ABSTRACT
Introduction
Objective was to describe the association between baseline characteristics and the number of Symptom Screening in Pediatrics Tool (SSPedi) assessments completed over an 8‐week period.
Methods
This was a sub‐analysis of a cluster randomized controlled trial among 10 sites that were randomized to the intervention group. Participants were English‐ or Spanish‐speaking pediatric patients 8–18 years of age newly diagnosed with cancer. Participants were prompted to complete SSPedi three times weekly for 8 weeks. The outcome was the number of SSPedi assessments completed during the 8‐week period. Factors associated with the number of assessments were determined using mixed effects Poisson regression.
Results
At the 10 intervention sites, 216 patients were included in the analysis. Among these participants, 129 (59.7%) were male, 112 (51.9%) were white, and 83 (38.4%) were Hispanic. The number of SSPedi assessments was significantly higher for participants 11–14 years (rate ratio (RR) 1.13, 95% confidence interval (CI) 1.02–1.25) and 15–18 years (RR 1.15, 95% CI 1.04–1.27) compared to 8–10 years. Participants completed more SSPedi assessments if they were Asian compared to white (RR 1.27, 95% CI 1.10–1.46), non‐Hispanic compared to Hispanic (RR 1.15, 95% CI 1.04–1.28) and from families with a household income ≥$60,000 (RR 1.12, 95% CI 1.03–1.21). Participants completed fewer SSPedi assessments if they had solid tumors compared to leukemia (RR 0.91, 95% CI 0.84–0.99).
Conclusion
Adherence to three‐times weekly SSPedi varied by age, race, ethnicity, cancer diagnosis, and family income. This information may facilitate interventions to support routine symptom screening in clinical practice.
Trial Registration: NCT04614662.
Keywords: adherence, oncology, pediatric, symptom screening
1. Introduction
Pediatric patients receiving cancer therapy commonly experience severely bothersome symptoms. Common symptoms include fatigue, changes in hunger, pain, and nausea [1]. These symptoms are rarely documented or treated [2]. To improve symptom control, patients benefit from the implementation of routine symptom screening and clinical practice guideline‐consistent care for symptom management [3]. Symptom screening might be useful as documentation and interventions for symptom management are poor, even when patients experience severely bothersome symptoms [2, 4].
In contrast to adult cancer patients, routine symptom screening is uncommon in pediatric patients. It is uncertain whether pediatric patients would be willing to self‐report symptoms on an ongoing basis. We recently completed a study where patients newly diagnosed with cancer were prompted to complete symptom screening three times weekly for 8 weeks [5, 6]. In this study, symptom screening, when combined with adapted care pathways for symptom management, improved symptom control across most symptoms [5, 6]. Symptom screening could be performed as outpatients or inpatients, mirroring pediatric cancer care settings.
For symptom screening to be effective, pediatric patients must be willing to provide repeated symptom scores. We used the 8‐week longitudinal study as an opportunity to understand participant characteristics associated with the number of Symptom Screening in Pediatrics Tool (SSPedi) assessments submitted. Consequently, the objective was to describe the association between baseline characteristics and the number of SSPedi assessments completed over an 8‐week period.
2. Materials and Methods
This was a sub‐analysis of a cluster randomized controlled trial in which 10 sites were randomized to the intervention group and 10 sites were randomized to the control group. This report focuses on participants enrolled at the 10 intervention sites [5]. The study was approved by the Western Institutional Review Board, each participating site's Institutional Review Board, and the Research Ethics Board at The Hospital for Sick Children. Informed consent and assent were obtained from participants and guardians as appropriate. The study was registered with clinicaltrials.gov (NCT04614662).
2.1. Eligibility
Participants were English‐ or Spanish‐speaking pediatric cancer patients 8–18 years of age with a plan for any cancer treatment. They had to have been newly diagnosed with cancer or have started cancer treatment within the 4 weeks prior to enrollment. Those with cognitive disabilities or visual impairments precluding routine symptom screening were excluded.
2.2. Study Procedures
Intervention sites adapted symptom management care pathway templates based upon clinical practice guidelines before the site was activated to participant enrollment. Once activated, patients were approached in the inpatient setting, outpatient setting, or remotely. Participants could consent to provide patient‐reported outcomes or to chart review only. For those who agreed to provide patient‐reported outcomes, demographic characteristics were obtained from the participant, family, or health record. Participants were set up to complete SSPedi on their personal device, which could be a phone, tablet, or computer. If a device was not available, a tablet was loaned to them for the study duration. SSPedi is a self‐report symptom screening and assessment tool that is reliable, valid, and responsive to change in pediatric patients receiving cancer treatments 8–18 years of age [7]. SSPedi is available in English, Spanish [8, 9] and French [10] although only the English and Spanish versions were used in this study. SSPedi completion requires about 1 min to complete.
We used a web application named Supportive care Prioritization, Assessment and Recommendations for Kids (SPARK) to enable participants to receive reminders, allow participants and healthcare professionals to track symptoms, and provide email alerts to the healthcare team [11, 12]. Participants received reminders to complete symptom screening by text or email according to their preference. They also could enable their guardian to receive a concurrent reminder to facilitate pediatric patient self‐report. They were reminded to complete symptom screening three times weekly for 8 weeks, but they could complete SSPedi on their own at any time outside of the reminders. Participants could choose the days of the week and the times of reminders. These days and times could be changed during the eight‐week period with the assistance of the local research team. If participants did not complete any SSPedi assessments after 1–2 weeks, the local research team reached out to the participant to identify if they were experiencing challenges such as forgetting their password. Each time participants completed SSPedi, an email alert was sent to their healthcare team if they reported at least one symptom that was severely bothersome, defined as a score of 3 or 4 on a 5‐point Likert scale ranging from 0 to 4. The healthcare team recipients were chosen by the site, and the recipients could change over time for individual participants.
To support symptom management, implementation materials such as posters, pens, and badges with QR codes linked to the site‐specific care pathways were created. The email alert based on severely bothersome symptoms included a link to these care pathways.
2.3. Statistical Analysis
The outcome for this analysis was the number of SSPedi assessments completed during the eight‐week period. We assessed the dependence of the number of SSPedi assessments completed on participant and guardian characteristics using a mixed effects Poisson regression model with a random effect for site. The model was fitted using maximum likelihood and included six participant characteristics (sex, age group, race, ethnicity, preferred language, and cancer diagnosis group) and three guardian characteristics (marital status, guardian employment status and high annual household income). Rate ratios (RR) for the number of SSPedi assessments completed per week were estimated for each level of a characteristic compared to its reference level, along with 95% confidence intervals (CI) and Wald‐test P values. Global tests of significance of each multi‐level characteristic were obtained from likelihood ratio tests comparing the full model (with all nine characteristics) to a model omitting that characteristic. As there were only 10 intervention sites, we did not evaluate site characteristics in the model. However, we described between‐site variability in average SSPedi adherence after consideration for site and adjustment for patient characteristics.
As a post hoc analysis, we also evaluated the impact of baseline SSPedi score quartile on adherence using the same mixed effects Poisson regression model. Tests of significance were two‐sided, and statistical significance was defined as p < 0.05. Statistical analysis was conducted using R 4.3.2.
3. Results
As previously reported, 217 participants were enrolled at intervention sites and agreed to provide patient‐reported outcomes [5, 6]. One participant came off the study prior to completing study observations and thus, 216 participants were included in this analysis [5]. Table 1 shows the demographic distribution of the cohort; 129 (59.7%) were male, 112 (51.9%) were white, and 83 (38.4%) were Hispanic or Latino.
TABLE 1.
Mean number of SSPedi assessments and their association with baseline characteristics (N = 216).
| Characteristic | N | Mean (SD) a | RR a (95% CI) | p | |
|---|---|---|---|---|---|
| vs. Reference | Group a | ||||
| Sex | |||||
| Female | 87 | 18.1 (8.0) | REF | NA | |
| Male | 129 | 17.6 (8.5) | 1.00 (0.93, 1.07) | 1.00 | |
| Age group in years | |||||
| 8–10 | 33 | 16.8 (8.4) | REF | — | 0.02 |
| 11–14 | 76 | 17.8 (8.0) | 1.13 (1.02, 1.25) | 0.02 | |
| 15–18 | 107 | 18.1 (8.5) | 1.15 (1.04, 1.27) | 0.008 | |
| Race | |||||
| American Indian or Alaska Native | 2 | 14.5 (17.7) | 1.13 (0.77, 1.67) | 0.52 | < 0.001 |
| Asian | 13 | 21.0 (7.5) | 1.27 (1.10, 1.46) | < 0.001 | |
| Black or African American | 13 | 16.2 (9.9) | 0.92 (0.78, 1.07) | 0.27 | |
| Native Hawaiian or Other Pacific Islander | 14 | 15.8 (6.3) | 0.92 (0.77, 1.11) | 0.39 | |
| White | 112 | 17.6 (8.4) | REF | — | |
| Unknown/prefer not to say | 40 | 18.0 (7.6) | 1.28 (1.13, 1.45) | < 0.001 | |
| More than one | 22 | 19.2 (9.0) | 1.14 (1.01, 1.29) | 0.03 | |
| Ethnicity | |||||
| Hispanic or Latino | 83 | 17.0 (8.5) | REF | — | 0.02 |
| Not Hispanic or Latino | 122 | 18.4 (8.1) | 1.15 (1.04, 1.28) | 0.009 | |
| Unknown/prefer not to say | 11 | 17.5 (9.3) | 1.01 (0.86, 1.19) | 0.90 | |
| Preferred language for patient‐reported outcomes | |||||
| English | 203 | 17.9 (8.3) | 1.12 (0.96, 1.31) | 0.15 | NA |
| Spanish | 13 | 16.2 (7.5) | REF | — | |
| Cancer diagnosis | |||||
| Leukemia | 87 | 18.2 (8.1) | REF | — | 0.03 |
| Lymphoma | 48 | 17.5 (8.5) | 0.92 (0.84, 1.01) | 0.08 | |
| Solid tumor | 73 | 17.0 (8.4) | 0.91 (0.84, 0.99) | 0.02 | |
| Brain tumor | 8 | 22.2 (8.1) | 1.09 (0.91, 1.30) | 0.37 | |
| Family composition | |||||
| Guardian married | 137 | 18.2 (8.3) | REF | NA | |
| Guardian not married | 79 | 17.1 (8.2) | 0.98 (0.91, 1.05) | 0.51 | |
| Guardian employment | |||||
| Full time or part time | 123 | 17.8 (8.2) | 0.93 (0.86, 1.00) | 0.05 | NA |
| Other | 93 | 17.9 (8.4) | REF | — | |
| Annual household income | |||||
| ≥ $60,000 | 77 | 19.2 (7.2) | 1.12 (1.03, 1.21) | 0.007 | NA |
| < $60,000 | 139 | 17.0 (8.7) | REF | — | |
Abbreviations: CI, confidence interval; NA, not applicable; RR, rate ratio; SD, standard deviation; SSPedi, Symptom Screening in Pediatrics Tool.
Mean number of assessments was only descriptive. Statistical association was determined using mixed effects Poisson regression adjusting for site as a random effect. p values for multi‐level characteristics were calculated from likelihood ratio tests.
Figure 1 shows a histogram describing the SSPedi assessment count distribution. Table 1 shows that the number of SSPedi assessments was significantly higher for participants 11–14 years (RR 1.13, 95% CI 1.02–1.25) and 15–18 years (RR 1.15, 95% CI 1.04–1.27) compared to 8–10 years. Participants completed more SSPedi assessments if they were Asian compared to white (RR 1.27, 95% CI 1.10–1.46), non‐Hispanic compared to Hispanic (RR 1.15, 95% CI 1.04–1.28), and from families with a household income ≥$60,000 (RR 1.12, 95% CI 1.03–1.21). Participants completed fewer SSPedi assessments if they had solid tumors compared to leukemia (RR 0.91, 95% CI 0.84–0.99). All multi‐level characteristics containing at least one statistically significant comparison to their reference group had statistically significant likelihood ratio tests. Table 1 also shows the mean number of SSPedi assessments for descriptive purposes.
FIGURE 1.
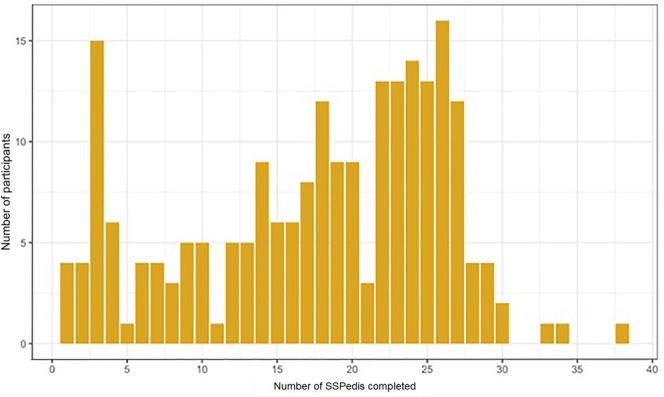
Bar graph showing number of SSPedi assessments completed. SSPedi, Symptom Screening in Pediatrics Tool.
Figure 2 shows substantial between‐site variability in average SSPedi adherence after consideration of site and adjustment for patient characteristics. Figure 3 shows the relationship between baseline SSPedi score on adherence. In the post hoc mixed effects Poisson regression analysis, baseline SSPedi quartile was not significantly associated with adherence (p = 0.38).
FIGURE 2.
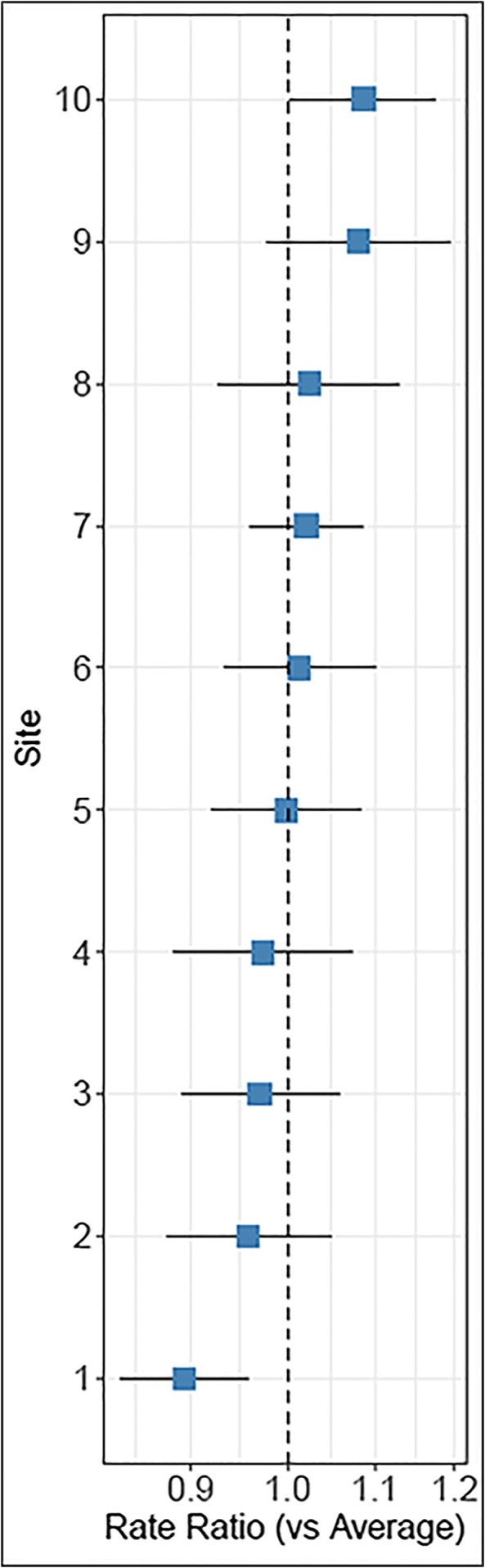
Number of SSPedi assessments completed by site compared to the average. SSPedi, Symptom Screening in Pediatrics Tool.
FIGURE 3.

Relationship between number of SSPedi assessments and Baseline Total SSPedi Score. SSPedi, Symptom Screening in Pediatrics Tool.
4. Discussion
We found that age, race, ethnicity, cancer diagnosis, and family income explained variability in SSPedi adherence over 8 weeks. More specifically, those with younger ages, Hispanic or Latino ethnicity, solid tumors, and households with lower family income completed significantly fewer SSPedi assessments. Asian participants completed significantly more SSPedi assessments compared to white participants.
Some studies have demonstrated high adherence rates to routine patient‐reported outcome monitoring among adult cohorts [13]. Factors identified to be associated with non‐adherence have included younger age, more comorbidities, absence of chemotherapy, and low physical functioning score at baseline [14]. A study of longitudinal symptom screening in adults showed that adherence decreased from 72% to 52% over a 10‐week period [15]. Nonetheless, a systematic review of chronic conditions outside of cancer was unable to conclude that any factor was consistently associated with non‐adherence [16].
In contrast to these reports, very few studies have evaluated factors associated with adherence in pediatric populations. Pediatric patients are expected to have additional challenges to symptom screening adherence compared to adult patients. Our previous study highlighted the importance of engaging with guardians to enable their child to self‐report SSPedi, particularly for younger children [17]. Thus, the finding that younger children reported fewer SSPedi assessments is not surprising. It is likely that independent completion of repeated SSPedi assessments will continue to be challenging among the youngest patients. It is for this reason that we created a novel structured dyadic approach to symptom screening that allows guardians to support their child while ensuring the child voices their perspectives first [18, 19, 20]. This instrument, named co‐SSPedi, is reliable, valid, and responsive to change [21].
It is also interesting that we showed that race, ethnicity, and household income were associated with SSPedi adherence. This finding suggests that specific interventions may be required to promote adherence among some patient sub‐groups. Such interventions will help to mitigate widening disparities that will arise without purposeful efforts. It is also interesting that there was heterogeneity in SSPedi adherence across sites. As this analysis adjusted for patient factors, it is more likely that this variability reflects site procedures, values, and possibly resources. While the number of sites did not permit evaluation of site characteristics on SSPedi adherence, interventions at the site level may also be helpful.
The increased adherence among leukemia participants compared to solid tumor participants may be related to increased inpatient stays and more frequent contact with the healthcare system in general for leukemia patients. While not statistically significant, it is interesting that brain tumor patients had qualitatively higher rates of SSPedi assessment completion. This observation may be related to symptom burden among this group. The low number of brain tumor patients may have been related to some of these patients receiving their primary care from non‐oncologists such as neurosurgeons. Nonetheless, the cancer group approach is likely too broad, and future work could evaluate SSPedi assessment adherence by specific diagnoses and treatment protocols.
A strength of this report is the ability to leverage a large database of longitudinal pediatric patient self‐report symptom screening assessments. Another strength is the multi‐center nature and the heterogeneity of the cohort. However, a limitation of the study is that we only instituted symptom screening for 8 weeks. Also, potentially important explanatory variables were not collected or were not available to the lead site. Future research should explicitly collect variables such as guardian receipt of reminders, access to information technology, and additional socioeconomic variables. Time‐dependent variables such as treatment intensity, concurrent chemotherapy, and inpatient or outpatient status could be included in this evaluation. Qualitative evaluation, which was not included as part of this study, might be particularly useful to identify if events such as emergency visits might increase or decrease SSPedi adherence.
5. Conclusion
Adherence to three‐times weekly SSPedi varied by age, race, ethnicity, and family income. This information may facilitate interventions to support routine symptom screening in clinical practice. Some sub‐populations might benefit from additional support, such as structured outreach to improve adherence to routine symptom screening.
Author Contributions
L. Lee Dupuis: conceptualization (equal), writing – original draft (equal), writing – review and editing (equal). Emily Vettese: conceptualization (equal), project administration (equal), writing – review and editing (equal). Catherine Aftandilian: conceptualization (equal), writing – review and editing (equal). Vibhuti Agarwal: conceptualization (equal), investigation (equal), writing – review and editing (equal). Christina Baggott: conceptualization (equal), investigation (equal), writing – review and editing (equal). Scott M. Bradfield: conceptualization (equal), writing – review and editing (equal). Nicole Crellin‐Parsons: conceptualization (equal), project administration (equal), writing – review and editing (equal). David R. Freyer: conceptualization (equal), investigation (equal), writing – review and editing (equal). Kara M. Kelly: conceptualization (equal), investigation (equal), writing – review and editing (equal). Allison A. King: conceptualization (equal), writing – review and editing (equal). Wade Kyono: conceptualization (equal), investigation (equal), writing – review and editing (equal). Ramamoorthy Nagasubramanian: conceptualization (equal), writing – review and editing (equal). Etan Orgel: conceptualization (equal), investigation (equal), writing – review and editing (equal). Michael E. Roth: conceptualization (equal), investigation (equal), writing – review and editing (equal). Farha Sherani: conceptualization (equal), investigation (equal), writing – review and editing (equal). Lolie Yu: conceptualization (equal), investigation (equal), writing – review and editing (equal). Allison C. Grimes: conceptualization (equal), writing – review and editing (equal). Melissa P. Beauchemin: conceptualization (equal), investigation (equal), writing – review and editing (equal). Lisa M. Klesges: conceptualization (equal), writing – review and editing (equal). George A. Tomlinson: conceptualization (equal), formal analysis (equal), writing – original draft (equal), writing – review and editing (equal). Lillian Sung: conceptualization (lead), writing – original draft (equal), writing – review and editing (equal).
Conflicts of Interest
The following authors declare conflicts of interest: E.O. consults with Jazz Pharmaceuticals.
Acknowledgements
The funding for this study was provided by a project grant from the Canadian Institutes of Health Research (PJT‐169165), and an R01 from the National Institutes of Health (R01CA251112). Care pathway development procedures and early SSPedi development work were supported by the Pediatric Oncology Group of Ontario. L.S. is supported by the Canada Research Chair in Pediatric Oncology Supportive Care.
Dupuis L. L., Vettese E., Aftandilian C., et al., “Factors Associated With Self‐Report Symptom Screening Adherence in Pediatric Cancer Patients,” Cancer Medicine 14, no. 14 (2025): e71053, 10.1002/cam4.71053.
Funding: This work was supported by Canadian Institutes of Health Research, PJT‐169165. National Institutes of Health, R01CA251112.
L. Lee Dupuis and Emily Vettese co‐first authors.
Data Availability Statement
The data that support the findings of this study are openly available at https://clinicaltrials.gov/.
References
- 1. Johnston D. L., Hyslop S., Tomlinson D., et al., “Describing Symptoms Using the Symptom Screening in Pediatrics Tool in Hospitalized Children With Cancer and Hematopoietic Stem Cell Transplant Recipients,” Cancer Medicine 7, no. 5 (2018): 1750–1755, 10.1002/cam4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hyslop S., Davis H., Duong N., et al., “Symptom Documentation and Intervention Provision for Symptom Control in Children Receiving Cancer Treatments,” European Journal of Cancer 109 (2019): 120–128, 10.1016/j.ejca.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 3. Dupuis L. L., Cook S., Robinson P. D., Tomlinson D., Vettese E., and Sung L., “Optimizing Symptom Control in Children and Adolescents With Cancer,” Pediatric Research 86, no. 5 (2019): 573–578, 10.1038/s41390-019-0516-3. [DOI] [PubMed] [Google Scholar]
- 4. Tomlinson D., Chakkalackal L., Calligan M., et al., “Symptom Documentation and Intervention in Paediatric Cancer Care‐Association With Severity: Observational Study,” BMJ Supportive & Palliative Care 13, no. e3 (2024): e1265–e1271, 10.1136/spcare-2022-003874. [DOI] [PubMed] [Google Scholar]
- 5. Dupuis L. L., Vettese E., Grimes A., et al., “Symptom Screening Linked to Care Pathways for Pediatric Patients With Cancer: A Cluster Randomized Trial,” (2024) In Press. [DOI] [PMC free article] [PubMed]
- 6. Dupuis L. L., Johnston D., Dix D., et al., “A Randomized Controlled Trial of Symptom Screening for Hospitalized Pediatric Patients With Cancer,” (2024) In Press. [DOI] [PMC free article] [PubMed]
- 7. Dupuis L. L., Johnston D. L., Baggott C., et al., “Validation of the Symptom Screening in Pediatrics Tool in Children Receiving Cancer Treatments,” Journal of the National Cancer Institute 110, no. 6 (2018): 661–668, 10.1093/jnci/djx250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gomez S., Salaverria C., Plenert E., et al., “Translating the Symptom Screening in Pediatrics Tool (SSPedi) Into Argentinian Spanish for Paediatric Patients Receiving Cancer Treatments, and Evaluating Understandability and Cultural Relevance in a Multiple‐Phase Descriptive Study,” BMJ Open 11, no. 4 (2021): e048287, 10.1136/bmjopen-2020-048287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plenert E., Grimes A., Sugalski A., et al., “Translating the Symptom Screening in Paediatrics Tool (SSPedi) Into North American Spanish and Among Spanish‐Speaking Children Receiving Cancer Treatments: Evaluating Understandability and Cultural Relevance in a Multiple‐Phase Descriptive Study,” BMJ Open 10, no. 11 (2020): e037406, 10.1136/bmjopen-2020-037406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Larouche V., Revon‐Riviere G., Johnston D., et al., “Translating the Symptom Screening in Pediatrics Tool (SSPedi) Into French and Among French‐Speaking Children Receiving Cancer Treatments, Evaluating Understandability and Cultural Relevance in a Multiple‐Phase Descriptive Study,” BMJ Open 10, no. 4 (2020): e035265, 10.1136/bmjopen-2019-035265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vettese E., Cook S., Soman D., et al., “Longitudinal Evaluation of Supportive Care Prioritization, Assessment and Recommendations for Kids (SPARK), a Symptom Screening and Management Application,” BMC Cancer 19, no. 1 (2019): 458, 10.1186/s12885-019-5662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook S., Vettese E., Soman D., et al., “Initial Development of Supportive Care Assessment, Prioritization and Recommendations for Kids (SPARK), a Symptom Screening and Management Application,” BMC Medical Informatics and Decision Making 19, no. 1 (2019): 9, 10.1186/s12911-018-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehmann J., de Ligt K. M., Tipelius S., et al., “Adherence to Patient‐Reported Symptom Monitoring and Subsequent Clinical Interventions for Patients With Multiple Myeloma in Outpatient Care: Longitudinal Observational Study,” Journal of Medical Internet Research 25 (2023): e46017, 10.2196/46017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gebert P., Hage A. M., Blohmer J. U., Roehle R., and Karsten M. M., “Longitudinal Assessment of Real‐World Patient Adherence: A 12‐Month Electronic Patient‐Reported Outcomes Follow‐Up of Women With Early Breast Cancer Undergoing Treatment,” Supportive Care in Cancer 32, no. 6 (2024): 344, 10.1007/s00520-024-08547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patt D., Wilfong L., Hudson K. E., et al., “Implementation of Electronic Patient‐Reported Outcomes for Symptom Monitoring in a Large Multisite Community Oncology Practice: Dancing the Texas Two‐Step Through a Pandemic,” JCO Clinical Cancer Informatics 5 (2021): 615–621, 10.1200/CCI.21.00063. [DOI] [PubMed] [Google Scholar]
- 16. Wiegel J., Seppen B., van der Leeden M., Esch M., de Vries R., and Bos W., “Adherence to Telemonitoring by Electronic Patient‐Reported Outcome Measures in Patients With Chronic Diseases: A Systematic Review,” International Journal of Environmental Research and Public Health 18, no. 19 (2021): 10161, 10.3390/ijerph181910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calligan M., Chakkalackal L., Dadzie G., et al., “Feasibility of Three Times Weekly Symptom Screening in Pediatric Cancer Patients,” BMC Cancer 23, no. 1 (2023): 4, 10.1186/s12885-022-10400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomlinson D., Tardif‐Theriault C., Schechter T., Dupuis L. L., and Sung L., “Randomized Trial of Dyadic‐Report vs Proxy‐Report and Self‐Report Symptom Assessment for Pediatric Patients Receiving Cancer Treatments,” Journal of the National Cancer Institute 116, no. 4 (2024): 588–595, 10.1093/jnci/djad251. [DOI] [PubMed] [Google Scholar]
- 19. Tomlinson D., Schechter T., Mairs M., et al., “Finalising the Administration of Co‐SSPedi, a Dyad Approach to Symptom Screening for Paediatric Patients Receiving Cancer Treatments,” BMJ Supportive & Palliative Care 13, no. e2 (2023): e469–e475, 10.1136/bmjspcare-2021-003169. [DOI] [PubMed] [Google Scholar]
- 20. Tomlinson D., Plenert E., Dadzie G., et al., “Discordance Between Pediatric Self‐Report and Parent Proxy‐Report Symptom Scores and Creation of a Dyad Symptom Screening Tool (Co‐SSPedi),” Cancer Medicine 9, no. 15 (2020): 5526–5534, 10.1002/cam4.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomlinson D., Dupuis L. L., Dix D., et al., “Validation of Co‐Symptom Screening in Pediatrics Tool: A Novel Dyadic Approach to Symptom Screening in Pediatric Patients Receiving Cancer Treatment,” Journal of the National Cancer Institute 116, no. 1 (2024): 160–166, 10.1093/jnci/djad181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available at https://clinicaltrials.gov/.


