Abstract
Background
Techniques to support secretion clearance for individuals with neuromuscular conditions and respiratory muscle weakness include mechanical insufflation–exsufflation and chest wall vibrations. Assessing the comparative efficacy of these techniques is challenging due to the absence of a core outcome set. We sought to describe outcomes and measurement instruments reported in studies of airway clearance techniques for individuals with neuromuscular conditions living in the community.
Methods
We conducted a scoping review of primary research studies. We searched six databases from inception to 22 February 2024. Two reviewers independently screened citations against the inclusion criteria and extracted data on outcomes and measurement characteristics. Outcomes were categorised according to the Core Outcome Measures in Effectiveness Trials (COMET) 38-domain taxonomy.
Results
We identified 75 eligible studies describing 55 outcomes. We grouped outcomes deemed overlapping and categorised them using the COMET 38-domain taxonomy, resulting in 34 distinct outcomes. Common physiological/clinical outcomes were cough strength (n=48 studies, 64%), lung volume (n=48, 64%) and insufflation capacity (n=22, 29%). The most common measurement tools for these outcomes were spirometer (n=38, 51%), peak flow meter (n=24, 32%) and pneumotachograph (n=20, 27%). The most common resource-use outcome was hospitalisation due to respiratory illness (n=13, 17%). Few studies reported on life impact outcomes, with the most common being comfort (n=6, 8%) and patient satisfaction (n=4, 5%).
Conclusion
We identified 34 outcomes from 75 studies, which were most commonly physiological/clinical, with resource-use and life impact outcomes being seldom reported. The number and range of outcomes and measures demonstrates the need for a core outcome set.
Shareable abstract
There is a wide range of outcomes and measures used in studies of interventions to promote secretion clearance in individuals with neuromuscular conditions, demonstrating the need to develop a core outcome set to allow better integration of trial results. https://bit.ly/40DrXCU
Introduction
Individuals with neuromuscular disorders often develop respiratory muscle weakness, involving both the inspiratory and expiratory muscles [1]. These neuromuscular disorders include congenital diseases such as the muscular dystrophies, and acquired diseases such as multiple sclerosis and amyotrophic lateral sclerosis. Cervical spinal cord injuries can also result in respiratory muscle weakness, due to interruption of the descending bulbospinal respiratory pathways [2]. In addition to respiratory muscle weakness, certain neuromuscular conditions can also lead to bulbar-innervated muscle dysfunction [3, 4]. The combination of respiratory and bulbar muscle weakness can lead to impaired secretion clearance [5] as these muscles are used to generate an effective cough [6]. Impaired secretion clearance can result in an increased risk of lower respiratory tract infections. This is significant because respiratory infection remains a common cause of death in individuals with neuromuscular disorders [7, 8].
To reduce the risk of respiratory infection, community-dwelling individuals with neuromuscular disorders are supported with interventions to augment secretion clearance. Methods used can be categorised into proximal (“cough augmentation”) or distal (“sputum mobilisation”) techniques [9]. Proximal techniques include assisted inspiration (e.g. air stacking, glossopharyngeal breathing, lung volume recruitment), assisted expiration (e.g. manually assisted cough) and assisted inspiration and expiration (e.g. mechanical insufflation–exsufflation) [9]. Distal techniques include high frequency chest wall oscillation or compression, and manual techniques (e.g. chest percussion and expiratory vibration) [9].
Secretion clearance used for individuals with a neuromuscular disorder varies in terms of type of technique, application of technique and equipment availability [10–12]. A frequently cited reason for this practice variation is the lack of evidence for the efficacy of these techniques [9, 13]. Furthermore, assessing the comparative efficacy of secretion clearance techniques is challenging due to the lack of consensus on which study outcomes to measure and which measurement instruments to use when designing a clinical trial. A key stakeholder-informed core outcome set (COS) would assist with homogeneity in the selection of trial outcomes in the future. A COS is an agreed set of outcomes which should be measured and reported in all trials undertaking an investigation into a particular area [14]. This set is agreed upon via item generation and consensus building activities using an expert panel including individuals living with the disease, caregivers and family members.
As the first step to informing a COS for interventional studies of airway clearance techniques for individuals with neuromuscular disease living in the community, our primary objective was to conduct a scoping review to identify outcomes reported in studies recruiting these individuals. Our secondary objective was to characterise the instruments used to measure the outcomes identified.
Methods
We conducted a preliminary search of PROSPERO, MEDLINE, the Cochrane Database of Systematic Reviews, and Joanna Briggs Institute Evidence Synthesis and did not find published or in-progress scoping reviews or systematic reviews on this topic. We searched the Core Outcome Measures in Effectiveness Trials (COMET) database and did not identify any ongoing COS relevant to this work.
We followed the scoping review methods published by Levac et al. [15], which built upon the original framework proposed by Arksey and O’Malley [16], alongside the amendments made by the Joanna Briggs Institute by Peters et al. [17]. This review was registered a priori on the Open Science Framework platform (https://doi.org/10.17605/OSF.IO/JKMG2). The COS was registered on the COMET database, (www.comet-initiative.org/Studies/Details/1987). Findings were reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) extension for Scoping Reviews (PRISMA-ScR) [18].
Inclusion criteria
We included primary research studies recruiting community-dwelling participants with a neuromuscular condition resulting in respiratory muscle weakness requiring an airway clearance technique. We included studies recruiting participants (both adult and paediatric) who were either using long-term tracheostomy ventilation, noninvasive ventilation, or self-ventilating (i.e. not using supplementary ventilation, noninvasive ventilation or long-term tracheostomy ventilation). Studies that recruited mixed populations with individuals without neuromuscular conditions were included if more than 50% of the population had neuromuscular conditions. We included studies that recruited individuals living at home or in long-term care facilities, but not those who were inpatient in hospital receiving acute medical care. Grey literature was included where appropriate.
Exclusion criteria
We excluded reviews, editorials and commentaries, as well as case reports, qualitative, and animal studies. For pragmatic reasons related to resourcing, we included only studies published in English.
Search strategy
Our search strategy (supplementary material) was informed by search strategies used in two previous Cochrane reviews on cough augmentation techniques in patients with respiratory failure [13, 19], and an initial search of MEDLINE (PubMed) and CINAHL (EBSCO). The finalised search strategy was conducted from database inception to 14 November 2024 in MEDLINE (PubMed), EMBASE, Web of Science and CINAHL (EBSCO). Each search strategy was validated by ensuring that a publication that we knew to be eligible for inclusion was identified by the search. We searched the World Health Organization International Clinical Trials Registry Platform (https://apps.who.int/trialsearch/) for ongoing or completed trials, and the Cochrane Library for systematic reviews. We screened reference lists of relevant reviews for additional studies. We searched OpenGrey for potential studies that are ongoing or had not been published via a traditional route.
Screening, data extraction and critical appraisal
Two independent reviewers (NS and CA) screened titles and abstracts against the inclusion criteria using EndNote X9 (Clarivate Analytics, PA, USA). All full texts of potentially eligible citations were retrieved and assessed by two independent reviewers (NS and CA) with a third author (LR) available for further discussion. We used a PRISMA flow diagram to present our search results [20].
Two independent reviewers (NS and CA) extracted data using a bespoke data extraction tool which was initially piloted with five studies and revised as needed. Data extracted comprised study characteristics (including country of study, clinical conditions, year of publication, types of participants and study design), a description of the intervention and comparator group (if applicable), outcomes verbatim as described and measurement instruments. We categorised outcomes using the COMET 38-domain taxonomy [21]. We summarised study characteristics, interventions, outcomes and measures reported in studies using descriptive statistics. Two authors (NS and CA) independently applied the Management of Otitis Media with Effusion in Children with Cleft Palate (MOMENT) criteria [22] to assess the quality of outcome reporting. This tool assesses how well primary and secondary outcomes have been identified and explained. Although it is not a validated assessment tool for outcome reporting, it has been used previously to assess the quality of describing and reporting outcomes in systematic and scoping reviews of the literature [23–30], and in the development of COS [31, 32]. The six questions (each scored as one point) include: 1) Was the primary outcome stated?; 2) Was the primary outcome clearly defined so that another researcher would be able to reproduce its measurement?; 3) Were the secondary outcomes clearly stated?; 4) Were the secondary outcomes clearly defined?; 5) Do the authors explain the choice of outcomes they have selected?; and 6) Were methods used to enhance quality of outcome measurement, if appropriate? This scoring system ranges from 0–6, with a score of ≥4 representing high-quality outcome reporting [22]. This assessment will facilitate an exploration of the relationship between reporting quality and the nature of the outcomes reported. Reporting on the quality of outcome identified will provide support behind which of these outcomes should be taken forward into COS development.
Results
We screened 8514 citations and included 75 studies meeting our inclusion criteria (figure 1). Of these, 42 (56%) recruited only adult participants, 5 (7%) only paediatric participants and 28 (37%) a mixed population. The age range included in the studies that included paediatric participants was 11 months to 15 years. Congenital muscular dystrophy was the most commonly included clinical condition (n=48, 64%). Most studies were conducted in Europe (n=31, 41%) or North America (n=25, 33%) between the years of 2010 to 2019 (n=33, 44%) (table 1). The most common study designs were cohort studies (n=44, 59%) with 13 (17%) randomised controlled trials.
FIGURE 1.
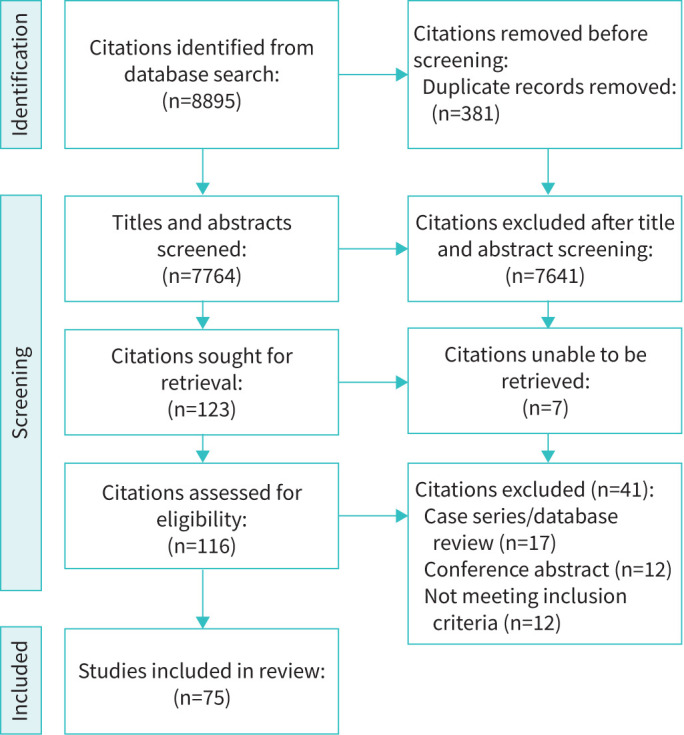
Flow diagram displaying literature search process and identification of studies included in this review.
TABLE 1.
Characteristics of included studies
| Country | Europe | 31 (41) |
| North America | 25 (33) | |
| Asia | 11 (15) | |
| Australasia | 5 (7) | |
| South America | 3 (4) | |
| Clinical condition | Congenital muscular dystrophy | 48 (64) |
| Spinal muscular atrophy | 29 (39) | |
| Motor neurone disease | 27 (36) | |
| Spinal cord injury | 16 (21) | |
| Myotonic dystrophy | 12 (16) | |
| Polio/poliomyelitis | 11 (15) | |
| Glycogen storage disease/metabolic disease | 11 (15) | |
| Peripheral system disorder | 10 (13) | |
| Cerebral palsy | 7 (9) | |
| Myasthenia gravis | 6 (8) | |
| Multiple sclerosis | 5 (7) | |
| Inflammatory myopathy | 2 (3) | |
| Other# | 12 (16) | |
| Publication | Before 1980 | 1 (1) |
| 1980–1989 | 1 (1) | |
| 1990–1999 | 3 (4) | |
| 2000–2009 | 27 (36) | |
| 2010–2019 | 33 (44) | |
| 2020–present | 10 (13) | |
| Participants | Adults | 42 (56) |
| Paediatrics | 5 (7) | |
| Mixed | 28 (37) | |
| Study design | Cohort | 44 (59) |
| Randomised controlled trial | 13 (17) | |
| Randomised crossover trial | 11 (15) | |
| Before/after | 4 (5) | |
| Case–control | 2 (3) | |
| Nonrandomised controlled trial | 1 (1) |
Data are presented as n (%) out of a total of 75 studies. Proportions do not always sum to 100% as individuals with multiple conditions were included in some studies. #: including Friedreich's ataxia, lissencephaly, Parkinson's disease, spinocerebellar degeneration, traumatic brain injury, static encephalopathy, cerebrovascular accident, spina bifida, familial dysautonomia, Lafora disease, osteogenesis imperfecta and chromosome 1 deletion.
The most commonly evaluated airway clearance techniques were breath stacking (including use of a lung volume recruitment bag) (n=34, 45%), manually assisted cough (n=30, 40%) and mechanical insufflation–exsufflation (n=25, 33%) (table 2). The treatment intervention was most frequently administered by clinicians, which included physiotherapists, respiratory therapists, physicians, nurses and technicians (n=25, 33%) followed by family members/carers (n=15, 20%).
TABLE 2.
Summary of interventions investigated in the studies included in this review
| Secretion clearance techniques | Breath stacking/air stacking, lung volume recruitment | 34 (45) |
| Manual assisted cough | 30 (40) | |
| Mechanical insufflation–exsufflation | 25 (33) | |
| High-frequency chest wall compression/high-frequency chest wall oscillation | 8 (11) | |
| Glossopharyngeal breathing | 5 (7) | |
| Manual insufflation | 5 (7) | |
| Positioning/postural drainage | 5 (7) | |
| Intermittent positive pressure breathing | 4 (5) | |
| External glottic device | 3 (4) | |
| Intrapulmonary percussive ventilation | 3 (4) | |
| Exsufflation only | 2 (3) | |
| Functional electrical stimulation | 2 (3) | |
| Inspiratory muscle training | 1 (1) | |
| Positive expiratory pressure/bubble positive expiratory pressure | 1 (1) | |
| Other# | 7 (9) | |
| Individual administering intervention | Clinician (physician, physiotherapist, respiratory therapist, nurse, technician) | 25 (33) |
| Family member/carer | 15 (20) | |
| Patient | 11 (15) | |
| Not reported | 33 (44) |
Data are presented as n (%) out of a total of 75 studies. Proportions do not always sum to 100% as multiple interventions were used in some studies and multiple individuals administered the intervention. #: includes incentive spirometry, proprioceptive neuromuscular facilitation, continuous positive airways pressure, noninvasive ventilation, expiratory flow accelerator, forced expiration technique and breathing exercises.
Outcomes
Data extraction identified 55 outcomes. Following iterative discussion, three authors (NMS, CA and LR) deemed 21 to be overlapping or similar (e.g. vital capacity and forced vital capacity). This resulted in 34 distinct outcomes in seven domains of the 38-domain COMET taxonomy (table 3). At least one physiological/clinical outcome was included in 69 (92%) studies. Outcomes in the life impact core domain were reported in 15 (20%) studies; outcomes in the resource-use domain were reported in 16 (21%) studies.
TABLE 3.
Outcomes reported in studies included in this scoping review. Outcomes are classified according to the 38-domain COMET taxonomy
| Core area | Outcome domain | Outcome | n (%) |
|---|---|---|---|
| Death | Mortality/survival | Survival | 4 (5) |
| Physiological/clinical | Physiological/clinical | Cough strength (e.g. peak cough flow) | 48 (64) |
| Lung volume (e.g. vital capacity, forced vital capacity, forced expiratory volume, expiratory reserve volume) | 48 (64) | ||
| Insufflation capacity (e.g. maximum insufflation capacity, lung insufflation capacity) | 22 (29) | ||
| Respiratory muscle strength (e.g. maximal inspiratory pressure, maximal expiratory pressure, sniff nasal inspiratory pressure) | 16 (21) | ||
| Oxygen levels (e.g. saturation of peripheral oxygen) | 15 (20) | ||
| Air flow (e.g. peak expiratory flow, forced expiratory flow) | 13 (17) | ||
| Respiratory rate | 8 (11) | ||
| Carbon dioxide levels | 6 (8) | ||
| Lung expansion/compliance | 5 (7) | ||
| Heart rate | 5 (7) | ||
| Airway pressure (e.g. peak inspiratory pressure, peak expiratory pressure, transpulmonary pressure) | 3 (4) | ||
| Cough volume | 2 (3) | ||
| Mean arterial pressure | 2 (3) | ||
| Airway resistance | 2 (3) | ||
| Other# | 14 (19) | ||
| Life impact | Global quality of life | Quality of life | 3 (4) |
| Functioning | Breathlessness | 3 (4) | |
| Impact on functional ability | 3 (4) | ||
| Job/school absence | 2 (3) | ||
| Fatigue | 1 (1) | ||
| Delivery of care | Comfort | 6 (8) | |
| Patient satisfaction | 4 (5) | ||
| Adherence to secretion clearance techniques | 3 (4) | ||
| Patient-reported cough strength | 2 (3) | ||
| Impact of delivering secretion clearance technique on caregiver | 2 (3) | ||
| Patient-reported effectiveness of secretion clearance | 1 (1) | ||
| Side effects of treatment | 1 (1) | ||
| Resource use | Resource use | Admissions to hospital due to respiratory illness | 13 (17) |
| Respiratory illnesses | 8 (11) | ||
| Supplementary secretion clearance treatments | 4 (5) | ||
| Use/duration of antibiotics | 4 (5) | ||
| Amount of medication for secretion clearance in a treatment session | 1 (1) | ||
| Doctor visits | 1 (1) | ||
| Adverse events | Adverse events/effects | Adverse events | 3 (4) |
Data are presented as n (%) out of a total of 75 studies. COMET: Core Outcome Measures in Effectiveness Trials [21]. Proportions do not always sum to 100% as multiple outcomes were used in some studies. #: including airway collapsibility, sputum weight, effective cough time, effective suctioning, rapid shallow breathing index, inspiratory/expiratory time, lung aeration, need for tracheostomy, invasive ventilation/noninvasive ventilation time, deposition of inhaled particles, ventilator-free breathing ability, pulmonary perfusion, seizures and sleep efficiency/sleep disordered breathing.
Physiological/clinical outcomes
Of the 16 physiological/clinical outcomes, cough strength (e.g. peak cough flow) (n=48 studies, 64%) [33–80] and lung volume (e.g. forced vital capacity, forced expiratory volume and expiratory reserve volume) (n=48, 64%) [33–37, 40, 42, 43, 46–48, 51, 52, 54–60, 66–73, 75, 77–95] were the most frequently reported outcomes. Other commonly reported outcomes were insufflation capacity (e.g. maximum insufflation capacity, lung inflation capacity) (n=22, 29%) [34, 35, 42, 45, 46, 52, 54–59, 63, 66, 67, 71, 73, 76, 77, 79, 87], respiratory muscle strength testing (n=16, 21%) [33, 43, 47, 51, 57–59, 68, 71, 72, 77–79, 90, 93, 96], oxygen levels (n=15, 20%) [47, 51, 64, 80, 82, 84–86, 88–90, 97–100] and air flow (e.g. peak expiratory flow (n=13, 17%)) [37, 47, 60, 66, 67, 80, 81, 83, 84, 88, 89, 95, 99].
Life impact outcomes
Six studies (8%) reported on the outcome of patient comfort [41, 45, 47, 63, 74, 101]; four (5%) reported on patient satisfaction [51, 52, 70, 89] and three (4%) reported on health-related quality of life [52, 69, 77, 89]. Breathlessness [80, 89, 90] and impact on functional ability [51, 77, 89] were each reported by three studies (4%).
Resource-use outcomes
13 studies (17%) reported on the number of hospital admissions due to respiratory illness [51, 69, 71, 77, 82, 84, 93, 100, 102–106] and eight studies (11%) reported on frequency of respiratory illnesses [51, 71, 77, 82, 84, 102, 105, 106].
Other resource-use outcomes included number of supplementary secretion clearance techniques (n=4, 5%) [71, 93, 101, 106] and use/duration of antibiotics (n=4, 5%) [77, 84, 93, 100].
Quality assessment
Of the 75 studies, we scored 51 (68%) as ≥4 on the MOMENT criteria indicating high-quality outcome reporting (table 4).
TABLE 4.
The quality of outcome reporting in each study included in this scoping review, assessed according to the MOMENT criteria
| Is the primary outcome clearly stated? | 46 (61) |
| Is the primary outcome clearly defined so that another researcher would be able to reproduce the measurement? | 46 (61) |
| Are the secondary outcomes clearly stated? | 64 (85) |
| Are the secondary outcomes clearly defined? | 64 (85) |
| Do the authors explain the use of the outcomes they have selected? | 36 (48) |
| Are methods used to enhance the quality of outcome measurement (e.g. repeated measurements, training) used if appropriate? | 46 (61) |
Data are presented as n (%) out of a total of 75 studies. MOMENT: Management of Otitis Media with Effusion in Children with Cleft Palate [22]. If the outcomes were stated and defined as a whole, but we were unable to determine the primary outcome, we included these data as part of secondary outcomes.
Measurement
Of the most common physiological/clinical outcomes, cough strength was measured using a peak flow meter in 22 (46%) studies [33–39, 43, 44, 46, 49, 50, 53–58, 60, 65, 69, 80], pneumotachograph in 15 (31%) studies [41, 45, 47, 63, 64, 66, 68, 70–72, 74–77, 79] and spirometer in 14 (29%) studies [40, 41, 48, 52, 59, 60, 62, 66–68, 70–72, 78]. Lung volume was measured using a spirometer in 32 (67%) studies [33–37, 40, 43, 46, 48, 52, 54–57, 59, 60, 66, 69, 71, 72, 78, 81, 83, 84, 87, 88, 90, 91, 93–95, 107] or a pneumotachograph in 10 (21%) studies [47, 66, 68, 70, 75, 77, 79, 86, 90, 91]. In the other outcome domains, patient comfort was measured by a visual analogue scale in five (83%) studies [41, 45, 47, 63, 74]; and the Visual Numeric Scale in one (17%) study [101]. Health-related quality of life was measured by the 36-item Short Form Survey (SF-36) [108] in two (67%) studies [52, 69] (table 5).
TABLE 5.
Reporting the instruments used to measure the outcomes reported in this scoping review
| Outcome domain | Outcome | Measurement instruments per outcome | n (%) |
|---|---|---|---|
| Mortality/survival | Survival | _ | |
| Physiological/clinical | Cough strength (e.g. peak cough flow) | Peak flow meter | 22 (46) |
| Pneumotachograph | 15 (31) | ||
| Spirometer | 14 (29) | ||
| Flow analyser | 2 (4) | ||
| Plethysmography | 1 (2) | ||
| Not reported | 1 (2) | ||
| Lung volume (e.g. vital capacity, forced vital capacity, forced expiratory volume, expiratory reserve volume) | Spirometer | 32 (67) | |
| Pneumotachograph | 10 (21) | ||
| Plethysmography | 3 (6) | ||
| Electrical impedance tomography | 3 (6) | ||
| Flow analyser | 1 (2) | ||
| Helium dilation | 1 (2) | ||
| Not reported | 3 (6) | ||
| Insufflation capacity (e.g. maximum insufflation capacity, lung insufflation capacity) | Spirometer | 14 (64) | |
| Pneumotachograph | 5 (23) | ||
| Plethysmography | 2 (9) | ||
| Flow analyser | 1 (5) | ||
| Helium dilation | 1 (5) | ||
| Calculation from ventilator | 1 (5) | ||
| Oxygen levels (e.g. saturation of peripheral oxygen) | Transcutaneous monitor | 13 (87) | |
| Plethysmography | 1 (7) | ||
| Polysomnography | 1 (7) | ||
| Air flow (e.g. peak expiratory flow, forced expiratory flow) | Spirometer | 8 (62) | |
| Pneumotachograph | 3 (23) | ||
| Peak flow meter | 2 (15) | ||
| Plethysmography | 1 (8) | ||
| Respiratory muscle strength (e.g. maximal inspiratory pressure, maximal expiratory pressure, sniff nasal inspiratory pressure) | Manometer | 10 (63) | |
| Electrometer | 2 (13) | ||
| Not described | 4 (25) | ||
| Respiratory rate | Pneumotachograph | 4 (50) | |
| Plethysmography | 2 (25) | ||
| Electrical impedance tomography | 1 (13) | ||
| Spirometer | 1 (13) | ||
| Transcutaneous monitor | 1 (13) | ||
| Airway pressure (e.g. peak inspiratory pressure, peak expiratory pressure, transpulmonary pressure) | Manometer | 3 (100) | |
| Carbon dioxide levels | Transcutaneous monitor | 3 (50) | |
| Capnography monitor | 3 (50) | ||
| Lung expansion/compliance | Pneumotachograph | 2 (40) | |
| Manometer | 2 (40) | ||
| Spirometer | 1 (20) | ||
| Pulse method | 1 (20) | ||
| From a previous study – unable to obtain details | 1 (20) | ||
| Heart rate | Transcutaneous monitor | 2 (40) | |
| Photoplethysmography | 1 (20) | ||
| Electrocardiogram | 1 (20) | ||
| Not reported | 1 (20) | ||
| Cough volume | Spirometer | 2 (100) | |
| Pneumotachograph | 1 (50) | ||
| Mean arterial pressure | Photoplethysmography | 2 (100) | |
| Airway resistance | Oscilloresistometry | 1 (50) | |
| Plethysmography | 1 (50) | ||
| Airway collapsibility | Spirometer | 1 (100) | |
| Sputum weight | Balance | 1 (100) | |
| Global quality of life | Quality of life | 36-Item Short Form Survey | 2 (67) |
| Sickness impact profile | 1 (33) | ||
| Assessment of quality of life 8-dimension | 1 (33) | ||
| Severe respiratory insufficiency questionnaire | 1 (33) | ||
| Sleep apnoea quality of life index | 1 (33) | ||
| Functioning | Breathlessness | Borg scale | 2 (67) |
| Baseline dyspnoea Index/transitional dyspnoea index | 1 (33) | ||
| Impact on functional ability | Amyotrophic lateral sclerosis functional rating scale - revised | 2 (67) | |
| Amyotrophic lateral sclerosis assessment questionnaire 5 | 1 (33) | ||
| Fatigue | Fatigue severity scale | 1 (100) | |
| Delivery of care | Comfort | Visual analogue scale | 5 (83) |
| Visual numeric scale | 1 (17) | ||
| Patient satisfaction | Questionnaire/survey | 3 (75) | |
| Visual analogue scale | 1 (25) | ||
| Patient-reported cough strength | Visual analogue scale | 2 (100) | |
| Patient-reported effectiveness of secretion clearance | Visual analogue scale | 1 (100) | |
| Impact of delivering secretion clearance technique on caregiver | Caregiver strain index | 1 (50) | |
| Purposely designed survey | 1 (50) | ||
| Adherence to secretion clearance techniques | Diary | 2 (67) | |
| Caregivers therapy adherence questionnaire | 1 (33) | ||
| Lung volume recruitment counter | 1 (33) |
Data are presented as n (%). Proportions do not always sum to 100% as multiple instruments were used in some studies to measure the same outcome and some studies do not report which measurement instruments were utilised. The denominators for the calculation are the number of studies who reported using the outcome.
Discussion
From this scoping review we identified 75 studies recruiting community-dwelling participants with a neuromuscular condition who received an airway clearance technique, with 34 distinct outcomes. When grouped according to the COMET 38-domain taxonomy, most studies included at least one physiological/clinical outcome. Few studies included life impact outcomes such as health-related quality of life, or outcomes concerning resource use such as hospital admission. There were no differences in outcomes selected in studies that recruited adult only, mixed adult and paediatric populations, and paediatric-only populations. However, no studies with a paediatric-only population included life impact outcomes.
The heterogeneous use of outcomes and their measurement included by studies identified in our scoping review indicates a lack of consensus on outcomes to include in research studies in this patient group. To help patients and clinicians make robust evidence-based decisions about secretion clearance management, clinical trial data must be collected in a manner to facilitate synthesis between multiple studies. This is of particular importance for neuromuscular diseases, because they tend to be rare, with patients living across vast geographical locations, so large high-powered randomised studies can be difficult to deliver [109].
Life impact outcomes can be considered synonymous with patient-reported outcomes (PROs). The importance of PROs is growing within health research and has become more widely recognised in recent years [110, 111]. PROs provide valuable insight into the impact of a medical condition and associated treatment on an individual and are a way of ensuring patient-centred care [112–114]. The inclusion of PROs in health research helps to ensure a more holistic view of a condition or treatment is achieved [111]. Secretion clearance interventions can be invasive and/or uncomfortable [9]. Moreover, there is insufficient evidence demonstrating the superiority of one technique over another [115, 116]. Therefore, including PROs is particularly important in this area of research. The data presented here demonstrate that life impact outcomes were only included in a small number of studies, none of which were solely in the paediatric population. The low inclusion of PROs is consistent with the wider literature. A review of all clinical trials registered on ClinicalTrials.gov between 2007 and 2013 revealed that only 27% included PROs. Of respiratory trials, 12% included PROs [117]. Another review of phase IV trials registered on ClinicalTrials.gov between 1999 and 2021 reported that 21% of studies included PROs [118]. Reasons provided for the poor uptake of PROs in study design included a lack of relevant PROs or PRO measures for specific disease groups, lack of consensus about the optimal PROs and how they should be measured, and the additional resources required to collect them. In addition, specifically in phase IV trials, it was also suggested that collecting PROs in the real-world context may be particularly difficult [118]. Most of the studies included in this scoping review were cohort studies. There may have been similar challenges incorporating PROs into these studies. The lack of PROs in paediatric studies may be in part due to the age of some participants who were too young to engage with these outcomes [119]. It is worth noting that we did not identify any studies focusing on caregivers’ perspectives on secretion clearance. In the community setting, individuals who require secretion clearance interventions often need support from a parent or caregiver. It would therefore be important to include their perspective on the efficacy of secretion clearance in future studies.
In the few studies that did identify PROs, patient comfort and satisfaction were reported the most frequently. None of the outcome measures used to assess these outcomes was validated, highlighting a need to develop validated tools to be able to assess PROs in the field of neuromuscular respiratory failure. This may facilitate an increase in the inclusion of PROs in future studies in this field. Health-related quality of life, breathlessness, impact on functional ability and patient-reported effectiveness were seldom reported. Perception of treatment techniques can in turn influence adherence, and subsequent treatment effectiveness [120]. Adherence rates to some airway clearance techniques have been found to be poor in the literature [59, 121–123]; thus, patient preference is an important factor to consider [115]. It should be noted that all the studies that included PROs were undertaken between 2003 and 2023. This may potentially indicate the changing landscape of outcome reporting and the move toward involvement of patients in health and research [111]. PROs are becoming increasingly more common in COS, with 73% of COS published to 2021, including life impact outcomes [124]. However, patient participation in the COS was a key factor in the inclusion of PROs. Only 62% of COS included a PRO when patients were not stakeholders in the COS process, compared with 86% when patients were participants [124]. The literature demonstrates that patients’ perspectives on outcomes often differ to that of clinicians’ [124, 125]. Patients focus on outcomes pertaining to the impact of a condition or treatment on their life, including functional ability and treatment burden, whereas clinicians focus on physiological outcomes or mortality [124, 126]. Patient involvement in COS is imperative to ensure outcomes of importance to patients remain central to the research process [124].
We also identified few studies including outcomes pertaining to healthcare utilisation and resource use. Studies designed to investigate these outcomes tend to be longitudinal, requiring more resources over a longer period of time than laboratory-based studies. As neuromuscular conditions tend to be rare, there may have been difficulties designing longitudinal studies with sufficient statistical power, within the resources available to researchers. Healthcare utilisation, such as hospital admission, can have significant consequences. These consequences include important cost implications and profound effects on health-related quality of life and patient satisfaction [127]. With healthcare needs often surpassing available resources [128] and competition for funding a feature in several healthcare systems [129–131], incorporating healthcare utilisation outcomes may be an important consideration for future research.
This scoping review adds to our understanding about the broad range of outcomes and outcome measures employed in this patient group and emphasises the need to harmonise objectives and data collection techniques among clinical studies. It will therefore be a useful contribution to the item generation phase of a future COS focused on studies investigating secretion clearance interventions being used in the community.
Strengths and limitations
Our review has several strengths. These include a rigorous search design based on previous Cochrane reviews, searching multiple databases, not limiting publication dates and having two independent authors screen citations. Additionally, we utilised the PRISMA-ScR reporting format. Outcomes were classified according to the 38-domain COMET taxonomy and study quality was assessed using the MOMENT criteria. A limitation of our review is the exclusion of studies published in languages other than English; this may have resulted in missed outcomes. In addition, this review has included studies that reported on individuals with a broad range of neuromuscular conditions. As different secretion clearance interventions have different degrees of effectiveness in different neuromuscular conditions, the outcomes identified in the study may not be universally applicable to all secretion clearance interventions, and all neuromuscular conditions. The COS should aim to be applicable to as wide a range of interventions and conditions as possible. It is important to note that 40% of the studies identified were published prior to 2010. The pattern of use of specific secretion clearance interventions may have changed over time, making the outcomes identified potentially less applicable to current practice. Nevertheless, it was important to identify all potential outcomes for inclusion in the COS, so that the COS development methodology can consider all of the available evidence and include the most appropriate outcomes [14].
Conclusions
In this scoping review we confirmed the heterogeneity of outcome selection and measurement in research on interventions to promote airway clearance in individuals with neuromuscular disease living in the community. Use of physiological and clinical outcomes in studies was more common, with few studies including outcomes relating to life impact and resource use. This outcome heterogeneity confirms the need for a COS to be developed with patients, relatives and healthcare professionals to ensure outcomes are clinically relevant and meaningful to all stakeholder groups.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: Literature search: N.M. Shah and C. Apps; data extraction: N.M. Shah and C. Apps; study design: N.M. Shah, C. Apps, R. Amin and L. Rose; manuscript preparation: N.M. Shah, C. Apps, R. Amin, G. Kaltsakas, N. Hart, P.B. Murphy and L. Rose; review of the manuscript: N.M. Shah, C. Apps, R. Amin, G. Kaltsakas, N. Hart, P.B. Murphy and L. Rose.
Conflict of interest: The authors declare no conflicts of interest in relation to this study.
Support statement: This work was funded by a grant from Muscular Dystrophy UK (22GRO-PG12-0602). The funder was not involved in the design or conduct of the study, or the preparation of this article. Funding information for this article has been deposited with the Open Funder Registry.
References
- 1.Ambrosino N, Carpene N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J 2009; 34: 444–451. doi: 10.1183/09031936.00182208 [DOI] [PubMed] [Google Scholar]
- 2.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med 2007; 30: 319–330. doi: 10.1080/10790268.2007.11753947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchinson D, Whyte K. Neuromuscular disease and respiratory failure. Pract Neurol 2008; 8: 229–237. doi: 10.1136/pn.2008.152611 [DOI] [PubMed] [Google Scholar]
- 4.Suárez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am J Phys Med Rehabil 2002; 81: 506–511. doi: 10.1097/00002060-200207000-00007 [DOI] [PubMed] [Google Scholar]
- 5.Shah NM, Murphy PB, Kaltsakas G. The adult multidisciplinary respiratory neuromuscular clinic. Breathe 2020; 16: 200121. doi: 10.1183/20734735.0121-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross BB, Gramiak R, Rahn H. Physical dynamics of the cough mechanism. J Appl Physiol 1955; 8: 264–268. doi: 10.1152/jappl.1955.8.3.264 [DOI] [PubMed] [Google Scholar]
- 7.Van Ruiten HJ, Marini Bettolo C, Cheetham T, et al. Why are some patients with Duchenne muscular dystrophy dying young: an analysis of causes of death in North East England. Eur J Paediatr Neurol 2016; 20: 904–909. doi: 10.1016/j.ejpn.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 8.Savic G, DeVivo MJ, Frankel HL, et al. Long-term survival after traumatic spinal cord injury: a 70-year British study. Spinal Cord 2017; 55: 651–658. doi: 10.1038/sc.2017.23 [DOI] [PubMed] [Google Scholar]
- 9.Chatwin M, Toussaint M, Goncalves MR, et al. Airway clearance techniques in neuromuscular disorders: a state of the art review. Respir Med 2018; 136: 98–110. doi: 10.1016/j.rmed.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 10.Rose L, Adhikari NK, Poon J, et al. Cough augmentation techniques in the critically ill: a Canadian national survey. Respir Care 2016; 61: 1360–1368. doi: 10.4187/respcare.04775 [DOI] [PubMed] [Google Scholar]
- 11.Swingwood E, Tume L, Cramp F. A survey examining the use of mechanical insufflation-exsufflation on adult intensive care units across the UK. J Intensive Care Soc 2020; 21: 283–289. doi: 10.1177/1751143719870121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczepanski R, Cox M, Hashmi-Greenwood M. UK survey of current cough augmentation management in patients with motor neurone disease. Eur Respir J 2020; 56: 1272. [Google Scholar]
- 13.Morrow B, Argent A, Zampoli M, et al. Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database Syst Rev 2021; 4: CD013170. doi: 10.1002/14651858.CD013170.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials 2017; 18: 280. doi: 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. doi: 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Social Res Methodol 2005; 8: 19–32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 17.Peters MDJ, Godfrey C, McInerney P, et al. Scoping reviews 2020. In: Aromataris E, Lockwood C, Porritt K, et al. eds. JBI Manual for Evidence Synthesis. JBI, 2024. [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19.Rose L, Adhikari NK, Leasa D, et al. Cough augmentation techniques for extubation or weaning critically ill patients from mechanical ventilation. Cochrane Database Syst Rev 2017; 1: Cd011833. doi: 10.1002/14651858.CD011833.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodd S, Clarke M, Becker L, et al. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018; 96: 84–92. doi: 10.1016/j.jclinepi.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harman NL, Bruce IA, Callery P, et al. MOMENT – Management of otitis media with effusion in cleft palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2013; 14: 70. doi: 10.1186/1745-6215-14-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leow HW, Tan EL, Black M. Reported outcomes for planned caesarean section versus planned vaginal delivery: a systematic review. Eur J Obstetrics Gynecol Reprod Biol 2021; 256: 101–108. doi: 10.1016/j.ejogrb.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 24.Russell G, Rana N, Watts R, et al. Reporting of outcomes and measures in studies of interventions to prevent and/or treat delirium in older adults resident in long-term care: a systematic review. Age Ageing 2022; 51: afac267. doi: 10.1093/ageing/afac267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stangl S, Popp M, Reis S, et al. Reported outcomes in patients with iron deficiency or iron deficiency anemia undergoing major surgery: a systematic review of outcomes. Syst Rev 2024; 13: 5. doi: 10.1186/s13643-023-02431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher K, Chant K, Mancini A, et al. The NeoPACE study: study protocol for the development of a core outcome set for neonatal palliative care. BMC Palliat Care 2023; 22: 203. doi: 10.1186/s12904-023-01326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong N, Cooper N, Yorke S, et al. Variation in outcome reporting in studies of fertility-sparing surgery for cervical cancer: a systematic review. BJOG 2023; 130: 163–175. doi: 10.1111/1471-0528.17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moussa R, Rada MP, Durnea C, et al. Outcome reporting in randomized controlled trials (RCTs) on the pharmacological management of idiopathic overactive bladder (OAB) in women; a systematic review for the development of core outcome sets (COS). Int Urogynecol J 2022; 33: 1243–1250. doi: 10.1007/s00192-021-05040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tellum T, Omtvedt M, Naftalin J, et al. A systematic review of outcome reporting and outcome measures in studies investigating uterine-sparing treatment for adenomyosis. Hum Reprod Open 2021; 2021: hoab030. doi: 10.1093/hropen/hoab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Qiu R, Li C, et al. Problems with the outcome measures in randomized controlled trials of traditional Chinese medicine in treating chronic heart failure caused by coronary heart disease: a systematic review. BMC Complement Med Ther 2021; 21: 217. doi: 10.1186/s12906-021-03378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dadouch R, Faheim M, Juando-Prats C, et al. Development of a Core Outcome Set for Studies on Obesity in Pregnant Patients (COSSOPP): a study protocol. Trials 2018; 19: 655. doi: 10.1186/s13063-018-3029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose L, Agar M, Burry LD, et al. Development of core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium (Del-COrS): study protocol. BMJ Open 2017; 7: e016371. doi: 10.1136/bmjopen-2017-016371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aslan GK, Gurses HN, Issever H, et al. Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: a randomized controlled trial. Clin Rehabil 2014; 28: 573–581. doi: 10.1177/0269215513512215 [DOI] [PubMed] [Google Scholar]
- 34.Bach JR, Bianchi C, Vidigal-Lopes M, et al. Lung inflation by glossopharyngeal breathing and “air stacking” in Duchenne muscular dystrophy. Am J Phys Med Rehabil 2007; 86: 295–300. doi: 10.1097/PHM.0b013e318038d1ce [DOI] [PubMed] [Google Scholar]
- 35.Bach JR, Mahajan K, Lipa B, et al. Lung insufflation capacity in neuromuscular disease. Am J Phys Med Rehabil 2008; 87: 720–725. doi: 10.1097/PHM.0b013e31817fb26f [DOI] [PubMed] [Google Scholar]
- 36.Bach JR, Smith WH, Michaels J, et al. Airway secretion clearance by mechanical exsufflation for post-poliomyelitis ventilator-assisted individuals. Arch Phys Med Rehabil 1993; 74: 170–177. [PubMed] [Google Scholar]
- 37.Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest 1993; 104: 1553–1562. doi: 10.1378/chest.104.5.1553 [DOI] [PubMed] [Google Scholar]
- 38.Bianchi C, Carrara R, Khirani S, et al. Independent cough flow augmentation by glossopharyngeal breathing plus table thrust in muscular dystrophy. Am J Phys Med Rehabil 2014; 93: 43–48. doi: 10.1097/PHM.0b013e3182975bfa [DOI] [PubMed] [Google Scholar]
- 39.Brito MF, Moreira GA, Pradella-Hallinan M, et al. Air stacking and chest compression increase peak cough flow in patients with Duchenne muscular dystrophy. J Bras Pneumonol 2009; 1: 973–979. doi: 10.1590/S1806-37132009001000005 [DOI] [PubMed] [Google Scholar]
- 40.Cesareo A, LoMauro A, Santi M, et al. Acute effects of mechanical insufflation-exsufflation on the breathing pattern in stable subjects with Duchenne muscular dystrophy. Respir Care 2018; 63: 955–965. doi: 10.4187/respcare.05895 [DOI] [PubMed] [Google Scholar]
- 41.Chatwin M, Ross E, Hart N, et al. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J 2003; 21: 502–508. doi: 10.1183/09031936.03.00048102 [DOI] [PubMed] [Google Scholar]
- 42.Choi WA, Park JH, Kim DH, et al. Cough assistance device for patients with glottis dysfunction and/or tracheostomy. J Rehabil Med 2012; 44: 351–354. doi: 10.2340/16501977-0948 [DOI] [PubMed] [Google Scholar]
- 43.Cleary S, Misiaszek JE, Kalra S, et al. The effects of lung volume recruitment on coughing and pulmonary function in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 111–115. doi: 10.3109/17482968.2012.720262 [DOI] [PubMed] [Google Scholar]
- 44.Cleary S, Misiaszek JE, Wheeler S, et al. Lung volume recruitment improves volitional airway clearance in amyotrophic lateral sclerosis. Muscle Nerve 2021; 10: 676–682. doi: 10.1002/mus.27417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Amo Castrillo L, Lacombe M, Bore A, et al. Comparison of two cough-augmentation techniques delivered by a home ventilator in subjects with neuromuscular disease. Respir Care 2019; 64: 255–261. doi: 10.4187/respcare.06259 [DOI] [PubMed] [Google Scholar]
- 46.Dohna-Schwake C, Ragette R, Teschler H, et al. IPPB-assisted coughing in neuromuscular disorders. Pediatr Pulmonol 2006; 41: 551–557. doi: 10.1002/ppul.20406 [DOI] [PubMed] [Google Scholar]
- 47.Fauroux B, Guillemot N, Aubertin G, et al. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest 2008; 133: 161–168. doi: 10.1378/chest.07-1615 [DOI] [PubMed] [Google Scholar]
- 48.Haviv L, Friedman H, Bierman U, et al. Using a sniff controller to self-trigger abdominal functional electrical stimulation for assisted coughing following cervical spinal cord lesions. IEEE Trans Neural Syst Rehabil Eng 2017; 25: 1461–1471. doi: 10.1109/TNSRE.2016.2632754 [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa Y, Bach JR, Komaroff E, et al. Cough augmentation in Duchenne muscular dystrophy. Am J Phys Med Rehabil 2008; 87: 726–730. doi: 10.1097/PHM.0b013e31817f99a8 [DOI] [PubMed] [Google Scholar]
- 50.Iskandar K, Sunartini, Nugrahanto AP, et al. Use of air stacking to improve pulmonary function in Indonesian Duchenne muscular dystrophy patients: bridging the standard of care gap in low middle income country setting. BMC Proc 2019; 13: Suppl. 11, 21. doi: 10.1186/s12919-019-0179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson CE, Moore DH, Kittrell P, et al. High-frequency chest wall oscillation therapy in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis 2006; 8: 60–64. doi: 10.1097/01.cnd.0000249872.29091.9a [DOI] [Google Scholar]
- 52.Kaminska M, Browman F, Trojan DA, et al. Feasibility of lung volume recruitment in early neuromuscular weakness: a comparison between amyotrophic lateral sclerosis, myotonic dystrophy, and postpolio syndrome. Pm R 2015; 7: 677–684. doi: 10.1016/j.pmrj.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 53.Kan AF, Butler JM, Hutchence M, et al. Teaching manually assisted cough to caregivers of children with neuromuscular disease. Respir Care 2018; 63: 1520–1527. doi: 10.4187/respcare.06213 [DOI] [PubMed] [Google Scholar]
- 54.Kang S, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil 2000; 79: 222–227. doi: 10.1097/00002060-200005000-00002 [DOI] [PubMed] [Google Scholar]
- 55.Kang SW, Bach JR. Maximum insufflation capacity. Chest 2000; 118: 61–65. doi: 10.1378/chest.118.1.61 [DOI] [PubMed] [Google Scholar]
- 56.Kang SW, Kang YS, Moon JH, et al. Assisted cough and pulmonary compliance in patients with Duchenne muscular dystrophy. Yonsei Med J 2005; 46: 233–238. doi: 10.3349/ymj.2005.46.2.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang SW, Shin JC, Park CI, et al. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord 2006; 44: 242–248. doi: 10.1038/sj.sc.3101835 [DOI] [PubMed] [Google Scholar]
- 58.Kang SW, Kang YS, Sohn HS, et al. Respiratory muscle strength and cough capacity in patients with Duchenne muscular dystrophy. Yonsei Med J 2006; 47: 184–190. doi: 10.3349/ymj.2006.47.2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz SL, Mah JK, McMillan HJ, et al. Routine lung volume recruitment in boys with Duchenne muscular dystrophy: a randomised clinical trial. Thorax 2022; 77: 805–811. doi: 10.1136/thoraxjnl-2021-218196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kikuchi K, Satake M, Terui Y, et al. Cough peak flow with different mechanically assisted coughing approaches under different conditions in patients with neuromuscular disorders. Phys Ther Res 2019; 22: 58–65. doi: 10.1298/ptr.E9978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SM, Choi WA, Won YH, et al. A comparison of cough assistance techniques in patients with respiratory muscle weakness. Yonsei Med J 2016; 57: 1488–1493. doi: 10.3349/ymj.2016.57.6.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacombe M, Bore A, Amo Castrillo LD, et al. Peak cough flow fails to detect upper airway collapse during negative pressure titration for Cough-Assist. Arch Phys Med Rehabil 2019; 100: 2346–2353. doi: 10.1016/j.apmr.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 63.Lacombe M, Del Amo Castrillo L, Bore A, et al. Comparison of three cough-augmentation techniques in neuromuscular patients: mechanical insufflation combined with manually assisted cough, insufflation-exsufflation alone and insufflation-exsufflation combined with manually assisted cough. Respiration 2014; 88: 215–222. doi: 10.1159/000364911 [DOI] [PubMed] [Google Scholar]
- 64.Lalmolda C, Prados H, Mateu G, et al. Titration of mechanical insufflation-exsufflation optimal pressure combinations in neuromuscular diseases by flow/pressure waveform analysis. Arch Bronconeumol (Engl Ed) 2019; 55: 246–251. doi: 10.1016/j.arbr.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 65.Lee SC, Park JH, Kang SW, et al. External control of exhalation for cough assistance: a method for patients with glottis dysfunction and/or tracheostomy. Arch Phys Med Rehabil 2009; 90: 1402–1407. doi: 10.1016/j.apmr.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 66.Marques TB, de Carvaldo Neves J, Portes LA, et al. Air stacking: effects on pulmonary function in patients with spinal muscular atrophy and in patients with congenital muscular dystrophy. J Bras Pneumol 2014; 40: 528–534. doi: 10.1590/S1806-37132014000500009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molgat-Seon Y, Hannan LM, Dominelli PB, et al. Lung volume recruitment acutely increases respiratory system compliance in individuals with severe respiratory muscle weakness. ERJ Open Res 2017; 3: 00135-2016. doi: 10.1183/23120541.00135-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mustfa N, Aiello M, Lyall RA, et al. Cough augmentation in amyotrophic lateral sclerosis. Neurology 2003; 61: 1285–1287. doi: 10.1212/01.WNL.0000092018.56823.02 [DOI] [PubMed] [Google Scholar]
- 69.Rafiq MK, Bradburn M, Proctor AR, et al. A preliminary randomized trial of the mechanical insufflator-exsufflator versus breath-stacking technique in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 448–455. doi: 10.3109/21678421.2015.1051992 [DOI] [PubMed] [Google Scholar]
- 70.Sancho J, Bures E, de La Asunción S. Effect of high-frequency oscillations on cough peak flows generated by mechanical in-exsufflation in medically stable subjects with amyotrophic lateral sclerosis. Respir Care 2016; 61: 1051–1058. doi: 10.4187/respcare.04552 [DOI] [PubMed] [Google Scholar]
- 71.Sancho J, Bures E, Ferrer S, et al. Usefulness of oscillations added to mechanical in-exsufflation in amyotrophic lateral sclerosis. Respir Care 2020; 65: 596–602. doi: 10.4187/respcare.07202 [DOI] [PubMed] [Google Scholar]
- 72.Sancho J, Servera E, Diaz J, et al. Efficacy of mechanical insufflation-exsufflation in medically stable patients with amyotrophic lateral sclerosis. Chest 2004; 125: 1400–1405. doi: 10.1378/chest.125.4.1400 [DOI] [PubMed] [Google Scholar]
- 73.Sarmento A, Resqueti V, Dourado-Junior M, et al. Effects of air stacking maneuver on cough peak flow and chest wall compartmental volumes of subjects with amyotrophic lateral sclerosis. Arch Phys Med Rehabil 2017; 98: 2237–46.e1. doi: 10.1016/j.apmr.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 74.Senent C, Golmard JL, Salachas F, et al. A comparison of assisted cough techniques in stable patients with severe respiratory insufficiency due to amyotrophic lateral sclerosis. Amyotroph 2011; 12: 26–32. doi: 10.3109/17482968.2010.535541 [DOI] [PubMed] [Google Scholar]
- 75.Shah NM, Apps C, Kaltsakas G, et al. The effect of pressure changes during mechanical insufflation-exsufflation on respiratory and airway physiology. Chest 2024; 165: 929–941. doi: 10.1016/j.chest.2023.10.015 [DOI] [PubMed] [Google Scholar]
- 76.Sheers NL, Berlowitz DJ, Dirago RK, et al. Rapidly and slowly progressive neuromuscular disease: differences in pulmonary function, respiratory tract infections and response to lung volume recruitment therapy (LVR). BMJ Open Respir Res 2022; 9: e001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheers NL, Howard ME, Rochford PD, et al. A randomized controlled clinical trial of lung volume recruitment in adults with neuromuscular disease. Ann Am Thorac Soc 2023; 20: 1445–1455. doi: 10.1513/AnnalsATS.202212-1062OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toussaint M, Boitano LJ, Gathot V, et al. Limits of effective cough-augmentation techniques in patients with neuromuscular disease. Respir Care 2009; 54: 359–366. [PubMed] [Google Scholar]
- 79.Toussaint M, Pernet K, Steens M, et al. Cough augmentation in subjects with Duchenne muscular dystrophy: comparison of air stacking via a resuscitator bag versus mechanical ventilation. Respir Care 2016; 61: 61–67. doi: 10.4187/respcare.04033 [DOI] [PubMed] [Google Scholar]
- 80.Winck JC, Goncalves MR, Lourenco C, et al. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest 2004; 126: 774–780. doi: 10.1378/chest.126.3.774 [DOI] [PubMed] [Google Scholar]
- 81.Casaulta C, Messerli F, Rodriguez R, et al. Changes in ventilation distribution in children with neuromuscular disease using the insufflator/exsufflator technique: an observational study. Sci Rep 2022; 12: 7009. doi: 10.1038/s41598-022-11190-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaisson KM, Walsh S, Simmons Z, et al. A clinical pilot study: high frequency chest wall oscillation airway clearance in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2006; 7: 107–111. doi: 10.1080/14660820600640570 [DOI] [PubMed] [Google Scholar]
- 83.Feigelson CI, Dickinson DG, Talner NS, et al. Glossopharyngeal breathing as an aid to the coughing mechanism in the patient with chronic poliomyelitis in a respirator. N Engl J Med 1956; 254: 611–613. doi: 10.1056/NEJM195603292541306 [DOI] [PubMed] [Google Scholar]
- 84.Giarraffa P, Berger KI, Chaikin AA, et al. Assessing efficacy of high-frequency chest wall oscillation in patients with familial dysautonomia. Chest 2005; 128: 3377–3381. doi: 10.1378/chest.128.5.3377 [DOI] [PubMed] [Google Scholar]
- 85.Guerin C, Vincent B, Petitjean T, et al. The short-term effects of intermittent positive pressure breathing treatments on ventilation in patients with neuromuscular disease. Respir Care 2010; 55: 866–872. [PubMed] [Google Scholar]
- 86.Jenkins HM, Stocki A, Kriellaars D, et al. Breath stacking in children with neuromuscular disorders. Pediatr Pulmonol 2014; 49: 544–553. doi: 10.1002/ppul.22865 [DOI] [PubMed] [Google Scholar]
- 87.Kim DH, Kang SW, Park YG, et al. Artificial external glottic device for passive lung insufflation. Yonsei Med J 2011; 52: 972–976. doi: 10.3349/ymj.2011.52.6.972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klefbeck B, Svartengren K, Camner P, et al. Lung clearance in children with Duchenne muscular dystrophy or spinal muscular atrophy with and without CPAP (continuous positive airway pressure). Exp Lung Res 2001; 27: 469–484. doi: 10.1080/019021401750414010 [DOI] [PubMed] [Google Scholar]
- 89.Lange DJ, Lechtzin N, Davey C, et al. High-frequency chest wall oscillation in ALS: an exploratory randomized, controlled trial. Neurology 2006; 67: 991–997. doi: 10.1212/01.wnl.0000237439.78935.46 [DOI] [PubMed] [Google Scholar]
- 90.Meric H, Falaize L, Pradon D, et al. Short-term effect of volume recruitment-derecruitment manoeuvre on chest-wall motion in Duchenne muscular dystrophy. Chron 2017; 14: 110–116. doi: 10.1177/1479972316674413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nygren-Bonnier M, Schiffer TA, Lindholm P. Acute effects of glossopharyngeal insufflation in people with cervical spinal cord injury. J Spinal Cord Med 2018; 41: 85–90. doi: 10.1080/10790268.2016.1275446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pigatto AV, Kao TJ, Mueller JL, et al. Electrical impedance tomography detects changes in ventilation after airway clearance in spinal muscular atrophy type I. Respir Physiolo Neurobiol 2021; 294: 103773. doi: 10.1016/j.resp.2021.103773 [DOI] [PubMed] [Google Scholar]
- 93.Reardon CC, Christiansen D, Barnett ED, et al. Intrapulmonary percussive ventilation vs incentive spirometry for children with neuromuscular disease. Arch Pediatr Adolesc Med 2005; 159: 526–531. doi: 10.1001/archpedi.159.6.526 [DOI] [PubMed] [Google Scholar]
- 94.Stehling F, Bouikidis A, Schara U, et al. Mechanical insufflation/exsufflation improves vital capacity in neuromuscular disorders. Chron Respir Dis 2015; 12: 31–35. doi: 10.1177/1479972314562209 [DOI] [PubMed] [Google Scholar]
- 95.Veldhoen ES, Vercoelen F, Ros L, et al. Short-term effect of air stacking and mechanical insufflation-exsufflation on lung function in patients with neuromuscular diseases. Chron Respir Dis 2022; 19: 14799731221094619. doi: 10.1177/14799731221094619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest 1993; 103: 166–169. doi: 10.1378/chest.103.1.166 [DOI] [PubMed] [Google Scholar]
- 97.Lagerkvist AL, Sten G, Westerberg B, et al. Positive expiratory pressure (PEP) treatment in children with multiple severe disabilities. Acta Paediatr 2005; 94: 538–542. doi: 10.1111/j.1651-2227.2005.tb01935.x [DOI] [PubMed] [Google Scholar]
- 98.Nitz J, Burke B. A study of the facilitation of respiration in myotonic dystrophy. Physiother Res Int 2002; 7: 228–238. doi: 10.1002/pri.262 [DOI] [PubMed] [Google Scholar]
- 99.Toussaint M, De Win H, Steens M, et al. Effect of intrapulmonary percussive ventilation on mucus clearance in Duchenne muscular dystrophy patients: a preliminary report. Respir Care 2003; 48: 940–947. [PubMed] [Google Scholar]
- 100.Yuan N, Kane P, Shelton K, et al. Safety, tolerability, and efficacy of high-frequency chest wall oscillation in pediatric patients with cerebral palsy and neuromuscular diseases: an exploratory randomized controlled trial. J Child Neurol 2010; 25: 815–821. doi: 10.1177/0883073809350223 [DOI] [PubMed] [Google Scholar]
- 101.Belli S, Cattaneo D, D'Abrosca F, et al. A pilot study on the non-invasive management of tracheobronchial secretions in tracheostomised patients. Clin Respir J 2019; 13: 637–642. doi: 10.1111/crj.13074 [DOI] [PubMed] [Google Scholar]
- 102.Bidiwala A, Volpe L, Halaby C, et al. A comparison of high frequency chest wall oscillation and intrapulmonary percussive ventilation for airway clearance in pediatric patients with tracheostomy. Postgrad Med 2017; 129: 276–282. doi: 10.1080/00325481.2017.1264854 [DOI] [PubMed] [Google Scholar]
- 103.Fitzgerald K, Dugre J, Pagala S, et al. High-frequency chest wall compression therapy in neurologically impaired children. Respir Care 2014; 59: 107–112. doi: 10.4187/respcare.02446 [DOI] [PubMed] [Google Scholar]
- 104.Moran FC, Spittle A, Delany C, et al. Effect of home mechanical in-exsufflation on hospitalisation and life-style in neuromuscular disease: a pilot study. J Paediatr Child Health 2013; 49: 233–237. doi: 10.1111/jpc.12111 [DOI] [PubMed] [Google Scholar]
- 105.Plioplys AV, Lewis S, Kasnicka I. Pulmonary vest therapy in pediatric long-term care. J Am Med Dir Assoc 2002; 3: 318–321. doi: 10.1016/S1525-8610(05)70548-X [DOI] [PubMed] [Google Scholar]
- 106.Sancho J, Bures E, Ferrer S, et al. Mechanical insufflation-exsufflation with oscillations in amyotrophic lateral sclerosis with home ventilation via tracheostomy. Respir Care 2021; 66: 378–383. doi: 10.4187/respcare.08145 [DOI] [PubMed] [Google Scholar]
- 107.Kang SW. Pulmonary rehabilitation in patients with neuromuscular disease. Yonsei Med J 2006; 47: 307–314. doi: 10.3349/ymj.2006.47.3.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ware JE,Jr, Sherbourne CD. The MOS 36-ltem Short-Form Health Survey (SF-36): I. conceptual framework and item selection. Med Care 1992; 30: 473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 109.Bach JR, Chiarello G, Weiss W, et al. Is there value in using randomized placebo-controlled trials in neuromuscular disease? Expert Rev Neurother 2021; 21: 5–7. doi: 10.1080/14737175.2020.1834854 [DOI] [PubMed] [Google Scholar]
- 110.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. Jama 2003; 290: 1624–1632. doi: 10.1001/jama.290.12.1624 [DOI] [PubMed] [Google Scholar]
- 111.Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights 2013; 6: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mercieca-Bebber R, King MT, Calvert MJ, et al. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018; 9: 353–367. doi: 10.2147/PROM.S156279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ciani O, Salcher-Konrad M, Meregaglia M, et al. Patient-reported outcome measures in core outcome sets targeted overlapping domains but through different instruments. J Clin Epidemiol 2021; 136: 26–36. doi: 10.1016/j.jclinepi.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 114.Oakes DB, Baker MJ, McLeod C, et al. Patient-reported outcome measures for paediatric acute lower respiratory infection studies. Eur Respir Rev 2023; 32: 220229. doi: 10.1183/16000617.0229-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belli S, Prince I, Savio G, et al. Airway clearance techniques: the right choice for the right patient. Front Med (Lausanne) 2021; 8: 544826. doi: 10.3389/fmed.2021.544826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest 2023; 164: 394–413. doi: 10.1016/j.chest.2023.03.011 [DOI] [PubMed] [Google Scholar]
- 117.Vodicka E, Kim K, Devine EB, et al. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007–2013). Contemp Clin Trials 2015; 43: 1–9. doi: 10.1016/j.cct.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 118.Maruszczyk K, Aiyegbusi OL, Cardoso VR, et al. Implementation of patient-reported outcome measures in real-world evidence studies: analysis of ClinicalTrials.gov records (1999–2021). Contemp Clin Trials 2022; 120: 106882. doi: 10.1016/j.cct.2022.106882 [DOI] [PubMed] [Google Scholar]
- 119.Bele S, Chugh A, Mohamed B, et al. Patient-reported outcome measures in routine pediatric clinical care: a systematic review. Front 2020; 8: 364. doi: 10.3389/fped.2020.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.WHO . Adherence to long-term therapies: evidence for action. Geneva, World Health Organization, 2003. [Google Scholar]
- 121.Sawnani H, Mayer OH, Modi AC, et al. Randomized trial of lung hyperinflation therapy in children with congenital muscular dystrophy. Pediatr Pulmonol 2020; 55: 2471–2478. doi: 10.1002/ppul.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chatwin M, Simonds AK. Long-term mechanical insufflation-exsufflation cough assistance in neuromuscular disease: patterns of use and lessons for application. Respir Care 2020; 65: 135–143. doi: 10.4187/respcare.06882 [DOI] [PubMed] [Google Scholar]
- 123.Mahede T, Davis G, Rutkay A, et al. Use of mechanical airway clearance devices in the home by people with neuromuscular disorders: effects on health service use and lifestyle benefits. Orphanet J Rare Dis 2015; 10: 54. doi: 10.1186/s13023-015-0267-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dodd S, Gorst SL, Young A, et al. Patient participation impacts outcome domain selection in core outcome sets for research: an updated systematic review. J Clin Epidemiol 2023; 158: 127–133. doi: 10.1016/j.jclinepi.2023.03.022 [DOI] [PubMed] [Google Scholar]
- 125.Armstrong MJ, Mullins CD, Gronseth GS, et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implement Sci 2018; 13: 55. doi: 10.1186/s13012-018-0745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sathanapally H, Sidhu M, Fahami R, et al. Priorities of patients with multimorbidity and of clinicians regarding treatment and health outcomes: a systematic mixed studies review. BMJ Open 2020; 10: e033445. doi: 10.1136/bmjopen-2019-033445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scalco RS, Quinlivan RM, Nastasi L, et al. Improving specialised care for neuromuscular patients reduces the frequency of preventable emergency hospital admissions. Neuromuscul Disord 2020; 30: 173–179. doi: 10.1016/j.nmd.2019.11.013 [DOI] [PubMed] [Google Scholar]
- 128.Janssen LMM, Drost R, Paulus ATG, et al. Aspects and challenges of resource use measurement in health economics: towards a comprehensive measurement framework. Pharmacoeconomics 2021; 39: 983–993. doi: 10.1007/s40273-021-01048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schut FT, Van de Ven WPMM. Rationing and competition in the Dutch health-care system. Health Econ 2005; 14: S59–S74. doi: 10.1002/hec.1036 [DOI] [PubMed] [Google Scholar]
- 130.de Pouvourville G. Hospital funding and competition. Eur J Health Econ 2004; 5: 3–5. doi: 10.1007/s10198-004-0230-9 [DOI] [PubMed] [Google Scholar]
- 131.Woolhandler S, Himmelstein DU. Competition in a publicly funded healthcare system. BMJ 2007; 335: 1126–1129. doi: 10.1136/bmj.39400.549502.94 [DOI] [PMC free article] [PubMed] [Google Scholar]


