Summary
Bone is a common site for metastasis of solid cancers. The diversity of histological and molecular characteristics of bone metastases (BMs) remains poorly studied. Here, we performed single-cell RNA sequencing on 42 BMs from eight cancer types, identifying three distinct ecosystem archetypes, each characterized by an enrichment of specific immune cells: macrophages/osteoclasts, regulatory/exhausted T cells, or monocytes. We validated these archetypes by immunostaining on tissue sections and bioinformatic analysis of bulk RNA sequencing/microarray data from 158 BMs across more than 10 cancer types. Interestingly, we found only a modest correlation between the BM archetypes and the tissues of origin; BMs from the same cancer type often fell into different archetypes, while BMs from different cancer types sometimes converged on the same archetype. Additional analyses revealed parallel immunosuppression and bone remodeling mechanisms, some of which were experimentally validated. Overall, we discovered unappreciated heterogeneity of BMs across different cancers.
Keywords: bone metastases, single-cell RNA sequencing, tumor microenvironment, immune archetypes, immunosuppression, immune evasion, convergent and divergent evolution
Graphical abstract

Highlights
-
•
Analyses of bone metastases from eight cancer types revealed three immune archetypes
-
•
Archetypes diverge on immune trajectories and features of tumor/stromal cells
-
•
The dominant cell type in each archetype undergoes convergent evolution
-
•
Regulatory networks converge on osteoclasts and Tregs to drive archetype formation
Liu et al. analyzed single-cell RNA sequencing from human bone metastases across eight cancer types, identifying three immune ecosystem archetypes. These archetypes demonstrate distinct yet convergent immune suppression and bone remodeling mechanisms, revealing previously unappreciated metastatic heterogeneity and suggesting targeted therapeutic strategies for bone metastases.
Introduction
Bone and bone marrow play multiple roles in normal physiology.1 The mineralized part of bone provides mechanistic support to the body, whereas the semifluid bone marrow is the birthplace of new blood and immune cells. In addition, bone modeling generates metabolic and endocrine impact that extends to the entire organism.2,3 Enriching cytokines and growth factors in the bone microenvironment provides congenial soil for normal stem cells and metastatic seeds.4
Indeed, bone is a common metastatic target of many solid cancer types. Upon arrival, the disseminated tumor cells (DTCs) often reside in the perivascular niche, and their complex interactions with endothelial cells alter the dormancy/proliferation status.5,6,7 On the other hand, the outgrowth of DTCs was associated with an osteogenic environment wherein cancer cells and osteogenic cells form intimate interactions mediated by heterotypic adherens and gap junctions.8,9 The transition of DTCs from the perivascular niche to the osteogenic niche may be coupled with the bone remodeling process.10 This previous knowledge highlights the importance of crosstalk among metastatic cells, endothelial cells, and osteogenic cells in early-stage bone metastasis.
To fully colonize bone, tumor cells evolve mechanisms to invade the mineralized bone matrix and evade immunosurveillance. Previous studies indicate that dysregulation of osteoclast and osteoblast activities, especially the hyperactive bone resorption driven by osteoclasts, underpin metastasis-induced bone remodeling.11,12,13,14 Accordingly, agents targeting osteoclasts, such as bisphosphonates and denosumab (anti-RANKL), have been widely used to treat bone metastases of various cancer types, including osteosclerotic prostate cancers.15 However, some cancers do not respond to these agents. Renal cancers, for instance, are notoriously resistant to bisphosphonates despite the similar osteolytic appearance,16 suggesting potentially osteoclast-independent mechanisms.
In addition to the ability to invade the foreign environment, exacerbated immunosuppression is another hallmark of metastases. Compared with visceral and brain metastases, bone metastasis is much less characterized due to the scarcity of clinical specimens.17,18 The immune landscape of bone is unique, as bone marrow is the site of immune cell production and is postulated to provide an immunoprivileged niche for adult stem cells.19 However, very little is known about the potential immunosuppressive mechanisms in bone metastases.
Prompted by these significant gaps in our knowledge, we have performed single-cell RNA sequencing (scRNA-seq) of 42 human bone metastases from eight cancer types. Interestingly, we identified three archetypes of ecosystems that do not completely coincide with organs of origin. We further characterized two of these archetypes represented by the majority of breast cancer and kidney cancer, respectively, and revealed distinctive parallel mechanisms of bone colonization and immune evasion. Our work demonstrates how different cancer types may colonize the same organ via a finite number of distinct mechanisms and provides the rationale to investigate completely different treatment strategies for certain bone metastases.
Results
Human bone metastases of different cancer types fall into three ecosystem archetypes with largely distinctive immune landscapes
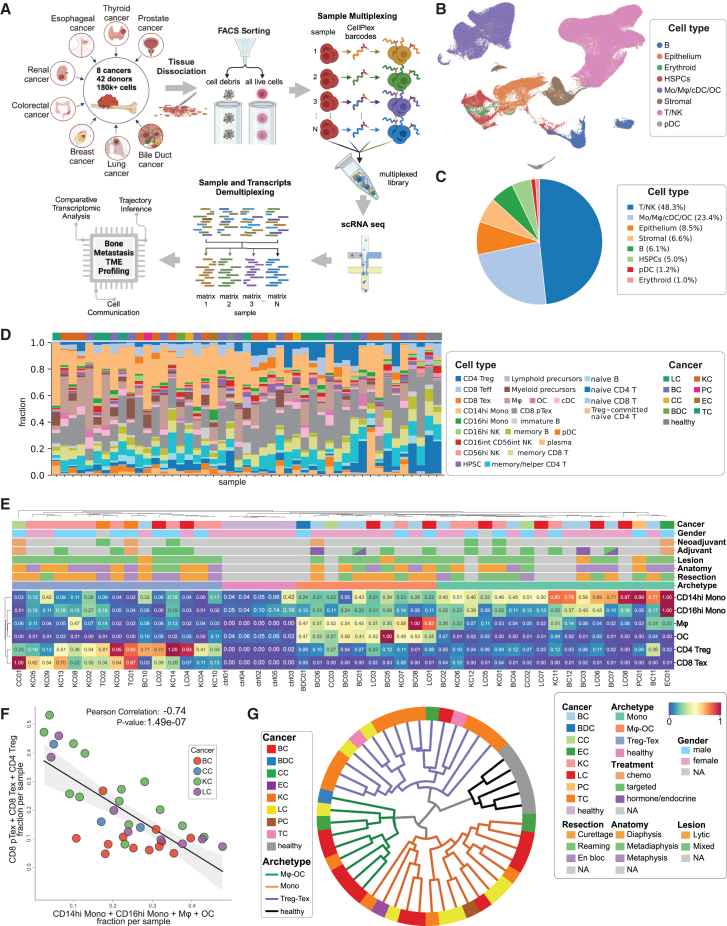
Fresh bone metastasis tissues were collected during orthopedic surgeries. The clinical information of all 42 patients, including prior treatments, is provided in the supplemental information (Table S1). A portion of each sample was reserved for subsequent sample-multiplexed scRNA-seq, and the remainder was preserved for other purposes. We used the cell hashing approach to combine 3–5 samples in each sequencing run to reduce the cost and batch effects. Detailed information on data preprocessing is provided in the Star Methods section and summarized in Figure 1A. H&E-stained tissue sections and accompanying pathology descriptions for representative samples are available for download from Zenodo (https://doi.org/10.5281/zenodo.14270976).
Figure 1.
Overview of study design and immune archetypes among patients
(A) Overview of the study workflow, illustrating the process from data collection to analysis. Forty-two patients and five healthy donors were included, resulting in more than 180,000 high-quality cells post-preprocessing and quality control (QC). (Illustration created with BioRender.)
(B) UMAP projection depicting major cell types.
(C) Pie chart details the proportion of each major cell type relative to the overall cell count.
(D) Stacked bar plot depicting the frequency of detailed immune cell types (as annotated in Figure S1C) across individual patients. Cancer types for each patient are annotated above the corresponding bars.
(E) Patient hierarchical clustering based on scaled (min-max standardized across patient data, ranging from 0 to 1) cell frequencies, revealing three immune archetypes: monocyte-enriched (Mono), macrophage/osteoclast-enriched (Mφ-OC), and regulatory/exhausted T cell-enriched (Treg-Tex), distinct from healthy control donors.
(F) Pearson correlation analysis of myeloid cell frequency against the/of immunosuppressive T cells (pTex, Tex, and Treg) in breast cancer (BC), kidney cancer (KC), colorectal cancer (CC), and lung cancer (LC) patients, revealing a significant inverse relationship.
(G) Circular hierarchical plot illustrating the classification of individual patients based on their immune archetypes, derived from (E). Cancer types are represented by colored blocks, while immune archetypes are denoted by branching colored lines.
To identify cell types, we assembled a reference cell database based on multiple previously published datasets (see method details for details) (Figure S1A; Table S2). We used support vector machine (SVM) to classify cells derived from bone metastases. This approach resulted in a robust assignment of each cluster of cells to specific cell types (Figures S1B and S1C), resulting in the annotation of 32 cell types (Figure S1D). We then inspected established marker genes by feature plots (Figures S1E and S2A) of major cell types by gene expression scores (Figure S2B) and numerically, which largely validated SVM annotation.
Two of the identified cell clusters caught our attention and warranted further clarification. One cluster selectively expresses multiple markers of osteoclasts (Figure S3A). Although matured osteoclasts have 3–100 nuclei and are often excluded from single-cell sequencing droplets due to their large size, osteoclast precursors and those relatively small ones (<50 μm in diameter) can still be captured. This seems to be the case in our dataset—the cluster annotated as osteoclasts indeed possesses a higher RNA content (Figure S3B), consistent with the osteoclast characteristics being the fusion of multiple precursors. Another cell cluster exhibited a transitory phenotype that appears to be intermediate between effector and exhausted CD8 T cells (Figure S3C), which aligns with observations in other studies.20 Following the previous reports, we denoted these cells "CD8 pTex" (precursor CD8 Tex).
Overall, we observed abundant immune cells (Figures 1B–1D). This may not be surprising, considering that bone marrow is the birthplace of immune cells. In contrast to bone metastases, previous studies suggest that metastases in other organs are immunologically "colder" than primary tumors.17,18 The heavy involvement of immune cells led us to conduct a more systematic investigation. An unbiased clustering of all bone metastases using frequencies of various immune cells revealed three major clusters that are characterized by enrichment of (1) macrophages/osteoclasts, (2) monocytes, or (3) regulatory/exhausted T cells (Figures 1E, S4A, and S4B). Indeed, the percentage of myeloid cells and T cells exhibited a strong negative correlation across all cancer types (Figure 1F) or in individual cancer types with relatively large sample sizes but was not observed in healthy control samples (Figure S4C).
Both myeloid and T regulatory cells are immunosuppressive and support evasion of immunosurveillance.21 However, their inverse correlation suggests parallel mechanisms selected by different tumors during their evolution to survive selective pressure exerted by the immune microenvironment in bone. Furthermore, the absence of osteoclasts in bone metastases also suggests alternative bone remodeling mechanisms. Overall, these data indicate previously uncharacterized archetypes of metastatic ecosystems that employ distinctive strategies to cope with challenges during bone colonization. We hereby refer to these subtypes by their characteristic contents, namely "Mφ-OC" (enrichment of macrophages and osteoclasts), "Mono" (enrichment of monocytes), and "Treg-Tex" (enrichment of regulatory CD4 and exhausted CD8 T cells), respectively.
Remarkably, we observed a complicated scenario when aligning different tumor types to the three ecosystem archetypes (Figures 1G and S4D). Whereas breast cancer bone metastases are classified as either Mφ-OC or Mono, kidney and lung cancer bone metastases exhibit all three archetypes. Specifically, most kidney cancer bone metastases belong to Treg-Tex that lack OC, but lung cancer metastases are evenly distributed across different archetypes. The three other cancer types were classified as Treg-Tex and Mono archetypes, although only one or two cases were included in each type. These observations suggest both convergent and divergent evolution pathways—cancers stemmed from different tissues of origin can evolve similar colonization mechanisms, and conversely, cancers of the same type also develop parallel mechanisms to colonize the same organ, bone.
Archetypes validation by immunofluorescence staining
We sought to validate the archetypes by examining key cell types that define these archetypes on tissue sections. Toward this end, immunofluorescence staining of CTSK, CD4 + FOXP3, and CD8 + TIM3 was performed to identify osteoclasts in a subset of randomly selected patients, Treg and Tex cells, respectively (Figures 2A and S5). The frequencies of these cells significantly differed among the archetypes determined by scRNA-seq data (Figure 2B), supporting the enrichment of osteoclasts in the Mφ-OC archetype and Treg and Tex cells in the Treg-Tex archetype. It is also notable that cancer cells in many metastases appeared to be diffusive among other cell types, which may explain the large proportion of immune and stromal cells in metastatic tissues reflected by scRNA-seq data.
Figure 2.
Immunofluorescence staining verifies distinct immune archetypes
(A) Representative fields selected from tissue section immunofluorescence (IF) staining of patients classified by archetypes (columns), highlighting three major cell types (rows). CTSK marks the OC population, CD4 and FOXP3 double-positive signals indicate CD4 Treg populations, and CD8A and TIM3 double-positive signals represent CD8 Tex populations.
(B) Cell counting from IF staining of tissue sections from 21 patients, grouped by archetypes: Mφ-OC (N = 6 patients), Treg-Tex (N = 8 patients), and Mono (N = 7 patients). Three major cell types were analyzed: OC cells were manually counted from entire tissue sections. Treg and Tex populations were quantified from selected fields (regions of interest based on CD4 or CD8A positivity, shown in Figure S2). Left: Proportion of OC cells out of total cells; Middle: Proportion of CD4 Tregs out of total CD4 T cells; Right: Proportion of CD8 Tex cells out of total CD8 T cells. (Significance test: One-way ANOVA, ∗p < 0.05; ∗∗p < 0.01).
Bone metastasis archetypes in previously published bulk transcriptomics data
To further validate our findings, we collected a total of 158 bone metastases from six previously published datasets,22,23,24,25,26,27 which were integrated by removing batch effects (Figure S6A). To delineate the contents of key cell types, we used single-sample gene set enrichment analysis (ssGSEA) to generate scores of cell type-specific gene expression signatures (Figure 3A; Table S3). The accuracy of this approach was first benchmarked using our scRNA-seq data (Figures S6B and S6C).
Figure 3.
Immune archetypes in published dataset
(A) Schematic of the workflow for cell type identification and frequency estimation. (Illustration created with BioRender.)
(B) Hierarchical clustering of 957 patients (columns) based on estimated cell types and their frequencies (rows).
(C) Subset analysis of 158 patients (from B) with cancer metastasis to the bones. Patients were clustered into three groups based on the frequencies of selected cell types: Monocytes, Mφ, OC, CD4 Treg, CD8 pTex, and CD8 Tex.
(D) Confusion matrix displaying the correlation clustering of matched patients based on the cell frequencies of selected cell types (from C), comparing the primary breast tumor microenvironment (TME) with their bone metastasis TME.
(E) Confusion matrix displaying patient clustering based on the frequencies of selected cell types, annotated with patients' progression-free survival (PFS).
(F) Correlation analysis of patients from (E), examining the relationship between PFS probability and the expression of Treg and Tex cell signatures (Treg/Tex infiltration). The analysis was conducted across different metastatic tissues (p values reported from Log Rank Test).
Unbiased hierarchical clustering was then used to examine potential archetypes using all immune cell types (Figure 3B) and revealed subsets of bone metastases (BMs) with clearly distinctive immune profiles. A closer examination confirmed the division of the three archetypes according to macrophages, osteoclasts, Treg, pTex, Tex, and monocytes (Figure 3C).
One of the six datasets (S-EPMC562187425) contains matched primary breast tumors, which failed to display similar archetypes (Figure 3D), indicating that the immune characteristics of BMs do not stem from primary tumors and are more likely to result from interactions with the bone microenvironment. Another dataset (GSE12464723) has clinical follow-up information. Unsupervised clustering of breast cancer BMs in this dataset results in two groups with high and low Treg/Tex (Figure 3E), respectively. Interestingly, the enrichment of Treg/Tex appears to be strongly associated with shortened progress-free survival (Figure 3F), suggesting that the immune archetypes are prognostic of clinical outcomes.
Taken together, the above analyses in the bulk transcriptome data validated the immune archetypes in a broader scope of clinical specimens and supported the biological and clinical importance of these archetypes.
Myeloid cells and T lymphocytes undergo distinct differentiation trajectories in different archetypes
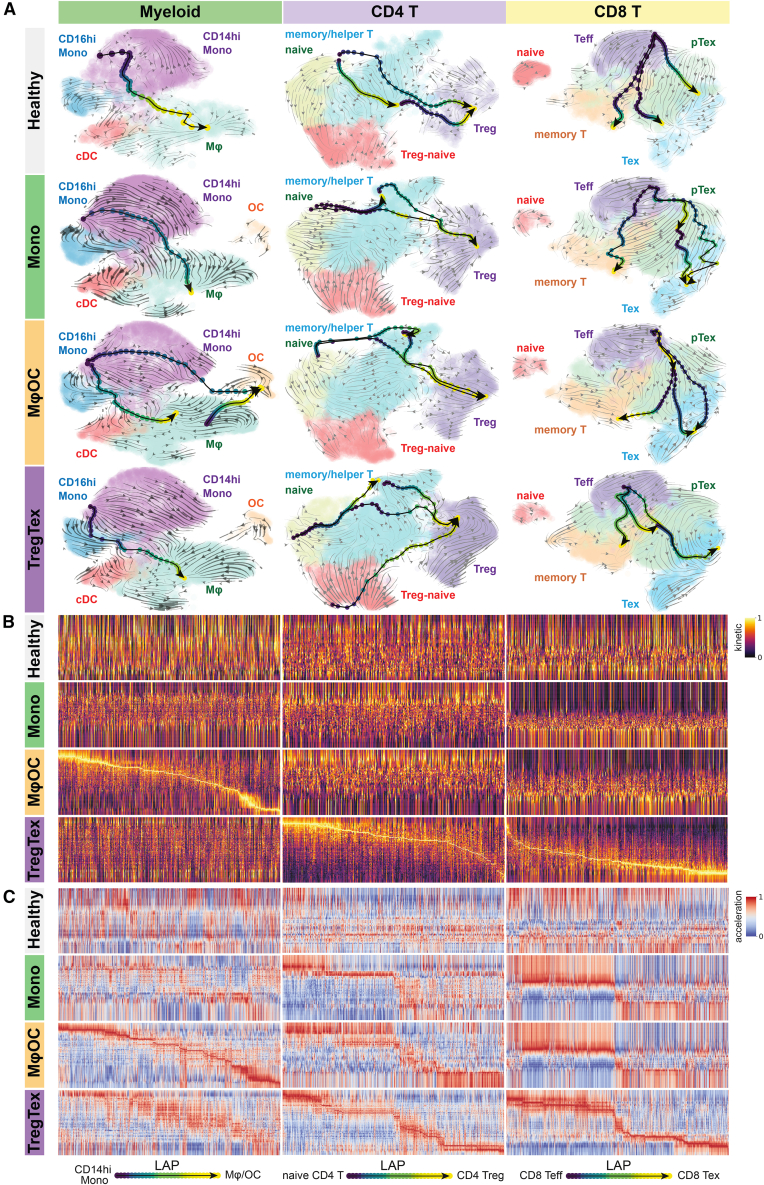
Since immune archetypes are delineated by myeloid cells and T lymphocytes, we hypothesized that these lineages may go through unique differentiation processes within each archetype. We utilized the Dynamo package for RNA velocity analysis,28 which leverages partially spliced RNA sequences to deduce the change rate of gene expression. The embedded least action path (LAP) principle was adopted to ascertain optimal lineage transition routes. Like the concept of "pseudotime," an LAP consists of an inferred sequence of differentiation statuses defined by expression kinetics of pertinent genes. We validated the accuracy of this approach using the published reference dataset29 (Figures S7A–S7D). The estimated differentiation trajectories exhibited striking differences among the three archetypes and healthy control samples. In the Mφ-OC archetype, our analysis suggested that both monocytes and macrophages bear transcriptomic similarities to osteoclast precursors30,31,32,33 (Figure 4A). In contrast, monocytes in other archetypes display limited differentiation trajectory toward osteoclasts (Figure 4A). Unique differentiation LAPs were also observed for CD4 and CD8 T cells in the Treg-Tex archetype. Specifically, a robust LAP of Treg maturation distinguishes CD4 T cells in the Treg-Tex archetype from those in other archetypes. Furthermore, the fate of CD8 T effector cells (Teff) appears to be heavily biased toward exhaustion as opposed to the development of T memory cells (Figure 4A). These archetype-specific trajectories can also be reflected by RNA transcription velocity and acceleration patterns along LAPs (Figures 4B and 4C; data provided in Table S4). We observed successional changes of genes defining consecutive differentiation stages for osteoclasts only in the Mφ-OC archetype and for Treg and Tex cells only in the Treg-Tex archetype. Similar analyses were also restricted to transcription factors, leading to the same conclusion (Figures S7E–S7G). Taken together, these data indicate that altered differentiation trajectories may play crucial roles in archetype development.
Figure 4.
Distinct differentiation routes of myeloid populations and T lymphocytes
(A) Trajectory inferences of myeloid and T cells (columns) across archetypes (rows). Streamlines in the background UMAP represent unbiased, calculated cell state transitions, while arrows and gradient-colored dots depict supervised least action paths (LAPs), directed from designated initiating cell populations to terminal cell populations: CD14hi Mono to Mϕ/OC (Myeloid), naive CD4 T to CD4 Treg (CD4 T), and CD8 Teff to CD8 Tex (CD8 T).
(B) Gene expression kinetics (RNA velocity). Clear and visible kinetic shifts indicate committed differentiation events.
(C) Gene expression accelerations (a derivative of RNA velocity). Distinct and visible acceleration shifts indicate committed differentiation potential.
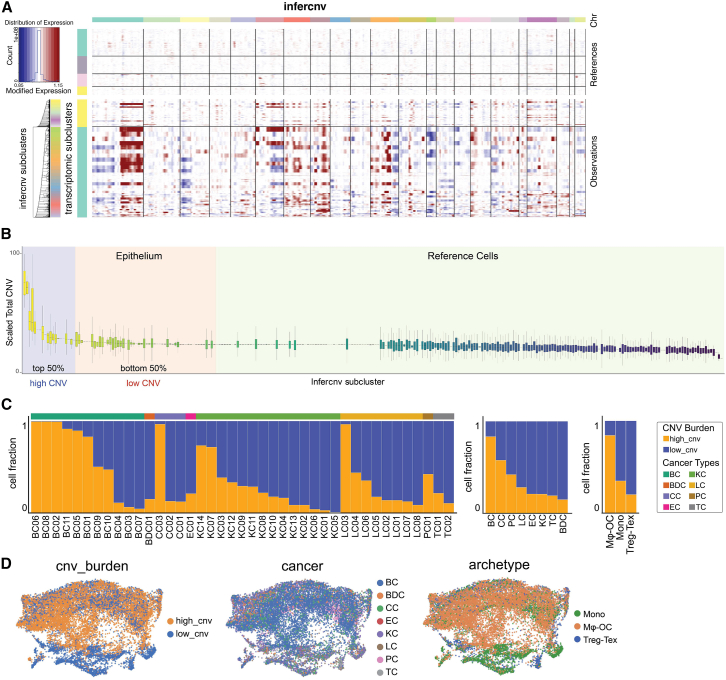
Genomic copy number alterations in cancer cells are associated with ecosystem archetypes
To distinguish epithelial cancer cells from normal cells, we used the infercnv34 method to deduce copy number alterations (CNAs) using single-cell transcriptome (Figure 5A). This approach successfully identified cancer cells as expected. We performed an unbiased transcriptomic comparison among cancer cells in different archetypes. Because lung cancers are more evenly distributed across the three archetypes, we restricted the analysis to this cancer type to eliminate differences by anatomical origins. Interestingly, HALLMARK pathways related to immune responses were expressed at a significantly lower level in the Mφ-OC archetype as compared with other archetypes (Table S5), which may partly explain the lack of lymphocytes in these BMs. In contrast, the Mφ-OC archetype is associated with the increased epithelial-mesenchymal transition (EMT) phenotype (Table S5), which is consistent with the previously reported connection between EMT and macrophage recruitment/polarization. A broader scope of pathways was also compared, and the output is provided in Table S5.
Figure 5.
Characterization of cancer epithelial cell CNV burden and its correlation with immune archetypes
(A) Epithelium copy number variation (CNV) estimation. Endothelium, CAR, OB, ACTA2+, and endothelium were selected as references in epithelium CNV estimations.
(B) Assessment of CNV load. Scaled CNV scores for each inferred subcluster (calculated by infercnv) were used to rank the subclusters. Cells in the top 50% were assigned to the high CNV group, while the remaining 50% were assigned to the low CNV group.
(C) Distribution of CNV scores across patients, cancer types, and immune archetypes, with the frequency of epithelial cells in high and low CNV burden groups displayed for each category.
(D) UMAP projection of epithelial CNV burden, cross-displayed by cancer types and immune archetypes.
Furthermore, we noticed a strikingly wide range of CNAs across individual BMs and divided all BMs into two categories with high and low CNAs, respectively (Figure 5B). The low CNA subset of BMs was more frequently observed in kidney cancers and the Treg-Tex archetype (Figures 5C and 5D), which is expected considering the relative scarcity of genomic copy number alterations in kidney cancers35 and that the Treg-Tex archetype is enriched in kidney cancer BMs (Figure 1G). We then compared cancer cells with high CNAs vs. those with low CNAs in each cancer type. We discovered increased MYC activity and oxidative phosphorylation in high CNA cells in all three cancer types, suggesting a potential connection among metabolism, oncogenic signaling, and genomic instability (Table S5).
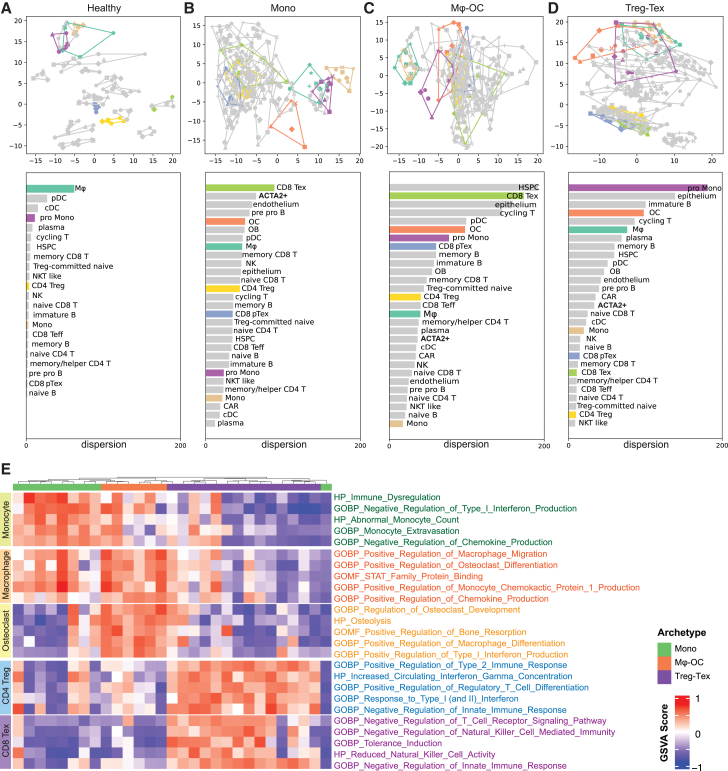
Evidence for convergent evolution of driver cell populations in each archetype
Having analyzed the divergence between different archetypes, we next examined the microenvironmental diversity within the same archetypes. Principal-component analysis (PCA) was performed on scRNA-seq profiles of individual patients and healthy control samples. Cells of the same type were then combined and subjected to PCA across patients in each immune archetype (Figure 6). In this analysis, the distance between any two points or the area of a polygon connecting multiple points indicates the degree of dissimilarity among the underlying data. Therefore, when all points of the same cell type were connected, the covered area reflects patient-to-patient variations of this cell type. Compared with their normal counterparts (Figure 6A), microenvironmental components of bone metastases exhibited dramatic variations in all three archetypes (Figures 6B–6D). Some variations even exceed those of epithelial cells (cancer cells). This observation indicates that metastatic seeds of different tumors may sculpt the bone marrow soil and induce significant phenotypic plasticity even within the same archetype.
Figure 6.
Convergent evolution of driver cell populations across archetypes
(A–D) PCA-based transcriptional variance across patients. Top: PCA plot showing each cell type per patient, with colors representing cell types and dots representing individual patients. Joint lines outline the dispersion of transcriptional variance for each cell type. Bottom: Ranked dispersion (in descending order of variance) for each cell type across patients within the corresponding immune archetype.
(E) Selected gene set variation analysis (GSVA) results comparing major cell types across immune archetypes.
Further investigation revealed that the defining components of each archetype typically exhibit lower diversity in this archetype compared with others. For instance, CD8+ Tex cells appear to be highly heterogeneous in Mono and Mφ-OC, but the heterogeneity is much reduced in Treg-Tex metastases. The same is true for Mφ and monocytes in Mφ-OC Mono, respectively (compare bars of the same color across Figures 6A–6D). These observations suggest that the dominant cell types of each ecosystem are under selective pressure from the microenvironment and evolve conserved or convergent functions that probably represent the driving force of bone colonization.
We then compared the archetype-defining cell types, monocytes, macrophage, osteoclasts, and T cells, across the three archetypes and identified pathways that distinguish these cells in the archetypes they dominate from their counterparts in other archetypes. The top pathways of each cell comparison are shown in Figure 6E and Table S6. Both monocytes and T cells are characterized by immunosuppressive activities in their corresponding archetypes. Osteoclast differentiation and bone remodeling are up-regulated in macrophages in the Mφ-OC archetype. In addition, both macrophages and osteoclasts can also inhibit anti-tumor immune responses.36,37 Interestingly, interferon-responsive pathways are differentially expressed in multiple cell types across the archetypes, which may reflect the increasingly appreciated, complex roles of these pathways dependent on different cell contexts.38 Taken together, these results suggest that different archetypes evolve to rely on different immunosuppressive cell types to escape immunosurveillance. Therefore, the convergent evolution of the dominant cell type in each archetype is partly driven by the selective pressure from the anti-tumor immunity.
Crosstalk between different cell types leads to divergent bone colonization and immuno-evasion mechanisms
We conducted cell-cell communication analyses to delineate mechanisms behind the evolution of different archetypes. Toward this end, we focused on comparing Mφ-OC and Treg-Tex archetypes, which appear to represent two opposite types of systems. Using the algorithm CellChat, we estimated the strengths of intercellular signaling in each cell type, either as receiver (expressing receptors) or sender (expressing ligands) (Figure 7A). Interestingly, in both Mφ-OC and Treg-Tex archetypes, cell types of mesenchymal origins, including ACTA2+ cells, OB, and CXCL12-abundant reticular cells (CARs), appear to be the most potent signal sources, whereas CD8 T cells are among the most prominent signal receivers (Figure 7A). This result is consistent with many previous studies supporting the roles of CARs.39 We subsequently quantified the relative probability of each signaling pathway across cell pairs within the Mφ-OC and Treg-Tex archetypes (Figure S8A; Table S7). We then identified and reported the top signaling pathways showing more than a 90% difference between the archetypes in Figure 7B, highlighting osteoclasts as the principal receptor cells in these pathways. To verify the roles of signaling molecules identified in Figure 7B, we conducted a series of in vitro assays to assess their impact on osteoclastogenesis. Initially, CD14+ monocytes were isolated and enriched from healthy human peripheral blood to serve as precursors for osteoclast induction (Figure 7C). We introduced RANKL and M-CSF to initiate osteoclastogenesis and supplemented the culture with seven selected signaling factors. The impact of these factors was assessed by measuring the expression of osteoclast signature genes, including TRAP (tartrate-resistant acid phosphatase), CTSK (cathepsin K), DC-STAMP (dendritic cell-specific transmembrane protein), and NFATC1 (nuclear factor of activated T cells c1), using qPCR (Figure 7D). Additionally, TRAP assays were conducted to visualize osteoclast formation (Figure S8Β). Our evaluations confirmed the roles of TWEAK (TNFSF12), COMP, and NRG1 in enhancing osteoclastogenesis, while SEMA4A, EFNA5, TNFSF10, and BMP8A suppressing osteoclastogenesis, likely through mechanisms mediated by macrophages.
Figure 7.
Bone stromal drives divergent bone colonization and immune evasion mechanisms
(A) Analysis of cell-cell communication signal flow. Outgoing signal strength is shown on the x axis and incoming signal strength on the y axis, comparing the Mφ-OC and Treg-Tex archetypes with healthy samples serving as references.
(B) Identification of key ligand-receptor pairs that differentially regulate the OC populations. This analysis compares the relative signaling strengths between the Mφ-OC and Treg-Tex archetypes, focusing on osteoclasts as the signal receivers (from Figure S5A).
(C) Schematic illustration of in vitro experimental validation for estimated signaling molecules. CD14+ monocytes isolated from human peripheral blood were enriched for osteoclastogenesis induction, with selected factors added to the culture medium to test their predicted roles in regulating differential osteoclastogenesis. Osteoclastogenesis was then evaluated by both qPCR and TRAP staining.
(D) qPCR analysis of osteoclast signature genes to validate differential osteoclastogenesis regulation by estimated signaling molecules. Each signaling factor was tested using graded concentrations: TWEAK (TNFSF12; 0.1, 1, 10 ng/μL), COMP (5, 50, 500 ng/μL), and NRG1 (10, 100, 1000 ng/μL), TNFSF10 (1, 10, 100 ng/μL), SEMA4A (1, 10, 100 ng/μL), EFNA5 (1, 10, 100 ng/μL), BMP8A (1, 10, 100 ng/μL). Each condition has five replicates. Statistical significance was assessed using one-way ANOVA, with significance levels: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Among the positive regulators, TWEAK was previously shown to promote osteoclastogenesis.40 NRG1 is an EGF-like ligand and stimulates EGFR signaling in osteoclasts.41,42 It has been noticed that EGFR signaling is biphasic, and low-dosage (0.06–10 ng/mL) and high-dosage (50–200 ng/mL) ligand treatments will lead to activation of different downstream effectors.43,44 Whereas low dosage stimulates most canonical pathways, high-dosage treatment additionally triggers STAT1 and STAT5 signaling.43,44 Interestingly, both STAT1 and STAT5 inhibit osteoclastogenesis.45,46 These previous studies explain our observation that NRG1 stimulated osteoclastogenesis at a low concentration, but the effects diminished as the concentration increased. COMP does not have known cognate receptors. However, it interacts with many other extracellular proteins, including integrins (e.g., αvβ3),47 many of which regulate osteoclastogenesis.48,49
Among the predicted negative regulators, although the roles of semaphorins have been studied in bone development, homeostasis, and disease,50 the specific functions of SEMA4A remain elusive. EFNA5 encodes ephrin A5, which is a ligand of EphB2. The latter is known to inhibit osteoclastogenesis.51 TNFSF10, a.k.a. TRAIL, induces osteoclast apoptosis and suppresses osteoclast activation.52 There is currently no study on the role of BMP8 in osteoclast biology (Figures S8C and S8D). Thus, it appears that the predicted osteoclastogenesis regulators are supported by experimental validation, and some of them confirmed previous studies in the literature. These regulators cooperatively lead to bifurcation between Mφ-OC and other ecosystems.
Discussion
The mechanism by which different cancer types colonize the same organ has not yet been systematically investigated. Our data revealed three archetypes of ecosystems characterized by different immune landscapes. The development of these ecosystems represents divergent evolution propelled by two major sources of selective pressure—immunosurveillance and confinement of mineralized bone tissues. For the former, different ecosystems develop parallel immunosuppressive mechanisms primarily based on T regulatory/exhausted cells, macrophages, or monocytes, respectively. For the latter, different ecosystems diverge on the driving force of metastasis-induced bone remodeling. Activated osteoclasts are enriched in the Mφ-OC type and potentially also the Mono type, encompassing almost all breast cancers. This is consistent with the large body of literature demonstrating the essential roles of osteoclasts in osteolytic bone metastases. However, the Treg-Tex type lacks osteoclasts and is unlikely to rely on osteoclasts for bone remodeling. Kidney cancer is the major cancer type in this class. In fact, evidence has emerged that kidney cancers invade bone matrix by inducing apoptosis of osteocytes rather than activating osteoclasts.53 In the clinic, osteoclast-targeting agents, such as bisphosphonates, are not as effective on kidney cancer bone metastasis as they are in other cancer types,16 consistent with the fact that osteoclasts are not the major driver of bone colonization.
Interestingly, besides the divergence across different cancer types, we also observed that individual bone metastases of the same cancer type evolve vastly different mechanisms. For example, the six lung cancer bone metastases examined in our study were evenly distributed across all three types of ecosystems. The determinants that sculpt metastatic evolution toward one ecosystem over the others remain to be investigated. One factor examined in our study is the number of CNAs (Figure 2): the Mφ-OC ecosystem is associated with high CNA, and this association can explain the diversity of breast cancer bone metastases. Further studies will be needed to establish causal links between the overall frequency of genetic variations and the bone metastasis ecosystem archetypes.
Conversely, different cancer types can evolve into similar ecosystems and utilize the same bone colonization mechanism. This may not be surprising considering the aforementioned selective pressure in the bone microenvironment (BME) that is commonly encountered by metastatic cancer cells. However, it is curious to ask why cancers from different tissues of origin end up choosing the same mechanism among three parallel options. Again, if the level of genomic variation is a key determinant, then how does the BME sense this variation and how does it trigger the development of a particular type of ecosystem. It is possible that DNA damage response pathways and/or tumor-specific antigens are involved in the divergence among different mechanisms, which warrants further investigations.
Our data suggest that the skeletal stromal cells (including skeletal stem cells, ACTA2+, and OB) are essential sources of signals in all bone metastases, although the specific signals and the major recipient cell types may differ between different ecosystems, as revealed by the cell-cell communication analyses. These disparate skeletal signals, together with the vastly varying immune milieu, appear to converge on opposite regulation of osteoclast activation: whereas the ACTA2+ cell-derived COMP, cancer cell-derived NRG1, the skeletal stem cell-derived CSF1 and CD4+ memory/helper T-derived TWEAK promote osteoclastogenesis in the Mφ-OC ecosystem, the exhausted T cells may inhibit the same process in the Treg-Tex ecosystem. Thus, the complicated, multi-cell type crosstalk seems to dictate whether the bone colonization is osteoclast dependent.
Immune cell infiltration has been considered a hallmark of immunogenic tumors that tend to be responsive to immune checkpoint blockade (ICB). Recent comparisons between matched human primary and metastatic tumors, few of which were from bone, suggest that metastasis is typically "colder" and even less immunogenic.17,18,54 Herein, our data revealed that bone metastases of many cancer types are in fact well infiltrated by immune cells. Despite their presence, however, these cytotoxic cells are blunted by different immunosuppressive mechanisms in different types of ecosystems.
Limitations of the study
The surgical specimens of bone metastases were cryopreserved before being subjected to scRNA-seq. Some cell types are sensitive to these procedures and may drop out. For example, neutrophils are abundant in the bone marrow but were not well represented in our dataset. This is a missed opportunity considering the essential roles of neutrophils in the tumor microenvironment. The treatment history of the bone metastases varies from patient to patient. Although we did not observe any correlation between specific treatments and the immune archetypes, different therapeutic effects of these treatments may still represent a confounding variable in our analysis. Only a small subset of bone metastases examined in our study have matched primary tumors or long-term clinical follow-up information, which provided compelling evidence supporting the bone specificity or prognostic value of the immune archetypes, respectively. These data highlight the need for similar analyses in more cancer types and on a larger scale. Finally, we performed in vitro osteoclastogenesis assays to assess the accuracy of our computational analyses. However, these assays are oversimplified, and future studies will be required to verify the potential roles of these predicted regulators in vivo using metastasis models.
Resource availability
Lead contact
For additional information or to request resources and reagents, please contact the lead contact, Xiang H.-F. Zhang, at xiangz@bcm.edu.
Materials availability
No new unique reagents were generated in this study.
Data and code availability
Raw single-cell RNA-seq data have been deposited in the NCBI GEO database under accession number GSE266330. The full analysis code is available on GitHub (https://github.com/xzhanglab/multi-cancer-bone-metastasis-scrnaseq) and archived on Zenodo (https://doi.org/10.5281/zenodo.14270976). Processed data and key intermediate outputs are also available at Zenodo.
Acknowledgments
We are grateful for the discussion and suggestions from Zhang Laboratory members. We thank Dr. William Hudson from Baylor College of Medicine for his valuable opinions regarding the analysis of single-cell trajectory predictions. Y.G. is supported by NCI K99CA279899. X.H.-F.Z. is supported by US Department of Defense DAMD W81XWH-21-1-0790, HT9425-23-1-0493, NCI CA183878, NCI CA251950, NCI CA277838-01A1, NCI CA271498, NCI U01CA252553, and Breast Cancer Research Foundation. This project was supported by the Cytometry and Cell Sorting Core at BCM with funding from the CPRIT Core Facility Support Award (CPRIT-RP180672) and the NIH (P30 CA125123, S10 RR024574, S10 OD025251), by the Pathology Core of Lester and Sue Smith Breast Center at BCM, by the Optical Imaging & Vital Microscopy (OiVM) core at BCM, by the Single Cell Genomics Core at BCM partially supported by NIH S10OD025240 and CPRIT RP200504, by the RNA In Situ Hybridization Core at BCM with funding from the PHS grant DK056338, and by the Integrated Microscopy core at BCM with funding from NIH (DK56338, CA125123, ES030285) and CPRIT (RP150578, RP170719).
Author contributions
X.H.-F.Z. and F.L. conceived the concept and designed the analytic strategies. F.L. performed the single-cell RNA sequencing and bioinformatic analysis. Y.D., X.H., and Z.X. collected the patients' samples and conducted flow cytometry, cell culturing, and imaging experiments. Y.D., T.P., Z.G., and R.L.S. aided in the collection of clinical samples. G.M. analyzed the patients' tissue sections and H&E stainings. L.W., L.Y., D.G.E., S.W., and S.A. assisted with experimental settings. H.L.C. aided in cell differentiation work. M.W.D., E.C., and other authors contributed to manuscript editing. X.H.-F.Z. supervised the research.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human peripheral blood | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| recombinant human M-CSF | Peprotech | Cat#AF-300-25 |
| recombinant human sRANKL | Peprotech | Cat#AF-310-01 |
| recombinant human TWEAK | Sino Biological | Cat#90094-C04H |
| recombinant human COMP | Sino Biological | Cat#10173-H08H |
| recombinant human NRG1 | Sino Biological | Cat#10658-H08H |
| recombinant human SEMA4A | Sino Biological | Cat#11756-H08H-100 |
| recombinant human EFNA5 | Sino Biological | Cat#10192-H08H-100 |
| recombinant human TNFSF10 | Sino Biological | Cat#10409-HNAE-100 |
| recombinant human BMP8A | MedChemExpress | Cat#HY-P700031AF |
| Mouse anti-Human CD4, 1:100 | ThermoFisher scientific | Cat#14-2444-82; RRID: AB_2572868 |
| Mouse anti-Human CD8a, 1:500 | ThermoFisher scientific | Cat#14-0008-82; RRID: AB_2572848 |
| Rabbit anti-Human TIM-3, 1:300 | Cell Signaling Technology | Cat#45208; RRID: AB_2716862 |
| Rabbit anti-Human FoxP3, 1:100 | Cell Signaling Technology | Cat# 98377; RRID: AB_2747370 |
| Rabbit anti-Human Cathepsin K, 1:200 | Abcam | Cat#ab19027; RRID: AB_2261274 |
| rat anti-human Cytokeratin 8, 1:200 | DSHB | Cat#TROMA-I; RRID:AB_531826 |
| rat anti-human Cytokeratin 19, 1:200 | DSHB | Cat#TROMA-III; RRID: AB_2133570 |
| Dako Retrieval Solution pH 9.0 | Agilent Technologies | Cat#S2367 |
| Dako Retrieval Solution Citrate pH 6.1 | Agilent Technologies | Cat#S1699 |
| Vector® TrueVIEW® Autofluorescence Quenching Kit | Vector Laboratories | Cat#SP-8500-15 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat#62249 |
| ProLong™ Gold Antifade Mountant | Invitrogen | Cat#P36934 |
| Donkey anti-mouse Alexa Fluor 488 1:500 | Jackson ImmunoResearch | Cat#715–545-151; RRID: AB_2341099 |
| Donkey anti-rabbit Alexa Fluor 555 1:500 | Invitrogen™ | Cat#A-31572; RRID: AB_162543 |
| Donkey anti-rat Alexa Fluor 647 1:500 | Jackson ImmunoResearch | Cat#712-605-153; RRID: AB_2340694 |
| Donkey anti-rat Alexa Fluor 488 1:500 | Jackson ImmunoResearch, | Cat#712-545-153; RRID: AB_2340684 |
| Donkey anti-rabbit Alexa Fluor 555 1:500 | Invitrogen™ | Cat#A-31572; RRID: AB_162543 |
| Critical commercial assays | ||
| Leukocyte Acid Phosphatase (TRAP) Kit | Sigma-Aldrich | Cat#387A |
| Direct-zol RNA Miniprep Kit | Zymo Research | Cat#R2052 |
| RevertAid First Strand cDNA Synthesis Kit | Thermo Fisher Scientific | Cat#K1622 |
| PowerUp™ SYBR™ Green Master Mix | Thermo Fisher Scientific | Cat#A25741 |
| Deposited data | ||
| Raw and processed patient scRNA-seq data | This study | GEO: GSE266330 |
| Patient information | This study | See Table S1 for details |
| Human scRNA-seq data for SVM training | Other studies | See Table S2 for details |
| Patient H&E staining and pathology descriptions | This study | Zenodo: https://doi.org/10.5281/zenodo.14270976 |
| Human prostate cancer bone metastasis scRNA-seq data | Kfoury et al.55 | GEO: GSE143791 |
| Human kidney cancer bone metastasis scRNA-seq data | Mei et al.56 | GEO: GSE202813 |
| Human neuroblastoma bone metastasis scRNA-seq data | Fetahu et al.57 | GEO: GSE216176 |
| Oligonucleotides | ||
| hCTSKF 5′-ACTCAAAGTACCCCTGTCTCAT-3 hCTSK-R 5′-CCACAGAGCTAAAAGCCCAAC-3′ |
This paper | N/A |
| hNFATC1-F 5′-CACCGCATCACAGGGAAGAC-3 hNFATC1-R 5′-GCACAGTCAATGACGGCTC-3 |
This paper | N/A |
| hGAPDH-F 5′-GTCGCTGTTGAAGTCAGAGG-3 hGAPDH-R 5′-GAAACTGTGGCGTGATGG-3 |
This paper | N/A |
| hDC-STAMP-F 5’-CCTTGCCACTCCACTAAGTGT-3’ hDC-STAMP-R 5’-CTCTGTGGTTGTTGCCATCTG-3’ |
This paper | N/A |
| hTRAP-F 5’-GACTGTGCAGATCCTGGGTG-3‘ hTRAP-R 5’-GGTCAGAGAATACGTCCTCAAAG-3’ |
This paper | N/A |
| Software and algorithms | ||
| 10x Genomics Software Cell Ranger v7.1.0 | Zheng et al.58 | https://kb.10xgenomics.com/hc/en-us |
| Seurat | Hao et al.59 | https://satijalab.org/seurat/ |
| Scanpy | Wolf et al.60 | https://scanpy-tutorials.readthedocs.io/en/latest/index.html |
| scVelo | Bergen et al.61 | https://scvelo.readthedocs.io/en/stable/ |
| velocyto | Manno et al.28 | https://velocyto.org/velocyto.py/tutorial/index.html |
| Dynamo | Qiu et al.62 | https://dynamo-release.readthedocs.io/en/latest/ |
| SVM-based cell type prediction | Abdelaal et al.63 Alquicira-Hernandez et al.64 |
https://powellgenomicslab.github.io/scPred/articles/introduction.html |
| Pseudo-bulk analysis pipeline | Piper et al.65 | https://github.com/hbctraining/scRNA-seq_online |
| ClusterProfiler GSEA and gene enrichment analysis | Yu et al.66 | https://guangchuangyu.github.io/software/clusterProfiler/ |
| GSVA analysis | Hänzelmann et al.67 | https://alexslemonade.github.io/refinebio-examples/03-rnaseq/pathway-analysis_rnaseq_03_gsva.html |
| ComBat | Johnson et al.68 | https://www.rdocumentation.org/packages/sva/versions/3.20.0/topics/ComBat |
| ssGSEA | Broad Institute | https://github.com/broadinstitute/ssGSEA2.0 |
| ImageJ | National Institute of Health | https://imagej.nih.gov/ij/ |
| Graphpad Prism 10.1.2 | GraphPad Software, Inc. | https://www.graphpad.com/scientificsoftware/prism/ |
| BioTek Gen5 Microplate Reader | Agilent Technologies | https://www.agilent.com/en/product/microplate-instrumentation/microplate-instrumentation-control-analysis-software/imager-reader-control-analysis-software |
| BioTek Gen5 Imager Software | Agilent Technologies | N/A |
| ZEN 3.4 (blue edition) | ZEISS | https://www.zeiss.com/microscopy/us/products/microscope-software.html |
| Scripts for data analysis | This paper | GitHub: https://github.com/xzhanglab/multi-cancer-bone-metastasis-scrnaseq; Zenodo: https://doi.org/10.5281/zenodo.14270976 |
| Other | ||
| RBC lysis buffer | Tonbo Biosciences | Cat#TNB-4300-L100 |
| human CD14+ magnetic microbeads | Miltenyi Biotec | Cat#130-050-201 |
| LS columns | Miltenyi Biotec | Cat#130-042-401 |
| LSM 780 | Zeiss | N/A |
| Axioscan.Z1 scanner | Zeiss | N/A |
| BioTek Cytation 5 | Agilent Technologies | N/A |
Method details
Patient and healthy samples
This study collected patient samples from MD Anderson Cancer Center with Dr. Robert L Satcher. The protocols for the collection and use of human bone metastasis samples were performed following the Declaration of Helsinki and approved by the Institutional Review Boards at Baylor College of Medicine (H-49396), The University of Texas MD Anderson Cancer Center (PA15-0225), and the University of Texas Medical Branch (H-46675). All the patients have provided written informed consent on using their samples for research purposes when undergoing orthopedic surgery. The selection of patient samples was driven by clinical necessity, focusing on individuals requiring surgical intervention. However, there were no restrictions on patient selection beyond this clinical consideration. Our sample collection was comprehensive, encompassing both single-cell sequencing and the generation of tissue sections for pathology studies as dictated by clinical need. Specifically, we acquired bone tissue regions from 34 patients, encompassing both metastasized tumors and bone tissues. These samples were predominantly sourced from long bones within the human body. Additionally, we obtained five healthy individual human bone marrow from Lonza (Catalog#: 1M-105). This approach allowed us to acquire a diverse range of samples tailored to the specific requirements of the patients, ultimately contributing to the comprehensiveness of our study.
Single-cell RNA library preparation and sequencing of freeze-thawed tissue samples
To optimize cost-effectiveness, we implemented a cell hashing strategy for processing and sequencing the patient samples collected over the years. Fresh tissues, ranging from 0.5 to 2 cm3, were finely minced into 1 mm3 pieces using scalpels and pestles in 24-well plates with 5 mL of DMEM. These tissues were then preserved in liquid nitrogen in a cell-stocking buffer comprising 10% DMSO mixed with 90% FBS in DMEM.
For each batch of samples, typically containing 3–4 specimens, we initiated the process by thawing the samples and washing them with PBS. The samples were digested in a dissociation solution containing 1 mg/mL of Collagenase II (Gibco, 17101-015) and 3 mg/mL of Dispase II (Roche, 13437500) in DMEM F12/HEPES medium (Gibco, 113300). In cases where hard bone tissues were present, mechanical grinding of the tissue was conducted before dissociation. The samples were incubated at 37°C for 30 min. Subsequently, the tissue suspension was filtered through a 70-μm strainer to ensure complete tissue grinding, and the strainer was rinsed and ground with DMEM to capture any remaining single cells. The flow-through was centrifuged at 450g for 5 min, and the supernatant was carefully removed. In instances where red blood cells (RBCs) were present in the pellet, 3–4 mL of 1×RBC lysis buffer (1:10 dilution of 10× RBC lysis buffer (MACS, 130-094-183) in Milli-Q water) was applied, with incubation on ice for 10 min. To halt and remove RBC lysis, samples were centrifuged at 450g for 5 min. The supernatant was discarded, and the cell pellet was washed with 3 mL of 4°C DMEM. After centrifuging at 450g for 5 min, the supernatant was discarded, and the cells were resuspended in a cold PBS solution (Sigma, D8537) containing 2% FBS (Gibco, 52567-01). Single cell suspension was sorted through the FACS sorting machine of DAPI (Invitrogen, R37606) negative and Ghost dye (Tonbo, 13-0868-T100) negative group to eliminate dead cells and cell debris further. The subsequent steps encompassed single-cell capture, barcoding, and library preparation, following the Chromium Next GEM Single Cell 3ʹ v3.1: Cell Multiplexing protocol (CG000383) and carried out with the assistance of the Single Cell Genomics Core at BCM. The final libraries, containing barcoded single-cell transcriptomes, were sequenced to a depth of 600 million (for transcripts) and 150 million (for cell barcodes) reads on the Novoseq 6000 system (Illumina) at the Genomic and RNA Profiling Core (GARP). Data processing was performed using the CASAVA 1.8.1 pipeline (Illumina), and sequence reads were converted into FASTQ files, cell CMO (Cell Multiplexing Oligo) barcoding matrices, and UMI read counts using the CellRanger software (10X Genomics).
Data preprocessing, quality control, and batch correction
We specifically used cellranger multi pipeline to process the multiplexed raw data. We generated multiplexing and count assays for each dataset and patient based on CellRanger outputs. All subsequent processing was conducted using Seurat packages (v.4.0 and v.5.0). Initially, we performed hashtag demultiplexing within the Seurat framework. A two-step doublet filtering process was applied to ensure data quality. First, cells were filtered based on the demultiplexing results and hashtag assignments, retaining only the singlets (cells identified as having single barcode labeling). Subsequently, cells with fewer than 200 detected genes or exhibiting a deviation of more than 2-fold from the median gene count were excluded. Cells with more than 10% mitochondrial genes were systematically filtered out to minimize mitochondrial gene contamination. We proceeded to normalize each dataset using the NormalizeData function’s default parameters. Following normalization, we utilized the FindVariableFeatures function with its default settings to identify variable genes within each normalized dataset. We employed the SelectIntegrationFeatures function to select the most 2,000 variable genes for integration. To identify anchors across all datasets, we compared various methods, including "rpca," "cca," and "harmony." We chose the "rpca" method due to its superior speed and efficiency.69 These anchors were then used to integrate the matrices using the IntegrateData function, specifying the parameter dims as 1:50. Under the integrated assay, we performed scaling and PCA analysis. Finally, we utilized UMAP for dimension reduction and visualizing the unsupervised clustering results of the data.
Machine learning-based cell type/state identification
We initially categorized the major cell groups (immune, epithelium, and stromal cells) based on the expression levels of conventional cell markers (immune: PTPRC/CD45 for pan-immune cells; CD3E/G for T cells; CD79A for B cells; CD68 for myeloid cells; EPCAM, KRT8/18/19 for epithelial cells; and PECAM, ACTA2, MYL9 and MYLK for stromal cells). For immune cell annotations, we employed support vector machines (SVMs),63,64 and we integrated published, well-annotated single-cell dataset from various dataset (GSE143791 55; GSE202813 56; GSE216176 57; GSE179346 70; E-MTAB-8884, E-MTAB-9139 (https://www.ebi.ac.uk/arrayexpress/), GSE175604,71 GSE120221,72 GSE193138,73 GSE159929,74 GSE181989,75 GSE159624,76 GSE130430,77 GSE139369,78 GSE165645,79 GSE128639,80 GSE185381,81 GSE166895,82 GSE133181,83 GSE169426,84 GSE135194,85 resulting in a reference dataset comprising over 670,000 immune cells and encompassing more than 40 cell types and cellular states (Table S2). Following cell type predictions in our dataset using the trained references, we manually validated classical cell marker expression.86 Regarding stromal cell populations, we primarily utilized a comprehensive single-cell study on bone stromal cells as our ref.87
To reinforce our annotations, we employed cell type marker panels sourced from the interactive web portal ToppCell88 (http://toppcell.cchmc.org/) for cross-validation purposes. To illustrate the results of this manual validation process, we computed the average expression levels of each reference gene set across all clusters within single-cell datasets, with the same resolution that was utilized for cell type predictions.
Epithelium CNV estimation and malignancy quantification
We utilized the infercnv34 R package to investigate subsets of epithelial cells. Notably, since normal bone tissues do not contain epithelial cells, the presence of epithelial cells in our dataset signifies their cancerous nature. We employed stromal and immune cells as reference cells to assess copy number changes in the epithelial cells (observation cells). To quantitively describe the malignancy of epithelial cells, we established a scoring system: assigning two marks for each allele with two or more copy number changes (including both addition and deletion), one mark for one, and 0 for neutral changes. This allowed us to compute the total scores for each infercnv subcluster. High malignant clusters were identified as those subclusters with a median score higher than the 75th percentile of the reference group, while the remaining clusters were classified as low malignant clusters. We then created the epithelial cell malignancy group in our dataset according to such classifications.
Determination of patient immunophenotypes
The primary drivers of three distinct immunophenotypes among cancer patients are the varying fractions of different cell types. We used an unbiased hierarchical clustering approach to uncover these patterns, utilizing the average linkage criterion. This method firstly grouped patients based on cluster fractions (cell number of each cluster divided by the total cell numbers, per patient) within each patient, the clusters are derived from previous unsupervised transcriptomic clustering. Subsequently, we assigned their cellular identities based on pre-determined cell type identities. The cell transcriptomic clusters employed for this analysis were generated at a high resolution (resolution = 5.0) from the FindClusters function, and we scaled (Z score) the cell fractions within each cluster across rows (patients). We verified the hierarchical clustering results by selectively plotting the fractions of designated cell types across the patient cohort (supervised clustering) to validate our findings.
Estimation of cell type frequencies from bulk/microarray data
Normalized datasets from six different research groups and platforms, including one microarray dataset, were used for analysis. We applied ComBat for batch correction (without covariates) to address batch effects across datasets. PCA was performed to assess the effectiveness of batch correction, and outliers were excluded before downstream analyses.
Cell type estimation was conducted using the ssGSEA approach. We selected the top 15 cell signature genes for cell type predictions. These markers come from our single-cell dataset, cross-validated with classical cell signature genes identified through IF staining, FACS experiments, or our customized single-cell reference dataset.
Cell signature gene set scores were calculated using ssGSEA and scaled across all patients for hierarchical clustering and heatmap visualization.
Trajectory inference
We utilized the Dynamo62 package and cross-validated the results in scVelo,28,61,89 which leverages RNA velocity to conduct trajectory inference. Our primary focus was on myeloid cells, CD4+ T cells, and CD8+ T cells. We first generated loom files from the CellRanger outputs and then applied these files along with the desired cell type-specific dataset for trajectory analysis. One notable advantage of the Dynamo package is its ability to perform unbiased calculations of the "unstable" cells within the designated lineages as the potential "root" cells. This feature is particularly valuable as it ensures a more objective analysis. Moreover, the trajectory analysis provided compelling evidence for the high accuracy of our cell type annotations. It is essential to highlight that cell type annotation and trajectory inference are distinct processes. However, when we combine the results of trajectory inference with our cell type annotations, they synergistically yield biologically meaningful insights. For instance, the trajectory analysis successfully established connections between precursor and mature cells, thus elucidating the fulfillment of cellular differentiation paths. Furthermore, we present the output from the Least Action Path (LAP) function, as it not only reveals estimated developmental routes but also highlights preferred paths in different immunophenotypes based on the thickness of the arrows. To ensure the robustness of our findings, we further validated our results using alternative packages such as scVelo28 and Monocle 3.90
Cell-cell communication analysis
We conducted cell-cell communication analysis using CellChat91 to compare the two major immunophenotypes. Our objective was to identify primary communication senders, receivers, and significantly different signaling pathways, focusing on the Mac-OC and Treg-Tex groups compared to healthy donors. To evaluate interaction strength differences between these immunophenotypes for various cell type pairs, we systematically assessed signaling pathways present in at least one group, totaling 190 out of 293 pathways in the CellChat database. We then created an integrated scatterplot to visualize the biased signaling pathways within each immunophenotype group across the entire signaling pathway landscape. The process involved calculating differences in communication probabilities between the two groups for each cell pair within each signaling pathway. Subsequently, we standardized these differences by scaling them as percentages relative to the range for each specific signaling pathway. This analysis allowed us to compile a data matrix for generating scatterplots, distinguishing immunophenotype-specific signaling pathways based on a 75% threshold. Signaling pathways with a positive relative difference above 75% were considered Mac-OC specific, while those with a negative relative difference below −75% were identified as Treg-Tex biased.
Transcriptomic differences across cancers and immunophenotypes
We employed PCA to assess transcriptomic variations within our dataset, categorizing cells by cancer types or immunophenotypes. The variance was computed based on the distribution of each cell type, grouped according to their respective cancer types or immunophenotypes.
Pseudo-bulk transcriptomic comparisons and gene enrichment analysis
We opted for pseudo-bulk analysis to compute differentially expressed genes (DEGs) since we aimed to assess cell type-specific expression differences at the population level. We followed these key steps.
-
(1)
First, we selected cells of interest corresponding to the desired cell type(s) before initiating the differential expression (DE) analysis.
-
(2)
Next, we obtained raw counts by applying quality control (QC) filtering to the designated cells intended for DE analysis.
-
(3)
We then integrated the counts and accompanying metadata at the sample level.
-
(4)
Subsequently, we performed the DE analysis using DESeq292 packages in R.
Following the acquisition of DEGs, we conducted Gene Set Enrichment Analysis (GSEA) using the ClusterProfiler66 R package. The pathway dataset utilized for this analysis was sourced from MSigDB (https://www.gsea-msigdb.org/gsea/msigdb/). We set up parameters with a stringent BH-adjusted p-value cutoff of 0.01 to select the top significant differentially regulated pathways.
To visualize the differences in pathway activity across different cell types, cancer types, or immunophenotypes, we utilized the AUCell93 R package to compute pathway activity scores. Visualization of these scores was achieved through violin plots using the AddModuleScore function. Significance statistics were calculated using the Wilcoxon rank-sum test.
Paraffin embedding, microtomy, and hematoxylin and eosin staining (H&E)
The bone metastasis samples were first fixed in 10% neutrally buffered formalin for up to 24 h at 4°C. They were then subjected to decalcification in 14% EDTA (pH 7.4) for about one week, with the decalcifying medium changed every 2–3 days. Subsequently, the tissues were delicately transferred into histology cassettes using tweezers and incubated in 70% ethanol before being sent to the pathology core at the breast center of BCM. There, the tissues underwent automated dehydration using ethanol, followed by clearing with xylenes and infiltration with paraffin using the state-of-the-art Sakura Tissue-Tek VIP Processor. Finally, manual embedding of the bone metastasis tissues was performed using the Sakura Tissue-Tek paraffin embedding center. The tissue blocks were cut into 4μm slices using Richard-Allan HM 315 microtomes. Afterward, the sliced slides underwent H&E staining with the Shandon Varistain 24-4 Automatic Slide Stainer.
Diagnostic consultation for H&E-Stained human bone metastasis samples
Histological features indicative of invasive carcinomas were discerned on H&E-stained slides of human bone metastasis by Dr. George Miles from the pathology core at the breast center of BCM.
Isolation of CD14+ monocytes from peripheral blood for osteoclastogenesis
The peripheral blood (PB) samples were sourced from healthy donors at the Gulf Coast Regional Blood Center in Houston, and collected using lithium heparin vacuum tubes. Red blood cell (RBC) lysis was done with RBC lysis buffer (Tonbo Biosciences, Cat#TNB-4300-L100). Subsequently, CD14+ monocytes were isolated from the lysed PB cells using human CD14+ magnetic microbeads (Miltenyi Biotec, Cat#130-050-201) with LS columns (Miltenyi Biotec, Cat#130-042-401). These isolated human CD14+ monocytes were cultured in α-MEM supplemented with 10% FBS and antibiotics. Osteoclastogenesis induction was achieved with 25 ng/mL recombinant human M-CSF (Peprotech, Cat#AF-300-25) and 50 ng/mL recombinant human sRANKL (Peprotech, Cat#AF-310-01) following established protocols.94 Additional treatment with recombinant human TWEAK protein (Sino Biological, Cat#90094-C04H) was added dose-dependently, as illustrated in the figure. TRAP staining was conducted using the Leukocyte Acid Phosphatase (TRAP) Kit (Sigma-Aldrich, Cat#387A) per the manufacturer’s instructions. The images were captured by using BioTek Cytation 5(Agilent Technologies). The concentration of molecules for in vitro osteoclastogenesis and TWEAK, COMP, and NRP1 treatment was set based on published studies.40,95,96
mRNA extraction and qRT-PCR
Direct-zol RNA Miniprep Kit (Zymo Research, R2052) was utilized to extract RNA according to the manufacturer’s guidelines. After RNA extraction, cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622). For real-time PCR analysis, PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, A25741) was employed on a CFX96 Real-Time PCR system (Biorad). Quantitative PCR analysis was performed with primer sequences in the key resources table.
Tissue preparation and immunofluorescence staining
Human patient tissue samples were paraffin-embedded and sectioned with assistance from the Breast Center Pathology Core at Baylor College of Medicine. The tissue slides were baked at 55°C for 2 h, dewaxed, and rehydrated following standard protocols. Antigen retrieval was performed using either Dako Retrieval Solution pH 9.0 (Agilent Technologies, S2367) or Dako Retrieval Solution Citrate pH 6.1 (Agilent Technologies, S1699) in a pressure cooker at 115°C for 10 min, followed by 90°C for 10 s, and subsequently cooled to room temperature. The slides were blocked with 10% donkey serum in 0.4% Triton X-100 PBS for 1 h at room temperature. Primary antibody incubation was conducted overnight at 4°C in 0.2% Triton X-100 PBS. After three washes with PBS, the slides were incubated with secondary antibodies for 1 h at room temperature. To reduce autofluorescence, the Vector TrueVIEW Autofluorescence Quenching Kit (Vector Laboratories, SP-8500-15) was applied according to the manufacturer’s instructions. Nuclear staining was performed using Hoechst 33342 (Thermo Fisher Scientific, 62249). Following additional washes, the slides were mounted with ProLong Gold Antifade Mountant (Invitrogen, P36934). Antigen retrieval conditions were specific to the antibodies used. Dako Retrieval Solution pH 6.1 was employed for CD4 and FoxP3, while Dako Retrieval Solution pH 9.0 was used for CD8a, TIM-3, and Cathepsin K. Cytokeratins 8 and 19 were compatible with both retrieval conditions. Detailed information on primary antibodies is provided in the key resources table.
Imaging and analysis
Imaging was conducted using a Zeiss LSM780 confocal microscope or the Zeiss Axioscan.Z1 scanner. Image analysis was performed using ZEN software (Zeiss) or ImageJ.
Published: May 23, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xgen.2025.100888.
Contributor Information
Robert L. Satcher, Email: rlsatcher@mdanderson.org.
Xiang H.-F. Zhang, Email: xiangz@bcm.edu.
Supplemental information
References
- 1.Florencio-Silva R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho M.S.H., Medcalf R.L., Livesey S.A., Traianedes K. The dynamics of adult haematopoiesis in the bone and bone marrow environment. Br. J. Haematol. 2015;170:472–486. doi: 10.1111/bjh.13445. [DOI] [PubMed] [Google Scholar]
- 4.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H., Almeida D., Koller A., Hajjar K.A., Stainier D.Y.R., et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price T.T., Burness M.L., Sivan A., Warner M.J., Cheng R., Lee C.H., Olivere L., Comatas K., Magnani J., Kim Lyerly H., et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito M., Mondal N., Greco T.M., Wei Y., Spadazzi C., Lin S.C., Zheng H., Cheung C., Magnani J.L., Lin S.H., et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019;21:627–639. doi: 10.1038/s41556-019-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Tian L., Liu J., Goldstein A., Bado I., Zhang W., Arenkiel B.R., Li Z., Yang M., Du S., et al. The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell. 2018;34:823–839.e7. doi: 10.1016/j.ccell.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Yu C., Gao X., Welte T., Muscarella A.M., Tian L., Zhao H., Zhao Z., Du S., Tao J., et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W., Xu Z., Hao X., He T., Li J., Shen Y., Liu K., Gao Y., Liu J., Edwards D.G., et al. Bone Metastasis Initiation Is Coupled with Bone Remodeling through Osteogenic Differentiation of NG2+ Cells. Cancer Discov. 2023;13:474–495. doi: 10.1158/2159-8290.CD-22-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman R.E., Croucher P.I., Padhani A.R., Clézardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat. Rev. Dis. Primers. 2020;6:83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 12.Guise T.A., Mohammad K.S., Clines G., Stebbins E.G., Wong D.H., Higgins L.S., Vessella R., Corey E., Padalecki S., Suva L., Chirgwin J.M. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y., Siegel P.M., Shu W., Drobnjak M., Kakonen S.M., Cordón-Cardo C., Guise T.A., Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 14.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackiewicz-Wysocka M., Pankowska M., Wysocki P.J. Progress in the treatment of bone metastases in cancer patients. Expert Opin. Investig. Drugs. 2012;21:785–795. doi: 10.1517/13543784.2012.679928. [DOI] [PubMed] [Google Scholar]
- 16.McKay R.R., Lin X., Perkins J.J., Heng D.Y.C., Simantov R., Choueiri T.K. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur. Urol. 2014;66:502–509. doi: 10.1016/j.eururo.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aftimos P., Oliveira M., Irrthum A., Fumagalli D., Sotiriou C., Gal-Yam E.N., Robson M.E., Ndozeng J., Di Leo A., Ciruelos E.M., et al. Genomic and Transcriptomic Analyses of Breast Cancer Primaries and Matched Metastases in AURORA, the Breast International Group (BIG) Molecular Screening Initiative. Cancer Discov. 2021;11:2796–2811. doi: 10.1158/2159-8290.CD-20-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Recio S., Hinoue T., Wheeler G.L., Kelly B.J., Garrido-Castro A.C., Pascual T., De Cubas A.A., Xia Y., Felsheim B.M., McClure M.B., et al. Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nat. Cancer. 2023;4:128–147. doi: 10.1038/s43018-022-00491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou L., Barnett B., Safah H., Larussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 20.Chu Y., Dai E., Li Y., Han G., Pei G., Ingram D.R., Thakkar K., Qin J.J., Dang M., Le X., et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 2023;29:1550–1562. doi: 10.1038/s41591-023-02371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Visser K.E., Joyce J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. doi: 10.1016/j.ccell.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Ylitalo E.B., Thysell E., Landfors M., Brattsand M., Jernberg E., Crnalic S., Widmark A., Hultdin M., Bergh A., Degerman S., Wikström P. A novel DNA methylation signature is associated with androgen receptor activity and patient prognosis in bone metastatic prostate cancer. Clin. Epigenetics. 2021;13:133. doi: 10.1186/s13148-021-01119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinn B.V., Fu C., Lau R., Litton J., Tsai T.H., Murthy R., Tam A., Andreopoulou E., Gong Y., Murthy R., et al. SET(ER/PR): a robust 18-gene predictor for sensitivity to endocrine therapy for metastatic breast cancer. NPJ Breast Cancer. 2019;5:16. doi: 10.1038/s41523-019-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson D.R., Wu Y.M., Lonigro R.J., Vats P., Cobain E., Everett J., Cao X., Rabban E., Kumar-Sinha C., Raymond V., et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. doi: 10.1038/nature23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priedigkeit N., Watters R.J., Lucas P.C., Basudan A., Bhargava R., Horne W., Kolls J.K., Fang Z., Rosenzweig M.Q., Brufsky A.M., et al. Exome-capture RNA sequencing of decade-old breast cancers and matched decalcified bone metastases. JCI Insight. 2017;2 doi: 10.1172/jci.insight.95703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haider M., Zhang X., Coleman I., Ericson N., True L.D., Lam H.M., Brown L.G., Ketchanji M., Nghiem B., Lakely B., et al. Epithelial mesenchymal-like transition occurs in a subset of cells in castration resistant prostate cancer bone metastases. Clin. Exp. Metastasis. 2016;33:239–248. doi: 10.1007/s10585-015-9773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X.H.F., Wang Q., Gerald W., Hudis C.A., Norton L., Smid M., Foekens J.A., Massagué J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lönnerberg P., Furlan A., et al. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurd N.S., He Z., Louis T.L., Milner J.J., Omilusik K.D., Jin W., Tsai M.S., Widjaja C.E., Kanbar J.N., Olvera J.G., et al. Early precursors and molecular determinants of tissue-resident memory CD8(+) T lymphocytes revealed by single-cell RNA sequencing. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aaz6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang D., Wan Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin. Immunopathol. 2019;41:551–563. doi: 10.1007/s00281-019-00754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roodman G.D. Regulation of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- 32.Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T.J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheven B.A., Visser J.W., Nijweide P.J. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986;321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 34.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoadley K.A., Yau C., Wolf D.M., Cherniack A.D., Tamborero D., Ng S., Leiserson M.D.M., Niu B., McLellan M.D., Uzunangelov V., et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–944. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madel M.B., Ibáñez L., Wakkach A., de Vries T.J., Teti A., Apparailly F., Blin-Wakkach C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019;10:1408. doi: 10.3389/fimmu.2019.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benci J.L., Xu B., Qiu Y., Wu T.J., Dada H., Twyman-Saint Victor C., Cucolo L., Lee D.S.M., Pauken K.E., Huang A.C., et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554.e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bok S., Yallowitz A.R., Sun J., McCormick J., Cung M., Hu L., Lalani S., Li Z., Sosa B.R., Baumgartner T., et al. A multi-stem cell basis for craniosynostosis and calvarial mineralization. Nature. 2023;621:804–812. doi: 10.1038/s41586-023-06526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J.S., Kwok S.K., Lim M.A., Oh H.J., Kim E.K., Jhun J.Y., Ju J.H., Park K.S., Park Y.W., Park S.H., et al. TWEAK promotes osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 2013;183:857–867. doi: 10.1016/j.ajpath.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J., Jia X., Xiao G., Kang Y., Partridge N.C., Qin L. EGF-like ligands stimulate osteoclastogenesis by regulating expression of osteoclast regulatory factors by osteoblasts: implications for osteolytic bone metastases. J. Biol. Chem. 2007;282:26656–26665. doi: 10.1074/jbc.M705064200. [DOI] [PubMed] [Google Scholar]
- 42.Yi T., Lee H.L., Cha J.H., Ko S.I., Kim H.J., Shin H.I., Woo K.M., Ryoo H.M., Kim G.S., Baek J.H. Epidermal growth factor receptor regulates osteoclast differentiation and survival through cross-talking with RANK signaling. J. Cell. Physiol. 2008;217:409–422. doi: 10.1002/jcp.21511. [DOI] [PubMed] [Google Scholar]
- 43.Krall J.A., Beyer E.M., MacBeath G. High- and low-affinity epidermal growth factor receptor-ligand interactions activate distinct signaling pathways. PLoS One. 2011;6 doi: 10.1371/journal.pone.0015945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., Meng J., Wang Z.Y. A switch role of Src in the biphasic EGF signaling of ER-negative breast cancer cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K.M., Park K.H., Hwang J.S., Lee M., Yoon D.S., Ryu H.A., Jung H.S., Park K.W., Kim J., Park S.W., et al. Inhibition of STAT5A promotes osteogenesis by DLX5 regulation. Cell Death Dis. 2018;9:1136. doi: 10.1038/s41419-018-1184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeliger C., Schyschka L., Kronbach Z., Wottge A., van Griensven M., Wildemann B., Vester H. Signaling pathway STAT1 is strongly activated by IFN-beta in the pathogenesis of osteoporosis. Eur. J. Med. Res. 2015;20:1. doi: 10.1186/s40001-014-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acharya C., Yik J.H.N., Kishore A., Van Dinh V., Di Cesare P.E., Haudenschild D.R. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol. 2014;37:102–111. doi: 10.1016/j.matbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Mao L., Wang L., Xu J., Zou J. The role of integrin family in bone metabolism and tumor bone metastasis. Cell Death Discov. 2023;9:119. doi: 10.1038/s41420-023-01417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duong L.T., Lakkakorpi P., Nakamura I., Rodan G.A. Integrins and signaling in osteoclast function. Matrix Biol. 2000;19:97–105. doi: 10.1016/s0945-053x(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 50.Kang S., Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin. Cell Dev. Biol. 2013;24:163–171. doi: 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C., Irie N., Takada Y., Shimoda K., Miyamoto T., Nishiwaki T., Suda T., Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Liao H.J., Tsai H.F., Wu C.S., Chyuan I.T., Hsu P.N. TRAIL inhibits RANK signaling and suppresses osteoclast activation via inhibiting lipid raft assembly and TRAF6 recruitment. Cell Death Dis. 2019;10:77. doi: 10.1038/s41419-019-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan T., Lin S.-C., Yu K.-J., Yu G., Song J.H., Lewis V.O., Bird J.E., Moon B., Lin P.P., Tannir N.M., et al. BIGH3 Promotes Osteolytic Lesions in Renal Cell Carcinoma Bone Metastasis by Inhibiting Osteoblast Differentiation. Neoplasia. 2018;20:32–43. doi: 10.1016/j.neo.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu L., Narloch J.L., Onkar S., Joy M., Broadwater G., Luedke C., Hall A., Kim R., Pogue-Geile K., Sammons S., et al. Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. J. Immunother. Cancer. 2019;7:265. doi: 10.1186/s40425-019-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kfoury Y., Baryawno N., Severe N., Mei S., Gustafsson K., Hirz T., Brouse T., Scadden E.W., Igolkina A.A., Kokkaliaris K., et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell. 2021;39:1464–1478.e8. doi: 10.1016/j.ccell.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mei S., Alchahin A.M., Tsea I., Kfoury Y., Hirz T., Jeffries N.E., Zhao T., Xu Y., Zhang H., Sarkar H., et al. Single-cell analysis of immune and stroma cell remodeling in clear cell renal cell carcinoma primary tumors and bone metastatic lesions. Genome Med. 2024;16:1. doi: 10.1186/s13073-023-01272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fetahu I.S., Esser-Skala W., Dnyansagar R., Sindelar S., Rifatbegovic F., Bileck A., Skos L., Bozsaky E., Lazic D., Shaw L., et al. Single-cell transcriptomics and epigenomics unravel the role of monocytes in neuroblastoma bone marrow metastasis. Nat. Commun. 2023;14:3620. doi: 10.1038/s41467-023-39210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng G.X.Y., Terry J.M., Belgrader P., Ryvkin P., Bent Z.W., Wilson R., Ziraldo S.B., Wheeler T.D., McDermott G.P., Zhu J., et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017;8 doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao Y., Stuart T., Kowalski M.H., Choudhary S., Hoffman P., Hartman A., Srivastava A., Molla G., Madad S., Fernandez-Granda C., Satija R. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024;42:293–304. doi: 10.1038/s41587-023-01767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergen V., Lange M., Peidli S., Wolf F.A., Theis F.J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 2020;38:1408–1414. doi: 10.1038/s41587-020-0591-3. [DOI] [PubMed] [Google Scholar]
- 62.Qiu X., Zhang Y., Martin-Rufino J.D., Weng C., Hosseinzadeh S., Yang D., Pogson A.N., Hein M.Y., Hoi Joseph Min K., Wang L., et al. Mapping transcriptomic vector fields of single cells. Cell. 2022;185:690–711.e45. doi: 10.1016/j.cell.2021.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelaal T., Michielsen L., Cats D., Hoogduin D., Mei H., Reinders M.J.T., Mahfouz A. A comparison of automatic cell identification methods for single-cell RNA sequencing data. Genome Biol. 2019;20:194. doi: 10.1186/s13059-019-1795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alquicira-Hernandez J., Sathe A., Ji H.P., Nguyen Q., Powell J.E. scPred: accurate supervised method for cell-type classification from single-cell RNA-seq data. Genome Biol. 2019;20:264. doi: 10.1186/s13059-019-1862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piper, M., Mistry, M., Liu, J., Gammerdinger, W., and Khetani, R. (2022). hbctraining/scRNA-seq_online: scRNA-seq Lessons from HCBC (first release). Zenodo. 10.5281/zenodo.5826256. [DOI]
- 66.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanzelmann S., Castelo R., Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 69.Luecken M.D., Büttner M., Chaichoompu K., Danese A., Interlandi M., Mueller M.F., Strobl D.C., Zappia L., Dugas M., Colomé-Tatché M., Theis F.J. Benchmarking atlas-level data integration in single-cell genomics. Nat. Methods. 2022;19:41–50. doi: 10.1038/s41592-021-01336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W., DeVilbiss A., Arreola M., Mathews T., Zhao Z., Martin-Sandoval M., Benegiamo G., Awani A., Goeminne L., Dever D., et al. Reticular Dysgenesis-associated Adenylate Kinase 2 deficiency causes failure of myelopoiesis through disordered purine metabolism. bioRxiv. 2021 doi: 10.1101/2021.07.05.450633. Preprint at. [DOI] [Google Scholar]
- 71.Luo Y., Xu C., Wang B., Niu Q., Su X., Bai Y., Zhu S., Zhao C., Sun Y., Wang J., et al. Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human T(reg) compartment. Nat. Commun. 2021;12:3913. doi: 10.1038/s41467-021-24213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oetjen K.A., Lindblad K.E., Goswami M., Gui G., Dagur P.K., Lai C., Dillon L.W., McCoy J.P., Hourigan C.S. Human bone marrow assessment by single-cell RNA sequencing, mass cytometry, and flow cytometry. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You G., Zhang M., Bian Z., Guo H., Xu Z., Ni Y., Lan Y., Yue W., Gong Y., Chang Y., et al. Decoding lymphomyeloid divergence and immune hyporesponsiveness in G-CSF-primed human bone marrow by single-cell RNA-seq. Cell Discov. 2022;8:59. doi: 10.1038/s41421-022-00417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liao J., Yu Z., Chen Y., Bao M., Zou C., Zhang H., Liu D., Li T., Zhang Q., Li J., et al. Single-cell RNA sequencing of human kidney. Sci. Data. 2020;7:4. doi: 10.1038/s41597-019-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tonglin H., Yanna Z., Xiaoling Y., Ruilan G., Liming Y. Single-Cell RNA-Seq of Bone Marrow Cells in Aplastic Anemia. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crinier A., Dumas P.Y., Escalière B., Piperoglou C., Gil L., Villacreces A., Vély F., Ivanovic Z., Milpied P., Narni-Mancinelli É., Vivier É. Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell. Mol. Immunol. 2021;18:1290–1304. doi: 10.1038/s41423-020-00574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang C., Siebert J.R., Burns R., Gerbec Z.J., Bonacci B., Rymaszewski A., Rau M., Riese M.J., Rao S., Carlson K.S., et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat. Commun. 2019;10:3931. doi: 10.1038/s41467-019-11947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Granja J.M., Klemm S., McGinnis L.M., Kathiria A.S., Mezger A., Corces M.R., Parks B., Gars E., Liedtke M., Zheng G.X.Y., et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat. Biotechnol. 2019;37:1458–1465. doi: 10.1038/s41587-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Coz C., Nguyen D.N., Su C., Nolan B.E., Albrecht A.V., Xhani S., Sun D., Demaree B., Pillarisetti P., Khanna C., et al. Constrained chromatin accessibility in PU.1-mutated agammaglobulinemia patients. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lasry A., Nadorp B., Fornerod M., Nicolet D., Wu H., Walker C.J., Sun Z., Witkowski M.T., Tikhonova A.N., Guillamot-Ruano M., et al. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat. Cancer. 2023;4:27–42. doi: 10.1038/s43018-022-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jardine L., Webb S., Goh I., Quiroga Londoño M., Reynolds G., Mather M., Olabi B., Stephenson E., Botting R.A., Horsfall D., et al. Blood and immune development in human fetal bone marrow and Down syndrome. Nature. 2021;598:327–331. doi: 10.1038/s41586-021-03929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hua P., Roy N., de la Fuente J., Wang G., Thongjuea S., Clark K., Roy A., Psaila B., Ashley N., Harrington Y., et al. Single-cell analysis of bone marrow-derived CD34+ cells from children with sickle cell disease and thalassemia. Blood. 2019;134:2111–2115. doi: 10.1182/blood.2019002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gill H., Leung A.Y.H., Kwong Y.L. Molecular and Cellular Mechanisms of Myelodysplastic Syndrome: Implications on Targeted Therapy. Int. J. Mol. Sci. 2016;17:440. doi: 10.3390/ijms17040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Z., Gao S., Diamond C., Kajigaya S., Chen J., Shi R., Palmer C., Hsu A.P., Calvo K.R., Hickstein D.D., et al. Sequencing of RNA in single cells reveals a distinct transcriptome signature of hematopoiesis in GATA2 deficiency. Blood Adv. 2020;4:2656–2670. doi: 10.1182/bloodadvances.2019001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J., Bardes E.E., Aronow B.J., Jegga A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baryawno N., Przybylski D., Kowalczyk M.S., Kfoury Y., Severe N., Gustafsson K., Kokkaliaris K.D., Mercier F., Tabaka M., Hofree M., et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell. 2019;177:1915–1932.e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin K., Bardes E.E., Mitelpunkt A., Wang J.Y., Bhatnagar S., Sengupta S., Krummel D.P., Rothenberg M.E., Aronow B.J. An interactive single cell web portal identifies gene and cell networks in COVID-19 host responses. iScience. 2021;24 doi: 10.1016/j.isci.2021.103115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gayoso A., Weiler P., Lotfollahi M., Klein D., Hong J., Streets A., Theis F.J., Yosef N. Deep generative modeling of transcriptional dynamics for RNA velocity analysis in single cells. Nat. Methods. 2024;21:50–59. doi: 10.1038/s41592-023-01994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin S., Guerrero-Juarez C.F., Zhang L., Chang I., Ramos R., Kuan C.H., Myung P., Plikus M.V., Nie Q. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aibar S., González-Blas C.B., Moerman T., Huynh-Thu V.A., Imrichova H., Hulselmans G., Rambow F., Marine J.C., Geurts P., Aerts J., et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riedlova P., Sood S., Goodyear C.S., Ansalone C. Differentiation of functional osteoclasts from human peripheral blood CD14+ monocytes. J. Vis. Exp. 2023;191 doi: 10.3791/64698. [DOI] [PubMed] [Google Scholar]
- 95.Wang C., Chen Y., Xiang H., Wu X., Tang Q., Ma X., Zhang L. ADAMTS7 degrades Comp to fuel BMP2-dependent osteogenic differentiation and ameliorate oncogenic potential in osteosarcomas. FEBS Open Bio. 2020;10:1856–1867. doi: 10.1002/2211-5463.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang W., Cheng Y., Zhou F., Wang L., Zhong L., Li H.T., Wang X., Dang S., Wang X. Neuregulin-1 protects cardiac function in septic rats through multiple targets based on endothelial cells. Int. J. Mol. Med. 2019;44:1255–1266. doi: 10.3892/ijmm.2019.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw single-cell RNA-seq data have been deposited in the NCBI GEO database under accession number GSE266330. The full analysis code is available on GitHub (https://github.com/xzhanglab/multi-cancer-bone-metastasis-scrnaseq) and archived on Zenodo (https://doi.org/10.5281/zenodo.14270976). Processed data and key intermediate outputs are also available at Zenodo.