SUMMARY
Ser/Thr protein phosphatase 1 (PP1) forms a large nuclear holoenzyme (with PNUTS, WDR82, and Tox4) whose emerging role is to regulate transcription. However, the role of Tox4, and its interplay with the other phosphatase subunits in this complex, is poorly understood. Here, we combine biochemical, structural, cellular, and in vivo experiments to show that, while tox4 is dispensable for viability, it is essential for fertility, having both PNUTS-dependent and -independent roles in Drosophila germline development. We also show that Tox4 requires zinc for PNUTS TFIIS N-terminal domain (TND) binding, and that it binds the TND on a surface distinct from that used by established TND-interacting transcriptional regulators. We also show that selective disruption of the PNUTS-Tox4 and the PNUTS-PP1 interaction is critical for normal gene expression and chromosomal dispersal during oogenesis. Together, these data demonstrate how interactions within the PNUTS-Tox4-PP1 phosphatase combine to tune transcriptional outputs driving developmental transitions.
In brief
Duncalf et al. investigate the evolutionarily conserved interaction between the PNUTS N-terminal domain and the TOX4 C-terminal domain, resolving the PNUTS:TOX4 complex crystal structure. Leveraging these insights, the authors define the functional significance of Tox4-PNUTS-PP1 interactions in Drosophila germline development, highlighting their cooperative role in developmentally controlled chromosome reorganization.
Graphical Abstract

INTRODUCTION
Multivalent binding proteins play vital roles in recruiting regulatory enzymes and effectors at defined cellular sites to ensure specific biological outcomes. During eukaryotic gene expression, multiprotein phosphatase-containing complexes are essential to control RNA polymerase II (RNAPII) at sites of transcription, particularly to ensure transcription cycle phase transitions, such as regulating the release from polymerase pausing during early elongation and ensuring productive mRNA synthesis.1,2 This is because many transcription factors are functionally regulated by (de)phosphorylation. In addition, the reversible phosphorylation of multiple residues in the RNAPII C-terminal domain (RNAPII-CTD) controls its association with distinct factors that serve to coordinate elongation with co-transcriptional RNA processing.3,4
Protein phosphatase 1 (PP1) is the most widely expressed and abundant serine/threonine phosphatase, with roles during multiple steps of mRNA synthesis, as well as many other unrelated processes such as protein synthesis, muscle contraction, and carbohydrate metabolism.5–8 It achieves high specificity by interacting with more than 200 distinct regulatory proteins.7,9–11 The PP1 nuclear targeting subunit (PNUTS, also known as PPP1R10/p99/FB19/CAT53), which is one of the most abundant regulatory proteins of PP1 in the nucleus,12,13 has emerged as an essential regulator of gene expression. PNUTS regulates multiple steps of mRNA synthesis to control RNAPII pause release,14,15 block promiscuous initiation,16,17 facilitate RNA splicing,18 and promote transcription termination.19,20 In addition to targeting RNAPII-CTD Ser5 for dephosphorylation by PP1,20–22 PNUTS also regulates the functionally critical phosphorylation state of transcription factors, SPT5,19 p5323, and MYC.24 PNUTS also regulates retinoblastoma (Rb) protein in response to cellular stress,25,26 and associates with chromatin to promote chromosome decondensation,27 mitotic exit,28 and DNA damage responses.29–32 How PNUTS, via PP1 scaffolding, mediates these distinct functions remains a largely open question.
Central to PNUTS function within the PNUTS:PP1 phosphatase holoenzyme complex is its role as protein interaction hub. PNUTS, a largely intrinsically disordered protein, contains multiple functional units/domains, in addition to its central PP1 binding domain (residues 394–433). These include a folded transcriptional elongation factor S-II N-terminal homology domain (TFIIS TND; residues 1–160), an intrinsically disordered region (IDR) WDR82-interaction domain, C-terminal RGG RNA-binding repeats (residues 850–900), and a zinc finger domain (residues 900–940) (Figure 1A). Its TND has emerged as a key transcription assembly module that interacts directly and reversibly with a set of cognate disordered ligands known as TND-interacting motifs (TIMs) present in the transcription factor MYC,33 as well as transcription elongation regulators including IWS1, SPT6, and PAF1.34 Via these domains, PNUTS scaffolds a constitutive complex containing Tox4, Wdr82, and PP1, hereafter referred to as the PTW:PP1 complex.35 PTW:PP1 complex formation allows for the recruitment of RNAPII-CTD Ser536 and stimulates the RNAPII-CTD dephosphorylation by PP1 in vivo.20 Wdr82 is a structured WD40 domain protein that binds PNUTS just C-terminal to its PP1 binding domain.20,35 Tox4 is predicted to be a largely unstructured member of the DNA-binding high-mobility group box protein family (Figure 1A), which has been reported to restrict RNAPII pause release and early elongation, promote late elongation, and facilitate transcriptional reinitiation.37,38 However, in the absence of molecular information, how PNUTS assembles Tox4 with other PTW:PP1 components into functional transcription complexes remains under-investigated.
Figure 1. Evolutionarily conserved interaction between PNUTS N-terminal domain and Tox4 C-terminal domain.
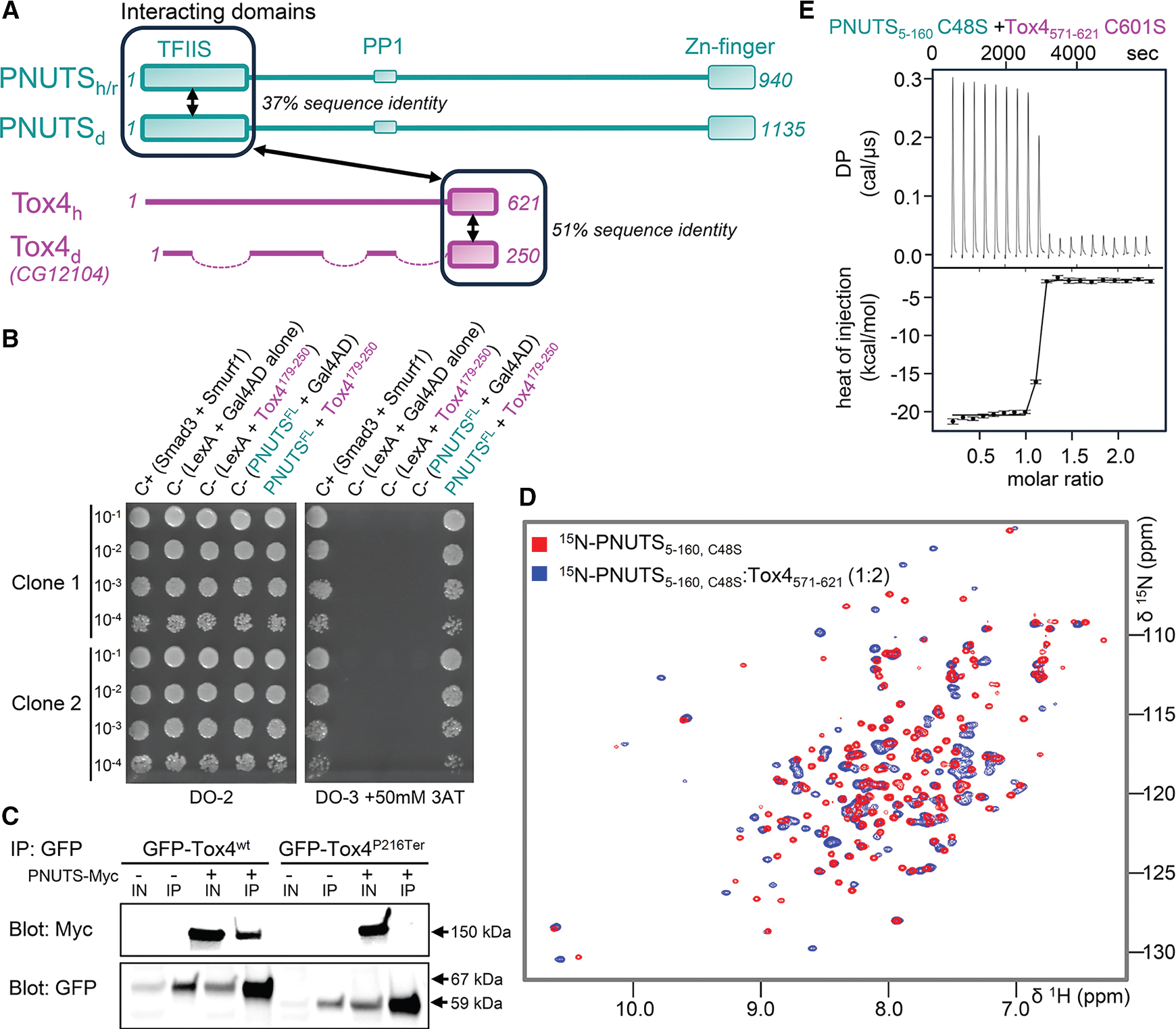
(A) Domain organization of mammalian (human/rat) and fly (Drosophila) PNUTS (aqua) and Tox4 (pink), with key domains labeled. Sequence identity between mammalian and fly for the PNUTS N-terminal TFIIS domain and the Tox4 C-terminal domain are indicated. Domains that interact between PNUTS and Tox4 are shown by a double arrowed line.
(B) Drosophila PNUTS interacts with Tox4 in the yeast two-hybrid system. PNUTS full-length bait (PNUTS1–1135-LexA DNA binding domain) and Tox4 prey (Tox4179–250-Gal4 activation domain) plasmids were tested in duplicate (clones 1 and 2) at several dilutions, as indicated. Auxotrophic growth on media without tryptophan and leucine (DO-2) or without tryptophan, leucine, and histidine with 50 mM 3-aminotriazol (DO-3+ 3-AT) is shown. C+, positive control with interacting bait and prey plasmids for Smad3 and Smurf1, respectively; C–, negative controls, as indicated.
(C) Binding of Myc-tagged PNUTS to GFP-tagged Tox4 requires the Tox4 C terminus (residues 216–250) in pull-downs from Drosophila S2R+ cells. Immunoblots show total protein extract (IN) and immunoprecipitated protein extract (IP) from S2R+ cells co-transfected with GFP-tagged wild-type (Tox4wt) or truncated (Tox4P216Term) Tox4, with or without myc-tagged PNUTS.
(D) 2D [1H,15N]HSQC spectrum of 15N-labeled PNUTS5–160 C48S alone (red) and in complex with Tox4571–621 (blue).
(E) Binding isotherm of PNUTS5–160 C48S with Tox4571–621 C601S.
Our previous data showed that the PNUTS:PP1 holoenzyme is necessary for developmental gene expression,21,39 but the identity of its interacting partners and their contribution to PNUTS function in vivo was not known. Here, we identify Drosophila Tox4 as a PNUTS binding protein, show that it binds zinc, and demonstrate that zinc binding is critical for the formation of the PNUTS:Tox4 complex. Our crystal structure of the Tox4: PNUTS complex shows that interaction between these proteins is extensive and, as we show using binding affinity measurements, constitutive, consistent with the previous observations that the PTW:PP1 complex is stable throughout the cell cycle. We also show that, while Drosophila tox4 is dispensable for viability, it is essential for fertility, having both PNUTS-dependent and -independent roles in Drosophila germline development. Finally, using structure-based mutagenesis, we show that integration of both functions by PNUTS is necessary for normal gene expression and germline development. Collectively, these findings provide insight into the function of Tox4, both independently and in coordination with other PTW:PP1 components, in shaping transcriptional programs during phases of cellular and tissue maturation.
RESULTS
Tox4:PNUTS binding is evolutionarily conserved in Drosophila
We identified Drosophila CG12104, which encodes the only Drosophila Tox4 homolog,40 in a yeast two-hybrid (Y2H) screen for Drosophila PNUTS-interacting proteins. Domain analysis of 61 recovered tox4 Y2H cDNA clones showed that the PNUTS-interacting region mapped to the Tox4 C terminus (residues 206–246) (Figure 1B), consistent with previous deletion co-immunoprecipitation studies showing that the C terminus of human Tox4 binds to the N terminus of PNUTS.35 These data confirm the evolutionarily conserved nature of PNUTS:Tox4 interactions. To further validate the importance of the Tox4 C terminus for binding, Drosophila S2R+ cells were transiently transfected with Myc-tagged Drosophila PNUTS and GFP-tagged Drosophila Tox4wt or GFP-Tox4P216Ter, which lacks the last 34 residues of Tox4. Blotting with an anti-GFP antibody confirmed expression of GFP-Tox4 in cell lysates. GFP-Toxwt, but not GFP-Tox4P216Ter, co-precipitated with Myc-tagged PNUTS (Figure 1C). Together, these data confirm that the C terminus of Drosophila Tox4 is required for PNUTS binding in Drosophila S2R+ cells.
The C-terminal domain of Tox4 is a zinc binding domain that binds constitutively to PNUTS
We next tested the interaction of the PNUTS TND with the C-terminal domain of Tox4 in vitro. PNUTS TND domain constructs (rat, aa 1–160, 5–160; Figure 1A) are readily expressed in E. coli. The high-quality 2D [1H,15N]HSQC spectrum of PNUTSTND (for NMR spectroscopy and X-ray crystallography, PNUTS construct 5–160 [C48S] was used throughout the study; Figure 1D) showed that the PNUTSTND is well folded. In contrast, while Tox4CTD (human Tox4571–621) purifies readily, its 2D [1H,15N]HSQC spectrum was of low quality with many peaks showing significant broadening (Figure S1). After exhausting canonical strategies to improve the NMR spectrum, we used the fold and function assignment system (FFAS)41 to identify proteins that are predicted to exhibit structural homology to Tox4CTD. FFAS predicted a weak homology to the MYND zinc binding domain (15% sequence identity) and the zinc finger HIT domain in the DEAD box polypeptide 59 (11% sequence identity), suggesting that Tox4CTD might also bind zinc. To test if Tox4CTD binds zinc, we measured the 2D [1H,15N]HSQC spectrum of the Tox4CTD in the presence of zinc, which substantially improved the spectrum (Figure S1), demonstrating that the fold of Tox4CTD is stabilized by zinc binding. We then titrated a 1:2 M ratio of zinc-loaded Tox4 to 15N-labeled PNUTSTND. The 2D [1H,15N]HSQC spectrum of PNUTSTND showed dramatic chemical shift perturbations (CSPs), confirming a direct interaction between the two proteins (Figure 1D). Finally, subsequent isothermal titration calorimetry (ITC) experiments showed that Tox4CTD and PNUTSTND bind tightly, with a KD = 0.3 ± 0.1 nM (Figures 1E and S2; Table S1).
Crystal structure of the PNUTSTND:Tox4CTD complex
To define how Tox4CTD binds PNUTSTND, we determined the crystal structure of the PNUTSTND:Tox4CTD complex (Figure 2A; Table S2; 2.1 Å resolution; PNUTSTND [5–160, C48S] and Tox4CTD [571–621, C601S], hereafter referred to as PNUTS: Tox4 complex). PNUTS adopts a nine α-helical bundle structure consistent with the PNUTSTND solution structures33,34; its structure is most similar to the IWS1/Spn1 protein (DALI Z scores = 13.4–13.8).42 PNUTS helices 5–9 form the TFIIS N-terminal domain (TND), which is a conserved scaffold enriched in transcription factors that mediates binding with TND-interacting motifs (TIMs) and is the domain that exhibits the greatest overlap with IWS1 (Figure 2A). This domain has previously been shown to bind directly to the MYC TIM (34VQPYF38) in addition to TIMs from other transcription factors (including SPT6 and PAF1).34 Helices 1–4, which pack tightly against the TFIIS domain to form a single compact fold, adopt a distinct α-helical bundle that is not present in other known TFIIS TND-containing proteins. It is this domain, referred to as the Tox4 binding domain (Tox4BD), that binds the Tox4CTD (Figure 2A).
Figure 2. Crystal structure of the PNUTS:Tox4 complex.
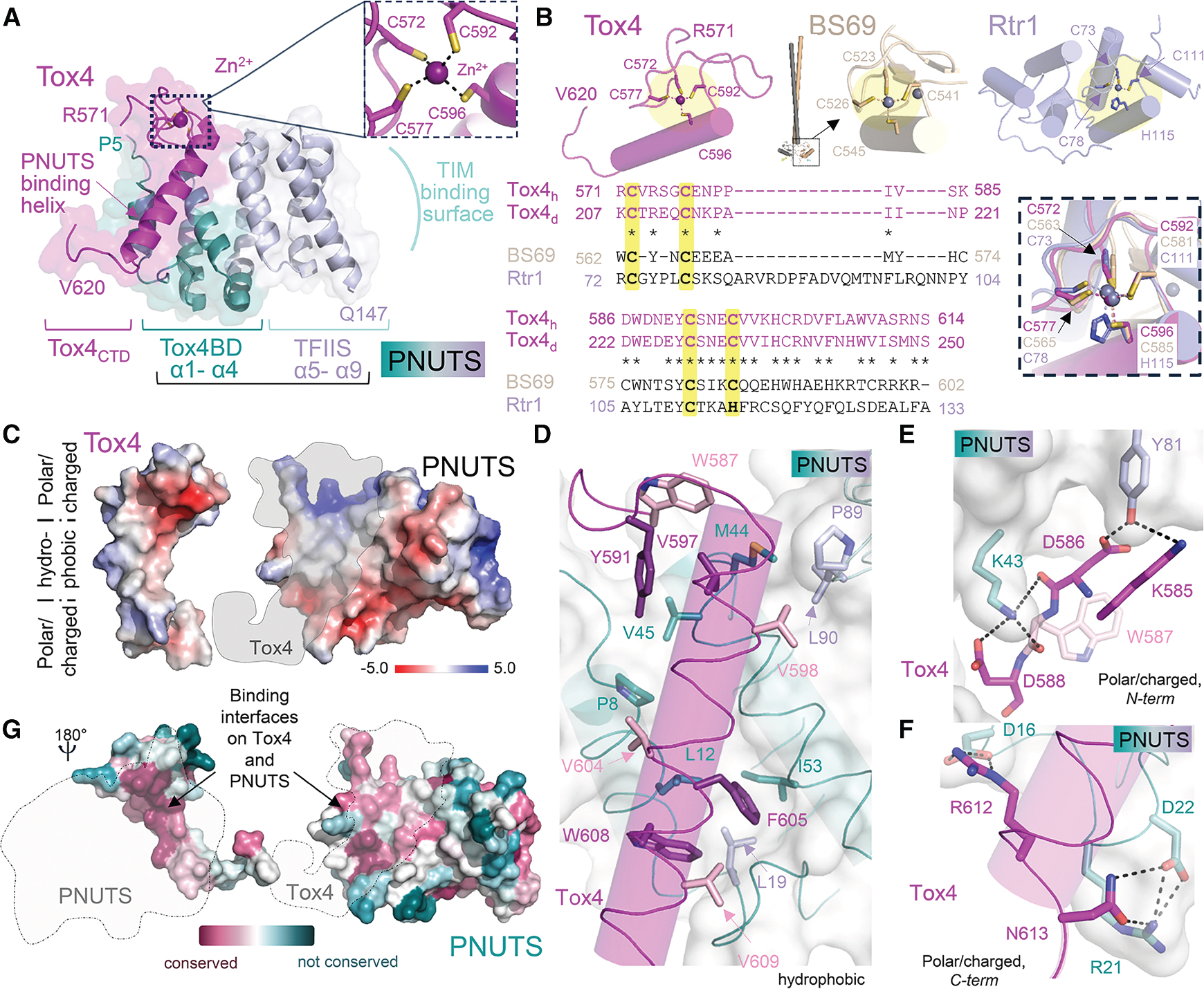
(A) Structure of the Tox4 (magenta) and PNUTS NTD (Tox4 binding domain [Tox4BD], helices ɑ1-ɑ4, dark teal; Tox4 TFIIS domain, helices ɑ5-ɑ9, light teal) complex. The Tox4 PNUTS binding helix and TND-interacting motif (TIM) surface on PNUTS are labeled. Dotted box is Tox4 zinc binding pocket, shown also in the inset with zinc binding residues labeled.
(B) A DALI structure similarity search identified RTR1 (lavender; PDB: 4FC8) and BS69 (gray; PDB: 5C2Y) as having weak similarity with the Tox4 C-terminal domain. Tox4, BS69, and Rtr1 structures, respectively, are shown at the top. BS69 is a dimer with the zinc binding domain at the end of a long coiled coil. Lower panel, structure-based sequence alignment of Tox4 (both the human and Drosophila sequence shown with identical residues indicated by an “*”), BS69, and Rtr1, with DALI Z scores reported, together with overlay of the zinc binding pockets with zinc binding residues labeled.
(C) Interaction surfaces of Tox4 and PNUTS showing the electrostatic potential surface, with the hydrophobic (middle) and polar/charged (upper/lower) regions labeled.
(D) Hydrophobic interactions between Tox4 (magenta/pink) and PNUTS (dark teal/light teal). Residues that become nearly completely buried upon complex formation are colored in dark shades.
(E) N-terminal polar/charged interaction surface, with hydrogen bonds/salt bridges between Tox4 and PNUTS shown as dashed lines.
(F) C-terminal polar/charged interaction surface, with inter- and intramolecular hydrogen bonds/salt bridges shown as dashed lines.
(G) Consurf analysis of Tox4 (left) and PNUTS (right), with the regions at the PNUTS:Tox4 interface indicated by gray dotted lines.
The Tox4CTD adopts a novel conformation, where aa 571–596 form a zinc binding loop in which Tox4 residues Cys572, Cys577, Cys592, and Cys596 coordinate a zinc ion, while residues 597–612 form a long α helix (the Tox4 PNUTS binding helix) (Figures 2A, 2B, and S3). A structure similarity search using DALI identified only weakly similar proteins (Z scores = 2.0–2.7), including RTR1, a putative atypical phosphatase that regulates the phosphorylation status of the RNAPII-CTD (Z score = 2.7), and the BS69/ZMYND11 protein that contains an MYND zinc binding domain (Z score = 2.2) (Figure 2B). While the zinc binding residues overlap between these proteins, the conformations of the intervening loops and the residues beyond the zinc binding domain differ, explaining the low similarity scores (Figure 2B).
The binding interface between Tox4 and PNUTS is extensive, with the Tox4 PNUTS binding helix interacting with PNUTS α helices α1 and α3 via hydrophobic and ionic interactions (Figure 2C). The hydrophobic interactions are toward the center of the Tox4-PNUTS interface (Figure 2D; Tox4: W587Tox4, Tyr591Tox4, Val597Tox4, Val598Tox4, Val604Tox4, Phe605Tox4, Trp608Tox4, V609Tox4; PNUTS: Pro8PNUTS, Leu12PNUTS, Leu19PNUTS, Met44PNUTS, Val45PNUTS, Ile53PNUTS, Pro89PNUTS, Leu90PNUTS). These interactions are bounded at both ends by polar/electrostatic contacts (Figures 2E and 2F; N-term: Lys585Tox4, Asp586Tox4, Asp588Tox4 with Lys43PNUTS, Tyr81PNUTS; C-term: Arg612Tox4, Asn613Tox4 with Asp16PNUTS, Arg21PNUTS, Asp22PNUTS). Finally, the aromatic ring of Trp587Tox4 forming an aromatic-sulfur interaction with Met44PNUTS (Figure 2D) and Lys585Tox4 forms a cation-π interaction with Trp81PNUTS (Figure 2E).
These interacting residues are conserved in Drosophila PNUTSTND and Tox4CTD sequences (Figures 2B and S4). To test if the residues that mediate binding are more extensively conserved, we performed a ConSurf analysis.43,44 The analysis showed that PNUTS residues in α helix 1, the α helix 1/2 linker region and α helix 3, which are key for forming the novel conserved interaction surface in the PNUTSTND that so far is exclusively used by Tox4, are highly conserved (Figure 2G). Likewise, the TOX4 residues that mediate PNUTS binding are equally conserved (Figure 2G). Finally, consistent with its nanomolar binding affinity, the complex buries 2,100 Å2 of solvent-accessible surface area. This tight, extensive interaction, coupled with that observed between PNUTS and PP1,25 explain why PNUTS, Tox4, and PP1 form a constitutive complex in cells.
Accumulation of Tox4 in the nucleus depends on PNUTS binding
To further investigate Tox4:PNUTS complex formation in cells, we examined the subcellular distribution of Tox4 and PNUTS. Drosophila S2R+ cells were transiently co-transfected with GFP-tagged Drosophila Tox4wt or Tox4P216Ter, alone or together with RFP- Drosophila PNUTS, and imaged using confocal microscopy. Single transfections revealed that GFP-Tox4wt and -Tox4P216Ter were distributed in both the nucleus and the cytoplasm, with a higher level of expression in the nucleus (Figure 3A). Upon co-transfection with RFP-PNUTS, GFP-Tox4wt became enriched in the nucleus and strongly co-localized with RFP-PNUTS (Manders’ coefficient = 0.945 ± 0.009) whereas GFP-Tox4P216Ter did not (Manders’ coefficient = 0.466 ± 0.010) (Figures 3B and 3C). We then examined the subcellular distribution of Drosophila Tox4 in vivo using GFP-tagged Tox4wt or Tox4P216Ter expressed from transgenic constructs under the control of the endogenous tox4 promoter. A difference in localization of GFP-tagged Tox4wt or Tox4P216Ter was clearly visible (Figure 3D). In nurse cells in the ovary, GFP-Tox4wt showed a 3.3-fold enrichment in nuclei relative to the cytoplasm (p < 0.001), whereas GFP-Tox4P216Ter showed a more diffuse distribution (mean nuclear/cytoplasmic ratio 1.31, Figures 3D and 3E). Taken together, these data confirm that PNUTS is critical for nuclear accumulation of Tox4.
Figure 3. PNUTS facilitates accumulation of Tox4 in the nucleus in vitro and in vivo.

(A) Maximal projection of stack of confocal images showing subcellular localization of ectopically expressed GFP-tagged Tox4 and RFP-tagged PNUTS throughout live S2R+ cells. In the absence of RFP-PNUTS (−), GFP-Tox4wt, and GFP-Tox4P216Term are found in both the nucleus and cytoplasm. In the presence of RFP-PNUTS (+), GFP-Tox4wt accumulates in the nucleus, whereas the distribution of GFP-Tox4P216Term is largely unaffected. Scale bars, 10 μm.
(B) Intensity histograms showing distribution of pairs of GFP and RFP voxel intensities of cells in (A) co-transfected with GFP-Tox4 (green) and RFP-PNUTS (magenta), revealing strong colocalization of RFP-PNUTS and GFP-Tox4wt but not GFP-Tox4P216Term.
(C) Plot showing mean ± SEM of Manders’ coefficient of the overlap between GFP and RFP in cotransfected cells. Means are derived from n = 3 experiments, shown on Beeswarm plots of individual measurements of repeated counts (n = 10 cells/biological replicate).
(D) Distribution of GFP-tagged Tox4 under the control of the endogenous tox4 promoter in Drosophila stage 10 egg chambers. Fluorescent signal from GFP-Tox4wt (green) is clearly evident in both (smaller) somatic and (larger, polyploid) nurse cell nuclei, costained for DNA with DAPI (blue). In contrast, the signal from GFP-Tox4P216Term can be seen less strongly in the nucleus and is diffusely localized throughout the cytoplasm.
(E) Quantification of nuclear/cytoplasmic ratio. Datapoints for individual nuclei, color coded by egg chamber, are shown together with mean ± SEM (n = 21 egg chambers, ~100 nuclei/genotype. Scale bars, 50 μm. Statistical significance was tested using pairwise t tests with Bonferroni p value correction. ***p < 0.001.
Tox4 and PNUTS-Tox4 binding are dispensable for viability
To establish the role(s) of the Tox4-PNUTS interaction, we tested if the Tox4:PNUTS complex can be disrupted by mutagenesis. Using the crystal structure, we engineered a PNUTS Tox4 binding domain dead variant (PNUTSTBD-dead) with the following mutations: K43EPNUTS (charge reversal to disrupt the salt bridge with D586Tox4), L12EPNUTS, and V45DPNUTS (hydrophobic residues that are completely buried at the PNUTS-Tox4 hydrophobic interface) (Figure 4A). PNUTSTBD-dead expressed and purified readily with subsequent ITC experiments demonstrating that it no longer binds Tox4 (Figure 4B; Table S1).
Figure 4. Tox4 binding to PNUTS is not essential for viability.

(A) PNUTS:Tox4 interaction interface highlighted the residue mutations to generate PNUTSTBD-dead—L12E, K43E, and V45D—shown in yellow.
(B) Representative binding isotherm of PNUTSTBD-dead with Tox4571–621 C601S.
(C) Results of complementation tests with genomic PNUTS transgenes to determine their ability to rescue larval lethality of PNUTS13B homozygous animals. A prediction of how often this genotype should be represented if there were no mutation (corresponding to a Mendelian ratio of 2:1 heterozygous/homozygous animals) is shown with a dotted line. Wild-type (PNUTSwt) and reduced-Tox4 binding mutants (PNUTSED and PNUTSE/ED) all rescued PNUTS13B homozygotes to adulthood (mean ± SEM is shown, n = 5).
(D) Quantitation of ratio of eggs laid by the indicated genotype of parental flies relative to control flies (PNUTS13B/13B; PNUTSwt). Egg laying in PNUTS13B/13B flies was strongly reduced by inducible PNUTSwt-flp-W726A and weakly reduced by PNUTSE/ED.
(E) Quantitation of ratio of eggs from the indicated genotype of parental flies that hatched relative to control. Hatching was reduced to a similar extent by PNUTSwt-flp-W726A and PNUTSE/ED. (D and E) Plots show overall mean ± SEM derived from means of n = 3 experiments, superimposed on Beeswarm plots of individual measurements of repeated counts for each of the indicated genotypes. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA.
To test the in vivo effect of disrupting the interaction between PNUTS and Tox4, we generated GFP-tagged Drosophila PNUTS rescue constructs, expressed under control of the endogenous PNUTS promoter, with the structurally derived and tested mutations in the Tox4 binding pocket (PNUTSED-GFP [K43E/V45D] and PNUTSE/ED-GFP [K43E/L12E/V45D]) (Figure S5). Expression of wild-type PNUTS-GFP in flies largely rescued a recessive lethal PNUTS null mutant (PNUTS13B)21 (Figure 4C). One or two copies of either PNUTSED or PNUTSE/ED also rescued the lethality of PNUTS loss of function (Figure 4C). PNUTSE/ED was at least as well tolerated as PNUTSED, and there was no significant difference in the extent of rescue between PNUTSE/ED and PNUTSwt. Together, these data show that PNUTS-Tox4 binding is dispensable for viability. When we tested the fertility of surviving adult female flies, we observed a modest but significant reduction in both egg laying (Figure 4D) and egg hatching (Figure 4E), indicating a reduction in egg production and viability.
Since PNUTS scaffolds both Tox4 and PP1 in a stable complex,35 we wondered whether PNUTS-PP1 binding was also necessary for female fertility. To test this, we used a conditional transgenic PNUTS allele (PNUTSwt-flp-W726A) that undergoes an irreversible transition from wild-type PNUTS (PNUTSwt) to a PP1 binding-incompetent version (PNUTSW726A)21 after FRT/flp-induced allele exchange (Figure S6A). When we specifically induced exchange of PNUTSwt to PNUTSW726A in all ovarian germline cells (Figure S6B), we found a much stronger reduction in egg laying than in mutant PNUTSE/ED animals, as well as a reduction in hatching (Figures 4D and 4E), suggesting that PNUTS:PP1 may have roles independent of Tox4 binding in germline development.
Tox4 is required for male and female fertility in Drosophila
To establish the in vivo requirement for tox4, we generated a null mutant allele of Drosophila tox4 by imprecise excision of a transposable P element inserted in the tox4 5′ untranslated region. We identified a strain (referred to as tox4null hereafter) that carries a 1,180 bp deletion of the tox4 transcription unit, including the translation start site and most of the coding sequence (Figure 5A). Unlike PNUTS null homozygous animals, which die during early larval development,21 tox4null/null animals survived fully to adulthood, although they did not readily thrive. This indicates that zygotic tox4 is dispensable for normal zygotic development, although early roles may be masked by perdurance of the maternal contribution.
Figure 5. Tox4 is required for male and female fertility in Drosophila.

(A) Genomic region of CG12104 (Drosophila tox4) showing exon-intron structure for CG12104 and location of flanking genes. Gray shading represents coding regions, unfilled boxes represent untranslated regions with arrows indicating direction of transcription. CG12104EY02201 contains a P element insertion in the 5′ untranslated region of tox4. A 1.2 kb deletion of the CG12104 coding sequence in tox4null resulting from imprecise excision of CG12104EY02201 is indicated, together with genomic sequence of the breakpoints. Also shown is the extent of genomic transgenes carrying either wild-type or mutant tox4 (tox4P216Term) transcription unit tagged at the N terminus with GFP. The base pair change in the transgenic GFP-tox4P215Term allele is shown.
(B) Quantitation of ratio of eggs laid by the indicated genotype of parental flies relative to controls (w1118), showing an 80% reduction in number in eggs from tox4null mothers, and transgenic rescue of this effect with GFP-tox4. (m) male and (f) female parental flies were crossed to w1118 flies. Plots show overall mean ± SEM derived from means of n = 3 experiments superimposed on Beeswarm plots of individual measurements of repeated egg counts over at least 3 days/genotype.
(C) Quantitation of ratio of eggs from the indicated genotype of parental flies that hatched relative to controls (w1118). Hatching was greatly reduced by loss of tox4 function in male and female parental flies. This was rescued by transgenic GFP-tox4, with GFP-tox4wt rescuing more strongly than GFP-tox4P215Term. Plots show overall mean ± SEM derived from means of n = 3 experiments, superimposed on Beeswarm plots of individual measurements of repeated counts for each of the indicated genotypes. ns, not significant; **p < 0.01, ***p < 0.001 by one-way ANOVA.
Reduced fecundity of tox4null animals prompted us to investigate whether fertility was reduced by tox4 loss of function. Egg production from inbred homozygous toxnull mutant animals was significantly reduced compared with the w1118 control (p < 0.0001), laying on average 79.0% ± 2.0% fewer eggs during the monitored period (Figure 5B). tox4null/null females exhibited the same reduced level of egg production when outbred to w1118 males, and few of the eggs that were laid went on to hatch (11.7% ± 2.8% of w1118 inbred control; p ≤ 0.0001, Figure 5C). No larvae emerged from eggs laid by inbred tox4null/null females. Eggs laid by w1118 females mated to homozygous tox4null males also showed a severe defect in hatching (p < 0.0001), although there were occasionally escapers that progressed to adulthood. This prompted us to examine the requirement of PNUTS binding for tox4 function in the germline using our genomic GFP-tagged Drosophila tox4 transgenes. GFP-tox4wt and -tox4P216Ter rescued the tox4 loss-of-function egg laying defect by 74.4% ± 3.6% and 59.2% ± 2.7%, respectively (Figure 5B). GFP-tox4P216Ter also showed a reduced ability to rescue hatching of embryos laid by tox4null mothers (57.9% ± 3.5% of controls), compared with GFP-tox4wt (85.7% ± 3.3% of controls, p < 0.0001). There was also a significant difference in the ability of GFP-tox4wt and GFP-tox4P216Ter to rescue sterility of tox4null males (p < 0.0001) (Figure 5C). Taken together, these data show that the role of tox4 in female and male fertility is partially dependent on PNUTS binding.
A shared role of TOX4, PNUTS, and PP1 results in a nurse cell chromosomal dispersal defect in ovaries
To understand the functional outcome of the interaction between Tox4 and PNUTS, we analyzed their role in the female germline. Surrounded by a layer of somatic follicle cells, germline nurse cells play an essential role in egg chamber maturation by providing nutrients and support to the developing oocyte. Normally, during the first five endocycles, polytene (64C) nurse cell chromosomes undergo condensin-mediated axial compaction and are visible as a “five-blob” structure up to stage 4 of oogenesis, with each blob representing one of the major chromosomal arms. At the end of the fifth cycle, a mitosis-like phase occurs as the association between somatically paired homologous chromosomes weakens and chromosomes disperse into 32 pairs resulting in a loss of the five-blob structure by stage 6 of oogenesis45,46 (Figure 6A). In tox4null ovaries at stage 6–7, chromosomes failed to fully disperse in nurse cell nuclei (mean non-dispersal 72.7% ± 4.4%), with a majority of nuclei remaining compacted at stage 10 of development (mean non-dispersal 59.0% ± 6.1%, Figure 6B). At stage 6–7, this phenotype was largely rescued by one transgenic copy of GFP-tox4wt (mean non-dispersal 7.6% ± 2.0%), but incompletely by the PNUTS binding mutant, GFP-tox4P216Ter (mean non-dispersal 55.5% ± 5.3%). Residual non-dispersal of chromosomes was still evident in 25.2% ± 5.1% of tox4null GFP-tox4P216Ter egg chambers at stage 10 (Figures 6C and S7). Temporary persistence of compacted nurse cell chromosomes was also observed in ovaries expressing PNUTSED or PNUTSE/ED in a PNUTS13B background (Figures 6C and S7). In PNUTSE/ED egg chambers, where the effect was stronger than PNUTSED, mean non-dispersal was 48.2% ± 4.8% at stage 6–7, decreasing to 29.4% ± 3.3% at stage 10.
Figure 6. Disruption of PNUTS-PP1 binding, loss of tox4 function, or loss of PNUTS-Tox4 binding results in chromosome dispersal phenotypes.

(A and B) Strings of egg chambers of different stages (numbered, up to stage 10) stained with DAPI, which labels both somatic follicular cell nuclei surrounding each chamber and large nurse cell germline nuclei. Lower panels show magnified images of representative nurse cell nuclei from different stages. (A) In w1118 control egg chambers, nurse cell chromosomes disperse throughout the nucleoplasm by stage 6. (B) In contrast, tox4null/tox4null nurse cell chromosomes fail to disperse, frequently retaining a “five-blob” structure until later stages of development (compare magnified images in A and B). Incomplete dispersal phenotypes were also observed at stage 10, with chromosomes decorating the nuclear periphery without dispersing completely throughout the nucleoplasm (arrow). Scale bars, 40 μm (ovarioles), 2 μm (magnified nuclei).
(C) Quantification of non-dispersed nurse cell chromosomes in egg chambers at stage 6–7, 8–9, and 10. Violin plots show mean percentage non-dispersed nurse cell chromosomes/egg chamber for each indicated condition (n > 15 egg chambers/stage/genotype). At stage 6–7, all conditions showed significant levels of non-dispersal except tox4null/tox4null rescued by GFP-tox4wt. GFP-tox4P216Term failed to substantially rescue dispersal phenotypes at this stage. In all conditions, the extent of non-dispersal decreased with developmental stage. However, significant non-dispersal was still observed for tox4null/tox4null (with or without GFP-tox4P216Term), and PNUTS13B/13B PNUTSEED-GFP at stage 10. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by Kruskal-Wallis test.
Next, we tested if PP1 is important for this nurse cell chromosomal dispersal defect using our inducible PNUTSwt-flp-W726A transgene. When we specifically induced exchange of PNUTSwt to PNUTSW726A in all ovarian germline cells, we observed persistence of compacted nurse cell chromosomes (mean non-dispersal 41.9% ± 5.3% at stage 6–7, Figures 6C and S6). Thus, considering the similarity in phenotypes observed upon loss of PNUTS-PP1 binding when compared with a loss of tox4 or of Tox4-PNUTS binding, it is likely that PNUTS, TOX4, and PP1 have a co-operative role in developmentally controlled chromosome reorganization.
Disruption of PP1 or Tox4 binding to PNUTS results in a common transcriptional response
To identify molecular signatures associated with loss of Tox4: PNUTS binding, we determined the total mRNA expression profiles from adult ovaries by RNA-seq. Principal-component analysis of the replicates (n = 3) showed close agreement between different samples of each line (Figure 7A). Divergent and common responses to loss of tox4 compared with Tox4-PNUTS binding mutants and controls, agrees with phenotypic analysis indicating that tox4 possesses both PNUTS-dependent and -independent roles during ovarian development. Strikingly, samples with loss of Tox4-PNUTS binding and loss of PP1-PNUTS binding clustered closely to one another, showing that these conditions share a common response.
Figure 7. Disruption of PP1 or Tox4 binding to PNUTS induces a common transcriptional signature.

(A) Principal-component analysis showing common response (PC1) to perturbation of PP1- or Tox4 binding to PNUTS, and divergent response (PC2) to tox4 loss of function, which together explain approximately 67% of the variance in gene expression. Data points for three independent biological repeats are shown together with the centroid in Euclidian space for each condition: w1118 (gray), tox4null/tox4null (red), tox4null/tox4null GFP-tox4wt (blue), PNUTS13B/13B ovoFLP>PNUTSwt-flp-W726A (purple), PNUTS13B/13B PNUTSED (green), and PNUTS13B/13B PNUTSE/ED (yellow).
(B) Plot showing number of differentially expressed (DE) genes compared with w1118 control (>1.5-fold over or under-expressed, padj <0.1) for the following conditions: (1) tox4null/null, (2) PNUTS13B/13B ovoFLP>PNUTSwt-flp-W726A, (3) PNUTS13B/13B with either PNUTSED or PNUTSE/ED. Overexpressed genes, red bar; underexpressed genes, blue bar.
(C) Plot showing percentage of overexpressed and underexpressed genes for each condition as (B) that were found in each quartile of normal expression level derived from read counts in w1118 control. Greater than 90% of underexpressed genes in each condition are in the top two quartiles for normal expression level, whereas >80% of overexpressed genes are more modestly expressed (2nd and 3rd quartiles).
(D and E) Gene Ontology (GO) enrichment for DE genes as (B) grouped by Boolean terms, as indicated. (D) Enrichment of transcription factors, for underexpressed (>1.5-fold) and modestly overexpressed (1.5- to 2.0-fold) genes among different conditions compared with w1118 control. The top two statistically significant GO categories (padj < 0.05) for transcription factors (black text)47 are shown in each case. (E) Enriched GO terms associated with biological functions among under-expressed genes.
(F and G) The PNUTS:PP1:WDR82:TOX4 complex. (F) The PNUTS:Tox4 complex (teal and magenta, respectively) bound to the MYC TIM (PDB: 7LQT; orange). (G) Cartoon illustrating the complex between Tox4 (magenta), PNUTS (teal), PP1 (yellow), WDR82 (blue), and the MYC TIM (orange). Domain interactions for which structures have been determined are shown as cartoon and/or surfaces. Folded interaction partners (WDR82) are shown as a shape, while residues predicted to be IDRs are shown as lines.
Differential gene expression analysis revealed underexpression of a large number of genes (Figure 7B; Table S3), which were in the top quartile of expression in w1118 controls (Figure 7C). Reduction in the level of highly expressed genes enriched in binding sites for the insulator element factors Chromator (Chro), BEAF-32, and CIP190 was common to PNUTS-PP1 binding, PNUTS-Tox4 binding, and tox4 loss-of-function mutations (Figures 7B–7D). Approximately twice as many genes were overexpressed than were underexpressed for all loss-of-function genotypes compared with the w1118 control (Figure 7B). Human Tox4 has previously been associated with release from proximal RNAPII pausing.37,38 Consistent with this, we found that modestly overexpressed (1.5- to 2.0-fold) genes were enriched for pausing factors such as the negative elongation factor, which associates with the GAGA-associated factor/Trl at many paused genes in Drosophila48,49 (Figure 7D).
To better define common and divergent properties of Tox4 and PNUTS:PP1, we extended a Boolean-logic-based approach to look at enrichment of functional Gene Ontology terms in the gene expression profiles of our different strains (Figure 7E; Table S4). Notably, among the divergent features, we found distinct enrichment of Gene Ontology terms for chromosome segregation and DNA replication among genes underexpressed in tox4 loss of function, but not in a Tox4 binding mutant of PNUTS. We also found unique enrichment of RNA processing and metabolic functions among genes underexpressed in the PNUTS PP1 binding, but not Tox4 binding mutant. In contrast, we found shared enrichment of terms for protein polymerization, Neddylation, and APC/C-mediated degradation across conditions, suggesting that these processes are commonly affected by Tox4:PNUTS:PP1.
DISCUSSION
PNUTS functions as a protein interaction hub that mediates interactions with multiple transcriptional regulators, integrating signals to modulate gene expression. The folded N-terminal domain of PNUTS is an important region of protein-protein interaction, being composed of an N-terminal Tox4BD and a C-terminal TND (TFIIS N-terminal domain) (Figure 2). The TND is a conserved domain recently identified to be enriched in transcription elongation factors such as TFIIS, ELOA, HRP2, and Spn1,34 whose function is to interact with short linear motifs present in cognate interacting partners, referred to as TIMs (TND-interacting motifs). Recent work has shown that the conserved PNUTS TND forms a binary interaction module that interacts with IDRs of several transcriptional factors including MYC (Figures 7F and 7G), IWS1, MED13-MED13L, EAF1, EAF2, PAF1, SPT6, and LEO1.33,34 Despite its role in binding TIMs, previous data35 and this work have shown that the PNUTSTND forms a constitutive complex with Tox4 in cells, suggesting that the Tox4:PNUTS complex must be compatible with dynamic TIM binding. Our data explain how this is achieved. Namely, Tox4 and the transcription factor TIMs bind on opposite surfaces of the PNUTSTND (Figures 7F and 7G).
Our data show that the PNUTS:Tox4 interaction is functionally conserved from flies to mammals. ConSurf analysis43,44 shows that the residues that mediate TIM binding, like those that mediate Tox4 binding, are conserved (PNUTS α helix 7 [Leu108, Lys109], α helix 8 [Ala114, Lys115, Lys118, Lys122], and α helix 9 [Ala133, Val137, Trp140, Met141, Ile144]). However, unlike PNUTSTND-TIM interactions, which have affinities in the micromolar range and explain the ability of different TIMs to readily displace one another, the PNUTSTox4BD-Tox4 affinity is subnanomolar. This suggests the interaction is constitutive in cells and that this interaction might be leveraged to target the transcription factors that bind the opposite side binding surface of the PNUTSTND to the PNUTS:Tox4:Wdr82 complex (Figure 7G).
The constitutive interaction between Tox4 and PNUTS in the PTW:PP1 complex35 implies they have overlapping roles. However, our functional data suggest tox4 and PNUTS also possess independent functions. Most noticeably, tox4, unlike PNUTS, is dispensable for viability in flies. Consequently, other components of the PNUTS protein interaction hub must be sufficient to mediate PNUTS’ essential functions during zygotic development. Moreover, we found that disruption of PNUTS-PP1 binding had a much greater effect on fertility than loss of binding of PNUTS to Tox4, suggesting distinct roles for PNUTS-PP1 during oogenesis. In line with this, we observed enrichment of RNA processing and energy metabolism terms for genes underexpressed in the PNUTS-PP1 binding, but not PNUTS-Tox4 binding mutant strain. These gene signatures may define a separate, Tox-independent role for PNUTS:PP1 that is required for viable egg production. Conversely, the fact that impaired nuclear accumulation in the absence of PNUTS binding does not completely abolish Tox4 function indicates that Tox4 has additional roles beyond its interaction with PNUTS.
Despite being dispensable for viability, tox4 is required for adult fertility. This requirement is shared by PNUTS:PP1, with loss of Tox4 or PP1 binding to PNUTS leading to similar nurse cell chromosome dispersal defects during oogenesis. Such phenotypes have previously been reported for mutations in transcription factors (E2F1 and DP50), a chromodomain protein (Rhino51), ribonucleoprotein genes (squid and hrb27C52), RNA helicases (P68/Rm6253), and splicing factors pUf68/hfp, Prp22/pea, snRNP-U1–70K, and U1-snRNA.54,55 These data highlight that the Tox4:PNUTS:PP1 complex may regulate the transcriptional programs driving chromosome rearrangements in this context. Nurse-cell chromosome dispersal has been suggested to facilitate rapid synthesis of proteins needed for the remainder of oogenesis from stage 6 onward.45 While human oogenesis does not involve chromosome dispersal in the same manner as in the Drosophila germline, significant reorganization of chromosome architecture occurs during human oocyte growth and maturation.56 These structural changes are associated with transcriptional regulation, contributing to the acquisition of the oocyte’s developmental competence.57,58
Previous studies have suggested that chromosome dispersal during Drosophila oogenesis involves a transient mitosis-like phase.45,46 Underexpressed gene signatures common to PNUTS-Tox4 and PNUTS-PP1 binding mutants were enriched in Neddylation factors and APC/C components that may contribute to the transition between such phases.59–61 We also observed underexpression of chromosome segregation genes in tox4 but not in the PNUTS Tox4 binding mutant, perhaps explaining the increased severity of the chromosomal dispersal phenotype and its persistence until later stages of egg chamber development in the tox4 null strain. Another aspect of the common transcriptional signature in non-Tox4 and non-PP1 binding mutant ovaries was the reduced RNA accumulation for highly expressed genes possessing binding sites for insulator factors, including Chromator and BEAF-32. This may reflect the defects in high-order chromosome structure observed in all the mutants and is consistent with evidence that developmentally controlled chromosomal reorganization, e.g., at the level of topologically associating domains, may be involved in transcriptional reprogramming.62–64
The contribution of different components of phosphatase-associated complexes to gene expression and function during development has long been an open question. Our findings demonstrate that recruitment of PP1 and Tox4 to PNUTS is necessary for normal gene expression and chromosomal dispersal during oogenesis. This reveals a mechanism whereby multiple motifs are used combinatorically to tune transcriptional outputs driving developmental transitions.
Limitations of the study
Drosophila was used as a model system to investigate functional consequences of Tox4-PNUTS binding, but whether the PNUTS complex controls germline development in other organisms was not tested. PNUTS is required for nuclear accumulation of Tox4 in Drosophila cells. However, whether this is due to changes in the half-life of Tox4, or due to altered nuclear import/export remains to be determined. Finally, RNA-seq experiments identified significant changes to developmental expression profiles in mutant strains. However, which genes are direct targets through which Tox:PNUTS:PP1 control chromosome dispersal remain to be identified.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Daimark Bennett (daimark.bennett@manchester.ac.uk).
Materials availability
Plasmids and all unique reagents generated in this study are available from the lead contact with a completed Materials Transfer agreement.
Data and code availability
Atomic coordinates and structure factors have been deposited in the Protein DataBank (PDB: 9CI7). RNA-seq data have been deposited in EMBL-EBI ArrayExpress: E-MTAB-13735.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact (daimark.bennett@manchester.ac.uk) upon request.
STAR★METHODS
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Saccharomyces cerevisiae strains L40ΔGal4 and HGX13 (Y187 ade2–101: loxP-kanMX-loxP), were used for yeast two hybrid screening as previously reported.78
S2R + Drosophila melanogaster cells (RRID:CVCL_Z831) used in this study were grown in complete Schneider’s Insect Medium (Sigma) with 10% heat inactivated Fetal Calf Serum (Gibco) and Penicillin-Streptomycin (Invitrogen) at 28°C.
All stocks of Drosophila melanogaster were grown and maintained at 18°C and raised at 25°C for experiments on standard fly food media (yeast 50 g/L, glucose 78 g/L, maize 72 g/L, agar 8 g/L, 10% nipagen in EtOH 27 mL/L and propionic acid 3 mL/L).
The following fly lines were used in this study, w1118 (isogenic line), PNUTS13B (ref. 21), ovo-FLP (RRID:BDSC_8705), tox4null (an imprecise excision, this study, of P[EPgy2]CG12104EY02201, RRID:BDSC_15089), GFP-Tox4wt (inserted at attP40, this study) GFP-Tox4P216Ter (inserted at attP40, this study), PNUTSwt-GFP (inserted at attP2, this study), PNUTSK42E,V44D-GFP (inserted at attP2, this study), PNUTSL10E,K42E,V44D-GFP (inserted at attP2, this study) and PNUTSwt-flp-W726A (inserted at attP2, this study).
METHOD DETAILS
Cloning and expression
PNUTS N-terminal domain (5–160, rat) was sub-cloned into pRP1b.65 Tox4 C-terminal domain (571–621, human) was sub-cloned into pTHMT vector containing an N-terminal His6-tag followed by maltose binding protein (MBP) and a tobacco etch virus (TEV) protease cleavage site. The PNUTS C48S and Tox4 C601S variants were generated using Quikchange II (Agilent Technologies) and sequence verified. PNUTS and Tox4 constructs were expressed in E. coli BL21 (DE3) (Agilent). Cells expressing PNUTS constructs were grown in Luria Broth in the presence of selective antibiotics at 37°C to an OD600 of ~0.8, and expression was induced by the addition of 1 mM isopropyl β-D- thiogalactoside (IPTG). Induction proceeded for ~18–20 h at 18°C prior to harvesting by centrifugation at 6,000 ×g. Cell pellets were stored at −80°C until purification. For NMR measurements, expression of uniformly 15N- and/or 13C-labeled PNUTS or Tox4 was achieved by growing cells in M9 minimal media containing 1 g/L 15NH4Cl and/or 4 g/L [13C]-D-glucose as the sole nitrogen and carbon sources, respectively. Tox4 C-terminal domain variants were expressed similarly, except 0.1 mM ZnSO4 was added to the Luria Broth or minimal media for proper folding of the protein.
Protein purification
PNUTS expressing E. coli cell pellets were resuspended in ice-cold lysis buffer (50 mM Tris pH 8.0, 0.5 M NaCl, 5 mM imidazole, 0.1% Triton X-100 containing EDTA-free protease inhibitor tablet [Roche]), lysed by high-pressure cell homogenization (Avestin C3 Emulsiflex) and centrifuged (35,000 ×g, 40 min, 4°C). The supernatant was loaded onto a HisTrap HP column (GE Healthcare) pre-equilibrated with Buffer A (50 mM Tris pH 8.0, 500 mM NaCl and 5 mM imidazole) and was eluted using a linear gradient of Buffer B (50 mM Tris pH 8.0, 500 mM NaCl, 500 mM imidazole). Fractions containing the protein were pooled and dialyzed overnight at 4°C (50 mM Tris pH 8.0, 500 mM NaCl, 0.5 mM TCEP) with TEV protease to cleave the His6-tag. The cleaved protein was incubated with Ni2+-NTA beads (Cytiva) and the flow-through was collected. The protein was concentrated and purified using size exclusion chromatography (SEC; Superdex 75 26/60) pre-equilibrated in NMR Buffer (20 mM MES pH 6, 150 mM NaCl, 5 mM DTT) or crystallization buffer (20 mM Tris pH 8.5, 150 mM NaCl, 0.1 mM ZnSO4, 5 mM TCEP). Fractions were pooled, concentrated to designated concentration for experiments or stored at −80°C. Tox4 was purified identically as PNUTS. except Tox4 was incubated with amylose resin to bind cleaved MBP, before being further purified via SEC (Superdex 75 26/60). The Tox4 NMR and crystallization buffers were identical (20 mM Tris pH 8.5, 150 mM NaCl, 0.1 mM ZnSO4, 0.5 mM TCEP). To form the PNUTS:Tox4 complex, purified PNUTS and Tox4C601S were mixed at 1: 2 ratio and purified via SEC (Superdex 75 26/60) in 20 mM Tris pH 8.5, 150 mM NaCl, 0.1 mM ZnSO4, 0.5 mM TCEP to form 1:1 complex used for crystallization.
Crystallization and structure determination
Pooled PNUTS:Tox4 complex in crystallization buffer (20 mM Tris pH 8.5, 150 mM NaCl, 0.1 mM ZnSO4, 0.5 mM TCEP) was concentrated to 7 mg/mL. Crystals were formed in 100 mM MES pH 6.5, 1 M LiCl, 15% PEG6K using hanging drop vapor diffusion at room temperature. Crystals were cryo-protected using paraffin oil and immediately flash frozen. Data was collected at SSRL beamline 12.2 at 100 K using a Pilatus 6M PAD detector at two wavelengths, 1.192 Å (remote) and 1.283 Å (inflection, zinc). Data were processed to 2.1 Å using XDS,66 Aimless68 and Truncate.67 The structure was phased using multiple anomalous dispersion (MAD) using the data from both wavelengths and the solve_structure script available at SSRL (http://smb.slac.stanford.edu/facilities/software/MAD_scripts/; script calls Solve and Resolve for phasing and initial model building). Two strong anomalous peaks for zinc were identified, one corresponding to the Tox4 zinc binding site and a second located at a crystal contact. The model of the complex was completed using iterative rounds of refinement in PHENIX and manual building using Coot.71 Data collection and refinement details are provided in Table S2.
Isothermal titration calorimetry
SEC was used to transfer PNUTS and Tox4 into ITC Buffer (20 mM Tris pH 8.5, 150 mM NaCl, 0.1 mM ZnSO4, 0.5 mM TCEP). Purified Tox4 was titrated into PNUTS using an Affinity-ITC at 25°C (TA). Data were analyzed using NITPIC, SEDPHAT and GUSSI.72,73
NMR spectroscopy
NMR data were collected on Bruker Avance 500, Avance Neo 600 and Avance IIIHD 850 MHz spectrometers equipped with TCI HCN Z-gradient cryoprobes at 298 K. NMR measurements of PNUTS or Tox4CTD were recorded using 15N-labeled protein at a final concentration of 0.1 mM in NMR buffer and 90% H2O/10% D2O. The PNUTS and Tox4CTD 1:2 complex was formed in Tox4 NMR buffer, and then buffer exchanged to PNUTS NMR buffer (20 mM MES pH 6, 150 mM NaCl, 5 mM DTT) for the NMR measurements. All NMR data were processed using TopSpin 3.5 or 4.05 (Bruker) and analyzed using ccpNMR.79
Yeast two-hybrid (Y2H) screen
6.1 × 107 Drosophila 3rd instar larval cDNA clones were screened in yeast using full-length PNUTS (amino acids 1–1135) protein as ‘bait’ fused to the LexA DNA binding domain in pB27 (a derivative of pBTM116)80 by Hybrigenics Inc. Selective medium without tryptophan, leucine and histidine was used to select bait and library plasmids and interactions between the expressed proteins, respectively. 3-aminotriazol (3-AT) an inhibitor of the HIS3 reporter gene, was used at concentrations up to 50 mM to test binding under increasing stringency in confirmatory assays.81 The prey fragment was cloned in frame with the Gal4 Activation Domain (AD) into plasmid pP6, (derived from pGADGH).82 The diploid yeast cells were obtained using a mating protocol with L40ΔGal4 (mata) and HGX13 (Y187 ade2–101: loxP-kanMX-loxP, matα) yeast strains.78
Drosophila DNA cloning and mutagenesis
cDNA constructs
The tox4 coding sequence was cloned from cDNA clone LP01188 (Berkley Drosophila Research Project) into pENTR Gateway entry vector and mutagenised to create a truncating Proline to translation stop mutation (Tox4P216Ter). Tox4wt and Tox4P216Ter were inserted into N-terminal GFP tagged S2 cell expression vector, pAGW for co-immunoprecipitation (co-IP) and co-localisation studies. PNUTS coding sequence conjugated to a C-terminal Myc tag in the Gateway entry vector pDONR221, was subcloned into pAW for co-IP experiments, and into pARW to create N-terminal RFP fusions for expression in S2 cells. Expression vectors were obtained from the Drosophila Genome Resource Center (Indiana, USA). Genomic constructs: A 3.3 kb fragment corresponding to the entire tox4 transcription unit (from 461bp upstream of the ATG to the end of the 3′UTR) was synthesised by GeneArt with the following changes: i) the ORF for eGFP was inserted before the transcription start site to allow visualisation of the expressed protein; ii) an attB site was added at the end of the sequence for ϕC31 site specific recombination83; iii) Flanking BamHI and NotI restriction sites were added for subcloning into the pCaSpeR4. A variant of this construct was generated that carried Tox4P216Ter. For Tox4-binding mutations in PNUTS, we modified an existing 9.1Kb genomic clone (pW8-PNUTS)21 to include a 5′ attB site and C-terminal eGFP tag, generating pW8-attB::PNUTSwtGFP. The Tox4 binding site was mutagenized via Gibson cloning to generate pW8-attB::PNUTSK42E, V44DGFP (PNUTSED) and pW8-attB: PNUTSL10E,K42E,V44DGFP (PNUTSEED). An inducible PP1 non-binding construct in PNUTS (PNUTSwt-flp-W726A) was derived from pW8-attB::PNUTSwtGFP to additionally include a duplicated mCherry-tagged 3′ exon harboring a W726A mutation, which significantly decreases the ability of PNUTS to bind to PP1.21 The wildtype and W726A-bearing exons, flanked with FRT sites, are irreversibly exchanged by FRT/FLP recombination.
Drosophila transgenesis
Site-specific integration into the Drosophila genome was performed by the Cambridge Fly Facility, University of Cambridge. pCaSpeR4:tox4wt and pCaSpeR4:tox4P216Ter were inserted into the attP40 landing site. pW8-attB::PNUTSwtGFP, pW8-attB::PNUTSEDGFP and pW8-attB::PNUTSEEDGFP were inserted into attP2. Generation of a Drosophila tox4 null allele: A null tox4 allele was generated through imprecise excision of P[EPgy2]CG12104EY02201. This was done by crossing the P element to P[Δ2–3], a stable source of P-transposase,84,85 and by screening progeny by PCR to look for deletions in the tox4 gene. Lesions were confirmed by Sanger sequencing.
Egg laying and fertility assay
10 female virgins were crossed to 6 males and allowed to mate for 48 h at 25°C on standard food before transferring onto apple juice agar media. After 24 h, crosses were transferred onto new plates and the first collection of eggs was discarded. Recording of egg counts began with the second plate. The crosses were changed every 24 h and the number of eggs counted. 48 h after egg collections, the number of larvae emerged was counted and the percentage of eggs hatched calculated. Counts from each collection (technical repeats) were used to derive mean measurements for a single experiment. Experiments were performed in triplicate.
Transfection of Drosophila S2R + cells
Cells at 50–60% confluency were transiently transfected with Effectene transfection reagent (Qiagen) according to the manufacturer’s protocol and assayed after 48–72 h. Cells were grown in 6-well plastic plates for immunoprecipitation experiments or poly-lysine treated 24-well glass-bottomed Sensoplates (Greiner) for cell imaging.
Co-immunoprecipitation and immunoblotting
Transfected S2R + cells were pelleted by centrifugation at 4000 rpm, resuspended in ChromoTek lysis buffer. All buffers were supplemented with 1mM PMSF and 1×Protease Inhibitor Cocktail (Sigma). After incubation on ice for 30 min with pipetting every 5 to 10 min, the lysate was centrifuged at 13,000rpm for 10 min at 4°C and the supernatant transferred to a pre-cooled 1.5mL tube on ice. The supernatant was diluted to 500μL with ice-cold ChromoTek dilution buffer and 20μL of GFP-Trap magnetic beads equilibrated in the same buffer before adding to the lysate. Samples were incubated for 2 h at 4°C on a rotating mixer set at 25 rpm before pelleting the beads using a DiaMag 1.5 magnetic separator (Diagenode) and the buffer removed before washing three times with ice-cold Chromotek wash buffer.
Immunostaining and image analysis
S2R+ cells: Cells were live imaged using a Cell Discoverer 7 with Airyscan (Zeiss) equipped with Plan Apochromat 20x/0.95 NA objective, taking a stack of optical sections, each 1μm thick, across every cell. 3D co-localisation and Manders’ coefficient analysis86 were carried out using Imaris. Adult Drosophila ovaries: After dissection in PBS, tissues were fixed for 30 min in 3.7% paraformaldehyde in PBS at room temperature and then washed three times in PBST (PBS with 0.1% Triton X-100) for 10 min. Staining was performed as previously described.87 Ovaries were stained with either Hoechst 33342 or DAPI. After mounting with VectaShield (Vector Laboratories) or ProLong Glass (ThermoFisher) mounting media, fixed tissues were imaged using an LSM710, LSM780, LSM880 (Zeiss) or Nikon A1-R (Nikon Instruments Inc) confocal microscope equipped with 405nm, 488nm, 561nm and 633nm lasers. Tissues were imaged with Plan Apochromat 20x/0.8NA or 40x/1.3NA (oil immersion) objectives. Staging of egg chambers was as previously described.88 Measurements of nuclear to cytoplasmic GFP ratio were done in ImageJ, after subtracting mean background signal intensity from the entire image. The mean GFP intensity from at least 4 nuclei/egg chamber was divided by the mean cytoplasmic signal from the corresponding egg chamber to calculate the ratio of nuclear to cytoplasmic GFP for stage 9–10 egg chambers.
RNA-sequencing of total RNA following rRNA depletion
Quantity and quality of RNA was quantified using Nanodrop ND-2000 (Thermo Fisher Scientific) and Agilent TapeStation system (Agilent), respectively. RNA-sequencing libraries were constructed using the Stranded Total RNA Prep. Ligation with Ribo-Zero Plus kit (Illumina, Inc.) according to the manufacturer’s protocol with modifications to improve the Ribosomal depletion. Briefly, supplemental oligonucleotide probes (listed in Table S5) were designed corresponding to the following Drosophila Ribosomal RNA sequences: 28S, https://www.ebi.ac.uk/ena/browser/view/Non-coding:M21017.1:3288..7232:rRNA; 18S, https://rnacentral.org/rna/URS000030AF9A/7227; 5.8S & 2S, https://www.ebi.ac.uk/ena/browser/view/V00236. Oligonucleotides were purchased lyophilised at 50 pmol/oligo (Integrated DNA Technologies Inc.) and resuspended in RNase-free water to provide a final pool concentration of 1 pmol/oligo. RiboZero Plus reactions were then supplemented (at the ‘Hybridize Probes’ step) with 1 μL of this supplementary oligo pool prior to the addition of 10 μL of the total RNA and continuation of the remaining steps of the RiboZero Plus protocol. 159 bp paired-end reads from biological triplicates for each condition were generated using the NovaSeq6000 system (Illumina). The quality of raw sequence was checked using FastQC. 45–58M reads were generated per sample; ~4% of reads mapped to rRNA after depletion steps described above. Adapters and low-quality bases were removed from the reads using Trimmomatic_0.36.75 The trimmer reads were aligned to the Drosophila genome version dm6. Counts per gene were calculated using the corresponding annotation with STAR_2.7.7a aligner.76 Highly expressed genes were defined as those with normalised read counts in the top two quartiles of the control. Normalisation, principal components analysis (PCA), and differential expression was calculated in DESeq. 2_1.20.0.74 Gene ontology and enrichment pathway analysis was performed using FlyenrichR47 and Metascape.77
QUANTIFICATION AND STATISTICAL ANALYSIS
For ITC experiments, data were analyzed with a one-site binding model assuming a binding stoichiometry of 1:1 using NITPIC, SEDPHAT and GUSSI. Statistical analyses (mean ± SEM; 3 replicates) of ITC data were completed using Microsoft Excel. For fluorescent imaging, images were captured by Zen (Zeiss) or NIS (Nikon) acquisition software. All images are either single optical sections or maximal intensity projections taken with a confocal microscope. Individual measurements are shown alongside mean values for each condition. Statistical tests (mean ± SEM; ≥3 replicates) were performed in Prism (Graphpad) using a student’s t-test, one-way ANOVA or Kruskal-Wallis test. Unpaired t-tests were used to compare the means of two unmatched groups. One-way ANOVA was used to compare three or more groups, with Tukey correction to compare every mean with every other mean, or Dunnett’s test to compare every mean to the control mean. In cases where data were not found to be normally distributed, we used a non-parametric Kruskal-Wallis test with Dunn’s multiple comparisons to compare the difference in the sum of ranks between conditions.
Supplementary Material
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2025.115693.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| GFP Polyclonal Antibody | Thermo Fisher Scientific | Cat# A-11122, RRID:AB_221569 |
| c-Myc Monoclonal Antibody (9E10) | Thermo Fisher Scientific | Cat# MA1-980, RRID:AB_558470 |
| Anti-mouse IgG, HRP-linked Antibody | Cell Signaling Technology | Cat# 7076, RRID:AB_330924 |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| E.coli BL21 (DE3) GOLD expression strain | Agilent | Cat #230132 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| ZnSO4 | Fisher | Z76-500 |
| PEG6K | Hampton Research | HR2-533 |
| β-D-thiogalactopyranoside (IPTG) | Gold Bio | Cat #367-93-1 |
| Complete Protease inhibitor tablets | Roche | Cat #14696200 |
| Kanamycin | Gold Bio | Cat #K-120-100 |
| Tris (2-carboxyethyl) phosphine hydrochloride (TCEP) | Gold Bio | Cat #TCEP-25 |
| 15N ammonium chloride 99% | Cambridge Isotope Laboratories | Cat #NLM-465-25 |
| HisTrap HP column | GE Healthcare | 17524801 |
| Ni Sepharose 6 Fast flow 1000mL | GE Healthcare | Cat #17-5318-04 |
| HiLoad 26/60 Superdex 75 pg | GE Healthcare | Cat #17-1070-01 |
| Luria Broth | Fisher | BP9722-5 |
| Schneider’s Insect Medium | Gibco | Cat# 21720-024 |
| GFP-Trap magnetic beads | Chromotek | Cat# gtmak |
| Effectene transfection reagent | Qiagen | Cat# 301425 |
| Fetal Bovine Serum (Heat Inactivated) | Gibco | Cat# A5256801 |
| Penicillin-Streptomycin | Sigma-Aldrich | Cat# P0781 |
| RIPA buffer | Thermo Fisher Scientific | Cat# 89900 |
| SIGMAFAST Protease Inhibitor Tablets | Sigma-Aldrich | Cat# S8820 |
| Laemmli buffer | Sigma-Aldrich | Cat# S3401 |
| ECL Prime Western Blotting Detection Reagent | Cytiva | Cat# RPN2232 |
| DAPI | Thermo Fisher Scientific | Cat# 62248 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat# 62249 |
| ProLong Diamond Antifade Mountant | Thermo Fisher Scientific | Cat# P36961 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Stranded Total RNA Prep, Ligation with Ribo-Zero Plus | Illumina | Cat# 20040525 |
|
| ||
| Deposited data | ||
|
| ||
| PDB (Structure of PNUTS:Tox4 complex) | This study | PDBID 9CI7 |
| EMBL-EBI ArrayExpress (RNA sequencing data) | This study | E-MTAB-13735 |
|
| ||
| Oligonucleotides | ||
|
| ||
| See Table S5 | ||
|
| ||
| Recombinant DNA | ||
|
| ||
| pRP1b-(rat) PNUTS5–160 | Peti and Page65 | N/A |
| pTHMT-(human) Tox4571–621 | This study | N/A |
| pAGW-Drosophila tox4wt | This study | N/A |
| pAGW-Drosophila tox4P216Ter | This study | N/A |
| pAW-Drosophila PNUTSwt | This study | N/A |
| pCaSpeR4-attB, GFP-Drosophila tox4wt | This study | N/A |
| pCaSpeR4-attB, GFP-Drosophila tox4P216Ter | This study | N/A |
| pw8-attB, Drosophila PNUTSwt-GFP | This study | N/A |
| pw8-attB, Drosophila PNUTSK42E,V44D-GFP | This study | N/A |
| pw8-attB, Drosophila PNUTSL10E,K42E,V44D-GFP | This study | N/A |
| pw8-attB, Drosophila pNUTSwt-flp-W726A | This study | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| Topspin 3.5/4.0.5 | Bruker | RRID:SCR_014227 |
| solve_structure script | SSRL | http://smb.slac.stanford.edu/facilities/software/MAD_scripts |
| XDS | Kabsch et al.66 | https://www.ccp4.ac.uk/ |
| Truncate | French and Wilson67 | https://www.ccp4.ac.uk/ |
| Aimless | Evans and Murshudov68 | RRID:SCR_015747 |
| Solve/Resolve | Terwilliger and Berendzen 69 | https://solve.lanl.gov/ |
| PHENIX | Zwart et al.70 | RRID:SCR_014224 |
| COOT | Emsley et al.71 | RRID:SCR_014222 |
| Pymol | Schrodinger, LLC | RRID:SCR_000305 |
| NITPIC | Scheuermann et al.72 | https://www.utsouthwestern.edu/labs/mbr/software/ |
| SEDPHAT | Zhao et al.73 | https://www.utsouthwestern.edu/labs/mbr/software/ |
| GUSSI | Scheuermann et al.72 | https://www.utsouthwestern.edu/labs/mbr/software/ |
| FIJI (ImageJ) | NIH | RRID:SCR_002285 |
| Zen (v 3.9) | Zeiss | RRID:SCR_013672 |
| Imaris (v 10) | Bitplane/Oxford Instruments | RRID:SCR_007370 |
| Prism (v 10) | Graphpad | RRID:SCR_002798 |
| FastQC | Babraham Bioinformatics | RRID:SCR_014583 |
| DESeq2 | Love et al.74 | RRID:SCR_015687 |
| Trimmomatic | Bolger et al.75 | RRID:SCR_011848 |
| STAR | Dobin et al.76 | RRID:SCR_004463 |
| Metascape | Zhou et al.77 | RRID:SCR_016620 |
| FlyEnrichR | Kuleschov et al.47 | https://maayanlab.doud/FlyEnrichr |
|
| ||
| Other | ||
|
| ||
| Cell Discoverer 7 with 20× objective | Zeiss | N/A |
| LSM880 with 20× objective | Zeiss | N/A |
| A1R confocal with 20× Plan-Apo objective | Nikon | N/A |
| 24 well Sensoplate | Greiner | Cat# 662892 |
| Coverslips No 1 | Scientific Laboratory Supplies | Cat# MIC3110 |
| ChemiDoc MP Imaging System | Biorad | 12003154 |
Highlights.
The PNUTS:Tox4 structure shows that Tox4 and transcription factors bind distinct surfaces of PNUTS
Drosophila Tox4 is vital for fertility, with some Tox4 fertility effects dependent on PNUTS
PNUTS-PP1/PNUTS-Tox4 are key for chromosomal dispersal and gene expression during oogenesis
ACKNOWLEDGMENTS
We thank the following University of Manchester core facilities for support: the Manchester Fly Facility, the Genomic Technologies and Bioinformatics Core Facility, the Gene Editing Unit, and the Bioimaging Core Facility. We thank Sanjai Patel, Haley Bennett, David Spiller, and Catherine Sutcliffe for technical assistance. We also thank the Liverpool Centre for Cell Imaging for assistance in the early phase of the project. L.D. and A.E.C. were funded by the BBSRC (nos. 976535 and 1510556). The work was also supported by the Medical Research Council UK (MR/K015931/1). We thank Dr. Yang Li for Tox4: PNUTS data collection and analysis. Crystallographic data were collected at the Stanford Synchrotron Radiation Lightsource. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02–76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH. This work was supported by grant 1R01GM144483 from the National Institute of General Medicine to W. P. and grant 1R01GM144379 from the National Institute of General Medicine to R.P.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Cossa G, Parua PK, Eilers M, and Fisher RP (2021). Protein phosphatases in the RNAPII transcription cycle: erasers, sculptors, gatekeepers, and potential drug targets. Genes Dev. 35, 658–676. 10.1101/gad.348315.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett D (2005). Transcriptional control by chromosome-associated protein phosphatase-1. Biochem. Soc. Trans. 33, 1444–1446. 10.1042/BST0331444. [DOI] [PubMed] [Google Scholar]
- 3.Buratowski S (2009). Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546. 10.1016/j.molcel.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harlen KM, and Churchman LS (2017). The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 18, 263–273. 10.1038/nrm.2017.10. [DOI] [PubMed] [Google Scholar]
- 5.Bollen M, and Beullens M (2002). Signaling by protein phosphatases in the nucleus. Trends Cell Biol. 12, 138–145. 10.1016/s0962-8924(01)02247-4. [DOI] [PubMed] [Google Scholar]
- 6.Ceulemans H, and Bollen M (2004). Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 84, 1–39. 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- 7.Bollen M, Peti W, Ragusa MJ, and Beullens M (2010). The extended PP1 toolkit: designed to create specificity. Trends Biochem. Sci. 35, 450–458. 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brautigan DL, and Shenolikar S (2018). Protein Serine/Threonine Phosphatases: Keys to Unlocking Regulators and Substrates. Annu. Rev. Biochem. 87, 921–964. 10.1146/annurev-biochem-062917-012332. [DOI] [PubMed] [Google Scholar]
- 9.Boens S, Szekér K, Van Eynde A, and Bollen M (2013). Interactor-guided dephosphorylation by protein phosphatase-1. Methods Mol. Biol. 1053, 271–281. 10.1007/978-1-62703-562-0_16. [DOI] [PubMed] [Google Scholar]
- 10.Heroes E, Lesage B, Görnemann J, Beullens M, Van Meervelt L, and Bollen M (2013). The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J. 280, 584–595. 10.1111/j.1742-4658.2012.08547.x. [DOI] [PubMed] [Google Scholar]
- 11.Peti W, Nairn AC, and Page R (2013). Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611. 10.1111/j.1742-4658.2012.08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen PB, Kwon YG, Nairn AC, and Greengard P (1998). Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J. Biol. Chem. 273, 4089–4095. 10.1074/jbc.273.7.4089. [DOI] [PubMed] [Google Scholar]
- 13.Jagiello I, Beullens M, Stalmans W, and Bollen M (1995). Subunit structure and regulation of protein phosphatase-1 in rat liver nuclei. J. Biol. Chem. 270, 17257–17263. 10.1074/jbc.270.29.17257. [DOI] [PubMed] [Google Scholar]
- 14.Kelley JR, Dimitrova E, Maciuszek M, Nguyen HT, Szczurek AT, Hughes AL, Blackledge NP, Kettenbach AN, and Klose RJ (2024). The PNUTS phosphatase complex controls transcription pause release. Mol. Cell 84, 4843–4861.e8. 10.1016/j.molcel.2024.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Song A, Tao B, Miao M, Luo YQ, Wang J, Yin Z, Xiao R, Zhou X, Shang XY, et al. (2024). The phosphatase PP1 sustains global transcription by promoting RNA polymerase II pause release. Mol. Cell 84, 4824–4842.e7. 10.1016/j.molcel.2024.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Estell C, Davidson L, Eaton JD, Kimura H, Gold VAM, and West S (2023). A restrictor complex of ZC3H4, WDR82, and ARS2 integrates with PNUTS to control unproductive transcription. Mol. Cell 83, 2222–2239.e5. 10.1016/j.molcel.2023.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Russo M, Piccolo V, Polizzese D, Prosperini E, Borriero C, Polletti S, Bedin F, Marenda M, Michieletto D, Mandana GM, et al. (2023). Restrictor synergizes with Symplekin and PNUTS to terminate extragenic transcription. Genes Dev. 37, 1017–1040. 10.1101/gad.351057.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cossa G, Roeschert I, Prinz F, Baluapuri A, Silveira Vidal R, Schülein-Völk C, Chang YC, Ade CP, Mastrobuoni G, Girard C, et al. (2020). Localized Inhibition of Protein Phosphatase 1 by NUAK1 Promotes Spliceosome Activity and Reveals a MYC-Sensitive Feedback Control of Transcription. Mol. Cell 77, 1322–1339.e11. 10.1016/j.molcel.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortazar MA, Sheridan RM, Erickson B, Fong N, Glover-Cutter K, Brannan K, and Bentley DL (2019). Control of RNA Pol II Speed by PNUTS-PP1 and Spt5 Dephosphorylation Facilitates Termination by a “Sitting Duck Torpedo” Mechanism. Mol. Cell 76, 896–908.e4. 10.1016/j.molcel.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landsverk HB, Sandquist LE, Bay LTE, Steurer B, Campsteijn C, Landsverk OJB, Marteijn JA, Petermann E, Trinkle-Mulcahy L, and Syljuåsen RG (2020). WDR82/PNUTS-PP1 Prevents Transcription-Replication Conflicts by Promoting RNA Polymerase II Degradation on Chromatin. Cell Rep. 33, 108469. 10.1016/j.celrep.2020.108469. [DOI] [PubMed] [Google Scholar]
- 21.Ciurciu A, Duncalf L, Jonchere V, Lansdale N, Vasieva O, Glenday P, Rudenko A, Vissi E, Cobbe N, Alphey L, and Bennett D (2013). PNUTS/PP1 regulates RNAPII-mediated gene expression and is necessary for developmental growth. PLoS Genet. 9, e1003885. 10.1371/journal.pgen.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu D, De Wever V, Derua R, Winkler C, Beullens M, Van Eynde A, and Bollen M (2018). A substrate-trapping strategy for protein phosphatase PP1 holoenzymes using hypoactive subunit fusions. J. Biol. Chem. 293, 15152–15162. 10.1074/jbc.RA118.004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Lim CJ, Min JK, Lee JK, Kim YM, Lee JY, Won MH, and Kwon YG (2007). Protein phosphatase 1 nuclear targeting subunit is a hypoxia inducible gene: its role in post-translational modification of p53 and MDM2. Cell Death Differ. 14, 1106–1116. 10.1038/sj.cdd.4402111. [DOI] [PubMed] [Google Scholar]
- 24.Dingar D, Tu WB, Resetca D, Lourenco C, Tamachi A, De Melo J, Houlahan KE, Kalkat M, Chan PK, Boutros PC, et al. (2018). MYC dephosphorylation by the PP1/PNUTS phosphatase complex regulates chromatin binding and protein stability. Nat. Commun. 9, 3502. 10.1038/s41467-018-05660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy MS, Hieke M, Kumar GS, Lewis GR, Gonzalez-DeWhitt KR, Kessler RP, Stein BJ, Hessenberger M, Nairn AC, Peti W, and Page R (2014). Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proc. Natl. Acad. Sci. USA 111, 4097–4102. 10.1073/pnas.1317395111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Leon G, Sherry TC, and Krucher NA (2008). Reduced expression of PNUTS leads to activation of Rb-phosphatase and caspase-mediated apoptosis. Cancer Biol. Ther. 7, 833–841. 10.4161/cbt.7.6.5839. [DOI] [PubMed] [Google Scholar]
- 27.Landsverk HB, Kirkhus M, Bollen M, Küntziger T, and Collas P (2005). PNUTS enhances in vitro chromosome decondensation in a PP1-dependent manner. Biochem. J. 390, 709–717. 10.1042/BJ20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher LA, Wang L, Wu L, and Peng A (2014). Phosphatase 1 nuclear targeting subunit is an essential regulator of M-phase entry, maintenance, and exit. J. Biol. Chem. 289, 23745–23752. 10.1074/jbc.M114.572149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landsverk HB, Mora-Bermúdez F, Landsverk OJB, Hasvold G, Naderi S, Bakke O, Ellenberg J, Collas P, Syljuåsen RG, and Küntziger T (2010). The protein phosphatase 1 regulator PNUTS is a new component of the DNA damage response. EMBO Rep. 11, 868–875. 10.1038/embor.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landsverk HB, Sandquist LE, Sridhara SC, Rødland GE, Sabino JC, de Almeida SF, Grallert B, Trinkle-Mulcahy L, and Syljuåsen RG (2019). Regulation of ATR activity via the RNA polymerase II associated factors CDC73 and PNUTS-PP1. Nucleic Acids Res. 47, 1797–1813. 10.1093/nar/gky1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murai J, and Pommier Y (2019). Phosphatase 1 Nuclear Targeting Subunit, a Novel DNA Repair Partner of PARP1. Cancer Res. 79, 2460–2461. 10.1158/0008-5472.CAN-19-0798. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Zhu S, Fisher LA, Wang L, Eurek NJ, Wahl JK 3rd, Lan L, and Peng A (2019). Phosphatase 1 Nuclear Targeting Subunit Mediates Recruitment and Function of Poly (ADP-Ribose) Polymerase 1 in DNA Repair. Cancer Res. 79, 2526–2535. 10.1158/0008-5472.CAN-18-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, Redel C, Ahlner A, Lemak A, Johansson-Åkhe I, Houliston S, Kenney TMG, Tamachi A, Morad V, Duan S, et al. (2022). The MYC oncoprotein directly interacts with its chromatin cofactor PNUTS to recruit PP1 phosphatase. Nucleic Acids Res. 50, 3505–3522. 10.1093/nar/gkac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cermakova K, Demeulemeester J, Lux V, Nedomova M, Goldman SR, Smith EA, Srb P, Hexnerova R, Fabry M, Madlikova M, et al. (2021). A ubiquitous disordered protein interaction module orchestrates transcription elongation. Science 374, 1113–1121. 10.1126/science.abe2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, You J, Dobrota E, and Skalnik DG (2010). Identification and characterization of a novel human PP1 phosphatase complex. J. Biol. Chem. 285, 24466–24476. 10.1074/jbc.M110.109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, and Skalnik DG (2008). Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A Histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol. Cell Biol. 28, 609–618. 10.1128/MCB.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Wu A, Wu Z, Wang T, Pan Y, Li B, Zhang X, and Yu M (2022). TOX4 facilitates promoter-proximal pausing and C-terminal domain dephosphorylation of RNA polymerase II in human cells. Commun. Biol. 5, 300. 10.1038/s42003-022-03214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Zhao R, Zhi J, Liu Z, Wu A, Yang Z, Wang W, Ni T, Jing L, and Yu M (2023). Tox4 regulates transcriptional elongation and reinitiation during murine T cell development. Commun. Biol. 6, 613. 10.1038/s42003-023-04992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett D, Lyulcheva E, Alphey L, and Hawcroft G (2006). Towards a comprehensive analysis of the protein phosphatase 1 interactome in Drosophila. J. Mol. Biol. 364, 196–212. 10.1016/j.jmb.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 40.O’Flaherty E, and Kaye J (2003). TOX defines a conserved subfamily of HMG-box proteins. BMC Genom. 4, 13. 10.1186/1471-2164-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu D, Jaroszewski L, Li Z, and Godzik A (2014). FFAS-3D: improving fold recognition by including optimized structural features and template re-ranking. Bioinformatics 30, 660–667. 10.1093/bioinformatics/btt578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holm L (2022). Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215. 10.1093/nar/gkac387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, and Ben-Tal N (2016). ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350. 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, and Ben-Tal N (2005). ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302. 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dej KJ, and Spradling AC (1999). The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development 126, 293–303. 10.1242/dev.126.2.293. [DOI] [PubMed] [Google Scholar]
- 46.Reed BH, and Orr-Weaver TL (1997). The Drosophila gene morula inhibits mitotic functions in the endo cell cycle and the mitotic cell cycle. Development 124, 3543–3553. 10.1242/dev.124.18.3543. [DOI] [PubMed] [Google Scholar]
- 47.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97. 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai SY, Chang YL, Swamy KBS, Chiang RL, and Huang DH (2016). GAGA factor, a positive regulator of global gene expression, modulates transcriptional pausing and organization of upstream nucleosomes. Epigenetics Chromatin 9, 32. 10.1186/s13072-016-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C, Li X, Hechmer A, Eisen M, Biggin MD, Venters BJ, Jiang C, Li J, Pugh BF, and Gilmour DS (2008). NELF and GAGA factor are linked to promoter-proximal pausing at many genes in Drosophila. Mol. Cell Biol. 28, 3290–3300. 10.1128/MCB.02224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Royzman I, Hayashi-Hagihara A, Dej KJ, Bosco G, Lee JY, and Orr-Weaver TL (2002). The E2F cell cycle regulator is required for Drosophila nurse cell DNA replication and apoptosis. Mech. Dev. 119, 225–237. 10.1016/s0925-4773(02)00388-x. [DOI] [PubMed] [Google Scholar]
- 51.Volpe AM, Horowitz H, Grafer CM, Jackson SM, and Berg CA (2001). Drosophila rhino encodes a female-specific chromo-domain protein that affects chromosome structure and egg polarity. Genetics 159, 1117–1134. 10.1093/genetics/159.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodrich JS, Clouse KN, and Schüpbach T (2004). Hrb27C, Sqd and Otu cooperatively regulate gurken RNA localization and mediate nurse cell chromosome dispersion in Drosophila oogenesis. Development 131, 1949–1958. 10.1242/dev.01078. [DOI] [PubMed] [Google Scholar]
- 53.Buszczak M, and Spradling AC (2006). The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes Dev. 20, 977–989. 10.1101/gad.1396306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Buskirk C, and Schüpbach T (2002). Half pint regulates alternative splice site selection in Drosophila. Dev. Cell 2, 343–353. 10.1016/s1534-5807(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 55.Klusza S, Novak A, Figueroa S, Palmer W, and Deng WM (2013). Prp22 and spliceosome components regulate chromatin dynamics in germ-line polyploid cells. PLoS One 8, e79048. 10.1371/journal.pone.0079048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, and Fadini R (2015). Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 21, 427–454. 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 57.Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, and Debey P (2003). Chromatin configuration and transcriptional control in human and mouse oocytes. Mol. Reprod. Dev. 64, 458–470. 10.1002/mrd.10233. [DOI] [PubMed] [Google Scholar]
- 58.Ducreux B, Ferreux L, Patrat C, and Fauque P (2023). Overview of Gene Expression Dynamics during Human Oogenesis/Folliculogenesis. Int. J. Mol. Sci. 25, 33. 10.3390/ijms25010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee CC, Li B, Yu H, and Matunis MJ (2018). Sumoylation promotes optimal APC/C Activation and Timely Anaphase. Elife 7, e29539. 10.7554/eLife.29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin L, Chen L, and Tran PT (2017). Fission yeast neddylation ligase Dcn1 facilitates cohesin cleavage and chromosome segregation at anaphase. Biol. Open 6, 844–849. 10.1242/bio.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson ER, Brown NG, Peters JM, Stark H, and Schulman BA (2019). Posing the APC/C E3 Ubiquitin Ligase to Orchestrate Cell Division. Trends Cell Biol. 29, 117–134. 10.1016/j.tcb.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozziconacci J, Merle M, and Lesne A (2020). The 3D Genome Shapes the Regulatory Code of Developmental Genes. J. Mol. Biol. 432, 712–723. 10.1016/j.jmb.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Bompadre O, and Andrey G (2019). Chromatin topology in development and disease. Curr. Opin. Genet. Dev. 55, 32–38. 10.1016/j.gde.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Vermunt MW, Zhang D, and Blobel GA (2019). The interdependence of gene-regulatory elements and the 3D genome. J. Cell Biol. 218, 12–26. 10.1083/jcb.201809040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peti W, and Page R (2007). Strategies to maximize heterologous protein expression in Escherichia coli with minimal cost. Protein Expr. Purif. 51, 1–10. 10.1016/j.pep.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 66.Kabsch W (2010). Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132. 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.French S, and Wilson K (1978). On the treatment of negative intensity observations. Acta Cryst 34, 517–525. [Google Scholar]
- 68.Evans PR, and Murshudov GN (2013). How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214. 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terwilliger TC, and Berendzen J (1999). Automated MAD and MIR structure solution. Acta Crystallogr. D Biol. Crystallogr. 55, 849–861. 10.1107/s0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zwart PH, Afonine PV, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, McKee E, Moriarty NW, Read RJ, Sacchettini JC, et al. (2008). Automated structure solution with the PHENIX suite. Methods Mol. Biol. 426, 419–435. 10.1007/978-1-60327-058-8_28. [DOI] [PubMed] [Google Scholar]
- 71.Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheuermann TH, and Brautigam CA (2015). High-precision, automated integration of multiple isothermal titration calorimetric thermograms: new features of NITPIC. Methods 76, 87–98. 10.1016/j.ymeth.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H, Piszczek G, and Schuck P (2015). SEDPHAT–a platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods 76, 137–148. 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bolger AM, Lohse M, and Usadel B (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, and Chanda SK (2019). Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523. 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fromont-Racine M, Rain JC, and Legrain P (1997). Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat. Genet. 16, 277–282. 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- 79.Skinner SP, Fogh RH, Boucher W, Ragan TJ, Mureddu LG, and Vuister GW (2016). CcpNmr AnalysisAssign: a flexible platform for integrated NMR analysis. J. Biomol. NMR 66, 111–124. 10.1007/s10858-016-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vojtek AB, and Hollenberg SM (1995). Ras-Raf interaction: two-hybrid analysis. Methods Enzymol. 255, 331–342. 10.1016/s0076-6879(95)55036-4. [DOI] [PubMed] [Google Scholar]
- 81.Bennett D, and Alphey L (2007). Yeast two-hybrid screens to identify Drosophila PP1-binding proteins. Methods Mol. Biol. 365, 155–179. 10.1385/1-59745-267-X:155. [DOI] [PubMed] [Google Scholar]
- 82.Bartel P, Chien CT, Sternglanz R, and Fields S (1993). Elimination of false positives that arise in using the two-hybrid system. Biotechniques 14, 920–924. [PubMed] [Google Scholar]
- 83.Groth AC, Fish M, Nusse R, and Calos MP (2004). Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166, 1775–1782. 10.1093/genetics/166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robertson HM, Preston CR, Phillis RW, Johnson-Schlitz DM, Benz WK, and Engels WR (1988). A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118, 461–470. 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan S, Lyulcheva E, Dean J, and Bennett D (2008). Mars promotes dTACC dephosphorylation on mitotic spindles to ensure spindle stability. J. Cell Biol. 182, 27–33. 10.1083/jcb.200712080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manders EMM, Verbeek FJ, and Aten JA (1993). Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 169, 375–382. 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 87.Badmos H, Cobbe N, Campbell A, Jackson R, and Bennett D (2021). Drosophila USP22/nonstop polarizes the actin cytoskeleton during collective border cell migration. J. Cell Biol. 220, e202007005. 10.1083/jcb.202007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spradling AC (1993). Developmental genetics of oogenesis. In The Development of Drosophila melanogaster, Bate M and Martinez Arias A, eds. (CSHL Press; ), pp. 1–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and structure factors have been deposited in the Protein DataBank (PDB: 9CI7). RNA-seq data have been deposited in EMBL-EBI ArrayExpress: E-MTAB-13735.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact (daimark.bennett@manchester.ac.uk) upon request.


