Abstract
Increased flux of glucose through the hexosamine biosynthetic pathway (HSP) is believed to mediate hyperglycemia-induced insulin resistance in diabetes. The end product of the HSP, UDPβ-N-acetylglucosamine (GlcNAc), is a donor sugar nucleotide for complex glycosylation in the secretory pathway and for O-linked GlcNAc (O-GlcNAc) addition to nucleocytoplasmic proteins. Cycling of the O-GlcNAc posttranslational modification was blocked by pharmacological inhibition of O-GlcNAcase, the enzyme that catalyzes O-GlcNAc removal from proteins, with O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc). PUGNAc treatment increased levels of O-GlcNAc and caused insulin resistance in 3T3-L1 adipocytes. Insulin resistance induced through the HSP by glucosamine and chronic insulin treatment correlated with increased O-GlcNAc levels on nucleocytoplasmic proteins. Whereas insulin receptor autophosphorylation and insulin receptor substrate 2 tyrosine phosphorylation were not affected by PUGNAc inhibition of O-GlcNAcase, downstream phosphorylation of Akt at Thr-308 and glycogen synthase kinase 3β at Ser-9 was inhibited. PUGNAc-induced insulin resistance was associated with increased O-GlcNAc modification of several proteins including insulin receptor substrate 1 and β-catenin, two important effectors of insulin signaling. These results suggest that elevation of O-GlcNAc levels attenuate insulin signaling and contribute to the mechanism by which increased flux through the HSP leads to insulin resistance in adipocytes.
Insulin resistance is a major pathogenic factor in both insulin-dependent and noninsulin-dependent diabetes mellitus (1, 2). Chronic hyperglycemia is the cause of most complications associated with diabetes (3) and leads to impaired insulin responsiveness in target tissues such as muscle and fat (4, 5). Although the molecular basis of pathologies associated with diabetes is complex (6), glucose-induced insulin resistance or “glucose toxicity” appears to be associated with an increased flux of glucose through the hexosamine biosynthetic pathway (HSP) (7–10).
In cultured adipocytes, Marshall et al. (8) first implicated the HSP in glucose-induced insulin resistance by demonstrating a requirement for the rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase (GFAT). Much subsequent work has established a role for HSP in mediating insulin resistance. Glucosamine, which enters the HSP downstream of GFAT, substitutes for high glucose in mediating desensitization of adipocytes and skeletal muscle to insulin-stimulated glucose uptake in vitro (8, 11). In vivo, glucosamine has been used as a model for inducing insulin resistance (12–14). Inhibitors of GFAT, such as azaserine, reverse hyperglycemia-induced insulin resistance but not glucosamine-induced insulin resistance (8). Furthermore, transgenic mice overexpressing GFAT in skeletal muscle and adipose tissue have decreased glucose disposal rates (9).
Several consequences of increased flux through the HSP may mediate insulin resistance. The end products of this pathway, UDP-β-N-acetylglucosamine (GlcNAc) and UDP-N-acetylgalactosamine, are substrates for the glycosylation of proteins and lipids. It has been suggested that in skeletal muscle an increased UDP-GlcNAc level is responsible for high glucose-induced insulin resistance (15). In addition to being used in the synthesis of classical complex glycosylation, UDP-GlcNAc is the donor sugar for a carbohydrate modification in which single GlcNAc moieties are enzymatically attached to Ser and Thr residues of cytosolic and nuclear proteins (O-linked GlcNAc, O-GlcNAc) (16, 17). O-GlcNAc transferase, the enzyme catalyzing the addition of O-GlcNAc, responds to the physiological range of UDP-GlcNAc concentrations occurring in cells (18–21). An increased level of UDP-GlcNAc, caused by insulin and glucosamine infusion, renders mice insulin-resistant and skeletal muscle proteins exhibit elevated O-GlcNAc levels (22).
O-GlcNAc is a regulatory posttranslational modification more analogous to phosphorylation than to other types of glycosylation (23–26). In some cases, O-GlcNAc competes with phosphorylation at specific sites (27–30), and a reciprocal relationship between phosphorylation and O-GlcNAc has been observed globally (31, 32). In models of hyperglycemia, insulin resistance has been attributed to postreceptor defects in insulin signaling (33). In view of the potential interplay between O-GlcNAc and phosphate in signaling cascades (26), we considered the possibility that aberrantly high levels of O-GlcNAc antagonize insulin signaling and promote insulin resistance.
O-GlcNAcase is a nucleocytoplasmic enzyme that catalyzes removal of O-GlcNAc from proteins (34–36). O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) is a GlcNAc analogue that potently inhibits O-GlcNAcase in cells (37). PUGNAc prevents cycling of O-GlcNAc on proteins, leading to globally elevated levels of this modification without significantly altering N-linked glycosylation or UDP-GlcNAc levels (37). Differentiated 3T3-L1 cells expressing the GLUT4 transporter are a well-characterized model system for the study of insulin resistance (38, 39). In this article, we show that inhibition of O-GlcNAcase by PUGNAc in 3T3-L1 adipocytes leads to insulin resistance. We examine the relationship between elevated O-GlcNAc levels and reduced activation of downstream effectors of the insulin receptor. Finally, the O-GlcNAc modification status of individual members of the insulin signaling cascade in insulin-responsive and insulin-resistant 3T3-L1 adipocytes is determined.
Methods
Cell Culture.
3T3-L1 preadipocytes were grown and differentiated into adipocytes as described (40) with minor modifications. Briefly, preadipocytes were differentiated in DMEM, 10% FBS with dexamethasone (390 ng/ml), insulin (1 μg/ml), and methylisobutylxanthine (115 μg/ml) for 48 h and then treated with insulin (1 μg/ml) for an additional 48 h. Adipocytes were used for experiments 8–11 days after addition of differentiation factors.
Antibodies and Reagents.
The anti-O-GlcNAc antibody used is a mouse monoclonal IgM from ascites designated 110.6 (41). For 110.6 immunoprecipitations, antibody was bound and covalently coupled by using dimethyl pimelimidate (Pierce) to agarose-conjugated anti-IgM (Sigma). For 110.6 Western blots, secondary antibody was horseradish peroxidase-conjugated goat anti-mouse IgM (μ chain-specific) (Sigma). In all cases, Western blots were developed with ECL reagent and imaged on hyperfilm (Amersham Pharmacia). Antibodies were obtained against the following antigens: phosphotyrosine (horseradish peroxidase-coupled PY20 mouse monoclonal IgG2a, from Santa Cruz Biotechnology), insulin receptor β subunit and GLUT4 (rabbit polyclonal IgG raised against the whole proteins), insulin receptor substrate (IRS) 2 and phospho-Akt Thr-308 (Upstate Biotechnology, Lake Placid, NY), Akt, glycogen synthase kinase 3β (GSK3β), and β-catenin (Santa Cruz Biotechnology), phospho-GSK3α/β Ser-21/9 (Cell Signaling, Beverly, MA), and IRS-1 (Transduction Laboratories, Lexington, NY). Recombinant human insulin was from Roche Diagnostics. PUGNAc (Carbogen, Aarau, Switzerland), [14C]-2-deoxyglucose (2-DOG, NEN), glucosamine and 2-DOG (Sigma), DMEM, serum, penicillin, and streptomycin (Life Technologies, Gaithersburg, MD) were commercially obtained.
Glucose Uptake.
All uptake assays were performed in triplicate from at least two separate sets of cells. Glucose uptake assays were performed as described (42), with minor modifications. Cells were washed twice with 5 ml of Krebs-Ringer's phosphate buffer (KRP: 128 mM NaCl/4.7 mM KCl/1.25 mM CaCl2/1.25 mM MgSO4/1 mM Na2HPO4 at pH 7.4), and acute insulin stimulation at indicated concentration was performed in 1 ml of KRP on cells in monolayer on 3-cm cell culture dishes (Falcon) for 7 min at 37°C. Fifty microliters of [14C]-2-DOG at 2.0 μCi/ml in 4 mM “cold” 2-DOG was added at the time of insulin stimulation. After stimulation, cells were washed two times in ice-cold PBS and lysed in 0.5 M NaOH containing 0.1% SDS, and total disintegrations per min taken up into cells were determined by liquid scintillation (Wallac 1410). Insulin-dependent 2-DOG DPM uptake was measured as unstimulated uptake subtracted from insulin-stimulated uptake.
Cell Treatments.
For PUGNAc treatment of cells, 3T3-L1 adipocytes were cultured for 16 h in DMEM containing 4 mM glucose in the absence of serum and containing PUGNAc (100 μM). After 16 h, cells were refed with identical media for 3 h. For glucosamine and/or chronic insulin treatment of cells, 3T3-L1 adipocytes were cultured for 16 h in DMEM containing 4 mM glucose in the absence of serum with 0.2% BSA (Sigma) and containing 5 mM glucosamine/5 mM Hepes, pH 7.6 and/or insulin (1 nM). After 16 h, cells were washed twice and refed with identical media used during 16-h treatment, but lacking insulin. Cells were then washed twice with Krebs-Ringer's phosphate buffer (KRP) and stimulated in KRP with various concentrations of insulin for the indicated time at 37°C.
Western Blotting and Immunoprecipitation.
Whole-cell lysates for Western blotting were obtained by scraping 3T3-L1 cells from 6-cm culture dishes in 400 μl of 1% SDS, transferring to Eppendorf tubes, and boiling for 10 min. Laemli buffer was added directly to lysates for SDS/PAGE. Lysates for immunoprecipitations were obtained by scraping cells in 1% Nonidet P-40 buffer containing 15 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, protease inhibitors, and 1 μM PUGNAc to inhibit deglycosylation during lysis. Protein concentrations in lysates were determined by using the Bio-Rad protein reagent. Immunoprecipitations with indicated antibodies were performed overnight at 4°C and captured with protein A/G Sepharose (Santa Cruz Biotechnology) unless otherwise indicated, washed in RIPA buffer (1% Nonidet P-40 buffer described above, but including 0.1% SDS, 0.25% sodium deoxycholate, and not containing protease inhibitors or PUGNAc), followed by washing in TBS. Proteins were boiled off Sepharose beads in Laemli buffer for SDS/PAGE. SDS/PAGE was performed with precast minigels (Bio-Rad), and separated proteins were transferred to poly(vinylidene difluoride) (Immobilon-P from Millipore), blocked at least 1 h in 4% BSA in 0.1% TBS-Tween20, and probed with indicated antibodies overnight at 4°C. After binding of appropriate horseradish peroxidase-coupled secondary antibodies, ECL detection was used. In cases where densitometry was used to quantitate Western blot signals, the Flourchem imaging system and software (Alpha Innotech, San Leandro, CA) were used. All immunoprecipitations and Western blots were performed at least in duplicate, and densitometry results shown are from one representative experiment.
Results
Elevated O-GlcNAc Modification of Proteins Leads to Insulin Resistance in 3T3-L1 Adipocytes.
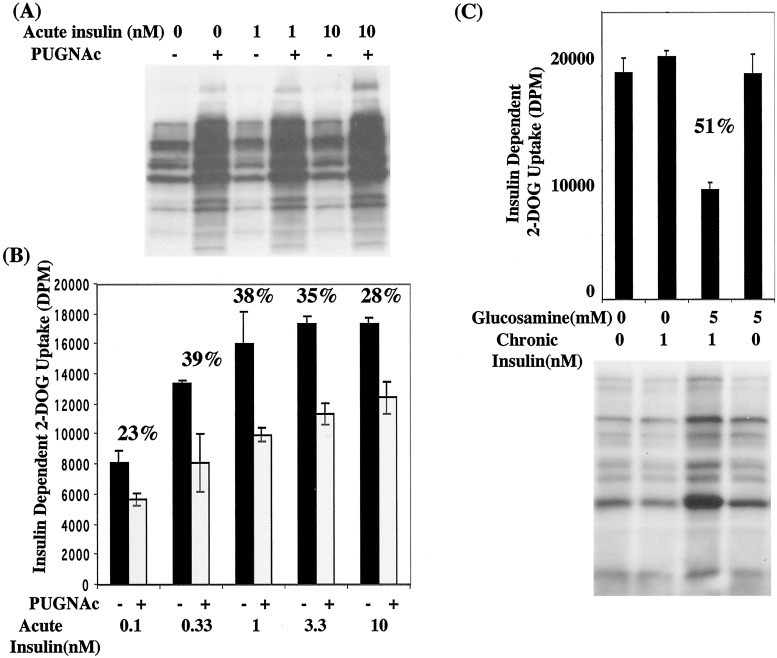
We initially tested the hypothesis that insulin resistance mediated by the HSP may be caused by elevated O-GlcNAc levels. Using PUGNAc, we specifically blocked cycling of the O-GlcNAc posttranslational modification through inhibition of O-GlcNAcase, the enzyme that catalyzes O-GlcNAc removal. PUGNAc treatment elevated O-GlcNAc levels as determined by Western blotting of whole-cell extracts with the O-GlcNAc-specific antibody 110.6 (Fig. 1A). This treatment did not affect total protein levels, and cells were fully viable and maintained adipocyte morphology (data not shown). We then examined the effect of elevated O-GlcNAc levels on insulin-stimulated glucose uptake by using radiolabeled 2-DOG. 3T3-L1 adipocytes in the absence or presence of PUGNAc were stimulated with a range of insulin concentrations and 2-DOG uptake was measured. Insulin concentrations ranged from physiological to pharmacological doses. At all levels of insulin tested, PUGNAc caused defective insulin-stimulated 2-DOG uptake, leading to levels of resistance as high as 39% (Fig. 1B). PUGNAc had no significant effect on basal 2-DOG uptake (data not shown) and insulin-dependent uptake ranged from 2- to 6-fold of basal depending on conditions.
Figure 1.
Elevated levels of O-GlcNAc in 3T3-L1 adipocytes is linked to insulin resistance. 3T3-L1 adipocytes in the absence or presence of PUGNAc (100 μM) were (A) stimulated with 0, 1, or 10 nM insulin for 10 min and 50 μg of whole-cell lysate per treatment was immunoblotted with a general anti-O-GlcNAc antibody (110.6) or (B) stimulated with various concentrations of insulin for 10 min and [14C]-2-DOG uptake was measured and plotted as insulin-dependent uptake. The percent resistance induced by PUGNAc at each insulin concentration is given numerically above the bar graph. (C) 3T3-L1 adipocytes in the presence of indicated concentrations of glucosamine and/or insulin were analyzed for [14C]-2-DOG uptake during a 10-min insulin stimulation (10 nM) or whole-cell lysates were immunoblotted with the 110.6 antibody.
In 3T3-L1 cells, glucosamine treatment and chronic insulin exposure are required to establish insulin resistance (38). As expected, neither glucosamine (5 mM) treatment alone or chronic insulin (1 nM) alone affected insulin-stimulated 2-DOG uptake, whereas in combination these treatments resulted in 51% insulin resistance (Fig. 1C). As measured by Western blotting of whole-cell extracts with the anti-O-GlcNAc antibody 110.6, chronic insulin alone also had no effect on O-GlcNAc levels and glucosamine alone only slightly elevated O-GlcNAc on some proteins. However, resistance induced by a combination of these treatments correlated with a dramatic increase in O-GlcNAc levels (Fig. 1C).
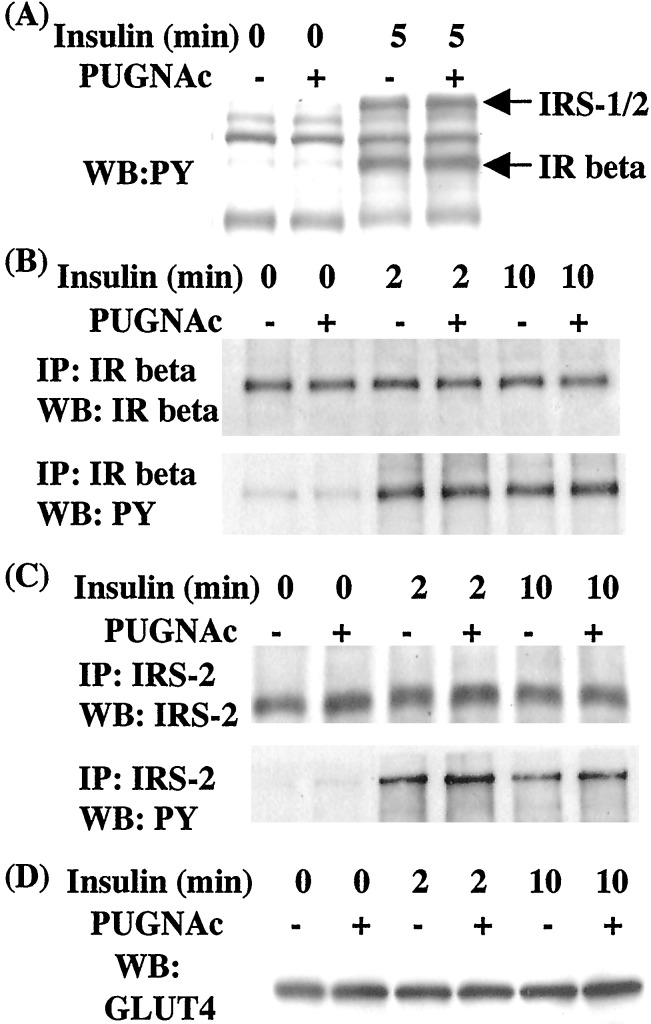
Early Insulin Receptor Signaling and GLUT4 Expression Are Normal in 3T3-L1 Cells Rendered Insulin-Resistant by PUGNAc.
We wanted to determine what steps in the insulin signaling pathway are defective during PUGNAc-induced insulin resistance. A critical receptor proximal signaling event is the tyrosine phosphorylation of IRS-1 and IRS-2 after their association with the activated insulin receptor (43). Phosphotyrosine blotting of crude extracts did not reveal PUGNAc-induced defects in tyrosine phosphorylation of the insulin receptor or IRS proteins in response to 10 nM acute insulin (Fig. 2A). Insulin receptor and phosphotyrosine Western blotting of immunoprecipitations with the insulin receptor β subunit revealed no PUGNAc-induced defects in expression levels or autophosphorylation in response to 10 nM insulin stimulation (Fig. 2B). Similarly, IRS-2 and phosphotyrosine Western blotting of IRS-2 immunoprecipitates displayed normal expression and phosphorylation in response to insulin under conditions in which PUGNAc caused insulin resistance (Fig. 2C). Western blotting of whole-cell extracts showed similar levels of GLUT4 in normal and PUGNAc-treated cells (Fig. 2D), indicating that insulin resistance induced by elevated O-GlcNAc is not caused by reduced expression of this insulin-responsive glucose transporter.
Figure 2.
PUGNAc does not affect insulin receptor or IRS protein tyrosine phosphorylation or GLUT4 levels. 3T3-L1 adipocytes were starved of growth factors in the absence or presence of PUGNAc (100 μM) and were acutely treated with 10 nM insulin for various times. (A) Whole-cell lysates were immunoblotted with antiphosphotyrosine (PY20) and positions of the insulin receptor β (IR) at 86 kDa and IRS proteins (IRS-1/2) at ≈180 kDa are indicated. (B) Insulin receptor (IR) β immunoprecipitates were immunoblotted with antibodies to either insulin receptor β or phosphotyrosine. (C) IRS-2 immuno-precipitates were immunoblotted with antibodies to either IRS-2 or phosphotyrosine. (D) Whole-cell lysates were immunoblotted with anti-GLUT 4 antibody. WB:PY, phosphotyrosine Western blot; IP, immunoprecipitation.
PUGNAc-Induced Elevation of O-GlcNAc Inhibits Insulin-Stimulated Akt Phosphorylation at Thr-308 and GSK3β Phosphorylation at Ser-9.
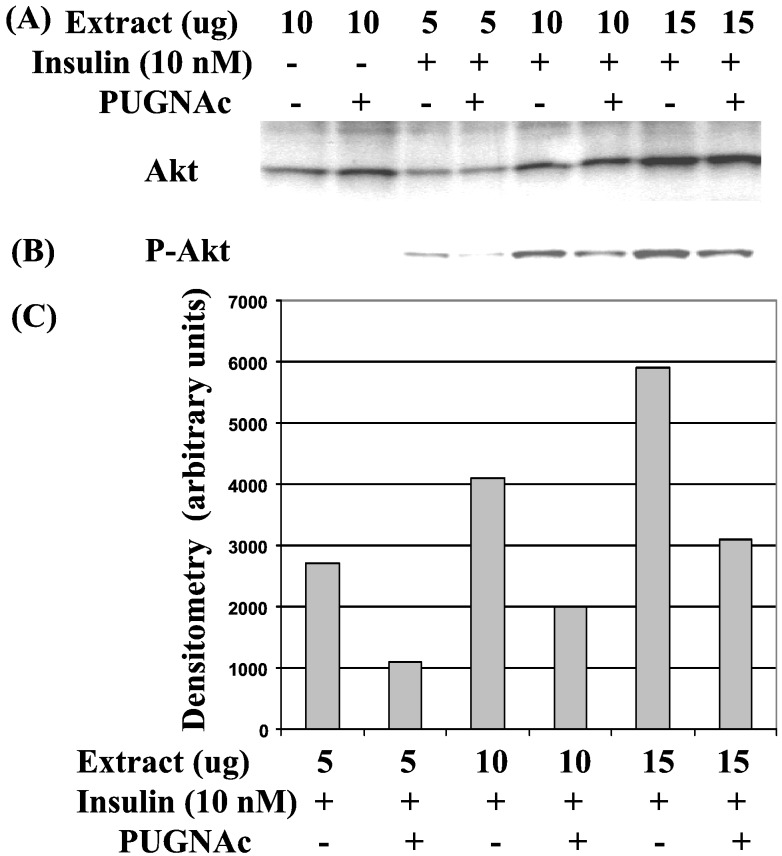
Next, we examined which downstream components of the insulin signaling pathway were negatively regulated by elevated O-GlcNAc. The phosphoinositide-dependent Ser/Thr protein kinase Akt (also known as protein kinase B, or PKB) has been implicated as a downstream effector controlling glucose uptake (44, 45), although this view has been challenged (46). Akt activation appears to require phosphorylation of Thr-308 by the PDK1 kinase (47). Western blotting with different amounts of extract from 3T3-L1 cells showed that whereas Akt protein levels remained unchanged in response to PUGNAc treatment, insulin-induced phosphorylation of Akt at Thr-308 was inhibited by PUGNAc (Fig. 3).
Figure 3.
PUGNAc-induced elevation of O-GlcNAc inhibits insulin-stimulated Akt phosphorylation at Thr-308. 3T3-L1 adipocytes were starved of growth factors in the absence or presence of PUGNAc (100 μM) and acutely stimulated with 10 nM insulin for 5 min. Various amounts of whole-cell lysates were immunoblotted with antibodies recognizing either (A) Akt or (B) phosphorylated Thr-308–Akt. (C) Densitometry of bands from phosphorylated Thr-308–Akt immunoblot.
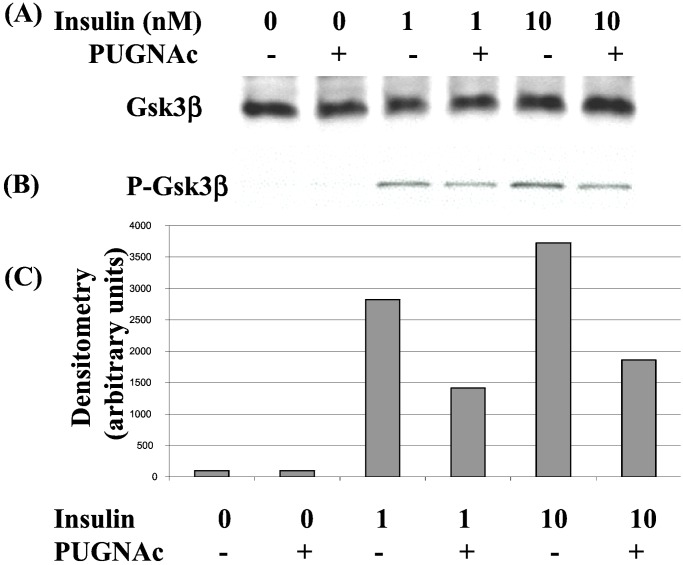
GSK3β, an upstream inhibitor of glycogen synthase, is inactivated by insulin (48), contributing to stimulation of glycogen synthesis. Although targets of Akt in signals regulating glucose transport are not fully understood, Akt activation leads to inhibition of GSK3β at least in part through its phosphorylation at Ser-9 (49, 50). Having observed a defect in Akt phosphorylation as a result of elevated O-GlcNAc levels, we next examined whether the downstream phosphorylation of GSK3β at Ser-9 was also inhibited. Whereas PUGNAc had no effect on GSK3β protein levels (Fig. 4A), Western blotting of extracts with a phospho-specific antibody showed that insulin-stimulated phosphorylation of GSK3β at Ser-9 was defective (Fig. 4 B and C). These findings indicate that a postreceptor mechanism through which elevated O-GlcNAc interferes with insulin signaling is acting at or upstream of Akt, and that the defect observed interferes with activation of Akt and its ability to signal to a downstream effector.
Figure 4.
Insulin-stimulated phosphorylation of GSK3β is reduced in PUGNAc-treated cells. 3T3-L1 adipocytes were starved of growth factors in the absence or presence of PUGNAc (100 μM) and stimulated with insulin at concentrations of 1 nM or 10 nM for 5 min. Whole-cell lysates were separated by SDS/PAGE and immunoblotted with either (A) anti-GSK3β or (B) an antibody recognizing GSK3β phosphorylated at Ser-9. (C) Densitometry of bands from phosphorylated Ser-9–GSK3β Western blot.
PUGNAc-Induced Insulin Resistance Is Associated with Increased O-GlcNAc Modification of IRS-1 and β-Catenin.
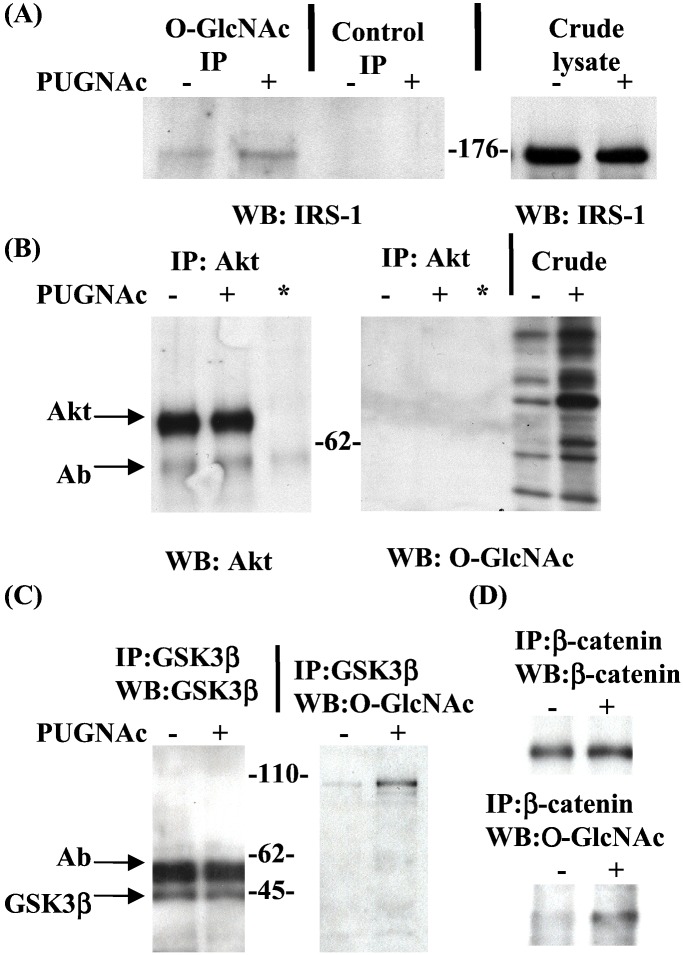
O-GlcNAc modification may negatively regulate components of the insulin signaling pathway. We thus examined which, if any, of the known proteins of the pathway are modified by O-GlcNAc. O-GlcNAc-containing proteins, immunoprecipitated from control or PUGNAc-treated 3T3-L1 cell lysates, were Western-blotted with a panel of antibodies against known mediators of the insulin receptor signaling pathway. Although defects in insulin-stimulated Akt and GSK3β phosphorylation were observed, we could not detect these proteins in O-GlcNAc immunoprecipitates (data not shown). Although phosphatidylinositol 3-kinase (PI3-kinase) activity is required for insulin-stimulated Akt activation (51), the p85 subunit of PI3-kinase was also not detectable in O-GlcNAc immunoprecipitates (data not shown). However, IRS-1, which binds both the insulin receptor and p85, and participates in the signaling pathway for Akt activation and glucose uptake (52), was detected in O-GlcNAc immunoprecipitates in a PUGNAc-dependent manner whereas total IRS-1 protein levels were unaffected (Fig. 5A).
Figure 5.
O-GlcNAc modification of IRS-1 and β-catenin increases in insulin-resistant 3T3-L1 adipocytes. 3T3-L1 adipocytes were starved of growth factors in the absence or presence of PUGNAc (100 μM). (A) Whole-cell lysates were immunoprecipitated with the general O-GlcNAc antibody 110.6 or anti-IgM agarose (control), separated by SDS/PAGE, and immunoblotted with an antibody to IRS-1. (B) Whole-cell lysates were immunoprecipitated with anti-Akt or (*) anti-Akt antibody in the absence of lysate as a negative control, separated by SDS/PAGE, and immunoblotted with anti-Akt or the general O-GlcNAc antibody 110.6. (C) Whole-cell lysates were immunoprecipitated with anti-GSK3β, separated by SDS/PAGE, and immunoblotted with anti-GSK3β or the general O-GlcNAc antibody 110.6. (D) Whole-cell lysates were immuoprecipitated with anti-β-catenin, separated by SDS/PAGE, and immunoblotted with anti-β-catenin or the general O-GlcNAc antibody 110.6. IP, immunoprecipitation; WB, Western blot.
Although the presence of increased nucleocytoplasmic O-GlcNAc in response to PUGNAc was confirmed on lysates, O-GlcNAc was not detected on either Akt or GSK3β immunoprecipitates (Fig. 5 B and C). A GSK3β coimmunoprecipitating protein running at approximately 110 kDa was detected and its state of O-GlcNAc modification increased in response to PUGNAc. The activity of GSK3β is thought to regulate the stability of the 110-kDa protein β-catenin by means of a tight complex also containing adenomatous polyposis coli protein and axin (53, 54). Immunoprecipitates of β-catenin were recognized on Western blots by the anti-O-GlcNAc antibody and immunoreactivity increased in response to PUGNAc (Fig. 5D).
Discussion
Several possible mechanisms for insulin resistance induced through the HSP have been proposed, including increased proteoglycan formation, glycosylphosphatidylinositol anchor biosynthesis, glycosylation of lipids, complex N-linked glycosylation, and increased O-GlcNAc modification of proteins (6). We have implicated elevated O-GlcNAc modification of nucleocytoplasmic proteins as the downstream mediator of insulin resistance caused by increased flux through the HSP. Although the combination of glucosamine entry into the HSP and chronic insulin are required to induce insulin resistance (8, 38), PUGNAc induced elevation of O-GlcNAc alone inhibits insulin-stimulated glucose uptake. Furthermore, only insulin resistance induced by the combination of chronic insulin and glucosamine treatment, but neither alone, markedly increased O-GlcNAc levels (Fig. 1C). The fact that exogenous glucosamine alone is able to elevate UDP-GlcNAc levels (55), the donor sugar nucleotide for O-GlcNAc modification, suggests that chronic insulin treatment acts on one or both of the enzymes (O-GlcNAc transferase and O-GlcNAcase) responsible for O-GlcNAc cycling. Consistent with our findings, rats rendered insulin-resistant by glucosamine and chronic insulin treatment exhibit elevated levels of O-GlcNAc modification in muscle (22), and the transcription factor Sp1 is more heavily modified by O-GlcNAc in hyperglycemic bovine aortic endothelial cells (56).
We next wanted to determine the molecular defects in insulin signaling responsible for PUGNAc-induced insulin resistance. No defect in insulin receptor β subunit or IRS tyrosine phosphorylation was observed after PUGNAc treatment (Fig. 2 A–C). This finding is consistent with studies showing that glucosamine- or hyperglycemia-induced insulin resistance is not associated with insulin receptor or IRS tyrosine phosphorylation defects (33, 57). Although it has been suggested that HSP-mediated insulin resistance is associated with reduced GLUT4 expression (38), Buse and colleagues (55) found that HSP-mediated insulin resistance at physiological levels of chronic insulin is not associated with reduced GLUT4 levels. Similarly, we found that PUGNAc has no effect on the expression level of GLUT4 (Fig. 2D). Next, elevated O-GlcNAc levels were shown to lead to a defect in insulin-stimulated phosphorylation of Akt at Thr-308 (Fig. 3) and phosphorylation of GSK3β at Ser-9 (Fig. 4). The finding of a defect at the level of Akt is consistent with defects in Akt activation in response to excessive flux through the HSP in retinal neurons (58) and adipocytes (59).
Having established that PUGNAc causes a defect in Akt activation (Figs. 3 and 4), the glycosylation status of members of the insulin signaling cascade was investigated. Our observation that IRS-1 is modified by O-GlcNAc in a PUGNAc-responsive manner (Fig. 5A) is in good agreement with Yki-Jarvinen and colleagues (57), who showed that IRS-1 is modified by O-GlcNAc in rat muscle. We were unable to demonstrate anti-O-GlcNAc antibody cross-reactivity with immunoprecipitated Akt and GSK3β (Fig. 5 B and C). However, this does not exclude the possibility that these molecules are modified by O-GlcNAc. The stoichiometry of the modification might have been below the limit of detection by this antibody, or the 110.6 antibody may have specificity that excludes recognition of a subset of O-GlcNAc-modified sites. In connection with this, Dennis and colleagues have determined that recombinant bacculovirus-expressed human Akt1 is modified by O-GlcNAc (J. W. Dennis, personal communication), and Lubas and Hanover (21) showed that GSK3β was an in vitro substrate for O-GlcNAc transferase. β-Catenin, an established GSK3β binding partner (53), was shown to be modified by O-GlcNAc in a PUGNAc-responsive fashion (Fig. 5D). Insulin was shown previously to stimulate the β-catenin pathway (60), and work by others suggests that β-catenin is modified by O-GlcNAc in MCF-7 cells (61). Thus, at least two known downstream effectors of the insulin receptor, IRS-1 and β-catenin, are modified by O-GlcNAc, and the extent of this modification was increased by provoking insulin resistance with PUGNAc.
Although this study was with adipocytes, excessive flux through the HSP has been shown to affect insulin signaling in a variety of other cell types as well as whole animal models (9–14, 56, 58, 59). Therefore, the modification of signaling proteins by O-GlcNAc should be considered a possible mechanism by which increased shunt through the HSP alters signal transduction systems. The mechanisms by which O-GlcNAc impinges on insulin signaling are no doubt complex. In addition to kinases that mediate insulin signaling, negative regulators of insulin signaling, such as the inositol 5′-phosphatase SHIP2 (62), should be considered as candidates for regulation by O-GlcNAc. Although O-GlcNAc may compete for insulin-stimulated phosphorylation sites on effector molecules, O-GlcNAc may also directly regulate components of insulin signaling.
Acknowledgments
We offer special thanks to Kathleen Anuzis, Paul Dowell, and Qi-Qun Tang for their help in maintenance of the 3T3-L1 adipocytes and technical assistance. This work is supported in part by National Institutes of Health Grants HD13563 and CA42486 (to G.W.H.) and DK38418 (to M.D.L.) and National Research Service Award Fellowships GM20528 (to K.V.) and CA83261 (to L.W.). Under a licensing agreement between Covance Research Products and The Johns Hopkins University, G.W.H. receives a share of royalties received by the university on sales of the CTD 110.6 antibody. The terms of this arrangement are managed by The Johns Hopkins University in accordance with its conflict-of-interest policies.
Abbreviations
- GlcNAc
β-N-acetylglucosamine
- O-GlcNAc
O-linked GLCNAc
- HSP
hexosamine biosynthetic pathway
- PUGNAc
O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate
- 2-DOG
2-deoxyglucose
- GSK3β
glycogen synthase kinase 3β
- IRS
insulin receptor substrate
References
- 1.Yki-Jarvinen H, Koivisto V. Ann Clin Res. 1984;16:74–83. [PubMed] [Google Scholar]
- 2.Beck-Nielsen H, Vaag A, Damsbo P, Handberg A, Nielsen O H, Henriksen J E, Thye-Ronn P. Diabetes Care. 1992;15:418–429. doi: 10.2337/diacare.15.3.418. [DOI] [PubMed] [Google Scholar]
- 3.Kruszynska Y T, Olefsky J M. J Invest Med. 1996;44:413–428. [PubMed] [Google Scholar]
- 4.Rossetti L, Smith D, Shulman G I, Papachristou D, DeFronzo R A. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hager S R, Jochen A L, Kalkhoff R K. Am J Physiol. 1991;260:E353–E362. doi: 10.1152/ajpendo.1991.260.3.E353. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Nature (London) 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Rossetti L, Giaccari A, De Fronzo R A. Diabetes Care. 1990;13:610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 8.Marshall S, Bacote V, Traxinger R R. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 9.Hebert L F, Daniels M C, Zhou J, Crook E D, Turner R L, Simmons S T, Neidigh J L, Zhu J S, Baron A D, McClain D A. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClain D A, Crook E D. Diabetes. 1996;45:1003–1009. doi: 10.2337/diab.45.8.1003. [DOI] [PubMed] [Google Scholar]
- 11.Robinson K A, Sens D A, Buse M G. Diabetes. 1993;42:1333–1346. doi: 10.2337/diab.42.9.1333. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giaccari A, Morviducci L, Zorretta D, Sbraccia P, Leonetti F, Caiola S, Buongiorno A, Bonadonna R C, Tamburrano G. Diabetologia. 1995;38:518–524. doi: 10.1007/BF00400719. [DOI] [PubMed] [Google Scholar]
- 14.Baron A D, Zhu J S, Weldon J, Maianu L, Garvey W T. J Clin Invest. 1995;96:2792–2801. doi: 10.1172/JCI118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart G W. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 17.Hanover J A. FASEB J. 2001;15:1865–1876. doi: 10.1096/fj.01-0094rev. [DOI] [PubMed] [Google Scholar]
- 18.Kreppel L K, Blomberg M A, Hart G W. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 19.Lubas W A, Frank D W, Krause M, Hanover J A. J Biol Chem. 1997;272:9316–9324. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- 20.Kreppel L K, Hart G W. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 21.Lubas W A, Hanover J A. J Biol Chem. 2000;275:10983–10988. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 22.Yki-Jarvinen H, Virkamaki A, Daniels M C, McClain D, Gottschalk W K. Metabolism. 1998;47:449–455. doi: 10.1016/s0026-0495(98)90058-0. [DOI] [PubMed] [Google Scholar]
- 23.Hart G W, Greis K D, Dong D, Blomberg M A, Chou T, Jiang M, Roquemore E P, Snow D M, Kreppel L K, Cole R N, et al. Adv Exp Med Biol. 1995;376:115–123. [PubMed] [Google Scholar]
- 24.Haltiwanger R S, Busby S, Grove K, Li S, Mason D, Medina L, Moloney D, Phillipsberg G, Scartozzi R. Biochem Biophys Res Commun. 1997;231:237–242. doi: 10.1006/bbrc.1997.6110. [DOI] [PubMed] [Google Scholar]
- 25.Vosseller K, Wells L, Hart G W. Biochimie. 2001;83:575–581. doi: 10.1016/s0300-9084(01)01295-0. [DOI] [PubMed] [Google Scholar]
- 26.Wells L, Vosseller K, Hart G W. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 27.Chou T, Hart G W, Dang C V. J Biol Chem. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 28.Kelly W G, Dahmus M E, Hart G W. J Biol Chem. 1993;268:10416–10424. [PubMed] [Google Scholar]
- 29.Arnold C S, Johnson G V, Cole R N, Dong D L, Lee M, Hart G W. J Biol Chem. 1996;271:28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 30.Comer F I, Hart G W. Biochemistry. 2001;40:7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 31.Comer F I, Hart G W. J Biol Chem. 2000;275:29179–29182. doi: 10.1074/jbc.R000010200. [DOI] [PubMed] [Google Scholar]
- 32.Lefebvre T, Alonso C, Mahboub S, Dupire M J, Zanetta J P, Caillet-Boudin M L, Michalski J C. Biochim Biophys Acta. 1999;1472:71–81. doi: 10.1016/s0304-4165(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 33.Lima F B, Thies R S, Garvey W T. Endocrinology. 1991;128:2415–2426. doi: 10.1210/endo-128-5-2415. [DOI] [PubMed] [Google Scholar]
- 34.Dong D L, Hart G W. J Biol Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 35.Gao Y, Wells L, Comer F I, Parker G J, Hart G W. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 36.Wells L, Gao Y, Mahoney J A, Vosseller K, Chen C, Rosen A, Hart G W. J Biol Chem. 2001;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 37.Haltiwanger R S, Grove K, Philipsberg G A. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 38.Thomson M J, Williams M G, Frost S C. J Biol Chem. 1997;272:7759–7764. doi: 10.1074/jbc.272.12.7759. [DOI] [PubMed] [Google Scholar]
- 39.Kaestner K H, Christy R J, Lane M D. Proc Natl Acad Sci USA. 1990;87:251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Student A K, Hsu R Y, Lane M D. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 41.Comer F I, Vosseller K, Wells L, Accavitti M A, Hart G W. Anal Biochem. 2001;293:169–177. doi: 10.1006/abio.2001.5132. [DOI] [PubMed] [Google Scholar]
- 42.Frost S C, Lane M D. J Biol Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 43.Kido Y, Nakae J, Accili D. J Clin Endocrinol Metab. 2001;86:972–979. doi: 10.1210/jcem.86.3.7306. [DOI] [PubMed] [Google Scholar]
- 44.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 48.Welsh G I, Proud C G. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 50.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 51.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 52.White M F. Recent Prog Horm Res. 1998;53:119–138. [PubMed] [Google Scholar]
- 53.Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 54.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 55.Nelson B A, Robinson K A, Buse M G. Diabetes. 2000;49:981–991. doi: 10.2337/diabetes.49.6.981. [DOI] [PubMed] [Google Scholar]
- 56.Du X L, Edelstein D, Rossetti L, Fantus I G, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patti M E, Virkamaki A, Landaker E J, Kahn C R, Yki-Jarvinen H. Diabetes. 1999;48:1562–1571. doi: 10.2337/diabetes.48.8.1562. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura M, Barber A J, Antonetti D A, LaNoue K F, Robinson K A, Buse M G, Gardner T W. J Biol Chem. 2001;276:43748–43755. doi: 10.1074/jbc.M108594200. [DOI] [PubMed] [Google Scholar]
- 59.Heart E, Choi W S, Sung C K. Am J Physiol. 2000;278:E103–E112. doi: 10.1152/ajpendo.2000.278.1.E103. [DOI] [PubMed] [Google Scholar]
- 60.Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel M J, Bertrand F, Cherqui G, Perret C, Capeau J. Oncogene. 2001;20:252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- 61.Zhu W, Leber B, Andrews D W. EMBO J. 2001;20:5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wada T, Sasaoka T, Funaki M, Hori H, Murakami S, Ishiki M, Haruta T, Asano T, Ogawa W, Ishihara H, Kobayashi M. Mol Cell Biol. 2001;21:1633–1646. doi: 10.1128/MCB.21.5.1633-1646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]