Abstract
Introduction
The pathophysiology of respiratory complications in post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) is poorly understood, but a high incidence of progressive pulmonary fibrosis was anticipated. Deupirfenidone (LYT-100) is a selectively deuterated form of pirfenidone that retains antifibrotic and anti-inflammatory activity but with improved tolerability. This study evaluated the safety and efficacy of deupirfenidone in PASC patients with respiratory complications.
Methods
Global, double-blind, randomised placebo-controlled trial evaluating 750 mg deupirfenidone twice daily versus placebo for 3 months in PASC patients with respiratory complications following hospitalisation for acute COVID-19 infection severe enough to necessitate supplemental oxygen (NCT04652518).
Results
185 patients were randomised and treated (95 with deupirfenidone, 90 with placebo), with 177 included in the modified intention-to-treat population. The mean age was 54.5 years, 62.7% were male and 10.7% had prior mechanical ventilation. The 6-min walk distance improved across both arms between baseline and day 91 (deupirfenidone 44.3 m (95% CI 24.8–63.8 m) versus placebo 48.8 m (95% CI 29.2–68.4 m); p=0.70). The most common treatment-emergent adverse events (TEAEs) for deupirfenidone versus placebo were nausea (9.5% versus 1.1%), upper abdominal discomfort (5.3% versus 2.2%) and dyspepsia (6.3% versus 1.1%). TEAEs leading to trial drug discontinuation were 11.6% for deupirfenidone and 4.4% for placebo. The proportion of discontinuations considered at least possibly related to treatment was 8.6% for deupirfenidone and 2.4% for placebo.
Discussion
Most patients with PASC and respiratory complications showed significant improvement over 91 days irrespective of treatment assignment. Deupirfenidone was well tolerated, with low rates of TEAEs, which supports further investigation in patients with idiopathic pulmonary fibrosis.
Shareable abstract
Most patients with respiratory complications and post-acute sequelae of SARS-CoV-2 infection showed significant improvement over 91 days, irrespective of treatment assignment. Deupirfenidone was well tolerated, with low rates of TEAEs. https://bit.ly/42wnW4Z
Introduction
COVID-19 is a heterogeneous condition caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. While most patients experience mild illness and spontaneous recovery, a small but important subgroup of those infected require hospitalisation because of pneumonia and other complications. Studies of survivors of two other severe coronavirus infections, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome coronavirus (MERS-CoV), raised concerns, supported by early data from the COVID-19 pandemic, that severe COVID-19 might trigger the development of pulmonary fibrosis in susceptible individuals [1–3]. Post-acute sequelae of SARS-CoV-2 infection (“post-acute COVID” (PASC)) have been reported, including persistent dyspnoea and radiological evidence of residual lung parenchymal abnormalities [4–10] with, in some cases, concomitant findings of fibrotic features on histopathology [11, 12]. The mechanisms driving the development of post-COVID-19 pulmonary fibrosis appear to be similar to those seen in idiopathic pulmonary fibrosis (IPF).
Antifibrotic therapy has been shown to slow disease progression in IPF and other forms of progressive pulmonary fibrosis, but the uptake of treatment has been limited by tolerability issues. Deupirfenidone (LYT-100) is a selectively deuterated form of pirfenidone. It has a differentiated pharmacokinetic profile from pirfenidone that has shown improved tolerability [13] while retaining the biochemical potency and specificity of pirfenidone.
The aim of this Phase II trial (NCT04652518) was to evaluate the safety and efficacy of deupirfenidone in patients with PASC and continued respiratory complications following hospitalisation for acute COVID-19 infection severe enough to necessitate supplemental oxygen. Based on modelling of escalating twice-daily doses of deupirfenidone, a 750-mg twice-daily dose was selected to approximate the exposure (area under the curve) of 801 mg of pirfenidone three times daily. Preliminary findings from this study were previously reported in abstract form [14].
Methods
Study design and eligibility
A randomised, double-blind, parallel-arm, placebo-controlled Phase II clinical trial was conducted at 24 study centres in Europe, the USA, Asia and South America. Eligible patients were aged 18–80 years with a positive SARS-CoV-2 test, had been hospitalised within the preceding 3 months with a need for respiratory support (including mechanical ventilation (MV), extracorporeal membrane oxygenation (ECMO) and oxygen administration) and had evidence of sequelae of COVID-19 pneumonia with involvement of two or more lung lobes (based on radiography or computed tomography (CT) scans). Patients had to have the ability to walk a minimum of 10 m (use of inhaled oxygen permitted) and have shortness of breath of grade 3 or higher on the modified Borg Dyspnoea Scale (mBDS). Considering the primary efficacy endpoint of change from baseline to day 91 in the 6-min walk test (6MWT) distance, and to avoid including patients who were too unwell, patients on MV, ECMO, noninvasive ventilation (NIV), high-flow nasal oxygen (HFNO) and/or other high-flow oxygen with inspiratory oxygen fraction (FIO2) of ≥35% and ≥8 L·m−1 within 72 h prior to screening were excluded. Ongoing use of steroids to treat non-COVID-19 related conditions was not permitted. Patients with pre-existing chronic respiratory conditions requiring medication were excluded, as were those with a history of IPF, lung cancer, pulmonary arterial hypertension, another interstitial lung disease or severe cardiac insufficiency (grade IV).
Study protocol
Following informed consent and eligibility assessment, patients completed a 6MWT and were randomised in a 1:1 ratio to deupirfenidone or matching placebo, stratified by baseline 6MWT distance, prior MV status and age (<65 versus ≥65). Patients could be randomised while hospitalised, at the time of discharge or post discharge. Standard of care was administered at the discretion of the investigators and according to local government and/or institutional guidelines. Double-blind treatment (750 mg of deupirfenidone or matching placebo) was administered (with or without food) orally twice daily for 91 days. Dose titration was allowed for tolerability at the discretion of the investigator.
Study endpoints
The primary efficacy endpoint was change from baseline to day 91 in 6MWT distance. The 6MWT was performed in accordance with the American Thoracic Society/European Respiratory Society guidelines and all sites were trained for standardisation [15]. Key secondary objectives included change in the Dyspnoea-12 [16] and semi-Quantitative Lung Fibrosis (QLF) scores. The analysis for Dyspnoea-12 was performed for the overall score (questions 1–12), the physical items score (questions 1–7) and the affective items score (questions 8–12). CT was performed, with QLF and computer-aided diagnosis QLF scores at days 0 and 91. All radiographic images were centrally read by trained radiologists who were blinded to treatment allocation but aware of image time point. CT images were scored for each of the five lung lobes on a scale of 0–5 for each of ground-glass opacity, fibrosis, consolidation and reticulation/bronchiectasis. A summary fibrosis score represented a sum of each component for all lung regions in total, using a scale ranging from 0 to 25. Other endpoints included changes from baseline in St George's Respiratory Questionnaire-I (SGRQ-I), mBDS, World Health Organization (WHO) Ordinal Scale for Clinical Improvement and quality-of-life assessment (36-item Short-Form Health Survey (SF-36)); pulmonary exacerbations, all-cause rehospitalisations, change in use of supplemental oxygen and all-cause mortality. Safety and tolerability were assessed via clinical and laboratory evaluations and treatment-emergent adverse events (TEAEs).
Statistical analysis
The sample size was based on the hypothesis that deupirfenidone would significantly improve the 91-day time–distance profile as measured by change from baseline in the 6MWT distance. Specifically, sample sizes of 84 per arm (total=168) provided 85% power to detect a difference of 30 m in the change from baseline in the 6MWT, using a mixed model for repeated measures (MMRM), an assumed standard deviation of 75 m, and alpha of 5%.
Analysis of the 6MWT was performed at each protocol-scheduled time point, i.e. baseline and days 14, 28, 56 and 91. The primary method of analysis utilised an MMRM. Fixed effects included treatment, visit, the treatment by visit interaction, the baseline value of the endpoint as a covariate and study centre. Outcomes at each time point were reported as adjusted least square (LS) means and standard errors (se). Categorical endpoints were assessed using a Cochran–Mantel–Haenszel (CMH) test, stratified by centre. Kaplan–Meier plots and log-rank tests assessed time to all-cause mortality (if sufficient mortality data existed) as well as time to discharge from hospital (in days) and other time-to-event parameters. TEAEs were tabulated by MedDRA system organ class and preferred term, while changes from baseline in clinical laboratory and CT parameters were derived. All inferential tests were two-sided with alpha=5%. The intention-to-treat (ITT) population was used for efficacy assessments, while the safety population was used for all safety endpoints.
Main results
Patient characteristics
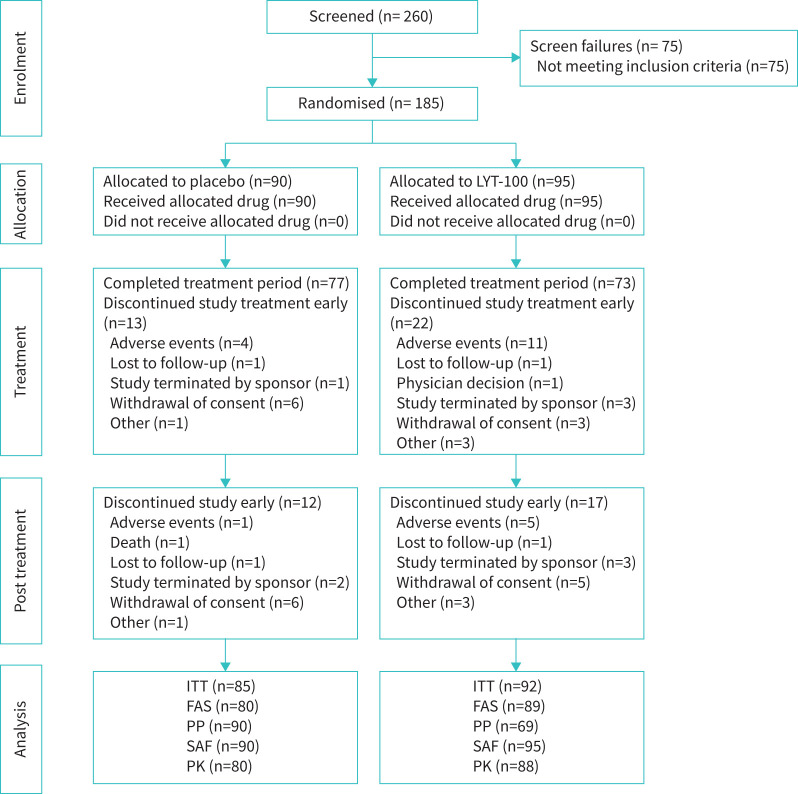
A total of 260 patients were screened from September 2020 to June 2022, of whom 185 were randomised (95 to deupirfenidone and 90 to placebo) and received at least one dose of study medication (figure 1). This group formed the safety population. Treatment was discontinued early by 22 (23.2%) deupirfenidone and 13 (14.4%) placebo patients, with 11 (11.6%) deupirfenidone and four (4.4%) placebo patients discontinuing due to TEAE. One patient (placebo) died; there were no deaths in the deupirfenidone arm.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. ITT population, N=177 (deupirfenidone (LYT-100) arm, n=92; placebo arm, n=85); safety analysis population, N=185 (deupirfenidone arm, n=95; placebo arm, n=90). LYT-100: deupirfenidone; ITT: intention to treat; FAS: full analysis set; PP: per protocol; SAF: safety analysis set; PK: pharmacokinetics.
The review of efficacy data at completion of the primary double-blinded portion of the study suggested that there was no clinically meaningful benefit with deupirfenidone treatment and additional studies were terminated (open-label extension study and Clinical Outcome Assessment Development sub-study). This study enrolled a total of 34 patients in Ukraine, and the war started during the study, thus complicating the collection of data from these patients.
Patient demographics were comparable between the treatment arms (table 1). Overall mean (sd) age was 54.7 (12.4) years, and 110 (62.1%) patients were male. The racial composition of the population included 140 (79.1%) patients who identified as white, 30 (16.9%) as Asian, three (1.7%) as Black or African American and four (2.3%) as other, and the majority of patients were not Hispanic or Latino (158 (89.3%) patients).
TABLE 1.
Demographics and baseline characteristics
| Deupirfenidone (n=92) | Placebo (n=85) | |
|---|---|---|
| Age, years | 56.6 (12.1) | 52.7 (132.5) |
| Sex, male | 60 (65.2%) | 50 (58.8%) |
| Ethnicity, non-Hispanic/Latino | 82 (89.1%) | 76 (89.4%) |
| Race, White | 79 (85.9%) | 61 (71.8%) |
| BMI, kg·m−2 | 30.1 (4.7) | 29.7 (5.2) |
| Days since hospital discharge | 34.3 (26.9) | 34.2 (29.1) |
| Days from COVID-19 diagnosis to first dose of study drug | 56.3 (28.2) | 58.1 (27.6) |
| Longest 6MWT distance, m | 352.2 (125.8) | 342.1 (114.1) |
| Prior ventilation/ECMO | 11 (12.0%) | 8 (9.4%) |
| mBDS | 3.9 (1.4) | 3.8 (1.4) |
| SpO2, % | 96.4 (1.5) | 95.9 (1.8) |
| Computed tomography quantitative lung fibrosis score, median (IQR) | 4 (1–5) | 4 (2–5) |
Data are presented as mean (sd) or n (%) unless otherwise noted. BMI: body mass index; 6MWT: 6-min walk test; ECMO: extracorporeal membrane oxygenation; mBDS: modified Borg Dyspnoea Scale; SpO2: peripheral oxygen saturation; IQR: interquartile range.
The mean (sd) time from COVID-19 diagnosis to first dose of study drug was 57.1 (27.8) days, indicating that the typical patient enrolled in this study received positive results for SARS-CoV-2 testing almost 2 months prior to the initiation of study treatment. The mean (sd) time since initial hospitalisation for COVID-19 relative to baseline/randomisation was 53.0 (28.7) days. The mean (sd) time since discharge from hospital for COVID-19 relative to baseline/randomisation was 34.3 (27.9) days). 21 patients were still hospitalised at their randomisation date. The majority of patients had not received prior MV or ECMO (158 (89.3%) patients). All patients received some type of respiratory support for COVID-19: low-flow O2, 76 (42.9%); other high-flow O2, 44 (24.9%); HFNO, 35 (19.8%); NIV, 13 (7.3%); and MV, 9 (5.1%). The mean (sd) mBDS score at baseline was 3.9 (1.4) (reflecting mild to moderate dyspnoea), and the average body mass index (BMI) (29.9 kg·m−2) reflected a slightly obese population.
6-min walk test distance
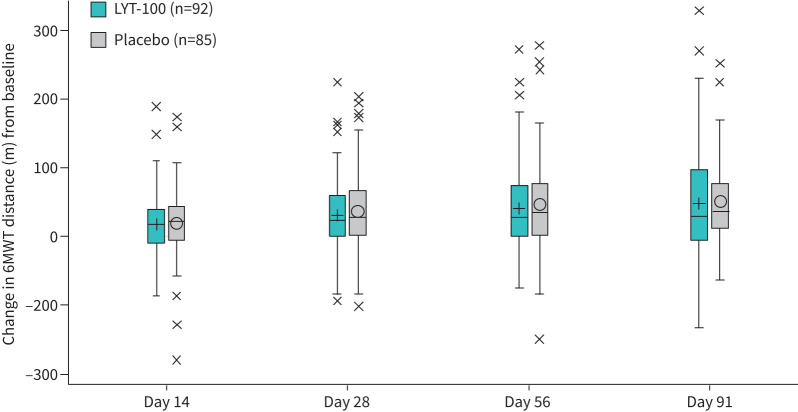
Baseline 6MWT distance (sd) averaged 352.2 (125.8) m in the deupirfenidone arm and 342.0 (114.1) m in the placebo arm, with the majority of patients able to walk >200 m (156 (88.1%)). By the end of the 91-day treatment period, patients in both arms had improved by a similar amount. For the deupirfenidone arm, the day-91 LS mean (se) change from baseline was 44.3 (10.0) m, while the placebo arm had a mean change of 48.8 (10.0) m. The pattern of improvements in the distance walked was similar between treatment arms, with no significant treatment differences noted (p=0.70). A box plot of the LS mean changes from baseline in 6MWT score from the combined results of the MMRM analysis is presented for the ITT population in figure 2.
FIGURE 2.
Change from baseline in 6-min walk test (6MWT) distance. Length of boxes=interquartile range (IQR) (distance between first or upper quartile (Q1) and third or lower quartile (Q3)); horizontal lines within boxes=group median; symbols within boxes=group mean; symbol values (i.e. “x”)=potential outliers; baseline=longest recorded test value prior to treatment administration. Whiskers extend to 1.5 times the IQR above Q1 (Q1−1.5*IQR) or below Q3 (Q3+1.5*IQR). LYT-100: deupirfenidone.
Dyspnoea-12 scores
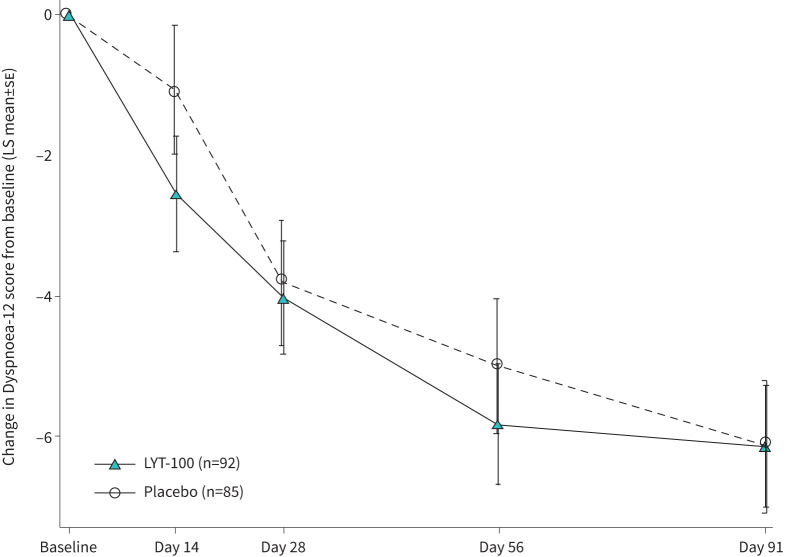
The key secondary endpoint was change from baseline to day 91 in the Dyspnoea-12. Both arms exhibited improved total Dyspnoea-12 scores (sd) at day 91, compared with baseline, but there were no meaningful differences between the deupirfenidone and placebo arms (−6.9 (7.18) and −7.3 (7.15), respectively; LS mean difference: 0.01 (95% CI −1.58–1.60); p=0.99). A line plot of the LS mean changes from baseline in the Dyspnoea-12 overall score is presented in figure 3. The difference in Dyspnoea-12 Physical Score at day 91, compared with baseline, between the two arms was 0.10 (95% CI −0.96–1.09; p=0.90) and the difference in affective score was −0.10 (95% CI −0.74–0.57; p=0.80).
FIGURE 3.
Least square (LS) mean (se) change from baseline in Dyspnoea-12 score. Combined results from mixed model for repeated measures (MMRM) analysis of multiple imputed datasets. LYT-100, deupirfenidone.
Quantitative scores on CT
CT was available to compare QLF scores between baseline and day 91 for 74 patients in the deupirfenidone arm and 71 patients in the placebo arm. The median scores for apparent fibrosis on chest CT (scale ranges from 0 to 25, with higher scores indicating worse fibrosis) were similarly low for deupirfenidone and placebo patients at baseline (4 (interquartile range (IQR) 1–5) and 4 (IQR 2–5), respectively). By day 91, median fibrosis scores decreased in most patients in both arms (1 (IQR 1–3) and 1 (IQR 0–3), respectively). At day 91, an improvement of at least 2 points was seen for ground-glass opacity (62 (83.8%) patients in the deupirfenidone arm and 55 (77.5%) patients in the placebo arm), reticulation/bronchiectasis (49 (66.2%) patients in the deupirfenidone arm and 44 (62.0%) patients in the placebo arm) and fibrosis (30 (40.5)% patients in the deupirfenidone arm and 33 (46.5%) patients in the placebo arm). The results of a CMH test show that the differences between the two treatment arms (deupirfenidone minus placebo) for the 2-point improvement was not statistically significant for any of the CT scan attributes.
Safety and tolerability
TEAEs were observed in 71 (74.7%) deupirfenidone and 43 (47.8%) placebo patients, with the majority of events judged by the investigator to be mild or moderate in severity (grade 1, 40.0% versus 27.8%; grade 2, 24.2% versus 15.6%; grade 3, 10.5% versus 3.3%; and grade 4/5, 0% versus 1.1%, respectively). There were three serious adverse events in each treatment arm. Discontinuation of study treatment due to TEAEs was low (deupirfenidone: 11.6%; placebo: 4.4%) in this population of patients with PASC.
Gastrointestinal (GI) disorders were the most frequent events, with 23 (24.2%) deupirfenidone and 10 (11.1%) placebo patients experiencing at least one GI disorder (table 2). Nausea was the most common event in the deupirfenidone arm (9.5%, compared with 1.1% in the placebo arm), followed by dyspepsia (6.3%, compared with 1.1% in the placebo arm) and upper abdominal pain (5.3%, compared with 2.2% in the placebo arm).
TABLE 2.
Overview of treatment-emergent adverse events (TEAEs)
| Deupirfenidone (n=95) | Placebo (n=90) | |
|---|---|---|
| Patients with ≥1 TEAE | 71 (74.7) | 43 (47.8) |
| Drug-related TEAEs | 38 (40.0) | 18 (20.0) |
| TEAEs by severity | ||
| Grade 1 (mild) | 38 (40.0) | 25 (27.8) |
| Grade 2 (moderate) | 23 (24.2) | 14 (15.6) |
| Grade 3 (severe) | 10 (10.5) | 3 (3.3) |
| Grade 4 (life-threatening) | 0 | 0 |
| Grade 5 (death) | 0 | 1 (1.1) |
| Drug-related severe TEAEs (≥grade 3) | 0 | 2 (2.2) |
| Serious TEAEs# | 3 (3.2) | 3 (3.3) |
| Drug-related serious TEAEs | 0 | 1 (1.1) |
| TEAEs leading to discontinuation¶ | 11 (11.6) | 4 (4.4) |
| TEAEs leading to dose reduction | 7 (7.4) | 2 (2.2) |
| TEAEs >5% in either arm | ||
| Nausea | 9 (9.5) | 1 (1.1) |
| Dyspepsia | 6 (6.3) | 1 (1.1) |
| Upper abdominal pain | 5 (5.3) | 2 (2.2) |
| Nasopharyngitis | 5 (5.3) | 1 (1.1) |
| Increased D-dimer | 5 (5.3) | 1 (1.1) |
| Hyperglycaemia | 3 (3.2) | 5 (5.6) |
| Headache | 5 (5.3) | 2 (2.2) |
| Cough | 5 (5.3) | 0 |
Data are presented as n (%). #: TEAEs that resulted in death, were immediately life-threatening, necessitated hospitalisation/prolongation of existing hospitalisation, resulted in persistent or significant disability/incapacity and/or resulted in a congenital abnormality/birth defect. ¶: Deupirfenidone arm: liver function test increase (n=1), pruritus (n=1), photosensitivity reaction (n=2), nail disorder (n=1), alanine aminotransferase increase (n=1), atrial fibrillation (n=1), mixed dementia (n=1), diarrhoea (n=1), dyspepsia (n=1), hepatic steatosis (n=1) and drug hypersensitivity (n=1) (note that one patient reported two of the symptoms). Placebo arm: anxiety (n=1), COVID-19 (n=1), dyspepsia (n=1) and abdominal pain (n=1).
Two deupirfenidone arm patients with elevated alanine aminotransferase levels at baseline discontinued the study drug due to elevated hepatic enzymes. One patient, diagnosed with hepatic steatosis, continued to have elevated liver enzymes consistent with this condition months after discontinuing deupirfenidone; in the second patient, elevated liver enzymes resolved following deupirfenidone discontinuation. One death occurred in the placebo arm due to worsening COVID-19 (unrelated to study treatment).
Discussion
We report the findings from a randomised, placebo-controlled trial evaluating the efficacy of antifibrotic therapy in patients with PASC with respiratory complications. At the time the study was designed there was speculation that COVID-19 infection would be associated with a high incidence of progressive pulmonary fibrosis. However, despite the fact that enrolled patients had multilobar COVID-19 pneumonia necessitating respiratory support, most patients had minimal apparent lung fibrosis at baseline and did not develop progression of lung fibrosis over 91 days. The majority of patients in both treatment arms showed clinically significant improvement in physiological parameters and quality of life; furthermore, on imaging assessment, a large proportion of patients in both arms showed improvement in ground-glass opacities and reticulation over the course of the study. It is therefore not surprising that deupirfenidone did not show an effect on the results of the 6MWT or Dyspnoea-12 over placebo in this patient population.
A few small studies of patients who suffered COVID-19 pneumonia early in the pandemic reported findings of fibrotic features on histopathology; however, others have reported diffuse alveolar damage with features of organising pneumonia, a pattern of injury that has capacity for improvement [17, 18]. This may explain the observed improvements in lung function and CT findings that have been reported in post-COVID-19 interstitial lung changes following corticosteroid treatment [6]. In some studies, a lower % predicted diffusing capacity of the lung for carbon monoxide (DLCO) has been associated with greater risk for persistent symptoms [5, 9], which may be related to vascular abnormalities [17, 19]. While the majority of individuals in our study showed marked clinical improvement over 91 days, approximately a quarter of patients in both treatment arms failed to show improvement. Globally, the burden of respiratory impairment from COVID-19 is high and has likely been driven by this subset of individuals who fail to improve over time. Further work is required to enable prospective identification of individuals at risk of persistent symptoms and progressive pulmonary fibrosis following hospitalisation with coronaviruses and other forms of viral pneumonia.
Similar to data from other smaller studies investigating the efficacy of antifibrotic therapies, nintedanib and pirfenidone [20], in this population of patients with PASC who did not have progressive pulmonary fibrosis, our study did not demonstrate an antifibrotic effect of deupirfenidone. However, care needs to be taken in applying these results more broadly. In designing the study early in the pandemic, we lacked clear data on how to best identify individuals at risk of progressive pulmonary fibrosis due to COVID-19 infection. Patients were selected based on a presumed risk of progressive pulmonary fibrosis and lung dysfunction due to severity of infection (hospitalisation and supplemental oxygenation) and persistence of respiratory symptoms. Given that three quarters of patients showed functional, physiological and imaging evidence of improvement over 91 days, it is clear that disease severity alone is not a predictor of the development of persistent or progressive lung fibrosis. It is to be hoped that data from the current study and from other longitudinal studies that have been conducted during the COVID-19 pandemic will enable more reliable identification of individuals at risk of progressive pulmonary fibrosis following severe viral infection. It remains plausible that antifibrotic therapy has the potential to modify outcomes in the subset of patients with persistent interstitial lung damage. Reliably identifying these individuals early in their disease course will be important if future trials of antifibrotic therapy are to be tested during outbreaks of viral pneumonia.
Pirfenidone is approved for the treatment of IPF. In the ASCEND study [21], pirfenidone was associated with a 45% reduction in the rate of forced vital capacity (FVC) decline, compared with placebo. However, the uptake of pirfenidone in clinical practice has been limited by tolerability issues. In the ASCEND study there was a significant burden of GI adverse events (diarrhoea 22.3%, anorexia 15.8%, dyspepsia 17.6% vomiting 12.9%, weight loss 12.6% and gastroesophageal reflux 11.9%), together with an appreciable burden of photosensitive rash (28.1%). In a study of healthy humans, the upper GI adverse events caused by pirfenidone appeared to relate to peak plasma levels [22]. Deupirfenidone is a deuterated form of pirfenidone; this chemical modification results in it being metabolised more slowly than pirfenidone, which attenuates peak exposure while maintaining overall exposure. In a double-blind, randomised, two-period crossover study in older healthy subjects, 550 mg of deupirfenidone three times daily showed bioequivalence in exposure (area under the curve) and a 24% lower peak serum concentration (Cmax) than 801 mg of pirfenidone three times daily. These pharmacokinetic differences were associated with a ∼50% reduced incidence of GI and nervous system adverse events [13]. Deupirfenidone appeared to be well tolerated in the current study, although the 750-mg twice-daily dose was associated with a higher Cmax than would be expected with 801 mg of pirfenidone three times daily. Most TEAEs reported in the deupirfenidone arm were mild or moderate, with a limited rate of treatment discontinuations and dose reductions (11.6% and 7.4%, respectively). The frequency of GI-related TEAEs was comparatively low (table 2).
COVID-19 respiratory disease is a multifaceted condition. This clinical study included several objectives (and corresponding endpoints) to evaluate deupirfenidone treatment efficacy. In other clinical trials evaluating fibrotic interstitial lung diseases, the primary outcome measure is typically FVC [23]. From a practical standpoint, factors such as aerosolisation risk and the need for pre-spirometry testing for COVID-19 would have made FVC a challenging endpoint to operationalise at the time of conduct of this study. Furthermore, in designing the study it was not clear that FVC would fully capture the limitations imposed by a systemic illness with important respiratory manifestations. For this reason, we chose the 6MWT distance as the endpoint for the trial. Although the study did not achieve the primary efficacy endpoint, it provides important lessons for designing similar trials in the future, including a clearer understanding of the dynamics of physiological measures in the context of recovery from serious respiratory illness.
This study was conducted at multiple investigational centres in eight countries, and thus reflects data from a wide range of patients and medical “best practices”, with patient visits sometimes limited due to local mandates in the healthcare facilities. The study started enrolment early in the pandemic, but required 18 months to fully enrol, and medical “best practices” to treat COVID-19 evolved as the general medical knowledge changed during those early months of the pandemic. Furthermore, the heterogeneity of the baseline/pre-treatment pulmonary fibrosis measures we observed in the enrolled population and a failure to enrich the study for those individuals with progressive pulmonary fibrosis may have led to more variable study outcomes. And finally, the war in Ukraine, a country with 19% of the enrolled patients, started in the middle of the study. The war posed substantial operational challenges, but did not materially affect the primary study outcomes.
In summary, our findings suggest that the majority of patients with PASC and respiratory complications will show significant clinical improvement over 91 days regardless of intervention. Deupirfenidone did not contribute to any additional improvement over and above that seen in the placebo arm. However, the safety and tolerability profile of deupirfenidone in this patient population supports the current investigation of two doses of deupirfenidone in a Phase IIb trial for the treatment of IPF (NCT05321420). Further studies are required to enable prospective identification of individuals with COVID-19 and other respiratory virus infections at risk of developing progressive pulmonary fibrosis.
Acknowledgements
We want to thank all of the participating patients, the Long COVID advocacy organisations that raised awareness about the study, and Alison Gara and Heather Paden (both at SSI Strategy, formerly at PureTech Health) for expert conduct of clinical operations.
Footnotes
Provenance: Submitted article, peer reviewed.
This clinical trial is prospectively registered with ClinicalTrials.gov as NCT04652518
Ethics statement: Central Institutional Review Board (IRB) approval was obtained along with site-level IRB approvals.
Author contributions: J. Krop, T.M. Maher and M.C. Chen conceptualised the study and designed the protocol. M. Harnett undertook the statistical analyses. T. Kulkarni and T.M. Maher drafted the main manuscript text. All authors read and approved the final manuscript.
Conflict of interest: T. Kulkarni received speaker/consultation fees from Boehringer Ingelheim Inc. and Veracyte, and consultation/advisory board fees from United Therapeutics Corp., Aileron, Avalyn and PureTech Inc. T.M. Maher, via his institution, received industry-academic funding from AstraZeneca and GlaxoSmithKline R&D, and consultancy/speaker fees from PureTech Inc., AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Fibrogen, Galapagos, GlaxoSmithKline R&D, IQVIA, Pliant, F. Hoffmann-La Roche, Ltd., Trevi and Veracyte. M.C. Chen and C.S. Graham are employed at PureTech Health, and J. Krop is a consultant for PureTech Health. The other authors have nothing to disclose. Support statement: This trial was sponsored by PureTech Health. Funding information for this article has been deposited with the Crossref Funder Registry.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
01142-2024.SUPPLEMENT
Data availability
Data sharing is not available.
References
- 1.Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8: 8. doi: 10.1038/s41413-020-0084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie L, Liu Y, Fan B, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res 2005; 6: 5. doi: 10.1186/1465-9921-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 2020; 214: 1072–1077. doi: 10.2214/AJR.20.22976 [DOI] [PubMed] [Google Scholar]
- 5.Stewart I, Jacob J, George PM, et al. Residual lung abnormalities after COVID-19 hospitalization: interim analysis of the UKILD post-COVID-19 study. Am J Respir Crit Care Med 2023; 207: 693–703. doi: 10.1164/rccm.202203-0564OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc 2021; 18: 799–806. doi: 10.1513/AnnalsATS.202008-1002OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76: 396–398. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellemons ME, Huijts S, Bek LM, et al. Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for COVID-19: a longitudinal study of respiratory, physical, and psychological outcomes. Ann Am Thorac Soc 2022; 19: 551–561. doi: 10.1513/AnnalsATS.202103-340OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20: 1135–1140. doi: 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Wang X, Xiong Y, et al. Correlation of autopsy pathological findings and imaging features from 9 fatal cases of COVID-19 pneumonia. Medicine (Baltimore) 2021; 100: e25232. doi: 10.1097/MD.0000000000025232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenko E, Korth CC, Harnett MD, et al. Deupirfenidone (LYT-100) reduces incidence of gastrointestinal (GI)-related adverse events (AEs) compared to pirfenidone at comparable exposure levels: a phase 1, double-blind, randomized crossover study in older healthy subjects. Am J Respir Crit Care Med 2022; 205: A5572 [Google Scholar]
- 14.Kulkarni T, Santiaguel J, Aul R, et al. Safety and tolerability of LYT-100 (deupirfenidone) in post-acute sequelae of SARS-CoV-2 (PASC) “long COVID” patients presenting with respiratory complications. Am J Respir Crit Care Med 2023; 207: A6293. doi: 10.1164/ajrccm-conference.2023.207.1 [DOI] [Google Scholar]
- 15.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 16.Yorke J, Moosavi SH, Shuldham C, et al. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax 2010; 65: 21–26. doi: 10.1136/thx.2009.118521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020; 396: 320–332. doi: 10.1016/S0140-6736(20)31305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Culebras M, Loor K, Sansano I, et al. Histological findings in transbronchial cryobiopsies obtained from patients after COVID-19. Chest 2022; 161: 647–650. doi: 10.1016/j.chest.2021.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Sun LX, Feng RE. Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43: 496–502. doi: 10.3760/cma.j.cn112147-20200311-00312 [DOI] [PubMed] [Google Scholar]
- 20.Shu Y, He L, Liu C. Impact of anti-fibrotic medications on post-COVID-19 pulmonary fibrosis: a systematic review and meta-analysis. Int J Infect Dis 2024; 147: 107193. doi: 10.1016/j.ijid.2024.107193 [DOI] [PubMed] [Google Scholar]
- 21.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. , ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 22.Rubino CM, Bhavnani SM, Ambrose PG, et al. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm Pharmacol Ther 2009; 22: 279–285. doi: 10.1016/j.pupt.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Raghu G, Ghazipura M, Fleming TR, et al. Meaningful endpoints for idiopathic pulmonary fibrosis (IPF) clinical trials: emphasis on ‘feels, functions, survives’. Report of a collaborative discussion in a symposium with direct engagement from representatives of patients, investigators, the National Institutes of Health, a Patient Advocacy Organization, and a Regulatory Agency. Am J Respir Crit Care Med 2024; 209: 647–669. doi: 10.1164/rccm.202312- [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material
01142-2024.SUPPLEMENT
Data Availability Statement
Data sharing is not available.