], [98], [99], [100], [101], [102]
Progressive HSC activation modifies their interactions with other cells, as shown in single cell-based ligand-receptor analysis.50,51,65 In addition to an overall increase in HSC interactions, there is a major shift in the pattern of interactions. HSC interactions with hepatocytes and LSECs, which maintain homeostasis and epithelial health and are characteristic of qHSCs, decrease during the progression of CLD. In parallel, HSC interactions with inflammatory cell types, cholangiocytes, and LSECs, as well as autocrine HSC-HSC interactions increase during CLD progression. Examples of interactions that decrease with HSC activation include those with hepatocytes, endothelial cells and Kupffer cells, mediated by HSC mediators HGF, RSPO3 and HSC-enriched BMP family members.31,32,46 One example of increased ligand-receptor interactions includes NTF3-NTRK3. NTF3-NTRK3 this interaction further amplifies the activation and and proliferation of HSC within fibrotic septa, where they are densely packed in proximity to one another and distrant form signals from hepatocyte- and Kupffer cell-derived signals.34 Together with the concomitant upregulation of type I collagen, αSMA and other ECM components during activation, there is a shift from beneficial, homeostatic HSC interactions toward fibro-pathogenic HSC interactions in liver disease and MASLD progression.39
Senescent HSCs
After many years of MASLD, during which HSCs have undergone many rounds of proliferation, they can undergo replicative senescence.103 Senescence limits their ability to proliferate and produce ECM and can thereby reduce further expansion and fibrosis,104,105 as well as long-term consequences such as the development of HCC.106 However, senescent HSCs are also characterised by an increased expression of inflammatory mediators, termed the senescence-associated secretory phenotype (SASP). A recent study has characterised the senescent features of HSCs’ SASP in MASH.103 A unique cluster of molecular markers define senescence in this population, underscoring that each cell type may have different components of a senescence signature. Regardless, SASP of HSCs is thought to provoke inflammation and the recruitment of inflammatory cells, thereby promoting the progression of chronic liver disease.107 Thus, clearance of senescent HSCs using chimeric antigen receptor (CAR) T cell therapies is a potential therapeutic strategy.108
Deactivated HSCs
Chronic liver diseases such as MASLD are characterised by alternating phases of disease progression and regression. During regression, activated HSCs may undergo cell death109 or revert to a deactivated or inactivated phenotype.110,111 Deactivated HSCs express a similar transcriptome as qHSCs but retain memory that renders them more prone to activation than their quiescent counterparts.110,111 Deactivated HSCs have been identified in MASLD.112 With HSCs constantly cycling between different states, it is possible that deactivated HSCs accumulate over time in chronic liver diseases, including in patients with MASLD.113 Moreover, deactivation leads to a higher expression of homeostatic and protective HSC mediators, characteristic of qHSCs, which may help to restore liver function,32 making drivers of HSC deactivation appealing therapeutic targets.39
How hepatocytes trigger HSC activation and liver fibrosis
Both animal studies and clinical trials have demonstrated that treating the underlying disease-driving metabolic abnormalities results in an improvement of liver fibrosis in MASLD.[114], [115], [116], [117], [118], [119], [120] Therefore, it is important to understand the mechanisms through which hepatocytes trigger HSC activation and liver fibrosis. The initial precipitant of HSC activation in MASLD results from signals emanating from stressed or dying steatotic hepatocytes.[121], [122], [123], [124] However, rather than directly causing HSC activation, several key events may need to occur simultaneously (Fig. 1), which engage multicellular networks that involve (i) signals from macrophages, in particular release of TGFβ; (ii) a loss of inhibitory signals from fenestrated LSECs; (iii) activation of latent TGFβ by thrombospondin 1 and integrin alpha V,125,126 facilitated through decreased expression of ECM1, a potent inhibitor of latent TGFβ activation;127,128 and (iv) additional signals from CD8+ T cells, intestinal B cells, regulatory T cells and potentially additional immune cell populations.30,83,84,129
In MASLD, hepatocyte stress and death can be triggered by a wide range of pathways that are mostly activated in response to altered quantities and qualities of lipids. Saturated free fatty acids, palmitate, the phospholipid lysophosphatidylcholine or cholesterol can promote fibrosis by directly triggering endoplasmic reticulum (ER) stress, profibrogenic signalling pathways, including Hedgehog, YAP/TAZ and NOTCH,122 or by causing lipotoxic hepatocyte death,130,131 which subsequently induces inflammation and fibrosis (Fig. 1). The accumulation of lipids leads to ER stress in hepatocytes and activation of the unfolded protein response in animal models and patients with MASLD.[132], [133], [134] While the unfolded protein response is initially an adaptive response, ER stress becomes maladaptive, inducing pro-inflammatory signalling pathways such as JNK, NF-kB, NLRP3, and driving the expression of caspase 2, which collectively trigger inflammation, fibrosis and hepatocyte death.[135], [136], [137] Hedgehog pathway activation tracks with liver injury and fibrosis in patients with MASLD, and hepatocyte ballooning has also been linked to the activation of HSCs via the secretion of sonic hedgehog.[138], [139], [140] YAP and TAZ expression are strongly increased in hepatocytes in mouse models and in patients with MASLD.141,142 The upregulation of TAZ is mediated by increased hepatocyte cholesterol via an ASTER-B/C-soluble adenylyl cyclase-RhoA-mediated pathway that suppresses β-TrCP-mediated TAZ degradation.143 TAZ activation in hepatocytes promotes MASLD-induced liver fibrosis and is mediated by enhanced secretion of profibrogenic mediators including Indian hedgehog.142 Hepatocyte-specific deletion of YAP reduces carbon tetrachloride-induced liver fibrosis in mice, but the contribution of YAP to MASLD-induced liver fibrosis was not tested in this study.141 NOTCH activation tracks with MASH severity in patients, and NOTCH loss- and gain-of-function studies in mice underscore hepatocyte NOTCH’s activity in promoting liver fibrosis.144 Through the induction of osteopontin as well as CCL2,144,145 NOTCH also triggers the expression of EphB2 in hepatocytes in mouse and human MASH.146 EphB2 promotes inflammation and fibrosis in MASLD as shown by loss- and gain-of-function studies in mice.146
Lipid overload can trigger hepatocyte death, including apoptosis, ferroptosis, necroptosis and pyroptosis, and drive disease progression in MASLD.122,123,147 In mice fed a methionine- and choline-deficient diet, global caspase 3 knockout had no effect on alanine aminotransferase (ALT) levels or NAFLD activity score (NAS), but did reduce fibrosis.148 GPX-4 (glutathione peroxidase 4) is essential to protect hepatocytes from ferroptosis as shown by constitutive or inducible knockout studies.149,150 Due to this pronounced effect, most studies on ferroptosis have relied on pharmacologic inhibition of ferroptosis, which improves MASLD and MASLD fibrosis.[151], [152], [153], [154] Notably, vitamin E, an antioxidant that blocks ferroptosis,155 not only extended the life-span of mice with hepatocyte-specific knockout of Gpx4, but also improved MASLD in the PIVENS trial.149,156 Cell death leads to a wide range of signalling pathways that promote inflammation and liver fibrosis in MASLD and other liver diseases.122,123 These include the recruitment of inflammatory cells, efferocytosis and TGFβ release, as well as the release of DAMPs, such as nuclear DNA, mitochondrial DNA, HMGB1, ATP, UDP-glucose, and apoptotic bodies. DAMPs may act on macrophages and HSCs to trigger fibrogenic signalling cascades (Fig. 1).157 Examples of DAMPs directly triggering the activation of HSCs include P2Y14 receptor ligands and HMGB1.122,124,158,159
In summary, lipid overload elicits a wide range of signalling cascades, the release of profibrogenic mediators, as well as cell death in hepatocytes, which may all serve as therapeutic targets in MASLD.
How macrophages modulate HSC activation, fibrosis and fibrosis regression in MASLD
Macrophages are highly plastic and exert various roles in tissue homeostasis, injury and repair.160,161 The pivotal role of macrophages in the development and resolution of MASLD make them attractive therapeutic targets. Hepatic macrophages are comprised of distinct subsets with differing origins and functions, including tissue-resident Kupffer cells and infiltrating monocyte-derived macrophages, both of which exhibit remarkable plasticity.162 Kupffer cells detect hepatocyte stress and injury signals – whether from neighbouring cells or systemic sources – activating inflammatory pathways, recruiting monocytes and other immune cells through chemokine signalling, and clearing cellular debris. Monocyte-derived macrophages contribute significantly to fibrogenesis but also participate in resolving fibrosis (Fig. 3).[163], [164], [165]
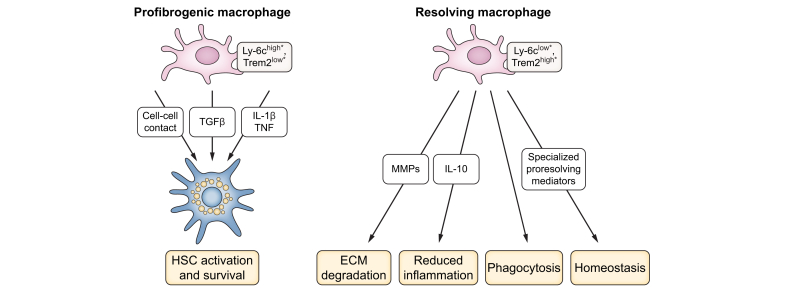
Fig. 3.
Macrophage states in MASLD.
During MASLD progression, profibrogenic macrophage subsets (∗Ly-6chigh Trem2low in mice, further characterisation in patients needed) promote HSC activation and survival through the secretion of TGFβ, pro-inflammatory mediators like IL-1β and TNF and through physical contact. During MASLD resolution, specific subsets of macrophages (∗Ly-6clow Trem2high in mice, further characterisation in patients needed) degrade ECM via high MMP expression and promote the return to homeostasis, additionally through phagocytes and the secretion of anti-inflammatory and pro-resolving lipid mediators. Shifting the macrophage balance from profibrogenic to pro-resolution may represent a strategy for the treatment of MASLD fibrosis. ECM, extracellular matrix; IL, interleukin-; MASLD, metabolic dysfunction-associated steatotic liver disease; MMPs, matrix metalloproteinases; TGFβ, transforming growth factor β; TNF, tumour necrosis factor.
Profibrogenic actions of macrophages
Liver macrophages with an inflammatory phenotype promote the progression of MASLD, with their accumulation correlating with disease severity in human biopsies.166 Advances in single-cell RNA sequencing have unveiled an unprecedented level of detail in the heterogeneity of hepatic immune cells, highlighting profound changes in myeloid cells and macrophages during MASLD progression, helping contextualise findings in a spatial orientation.167 From a spatial perspective, the occurrence of hepatic “crown-like structures” (i.e. macrophages surrounding dead or dying hepatocytes) as well as bile duct-associated macrophages have been linked to MASH with fibrosis progression in mice and humans, and both phenomena relate to infiltrating monocyte-derived macrophages.168,169 Among Kupffer cell subsets, the CD206hi ESAM+ population has been implicated in fatty acid metabolism, potentially driving MASH progression.170
Single-cell analyses have underscored the critical role of monocyte- or bone marrow-derived macrophages in MASH. Monocyte-derived macrophages can replace Kupffer cells, while adopting phenotypes such as "lipid-associated macrophages" (LAMs) or "scar-associated macrophages" (SAMs) in MASH, characterised by markers like TREM2, CD9, and osteopontin.[170], [171], [172] These hepatic MASH-associated macrophages thereby share many phenotypic markers with LAMs in adipose tissue.173
Mouse models have provided foundational insights into the functions of these macrophage subsets. Contrary to initial assumptions, TREM2+ macrophages associated with MASH, which were thought to promote inflammation and fibrosis, instead were found to mitigate inflammation and even support fibrosis resolution.174,175 Interestingly, the resolving phenotype of TREM2+ macrophages in hepatic fibrosis regression applies to both recruited and resident macrophage subsets that cooperate in tissue repair.176 In addition, the Notch-RBPJ signalling pathway can regulate monocyte differentiation into inflammatory (and fibrogenic) macrophages in MASLD models, with RBPJ deficiency promoting reparative responses.177 In advanced fibrosis, however, the loss of Kupffer cells and their replacement by monocyte-derived macrophages impair essential homeostatic functions.178
Although many findings are based on mouse models, human liver single-cell RNA-seq data have identified SAMs as a distinct population residing within fibrotic niches in cirrhotic livers.179 Proteo-genomic studies combined with spatial mapping reveal that LAMs (and SAMs) typically localise near intrahepatic bile ducts in healthy conditions, but migrate towards steatotic areas in MASLD, driven by HSC-derived CCL2 chemokine signalling.180
Macrophages are considered essential for HSC activation. The genetic or pharmacologic depletion of macrophages has demonstrated a strong reduction of HSC activation and liver fibrosis.163,181,182 It is likely that the main effects of macrophages on HSC activation and fibrosis result from the release of TGFβ (Fig. 3).183 In MASH, the release of TGFβ by macrophages requires the macrophage c-mer tyrosine kinase (MerTK) receptor and mice lacking MerTK or humans with hypomorphic MERTK variants are protected from MASH fibrosis.184 Recent studies suggest that contact-dependent signals between macrophages and fibroblasts create mechanical stress that allows full-blown TGFβ-mediated fibroblast activation in soft environments185 and may, therefore, be crucial for fibroblast activation in the early stages of MASH, where livers are still mechanically soft. Furthermore, macrophages provide survival signals to HSCs via IL-1β and tumour necrosis factor (TNF).186 Together, the activation and survival signals significantly contribute to maintaining a pool of activated HSCs that promote fibrosis in MASH. Besides HSCs, macrophages also interact with other immune cells.168 For instance, in MASH, activated platelets interact with hepatic macrophages, exacerbating inflammation, and indirectly, fibrosis.187 Intestinal B cells interacting with hepatic macrophages via Fc-receptor γ further amplify metabolic T cell activation and fibrosis in a microbiota- and antigen-independent fashion.83
Fibrosis resolution by macrophages
The restorative properties of macrophages make them highly attractive for the treatment of liver fibrosis.168 Genetic depletion of macrophages has not only revealed a role for macrophages in hepatic fibrogenesis but also during the recovery phase, demonstrating a failure of ECM degradation in the absence of macrophages.163 Macrophage-expressed matrix metalloproteinases (MMPs), including MMP-9, MMP-12 and MMP-13, constitute major effectors contributing to the degradation of collagen during resolution stages.40,188,189
Fibrolytic macrophages are phenotypically distinct from fibrogenic macrophages and are characterised by low expression of Ly-6C(lo), enrichment of Trem2, and an M2-like phenotype in mice.40,175 Efferocytosis of dead hepatocytes mediates phenotypic shifts in macrophages, including an upregulation of anti-inflammatory IL-10 and pro-resolution lipid mediators, as well as feed-forward loops that increase the phagocytotic and efferocytotic capacity of macrophages (Fig. 3).124 Moreover, macrophages interact with neutrophils to promote tissue repair.190 The therapeutic potential of fibrolytic macrophages has been demonstrated in mice and is currently being investigated in patients.41,191 Beyond their pure fibrolytic actions, Ly-6C(lo) Trem2(hi) macrophages may also exert other restorative functions,192 consistent with a key role for macrophages in tissue homeostasis.160
Open questions about antifibrotic therapies in MASH
Whereas treatment of other chronic liver diseases (e.g. chronic HCV infection), have clearly defined endpoints such as viral eradication, MASLD represents a challenge due to the wider range of hepatic and extrahepatic clinical endpoints, including cardiovascular mortality, and its multifactorial pathophysiology that includes genetic and behavioural factors, unique disease subtypes, as well as complex disease-driving interactions between multiple cell types and organs.1,2,73,193 Several cell types, including hepatocytes, immune cells and HSCs, as well as mechanisms controlling food intake, metabolism and energy expenditure, represent potential therapeutic targets in MASLD.1,15,194 Moreover, treatment for a disease such as MASLD may require lifelong therapy.
Therapeutic concepts and clinical endpoints
Conceptually, the treatment of MASLD in early stages, where metabolic abnormalities dominate, may differ from more advanced stages, in which fibrosis and parenchymal extinction are characteristic (Fig. 4). Notably, data from recent positive phase III trials show that only 25-37% of patients respond to current MASLD therapies (vs. 12-22.5% in the placebo groups) when including fibrosis improvement as an endpoint,117,195 suggesting the need for individualised or combination therapies to improve response rates. Currently accepted primary endpoints for phase III trials in MASLD constitute: (i) the resolution of steatohepatitis without worsening of fibrosis and (ii) the regression of liver fibrosis by at least one stage without worsening of steatohepatitis.196 Some trials in patients with advanced fibrosis (e.g. F3) also seek to demonstrate lack of progression to cirrhosis rather than regression, which is considered by regulatory agencies as a ‘hard endpoint’. In clinical practice, treatments will likely be patient-specific and will need to consider long-term outcomes, which are not only determined by beneficial effects of treatments on MASH resolution and fibrosis improvement but also on cardiovascular mortality (Table 1).
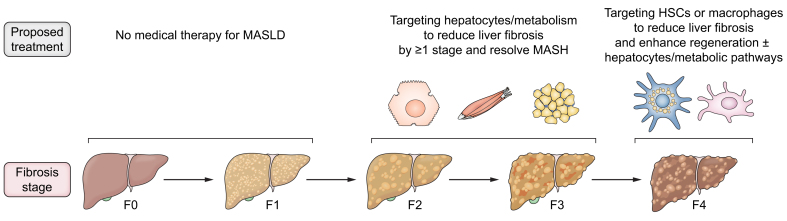
Fig. 4.
Stage-specific therapeutic concepts in MASLD.
While early stages (F0-F1 fibrosis) may not require medical therapy, encouraging data suggest that hepatocyte- and metabolism-directed therapies may not only improve the underlying metabolic abnormalities but also achieve reversal of fibrosis by ≥1 stage in subsets of patients with F2-F3 fibrosis. In patients with cirrhosis (stage F4), hepatocyte- and metabolism-directed therapies alone seem to have little efficacy in reversing fibrosis. Instead, HSC- and macrophage-directed therapies may be more appropriate for patients with F4 fibrosis, possibly in combination with hepatocyte- and metabolism-directed therapies. HSC, hepatic stellate cell; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease.
Table 1.
Summary of antifibrotic therapies tested in clinical trials that target hepatocytes, HSCs or macrophages.
| Drug class | Clinical development | Fibrosis reduction by ≥1 stage without worsened MASH | MASH improvement without worsening of fibrosis | Effects on cardiometabolic health |
|---|---|---|---|---|
| Targeting hepatocytes and/or metabolism | ||||
| THRβ agonists | ||||
| Resmetirom | FDA-approved for patients with MASH and F2-F3 fibrosis (NCT03900429) | 24.2% (80 mg) and 25.9% (100 mg) vs. 14.2% (placebo) after 52 weeks117 | 25.9% (80 mg) and 29.9% (100 mg) vs. 9.7% in placebo117 | Reduced LDL-C, apoB and TG; no alterations in HbA1c117,202,225 |
| VK2809 | Phase IIb trial in patients with MASH and F1-F3 fibrosis (NCT04173065) | 44%-57% (across doses) vs. 34% (placebo) after 52 weeks206 | 63%-75% (across doses) vs. 29% (placebo) after 52 weeks206 | Not reported |
| FGF19 agonists | ||||
| Aldafermin | ALPINE 2/3 in patients with MASH and F2-F3 fibrosis stage (NCT03912532) | 31% (0.3 mg daily), 15% (1 mg daily), 30% (3 mg daily) vs. 19% (placebo) after 24 weeks207 | 11% (0.3 mg daily), 18%, (1 mg daily), 22% (3 mg daily) vs. 6% (placebo) after 24 weeks207 | Significantly decreased body weight and serum TG but unaltered HbA1c in 3 mg group207 |
| ALPINE 4 in patients with compensated MASH cirrhosis (NCT04210245) | 16% (1 mg daily) and 20% (3 mg daily) vs. 13% (placebo) after 48 weeks191 | Not determined191 | ||
| FGF21 analogues | ||||
| Efruxifermin | Phase IIb in patients with MASH and F2-F3 fibrosis, completed (NCT04767529) Phase IIb trial in patients with compensated liver cirrhosis (F4 fibrosis; NCT05039450) |
39%∗ (28 mg) and 41%∗ (50 mg) vs. 20% (placebo) at week 24118 ∗liver biopsy patients only 18% (28 mg) 19% (50 mg) vs 13% (placebo) at week 36 (primary endpoint); 21% (28 mg) and 29% (50 mg) vs. 11% (placebo) at week 96 (secondary endpoint)208 |
43% (28 mg) and 60% (50 mg) vs. 14% in placebo at week 24118 42% (28 mg and 50 mg) vs. 13% in placebo at week 96208 |
Reduced body eight, insulin resistance and hyperlipidaemia118 Great improvement in HDL and non-HDL cholesterol vs. placebo208 |
| Pegozafermin | Phase IIb in patients with MASH and F2-F3 fibrosis, completed (NCT04929483) | 22% (15 mg weekly), 26% (30 mg weekly), 27% (44 mg biweekly) vs. 7% (placebo) at week 24119 | 37% (15 mg weekly), 23% (30 mg weekly), 26% (44 mg biweekly) vs. 2% (placebo) at week 24119 | Improved serum TG, and HDL-C119 |
| Efimosfermin | Phase IIb in patients with MASH and F2-F3 fibrosis (NCT04880031) | 45.2% (300 mg monthly) vs. 20.6% (placebo) after 24 weeks120 | 67.7% (300 mg monthly) vs. 29.4% (placebo) after 24 weeks120 | Cardiometabolic data not yet published120 |
| FASN inhibitors | ||||
| Denifanstat | Phase IIb in patients with F2-F3 fibrosis, completed (NCT04906421) | 41% (50 mg daily) vs. 18% (placebo) after 52 weeks209 | 26% (50 mg daily) vs. 11% (placebo) after 52 weeks209 | Significant reduction in TG but not in LDL-C (58% of patients were also on statins) |
| Pan-PPAR agonist | ||||
| Lanifibranor | Phase IIb in patients with non-cirrhotic MASH, completed (NCT03008070) | 34% (800 mg daily), 48% (1,200 mg daily), vs. 29% (placebo) after 6 months210 | 39% (800 mg daily), 49% (1,200 mg daily), vs. 22% (placebo) after 6 months210 | Improved TG, HDL-C, apolipoproteins, insulin, HOMA-IR, HbA1c and diastolic BP, independent of diabetes status226 |
| GLP-1RA | ||||
| Liraglutide | Phase II (LEAN) trial in patients with MASH and F0-F4 fibrosis stage, completed (NCT01237119) | Non-significant improvement 26% (1.8 mg daily) vs. 14% (placebo) at week 4862 | 39% (1.8 mg daily) vs. 9% (placebo) MASH resolution at 48 weeks62 | Weight loss, improved glucose and Hb1Ac, reduced cardiovascular death, myocardial infarction and stroke208,409,227 |
| Semaglutide | Phase II trial in patients with MASH and F2-3 fibrosis (amended to F1-F3), completed | 49% (0.1 mg daily) 32% (0.2 mg daily), 43% (0.4 mg daily) vs. 33% (placebo) at week 72, p = 0.48211 | 40% (0.1 mg daily) 36% (0.2 mg daily), 59% (0.4 mg) vs. 17% (placebo) at week 72211 | Weight loss, improved HbA1c, blood pressure, TG and HDL-C; reduced heart failure, cardiovascular death, myocardial infarction and stroke in obesity with and without diabetes228,229,[230], [231], [232], [233], [234], [235] |
| Phase III trial (ESSENCE) in patients with MASH and F2-3 fibrosis, completed (NCT04822181) | 37% (2.4 mg weekly) vs. 22.5% (placebo), interim analysis at week 72195 | 62.9% (2.4 mg weekly) vs. 34.1% (placebo), interim analysis at week195 | ||
| Dulaglutide | Open label randomised controlled trial in patients with MASLD and type 2 diabetes, completed (NCT03590626) | No biopsy212 | No biopsy212 | Weight loss, body weight (p = 0.011), decrease in HbA1c (p = 0.039) and TG levels212,236 |
| Dual GLP-1/glucagon agonists | ||||
| Survodutide | Phase II trial in patients with MASH and F1-F3 fibrosis, completed (NCT04771273) | 34% (2.4 mg weekly) 36% (4.8 mg weekly), 34% (6 mg weekly) vs. 22% (placebo) at week 48213 | 47% (2.4 mg weekly) 62% (4.8 mg weekly), 43% (6 mg weekly) vs. 14% (placebo) at week 48213 | Decreased LDL-C, TG and HbA1c (significance not evaluated);213 ongoing evaluation in the Synchronize trials237,238 |
| Cotadutide | Phase II trial in patients with MASH and F1-F3 fibrosis, completed (NCT04019561) | No biopsy214 | No biopsy214 | Reduced body weight, HbA1c and TG214,239,240 |
| Dual GIP/GLP-1 agonists | ||||
| Tirzepatide | Phase II trial in patients with MASH and F2-F3 fibrosis, completed (NCT04166773) | 55% (5 mg weekly) 51% (10 mg weekly), 51% (15 mg weekly) vs. 30% (placebo) at week 48.215 | 44% (5 mg weekly) 56% (10 mg weekly), 62% (15 mg weekly) vs. 10% (placebo) at week 48.215 | Reduced body weight, improved TG, HbA1c and reduced death from cardiovascular causes215,241,242,[243], [244], [245], [246] |
| Triple GIP/glucagon/GLP-1 agonists | ||||
| Retatrutide | Phase II study in obese or overweight patients with weight-related complications other than type 2 diabetes, completed (NCT04881760) | No biopsy216 | No biopsy216 | Reduction in body weight, TG, LDL-C and HbA1c216,247,248 |
| SGLT-2 inhibitors | ||||
| Empagliflozin | Phase II study in patients with MASLD without diabetes mellitus, completed (NCT04642261) | Not determined | Not determined Note: Greater reduction in steatosis224 |
Fewer cardiovascular events and death in type 2 diabetes249 |
| FXR agonists | ||||
| Obitecholic acid | Phase III trial in patients with MASH and F2-F3 fibrosis or F1 fibrosis with additional risk factors (NCT02548351), completed | 22.4% (25 mg daily) vs. 9.6% (placebo) after 18 months, p <0.0001.217 | 6.5% (25 mg daily) vs. 3.5% (placebo) after 18 months, p = 0.093217 | Elevated LDL-C, decreased HDL-C, increased HOMA-IR250,217 |
| Cilofexor | Phase IIb trial in patients with MASH and F3-F4 fibrosis (NCT02548351), completed | 12% (30 mg daily) vs. 11% (placebo) at week 48218 | 0% (30 mg daily) vs. 0% (placebo) at week 48218 | No changes in serum lipids or HbA1c218 |
| ACC inhibitor | ||||
| Firsocostat | Phase IIb trial in patients with MASH and F3-F4 fibrosis (NCT02548351), completed | 12% (30 mg daily) vs. 11% (placebo) at week 48218 | 2.9% (30 mg daily) vs. 0% (placebo) at week 48218 | Increased TG and VLDL-C, no change in HbA1c218 |
| Kinase inhibition | ||||
| Selonsertib (ASK1 inhibitor) | Phase III in patients with MASH and F3 fibrosis (STELLAR-3, NCT03053050) | 12% (75 mg weekly), 10% (125 mg weekly) vs. 13% (placebo) at week 48 in F3 patients219 | No effect on MASH resolution | Not reported in detail |
| Phase III trial in patients with MASH and F4 fibrosis (STELLAR-4, NCT03053063) | 13% (75 mg weekly), 14% (125 mg weekly) vs. 13% (placebo) at week 48 in F4 patients219 | |||
| Hepatocyte-directed oligonucleotides | ||||
| ION224 (DGAT2 antisense) | Phase II trial in patients with MASH and F2-F3 fibrosis (NCT04932512), analysis in F3 subcohort | 46.2% (90 mg or 120 mg, monthly) vs. 30.8% (placebo) after 51 weeks.220 | 30.8% (90-120 mg) vs. 15.4% (placebo)220 | Improvement in HbA1c |
| GSK4532990 (HSD17B13 siRNA | Phase IIb study in patients with MASH and F3-F4 fibrosis (NCT05583344), ongoing | No data yet | No data yet | No data |
| ARO-HSD (HSD17B13 siRNA) | Phase I/IIa study in healthy volunteers and patients with MASH (NCT04202354), completed | Not evaluated | Not evaluated Note: ALT decreased by 42% in the highest does |
No data |
| ION455/AZD7503 (HSD17B13 ASO) | Phase I study in patients with MASLD or MASH (NCT05560607) | Not evaluated | Not evaluated | No data |
| ALN-HSD (HSD17B13 siRNA) | Phase II study in patients with MASH and genetic risk factors (NCT05519475), ongoing | No data yet | No data yet | No data yet |
| JNJ-75220795 (PNPLA3 siRNA) | Phase I study in patients with MASLD (NCT04844450), completed | Not evaluated | Not evaluated | No data |
| ALN-PNP (PNPLA3 siRNA) | Phase I study in patients with MASLD (NCT05039710) terminated | Not evaluated | Not evaluated | No data |
| AZD2693 (PNPLA3 ASO) | Phase IIb study in PNPLA3 I148M carriers with F2-F3 MASH (NCT05809934), ongoing | No data yet | No data yet | No data |
| AMG 609 (PNPLA3-I148M siRNA) | Phase I study in patients with MASLD carrying the PNPLA3 I148M allele (NCT04857606), completed | Not evaluated | Not evaluated | No data |
| Targeting HSCs and fibrosis | ||||
| HSC targeting | ||||
| BMS-986263 (HSC-targeted HSP47 siRNA) | Phase II trial in patients with MASH and compensated cirrhosis (NCT04267393), discontinued | Lacking efficacy (data not published) | Not published | Not reported |
| Reducing ECM stiffness | ||||
| Simtuzumab (Loxl2 inhibitor) | Phase IIb trial in patients with MASH and bridging fibrosis (NCT01672866) | 33.9% (75 mg weekly), 34.3 % (125 mg weekly) vs. 39.1% (placebo) at week 96 in patients with bridging fibrosis | Not assessed | Not reported |
| Phase IIb trial in patients with MASH and compensated cirrhosis (NCT01672879) | 11.8% (200 mg biweekly), 20.3 % (700 mg biweekly) vs. 14.7% (placebo) at week 96 in patients with compensated cirrhosis | |||
| Reducing HSC activation and contraction | ||||
| Belapectin (Galectin 3 inhibitor) |
Phase IIb trial in patients with MASH cirrhosis NCT02462967) | No difference in fibrosis or HVPG but improved HVPG in a subgroup without varices221 | Not investigated in NCT02462967 | Not reported |
| Phase IIb/III trial ongoing (NCT02421094) |
Phase IIb/III trial ongoing |
Phase IIb/3 trial ongoing |
||
| Targeting macrophages | ||||
| Blocking of chemokine signals | ||||
| Cenicriviroc (CCR2/CCR5 inhibitor) | Phase IIb in patients with MASH and F1-F3 fibrosis (NCT02217475) | 20% (150 mg daily) vs. 10.4% (placebo) at year 1 in phase 2b222 | No effect on MASH resolution | Neutral to cardiometabolic biomarkers |
| Phase III in patients with MASH and F2-F3 fibrosis (NCT03028740) | 22.3% (150 mg daily) vs. 25.5% (placebo) (placebo)223 | |||
| Cell therapy | ||||
| Autologous macrophage therapy | Phase I in cirrhosis of any aetiology and MELD score 10-16 and phase II in compensated cirrhosis with MELD score 10-17 (ISRCTN10368050) | Signal of MELD score stabilization/reduction, no fibrosis data and a non-significant reduction in MELD score42,43 | Not reported | Not reported in detail |
ALT, alanine aminotransferase; ASO, antisense oligonucleotide; BP, blood pressure; ECM, extracellular matrix; HSC, hepatic stellate cell; HVPG, hepatic venous pressure gradient; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; MELD, model for end-stage liver disease; siRNA, small-interfering RNA; TG, triglyceride.
Remarkably, there are no studies linking the level of histologic activity with fibrosis regression; even a potential link between inflammation and fibrosis progression is not well established. Moreover, the extent of inflammation per se does not correlate with outcomes in MASH, only fibrosis does.8 Similarly, there are limited data regarding regional differences in rates of fibrosis regression (e.g. septal vs. perisinusoidal). A key question is what is the “point of no return” for MASH fibrosis? The limited data available in HCV suggests that regression in HCV following cure is unlikely in the presence of elevated hepatic venous pressure gradient.197 Nonetheless, a recent study from India suggests that even some patients with decompensated cirrhosis can recompensate following HCV cure.198 It remains to be determined if similar prospects for regression apply to MASH fibrosis following effective therapies.
Which patients should be treated and for how long?
Patients with MASLD and ≥F2 fibrosis stage or a NAS ≥4 are at the highest risk of progression and hepatic decompensation, and therefore represent the group of patients who will benefit most from current therapies.12,15 Accordingly, EASL-EASD-EASO Clinical Practice Guidelines recommend that adults with non-cirrhotic MASH and ≥ stage F2 liver fibrosis should be considered for a MASH-targeted treatment with resmetirom, the first FDA-approved treatment for MASH, whereas there are no recommended MASH-targeted pharmacotherapies for the cirrhotic stage.16
Beyond the fibrosis stage and NAS, integration of additional criteria such as polygenic risk scores may further identify patients at risk.199 For example, integrating the polygenic risk score-hepatic fat content, which integrates genetic variants in patatin-like phospholipase domain-containing protein 3 (PNPLA3), TM6SF2, MBOAT7, and GCKR, can further stratify risk in patients with MASLD and may be used to identify individuals that may benefit most from therapeutic interventions.199,200 Consistent with the loss of steatosis and metabolic alteration and a predominance of ECM accumulation in cirrhosis, most current trials targeting hepatocytes and metabolism are focusing on F2-F3 stages,118,119,201,202 whereas trials of direct antifibrotics are focusing on F3-F4 stages.203 However, for MASH-associated compensated cirrhosis (i.e. F4 stage), clinical trials are increasingly moving away from histological fibrosis improvement as an endpoint and are instead focusing on non-invasive fibrosis tests (e.g. ELF), portal pressure measurement and clinical outcomes.204
For all MASLD therapies, the effects on systemic health and overall survival are a key consideration, because mortality in patients with MASLD is significantly driven by cardiovascular events.205 Recent studies have suggested that MASLD can be divided into two distinct subgroups, presenting either with an aggressive disease limited to the liver, or a more systemic disease associated with a higher risk for cardiometabolic disease.38,193 While one could speculate that these forms may require distinct treatments, (e.g. focusing on the prevention of fibrosis progression in the liver-specific form and the prevention of cardiovascular disease in the cardiometabolic form), links between MASLD, including fibrosis, and cardiovascular health are complex and further studies are warranted. Likewise, it is uncertain how long patients will require treatment for MASLD fibrosis, but most assume it will be lifelong unless disease drivers are mitigated. It can be envisioned that, after achieving fibrosis reduction, therapies could be shifted towards interventions that maintain metabolic health and thereby halt recurrent or progressive MASLD and cardiovascular disease. A recent study, in which patients with compensated liver cirrhosis were treated with FGF21 analogue efruxifermin, suggests that prolonged treatment may be required to achieve fibrosis regression in advanced disease stages.208
Will direct antifibrotics ever be successful or should metabolic pathways be the primary target?
Targeting disease-driving metabolic abnormalities in hepatocytes or the multi-organ crosstalk that regulates hepatocytes in MASLD likely represent the most efficient and straightforward therapeutic approaches for MASLD as they can reduce fibrosis by improving the underlying disease, as discussed later in this review. To date, direct antifibrotic therapies have not yet demonstrated clinical success. Notably, phase II clinical trials assessing antagonism of lysyl oxidase like 2 (LOXL2)251 or the collagen chaperone heat shock protein 47 (HSP47) in HSCs (NCT04267393) have not been successful in patients with MASH and F3-F4 fibrosis. However, rapid progress in refining therapeutic targets on HSCs, and an increasing understanding of matrix synthesis and degradation augur well for eventual success. In the following sections, the current state of direct and indirect antifibrotic therapies will be discussed. In this review, direct antifibrotics are considered drugs that target ECM-producing HSCs or ECM synthesis or degradation directly, whereas indirect antifibrotics are those that achieve fibrosis reduction via indirect mechanisms such as alterations in hepatocytes, other metabolically active tissues or macrophages, thereby altering the production or degradation of ECM by other cells.
Direct antifibrotic therapies targeting HSCs in MASH – emerging strategies
The recognition that activated HSCs are the major source of ECM in MASH has led to efforts to either deactivate these cells, clear them, or inhibit specific features to attenuate their fibrogenic activity. While no direct antifibrotics are yet approved for clinical usage, mounting preclinical evidence suggests that such an approach will be effective, especially if combined with therapies to attenuate the upstream metabolic dysregulation associated with MASH, as described in the preceding section. In this section, we review those targets and potential therapies that directly engage HSCs and are either novel, most promising or nearest to advanced clinical trials in patients with MASH. It is not intended to be an exhaustive list, but rather is representative of the diverse mechanisms of action and approaches to target HSCs in MASH.
With the recent discoveries that among activated HSCs there are functionally and genetically distinct subtypes,31,34,60,112,252,253 efforts to target an antifibrotic molecule to all activated stellate cells may not be as effective as only targeting those subsets that are clearly promoting fibrosis. This more nuanced approach has begun to take root with recent efforts to deplete only a senescent subset of HSCs (see “Cell therapies to treat fibrosis”). Still, current strategies assume that most activated HSCs share sufficient common features that make them all viable therapeutic targets, a conclusion borne out by a recent study documenting strong similarities in the activate HSC transcriptome across different aetiologies of liver disease.254
TGFβ
The cytokine TGFβ has long been recognised as the most potent signal driving fibrosis in all tissues, and remains the most important antifibrotic target in HSCs and other fibrogenic cell populations.255 However, its pleiotropic activities, multiple modes of activation and diverse signalling pathways that are cell type- or cell state-specific make it a challenging target. Moreover, systemic antagonism of TGFβ is not safe, because the inhibition of its developmental, antiproliferative, anti-apoptotic, and anti-inflammatory activities can disrupt tissue homeostasis and promote inflamamtion, autoimmunity and cancer.[255], [256], [257], [258] Therefore, TGFβ antagonists are sought that antagonise only its fibrogenic activity while preserving other functions. One strategy seeks to inhibit cell surface integrins that contribute to TGFβ activation at the cell membrane, which underlies the promise of using a small molecule (bexotegrast, PLN-74809) that blocks the activity of αvβ1 and αvβ6 in pulmonary fibrosis259 and primary sclerosing cholangitis (ClinicalTrials.gov ID NCT04480840). As noted, however, mechanisms of TGFβ activation can vary across tissues – this feature may be beneficial by restricting antagonism only to tissues of interest, or detrimental by limiting the scope of inhibition when more than one tissue is fibrotic.
An exciting new approach has leveraged the discovery that latent TGFβ is complexed with different proteins, each of which mediates different activities of the cytokine. Specifically, whereas release of latent TGFβ from either GARP (encoded by LRRC33) largely regulates its immunogenic activity,260,261 its binding to latent TGFβ binding protein (LTBP) controls its fibrogenic activity.262 With this knowledge, investigators have developed an antibody that only prevents the release of LTBP-bound TGFβ262 but does not block the release of TGFβ from GARP or LRRC33, thereby inhibiting fibrosis while preserving TGFβ’s immunoregulatory and other activities.263 As proof-of-principle, this antibody attenuates progression of renal fibrosis in two mechanistically distinct mouse models,263 but no studies in MASH models have been reported yet. However, a recent study also showed a key role for GARP on HSCs.227
Another approach to antagonising TGFβ activity distinguishes between the differential fibrogenic activities of the three major TGFβ isoforms, TGFβ1, TGFβ2 and TGFβ3. A recent study indicates that most of TGFβ’s fibrogenic activity can be ascribed to TGFβ2 and TGFβ3,264 whose antagonism avoids the liabilities of inhibiting TGFβ1; this strategy shows promise in systemic sclerosis but has not yet been explored in liver fibrosis.265
Cell therapies to treat fibrosis
With increasing knowledge about the unique features of different HSC subtypes, the prospect of deleting specific HSC populations using engineered T cells has emerged. CAR T cells were first developed to treat haematologic malignancies because the neoplasms express unique cell surface markers that are accessible within the circulation. To generate CAR T cells, DNA constructs encoding transmembrane chimeric receptors are transduced into T cells; their general structure includes an antigen binding domain on the cell surface linked to an intracellular domain that activates T cells upon ligand engagement.266 Based on the antigen binding specificity, these cytolytic T cells can, in principle, target any accessible cell that expresses the cognate receptor recognised by the CAR T cell. There has been rapid progress since the initial development of CAR T cells, both in their targeting efficiency, specificity, safety and potency, as well as the types of immune cells that can be engineered to express CARs, including regulatory T cells and macrophages.[267], [268], [269] Additionally, safety concerns – specifically, a cytokine release syndrome that may occur after CAR T cell administration – are less common through refined treatment regimens and prompt intervention.
CAR T cells are now being developed for a growing number of indications beyond haematologic malignancies, including solid tumours, autoimmunity and, most recently, senescence and fibrosis. Studies have implicated HSCs as drivers of liver injury and inflammation, leading to an effort to identify cell surface markers of senescence in this cell type to target them for CAR T-mediated clearance.
The markers and roles of HSC senescence have been debated, with some studies suggesting that they promote regeneration and limit injury,105,270 while more recent reports suggest that senescent HSCs are pro-inflammatory, pro-fibrotic and carcinogenic.[271], [272], [273] Part of the confusion may arise from varied definitions of the senescence phenotype. Efforts to establish a universal signature of senescence have been elusive, and each tissue and cell type may have a different repertoire of senescence-associated cell receptors, including HSCs.
Recently, the phenotype and ontogeny of senescent HSCs in mouse and human MASH have been extensively characterised, identifying the expression of some canonical senescence markers such as p21, p16, and β-galactosidase activity, as well as other more restricted cell surface receptors such as CD206.103,274 In this study, RNA pseudotime velocity analysis has established that senescent HSCs are derived from conventional activated HSCs,103 consistent with the idea that as injury becomes chronic, activated HSCs can progressively acquire senescent features.
A pioneering study combined the knowledge of CAR T production with senescence biology to seek markers of senescence in HSCs.108 Using an informatics-based approach, the cell surface protein urokinase plasminogen activated receptor (uPAR) was identified as one such marker, and administration of uPAR-directed CAR T cells in a murine model of MASH attenuated fibrosis, cleared senescent cells and improved serum albumin levels.108 While uPAR expression has traditionally been ascribed to macrophages and neutrophils, the receptor is indeed restricted to HSCs in early murine MASH, with macrophages also expressing this antigen as the disease advances.103 Whether uPAR is the ideal target for hepatic fibrosis treatment by CAR T cells remains to be established, but its expression on HSCs is lower and less specific than several other cell surface markers, for example CD206.103 Interestingly, there is also an ongoing trial of the natural flavonoid quercetin and kinase inhibitor dasatinib for MASH (ClinicalTrials.gov ID, NCT05506488), which, when given together, display senolytic activity in adipose tissue and improve metabolic function in old age.275
Complementary to the CAR T cell approach to clear senescent HSCs, studies by Epstein and colleagues developed engineered CAR T cells to target only cells expressing fibroblast activating protein 1 (FAP-1), which is a cell surface receptor that marks fibrogenic cells in several tissues, including the heart and joints, among others.[276], [277], [278], [279] Administration of CAR T cells that were transduced ex vivo reduced fibroblast numbers and fibrosis, and improved cardiac function in a model of chronic cardiac injury.276 These findings have established a target that does not rely on senescence and is more specific than uPAR.
A remarkably elegant strategy by the same group built upon the conventional CAR T cell approach, instead developing a method of in vivo programming of CAR T cells.280,281 To do so, mRNA designed to programme T cells into CAR T cells is delivered by lipid nanoparticles that target T cells in vivo, instructing them to express a CAR directed to FAP on the fibroblast cell surface, yielding similar therapeutic benefit in the heart as conventional CAR T cells. This in vivo methodology has at least two distinct advantages. First, therapeutic CAR T-generating nanoparticles can be produced in advance and therefore available immediately as an “off the shelf” technology, greatly expanding their availability beyond only facilities that can generate ex vivo CAR T cells onsite. Second, the use of RNA-expressing lipid nanoparticles avoids integration of genetic material into the cell genome, thereby enabling titration of CAR T cell activity and allowing for discontinuation or repeat administration, while avoiding unrestrained HSC clearance. This in vivo methodology has also been employed to target FAP-expressing cells in the liver,282 complemented by studies using FAP imaging to quantify fibrosis.283 Importantly, transient induction of anti-FAP CAR T cells significantly reduced fibrosis in MASH by depleting pro-fibrogenic HSCs.282 Moreover, anti-FAP CAR T cell therapy modulates immune cells, endothelial cells and hepatocytes in a non-cell autonomous manner, mitigating inflammation and restoring hepatic homeostasis.282
While these reports underscore the potential benefit of selectively depleting HSC populations to reduce fibrosis, their complete elimination is potentially risky. Studies in mice have demonstrated that when 90-99% of HSCs are depleted using either a cell therapy similar to CAR T cells or diphtheria toxins, the livers fail to maintain proliferation and regeneration due to the loss of paracrine signals from HSCs that support hepatocyte replication,32,48 highlighting the importance of HSCs in maintaining liver homeostasis, as discussed above. The findings indicate that selective clearance of only those HSC populations that promote fibrosis or transient depletion strategies represent more rational approaches than total HSC clearance.
Chemokine and cytokine antagonism
In addition to TGFβ, a number of other growth factors and chemokine targets are being pursued, including IL-11, CCN2, and CCL24.
A growing body of work, primarily from the laboratory of Prof. Stuart Cook in Singapore, has strongly implicated IL-11 as a target for hepatic fibrosis, including MASH. The cytokine has remarkably pleiotropic activity towards epithelial cells and mesenchymal cells across a number of tissues, including the liver, kidney and heart, among others.267,279,[284], [285], [286], [287] In the liver, antagonism or knockout of IL-11 attenuates HSC activation and also reduces steatosis and metabolic derangements within hepatocytes in MASH.288 Thus, a neutralising antibody to IL-11 has significant potential in attenuating its injurious and pro-inflammatory effects, and a phase I trial establishing its safety has been completed, the results of which are awaited (ClincalTrials.gov ID, NCT05658107).
CCL24 is a circulating chemokine produced by epithelial cells and fibroblasts, which binds to its cognate receptor C-C motif chemokine receptor (CCR)3, to promote inflammation, cell trafficking and fibrosis.289 Serum levels of CCL24 correlate with severity of fibrosis, which is especially elevated in patients with primary sclerosing cholangitis. CCL24 levels also correlate with stage of disease in systemic sclerosis.290 A monoclonal antibody to CCL24 is efficacious in several animal models of liver disease,291 prompting its evaluation in early clinical trials. A completed phase IB trial in patients with MASLD demonstrated good tolerability and improvement in several serum markers of collagen turnover and inflammation.289 These encouraging results in the liver have established the rationale for continued clinical testing in patients with MASH, and a phase IIa randomised, placebo-controlled trial is underway (ClinicalTrials.gov ID NCT05824156).
HSC-directed oligonucleotide and drug therapies
HSCs express surface markers such as PDGF receptors, retinol binding proteins, mannose-6-phosphate/insulin-like growth factor-II receptor, fibroblast growth factor receptor, integrins, FAP and Fn14 that enable HSC-directed delivery of cargo to ameliorate liver fibrosis and its consequences such as portal hypertension.[292], [293], [294], [295], [296], [297], [298], [299], [300] HSC-targeted approaches may enable selective delivery of drugs and oligonucleotides. The latter can be used to silence or activate gene expression via small-interfering RNA (siRNA), self-amplifying RNA and CRISPR-based methods. Based on promising data on HSC-selective delivery of Hsp47 siRNA via vitamin A-coupled liposomes in a rat model of cirrhosis,294 phase II clinical trials were started in patients with advanced fibrosis or compensated cirrhosis related to eradicated HCV infection or compensated cirrhosis related to MASH. Hsp47 is a collagen chaperone and its inhibition not only alters collagen expression and alignment but also promotes HSC death due to intracellular collagen misfolding.294 In patients with eradicated HCV infection and F3-F4 fibrosis (NCT03420768), BMS-986263 led to an improvement in fibrosis by ≥1 stage in 17-21% compared to 13% in the placebo group and a reduction of HSP47 mRNA in most patients in the higher dose group.203 However, the effects of BMS-986263 on target gene expression were disappointing, showing only a reduction of 5.9% in HSP47 mRNA and 10.1% in HSP47 protein levels.203 Notably, the phase II trial of BMS-986263 in patients with compensated MASH cirrhosis was terminated due to a lack of efficacy (NCT04267393). It is possible that the low target gene reduction, possibly due to suboptimal delivery to HSCs, contributed to the low efficacy. Moreover, killing activated HSCs rather than reverting them to their hepatoprotective, quiescent state might have also contributed to the insufficient therapeutic efficacy. Further clinical development of BMS-986263 is uncertain. However, other HSC-targeted delivery systems and cargo have not yet been tested clinically and may hold great promise, especially for patients in F4 fibrosis stage, if efficient delivery and/or siRNA-mediated target gene suppression can be achieved.
Cell surface proteins, matrix modulators and receptors
Belapectin is a novel complex carbohydrate that antagonises galectin-3, which has been implicated in hepatic inflammation, HSC activation and fibrosis.301,302 Galectin-3 expression increases during HSC activation but has also been associated with expression on macrophages, inflammation and steatosis303 .304 In an advanced fibrosis model in mice, belapectin was highly effective and even led to regression of cirrhosis.221 In patients with MASH cirrhosis, a phase II trial did not show benefit in liver fibrosis or portal hypertension, with the exception of the subgroup of patients without oesophageal varices at baseline, who had significant decreases in the hepatic veinous pressure gradient and fewer new varices.305 A clinical trial is underway in advanced cirrhosis. This phase IIb/III NAVIGATE study is an ongoing global, adaptive, randomised, placebo-controlled, double-blind trial in patients with portal hypertension (ClinicalTrials.gov ID NCT02421094), based on its efficacy in reducing hepatic venous pressure gradient in a subset of patients with cirrhosis.306 Belapectin’s mechanism of action in vivo is not clear, but it could involve inhibiting the vasoconstrictive activity of activated HSCs that otherwise contributes to portal hypertension.
A monoclonal antibody targeting the non-junctional domain of the claudin-1 receptor expressed on hepatocytes is potently antifibrotic in organoids, cell culture and multiple mouse models of hepatic fibrosis.307 While claudin-1 is not expressed on HSCs, the antibody’s efficacy could in part be due to abrogation of hepatocyte–HSC interactions that promote fibrosis. Ongoing clinical studies are anticipated.
Inhibition of LOXL2, an enzyme catalysing the cross linking and stabilisation of fibrillar collagen, has been considered as an attractive target for an inhibitory antibody based on very promising animal studies;308 however, a clinical trial showed no efficacy in patients with MASH and advanced fibrosis or cirrhosis.251 Despite this failure, there remains interest in small molecule inhibitors of all LOXL enzymes,309 which would overcome concerns that the therapeutic antibody was too large to reach the collagen fibrils, whereas a small molecule is not. Moreover, a recent study indicates that in pulmonary fibrosis, the dominant LOXL isotype is LOXL4,310 suggesting that a pan LOXL inhibitor that antagonises this enzyme might be more effective than inhibiting only LOXL2 in liver fibrosis.
Metabolic modulators
While most metabolic therapies for MASH primarily target steatosis and metabolic dysregulation in hepatocytes, at least four agents also have direct antifibrotic activity towards HSCs in vivo: 1) aramchol; 2) a fatty acid synthase inhibitor (denifanstat); 3) an antagonist to PNPLA3, and; 4) a structurally engineered fatty acid (icosabutate). Aramchol is an oral fatty acid-bile acid conjugate that reduces liver fat and improves insulin resistance in experimental MASH, which has led to a phase II trial showing good safety and tolerability, as well as efficacy in reducing liver fat and fibrosis in patients with MASH.311 On a cellular level, incubation of HSCs with aramchol is directly antifibrotic through inhibition of steroyl CoA desaturase-1.312 A similar inhibitory effect of denifanstat, a fatty acid synthase inhibitor, on steatosis, as well as a direct antifibrotic effect has been demonstrated in cultured HSCs,209 complementing promising phase II results in MASH.313 Prior studies have demonstrated the dependence of HSC activation on autophagic degradation of fatty acids,314 so denifanstat effectively deprives the HSCs of a source of fuel for activation. A structurally engineered fatty acid, isosabutate, is a more potent form of omega-3 fatty acid, which has been developed to target metabolic, inflammatory and fibrotic pathways in MASH.315 In immortalised human HSCs (LX-2 cells), the agent is significantly anti-proliferative.316,317
A polymorphism in the PNPLA3 gene (I148M) was the first gene variant linked to the risk of MASH. While most of the underlying biology of this disease-associated variant has been ascribed to its role in hepatocytes, it is also expressed in HSCs, where the variant gene increases fibrogenic activity.318,319 However, ongoing clinical trials testing the efficacy of PNPLA3 knockdown are focusing on hepatocyte-directed delivery, as discussed in the following sections.
Intracellular targets
A growing body of evidence, much of it from the laboratory of Dr. AM Diehl, has established hedgehog signalling as a potential antifibrotic target. This nuclear transcription factor has pleiotropic roles including in embryonic development and cancer.320 In the liver, hedgehog signalling in HSCs is pro-fibrotic through multiple pathways and transcriptional targets including Smo, Ptc, Gli1 and Gli2;[321], [322], [323], [324], [325] hedgehog signalling also promotes senescence of hepatocytes, which is pro-inflammatory.326 Crosstalk with other signalling pathways including Notch and Yap further diversifies the range of activities downstream of hedgehog signalling.323,327 In a study relevant to human MASH, a pathway of TAZ-driven secretion of Indian hedgehog by hepatocytes leads to paracrine activation of HSCs in mouse MASH.142
Despite the solid data implicating hedgehog in HSC activation and fibrosis, current clinical inhibitors are restricted to trials across a range of malignancies, especially haematologic cancers and basal cell carcinoma, as tolerability of these drugs for a non-malignant indication is not acceptable. Development of better tolerated inhibitors could refocus interest on hedgehog as a target for MASH fibrosis.328
VAP-1
Vascular adhesion protein-1 (VAP-1) is a cell surface glycoprotein molecule primarily expressed by sinusoidal endothelial cells that promotes infiltration and retention of leukocytes in the liver. In addition to promoting adhesion of leukocytes, VAP-1 has amine oxidase activity, catalysing the oxidative deamination of primary amines to generate hydrogen peroxide and aldehydes. Inhibitors of VAP-1 seek to neutralise either its adhesive and/or enzymatic activities. In addition to their effects on endothelial-leukocyte interactions, which may indirectly affect fibrosis, VAP-1 inhibitors also have direct antifibrotic activity towards HSCs.329,330 Promising results in animal studies have led to a phase I study in MASH, demonstrating adequate safety (ClinicalTrials.gov ID NCT04897594).
Nuclear receptor ligands
Whereas the sole approved drug in MASH, resmetirom, a thyroid hormone receptor-β (THRβ)-selective agonist, has no established direct antifibrotic activity, two other classes of nuclear receptors have some direct action on HSCs. Farnesoid X receptor (FXR) agonists were among the first drug types showing potential benefit in MASH, with reports of direct antifibrotic activity in isolated HSCs and mouse models through synergy with the nuclear receptor peroxisome proliferator-activated receptor (PPAR)γ.331 However, it is likely that the primary effect of FXR and PPARγ agonists on fibrosis is through their benefit in attenuating hepatocellular injury and dysregulation. Unfortunately, safety concerns about obeticholic acid, an FXR agonist, led the FDA to decline its approval for clinical usage in MASH. In contrast, a pan-PPAR agonist, lanifibranor, has shown promise based on phase II trials, with its PPARγ component potentially contributing to the reduction of fibrosis seen in this trial226,332 (ClinicalTrials.gov ID NCT03008070). Based on these promising results, a phase III trial is underway in MASH (ClinicalTrials.gov ID NCT04849728).
ASK1
While the primary activity of apoptosis signal-regulating kinase 1 (ASK1) has been focused on hepatocytes, as discussed later, additional direct antifibrotic activity is possible. Pharmacologic ASK1 inhibition can directly reduce HSC activation333,334 by suppressing profibrogenic pathways, such as p38 and JNK.335,336
Indirect antifibrotics targeting hepatocytes and metabolic pathways in MASH – current and emerging therapies
Treatment of the underlying disease usually represents the most effective therapeutic approach. Accordingly, recent clinical trials in MASLD show that targeting metabolic pathways may improve liver steatosis, injury and fibrosis, whereas others affect steatosis without significantly affecting fibrosis. The mechanisms of fibrosis reduction by these therapies have not been investigated in detail, but it is likely that an improvement of metabolic parameters, amongst others, leads to reduced hepatocyte death and less inflammation, thereby decreasing HSC activation and fibrogenesis.
THR agonists
The THR is a nuclear receptor that regulates cell growth and metabolism and exists in two subtypes. THRα plays a key role in regulating heart rate and hypertrophy.337 In contrast, THRβ is highly enriched in the liver, where it regulates hepatic lipid and carbohydrate metabolism.338 Therefore, selective THRβ agonists primarily target the liver, inducing hepatic fatty acid oxidation and reducing steatosis and hyperlipidaemia.339 Resmetirom, a THRβ-selective agonist, is the first approved by the FDA for the treatment of MASH with moderate to advanced fibrosis.206 In phase III trials, resmetirom not only reduced triglycerides, LDL cholesterol, hepatic fat and the NAS in patients with MASH but also reduced liver stiffness.117,202 A highly significant reduction of the fibrosis score by stage without worsening of the NAS was achieved in 24.2% of the patients in the 80 mg and 25.9% of those in the 100 mg resmetirom group compared with 14.2% in the placebo group. VK2809, another THRβ agonist, has shown similar effects on the liver, with a reduced liver lipid content at 12 weeks and MASH resolution with no worsening of fibrosis in 69% across different doses (vs. 29% for placebo) as well as improvement in fibrosis by ≥1-stage with no worsening of MASH in 51% of patients across doses (vs. 34% for placebo) in a recent phase IIb trial (NCT02927184).340 Thus, despite the significant effects on fibrosis, it seems that only a subset of patients experience a reduction of liver fibrosis, at least after 1 year of treatment. Further studies are needed to determine why the resolution of MASH does not translate to a reduction in fibrosis in some patients. Ongoing trials are investigating effects on patients with MASLD and compensated cirrhosis.341
FGF21 analogues
Fibroblast growth factor (FGF) 21, a hormone-like protein within the FGF superfamily, regulates glucose and lipid metabolism and energy homeostasis in the liver.201,342 FGF21 expression is induced by the ingestion of large amounts of carbohydrates and fructose, in particular, as well as alcohol and FXR activation, and exerts pleiotropic effects that collaboratively protect hepatocytes from injury. FGF21 actions include the induction of HNF4α, protection from ER stress and apoptosis.201 Furthermore, FGF21 exerts systemic effects through adipose tissue and other organs that improve MASLD, such as alterations in food intake and metabolism.201 Notably, FGF21 induces the secretion of adiponectin, an antifibrotic and anti-inflammatory adipokine,343 from adipose tissue.344 FGF21 may also exert effects on HSCs and macrophages.201,345,346 These pleiotropic activities have generated substantial interest in using FGF21 to treat MASLD and other metabolic disorders. The main limitation of using FGF21 is its short half-life of 0.5-2 h, caused by FAP-mediated cleavage,347 which has led to the development of FGF21 analogues.
FGF21 analogues, including efruxifermin, pegozafermin, and efimosfermin, are being investigated for their therapeutic potential in MASLD and liver fibrosis. Efruxifermin, an Fc-fusion FGF21 analogue, achieved an improvement in histological liver fibrosis by ≥1 stage without worsening of MASH in 39-41% of patients with MASLD and F2 or F3 fibrosis (vs. 20% for placebo) in addition to reductions in liver fat, serum ALT and ELF score in the phase IIb HARMONY trial.118 Pegozafermin, a long-acting glycopegylated FGF21 analogue, led to a ≥1 stage improvement in liver fibrosis without worsening of MASH in 22-27% of patients (vs. 7% for placebo) in a phase IIb trial of patients with MASH and F2 or F3 fibrosis.119 Efimosfermin, a long-acting IgG-FGF21 fusion protein, treatment resulted in fibrosis improvement of ≥1 stage without worsening of MASH in 45% of patients vs. 21% in the placebo group.120 The failure of pegbelfermin, a pegylated FGF21 analogue, to statistically significantly increase the number of patients achieving a ≥1 stage fibrosis improvement without worsening of MASH in the FALCON 1 and FALCON 2 trials in F3 and F4, respectively,348,349 suggest that the effects of FGF21 analogues may be stronger in patients with earlier stages of fibrosis where metabolic alterations, the primary target of FGF21, dominate. A recent study in patients with compensated liver cirrhosis (stage 4 fibrosis), suggesting an improvement in liver fibrosis without worsening of MASH at 96 weeks but not 36 weeks of efruxifermin treatment, requires further confirmation.208
FASN inhibitors
De novo lipogenesis contributes to the development of MASH.1 Fatty acid synthase (FASN) is a lipogenic enzyme with a key role in de novo lipogenesis and the production of palmitate. Accumulation of lipids and toxic lipid moieties including palmitate, in particular, triggers lipotoxicity in hepatocytes, thereby provoking immune cell infiltration, inflammation and fibrogenesis. Accordingly, hepatocyte-specific knockout of FASN improved steatosis but not ALT levels in genetic mouse models of MASLD induced by ob/ob deficiency or melanocortin-4 receptor deficiency.350 However, in some settings, hepatocyte-specific knockout exacerbated hyperglycaemia in ob/ob mice.350 The effect of FASN inhibition was first investigated in a 10-day trial in obese patients, which showed reduced de novo lipogenesis and serum ALT levels.351 In a phase IIa trial, the FASN inhibitor denifanstat (previously TVB-2640) reduced liver fat and improved metabolic, pro-inflammatory and fibrotic markers.352 In a subsequent phase IIb trial, denifanstat led to a fibrosis improvement of ≥1 stage in 41% of patients with MASLD and F2 or F3 fibrosis compared to 18% of patients in the placebo arm, while more frequently leading to an improvement of the NAS by >2 points and resolution of steatohepatitis without worsening of fibrosis compared to placebo.313
Pan-PPAR agonists
PPARs are nuclear receptors which regulate the transcription of genes related to metabolism, inflammation, and cell differentiation (see above). Three types of PPARs have been identified: PPARα, PPARβ/δ and PPARγ. PPARs are activated by free fatty acids and their derivatives. The three PPARs are expressed in multiple cells of the liver, including hepatocytes, HSCs, LSECs and Kupffer cells, as well as in adipose tissue. The key role of PPARα in hepatocyte metabolism has been reinforced by the aggravation of MASLD by hepatocyte-specific PPARα knockout.353,354 Hepatocyte-expressed PPARδ has a role in diurnal hepatic lipogenesis and peripheral fatty acid use.355 Cell-specific expression of PPARy has revealed roles in multiple liver cell types, including the negative regulation of HSC activation, the promotion of steatosis in hepatocytes and the protection from hepatic inflammation and damage via myeloid-specific expression.356,357
The wide range of effects on metabolism, inflammation and fibrosis has raised interest in utilising PPAR agonists for the treatment of MASLD. While PPARα, PPARy, PPAR-δ and dual PPAR-α and PPAR-δ agonists have shown efficacy in a wide range of metabolic diseases, as well as in primary biliary cholangitis,358,210 the pan-PPAR agonist lanifibranor appears to be the only PPAR-based therapy with substantial efficacy in patients with MASLD.332 In addition to improving MASH without worsening fibrosis and cardiometabolic health, lanifibranor led to an improvement of ≥1 fibrosis stage without worsening of MASH in 34-48% of patients compared to 29% in the placebo group, but statistical significance was not demonstrated.332,359
Incretin mimetics
Incretins are hormones that are released from the enteroendocrine cells in the ileum and colon in response to orally ingested glucose, stimulating insulin secretion more potently than after intravenous injection of the same amount of glucose. Glucose-dependent insulinotropic polypeptide (GIP), produced by intestinal K cells and glucagon-like peptide 1 (GLP-1), produced by intestinal L cells, represent the two most relevant incretins. GLP-1 is derived from proglucagon, which can either be turned into glucagon or GLP-1 in a cell type-specific manner.360 GLP-1 not only increases pancreatic insulin secretion but also reduces food intake through effects on gastrointestinal transit and the central nervous system. GIP is derived from its precursor, pro-GIP. Through its receptor, GIP potentiates glucose-induced insulin secretion. Glucagon is released from alpha cells and counteracts many effects of insulin. However, in combination with GLP-1 receptor agonism, glucagon receptor agonism exerts beneficial effects on the liver, such as increased hepatic glycogenolysis and gluconeogenesis, as well as increased fatty acid oxidation and inhibited lipogenesis without its negative insulin-agonistic effects, which appear to be absent in the presence of GLP-1 receptor agonism.360,361 Additional beneficial effects of glucagon receptor agonism, such as increased lipolysis, thermogenesis and energy expenditure, are mediated by the adipose tissue.360 Based on these findings, single GLP-1 agonists, as well as combinations of GLP-1, GIP and glucagon receptor agonists, have been investigated for metabolic diseases, including type 2 diabetes mellitus, obesity and MASLD.362
GLP-1 receptor agonists
GLP-1 receptor agonists (GLP-1RAs), including liraglutide, semaglutide, and dulaglutide have been evaluated for their antifibrotic activity in patients with MASLD. In multiple phase II trials, liraglutide, semaglutide and dulaglutide treatment for up to 72 weeks decreased body weight, hepatic steatosis and markers of liver injury in patients with MASLD and F1-F3 fibrosis, but did not lead to significant improvements in liver fibrosis of ≥1-stage without worsening of MASH.[212], [363], [211], [364] However, recent retrospective studies in patients with type 2 diabetes have reported a decrease in liver-related outcomes, including cirrhosis, hepatic decompensation and hepatocellular carcinoma with GLP-1RAs compared to other glucose-lowering drugs.[365], [366], [367], [368], [369], [213] In support of this are first data from the phase III ESSENCE trial (NCT04822181), demonstrating that semaglutide not only induces MASH resolution without worsening of fibrosis (alongside significant reductions of ALT, AST and gamma-glutamyltransferase), but also leads to a significant improvement in liver fibrosis without worsening of MASH in 37% of patients vs. 22.5% in the placebo arm based on intention-to-treat analysis.195
Dual and triple GLP-1/GIP/glucagon receptor agonists
The dual GLP-1/glucagon receptor agonist survodutide led to an improvement in MASH with no worsening of fibrosis and a decrease in liver fat content in a phase II trial in patients with MASLD and F1-F3 fibrosis.214 Notably, survodutide ameliorated fibrosis by at least one stage in 34-36% of patients without worsening of MASH compared to 22% in the placebo group.214 The dual GLP-1/glucagon receptor agonist cotadutide improved non-invasive markers of fibrogenesis, such as Pro-C3, in addition to ameliorating steatosis and serum levels of ALT and AST.370 Trials of other glucagon receptor–GLP-1 receptor dual agonists, such as SAR425899 and NNC9204-1177, have been halted due to side effects, which may be higher for drugs with high relative activation of the glucagon receptor.[371], [372], [215] The dual GLP-1/GIP receptor agonist tirzepatide achieved a ≥1 stage improvement in fibrosis in 51-55% of patients vs. 30% in the placebo group as well as MASH resolution without worsening of fibrosis.216 In a phase IIa study, the triple GLP-1/GIP/glucagon agonist retatrutide normalised liver fat content in up to 86% of patients with MASLD and significantly reduced non-invasive markers of fibrosis such as Pro-C3 but not FIB-4 or ELF.228 Besides their beneficial effect on liver fat, injury and fibrosis, single, dual and triple GLP-1/GIP receptor agonists improve cardiovascular mortality, a key determinant of outcomes in patients with MASLD, with or without type 2 diabetes.[229], [208], [373], [241], [242], [374] Furthermore, single, dual and triple GLP-1/GIP/glucagon receptor agonists appear to have a good safety profile, eliciting few adverse effects, which are mostly gastrointestinal.360
FXR agonists
FXR is a bile acid-activated nuclear transcription factor highly expressed in the liver and the ileum. FXR regulates the synthesis and enterohepatic circulation of bile acids as well as lipid and glucose metabolism. Notably, the downregulation or knockout of FXR was associated with increased steatosis and MASH development in mouse models.[375], [376], [377] Some effects of FXR on the liver, such as downregulation of bile acid synthesis, are mediated by FXR-expressing enterocytes in the ileum, which release FGF 15/19, leading to downregulation of CYP7A1 in hepatocytes via FGF receptor 4. Moreover, ileal FXR activation also leads to the release of FGF21, which affects food intake and metabolism via the central nervous system and adipose tissue. In the liver, hepatocytes and HSCs constitute the main FXR-expressing cell types. Hepatocyte and intestinal FXR appear to exert complementary functions through distinct sets of target genes.378 HSC-specific knockout of FXR has been associated with a decrease in biliary fibrosis.250 The profound phenotype of FXR knockout mice and its key functions in bile acid, cholesterol and metabolic regulation made FXR a relevant therapeutic target for MASLD. Indeed, the phase II FLINT trial demonstrated strong effects of the FXR agonist obeticholic acid (OCA) on the NAS but also an increase in LDL cholesterol in patients with non-cirrhotic MASLD.379 This lipid imbalance could be mitigated by the addition of atorvastatin, as shown in the CONTROL trial.380 In the phase III REGENERATE trial, patients with MASLD and F2-F3 fibrosis were treated with OCA for 72 weeks. The primary endpoint of fibrosis improvement without worsening of MASH was achieved in 18-23% of OCA-treated patients compared to 12% in the placebo group, but the primary endpoint of MASH resolution was not met.217,381 Thus, OCA may achieve antifibrotic effects in MASLD that are disconnected from its effect on metabolic alterations. Furthermore, more than half of patients experienced pruritus. Several other FXR agonists, mostly non-steroidal, have been investigated in animal models and clinical trials, including PX-104, cilofexor, TERN 101, tropifexor, MET-409 and vonafexor, and have shown substantial improvements in steatohepatitis and fibrosis in mice.382 However, clinical trials did not clearly demonstrate advantages over OCA, with pruritus remaining a common side effect.382
Long-chain omega-3 fatty acids
Long-chain omega-3 fatty acids are involved in multiple pathways of relevance to MASH. Icosabutate, a structurally modified omega-3 fatty acid, led to significant decreases in MASH and fibrosis biomarkers independent of fibrosis stage and disease severity in a phase IIb clinical trial (NCT04052516). Besides higher rates of MASH resolution without worsening of fibrosis, a ≥2-point decrease in the NAS and improvement in markers of liver injury, inflammation and fibrosis, icosabutate was associated with a significantly higher frequency of ≥1 stage fibrosis improvement (based on both conventional and AI-assisted histopathology) without worsening of MASH, compared to placebo.383
SGLT2 inhibitors
Sodium-glucose cotransporter-2 (SGLT2) is expressed in the human kidney, where it promotes the reabsorption of filtered glucose.384 SGLT2 inhibitors lead to urinary loss of glucose and can thereby improve type 2 diabetes mellitus and decrease complications such as cardiovascular death and stroke.385 With insulin resistance and type 2 diabetes mellitus closely associated with MASLD, SGLT2 inhibitors, including empagliflozin, dapagliflozin, canagliflozin and ertugliflozin, have been investigated in patients with MASLD in clinical trials.[224], [386], [387], [388], [389], [390] Moreover, several studies have reported a decrease in liver fibrosis with SGLT2 inhibitors in animal models of MASLD.[391], [392], [393]
In clinical trials, SGLT2 inhibitors reduced body weight, improved dyslipidaemia and decreased liver fat content and markers of liver injury, such as ALT and gamma-glutamyltransferase, while they reduced non-invasive measurements of fibrosis, including liver stiffness, FIB-4 and the NAFLD fibrosis score, in some388,389 but not all studies.387,390 Importantly, histological fibrosis was not determined in these trials. As glucose transporters and glycolytic enzymes are upregulated in HSCs during their fibrogenic activation, SGLT2 inhibitors may reduce HSC activation and fibrogenesis, although this has not been extensively experimentally demonstrated.194 In summary, the effects of SGLT2 inhibitors on fibrosis in MASLD remain to be proven.
ASK1 inhibition
ASK1 is a MAP3K (mitogen-activated kinase kinase kinase) that is activated in many cell types by oxidant and cellular stress, and by the response to injury.333 ASK1 is activated by intracellular TNFα and ER stress and it goes on to activate the P38/JNK pathway, resulting in cell death.394 Hepatocyte-specific knockout of ASK1 reduced steatosis and inflammation in a high-fat diet mouse model of MASH.395 Likewise, hepatocyte-specific knockout of Traf6 activated ASK1 in hepatocytes, increasing hepatocyte death and, subsequently, HSC activation and liver fibrosis.396 There was great enthusiasm following a positive phase II trial219 of selonsertib, a small molecule ASK-1 inhibitor, yet the findings did not hold up in a larger phase III trial in patients with advanced fibrosis,397 significantly tempering interest in further development. Still, the central role of ASK-1 in MASH fibrosis is compelling and further efforts to improve the potency and selectivity of ASK-1 inhibitors are ongoing.
ACC inhibition
Acetyl-coenzyme A carboxylase (ACC) catalyses the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting and first reaction in de novo lipogenesis.398 ACC1 and ACC2 are highly enriched in hepatocytes. Genetic silencing or pharmacologic inhibition of ACC1 and ACC2 have been shown to reduce hepatic steatosis and improve metabolic parameters.399,400 Firsocostat is a liver-directed ACC1/ACC2 inhibitor that is a substrate for the hepatic organic anion-transporting polypeptide transporter and thereby achieves high hepatic concentrations.218 However, firsocostat was not associated with a significant reduction of liver fibrosis stage without worsening of MASH or improvement of MASH without worsening of liver fibrosis in a phase II trial.401
Hepatocyte-directed oligonucleotide therapeutics
The ability to deliver specific oligonucleotides to hepatocytes, e.g. via N-acetylgalactosamine (GalNAc) conjugation, has created countless possibilities to therapeutically modulate hepatocyte gene expression for therapeutic purposes. Several therapies based on GalNAc-mediated oligonucleotide delivery to hepatocytes are already FDA approved.[402], [403], [404] Besides GalNAc, additional bioconjugations as well as lipid nanoparticles and viral vectors can be utilised for specific delivery to the liver and to hepatocytes.405 Targeting key hepatocyte pathways that contribute to the progression of MASLD and the development of liver fibrosis is an area of intense clinical and preclinical evaluation.
Hepatocyte-directed oligonucleotide therapeutics in clinical trials
Therapies based on antisense oligonucleotide (ASO)- or siRNA-mediated silencing of common drivers of MASLD, including PNPLA3, diacylglycerol acyltransferase 2 (DGAT2) and 17β-hydroxysteroid dehydrogenase 13 (HSD17B13) are currently being evaluated in clinical trials (Table 1). The PNPLA3 I148M allele is associated with MASLD development and progression to fibrosis and cirrhosis.406,407 Anti-PNPLA3 ASO-GalNAc ameliorated steatohepatitis and fibrosis in a MASLD model in Pnpla3 I148M knock-in mice.408 Several phase I and phase II trials studying the effects of siRNA against PNPLA3 (JNJ-75220795, ALN-PNP), ASOs against PNPLA3 (AZD2693) or siRNA against PNPLA3-I148M (AMG 609) in patients with MASLD carrying the PNPLA3 I148M allele have either been completed or are ongoing (NCT04844450, NCT05039710, NCT05809934, NCT056482140), with first analyses suggesting a reduction of liver fat content for JNJ-75220795.409
DGAT2 catalyses the final step in the synthesis of triglycerides from diacylglycerol and long-chain fatty acyl-CoAs.410 The key pathogenic role of triglyceride accumulation in MASLD pathogenesis, as well as the strong effects of DGAT2 ASOs on hepatic steatosis in rodent models of MASLD, support the concept of therapeutic DGAT2 silencing for MASLD treatment.[411], [412], [413] However, it should be noted that DGAT2 ASOs aggravated hepatic inflammation and fibrosis in the methionine- and choline-deficient diet model of MASLD, suggesting potentially dichotomous effects mediated by elevated free fatty acids.412 A phase II clinical trial examining the effects of ASOs against DGAT2 (ION224) in patients with MASH (NCT04932512) demonstrated an improvement in the NAS without worsening fibrosis.220 Moreover, analysis in a subcohort of patients with MASH and F3 fibrosis suggest an improvement of fibrosis in patients treated with ION224 compared to placebo (46.2% vs. 30.8%).414 HSD17B13 encodes a lipid droplet-associated protein highly expressed in hepatocytes, which is upregulated in MASLD liver samples.[415], [416], [417], [418] In mice, HSD17B13 overexpression triggers fatty liver.418 Conversely, patients with an HSD17B13 loss-of-function variant show a reduced risk of progressing to MASH and cirrhosis.419,420 Several phase I and II clinical studies of GalNAc-conjugated siRNAs (ALN-HSD and ARO-HSD) or an ASO (ION455) against HSD17B13 are currently running (NCT05519475, NCT04202354, NCT05143905 and NCT05560607). First data show lowered hepatic HSD17B13 expression and an over 40% decrease in serum ALT level in patients with MASH treated with ARO-HSD.421 In summary, clinical trials examining hepatocyte-directed oligonucleotides for PNPLA3, DGAT2 and HSD17B13 are promising, but future studies need to determine their effects on liver fibrosis in long-term clinical trials.
Experimental hepatocyte-directed oligonucleotide therapeutics
A wide range of preclinical studies have explored targets in hepatocytes for oligonucleotide-based therapies in MASLD, including STK25, MST3, ADGRF1, PCSK7, nicastrin, MCJ, AEG-1, TAZ, miR-132, miR-22 and miR-33 (reviewed in405). Among these, silencing of MST3 and TAZ inhibits MASLD-induced fibrosis in preclinical mouse models. MST3 is a kinase that localises to intracellular lipid droplets in hepatocytes, where it regulates ectopic fat accumulation.422 MST3 expression correlates with hepatic lipid content, lobular inflammation and ballooning in human MASH422 and Mst3-targeting ASOs ameliorated liver steatosis, inflammation, fibrosis, and hepatocellular damage in a mouse model of MASL, mediated by suppressed lipogenic gene expression and ACC.423 The YAP homologue TAZ was increased in MASH livers from mice and patients.142 Hepatocyte-specific TAZ silencing ameliorated hepatic inflammation, hepatocyte death, and fibrosis in mouse models of MASH, which was mediated by the repression of fibrogenic mediator Indian hedgehog.142 GalNAc-conjugated TAZ siRNA could prevent and reverse MASH fibrosis in mouse models of MASL.424
Combination therapies
Combining drugs with distinct mechanisms of action or targeting distinct cell types may improve response rates.15 Due to the lack of established antifibrotic drugs, most combinations have sought to target different disease-driving metabolic pathways.15 In the phase IIa DUET trial (NCT05415722), the combination of TERN-501, a THR-β agonist, with TERN-101, an FXR agonist, reduced both liver fat content and fibro-inflammation in non-cirrhotic MASH but was no more effective than TERN-501 monotherapy based on corrected T1-weighted imaging, as a marker of fibro-inflammation. Combinations of semaglutide, a GLP-1RA, with firsocostat, an FXR agonist, and/or cilofexor, an ACC inhibitor, resulted in further improvement in liver steatosis and serum ALT compared with semaglutide alone, but the combinations showed only trends or no differences based on several fibrosis measures.425 In the TANDEM study, the combination of tropifexor or cenicriviroc did not result in substantial improvements in ALT or fibrosis stage compared to monotherapy in patients with F2-3 fibrosis.426 In the ATLAS trial, the combination of the FXR agonist cilofexor with the ACC inhibitor firsocostat suppressed lobular inflammation and ballooning and achieved a non-significant trend toward fibrosis stage improvement.401
In summary, further studies are needed to uncover combinations that achieve a more substantial fibrosis reduction in patients with MASLD. Rather than combining drugs with different modes-of-action to alter hepatocyte metabolism, it may be more effective to combine therapies that target different cell types with key roles in MASLD, for example hepatocyte-targeted therapies combined with either direct antifibrotics or therapies that target macrophages or inflammation. Additionally, high throughput screening could unveil effective combinations , even if the mechanisms underlying their synergy are not clear.
Direct and indirect antifibrotic therapies targeting macrophages in MASLD – emerging therapies
Very similar to the fibrogenic activation of HSCs, ameliorating hepatic injury in MASLD by targeting metabolic pathways will also have indirect salutary effects on inflammatory macrophages. Nonetheless, different direct “macrophage targeting” therapies have been explored, but with no clear clinical benefit yet.223 Because pathogenic liver macrophages in MASH often originate from infiltrating monocytes, the inhibition of their recruitment is a potential therapeutic strategy to reduce liver inflammation. Chemokine receptors CCR2 and CCR5 play central roles in this recruitment process. The dual CCR2/CCR5 inhibitor cenicriviroc, which effectively reduces inflammatory monocyte recruitment to the injured liver, demonstrated promise in preclinical studies and phase II trials but failed to show significant antifibrotic efficacy in a phase III trial.[427], [428], [429] Other agents targeting monocyte recruitment, such as inhibitors of CCR2, CCL2, and CCL5, as well as VEGF-neutralising antibodies, are under development but have not yet established clinical efficacy.194 Another therapeutic approach involves modulating macrophage activation by inhibiting responses to pathogen-associated molecular patterns, DAMPs, or Toll-like receptors. As noted above, while the ASK-1 inhibitor selonsertib did not demonstrate sufficient antifibrotic effects in a large clinical trial397 next-generation ASK-1 antagonists are still undergoing evaluation. Targets such as NLRP3 inflammasome activation and ASK-1 have also been explored.183
Future therapeutic strategies may aim to shift macrophages from a pro-inflammatory to a restorative phenotype. This approach leverages the inherent plasticity of macrophages to facilitate their transition from an inflammation-inducing state to one that promotes tissue repair and exhibits direct antifibrotic properties.430 Emerging treatments targeting macrophage metabolism, such as inhibiting glycolysis or fatty acid oxidation pathways, have the potential to drive this phenotypic shift.26
A proof-of-concept study and a recent phase 2 open-label randomized controlled trial have demonstrated the safety, feasibility and potential efficacy of cell therapy using ex vivo-matured autologous monocyte-derived macrophages, delivered via peripheral infusion, in patients with cirrhosis.42,43 In preclinical models, cell therapy with alternatively activated macrophages resolved necrosis following acute liver injury, and even ameliorated fibrosis.41,225 Furthermore, the development of CAR macrophages – with the ability to mount antifibrotic T cell immunity – has been effective in reducing experimental fibrosis (see “Cell therapies in fibrosis”, above).269 These advances in macrophage reprogramming offer promising avenues for reversing liver fibrosis and supporting liver regeneration, paving the way for novel treatments for chronic liver diseases.
Summary and outlook
Deeper understanding of its pathophysiology, combined with strong collaborative efforts between the research community, regulatory agencies and industry, has led to an expanding landscape of clinical trials in MASLD. As might be expected, treatments that correct the disease-driving underlying metabolic derangements rather than late-stage sequelae, such as HSC activation and fibrosis, appear at present to be the most effective in improving MASH, including fibrosis. At the same time, FDA-approved treatments such as the THRβ agonist resmetirom and promising therapies, such as incretin mimetics, FGF21 analogues and pan-PPAR agonists, ameliorate fibrosis only in some patients and efficacy has not been demonstrated in the F4 fibrosis stage. Thus, development of effective antifibrotic drugs, as well as combination or sequential therapies, remains a high priority. Recent breakthroughs in MASLD therapies signal just the beginning of the path towards therapeutic success.
Abbreviations
ACC, acetyl-coenzyme A carboxylase; ALT, alanine aminotransferase; ASO, antisense oligonucleotide; ASK1, apoptosis signal-regulating kinase 1; AST, aspartate aminotransferase; BMP, bone morphogenic protein; CAR, chimeric antigen receptor; CCL, C–C motif chemokine ligand; CCR, C-C motif chemokine receptor; DAMPs, damage-associated molecular patterns; DGAT2, diacylglycerol acyltransferase 2; ECM, extracellular matrix; ELF, enhanced liver fibrosis; ER, endoplasmic reticulum; FAP(-1), fibroblast activating protein(-1); FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GalNAc, N-acetylgalactosamine; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; GLP-1RA, GLP-1 receptor agonists; HCC, hepatocellular carcinoma; HGF, hepatocyte growth factor; (q)HSCs, (quiescent) hepatic stellate cells; HSD17B13, 17β-hydroxysteroid dehydrogenase 13; HSP47, heat shock protein 47; IL, interleukin-; KCs, Kupffer cells; LAMs, lipid-associated macrophages; LOXL2, lysyl oxidase like 2; LSECs, liver sinusoidal endothelial cells; LTBP, latent TGFβ binding protein; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; MMPs, matrix metalloproteinases; NAS, NAFLD activity score; NTF3, neurotrophin-3; OCA, obeticholic acid; PDGF, platelet-derived growth factor; PNPLA3, patatin-like phospholipase domain-containing protein 3; PPAR, peroxisome proliferator-activated receptor; RSPO3, R-spondin 3; SAMs, scar-associated macrophages; SASP, senescence-associated secretory phenotype; SGLT2, sodium-glucose cotransporter-2; siRNA, small-interfering RNA; TAZ, TAZ, transcriptional co-activator with a PDZ-binding motif; TGF-β, transforming growth factor-β; THR, thyroid hormone receptor; TNF, tumour necrosis factor; uPAR, urokinase plasminogen activated receptor; VAP-1, vascular adhesion protein-1; YAP, Yes-associated protein.
Financial support
R.F.S. was supported by 5R01DK128955, 5R01CA262424 and The Columbia University Digestive and Liver Disease Research Center (5P30DK132710). S.F. was supported by 5R01DK128289 and 5R44DK125191. F.T. was supported by DFG projects Ta434/8-1 and CRC1382 Project-ID 403224013.
Authors’ contributions
R.F.S., F.T., A.S. and S.L.F. conceptualized, searched the literature and drafted the manuscript, R.F.S. generated figures and tables.
Conflicts of interest
R.F.S., F.T., A.S. and S.L.F. declare no conflicts of interest. F.T. reports research funding from AstraZeneca, MSD, Gilead, Agomab (fundings to his institution); consulting fees from AstraZeneca, Gilead, GSK, Abbvie, BMS, Ipsen, Pfizer, Novartis, Novo Nordisk, Madrigal, MSD, Sanofi, Boehringer; payment or honoraria from Gilead, AbbVie, Falk, Merz, Intercept, Sanofi, Astra Zeneca, Boehringer; support for attending meetings and/or travel from Gilead; participation in Advisory Boards from Sanofi, MSD and Pfizer. S.L.F has relationships with the companies listed below; however, these activities are unrelated to the content of this article: Consulting: 89 Bio, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, ChemomAb, Foresite Laboratories, Gordian Biotechnology, Glycotest, Glympse Bio, Hepgene, In sitro, Junevity, Korro Bio, Kriya, Laekna, Lerna Therapeutics, Macomics, Mediar, Merck, Morphic Therapeutics, North Sea Therapeutics, Ochre Bio, Overtone Therapeutics, Pfizer Pharmaceuticals, Pliant, Prosciento, RAPT, Sagimet, Satellite Bio, Seal Rock, Scholar Rock, Sunbird Bio, Surrozen, Takeda, Variant Bio. Stock options: Escient, Galectin, Galmed, Genfit, Gordian Biotechnology, Hepgene, Junevity, Lifemax, Metacrine, Morphic Therapeutics, North Sea, Ochre Bio, Therapeutics, Scholar Rock, and Sunbird Bio. Research Activities with Commercial Entities: Abalone Bio (SBIR Grant) and Novo Nordisk.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2025.101421.
Contributor Information
Robert F. Schwabe, Email: rfs2102@cumc.columbia.edu.
Frank Tacke, Email: frank.tacke@charite.de.
Scott L. Friedman, Email: Scott.Friedman@mssm.edu.
[Appendix A]. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Israelsen M., Francque S., Tsochatzis E.A., et al. Steatotic liver disease. Lancet. 2024;404:1761–1778. doi: 10.1016/S0140-6736(24)01811-7. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2021 US Obesity Forecasting Collaborators National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404:2278–2298. doi: 10.1016/S0140-6736(24)01548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Golabi P., Paik J.M., et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amini-Salehi E., Letafatkar N., Norouzi N., et al. Global prevalence of nonalcoholic fatty liver disease: an updated review meta-analysis comprising a population of 78 million from 38 countries. Arch Med Res. 2024;55 doi: 10.1016/j.arcmed.2024.103043. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., El-Shabrawi M., Baur L.A., et al. An international multidisciplinary consensus on pediatric metabolic dysfunction-associated fatty liver disease. Med. 2024;5:797–815 e792. doi: 10.1016/j.medj.2024.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Lee E.J., Choi M., Ahn S.B., et al. Prevalence of nonalcoholic fatty liver disease in pediatrics and adolescents: a systematic review and meta-analysis. World J Pediatr. 2024;20:569–580. doi: 10.1007/s12519-024-00814-1. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P., Kleiner D.E., Dam-Larsen S., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397 e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baratta F., Pastori D., Angelico F., et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18:2324–2331 e2324. doi: 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Dulai P.S., Singh S., Patel J., et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagstrom H., Nasr P., Ekstedt M., et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal A.J., Van Natta M.L., Clark J., et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vali Y., Lee J., Boursier J., et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8:714–725. doi: 10.1016/S2468-1253(23)00017-1. [DOI] [PubMed] [Google Scholar]
- 14.Tincopa M.A., Loomba R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol. 2023;8:660–670. doi: 10.1016/S2468-1253(23)00066-3. [DOI] [PubMed] [Google Scholar]
- 15.Tincopa M.A., Anstee Q.M., Loomba R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024;36:912–926. doi: 10.1016/j.cmet.2024.03.011. [DOI] [PubMed] [Google Scholar]
- 16.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD) J Hepatol. 2024;81:492–542. doi: 10.1016/j.jhep.2024.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal A.J., Foucquier J., Younossi Z.M., et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J Hepatol. 2023;78:247–259. doi: 10.1016/j.jhep.2022.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunt E.M., Kleiner D.E., Wilson L.A., et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdurrachim D., Lek S., Lin Ong C.Z., et al. Utility of AI digital pathology as an aid for pathologists scoring fibrosis in MASH. J Hepatol. 2025;82:898–908. doi: 10.1016/j.jhep.2024.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Ratziu V., Hompesch M., Petitjean M., et al. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: current status and future directions. J Hepatol. 2024;80:335–351. doi: 10.1016/j.jhep.2023.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison S.A., Ratziu V., Magnanensi J., et al. NIS2+, an optimisation of the blood-based biomarker NIS4(R) technology for the detection of at-risk NASH: a prospective derivation and validation study. J Hepatol. 2023;79:758–767. doi: 10.1016/j.jhep.2023.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Hudson D., Afzaal T., Bualbanat H., et al. Modernizing metabolic dysfunction-associated steatotic liver disease diagnostics: the progressive shift from liver biopsy to noninvasive techniques. Therap Adv Gastroenterol. 2024;17 doi: 10.1177/17562848241276334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiele M., Villesen I.F., Niu L., et al. Opportunities and barriers in omics-based biomarker discovery for steatotic liver diseases. J Hepatol. 2024;81:345–359. doi: 10.1016/j.jhep.2024.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Tilg H., Adolph T.E., Trauner M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700–1718. doi: 10.1016/j.cmet.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Peiseler M., Schwabe R., Hampe J., et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–1160. doi: 10.1016/j.jhep.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Horn P., Tacke F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024;36:1439–1455. doi: 10.1016/j.cmet.2024.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Lanceta A., Medrano-Bosch M., Fundora Y., et al. RNF41 orchestrates macrophage-driven fibrosis resolution and hepatic regeneration. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.abq6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace S.J., Tacke F., Schwabe R.F., et al. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf M.J., Adili A., Piotrowitz K., et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Dudek M., Pfister D., Donakonda S., et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 31.Filliol A., Saito Y., Nair A., et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature. 2022;610:356–365. doi: 10.1038/s41586-022-05289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugimoto A., Saito Y., Wang G., et al. Hepatic stellate cells control liver zonation, size and functions via R-spondin 3. Nature. 2025;640:752–761. doi: 10.1038/s41586-025-08677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulsen K.L., Cajigas-Du Ross C.K., Chaney J.K., et al. Role of the chemokine system in liver fibrosis: a narrative review. Dig Med Res. 2022;5 doi: 10.21037/dmr-21-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Li K., Pickholz E., et al. An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.add3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang D.M., Sun W., Ning B.F., et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2018;67:1704–1715. doi: 10.1136/gutjnl-2016-313392. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z., Xu M.J., Cai Y., et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5:399–413. doi: 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romeo S., Sanyal A., Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab. 2020;31:35–45. doi: 10.1016/j.cmet.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Jamialahmadi O., De Vincentis A., Tavaglione F., et al. Partitioned polygenic risk scores identify distinct types of metabolic dysfunction-associated steatotic liver disease. Nat Med. 2024;30:3614–3623. doi: 10.1038/s41591-024-03284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwabe R.F., Brenner D.A. Hepatic stellate cells: balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nat Rev Gastroenterol Hepatol. 2025 doi: 10.1038/s41575-025-01068-6. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran P., Pellicoro A., Vernon M.A., et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas J.A., Pope C., Wojtacha D., et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 42.Moroni F., Dwyer B.J., Graham C., et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019;25:1560–1565. doi: 10.1038/s41591-019-0599-8. [DOI] [PubMed] [Google Scholar]
- 43.Brennan P.N., MacMillan M., Manship T., et al. Autologous macrophage therapy for liver cirrhosis: a phase 2 open-label randomized controlled trial. Nat Med. 2025;31:979–987. doi: 10.1038/s41591-024-03406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blaner W.S., O'Byrne S.M., Wongsiriroj N., et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shmarakov I.O., Jiang H., Yang K.J., et al. Hepatic retinoid stores are required for normal liver regeneration. J Lipid Res. 2013;54:893–908. doi: 10.1194/jlr.M029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao D., Huang Z., Li X., et al. GDF2 and BMP10 coordinate liver cellular crosstalk to maintain liver health. Elife. 2024;13 doi: 10.7554/eLife.95811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen D., Jensen J.E.R., Andersen C.A.T., et al. Hepatic stellate cells regulate liver fatty acid utilization via plasmalemma vesicle-associated protein. Cell Metab. 2025;37:917–986. doi: 10.1016/j.cmet.2025.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Trinh V.Q., Lee T.F., Lemoinne S., et al. Hepatic stellate cells maintain liver homeostasis through paracrine neurotrophin-3 signaling that induces hepatocyte proliferation. Sci Signal. 2023;16 doi: 10.1126/scisignal.adf6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wake K. Hepatic stellate cells: three-dimensional structure, localization, heterogeneity and development. Proc Jpn Acad Ser B Phys Biol Sci. 2006;82:155–164. doi: 10.2183/pjab.82.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong X., Kuang H., Ansari S., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell. 2019;75:644–660 e645. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z.Y., Keogh A., Waldt A., et al. Single-cell and bulk transcriptomics of the liver reveals potential targets of NASH with fibrosis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dobie R., Wilson-Kanamori J.R., Henderson B.E.P., et al. Single-cell transcriptomics uncovers zonation of function in the mesenchyme during liver fibrosis. Cell Rep. 2019;29:1832–1847 e1838. doi: 10.1016/j.celrep.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desroches-Castan A., Tillet E., Bouvard C., et al. BMP9 and BMP10: two close vascular quiescence partners that stand out. Dev Dyn. 2022;251:178–197. doi: 10.1002/dvdy.395. [DOI] [PubMed] [Google Scholar]
- 54.Cassiman D., Denef C., Desmet V.J., et al. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- 55.Friedman S.L., Roll F.J., Boyles J., et al. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mederacke I., Hsu C.C., Troeger J.S., et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W., He H., Wang T., et al. Single-cell transcriptomic analysis reveals a hepatic stellate cell-activation roadmap and myofibroblast origin during liver fibrosis in mice. Hepatology. 2021;74:2774–2790. doi: 10.1002/hep.31987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 59.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cogliati B., Yashaswini C.N., Wang S., et al. Friend or foe? The elusive role of hepatic stellate cells in liver cancer. Nat Rev Gastroenterol Hepatol. 2023;20:647–661. doi: 10.1038/s41575-023-00821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong M.J., Gaunt P., Aithal G.P., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 63.Rockey D.C. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459–479. doi: 10.1016/j.cld.2006.08.017. vii-viii. [DOI] [PubMed] [Google Scholar]
- 64.Affo S., Filliol A., Gores G.J., et al. Fibroblasts in liver cancer: functions and therapeutic translation. Lancet Gastroenterol Hepatol. 2023;8:748–759. doi: 10.1016/S2468-1253(23)00111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H.Y., Rosenthal S.B., Liu X., et al. Multi-modal analysis of human hepatic stellate cells identifies novel therapeutic targets for metabolic dysfunction-associated steatotic liver disease. J Hepatol. 2025;82:882–897. doi: 10.1016/j.jhep.2024.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aycock R.S., Seyer J.M. Collagens of normal and cirrhotic human liver. Connect Tissue Res. 1989;23:19–31. doi: 10.3109/03008208909103901. [DOI] [PubMed] [Google Scholar]
- 67.Rojkind M., Giambrone M.A., Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–719. [PubMed] [Google Scholar]
- 68.Naba A., Clauser K.R., Whittaker C.A., et al. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer. 2014;14:518. doi: 10.1186/1471-2407-14-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karsdal M.A., Nielsen S.H., Leeming D.J., et al. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Fan W., Bradford T.M., Torok N.J. Metabolic dysfunction-associated liver disease and diabetes: matrix remodeling, fibrosis, and therapeutic implications. Ann N Y Acad Sci. 2024;1538:21–33. doi: 10.1111/nyas.15184. [DOI] [PubMed] [Google Scholar]
- 71.Sun Z., Chen G. Impact of heterogeneity in liver matrix and intrahepatic cells on the progression of hepatic fibrosis. Tissue Cell. 2024;91 doi: 10.1016/j.tice.2024.102559. [DOI] [PubMed] [Google Scholar]
- 72.Chen W., Yang A., Jia J., et al. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology. 2020;72:729–741. doi: 10.1002/hep.31236. [DOI] [PubMed] [Google Scholar]
- 73.Chen W., Sun Y., Chen S., et al. Matrisome gene-based subclassification of patients with liver fibrosis identifies clinical and molecular heterogeneities. Hepatology. 2023;78:1118–1132. doi: 10.1097/HEP.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desai S.S., Tung J.C., Zhou V.X., et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64:261–275. doi: 10.1002/hep.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishikawa T., Bell A., Brooks J.M., et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015;125:1533–1544. doi: 10.1172/JCI73137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young K.M., Reinhart-King C.A. Cellular mechanosignaling for sensing and transducing matrix rigidity. Curr Opin Cell Biol. 2023;83 doi: 10.1016/j.ceb.2023.102208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coelho N.M., McCulloch C.A. Mechanical signaling through the discoidin domain receptor 1 plays a central role in tissue fibrosis. Cell Adh Migr. 2018;12:348–362. doi: 10.1080/19336918.2018.1448353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeltz C., Kusche-Gullberg M., Heljasvaara R., et al. Novel roles for cooperating collagen receptor families in fibrotic niches. Curr Opin Cell Biol. 2023;85 doi: 10.1016/j.ceb.2023.102273. [DOI] [PubMed] [Google Scholar]
- 79.Carter J.K., Tsai M.C., Venturini N., et al. Stellate cell-specific adhesion molecule protocadherin 7 regulates sinusoidal contraction. Hepatology. 2024;80:566–577. doi: 10.1097/HEP.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taura K., De Minicis S., Seki E., et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 81.Dirscherl K., Schlapfer M., Roth Z., graggen B., et al. Hypoxia sensing by hepatic stellate cells leads to VEGF-dependent angiogenesis and may contribute to accelerated liver regeneration. Sci Rep. 2020;10:4392. doi: 10.1038/s41598-020-60709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Felli E., Nulan Y., Selicean S., et al. Emerging therapeutic targets for portal hypertension. Curr Hepatol Rep. 2023;22:51–66. doi: 10.1007/s11901-023-00598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotsiliti E., Leone V., Schuehle S., et al. Intestinal B cells license metabolic T-cell activation in NASH microbiota/antigen-independently and contribute to fibrosis by IgA-FcR signalling. J Hepatol. 2023;79:296–313. doi: 10.1016/j.jhep.2023.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carter J.K., Friedman S.L. Hepatic stellate cell-immune interactions in NASH. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.867940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khazali A.S., Clark A.M., Wells A. Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. Br J Cancer. 2018;118:566–576. doi: 10.1038/bjc.2017.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marra F., Valente A.J., Pinzani M., et al. Cultured human liver fat-storing cells produce monocyte chemotactic protein-1. Regulation by proinflammatory cytokines. J Clin Invest. 1993;92:1674–1680. doi: 10.1172/JCI116753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwabe R.F., Bataller R., Brenner D.A. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 88.Fujita T., Soontrapa K., Ito Y., et al. Hepatic stellate cells relay inflammation signaling from sinusoids to parenchyma in mouse models of immune-mediated hepatitis. Hepatology. 2016;63:1325–1339. doi: 10.1002/hep.28112. [DOI] [PubMed] [Google Scholar]
- 89.Hellerbrand C., Wang S.C., Tsukamoto H., et al. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996;24:670–676. doi: 10.1002/hep.510240333. [DOI] [PubMed] [Google Scholar]
- 90.Horst A.K., Neumann K., Diehl L., et al. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. 2016;13:277–292. doi: 10.1038/cmi.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vinas O., Bataller R., Sancho-Bru P., et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 92.Winau F., Hegasy G., Weiskirchen R., et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Ichikawa S., Mucida D., Tyznik A.J., et al. Hepatic stellate cells function as regulatory bystanders. J Immunol. 2011;186:5549–5555. doi: 10.4049/jimmunol.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schildberg F.A., Wojtalla A., Siegmund S.V., et al. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology. 2011;54:262–272. doi: 10.1002/hep.24352. [DOI] [PubMed] [Google Scholar]
- 95.Sumpter T.L., Dangi A., Matta B.M., et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189:3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charles R., Chou H.S., Wang L., et al. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation. 2013;96:17–24. doi: 10.1097/TP.0b013e318294caae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen C.H., Kuo L.M., Chang Y., et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 98.Yu M.C., Chen C.H., Liang X., et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 99.Jiang G., Yang H.R., Wang L., et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation. 2008;86:1492–1502. doi: 10.1097/TP.0b013e31818bfd13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y., Lu L., Qian S., et al. Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J Immunol. 2016;196:1617–1625. doi: 10.4049/jimmunol.1501737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dunham R.M., Thapa M., Velazquez V.M., et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou H.S., Hsieh C.C., Yang H.R., et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yashaswini C.N., Qin T., Bhattacharya D., et al. Phenotypes and ontogeny of senescent hepatic stellate cells in metabolic dysfunction-associated steatohepatitis. J Hepatol. 2024;81:207–217. doi: 10.1016/j.jhep.2024.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schnabl B., Purbeck C.A., Choi Y.H., et al. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 105.Krizhanovsky V., Yon M., Dickins R.A., et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lujambio A., Akkari L., Simon J., et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Engelmann C., Tacke F. The potential role of cellular senescence in non-alcoholic fatty liver disease. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amor C., Feucht J., Leibold J., et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iredale J.P., Benyon R.C., Pickering J., et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kisseleva T., Cong M., Paik Y., et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Troeger J.S., Mederacke I., Gwak G.Y., et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083 e1022. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rosenthal S.B., Liu X., Ganguly S., et al. Heterogeneity of HSCs in a mouse model of NASH. Hepatology. 2021;74:667–685. doi: 10.1002/hep.31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bougueon M., Legagneux V., Hazard O., et al. A rule-based multiscale model of hepatic stellate cell plasticity: critical role of the inactivation loop in fibrosis progression. PLoS Comput Biol. 2024;20 doi: 10.1371/journal.pcbi.1011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui A., Li J., Ji S., et al. The effects of B1344, a novel fibroblast growth factor 21 analog, on nonalcoholic steatohepatitis in nonhuman primates. Diabetes. 2020;69:1611–1623. doi: 10.2337/db20-0209. [DOI] [PubMed] [Google Scholar]
- 115.Kannt A., Wohlfart P., Madsen A.N., et al. Activation of thyroid hormone receptor-beta improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br J Pharmacol. 2021;178:2412–2423. doi: 10.1111/bph.15427. [DOI] [PubMed] [Google Scholar]
- 116.Tolbol K.S., Kristiansen M.N., Hansen H.H., et al. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J Gastroenterol. 2018;24:179–194. doi: 10.3748/wjg.v24.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harrison S.A., Bedossa P., Guy C.D., et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497–509. doi: 10.1056/NEJMoa2309000. [DOI] [PubMed] [Google Scholar]
- 118.Harrison S.A., Frias J.P., Neff G., et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8:1080–1093. doi: 10.1016/S2468-1253(23)00272-8. [DOI] [PubMed] [Google Scholar]
- 119.Loomba R., Sanyal A.J., Kowdley K.V., et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389:998–1008. doi: 10.1056/NEJMoa2304286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Once-monthly efimosfermin alfa (Bos580) in metabolic dysfunction- associated steatohepatitis with F2/F3 fibrosis: results from a 24 week, randomized, double-blind, placebo-controlled, phase 2 trial. Gastroenterol Hepatol (NY) 2024;20:15–16. [PMC free article] [PubMed] [Google Scholar]
- 121.Hirsova P., Gores G.J. Ballooned hepatocytes, undead cells, sonic hedgehog, and vitamin E: therapeutic implications for nonalcoholic steatohepatitis. Hepatology. 2015;61:15–17. doi: 10.1002/hep.27279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwabe R.F., Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi H., Moore M.P., Wang X., et al. Efferocytosis in liver disease. JHEP Rep. 2024;6 doi: 10.1016/j.jhepr.2023.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kondou H., Mushiake S., Etani Y., et al. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39:742–748. doi: 10.1016/s0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]
- 126.Henderson N.C., Arnold T.D., Katamura Y., et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fan W., Liu T., Chen W., et al. ECM1 prevents activation of transforming growth factor beta, hepatic stellate cells, and fibrogenesis in mice. Gastroenterology. 2019;157:1352–1367 e1313. doi: 10.1053/j.gastro.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 128.Link F., Li Y., Zhao J., et al. ECM1 attenuates hepatic fibrosis by interfering with mediators of latent TGF-beta1 activation. Gut. 2025;74:424–439. doi: 10.1136/gutjnl-2024-333213. [DOI] [PubMed] [Google Scholar]
- 129.Savage T.M., Fortson K.T., de Los Santos-Alexis K., et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity. 2024;57:303–318 e306. doi: 10.1016/j.immuni.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hirsova P., Ibrabim S.H., Gores G.J., et al. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Horn C.L., Morales A.L., Savard C., et al. Role of cholesterol-associated steatohepatitis in the development of NASH. Hepatol Commun. 2022;6:12–35. doi: 10.1002/hep4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rutkowski D.T., Wu J., Back S.H., et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soon R.K., Jr., Yan J.S., Grenert J.P., et al. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology. 2010;139:1730–1739. doi: 10.1053/j.gastro.2010.07.046. 1739 e1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Puri P., Mirshahi F., Cheung O., et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 135.Lebeaupin C., Vallee D., Hazari Y., et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 136.Kim J.Y., Garcia-Carbonell R., Yamachika S., et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell. 2018;175:133–145 e115. doi: 10.1016/j.cell.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ajoolabady A., Kaplowitz N., Lebeaupin C., et al. Endoplasmic reticulum stress in liver diseases. Hepatology. 2023;77:619–639. doi: 10.1002/hep.32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi S.S., Omenetti A., Syn W.K., et al. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guy C.D., Suzuki A., Zdanowicz M., et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rangwala F., Guy C.D., Lu J., et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mooring M., Fowl B.H., Lum S.Z.C., et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology. 2020;71:1813–1830. doi: 10.1002/hep.30928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang X., Zheng Z., Caviglia J.M., et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang X., Cai B., Yang X., et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986 e967. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu C., Kim K., Wang X., et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang J., Postigo-Fernandez J., Kim K., et al. Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis. JCI Insight. 2023;8 doi: 10.1172/jci.insight.165369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xiao Y., Batmanov K., Hu W., et al. Hepatocytes demarcated by EphB2 contribute to the progression of nonalcoholic steatohepatitis. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.adc9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Afonso M.B., David J.C., Alves M.I., et al. Intricate interplay between cell metabolism and necroptosis regulation in metabolic dysfunction-associated steatotic liver disease: a narrative review. Metabolism. 2024;158 doi: 10.1016/j.metabol.2024.155975. [DOI] [PubMed] [Google Scholar]
- 148.Thapaliya S., Wree A., Povero D., et al. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59:1197–1206. doi: 10.1007/s10620-014-3167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carlson B.A., Tobe R., Yefremova E., et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 2016;9:22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Coillie S., Van San E., Goetschalckx I., et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat Commun. 2022;13:1046. doi: 10.1038/s41467-022-28718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li L., Zhu Z. Pharmacological modulation of ferroptosis as a therapeutic target for liver fibrosis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peleman C., Francque S., Berghe T.V. Emerging role of ferroptosis in metabolic dysfunction-associated steatotic liver disease: revisiting hepatic lipid peroxidation. EBioMedicine. 2024;102 doi: 10.1016/j.ebiom.2024.105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peleman C., Hellemans S., Veeckmans G., et al. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 2024;31:1113–1126. doi: 10.1038/s41418-024-01348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsurusaki S., Tsuchiya Y., Koumura T., et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. doi: 10.1038/s41419-019-1678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hinman A., Holst C.R., Latham J.C., et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sanyal A.J., Chalasani N., Kowdley K.V., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783 e764. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ge X., Arriazu E., Magdaleno F., et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology. 2018;68:2380–2404. doi: 10.1002/hep.30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mederacke I., Filliol A., Affo S., et al. The purinergic P2Y14 receptor links hepatocyte death to hepatic stellate cell activation and fibrogenesis in the liver. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abe5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guilliams M., Scott C.L. Liver macrophages in health and disease. Immunity. 2022;55:1515–1529. doi: 10.1016/j.immuni.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 163.Duffield J.S., Forbes S.J., Constandinou C.M., et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramachandran P., Iredale J.P. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56:1417–1419. doi: 10.1016/j.jhep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 165.Horn P., Tacke F. Liver macrophage diversity in health and disease. Results Probl Cell Differ. 2024;74:175–209. doi: 10.1007/978-3-031-65944-7_7. [DOI] [PubMed] [Google Scholar]
- 166.Guillot A., Winkler M., Silva Afonso M., et al. Mapping the hepatic immune landscape identifies monocytic macrophages as key drivers of steatohepatitis and cholangiopathy progression. Hepatology. 2023;78:150–166. doi: 10.1097/HEP.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 167.Matchett K.P., Paris J., Teichmann S.A., et al. Spatial genomics: mapping human steatotic liver disease. Nat Rev Gastroenterol Hepatol. 2024;21:646–660. doi: 10.1038/s41575-024-00915-2. [DOI] [PubMed] [Google Scholar]
- 168.Guillot A., Tacke F. Liver macrophages revisited: the expanding universe of versatile responses in a spatiotemporal context. Hepatol Commun. 2024;8 doi: 10.1097/HC9.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Simon-Codina B., Cacho-Pujol J., Moles A., et al. Reprogramming macrophages to treat liver diseases. Hepatology. 2024 doi: 10.1097/HEP.0000000000001160. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 170.Bleriot C., Barreby E., Dunsmore G., et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101–2116 e2106. doi: 10.1016/j.immuni.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 171.Remmerie A., Martens L., Thone T., et al. Osteopontin expression identifies a subset of recruited macrophages distinct from kupffer cells in the fatty liver. Immunity. 2020;53:641–657 e614. doi: 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seidman J.S., Troutman T.D., Sakai M., et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52:1057–1074 e1057. doi: 10.1016/j.immuni.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jaitin D.A., Adlung L., Thaiss C.A., et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178:686–698 e614. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hendrikx T., Porsch F., Kiss M.G., et al. Soluble TREM2 levels reflect the recruitment and expansion of TREM2(+) macrophages that localize to fibrotic areas and limit NASH. J Hepatol. 2022;77:1373–1385. doi: 10.1016/j.jhep.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 175.Ganguly S., Rosenthal S.B., Ishizuka K., et al. Lipid-associated macrophages' promotion of fibrosis resolution during MASH regression requires TREM2. Proc Natl Acad Sci U S A. 2024;121 doi: 10.1073/pnas.2405746121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Ponti F.F., Bujko A., Liu Z., et al. Spatially restricted and ontogenically distinct hepatic macrophages are required for tissue repair. Immunity. 2025;58:362–380 e310. doi: 10.1016/j.immuni.2025.01.002. [DOI] [PubMed] [Google Scholar]
- 177.Guo W., Li Z., Anagnostopoulos G., et al. Notch signaling regulates macrophage-mediated inflammation in metabolic dysfunction-associated steatotic liver disease. Immunity. 2024;57:2310–2327 e2316. doi: 10.1016/j.immuni.2024.08.016. [DOI] [PubMed] [Google Scholar]
- 178.Peiseler M., Araujo David B., Zindel J., et al. Kupffer cell-like syncytia replenish resident macrophage function in the fibrotic liver. Science. 2023;381 doi: 10.1126/science.abq5202. [DOI] [PubMed] [Google Scholar]
- 179.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guilliams M., Bonnardel J., Haest B., et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396 e338. doi: 10.1016/j.cell.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rivera C.A., Bradford B.U., Hunt K.J., et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 182.Seki E., De Minicis S., Osterreicher C.H., et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 183.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 184.Cai B., Dongiovanni P., Corey K.E., et al. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2020;31:406–421 e407. doi: 10.1016/j.cmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ezzo M., Spindler K., Wang J.B., et al. Acute contact with profibrotic macrophages mechanically activates fibroblasts via alphavbeta3 integrin-mediated engagement of Piezo1. Sci Adv. 2024;10:eadp4726. doi: 10.1126/sciadv.adp4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pradere J.P., Kluwe J., De Minicis S., et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Malehmir M., Pfister D., Gallage S., et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PubMed] [Google Scholar]
- 188.Fallowfield J.A., Mizuno M., Kendall T.J., et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 189.Adhyatmika A., Putri K.S., Beljaars L., et al. The elusive antifibrotic macrophage. Front Med (Lausanne) 2015;2:81. doi: 10.3389/fmed.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Feng D., Xiang X., Guan Y., et al. Monocyte-derived macrophages orchestrate multiple cell-type interactions to repair necrotic liver lesions in disease models. J Clin Invest. 2023;133 doi: 10.1172/JCI166954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rinella M.E., Lieu H.D., Kowdley K.V., et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology. 2024;79:674–689. doi: 10.1097/HEP.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Starkey Lewis P.J., Moroni F., Forbes S.J. Macrophages as a cell-based therapy for liver disease. Semin Liver Dis. 2019;39:442–451. doi: 10.1055/s-0039-1688502. [DOI] [PubMed] [Google Scholar]
- 193.Raverdy V., Tavaglione F., Chatelain E., et al. Data-driven cluster analysis identifies distinct types of metabolic dysfunction-associated steatotic liver disease. Nat Med. 2024;30:3624–3633. doi: 10.1038/s41591-024-03283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tacke F., Puengel T., Loomba R., et al. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. 2023;79:552–566. doi: 10.1016/j.jhep.2023.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Newsome P., Sanyal A., Kliers I., et al. American Association for the Study of Liver Diseases; 2024. Semaglutide in metabolic dysfunction-associated steatohepatitis (MASH]. The Liver Meeting®. San Diego; 2024. [Google Scholar]
- 196.Rinella M.E., Tacke F., Sanyal A.J., et al. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71:823–833. doi: 10.1016/j.jhep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 197.Mauro E., Crespo G., Montironi C., et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683–1694. doi: 10.1002/hep.29557. [DOI] [PubMed] [Google Scholar]
- 198.Premkumar M., Dhiman R.K., Duseja A., et al. Recompensation of chronic hepatitis C-related decompensated cirrhosis following direct-acting antiviral therapy: prospective cohort study from a hepatitis C virus elimination program. Gastroenterology. 2024;167:1429–1445. doi: 10.1053/j.gastro.2024.08.018. [DOI] [PubMed] [Google Scholar]
- 199.Moretti V., Romeo S., Valenti L. The contribution of genetics and epigenetics to MAFLD susceptibility. Hepatol Int. 2024;18:848–860. doi: 10.1007/s12072-024-10667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Vincentis A., Tavaglione F., Jamialahmadi O., et al. A polygenic risk score to refine risk stratification and prediction for severe liver disease by clinical fibrosis scores. Clin Gastroenterol Hepatol. 2022;20:658–673. doi: 10.1016/j.cgh.2021.05.056. [DOI] [PubMed] [Google Scholar]
- 201.Harrison S.A., Rolph T., Knot M., et al. FGF21 agonists: an emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J Hepatol. 2024;81:562–576. doi: 10.1016/j.jhep.2024.04.034. [DOI] [PubMed] [Google Scholar]
- 202.Harrison S.A., Taub R., Neff G.W., et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919–2928. doi: 10.1038/s41591-023-02603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lawitz E.J., Shevell D.E., Tirucherai G.S., et al. BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial. Hepatology. 2022;75:912–923. doi: 10.1002/hep.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pericas J.M., Anstee Q.M., Augustin S., et al. A roadmap for clinical trials in MASH-related compensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2024;21:809–823. doi: 10.1038/s41575-024-00955-8. [DOI] [PubMed] [Google Scholar]
- 205.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 206.Alshehade S.A. Resmetirom's approval: highlighting the need for comprehensive approaches in NASH therapeutics. Clin Res Hepatol Gastroenterol. 2024;48 doi: 10.1016/j.clinre.2024.102377. [DOI] [PubMed] [Google Scholar]
- 207.Harrison S.A., Abdelmalek M.F., Neff G., et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2022;7:603–616. doi: 10.1016/S2468-1253(22)00017-6. [DOI] [PubMed] [Google Scholar]
- 208.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 209.O'Farrell M., Duke G., Crowley R., et al. FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci Rep. 2022;12 doi: 10.1038/s41598-022-19459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kowdley K.V., Bowlus C.L., Levy C., et al. Efficacy and safety of elafibranor in primary biliary cholangitis. N Engl J Med. 2024;390:795–805. doi: 10.1056/NEJMoa2306185. [DOI] [PubMed] [Google Scholar]
- 211.Loomba R., Abdelmalek M.F., Armstrong M.J., et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–522. doi: 10.1016/S2468-1253(23)00068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khoo J., Hsiang J.C., Taneja R., et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019;39:941–949. doi: 10.1111/liv.14065. [DOI] [PubMed] [Google Scholar]
- 213.Yang C.T., Yao W.Y., Yang C.Y., et al. Lower risks of cirrhosis and hepatocellular carcinoma with GLP-1RAs in type 2 diabetes: a nationwide cohort study using target trial emulation framework. J Intern Med. 2024;295:357–368. doi: 10.1111/joim.13751. [DOI] [PubMed] [Google Scholar]
- 214.Sanyal A.J., Bedossa P., Fraessdorf M., et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N Engl J Med. 2024;391:311–319. doi: 10.1056/NEJMoa2401755. [DOI] [PubMed] [Google Scholar]
- 215.Adams B. GI toxicity hits midstage Sanofi GLP-1 drug as patients drop out. 2018. https://www.fiercebiotech.com/biotech/gi-toxicity-hits-midstage-poc-sanofi-glp-1-drug-as-patients-drop-out cited; Available from:
- 216.Loomba R., Hartman M.L., Lawitz E.J., et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391:299–310. doi: 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 217.Younossi Z.M., Ratziu V., Loomba R., et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 218.Alkhouri N., Lawitz E., Noureddin M., et al. GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH) Expert Opin Investig Drugs. 2020;29:135–141. doi: 10.1080/13543784.2020.1668374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Loomba R., Lawitz E., Mantry P.S., et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology. 2018;67:549–559. doi: 10.1002/hep.29514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ionis, Pharmaceuticals Ionis announces positive results from Phase 2 study of ION224, an investigational medicine demonstrating clinical efficacy in the treatment of NASH/MASH. 2024. https://ir.ionispharma.com/news-releases/news-release-details/ionis-announces-positive-results-phase-2-study-ion224 cited; Available from:
- 221.Traber P.G., Chou H., Zomer E., et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PloS one. 2013;8 doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Friedman S.L., Ratziu V., Harrison S.A., et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vonderlin J., Chavakis T., Sieweke M., et al. The multifaceted roles of macrophages in NAFLD pathogenesis. Cell Mol Gastroenterol Hepatol. 2023;15:1311–1324. doi: 10.1016/j.jcmgh.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cusi K., Bril F., Barb D., et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:812–821. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 225.Starkey Lewis P., Campana L., Aleksieva N., et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J Hepatol. 2020;73:349–360. doi: 10.1016/j.jhep.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Francque S., Szabo G., Abdelmalek M.F., et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 227.Gilbert M.P., Bain S.C., Franek E., et al. Effect of liraglutide on cardiovascular outcomes in elderly patients: a post hoc analysis of a randomized controlled trial. Ann Intern Med. 2019;170:423–426. doi: 10.7326/M18-1569. [DOI] [PubMed] [Google Scholar]
- 228.Sanyal A.J., Kaplan L.M., Frias J.P., et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30:2037–2048. doi: 10.1038/s41591-024-03018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 230.Kosiborod M.N., Abildstrom S.Z., Borlaug B.A., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 231.Kosiborod M.N., Petrie M.C., Borlaug B.A., et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med. 2024;390:1394–1407. doi: 10.1056/NEJMoa2313917. [DOI] [PubMed] [Google Scholar]
- 232.Ryan D.H., Lingvay I., Deanfield J., et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat Med. 2024;30:2049–2057. doi: 10.1038/s41591-024-02996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Borlaug B.A., Kitzman D.W., Davies M.J., et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med. 2023;29:2358–2365. doi: 10.1038/s41591-023-02526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Husain M., Birkenfeld A.L., Donsmark M., et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 235.Wilding J.P.H., Batterham R.L., Calanna S., et al. Once-Weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 236.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 237.Kosiborod M.N., Platz E., Wharton S., et al. Survodutide for the treatment of obesity: rationale and design of the SYNCHRONIZE cardiovascular outcomes trial. JACC Heart Fail. 2024;12:2101–2109. doi: 10.1016/j.jchf.2024.09.004. [DOI] [PubMed] [Google Scholar]
- 238.Wharton S., le Roux C.W., Kosiborod M.N., et al. Survodutide for treatment of obesity: rationale and design of two randomized phase 3 clinical trials (SYNCHRONIZE-1 and -2) Obesity (Silver Spring) 2025;33:67–77. doi: 10.1002/oby.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ambery P., Parker V.E., Stumvoll M., et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607–2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
- 240.Nahra R., Wang T., Gadde K.M., et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care. 2021;44:1433–1442. doi: 10.2337/dc20-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kosiborod M.N., Deanfield J., Pratley R., et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet. 2024;404:949–961. doi: 10.1016/S0140-6736(24)01643-X. [DOI] [PubMed] [Google Scholar]
- 242.Packer M., Zile M.R., Kramer C.M., et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. 2025;392:427–437. doi: 10.1056/NEJMoa2410027. [DOI] [PubMed] [Google Scholar]
- 243.Borlaug B.A., Zile M.R., Kramer C.M., et al. Effects of tirzepatide on circulatory overload and end-organ damage in heart failure with preserved ejection fraction and obesity: a secondary analysis of the SUMMIT trial. Nat Med. 2024 doi: 10.1038/s41591-024-03374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Del Prato S., Kahn S.E., Pavo I., et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 245.Garvey W.T., Frias J.P., Jastreboff A.M., et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613–626. doi: 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- 246.Jastreboff A.M., le Roux C.W., Stefanski A., et al. Tirzepatide for obesity treatment and diabetes prevention. N Engl J Med. 2024 doi: 10.1056/NEJMoa2410819. [DOI] [PubMed] [Google Scholar]
- 247.Jastreboff A.M., Kaplan L.M., Frias J.P., et al. Triple-hormone-Receptor agonist retatrutide for obesity - a phase 2 trial. N Engl J Med. 2023;389:514–526. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 248.Rosenstock J., Frias J., Jastreboff A.M., et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402:529–544. doi: 10.1016/S0140-6736(23)01053-X. [DOI] [PubMed] [Google Scholar]
- 249.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 250.Garrido A., Kim E., Teijeiro A., et al. Histone acetylation of bile acid transporter genes plays a critical role in cirrhosis. J Hepatol. 2022;76:850–861. doi: 10.1016/j.jhep.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Harrison S.A., Abdelmalek M.F., Caldwell S., et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 252.Yamagata K., Takasuga S., Tatematsu M., et al. FoxD1 expression identifies a distinct subset of hepatic stellate cells involved in liver fibrosis. Biochem Biophys Res Commun. 2024;734 doi: 10.1016/j.bbrc.2024.150632. [DOI] [PubMed] [Google Scholar]
- 253.Khan M.A., Fischer J., Harrer L., et al. Hepatic stellate cells in zone 1 engage in capillarization rather than myofibroblast formation in murine liver fibrosis. Sci Rep. 2024;14 doi: 10.1038/s41598-024-69898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Merens V., Knetemann E., Gürbüz E., et al. Hepatic stellate cell single cell atlas reveals a highly similar activation process across liver disease aetiologies. J Hep Reports. 2024;7:101223. doi: 10.1016/j.jhepr.2024.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Massague J., Sheppard D. TGF-beta signaling in health and disease. Cell. 2023;186:4007–4037. doi: 10.1016/j.cell.2023.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Anderton M.J., Mellor H.R., Bell A., et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol. 2011;39:916–924. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 257.Danielpour D. Advances and challenges in targeting TGF-beta isoforms for therapeutic intervention of cancer: a mechanism-based perspective. Pharmaceuticals (Basel) 2024;17 doi: 10.3390/ph17040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dickson M.C., Martin J.S., Cousins F.M., et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 259.Lancaster L., Cottin V., Ramaswamy M., et al. Bexotegrast in patients with idiopathic pulmonary fibrosis: the INTEGRIS-IPF clinical trial. Am J Respir Crit Care Med. 2024;210:424–434. doi: 10.1164/rccm.202403-0636OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lienart S., Merceron R., Vanderaa C., et al. Structural basis of latent TGF-beta1 presentation and activation by GARP on human regulatory T cells. Science. 2018;362:952–956. doi: 10.1126/science.aau2909. [DOI] [PubMed] [Google Scholar]
- 261.Duan Z., Lin X., Wang L., et al. Specificity of TGF-beta1 signal designated by LRRC33 and integrin alpha(V)beta(8) Nat Commun. 2022;13:4988. doi: 10.1038/s41467-022-32655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lack J., O'Leary J.M., Knott V., et al. Solution structure of the third TB domain from LTBP1 provides insight into assembly of the large latent complex that sequesters latent TGF-beta. J Mol Biol. 2003;334:281–291. doi: 10.1016/j.jmb.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 263.Jackson J.W., Frederick C.S., Jr., Pal A., et al. An antibody that inhibits TGF-beta1 release from latent extracellular matrix complexes attenuates the progression of renal fibrosis. Sci Signal. 2024;17 doi: 10.1126/scisignal.adn6052. [DOI] [PubMed] [Google Scholar]
- 264.Sun T., Huang Z., Liang W.C., et al. TGFbeta2 and TGFbeta3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abe0407. [DOI] [PubMed] [Google Scholar]
- 265.Sun T., Vander Heiden J.A., Gao X., et al. Isoform-selective TGF-beta3 inhibition for systemic sclerosis. Med. 2024;5:132–147 e137. doi: 10.1016/j.medj.2023.12.011. [DOI] [PubMed] [Google Scholar]
- 266.Hoydahl L.S., Berntzen G., Loset G.A. Engineering T-cell receptor-like antibodies for biologics and cell therapy. Curr Opin Biotechnol. 2024;90 doi: 10.1016/j.copbio.2024.103224. [DOI] [PubMed] [Google Scholar]
- 267.Baker D.J., Arany Z., Baur J.A., et al. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023;619:707–715. doi: 10.1038/s41586-023-06243-w. [DOI] [PubMed] [Google Scholar]
- 268.Posey A.D., Jr., Young R.M., June C.H. Future perspectives on engineered T cells for cancer. Trends Cancer. 2024;10:687–695. doi: 10.1016/j.trecan.2024.05.007. [DOI] [PubMed] [Google Scholar]
- 269.Dai H., Zhu C., Huai Q., et al. Chimeric antigen receptor-modified macrophages ameliorate liver fibrosis in preclinical models. J Hepatol. 2024;80:913–927. doi: 10.1016/j.jhep.2024.01.034. [DOI] [PubMed] [Google Scholar]
- 270.Cheng N., Kim K.H., Lau L.F. Senescent hepatic stellate cells promote liver regeneration through IL-6 and ligands of CXCR2. JCI Insight. 2022;7 doi: 10.1172/jci.insight.158207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Du K., Maeso-Diaz R., Oh S.H., et al. Targeting YAP-mediated HSC death susceptibility and senescence for treatment of liver fibrosis. Hepatology. 2023;77:1998–2015. doi: 10.1097/HEP.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yamagishi R., Kamachi F., Nakamura M., et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci Immunol. 2022;7 doi: 10.1126/sciimmunol.abl7209. [DOI] [PubMed] [Google Scholar]
- 273.Wu Z., Xia M., Wang J., et al. Extracellular vesicles originating from steatotic hepatocytes promote hepatic stellate cell senescence via AKT/mTOR signaling. Cell Biochem Funct. 2024;42 doi: 10.1002/cbf.4077. [DOI] [PubMed] [Google Scholar]
- 274.Zhang M., Serna-Salas S., Damba T., et al. Hepatic stellate cell senescence in liver fibrosis: characteristics, mechanisms and perspectives. Mech Ageing Dev. 2021;199 doi: 10.1016/j.mad.2021.111572. [DOI] [PubMed] [Google Scholar]
- 275.Islam M.T., Tuday E., Allen S., et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell. 2023;22 doi: 10.1111/acel.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Aghajanian H., Kimura T., Rurik J.G., et al. Author Correction: targeting cardiac fibrosis with engineered T cells. Nature. 2019;576 doi: 10.1038/s41586-019-1761-7. [DOI] [PubMed] [Google Scholar]
- 277.Amrute J.M., Luo X., Penna V., et al. Targeting immune-fibroblast cell communication in heart failure. Nature. 2024;635:423–433. doi: 10.1038/s41586-024-08008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhao X., Lin J., Liu M., et al. Targeting FAP-positive chondrocytes in osteoarthritis: a novel lipid nanoparticle siRNA approach to mitigate cartilage degeneration. J Nanobiotechnology. 2024;22:659. doi: 10.1186/s12951-024-02946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Basalova N., Alexandrushkina N., Grigorieva O., et al. Fibroblast activation protein alpha (FAPalpha) in fibrosis: beyond a perspective marker for activated stromal cells? Biomolecules. 2023;13 doi: 10.3390/biom13121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rurik J.G., Tombacz I., Yadegari A., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Friedman S.L. Fighting cardiac fibrosis with CAR T cells. N Engl J Med. 2022;386:1576–1578. doi: 10.1056/NEJMcibr2201182. [DOI] [PubMed] [Google Scholar]
- 282.Yashaswini C.N., Cogliati B., Qin T., et al. In vivo anti-FAP CAR T therapy reduces fibrosis and restores liver homeostasis in metabolic dysfunction-associated steatohepatitis. bioRxiv. 2025 2025.02.25.640143. [Google Scholar]
- 283.Eriksson O., Velikyan I. Radiotracers for imaging of fibrosis: advances during the last two decades and future directions. Pharmaceuticals (Basel) 2023;16 doi: 10.3390/ph16111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cook S.A. The pathobiology of interleukin 11 in mammalian disease is likely explained by its essential evolutionary role for fin regeneration. J Cardiovasc Transl Res. 2023;16:755–757. doi: 10.1007/s12265-022-10351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cook S.A. Understanding interleukin 11 as a disease gene and therapeutic target. Biochem J. 2023;480:1987–2008. doi: 10.1042/BCJ20220160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Widjaja A.A., Viswanathan S., Shekeran S.G., et al. Targeting endogenous kidney regeneration using anti-IL11 therapy in acute and chronic models of kidney disease. Nat Commun. 2022;13:7497. doi: 10.1038/s41467-022-35306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Widjaja A.A., Chothani S., Viswanathan S., et al. IL11 stimulates IL33 expression and proinflammatory fibroblast activation across tissues. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23168900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Widjaja A.A., Singh B.K., Adami E., et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology. 2019;157:777–792. doi: 10.1053/j.gastro.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 289.Mor A., Friedman S., Hashmueli S., et al. Targeting CCL24 in inflammatory and fibrotic diseases: rationale and results from three CM-101 phase 1 studies. Drug Saf. 2024;47:869–881. doi: 10.1007/s40264-024-01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.De Lorenzis E., Mor A., Ross R.L., et al. Serum CCL24 as a biomarker of fibrotic and vascular disease severity in systemic sclerosis. Arthritis Care Res (Hoboken) 2024;76:1269–1277. doi: 10.1002/acr.25344. [DOI] [PubMed] [Google Scholar]
- 291.Segal-Salto M., Barashi N., Katav A., et al. A blocking monoclonal antibody to CCL24 alleviates liver fibrosis and inflammation in experimental models of liver damage. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2019.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bansal R., Prakash J., Post E., et al. Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology. 2011;54:586–596. doi: 10.1002/hep.24395. [DOI] [PubMed] [Google Scholar]
- 293.Bansal R., Poelstra K. Hepatic stellate cell targeting using peptide-modified biologicals. Methods Mol Biol. 2023;2669:269–284. doi: 10.1007/978-1-0716-3207-9_17. [DOI] [PubMed] [Google Scholar]
- 294.Sato Y., Murase K., Kato J., et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 295.Klein S., Van Beuge M.M., Granzow M., et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 296.Lee J., Byun J., Shim G., et al. Fibroblast activation protein activated antifibrotic peptide delivery attenuates fibrosis in mouse models of liver fibrosis. Nat Commun. 2022;13:1516. doi: 10.1038/s41467-022-29186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Moreno M., Gonzalo T., Kok R.J., et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942–952. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- 298.Huang L., Xie J., Bi Q., et al. Highly selective targeting of hepatic stellate cells for liver fibrosis treatment using a d-enantiomeric peptide ligand of Fn14 identified by mirror-image mRNA display. Mol Pharm. 2017;14:1742–1753. doi: 10.1021/acs.molpharmaceut.6b01174. [DOI] [PubMed] [Google Scholar]
- 299.Kurniawan D.W., Booijink R., Pater L., et al. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J Control Release. 2020;328:640–652. doi: 10.1016/j.jconrel.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 300.Schnittert J., Bansal R., Storm G., et al. Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv Drug Deliv Rev. 2018;129:37–53. doi: 10.1016/j.addr.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 301.Henderson N.C., Mackinnon A.C., Farnworth S.L., et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Henderson N.C., Mackinnon A.C., Farnworth S.L., et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dragomir A.C., Sun R., Choi H., et al. Role of galectin-3 in classical and alternative macrophage activation in the liver following acetaminophen intoxication. J Immunol. 2012;189:5934–5941. doi: 10.4049/jimmunol.1201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–1345 e1335. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 306.Al Attar A., Antaramian A., Noureddin M. Review of galectin-3 inhibitors in the treatment of nonalcoholic steatohepatitis. Expert Rev Clin Pharmacol. 2021;14:457–464. doi: 10.1080/17512433.2021.1894127. [DOI] [PubMed] [Google Scholar]
- 307.Roehlen N., Saviano A., El Saghire H., et al. A monoclonal antibody targeting nonjunctional claudin-1 inhibits fibrosis in patient-derived models by modulating cell plasticity. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abj4221. [DOI] [PubMed] [Google Scholar]
- 308.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chitty J.L., Yam M., Perryman L., et al. A first-in-class pan-lysyl oxidase inhibitor impairs stromal remodeling and enhances gemcitabine response and survival in pancreatic cancer. Nat Cancer. 2023;4:1326–1344. doi: 10.1038/s43018-023-00614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ma H.Y., Li Q., Wong W.R., et al. LOXL4, but not LOXL2, is the critical determinant of pathological collagen cross-linking and fibrosis in the lung. Sci Adv. 2023;9 doi: 10.1126/sciadv.adf0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ratziu V., Yilmaz Y., Lazas D., et al. Aramchol improves hepatic fibrosis in metabolic dysfunction-associated steatohepatitis: results of multimodality assessment using both conventional and digital pathology. Hepatology. 2025;81:932–946. doi: 10.1097/HEP.0000000000000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bhattacharya D., Basta B., Mato J.M., et al. Aramchol downregulates stearoyl CoA-desaturase 1 in hepatic stellate cells to attenuate cellular fibrogenesis. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Loomba R., Bedossa P., Grimmer K., et al. Denifanstat for the treatment of metabolic dysfunction-associated steatohepatitis: a multicentre, double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2024;9:1090–1100. doi: 10.1016/S2468-1253(24)00246-2. [DOI] [PubMed] [Google Scholar]
- 314.Hernandez-Gea V., Ghiassi-Nejad Z., Rozenfeld R., et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Fraser D.A., Harrison S.A., Schuppan D. Icosabutate: targeting metabolic and inflammatory pathways for the treatment of NASH. Expert Opin Investig Drugs. 2022;31:1269–1278. doi: 10.1080/13543784.2022.2159804. [DOI] [PubMed] [Google Scholar]
- 316.Stokman G., van den Hoek A.M., Denker Thorbekk D., et al. Dual targeting of hepatic fibrosis and atherogenesis by icosabutate, an engineered eicosapentaenoic acid derivative. Liver Int. 2020;40:2860–2876. doi: 10.1111/liv.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.van den Hoek A.M., Pieterman E.J., van der Hoorn J.W., et al. Icosabutate exerts beneficial effects upon insulin sensitivity, hepatic inflammation, lipotoxicity, and fibrosis in mice. Hepatol Commun. 2020;4:193–207. doi: 10.1002/hep4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bruschi F.V., Claudel T., Tardelli M., et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology. 2017;65:1875–1890. doi: 10.1002/hep.29041. [DOI] [PubMed] [Google Scholar]
- 319.Caon E., Martins M., Hodgetts H., et al. Exploring the impact of the PNPLA3 I148M variant on primary human hepatic stellate cells using 3D extracellular matrix models. J Hepatol. 2024;80:941–956. doi: 10.1016/j.jhep.2024.01.032. [DOI] [PubMed] [Google Scholar]
- 320.Briscoe J., Therond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 321.Dutta R.K., Jun J., Du K., et al. Hedgehog signaling: implications in liver pathophysiology. Semin Liver Dis. 2023;43:418–428. doi: 10.1055/a-2187-3382. [DOI] [PubMed] [Google Scholar]
- 322.Li T., Leng X.S., Zhu J.Y., et al. Suppression of hedgehog signaling regulates hepatic stellate cell activation and collagen secretion. Int J Clin Exp Pathol. 2015;8:14574–14579. [PMC free article] [PubMed] [Google Scholar]
- 323.Du K., Hyun J., Premont R.T., et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. 2018;154:1465–1479 e1413. doi: 10.1053/j.gastro.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Michelotti G.A., Xie G., Swiderska M., et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Verdelho Machado M., Diehl A.M. The hedgehog pathway in nonalcoholic fatty liver disease. Crit Rev Biochem Mol Biol. 2018;53:264–278. doi: 10.1080/10409238.2018.1448752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Jun J.H., Du K., Dutta R.K., et al. The senescence-associated secretome of Hedgehog-deficient hepatocytes drives MASLD progression. J Clin Invest. 2024;134 doi: 10.1172/JCI180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Xie G., Karaca G., Swiderska-Syn M., et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nair B., Kamath A.J., Pradeep G., et al. Unveiling the role of the Hedgehog signaling pathway in chronic liver disease: therapeutic insights and strategies. Drug Discov Today. 2024;29 doi: 10.1016/j.drudis.2024.104064. [DOI] [PubMed] [Google Scholar]
- 329.Weston C.J., Shepherd E.L., Claridge L.C., et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. 2015;125:501–520. doi: 10.1172/JCI73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Torok N.J. Vascular adhesion protein 1 in nonalcoholic steatohepatitis: a novel biomarker? Hepatology. 2015;62:1313–1315. doi: 10.1002/hep.27942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schumacher J.D., Kong B., Wu J., et al. Direct and indirect effects of fibroblast growth factor (FGF) 15 and FGF19 on liver fibrosis development. Hepatology. 2020;71:670–685. doi: 10.1002/hep.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cooreman M.P., Butler J., Giugliano R.P., et al. The pan-PPAR agonist lanifibranor improves cardiometabolic health in patients with metabolic dysfunction-associated steatohepatitis. Nat Commun. 2024;15:3962. doi: 10.1038/s41467-024-47919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Schuster-Gaul S., Geisler L.J., McGeough M.D., et al. ASK1 inhibition reduces cell death and hepatic fibrosis in an Nlrp3 mutant liver injury model. JCI Insight. 2020;5 doi: 10.1172/jci.insight.123294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yoon Y.C., Fang Z., Lee J.E., et al. Selonsertib inhibits liver fibrosis via downregulation of ASK1/MAPK pathway of hepatic stellate cells. Biomol Ther (Seoul) 2020;28:527–536. doi: 10.4062/biomolther.2020.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Furukawa F., Matsuzaki K., Mori S., et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 336.Kluwe J., Pradere J.P., Gwak G.Y., et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Razvi S., Jabbar A., Pingitore A., et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71:1781–1796. doi: 10.1016/j.jacc.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 338.Polyzos S.A., Targher G. Hepatic thyroid hormone receptor-beta signalling: mechanisms and recent advancements in the treatment of metabolic dysfunction-associated steatohepatitis. Diabetes Obes Metab. 2025;27:1635–1647. doi: 10.1111/dom.16117. [DOI] [PubMed] [Google Scholar]
- 339.Zucchi R. Thyroid hormone analogues: an update. Thyroid. 2020;30:1099–1105. doi: 10.1089/thy.2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Viking Therapeutics. VK2809 selective thyroid receptor-β agonist. 2024. https://vikingtherapeutics.com/pipeline/metabolic-disease-program/vk2809/ cited; Available from:
- 341.Harrison S.A., Ratziu V., Anstee Q.M., et al. Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2024;59:51–63. doi: 10.1111/apt.17734. [DOI] [PubMed] [Google Scholar]
- 342.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 343.Park P.H., Sanz-Garcia C., Nagy L.E. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Curr Pathobiol Rep. 2015;3:243–252. doi: 10.1007/s40139-015-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Holland W.L., Adams A.C., Brozinick J.T., et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xu P., Zhang Y., Liu Y., et al. Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-beta/smad2/3 and NF-kappaB signaling pathways. Toxicol Appl Pharmacol. 2016;290:43–53. doi: 10.1016/j.taap.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 346.Lin X.L., He X.L., Zeng J.F., et al. FGF21 increases cholesterol efflux by upregulating ABCA1 through the ERK1/2-PPARgamma-LXRalpha pathway in THP1 macrophage-derived foam cells. DNA Cell Biol. 2014;33:514–521. doi: 10.1089/dna.2013.2290. [DOI] [PubMed] [Google Scholar]
- 347.Zhen E.Y., Jin Z., Ackermann B.L., et al. Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem J. 2016;473:605–614. doi: 10.1042/BJ20151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Loomba R., Sanyal A.J., Nakajima A., et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and stage 3 fibrosis (FALCON 1): a randomized phase 2b study. Clin Gastroenterol Hepatol. 2024;22:102–112 e109. doi: 10.1016/j.cgh.2023.04.011. [DOI] [PubMed] [Google Scholar]
- 349.Abdelmalek M.F., Sanyal A.J., Nakajima A., et al. Pegbelfermin in patients with nonalcoholic steatohepatitis and compensated cirrhosis (FALCON 2): a randomized phase 2b study. Clin Gastroenterol Hepatol. 2024;22:113–123 e119. doi: 10.1016/j.cgh.2023.04.012. [DOI] [PubMed] [Google Scholar]
- 350.Matsukawa T., Yagi T., Uchida T., et al. Hepatic FASN deficiency differentially affects nonalcoholic fatty liver disease and diabetes in mouse obesity models. JCI Insight. 2023;8 doi: 10.1172/jci.insight.161282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Syed-Abdul M.M., Parks E.J., Gaballah A.H., et al. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males With Metabolic Abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 352.Loomba R., Mohseni R., Lucas K.J., et al. TVB-2640 (FASN inhibitor) for the treatment of nonalcoholic steatohepatitis: FASCINATE-1, a randomized, placebo-controlled phase 2a trial. Gastroenterology. 2021;161:1475–1486. doi: 10.1053/j.gastro.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 353.Montagner A., Polizzi A., Fouche E., et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Regnier M., Polizzi A., Smati S., et al. Hepatocyte-specific deletion of Pparalpha promotes NAFLD in the context of obesity. Sci Rep. 2020;10:6489. doi: 10.1038/s41598-020-63579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liu S., Brown J.D., Stanya K.J., et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lee S.M., Muratalla J., Sierra-Cruz M., et al. Role of hepatic peroxisome proliferator-activated receptor gamma in non-alcoholic fatty liver disease. J Endocrinol. 2023;257 doi: 10.1530/JOE-22-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Liu X., Xu J., Rosenthal S., et al. Identification of lineage-specific transcription factors that prevent activation of hepatic stellate cells and promote fibrosis resolution. Gastroenterology. 2020;158:1728–1744 e1714. doi: 10.1053/j.gastro.2020.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Hirschfield G.M., Bowlus C.L., Mayo M.J., et al. A phase 3 trial of seladelpar in primary biliary cholangitis. N Engl J Med. 2024;390:783–794. doi: 10.1056/NEJMoa2312100. [DOI] [PubMed] [Google Scholar]
- 359.Francque S.M., Bedossa P., Ratziu V., et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 360.Targher G., Mantovani A., Byrne C.D., et al. Recent advances in incretin-based therapy for MASLD: from single to dual or triple incretin receptor agonists. Gut. 2025;74:487–497. doi: 10.1136/gutjnl-2024-334023. [DOI] [PubMed] [Google Scholar]
- 361.Harrison S.A., Browne S.K., Suschak J.J., et al. Effect of pemvidutide, a GLP-1/glucagon dual receptor agonist, on MASLD: a randomized, double-blind, placebo-controlled study. J Hepatol. 2025;82:7–17. doi: 10.1016/j.jhep.2024.07.006. [DOI] [PubMed] [Google Scholar]
- 362.Newsome P.N., Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79:1557–1565. doi: 10.1016/j.jhep.2023.07.033. [DOI] [PubMed] [Google Scholar]
- 363.Kuchay M.S., Krishan S., Mishra S.K., et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial) Diabetologia. 2020;63:2434–2445. doi: 10.1007/s00125-020-05265-7. [DOI] [PubMed] [Google Scholar]
- 364.Newsome P.N., Buchholtz K., Cusi K., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 365.Bea S., Ko H.Y., Bae J.H., et al. Risk of hepatic events associated with use of sodium-glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists, and thiazolidinediones among patients with metabolic dysfunction-associated steatotic liver disease. Gut. 2025;74:284–294. doi: 10.1136/gutjnl-2024-332687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Engstrom A., Wintzell V., Melbye M., et al. Association of glucagon-like peptide-1 receptor agonists with serious liver events among patients with type 2 diabetes: a Scandinavian cohort study. Hepatology. 2024;79:1401–1411. doi: 10.1097/HEP.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 367.Kanwal F., Kramer J.R., Li L., et al. GLP-1 receptor agonists and risk for cirrhosis and related complications in patients with metabolic dysfunction-associated steatotic liver disease. JAMA Intern Med. 2024;184:1314–1323. doi: 10.1001/jamainternmed.2024.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Wang L., Berger N.A., Kaelber D.C., et al. Association of GLP-1 receptor agonists and hepatocellular carcinoma incidence and hepatic decompensation in patients with type 2 diabetes. Gastroenterology. 2024;167:689–703. doi: 10.1053/j.gastro.2024.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wester A., Shang Y., Toresson Grip E., et al. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. 2024;73:835–843. doi: 10.1136/gutjnl-2023-330962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Shankar S.S., Daniels S.J., Robertson D., et al. Safety and efficacy of novel incretin Co-agonist cotadutide in biopsy-proven noncirrhotic MASH with fibrosis. Clin Gastroenterol Hepatol. 2024;22:1847–1857 e1811. doi: 10.1016/j.cgh.2024.04.017. [DOI] [PubMed] [Google Scholar]
- 371.Eriksson O., Haack T., Hijazi Y., et al. Receptor occupancy of dual glucagon-like peptide 1/glucagon receptor agonist SAR425899 in individuals with type 2 diabetes. Sci Rep. 2020;10 doi: 10.1038/s41598-020-73815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Friedrichsen M.H., Endahl L., Kreiner F.F., et al. Results from three phase 1 trials of NNC9204-1177, a glucagon/GLP-1 receptor co-agonist: effects on weight loss and safety in adults with overweight or obesity. Mol Metab. 2023;78 doi: 10.1016/j.molmet.2023.101801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Deanfield J., Verma S., Scirica B.M., et al. Semaglutide and cardiovascular outcomes in patients with obesity and prevalent heart failure: a prespecified analysis of the SELECT trial. Lancet. 2024;404:773–786. doi: 10.1016/S0140-6736(24)01498-3. [DOI] [PubMed] [Google Scholar]
- 374.Sattar N., McGuire D.K., Pavo I., et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28:591–598. doi: 10.1038/s41591-022-01707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bjursell M., Wedin M., Admyre T., et al. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kong B., Luyendyk J.P., Tawfik O., et al. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Xiong X., Wang X., Lu Y., et al. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60:847–854. doi: 10.1016/j.jhep.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 378.Kim I., Ahn S.H., Inagaki T., et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 379.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Pockros P.J., Fuchs M., Freilich B., et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 381.Sanyal A.J., Ratziu V., Loomba R., et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J Hepatol. 2023;79:1110–1120. doi: 10.1016/j.jhep.2023.07.014. [DOI] [PubMed] [Google Scholar]
- 382.Adorini L., Trauner M. FXR agonists in NASH treatment. J Hepatol. 2023;79:1317–1331. doi: 10.1016/j.jhep.2023.07.034. [DOI] [PubMed] [Google Scholar]
- 383.Northsea Therapeutics. Icosabutate, Metabolic dysfunction-associated steatohepatitis (MASH) 2024. https://northseatherapeutics.com/en/programs/icosabutate/ cited; Available from:
- 384.Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38. doi: 10.1016/j.cmet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 385.Xu B., Li S., Kang B., et al. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022;21:83. doi: 10.1186/s12933-022-01512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Cheung K.S., Ng H.Y., Hui R.W.H., et al. Effects of empagliflozin on liver fat in patients with metabolic dysfunction-associated steatotic liver disease without diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Hepatology. 2024;80:916–927. doi: 10.1097/HEP.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 387.Inoue M., Hayashi A., Taguchi T., et al. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2019;10:1004–1011. doi: 10.1111/jdi.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Khaliq A., Badshah H., Shah Y., et al. The effect of ertugliflozin in patients with nonalcoholic fatty liver disease associated with type 2 diabetes mellitus: a randomized controlled trial. Medicine (Baltimore) 2024;103 doi: 10.1097/MD.0000000000040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Chehrehgosha H., Sohrabi M.R., Ismail-Beigi F., et al. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Ther. 2021;12:843–861. doi: 10.1007/s13300-021-01011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Taheri H., Malek M., Ismail-Beigi F., et al. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv Ther. 2020;37:4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Hayashizaki-Someya Y., Kurosaki E., Takasu T., et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015;754:19–24. doi: 10.1016/j.ejphar.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 392.Nishimura N., Kitade M., Noguchi R., et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, ameliorates the development of liver fibrosis in diabetic Otsuka Long-Evans Tokushima fatty rats. J Gastroenterol. 2016;51:1141–1149. doi: 10.1007/s00535-016-1200-6. [DOI] [PubMed] [Google Scholar]
- 393.Qiang S., Nakatsu Y., Seno Y., et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104. doi: 10.1186/s13098-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Brenner C., Galluzzi L., Kepp O., et al. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 395.Wang P.X., Ji Y.X., Zhang X.J., et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:439–449. doi: 10.1038/nm.4290. [DOI] [PubMed] [Google Scholar]
- 396.Wang Y., Wen H., Fu J., et al. Hepatocyte TNF receptor-associated factor 6 aggravates hepatic inflammation and fibrosis by promoting lysine 6-linked polyubiquitination of apoptosis signal-regulating kinase 1. Hepatology. 2020;71:93–111. doi: 10.1002/hep.30822. [DOI] [PubMed] [Google Scholar]
- 397.Harrison S.A., Wong V.W., Okanoue T., et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 398.Harwood H.J., Jr. Treating the metabolic syndrome: acetyl-CoA carboxylase inhibition. Expert Opin Ther Targets. 2005;9:267–281. doi: 10.1517/14728222.9.2.267. [DOI] [PubMed] [Google Scholar]
- 399.Savage D.B., Choi C.S., Samuel V.T., et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Harriman G., Greenwood J., Bhat S., et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci U S A. 2016;113:E1796–E1805. doi: 10.1073/pnas.1520686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Loomba R., Noureddin M., Kowdley K.V., et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology. 2021;73:625–643. doi: 10.1002/hep.31622. [DOI] [PubMed] [Google Scholar]
- 402.Balwani M., Sardh E., Ventura P., et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 403.Ledford H. Gene-silencing technology gets first drug approval after 20-year wait. Nature. 2018;560:291–292. doi: 10.1038/d41586-018-05867-7. [DOI] [PubMed] [Google Scholar]
- 404.Shah V.N., Pyle L. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;385:e69. doi: 10.1056/NEJMc2107661. [DOI] [PubMed] [Google Scholar]
- 405.Li S., Xiong F., Zhang S., et al. Oligonucleotide therapies for nonalcoholic steatohepatitis. Mol Ther Nucleic Acids. 2024;35 doi: 10.1016/j.omtn.2024.102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Mann J.P., Anstee Q.M. NAFLD: PNPLA3 and obesity: a synergistic relationship in NAFLD. Nat Rev Gastroenterol Hepatol. 2017;14:506–507. doi: 10.1038/nrgastro.2017.74. [DOI] [PubMed] [Google Scholar]
- 407.Stender S., Kozlitina J., Nordestgaard B.G., et al. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842–847. doi: 10.1038/ng.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Linden D., Ahnmark A., Pingitore P., et al. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol Metab. 2019;22:49–61. doi: 10.1016/j.molmet.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Fabbrini E., Rady B., Koshkina A., et al. Phase 1 trials of PNPLA3 siRNA in I148M homozygous patients with MAFLD. N Engl J Med. 2024;391:475–476. doi: 10.1056/NEJMc2402341. [DOI] [PubMed] [Google Scholar]
- 410.Zammit V.A. Hepatic triacylglycerol synthesis and secretion: DGAT2 as the link between glycaemia and triglyceridaemia. Biochem J. 2013;451:1–12. doi: 10.1042/BJ20121689. [DOI] [PubMed] [Google Scholar]
- 411.Choi C.S., Savage D.B., Kulkarni A., et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 412.Yamaguchi K., Yang L., McCall S., et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 413.Yu X.X., Murray S.F., Pandey S.K., et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 414.Loomba R., Morgan E., Yousefi K., et al. Novel DGAT antisense inhibitor demonstrates significant histological benefit in biopsy-proven mash patients with advanced liver fibrosis stage F3: subset analysis of a 51-week multicenter randomized double-blind placebo-controlled phase 2 trial. Hepatology. 2024;80:S1–S2011. [Google Scholar]
- 415.Su W., Mao Z., Liu Y., et al. Role of HSD17B13 in the liver physiology and pathophysiology. Mol Cell Endocrinol. 2019;489:119–125. doi: 10.1016/j.mce.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 416.Zhang H.B., Su W., Xu H., et al. HSD17B13: a potential therapeutic target for NAFLD. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.824776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ma Y., Belyaeva O.V., Brown P.M., et al. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Su W., Wang Y., Jia X., et al. Comparative proteomic study reveals 17beta-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2014;111:11437–11442. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Abul-Husn N.S., Cheng X., Li A.H., et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Luukkonen P.K., Tukiainen T., Juuti A., et al. Hydroxysteroid 17-beta dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Mak L.Y., Gane E., Schwabe C., et al. A phase I/II study of ARO-HSD, an RNA interference therapeutic, for the treatment of non-alcoholic steatohepatitis. J Hepatol. 2023;78:684–692. doi: 10.1016/j.jhep.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 422.Cansby E., Kulkarni N.M., Magnusson E., et al. Protein kinase MST3 modulates lipid homeostasis in hepatocytes and correlates with nonalcoholic steatohepatitis in humans. FASEB J. 2019;33:9974–9989. doi: 10.1096/fj.201900356RR. [DOI] [PubMed] [Google Scholar]
- 423.Caputo M., Kurhe Y., Kumari S., et al. Silencing of STE20-type kinase MST3 in mice with antisense oligonucleotide treatment ameliorates diet-induced nonalcoholic fatty liver disease. FASEB J. 2021;35 doi: 10.1096/fj.202002671RR. [DOI] [PubMed] [Google Scholar]
- 424.Wang X., Sommerfeld M.R., Jahn-Hofmann K., et al. A therapeutic silencing RNA targeting hepatocyte TAZ prevents and reverses fibrosis in nonalcoholic steatohepatitis in mice. Hepatol Commun. 2019;3:1221–1234. doi: 10.1002/hep4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Alkhouri N., Herring R., Kabler H., et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: a randomised, open-label phase II trial. J Hepatol. 2022;77:607–618. doi: 10.1016/j.jhep.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 426.Anstee Q.M., Lucas K.J., Francque S., et al. Tropifexor plus cenicriviroc combination versus monotherapy in nonalcoholic steatohepatitis: results from the phase 2b TANDEM study. Hepatology. 2023;78:1223–1239. doi: 10.1097/HEP.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Anstee Q.M., Neuschwander-Tetri B.A., Wai-Sun Wong V., et al. Cenicriviroc lacked efficacy to treat liver fibrosis in nonalcoholic steatohepatitis: AURORA phase III randomized study. Clin Gastroenterol Hepatol. 2024;22:124–134 e121. doi: 10.1016/j.cgh.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 428.Krenkel O., Puengel T., Govaere O., et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 429.Ratziu V., Sanyal A., Harrison S.A., et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72:892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 430.Chen S., Saeed A., Liu Q., et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.