Abstract
Holocarboxylase synthetase (HCS) catalyzes the covalent attachment of biotin to five biotin-dependent carboxylases in human cells. Multiple carboxylase deficiency (MCD) is a life-threatening disease characterized by the lack of carboxylase activities because of deficiency of HCS activity. Here, we report the obligatory participation of HCS in the biotin-dependent stimulation of the level of HCS mRNA and those of acetyl-CoA carboxylase and the α subunit of propionyl-CoA carboxylase in human cells. Fibroblasts from patients with MCD are unable to increase HCS mRNA in response to biotin unless the vitamin concentration is raised 100-fold, in keeping with mutations that cause a reduced affinity for biotin by the mutant enzyme. The outcome is deficient synthesis of biotinyl-5′-AMP, the active form of the vitamin in the biotinylation reaction. HCS and carboxylase mRNA levels in normal and MCD fibroblasts and HepG2 cells can be restored by the addition of the cGMP analogue, 8-Br-cGMP, and can be abolished by the addition of inhibitors of the soluble form of guanylate cyclase. We propose a regulatory role for biotin in the control of HCS and carboxylase mRNA levels through a signaling cascade that requires HCS, guanylate cyclase, and cGMP-dependent protein kinase.
Biotin is a water-soluble vitamin found in all organisms that functions as a cofactor of enzymes known as biotin-dependent carboxylases (1). The covalent addition of biotin to these proteins is catalyzed by biotin ligases, which in prokaryotes are known as the BirA protein (2, 3) and in eukaryotes as holocarboxylase synthetase (HCS) (1). For both BirA and HCS, biotin addition occurs as an ATP-dependent, two-step reaction that, in the first step, involves synthesis of the intermediate, biotinyl-5′-AMP (B-AMP) (4, 5). In the second step, B-AMP is used to transfer biotin, with release of AMP, to a specific lysine residue in a highly conserved region in apocarboxylases (6–10).
Most of what we know about biotin and the role of HCS in human metabolism comes from the study of patients with deficiency of HCS activity. These patients, also described as having the neonatal form of multiple carboxylase deficiency (MCD), have life threatening ketoacidosis and organic acidemia (11). They have reduced activity of all biotin-dependent caboxylases: propionyl-CoA carboxylase (PCC), pyruvate carboxylase (PC), methylcrotonyl-CoA carboxylase (MCC), and acetyl-CoA carboxylase (ACC) (12–14). In these patients, the potentially lethal disruption of gluconeogenesis, fatty acid metabolism, and amino acid catabolism can be reversed with pharmacological doses of biotin. The HCS of most affected individuals studied have a reduced affinity for biotin with the Km of the mutant enzyme elevated 3–70 times over the value for the normal enzyme (15, 16). Most patients have mutations in the biotin-binding domain of HCS (16, 17). The outcome is a strong reduction in the ability of the cells to produce B-AMP (18).
A remarkable difference between bacterial and eukaryotic biotin ligases is the ability of the BirA protein to use biotin as a transcriptional corepressor of the five genes that form the biotin operon (19). When bacteria are grown in a biotin-rich medium, B-AMP accumulates in the cell and remains bound to BirA. The complex, BirA-B–AMP, recognizes a specific DNA sequence in the promoter of the biotin operon through a H-L-H domain located in the N-terminal half of the protein and represses transcription of the genes involved in biotin synthesis (20).
In recent years, it has been suggested that biotin may play a role in other cellular events in eukaryotic organisms such as transcriptional or translational regulation or enhancement of the activity of different hepatic enzymes. In these studies, biotin seems to augment the enzymatic activity of glucokinase and the transcription of its gene in rats in vivo, pancreatic β-cells and rat hepatocytes in culture (21–23). Similarly, mRNA levels for 6-phosphofructokinase are increased after administration of the vitamin to biotin-starved rats (24, 25). In human hepatocyte culture, biotin seems to be required to promote translation of the asialoglycoprotein receptor (ASGR) mRNA (26). Significantly, a recent report showed that biotin deficiency in rat reduces the protein concentration and activity of the carboxylases, PCC and PC, and the mRNA level of HCS, the enzyme responsible for their biotinylation (27).
Given the high structural and functional similarities between HCS and the BirA protein (9, 10, 28), we investigated the possibility that HCS may have a role in determining mRNA levels of different genes in eukaryotic cells in response to biotin. In this report we show that in human cells, HCS is required to mediate the biotin-dependent increase in mRNA levels of HCS, ACC-1, and the α subunit of PCC (PCCA). We suggest that the mechanism responsible for the biotin effect on mRNA requires activation of the soluble form of guanylate cyclase (sGC) and of the cGMP-dependent protein kinase (PKG).
Materials and Methods
Materials.
Biotin, 8-bromo-cyclic guanosine monophosphate (8-Br-cGMP), 1-H (1,2,4)oxadiazolo-[4,3-a]quinaxolin-1-one (ODQ), and actinomycin-D were purchased from Sigma. Rp-8(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphorothioate (Rp-8-pCPT-cGMPS) was from Biolog, San Diego, CA. The human hepatoblastoma cell line, HepG2, was obtained from the American Type Cell Collection. Normal human fibroblasts and fibroblasts from patients with MCD (MCD-MK and MCD-VE) were kindly provided by R. Gravel (University of Calgary, Alberta, Canada; ref. 16).
Reverse Transcription–PCR.
Total RNA was isolated from cultured cells by using the Trizol reagent method (GIBCO/BRL, Life Technology, Heidelberg, Germany). RNA was treated for 1h at 37°C with 6 units of ribonuclease-free DNase in 100 mM Tris⋅HCl, pH 7.5 and 50 mM MgCl2 in the presence of 2 U/μl placental RNase inhibitor. The RNA concentration was determined by absorbance at 260 nm, and the integrity of the RNA samples was confirmed by electrophoresis through 1% agarose. Next, 5 μg of total RNA were converted to cDNA by using Superscript II reverse transcriptase (GIBCO/BRL) and 0.8 μM of specific oligonucleotides for each of the genes tested. A control reaction without reverse transcriptase was performed for each RNA sample to verify that the subsequent PCR did not proceed from contaminating genomic DNA. For PCR amplification of the cDNAs, sense and antisense primers were designed to amplify 200- to 500-bp RNA fragments. This amplification was accomplished by incubating 1 μl of the resulting cDNA in a 30 μl reaction volume (50 mM KCl/150 mM MgCl2/10 mM Tris⋅HCl, pH 9.0) containing 100 pmol of specific sense and antisense primers and 0.3 μl of Taq polymerase (Perkin-Elmer). The oligonucleotides used were: HCS: 5′-CCC GAG CTC CGT CTC CTG GAT CGG-3′ and 5′-CCC AAG CCT TTT ACC GCC GTT TGG GGA-3′ [melting temperature (Tm) = 58°C]; ACC-1: 5′-GAT GTA CAT CGG CTG AGT GA-3′ and 5′-ATC CAT TCA TTA CAT TGA CC-3′ (Tm = 58°C); ACC-2: 5′-CCT AAA GGT GAC CCG GAG T-3′ and 5′-AAA AAG CCA CTC ATG ACG TT-3′(Tm = 60°C); PCCA: 5′-CCC CGA TGC CCG GAG GTG GT-3′ and 5′-TAT TTC CAG CTC CAG AGC AG-3′ (Tm = 60°C); β-actin: 5′-GGG TCA GAA TTC CTA TG-3′ and 5′-GGT CTC AAA CAT GAT CTG GG-3′ (Tm = 58°C).
Fluorescence Densitometry.
PCR products were separated on 1% agarose gels and stained with ethidium bromide. The amount of PCR product was determined by densitometry by using a Fluor-S-imager (Bio-Rad). The procedure was validated in prior studies by PCR amplification of different concentrations of cDNA fragments of HCS, PCCA, and β-actin (data not shown). The number of PCR cycles was also varied and plotted against fluorescence intensity to ensure that experiments were done within the exponential phase. For every experiment, the constitutive β-actin mRNA was used as the reference cellular transcript. It was present at equivalent levels in all RNA samples (Figs. 1 and 2).
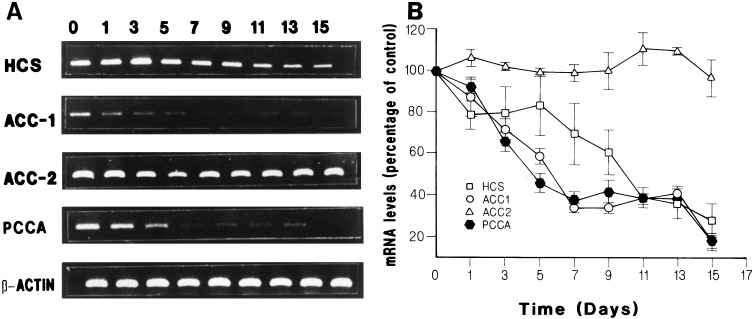
Figure 1.
Time course effect of biotin deficiency on mRNA levels. HepG2 cells were grown in a biotin-free medium. Total RNA was isolated at different times and HCS, ACC-1, ACC-2, and PCCA mRNAs were amplified by reverse transcription PCR and quantitated by densitometry as described in Materials and Methods. (A) representative experiment showing the effect of time of biotin restriction on the mRNA levels for HCS, ACC-1, ACC-2, and PCCA. (B) Summary of the results obtained in three different experiments presented as mean ± SE. Lane numbers correspond to days in biotin-deficient medium.
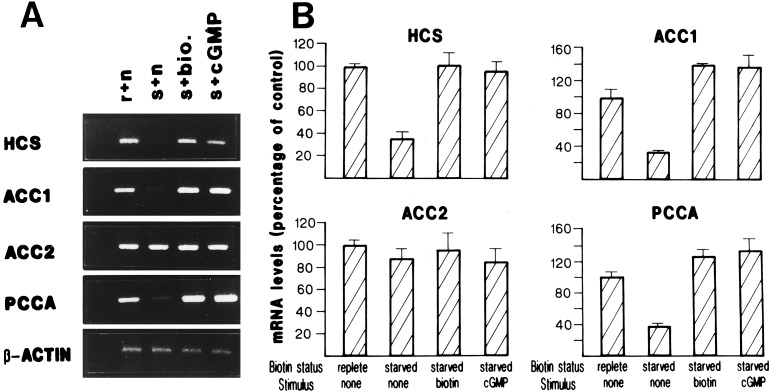
Figure 2.
Effect of biotin and cGMP on mRNA levels. HepG2 cells cultivated 14 days in a biotin-free medium were stimulated with 1 μM biotin or 1 mM 8-Br-cGMP. HCS, ACC-1, ACC-2, PCCA, and β-actin mRNA were determined as described in Materials and Methods. (A) Representative experiment showing mRNA levels in biotin-replete cells (r), biotin-starved cells (s), biotin-starved cells stimulated with biotin (s + bio), and biotin-starved cells stimulated with 8-Br-cGMP (s + cGMP). (B) Summary of the results obtained from three different experiments. Results are presented as mean ± SE.
Effect of Biotin Deficiency on mRNA Levels.
Human HepG2 cells were grown at 37°C with 5% CO2 in 5 ml of α-MEM medium containing high glucose levels (GIBCO/BRL) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. For biotin starvation experiments, the cells were grown in biotin-free MEM medium supplemented with 10% dialyzed FBS (GIBCO/BRL) to maximally reduce the biotin levels. HepG2 cells were cultivated in this medium for up to 20 days. Total RNA was isolated at various times, and the mRNA levels for HCS, ACC-1, ACC-2, PCCA, and β-actin were determined by fluorescence densitometry as described above. After 15 days in biotin-free medium, the cells cultures were 70–80% confluent and microscopic analysis showed normal morphology.
Effect of Biotin and cGMP on mRNA Levels in Biotin-Starved Cells.
HepG2 cells grown in biotin free medium for 15 days were stimulated with 1 μM biotin or 1 mM 8-Br-cGMP, a non hydrolizable analogue of cGMP. Cells were harvested after 2, 6, 12, or 24 h, and the mRNA levels for the different genes were determined as described above. To determine the involvement of sGC on mRNA levels, biotin-starved HepG2 cells were treated with 50 μM ODQ, a specific inhibitor of sGC, for 3 h (29). After this period, 1 μM biotin was added to the medium for 24 h, and the effect on mRNA levels was compared with biotin-deficient cells stimulated by biotin without ODQ and cells grown continuously in normal medium (control cells).
Biotin Stimulation of mRNA Levels After Inhibition of RNA Polymerase II.
Biotin-starved HepG2 cells were treated with 10 μM actinomycin-D for 1 h (30). After this time, biotin was added to the medium to a final concentration of 1 μM and total RNA was isolated 24 h later. mRNA levels were compared with data obtained from cells treated with actinomycin-D without biotin and cells not treated with the RNA polymerase II inhibitor.
Effect of cGMP-Dependent Protein Kinase Inhibition.
HepG2 cells grown in biotin-free medium for 15 days were treated with 10 μM Rp-8-pCPT-cGMPS, a specific inhibitor of cGMP-dependent protein kinase (29) for 1 h. After this period, 1 μM biotin (final) was added to the medium, and growth was continued for 24 h. Total RNA was isolated and the mRNA levels were determined as above.
Effect of Biotin and cGMP on HCS mRNA Levels in Fibroblast Cultures.
Normal fibroblasts and fibroblasts from patients with MCD were incubated in biotin-free medium for 15 days. Cells were treated with 0, 0.01, 0.1, or 1.0 μM biotin for 24 h. Total RNA was isolated, and the level of HCS mRNA was determined by fluorescence densitometry of reverse transcription PCR products. Similar experiments were performed on these cell lines using 0.01, 0.1, or 1.0 mM 8-Br-cGMP.
Statistical Analysis.
All experiments were done in triplicate and at least three different times with different RNA samples as the source of RNA for the reverse transcription PCR. Results of biotin starvation on mRNA were normalized to β-actin mRNA and expressed as a percentage of mRNA levels observed in cells grown in biotin-replete medium. Data are presented as mean of three different experiments ± SE.
Results
Effect of Biotin Starvation on mRNA Levels in HepG2 Cells.
To establish a model for investigation of the effect of biotin on mRNA levels, we made use of the hepatoblastoma line, HepG2, which has been used in studies of the regulation of ASGR translation by biotin. In our studies, we first determined the time course of developing biotin deficiency on HCS, PCCA, ACC-1, and ACC-2 mRNA levels in HepG2 cells grown in a biotin-free medium for up to 15 days. Total RNA was isolated at various times, and the amount of mRNA was determined as described in Materials and Methods (see Fig. 1A for a representative experiment). Biotin starvation resulted in a gradual decrease in the levels of HCS, PCCA, and ACC-1 mRNA to 17–28% of starting levels (Fig. lB). ACC-1 and PCCA mRNAs were more rapidly affected, showing less than 40% of starting levels by 7 days, whereas 11 days of treatment were required to observe a similar reduction of HCS mRNA level (Fig. 1B). No change was observed in ACC-2 or β-actin mRNA levels during the course of the experiment. Biotin-starved cells showed normal morphology and growth rate throughout the experiment. However, longer incubation of cells in biotin-free medium resulted in an increase in cell death, and the effects on mRNA levels became irreversible (see below) after 20 days. For these reasons, experiments to determine the effect of biotin and second messengers on HCS and carboxylase mRNA levels were performed in cells grown for 14 or 15 days in biotin-free medium.
Effect of Biotin Supplementation on HCS and Carboxylase mRNA Levels.
To determine whether the reduction observed in mRNA levels was specific to biotin starvation, we incubated HepG2 cells, previously made biotin-deficient for 14 days, in medium containing 1.0 μM biotin for 2, 6, 12, or 24 h. Although short incubations with biotin produced significant increments of HCS mRNA levels (data not shown), it took up to 24 h to observe restoration of mRNA to levels comparable to those found in normal cells (Fig. 2 A and B). Biotin supplementation had a more pronounced effect on ACC-1 and PCCA mRNAs compared with initial levels. In the case of ACC-1, biotin supplementation not only restored the mRNA level but showed a 40% increase above the results obtained with biotin-replete conditions. Similarly, PCCA mRNA rose to 30% over biotin-replete conditions. HCS was restored to initial levels only. In contrast, there was no significant change in ACC-2 or β-actin mRNA levels after biotin stimulation (Fig. 2 A and B).
Effect of cGMP on mRNA Levels in Biotin-Starved Cells.
To explore whether biotin-dependent recovery of mRNA levels involves a signal transduction pathway, we compared the effect of biotin and cGMP on recovery of HCS, ACC-1, and PCCA mRNA levels. Biotin-starved HepG2 cells were stimulated with 1.0 mM 8-Br-cGMP. This treatment resulted in the normalization of HCS mRNA to the initial biotin-replete level, comparable to that obtained by adding back biotin (Fig. 2 A and B). Interestingly, PCCA and ACC-1 mRNA levels were restored to 35–38% over initial levels (Fig. 2 A and B). Here too, the recovery mimicked the effect of biotin addition to the biotin-starved cells. Finally, treatment with 8-Br-cGMP had no effect on ACC-2 or actin mRNA levels (Fig. 2 A and B). Simultaneous addition of biotin and 8-Br-cGMP did not produce an additive or synergistic change in mRNA levels. Instead, they produced mRNA changes essentially identical to those obtained with either substance alone (data not shown).
sGC Mediates Biotin Effect on mRNA Levels.
Given the apparent involvement of cGMP as a mediator of the biotin effect on HCS, ACC-1, and PCCA mRNA levels, we determined whether inhibition of sGC would affect their biotin-dependent recovery. Vitamin-starved HepG2 cells were stimulated for 24 h with biotin in the presence of ODQ as described in Materials and Methods (37), and the mRNA levels for HCS, ACC-1, and PCCA were compared with those obtained with biotin-starved cells and biotin-starved cells treated for 24 h with biotin. As shown in Table 1, biotin starvation (Bio Def) reduced the mRNA levels of HCS, ACC-1, and PCCA to 34–38% compared with control cells. Addition of biotin to the medium (Bio Def + Bio) again resulted in a recovery of the mRNA levels to within normal values for HCS and above normal values for ACC-1 and PCC-1 (Table 1). In contrast, incubation of HepG2 cells with biotin and ODQ (ODQ + Bio) prevented the biotin-dependent recovery of HCS, ACC-1, and PCCA mRNAs levels. The results obtained when sGC was inhibited were HCS 11%, ACC-1 36% and PCCA 33% (Table 1). This experiment points to sGC involvement in stimulating the restoration of mRNA levels by biotin or cGMP. The data also confirmed that ACC-2 mRNA seems to be unaffected in a significant way by biotin starvation or biotin supplementation.
Table 1.
Effect of inhibition of soluble guanylate cyclase, cGMP-dependent protein kinase, and RNA polymerase II in mRNA levels
| mRNA | Bio Def, % | Bio Def + Bio, % | ODQ + Bio, % | PKGi + Bio, % | Act D + Bio, % |
|---|---|---|---|---|---|
| HCS | 36 ± 5.0 | 103 ± 9.87 | 11 ± 0.68 | 52 ± 2.51 | 25 ± 6.0 |
| ACC-1 | 34 ± 1.15 | 140 ± 2.21 | 36 ± 5.0 | 26 ± 9.53 | 14 ± 7.24 |
| PCCA | 38 ± 3.54 | 130 ± 6.61 | 33 ± 3.60 | 21 ± 11.1 | 34 ± 5.03 |
| ACC-2 | 88 ± 8.96 | 95 ± 2.31 | ND | ND | 19 ± 2.0 |
Biotin-starved HepG2 cells were stimulated with biotin in the presence of ODQ (ODQ + Bio), Rp-cGMPS (PKGi + Bio), or actinomycin-D. The results are shown as percentage of values obtained in cells grown in normal medium and compared to biotin-deficient cells (Bio Def) and biotin-deficient cells stimulated with biotin without inhibitors (Bio Def + Bio). ND, not determined. Results are from three different experiments shown as mean ± SE.
The cGMP-Dependent Protein Kinase May Be Involved in Biotin-Dependent Recovery of mRNA Levels As the Target of sGC Activity.
To assess whether the biotin effect occurs through a PKG (29), we incubated biotin-starved HepG2 cells in a medium containing the PKG inhibitor, Rp-cGMPS (PKGi). Because biotin and cGMP affected only HCS, ACC-1, and PCCA mRNA levels, ACC-2 was not included in these experiments. Although stimulation of HepG2 cells with 1 μM biotin was sufficient to restore mRNA levels to normal or greater than normal values, incubation of the cells in the presence of biotin and the inhibitor (Rp-cGMPS + Bio) severely limited the recovery of mRNA levels, with a small increases for HCS or small decreases for the other mRNAs (Table 1).
Actinomycin-D Prevents Biotin-Dependent Recovery of mRNA Levels.
Recovery of HCS, ACC-1, and PCCA mRNA levels in response to biotin was studied in the presence of actinomycin D. Biotin-starved HepG2 cells were incubated for 1 h in the presence of actinomycin D. Then biotin (1.0 μM) was added to half the samples, with the remaining samples serving as actinomycin D controls, and incubation continued for 24 h (Table 1). These data showed that mRNA levels for HCS, ACC-1, ACC-2, and PCCA fell to below the levels obtained in biotin-free medium in the samples containing actinomycin-D. Their levels likely reflect incomplete decay of remaining mRNA after the addition of inhibitor. The addition of biotin produced no effect on mRNA levels, with the results essentially identical to the actinomycin-D-only controls (Table 1).
Holocarboxylase Synthetase Activity Is Required for Biotin-Dependent Recovery of HCS mRNA Levels.
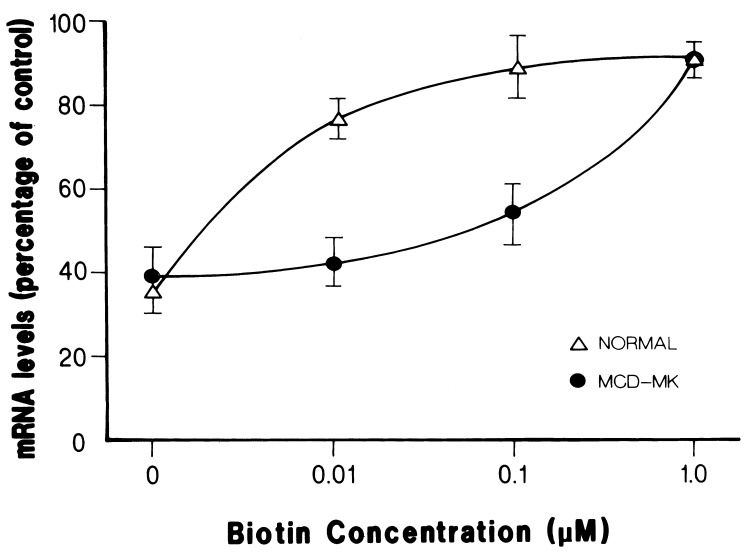
Because biotin is the substrate of HCS, we studied whether this enzyme is involved in the biotin-dependent recovery of mRNA levels. The effect of biotin concentration on HCS, ACC-1, and PCCA mRNA levels was determined in normal fibroblasts versus fibroblasts with mutations in HCS that affect the affinity of the enzyme for biotin. Because the results for the three mRNAs were similar, we decided to focus on HCS mRNA. Two mutant cell lines were used, MCD-MK, homozygous for R508W, and MCD-VE with the mutations L216R and V363D (16). Preliminary experiments on the biotin effect on HCS mRNA levels in MK and VE cells show basically identical patterns of response to the vitamin (data not shown). For this reason and the fact that MCD-MK cells provided us with a homogeneous genetic background, we decided to use this cell line to examine the role of HCS in mRNA levels in response to biotin. Fig. 3 shows the effect of different concentrations of biotin on the recovery of HCS mRNA levels after biotin starvation of control and mutant fibroblasts. Biotin starvation reduced the HCS mRNA level in both normal and MCD-MK cells to ≈35% of control values after 14 days of treatment. The addition of 0.01 μM biotin to the cultures increased the HCS mRNA level to 78% of the biotin-replete level in normal fibroblasts, whereas the MCD-MK cells did not show a significant change (38%). A further 10-fold increment in biotin concentration (0.1 μM) resulted in an increase in HCS mRNA levels to 87% in normal fibroblasts and 53% in MCD-MK cells. It was only when biotin was raised to 1.0 μM that similar HCS mRNA levels were observed in normal and MCD-MK cells (118% and 93%, respectively).
Figure 3.
Effect of biotin concentration on HCS mRNA levels in normal and MCD fibroblasts. Normal and MCD cells were grown in biotin free medium for 14 days. After this period, the cells were stimulated with 0.01 μM, 0.1 μM, and 1.0 μM biotin for 24 h. HCS mRNA levels were determined as described in Materials and Methods. Results are presented as percentage of mRNA values observed in cells grown in biotin-containing medium and normalized to β-actin mRNA levels. Data are from three different experiments and shown as mean ± SE.
cGMP Effect on Recovery of HCS mRNA Levels Is Normal in MCD Cells.
We further examined whether the cGMP-dependent recovery of HCS mRNA levels was also affected as a result of mutation in HCS. Different concentrations of 8-Br-cGMP (0.01 mM, 0.1 mM, and 1.0 mM) were used to stimulate normal and mutant fibroblasts (MCD-MK). Both cell lines showed no change in HCS mRNA levels when incubated with 0.01 mM and 0.1 mM 8-Br-cGMP (data not shown). The highest concentration of 8-Br-cGMP, 1.0 mM, produced a recovery of HCS mRNA to 117% of biotin-replete levels in normal cells versus 92% in MCD-MK cells (Table 2).
Table 2.
Effect of different concentrations of cGMP on HCS mRNA levels in normal and MCD cells
| Fibroblasts | Bio Def, % | Bio Def + cGMP, % |
|---|---|---|
| Normal | 34 ± 7.6 | 117 ± 1.5 |
| MCD-MK | 38 ± 12.6 | 92 ± 9.24 |
Biotin-starved MCD cells and normal fibroblasts were stimulated with 1.0 mM 8-Br-cGMP. Data are from three different experiments and are shown as mean ± SE.
Discussion
Numerous studies have demonstrated that biotin, in addition to its role as the cofactor of biotin-dependent carboxylases, also acts to influence gene expression at the transcriptional and translational level. Most of these findings have been made for rat liver proteins, including roles for biotin in the transcriptional regulation and translational stimulation of ASGR and insulin receptor. Recent studies also showed that biotin up-regulates HCS mRNA levels and maintains the level of at least two carboxylases in rat liver. In this study, we have examined a role for HCS in biotin-mediated gene regulation and showed that action of this enzyme, which functions in the activation of biotin to B-AMP and biotin transfer to apocarboxylases, is an intermediate in the biotin stimulation of HCS and carboxylase mRNA levels in cultured human fibroblasts. We show further, in human fibroblasts and HepG2 cells, that biotin stimulation of mRNA levels requires cGMP and the participation of sGC, and that the cGMP effect may be mediated through PKG.
The impact of biotin withdrawal from HepG2 cells was a gradual reduction of the mRNA levels of HCS, ACC-1, and PCCA to about one-third of starting levels, whereas ACC-2 mRNA was unaffected. This reduction took as long as 2 weeks, which likely reflected a slow clearing and catabolism of biotin. The re-addition of biotin produced a dramatic recovery of mRNA levels within 24 h, which for ACC-1 and PCC, exceeded starting levels. We did not distinguish formally between an effect of biotin on transcription rate or RNA processing or stability. Significantly, incubation of biotin-starved HepG2 cells in the presence of actinomycin-D for 24 h reduced mRNA levels to below biotin-starvation levels, and biotin did not protect against this reduction. If the role of biotin, or a downstream product, were to protect mRNA from decay, then the addition of biotin to the actinomycin-D experiment should have slowed the rate of loss. ACC-2 mRNA, which remained essentially unchanged during biotin-starvation, also fell to a low level after actinomycin-D addition, which would be expected if transcription were successfully blocked. We surmise, therefore, that the mRNA levels remaining after 24 h of actinomycin-D exposure reflected their decay rates, including ACC-2, and these were unaffected by biotin addition. These results suggest that the effect of biotin is to stimulate transcription, although confirmation will require direct examination of transcription rates.
The mechanism of biotin regulation of mRNA levels remains unknown, although our experiments point to a cGMP-PGK pathway. First, cGMP could mimic precisely the biotin effect on mRNA levels. Second, use of ODQ, an inhibitor of sGC, blocked the biotin effect. Third, Rp-cGMPS, an inhibitor of PKG, permitted only minimal recovery of HCS and carboxylase mRNA levels. These findings are similar to those made by Stockert and colleagues (29) in their investigation of biotin regulation of ASGR expression. Our experiments were modeled on those of Stockert, and the involvement of sGC and PGK was also demonstrated in his study. Interestingly, however, those studies support a role for biotin in controlling the rate of translation of ASGR rather than transcription, as suggested here. It is possible that multiple mechanisms are involved, but it would be prudent to look for a unifying mechanism for the action of biotin on gene expression.
Key to our studies was the use of MCD cells to distinguish between a direct effect of biotin and one in which biotin had to be metabolized to produce an effect on transcription. MCD-MK cells are homozygous for the mutation R508W, which is a strikingly biotin-responsive mutation in vitro (16) and is a frequent mutation detected in patients with biotin-responsive MCD (16, 31, 32). Incubation of biotin-starved normal and mutant cells with biotin showed that 10- to 100-fold higher biotin concentration was required by the mutant cells to restore HCS mRNA to similar levels. By comparing the capacity of biotin versus 8-Br-cGMP to stimulate HCS mRNA levels in these cell lines, we demonstrated both the essential contribution of HCS and the downstream role of cGMP in these cells, which was unaffected by the presence of mutant HCS. The immediate product of HCS interaction with biotin is the generation of B-AMP. It, in turn, is the substrate for biotin transfer to apocarboxylases, completed as the second half reaction of HCS. We suggest that B-AMP is a good candidate for a biotin-based regulatory molecule.
Our studies also raise the possibility that HCS itself may participate as a regulatory molecule. There is precedent for this hypothesis in the behavior of its bacterial counterpart, BirA, which, in addition to its biotin ligase role, is also the repressor of the biotin operon in Escherichia coli. Mechanistically, the complex, BirA–B-AMP, acts as a direct transcriptional repressor of the genes of the biotin operon (6). Its DNA-binding domain is in the N-terminal half of the protein. It is attractive to consider an analogous complex in eukaryotes between HCS and B-AMP that might have a regulatory function. For example, it has been shown that the biotin ligase function of HCS resides in the C-terminal half of the protein (38). Although the N-terminal half does not contain a recognizable DNA-binding domain, as found for BirA, it may contain sequences that are involved, perhaps as an HCS–B-AMP complex, in the activation of the sGC–cGMP-dependent protein kinase pathway. Additional studies will be required to resolve its role in the regulation of gene expression.
A significant issue in the reduction of HCS and carboxylase mRNA levels is the role of impaired carboxylases during biotin deficiency. It is possible that metabolites accumulating because of reduced activity of the carboxylases are responsible for depressing mRNA levels, which, with their disappearance after biotin replenishment, allows the mRNAs to return to starting levels. However, if this were true, deficiency of individual carboxylases, such as deficiency of PCC activity in propionic acidemia (33), would be expected to result in impairment of synthesis of other carboxylases or, in particular, of HCS, if mRNA and consequently protein levels were depressed. Also, patients would be expected to show accumulation not only of the defective enzyme substrate, but ultimately of other carboxylase substrates. To our knowledge, none of these outcomes has ever been described (11, 33).
A previous report showed that biotin deficiency in rat results in a reduction in HCS mRNA levels, but leaves PC and PCC mRNA unaffected (27). In this study we show that biotin deficiency in human HepG2 cells reduces HCS, PCC, and ACC-1 mRNA levels. The difference in the response in PCC mRNA levels to biotin in rat and human hepatocytes could be explained by technical and experimental model differences or the presence of a species-specific effect of biotin on carboxylase expression. However, our results seem to be confirmed by previous observations made in patients with colorectal cancer. Primary adenocarcinoma cells showed lower biotin concentration in comparison with normal mucosa cells and a reduction of transcript levels of the PCCA and PCCB genes (34).
In summary, we have proposed that biotin acts on mRNA levels through a signaling cascade that requires the action of HCS, sGC, and the cGMP-dependent protein kinase. A role for HCS in control of carboxylase gene expression has implications for the treatment of MCD. It is possible that the clinical and biochemical deficits in MCD patients reflect the combined effect of the low affinity of mutant HCS for biotin and the concomitant reduction in carboxylase and HCS mRNAs levels, in effect an exacerbation of the disease process. Conversely, our findings suggest that the dramatic response of patients to pharmacological doses of biotin represents the combined effect of overcoming the reduced affinity of the mutant enzyme as well as elevating enzyme levels through the mRNA increase.
Acknowledgments
We thank Drs. Roy A. Gravel and Monica Narang (University of Calgary) for invaluable discussion and for comments to the manuscript and Rafael Cervantes Roldán for technical assistance. These studies were supported by grants of Consejo Nacional de Ciencia y Tecnología and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica from Universidad Nacional Autónoma de México. R.S.S.-V. and D.P.-A. are recipients of a scholarship from Consejo Nacional de Ciencia y Tecnología.
Abbreviations
- HCS
holocarboxylase synthetase
- ACC
acetyl-CoA carboxylase
- PCCA
α subunit of propionyl-CoA carboxylase
- MCD
multiple carboxylase deficiency
- B-AMP
biotinyl-5′-AMP
- sGC
soluble guanylate cyclase
- PKG
cGMP-dependent protein kinase
- ODQ
1-H[1,2,4]oxadiazolo-[4,3-a]quinaxolin-1-one
- 8-Br-cGMP
8-bromo-cyclic guanosine monophosphate
- Rp-cGMPS
Rp-8(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphorothioate
- ASGR
asialoglycoprotein receptor
References
- 1.Wood H G, Barden R E. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg M A, Prakash O, Hsiung S C. J Biol Chem. 1982;257:15167–15173. [PubMed] [Google Scholar]
- 3.Cronan J E., Jr Cell. 1989;58:427–429. doi: 10.1016/0092-8674(89)90421-2. [DOI] [PubMed] [Google Scholar]
- 4.Sundaram T K, Cazzulo J J, Kornberg H L. Arch Biochem Biophys. 1971;143:609–616. doi: 10.1016/0003-9861(71)90246-3. [DOI] [PubMed] [Google Scholar]
- 5.Cazzulo J J, Sundaram T K, Dilks S N, Kornberg H L. Biochem J. 1971;122:653–661. doi: 10.1042/bj1220653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman-Smith A, Cronan J E, Jr, John E. Trends Biochem Sci. 1999;24:359–363. doi: 10.1016/s0968-0004(99)01438-3. [DOI] [PubMed] [Google Scholar]
- 7.León-Del-Río A, Gravel R A. J Biol Chem. 1994;269:22964–22968. [PubMed] [Google Scholar]
- 8.Cronan J E., Jr J Biol Chem. 1990;265:10327–10333. [PubMed] [Google Scholar]
- 9.León-Del Río A, Leclerc D, Akerman B, Wakamatsu N, Gravel R A. Proc Natl Acad Sci USA. 1995;92:4626–4630. doi: 10.1073/pnas.92.10.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Aoki Y, Chiba Y, Iwamatsu A, Kishino T, Niikawa N, Matsubara Y, Narisawa K. Nat Genet. 1994;8:122–128. doi: 10.1038/ng1094-122. [DOI] [PubMed] [Google Scholar]
- 11.Wolf B. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Kinzler K W, Vogelstein B, Sly W S, Valle D, Childs B, editors. Vol. 3. New York: McGraw–Hill Professional; 2001. pp. 3935–3961. [Google Scholar]
- 12.Bartlett K, Ghneim H K, Stirk J H, Wastell H J, Sherratt H S, Leonard J V. Ann NY Acad Sci. 1985;447:235–251. [PubMed] [Google Scholar]
- 13.Sherwood W G, Saunders M, Robinson B H, Brewster T, Gravel R A. J Pediatr. 1982;101:546–550. doi: 10.1016/s0022-3476(82)80697-5. [DOI] [PubMed] [Google Scholar]
- 14.Sweetman L, Nyhan W L, Sakati N A, Ohlsson A, Mange M S, Boychuk R B, Kaye R. J Inherit Metab Dis. 1982;5:49–53. doi: 10.1007/BF01799754. [DOI] [PubMed] [Google Scholar]
- 15.Burri B J, Sweetman L, Nyhan W L. Am J Hum Genet. 1985;373:26–337. [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis L, Campeau E, Leclerc D, Gravel R A. Mol Genet Metab. 1999;66:80–90. doi: 10.1006/mgme.1998.2785. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto O, Suzuki Y, Li X, Aoki Y, Hiratsuka M, Sourmala T, Baumgartner E R, Gibson K M, Narisawa K. Pediatr Res. 1999;46:671–676. doi: 10.1203/00006450-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Morita J, Thuy L P, Sweetman L. Mol Genet Metab. 1998;64:250–255. doi: 10.1006/mgme.1998.2700. [DOI] [PubMed] [Google Scholar]
- 19.Barker D F, Campbell A M. J Mol Biol. 1981;146:469–492. doi: 10.1016/0022-2836(81)90043-7. [DOI] [PubMed] [Google Scholar]
- 20.Buoncristiani M R, Howard P K, Otsuka A J. Gene. 1986;44:255–261. doi: 10.1016/0378-1119(86)90189-7. [DOI] [PubMed] [Google Scholar]
- 21.Borboni P, Magnaterra R, Rabini R A, Staffolani R, Porzio O, Sesti G, Fusco A, Mazzanti L, Lauro R, Marlier L N. Acta Diabetol. 1996;33:154–158. doi: 10.1007/BF00569427. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan J, Dakshinamurti K. J Biol Chem. 1991;266:10035–10038. [PubMed] [Google Scholar]
- 23.Spence J T, Koudelka A P. J Biol Chem. 1984;259:6393–9386. [PubMed] [Google Scholar]
- 24.Deodhar A D, Mistry S P. Life Sci II. 1970;9:581–588. doi: 10.1016/0024-3205(70)90166-9. [DOI] [PubMed] [Google Scholar]
- 25.Dakshinamurti K, Tarrago-Litvak L, Hong H C. Can J Biochem. 1970;48:493–500. doi: 10.1139/o70-079. [DOI] [PubMed] [Google Scholar]
- 26.Collins J C, Paietta E, Green R, Morell A G, Stockert R J. J Biol Chem. 1988;263:11280–11283. [PubMed] [Google Scholar]
- 27.Rodriguez-Melendez R, Cano S, Mendez S T, Velazquez A. J Nutr. 2001;131:1909–1913. doi: 10.1093/jn/131.7.1909. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett K, Gompertz D. Lancet. 1976;2:804. doi: 10.1016/s0140-6736(76)90640-1. [DOI] [PubMed] [Google Scholar]
- 29.De La Vega L A, Stockert J R. Am J Physiol. 2000;279:C2037–C2042. doi: 10.1152/ajpcell.2000.279.6.C2037. [DOI] [PubMed] [Google Scholar]
- 30.Pisarev M A, de Pisarev D L. Acta Endocrinol. 1977;84:297–302. doi: 10.1530/acta.0.0840297. [DOI] [PubMed] [Google Scholar]
- 31.Dupuis L, Leon-Del-Rio A, Leclerc D, Campeau E, Sweetman L, Saudubray J M, Herman G, Gibson K M, Gravel R A. Hum Mol Genet. 1996;5:1011–1016. doi: 10.1093/hmg/5.7.1011. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Aoki Y, Li X, Sakamoto O, Hiratsuka M, Gibson K M, Kure S, Narisawa K, Matsubara Y, Suzuki Y. J Hum Genet. 2000;45:358–362. doi: 10.1007/s100380070008. [DOI] [PubMed] [Google Scholar]
- 33.Wolf B, Hsia Y E, Sweetman L, Gravel R A, Harris D J, Nyhan W L. J Pediatr. 1981;99:835–846. doi: 10.1016/s0022-3476(81)80004-2. [DOI] [PubMed] [Google Scholar]
- 34.Cherbonnel-Lasserre C L, Linares-Cruz G, Rigaut J P, Sabatier L, Dutrillaux B. Int J Cancer. 1997;72:768–775. doi: 10.1002/(sici)1097-0215(19970904)72:5<768::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Ha J, Lee J K, Kim K S, Witters L A, Kim K H. Proc Natl Acad Sci USA. 1996;93:11466–11470. doi: 10.1073/pnas.93.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Elheiga L, Almarza-Ortega D B, Baldini A, Wakil S J. J Biol Chem. 1997;272:10669–10677. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y C, Martin E, Murad F. Proc Natl Acad Sci USA. 2000;97:10763–10768. doi: 10.1073/pnas.190333697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campeau E, Gravel R A. J Biol Chem. 2001;276:12310–12316. doi: 10.1074/jbc.M009717200. [DOI] [PubMed] [Google Scholar]