Abstract
Diabetic peripheral neuropathy (DPN) poses significant clinical challenges due to progressive nerve degeneration and vascular insufficiency. To address both neural and vascular complications simultaneously, we employed an mRNA-based protein replacement therapy. In this study, leveraging mRNA template design, structure-based screening identified NGFR100W as a variant dissociating neuroprotective and nociceptive functions, demonstrating enhanced neuritogenic activity without pain sensitization. Additionally, transcriptome analysis of NGF mutants versus wild type further reveals the potential mechanism by which NGFR100W uncouples neuroprotective and nociceptive pathways. We cotransfected chemically modified NGFR100W mRNA and vascular endothelial growth factor A (VEGFA) mRNA, and the conditioned media collected from this transfection promoted endothelial cell migration, tubulogenesis, and neurite outgrowth. In a diabetic mouse model, combination therapy with lipid nanoparticle codelivery of NGFR100W and VEGFA mRNA significantly improved blood flow in the plantar region and mitigated nerve function decline compared with monotherapy. Histological analysis showed increased microvessel formation and higher intraepidermal nerve fiber density in treated mice. Our findings highlight the therapeutic potential of NGFR100W and VEGFA mRNA coadministration for DPN, suggesting that protein supplementation via mRNA could offer a novel strategy for clinical intervention in some chronic medical conditions.
Article Highlights
We aimed to develop a dual-targeted mRNA-based therapy to address both neural degeneration and vascular insufficiency in diabetic peripheral neuropathy.
We identified NGFR100W as a mutation that enhances neuritogenic activity without pain sensitization and investigated its transcriptome to explore its ability to uncouple neuroprotective and nociceptive pathways.
Combination therapy using lipid nanoparticles for codelivery of NGFR100W and VEGFA mRNA improved blood flow, increased microvessel formation, and preserved nerve function in a diabetic mouse model.
This approach, which combines structure-based design and mRNA therapy, offers a novel strategy for decoupling protein functions and developing therapeutic molecules with specific functionalities.
Graphical Abstract
Introduction
Diabetes is a growing global health issue, with rising prevalence each year (1). Beyond blood glucose problems, patients often face severe complications, including diabetic peripheral neuropathy (DPN) (2,3). Characterized by nerve damage and vascular insufficiency in the extremities, DPN causes pain, numbness, and sensory loss, leading to ulcers and even amputations (4,5). Current treatments, such as blood glucose control, wound care, and neuroprotection (6), fall short in addressing DPN’s complexity. There is an urgent need for therapies that simultaneously target neural and vascular damage to improve outcomes.
In patients with DPN, the depletion of trophic factors at the extremities fails to protect nerves and blood vessels (7). Supplementing vascular endothelial growth factor A (VEGFA) can stimulate angiogenesis, promote vascular growth, and improve local blood flow in diabetic patients (8,9). Nerve growth factor (NGF) promotes the growth and differentiation of neurons and has the potential to treat DPN by stimulating the growth of peripheral nerve fibers (10). However, its clinical application is limited because of pain-inducing effects at therapeutic doses (11). Our previous research, based on the NGFR100W mutant found in patients with hereditary sensory and autonomic neuropathy type V (12,13), revealed that it retains the neurotrophic properties of NGF without inducing pain (14), making it a promising option for treating DPN.
In this study, we employed an mRNA-based protein replacement therapy to increase local levels of NGF and VEGFA in a diabetic mouse model, aiming to protect peripheral nerves and microvessels and evaluate its safety. Additionally, we designed specific mutations at the interaction interface between NGF and its receptor to screen for NGF mutants that decouple neurotrophic and pain-inducing pathways. Through RNA sequencing (RNA-seq), we preliminarily explored the molecular mechanisms underlying this functional decoupling. This study aims to provide a potential therapeutic strategy and a molecular mechanistic foundation for the treatment of DPN.
Research Design and Methods
Reagents and Antibodies
The following antibodies were used in this study: anti-HA (C29F4) Tag, anti-V5 tag, and anti-VEGFA (#3724, #13202s, and #65373s, respectively; Cell Signaling Technology); anti-tubulin and anti-PGP9.5 antibodies (#66031 and #14730-1-AP, respectively; Proteintech); anti-GAP43 (ET1610-94; HUABIO); anti-tubulin βIII (#Ab-78078; Abcam); and anti-CD31 (#GB113151; Servicebio). Lipofectamine 2000 was purchased from Thermo Fisher Scientific.
mRNA In Vitro Transcription
mRNAs were generated via T7 RNA polymerase-mediated in vitro transcription (IVT). Codon-optimized open reading frames with 5′ and 3′ UTRs were synthesized by General Bio Co., Ltd., subcloned into the pSG5L or pcDNA 3.1 vector, and amplified by PCR for IVT. The Cap1 analog (EzCap AG; APExBIO) was added to the IVT reaction to cap the 5′ end, with uridine replaced by the m1Ψ analog. A poly(A) tail was added using a poly(A) tailing kit (K1053; APExBIO). The DNA template was degraded by treatment with DNase I (New England BioLabs) at 37°C for 15 min, and the mRNA was purified with RNA Clean & Concentrator-25 (Zymo Research), dissolved in RNase-free ddH2O, and quantified using a UV spectrometer (15).
Lipid Nanoparticle Formulation and Assembly
Lipid nanoparticle (LNP) formulations were prepared by dissolving lipids in ethanol at a molar ratio of 50:10:38.5:1.5 (SM-102:1,2-distearoyl-sn-glycero-3-phosphocholine:cholesterol:PEG2000-DMG). The lipid mixture was combined with an equal volume of mRNA solution in 50 mmol/L citrate buffer (pH 3.0) using NanoAssemblr Ignite (Precision NanoSystem). The resulting formulations were diluted twofold with the same citrate buffer, dialyzed against PBS (pH 7.4) for 15 h, concentrated using Amicon Ultra filters (Millipore Sigma), and filtered through a 0.22-µm filter. The particle sizes and polydispersity index (PDI) were assessed using Zetasizer (Malvern Panalytical). mRNA encapsulation and concentration were assessed using the Quant-iT RiboGreen RNA assay (Thermo).
mRNA Transfection
PC12, HEK293T, or C2C12 cells were seeded in six-well plates and transfected with 2 µg mRNA using Lipofectamine 2000 (11668019; Thermo) at a 1:2 ratio when cell density reached 60–80%. Supernatants were collected 24 h posttransfection for analysis.
RNA-seq and RT–Quantitative PCR
Total RNA was extracted using the TRIzol method. Library construction and sequencing were performed at Shanghai Sinomics Corporation using the TruSeq RNA Sample Preparation Kit (Illumina). Data visualization was created using online platform https://www.bioinformatics.com.cn.
PC12 cells were transfected with 2 µg EGFP, NGFR100W, or NGFWT mRNA per well in a six-well plate. After 24 h, RNA was extracted using the EZ-press RNA Purification Kit (B0004dp), quantified, and analyzed by PCR using ChamQ Blue Universal SYBR quantitative PCR (qPCR) Master Mix (Q312). Primer sequences are shown in Supplementary Table 1. qPCR data were analyzed using the 2−ΔΔCT method.
PC12 Differentiation Assay
PC12 cells were cultured on collagen-coated six-well plates in RPMI complete medium for 24 h. NGFWT and mutant mRNAs were transfected using Lipo2K at a 2:1 ratio with 2 µg mRNA. After transfection, cells were switched to differentiation media (0.5% horse serum with antibiotics) for 24 h. mRNA retransfection occurred every 48 h during differentiation, and images were captured at 96 h. Neurite lengths were measured using ImageJ.
Preparation of Conditioned Medium
C2C12 cells were transfected with NGFR100W or VEGFA mRNA in serum- and antibiotic-free medium. After 24 h, the supernatant was collected, filtered through a 0.45-µm filter, and stored at −80°C. Western blot confirmed protein expression. The conditioned medium was used within 2 weeks, with FBS or other factors added as needed.
Scratch Assay
Human umbilical vein endothelial cells (HUVECs) were seeded in 12-well plates and scratched with a 200-µL pipette tip once 80–100% confluence was reached. After washing with PBS, baseline images were taken under a microscope (Leica DMi8). Conditioned medium (1% FBS) was added, and photographs were taken 12 h later to assess cell migration. Data were analyzed using ImageJ.
Tube Formation Assay
Matrigel (356234) was spread in a 24-well plate and incubated at 4°C overnight, then at 37°C for 30 min to solidify. HUVECs (150,000 cells per well) in conditioned media (1% FBS) were seeded and incubated for 4–6 h at 37°C. Tube formation was observed under a microscope and analyzed using ImageJ.
Animals
Sixteen-week-old male BKS Cg-Dock7m+/+ Leprdb/J mice (referred to as db/db) mice were used as a type 2 diabetes model for therapeutic studies. Age-matched male heterozygous db/m mice, a nonpenetrant genotype, were used as control group (16,17). The animals were purchased from Changzhou Cavens Laboratory Animal Co., Ltd., strain number C000110. All animal experiments were conducted under strict regulations and in a pathogen-free environment at the Shanghai Jiao Tong University School of Medicine animal facility, with protocol approval from Animal Care and Use Committee of the Shanghai Jiao Tong University School of Medicine (Shanghai, China).
We administered mRNA-LNP via plantar injection in mice to express the corresponding proteins for the treatment of DPN. During the formal treatment period, mice received 10 µL 2-µg mRNA-LNP every other day for the first 14 days (weeks 16–18). From day 14 (week 18) to day 35 (week 21), they received 10 µL 3-µg mRNA-LNP twice weekly. No interventions were performed from day 35 to day 42 (week 22), to maintain stable physiological conditions. Nociceptive responses were assessed on day 42 to evaluate therapeutic outcomes.
Dorsal Root Ganglion Isolation
Diabetic mice (≥20 weeks of age) and controls were euthanized and perfused with cold PBS. Dorsal root ganglia (DRGs) were isolated, placed in ice-cold DMEM and Ham’s F-12 Nutrient Mixture (DMEM/F12), and digested at 37°C for 30 min in PBS with DNase I (0.1 mg/mL) (10104159001; Roche Diagnostics), trypsin (0.4 mg/mL) (15090046; Thermo), and collagenase I (1 mg/mL) (17100017; Thermo). Digestion was halted with DMEM/F12 + 10% FBS. DRGs were triturated, filtered through a 70-µm mesh, centrifuged, and resuspended in DMEM/F12 complete or conditioned medium. Cells were seeded on poly-D-lysine–coated slides, washed with PBS, and cultured at 37°C.
Immunofluorescence of DRG and Frozen Sections
DRG cultures were fixed (4% paraformaldehyde, 15 min), permeabilized (0.2% Triton X-100, 10 min), blocked (2% BSA + 2% normal goat serum, 2 h), and incubated with anti-βIII-tubulin (overnight, 4°C), followed by Alexa Fluor 647 secondary antibody (1:400) (A21235; Thermo). Axon length was quantified (ImageJ). For frozen sections, footpad skin was fixed (methanol:acetone, 1:1), dehydrated (20% sucrose), embedded in optimal cutting temperature compound, and sectioned (25 µm). After glycine treatment and blocking (5% BSA + 10% normal goat serum + 0.5% Triton X–100, 1.5 h), PGP9.5 antibody was applied, followed by Alexa Fluor 647 secondary and DAPI. intraepidermal nerve fiber density (IENFD) was analyzed (ImageJ).
Measurement of Pain Threshold in Mice
Mice were acclimated to the equipment for 30 min daily over 1 week. Thermal nociceptive thresholds were assessed using a hot plate (XR1700; Shanghai Xinrun Chemical Company) (55 ± 0.5°C). The latency to nociceptive behaviors (e.g., paw licking or shaking) was recorded as the thermal pain response time. Mice were removed after 30 s if no response occurred. In the von Frey assay, filaments of varying forces were applied to the hind paw, and a positive response indicated sensitivity. The “up-down” method was used to measure the mechanical force required for paw withdrawal (18). All trials were video-recorded for precise response time determination. Tests were conducted in a double-blind manner.
Establishment and Measurement of Lower Limb Ischemia Model
Under isoflurane anesthesia, the femoral artery of db/m mice was exposed by incising the skin and separating muscle tissue. The artery was ligated proximally at the inguinal ligament and distally above the knee, then excised (19). After surgery, the muscle tissue was returned to its original position, and the skin was sutured. After 24 h, blood flow was measured using a laser Doppler meter (MoorLDI2-HIR; Moor Instruments); anesthesia depth and room temperature were carefully controlled to avoid impacting blood flow or causing temperature extremes. An 80% reduction compared with the healthy limb confirmed successful surgery (20).
Safety Detection
Serum and plantar muscle tissues were collected from db/db mice at baseline, day 1, and day 14 postinjection of either empty LNP or NGFR100W+VEGFA mRNA-LNP. Serum levels of IL-6 and TNF-α were quantified using commercial ELISA kits (IL-6: 431304; BioLegend) (TNF-α: 430904; BioLegend). Hematoxylin and eosin (H-E) staining was performed on muscle tissue sections to assess histopathological changes at each time point.
After administration, mice were anesthetized with isoflurane for cardiac blood collection. Serum AST and ALT levels were measured using colorimetric assay kits (Elabscience Biotechnology), creatinine levels with a reagent kit (Nanjing Jiancheng Bioengineering Institute), and blood urea nitrogen levels with a colorimetric kit (Nanjing Jiancheng Bioengineering Institute) to assess liver and kidney function. Liver, kidney, and footpad tissues were collected, fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with H-E to assess tissue structure and compare with the PBS group.
Statistical Analysis
All statistical analyses and graphs were generated using GraphPad Prism 9. One-way ANOVA was used for multiple group comparisons, except where a two-sided t test was specifically applied. Error bars represent the SD. A significance level of P < 0.05 was established, with significant values in the figures denoted as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Data and Resource Availability
All data generated or analyzed during the current study have been included in this article and the Supplementary Material.
Results
Design and Selection of Appropriate NGF Mutants Based on Protein Structure and Function
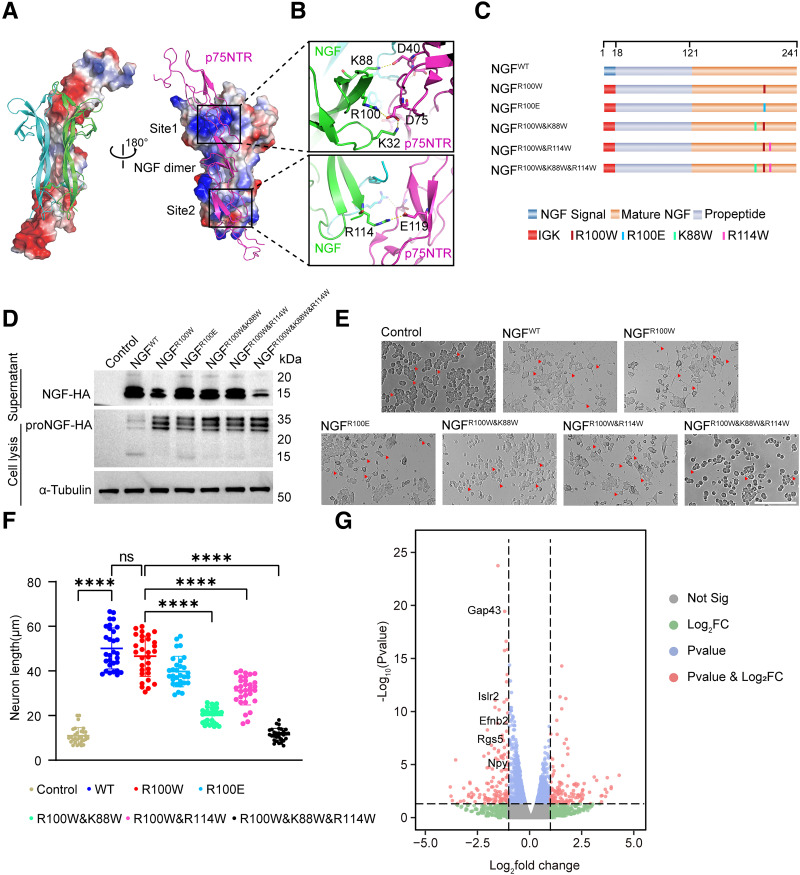
In our previous study, replacing the signal peptide sequence of NGFR100W with the Ig κ-leader sequence enhanced its secretion and alleviated peripheral neuropathy in mice (14). To further optimize painless NGF for treating DPN, we performed a structure-based design of NGF mutants through analyzing the NGF-p75NTR receptor complex (Protein Data Bank code 1sg1) (21). Electrostatic surface potential diagrams showed that the interaction surface of p75NTR is rich in negative charge, which matches the positive charge of the NGF dimer, and they are tethered together through two spatially separated binding sites (Fig. 1A). In site 1, three residues of NGF—K88, R100, and K32—formed salt bridge interactions with D40 and D75 from p75NTR. Besides site 1, site 2 consists of R114NGF-E119p75NTR salt bridge interaction (Fig. 1B). Considering the painless NGFR100W was due to weak activation of the p75NTR receptor, we introduced K88W and R114W mutations to NGFR100W to further disrupt the interaction of p75NTR (Fig. 1C). Using codon-optimized DNA templates, NGF mutants tagged with hemagglutinin (HA) mRNA were synthesized with m1Ψ nucleotide modification via IVT and transfected into HEK293T cells. Both proNGF (29 kDa) in cell lysates and mature NGF (15 kDa) in the supernatant were detected (Fig. 1D). Most NGF mutants had secretion levels similar to NGFR100W, except NGF-3Muts, which had lower mature NGF secretion. The axon growth ability of NGF mutants was assessed using PC12 cells. NGFR100W showed the best differentiation-promoting function, comparable to wild-type NGF(NGFWT). NGFR100W&R114W had a slightly better effect on promoting differentiation than NGFR100W&K88W. NGFR100W&K88W&R114W had almost no function in promoting neurite outgrowth (Fig. 1E and F).
Figure 1.
Structure-based NGF mutants design and screen. A: Overall structure of human NGF-p75NTR complex, Protein Data Bank code: 1sg1. The NGF monomers are colored green and blue, and p75NTR is colored violet. The electrostatic surface potential diagrams of p75NTR (left) and NGF dimer (right) are presented. The binding sites are marked by black boxes. B: The binding site of NGF and p75NTR. Residues involved in binding are shown by sticks. C: Schematic of NGF mutants mRNA design. The leading signal, propeptide region, mature NGF, and mutants are indicated in different colors. D: Immunoprecipitation and Western blot analyses showing the protein expression of NGFR100W mutants mRNA transfected into HEK293T cells. ProNGF was detected in whole cell lysate. HA-tagged mature NGF proteins in cell culture supernatant were immunoprecipitated using anti-HA magnetic beads followed by Western blot with anti-HA antibody. E: PC12 differentiation assay with nontransfected cells as a negative control. NGFWT, NGFR100W, NGFR100E, NGFR100W&K88W, NGFR100W&R114W, and NGFR100W&K88W&K114W mutants mRNA were transfected into PC12 cells. The red triangles indicate elongated neurites. Nerve growth length was viewed under a microscope. Scale bar, 200 µm. F: Nerve growth length statistics of differentiated PC12 cells. Error bars represent the SD (n = 30). P values were calculated using one-way ANOVA. ****P < 0.0001; ns, not significant. G: The differentially expressed genes between NGFR100W and NGFWT in a volcano plot. Significance threshold for P value: 0.05; fold change threshold: 2.
To verify the mutation effect of NGF, we performed three-dimensional structure prediction of the NGFR100W&K88W&R114W and p75NTR receptor using Alphafold3 (Supplementary Fig. 1A) (22). The analysis revealed that the K88W and R100W mutations at site 1 effectively disrupted the salt bridges with D41 and D75 of p75NTR, while K114W at site 2 maintained a weak interaction (Supplementary Fig. 1B).
To analyze the mechanisms behind the differences in nerve growth and pain, we performed RNA-seq on PC12 cells transfected with NGFR100W and NGFWT mRNA. Differentially expressed genes were displayed using a volcano plot (Fig. 1G). Among the numerous differentially expressed genes, some nerve growth–related genes, such as Gap43 and Islr2, showed decreased expression in NGFR100W compared with NGFWT, indicating that NGFR100W is slightly less effective in promoting nerve growth. Additionally, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses also showed variations in nerve growth pathways (Supplementary Fig. 1C and D). Additionally, pain-related genes Efnb2, Rgs5, and Npy were downregulated in NGFR100W, confirmed by RT-qPCR (Supplementary Fig. 1E). Considering the role of p75NTR interaction in nerve growth, we used NGFR100W for diabetic peripheral neuropathy.
In Vivo and In Vitro Expression of mRNA Packaged in LNPs
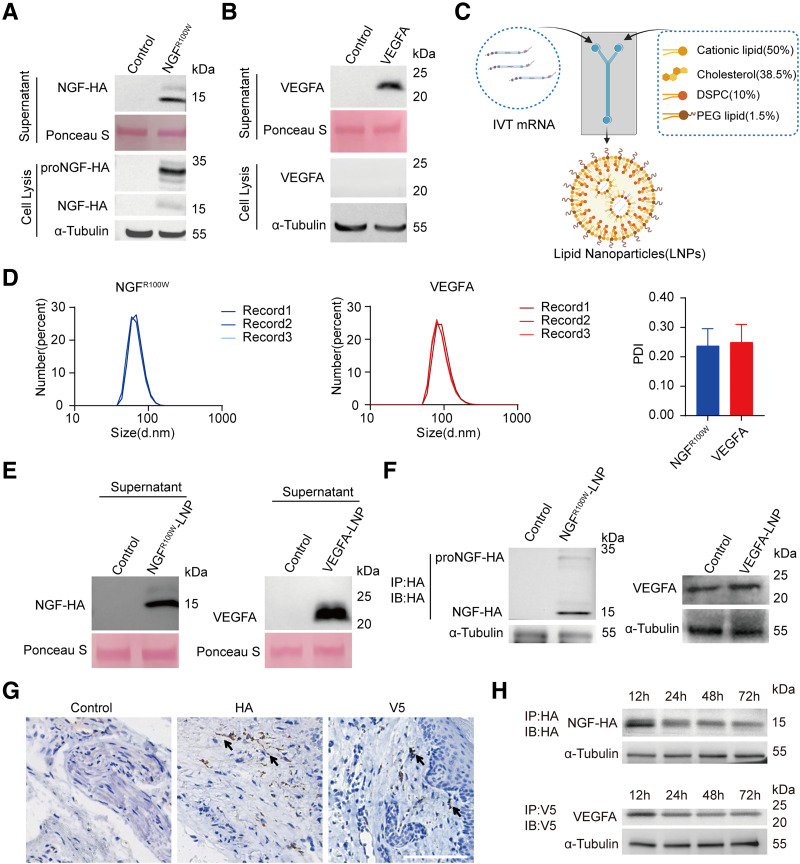
The lack of trophic factors at the extremities in DPN patients fails to protect nerves and blood vessels. We hypothesized that combining NGF and VEGFA therapy could promote nerve growth, improve blood flow, and support nerve regeneration. Using codon-optimized DNA templates, VEGFA mRNA was synthesized with m1Ψ modification via IVT. NGFR100W and VEGFA mRNA were transfected into C2C12 cells, resulting in the secretion of mature NGF (15 kDa) and VEGFA (22 kDa) proteins, along with proNGF (29 kDa) in cell lysates (Fig. 2A and B). To enhance mRNA stability and delivery, LNPs were used, with a particle size of ∼70 nm and PDI of 0.2–0.3 (Fig. 2C and D). Transfection of HEK293T cells with LNPs carrying both mRNAs led to high transfection efficiency and abundant protein secretion (Fig. 2E).
Figure 2.
Expression of NGFR100W mRNA and VEGFA mRNA in vivo and in vitro. A: Immunoprecipitation and Western blot analysis of HA-tagged NGFR100W in C2C12 cells. NGFR100W mRNA was transfected into C2C12 cells, lysed, and immunoprecipitated with an HA antibody. ProNGF was detected during cell lysis (bottom), and mature NGF was detected in the supernatant (top). Ponceau S was used as a reference for the supernatant. B: VEGFA mRNA was transfected into C2C12 cells, lysed, and immunoprecipitated with a VEGFA antibody. Western blot analysis was performed on both the filtered supernatant (top) and the cell lysis (bottom). VEGFA protein was detectable in the supernatant. Ponceau S was used as a reference for the supernatant. C: Diagram of LNP packaging. The percentages in parentheses indicate the molar ratios of lipids. DSPC, 1,2-distearoyl-sn-glycero-3-phosphocholine; PEG, polyethylene glycol. Created with BioRender.com. D: Particle size distribution and PDI of LNPs; d.nm refers to diameter (nm). Error bars represent the SD (n = 3). E: LNP-packaged VEGFA and NGFR100W mRNA were transfected into HEK293T cells. Twenty-four hours posttransfection, the supernatant was collected for Western blot analysis, with NGFR100W immunoprecipitated with an HA antibody and VEGFA incubated with a VEGFA antibody. Ponceau S was used as a reference. F: LNPs encapsulating NGFR100W and VEGFA mRNA were administered via intramuscular injection into the plantar region of the mice. The footpad tissues were harvested, lysated, and enriched with HA beads followed by immunoblotting with HA antibodies (left). The muscle lysates from mice injected with VEGFA mRNA-LNP were directly subjected to Western blot analysis, using VEGFA antibodies for detection (right). α-Tubulin antibody was used as an internal control. G: Mice were injected with NGFR100W-HA mRNA-LNP and VEGFA-V5 mRNA-LNP in the plantar region, and tissues were harvested 18 h postinjection. IHC staining was performed using HA and V5 antiboby (left), HA antibody (middle) and V5 antibody (right). Scale bar, 100 µm. H: Mice were injected with NGFR100W HA mRNA-LNP and VEGFA-V5 mRNA-LNP in the plantar region, and tissues were collected at 12, 24, 48, and 72 h postinjection for further analysis. The footpad tissues were harvested, lysated, and enriched with HA beads followed by immunoblotting with HA antibodies (top), or lysated and enriched with V5 beads followed by immunoblotting with V5 antibodies (bottom). α-Tubulin antibody was used as an internal control. IP, immunoprecipitation; IB, immunoblotting.
To evaluate mRNA expression in diabetic mice, LNPs containing NGFR100W or VEGFA mRNA were injected into the plantar muscle (Supplementary Fig. 2A). Twelve hours later, the HA-tagged NGF precursor (29 kDa) and mature NGFR100W (15 kDa) were detected via HA immunoprecipitation enrichment and Western blot. Direct analysis showed increased VEGFA expression, confirming successful mRNA expression of both NGFR100W and VEGFA in diabetic mice (Fig. 2F).
To assess LNP uptake and potential nerve fiber transfection, Cre mRNA-LNPs were injected into Ai14 (Rosa26-LSL-tdTomato) mice. After 3 days, red fluorescence indicated muscle cells as the main LNP uptake site (Supplementary Fig. 2B). Additionally, to distinguish between NGFR100W and VEGFA, an HA tag was added to NGFR100W, and a V5 tag was added to VEGFA. Immunohistochemical (IHC) staining 18 h after NGF mRNA-LNP and VEGFA mRNA-LNP injection confirmed HA-tagged NGF and V5-tagged VEGFA secretion and tissue diffusion (Fig. 2G). These findings suggest a paracrine mechanism where muscle cells absorb LNPs, and express and secrete NGF and VEGFA into the interstitial space for uptake by nerve fibers and endothelial cells.
To evaluate the temporal expression profile of the proteins, plantar muscle tissues were collected 12, 24, 48, and 72 h after LNP injection. HA and V5 immunoprecipitation showed that NGFR100W and VEGFA expression peaked at 12 h and gradually decreased, but was still detectable at 72 h (Fig. 2H). Serum analysis at 24 h showed no significant increase in circulating VEGFA, and undetectable NGF levels (Supplementary Fig. 2C), collectively indicating minimal systemic protein elevation.
NGFR100W and VEGFA mRNA Promote Angiogenesis and Neurite Growth at the Cellular Level
NGF promotes neuronal growth and stimulates angiogenesis (23), while VEGFA facilitates angiogenesis and neural repair (24,25) by interacting with VEGFR-2 on neurons and Schwann cells (24,26). To measure the synergistic effect of NGFR100W and VEGFA in promoting angiogenesis, we used HUVECs to mimic angiogenesis. A conditioned medium containing both proteins was prepared for the assay. Scratch assays with HUVECs showed that migration rates were around 0.1 for the control and EGFP groups, 0.2 to 0.3 for the single-agent group, and 0.3 to 0.4 for the combination treatment group (Fig. 3A and C), indicating faster migration with combination therapy after 12 h. In tube formation assays, after 4 h of conditioned medium treatment, the combination group reached 16,000 µm in tube length, surpassing the 13,000–14,000 µm in single-agent groups and 12,000–13,000 µm in control and EGFP groups (Fig. 3B and D). These results suggest that NGFR100W and VEGFA together enhance angiogenesis and tube formation.
Figure 3.
Combined application of VEGFA and NGFR100W effectively promotes angiogenesis and neurite outgrowth. A: Representative images of scratch assay migration results under different treatments in HUVEC cells. HUVEC cells were incubated with conditioned medium, containing control medium without target protein, EGFP, VEGFA, NGFR100W, and VEGFA+NGFR100W (left to right). After a 12-h treatment, the scratch widths were photographed (bottom). The initial scratch width at 0 h was taken as control (top). Scale bar, 500 µm. B: Representative images of tube formation assay results in HUVEC cells incubated with control medium without target protein, EGFP, VEGFA, NGFR100W, and VEGFA+NGFR100W (left to right) for 4 h. Scale bar, 500 µm. C: Quantitative analysis of scratch assay results in HUVEC cells corresponding to A. Error bars represent the SD (n = 5). P values were calculated using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. D: Quantitative analysis of tube formation assay results in HUVEC cells corresponding to B. Error bars represent the SD (n = 4). P values were calculated using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001. E: Representative images of primary cultured DRG cells from db/db mice (left) and db/m mice (right). DRG cells were cultured in conditioned media containing control without target protein, EGFP, VEGFA, NGFR100W, and VEGFA+NGFR100W (top to bottom) for 48 h. Immunofluorescence staining was performed using tubulin βIII. Scale bar, 100 µm. F: Quantitative analysis of E. Results were normalized to cell counts. Error bars represent the SD (db/m group, n = 15; db/db group, n = 20). P values were calculated using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To assess the effects of NGFR100W and VEGFA on axonal promotion under diabetic conditions, DRGs were isolated from 22-week-old healthy control mice and diabetic mice for primary cell culture. Neurite outgrowth was assessed with conditioned media from various treatments. The combination group showed an average neurite length of 3,500 ± 500 µm, compared with 2,500 ± 500 µm in single-agent groups and 2,000 µm in control and EGFP groups (Fig. 3E and F, left). Similar results were observed in healthy mice (Fig. 3E and F, right), indicating the combination treatment has a more pronounced effect on neurite growth.
To assess the effects of combined NGFR100W and VEGFA treatment on DRG growth in db/db mice, primary DRG cells were isolated from 22-week-old db/db mice treated with the combination therapy and cultured in vitro. Neurite outgrowth assay showed that neurite lengths of treated db/db DRG neurons were similar to those of untreated db/db and db/m control mice (Supplementary Fig. 3), indicating that local growth factor supplementation does not significantly alter the intrinsic growth properties of DRG neurons in vivo.
Neuroprotective and Perfusion Effects of NGFR100W and VEGFA in Diabetic Mice
Previous studies show that NGFR100W, unlike NGFWT, does not induce thermal or mechanical hyperalgesia in mice. To assess whether this property was preserved in combination therapy, we intramuscularly injected empty LNP, NGFWT+VEGFA mRNA-LNP, or NGFR100W+VEGFA mRNA-LNP into the plantar muscle of healthy mice. Pain thresholds were measured 24 h postinjection. The mechanical pain thresholds of the empty LNP group and the NGFR100W+VEGFA group were both 4.4 ± 0.1 g, while the NGFWT+VEGFA group had a significantly lower threshold of 4.1 ± 0.1 g. Similarly, thermal pain latency was 8.75 s for empty LNP, 8.25 s for NGFR100W+VEGFA, and 6.0 s for NGFWT+VEGFA (Supplementary Fig. 4A). NGFWT+VEGFA significantly reduced pain thresholds, while NGFR100W+VEGFA maintained thresholds similar to the empty LNP group, indicating that VEGFA coadministration does not compromise the “painless” properties of NGFR100W.
To assess the restorative effects of NGFR100W and VEGFA on peripheral blood vessels and nerves in diabetic mice with peripheral neuropathy, we employed BKS-db/db mice, a type 2 diabetes model (17). These mice had significantly higher body weight and blood glucose levels than db/m controls (Supplementary Fig. 4B). After confirming diabetes onset, LNPs containing NGFR100W and VEGFA mRNA were injected into the plantar region (Fig. 4A). At 16 weeks of age, blood flow was measured using Doppler imaging, while nerve function was assessed with the von Frey test for mechanical pain thresholds and the hot plate test for thermal pain thresholds. The average blood flow in db/m mice can exceed 600, while it is only around 300 in db/db mice (Fig. 4B). Thermal pain thresholds measured by the hot plate test showed no significant differences between db/db and db/m mice at 16 weeks (Fig. 4C, left). However, the von Frey test revealed that db/db mice had a lower mechanical pain threshold, indicating impaired blood supply and heightened sensitivity due to peripheral nerve damage (Fig. 4C, right). After baseline measurements, LNPs were administered (Fig. 4A), and blood flow was recorded. In the control group, blood flow steadily declined between weeks 16 and 19, with a drop from 300 to 150–250, and single-agent treatments failed to reverse this trend (Fig. 4D and Supplementary Fig. 4C). In contrast, the combination treatment maintained blood flow and showed significant improvements by week 19 (Fig. 4E). These indicated that, in the highly inflammatory pathological state of diabetes (27), while NGF or VEGFA alone cannot reverse microvascular damage in diabetes, the combination treatment offers better vascular protection.
Figure 4.
Effects of combined application of VEGFA and NGFR100W on blood flow perfusion and nerve function. A: Diagram of administration and detection protocol in diabetic mice. Created with BioRender.com. B: Representative images (left) and quantitative analysis (right) of baseline blood flow measured at 16 weeks in db/db mice and db/m mice. Error bars represent the SD (db/m group, n = 10; db/db group, n = 50). P values were calculated using two-sided t test. ****P < 0.0001. C: Baseline pain threshold was measured using hot plate (left) and von Frey monofilaments (right) in db/db mice and db/m mice at 16 weeks of age. Error bars represent the SD (n = 5). P values were calculated using two-sided t test. *P < 0.05; ns, not significant. D: Representative images of blood flow assessment during the administration process. PBS, empty LNP, VEGFA mRNA-LNP, NGFR100W mRNA-LNP, and VEGFA+NGFR100W mRNA-LNP (left to right) were injected into the mouse paw pads. The blood flow in the paw pads was measured on the first day, the 14th day, and the 21st day postinjection. E: Quantitative analysis of blood flow supply status in different groups of mice at 21 days postadministration. Error bars represent the SD (n = 10). P values were calculated using one-way ANOVA. *P < 0.05, **P < 0.01. F: Following the injection of PBS, empty LNP, VEGFA mRNA-LNP, NGFR100W mRNA-LNP, and VEGFA+NGFR100WmRNA-LNP into the mouse paw pads according to A. At week 22, thermal (left) and mechanical (right) pain thresholds in mice were measured using the von Frey test and hot plate test, respectively. Error bars represent the SD (n = 10). P values were calculated using one-way ANOVA. *P < 0.05. ***P < 0.001, ****P < 0.0001; ns, not significant.
To assess neovascularization, we performed femoral artery ligation and excision surgery on healthy mice, followed by treatment (Supplementary Fig. 4D). While the single-drug treatment using either NGFR100W or VEGFA promote neovascularization and partially accelerate blood flow recovery in healthy mice (Supplementary Fig. 4E and F), the combination treatment group restored nearly 90% of blood flow by the seventh day, showing significantly faster recovery. This suggests that combining NGFR100W and VEGFA enhances neovascularization more effectively than single treatments.
To assess nerve function in mice, the hot plate and von Frey tests were used to measure thermal and mechanical pain thresholds. At 22 weeks, thermal pain latency was 7.03 ± 1 s in db/m mice, 9.50 ± 2 s in PBS-treated db/db mice, and 7.86 ± 2 s in NGFR100W+VEGFA-treated db/db mice (Fig. 4F, left). Mechanical pain thresholds were 4.39 ± 0.1 g for db/m mice, 4.66 ± 0.2 g for PBS-treated db/db mice, and 4.38 ± 0.2 g for NGFR100W+VEGFA-treated db/db mice (Fig. 4F, right). These results suggest that the combined treatment helps maintain normal pain sensitivity, potentially protecting against nerve damage.
Neuroprotective and Vascular Effects of NGFR100W and VEGFA mRNA at the Tissue Level
In both behavioral and blood flow assessments, the combined mRNA treatment facilitated the recovery of nerve function and blood supply. To assess structural changes and protection of distal limbs, we analyzed tissue samples posttreatment. CD31 IHC staining of plantar tissues showed a significant increase in vascular density in the combination group (Fig. 5A and C). While single treatments increased vascular density, their efficacy was relatively limited. Histological analysis showed severe vascular loss in the control group, while the combination group maintained vessel density, especially in muscle interstitial spaces and small dermal vessels—key areas affected by DPN (Fig. 5A). Despite increased vascular density, single treatments failed to sustain local blood flow.
Figure 5.
Protective and safety effects of combined application of VEGFA and NGFR100W. A: Representative images of CD31 IHC staining of plantar tissues from 22-week-old db/m and db/db mice. At this time point, db/db mice received 12 doses of PBS, empty LNP, VEGFA mRNA-LNP, NGFR100W mRNA-LNP, or VEGFA+NGFR100W mRNA-LNP injected into the paw pads, while untreated db/m mice served as controls. Scale bar, 200 µm. B: PGP9.5 immunofluorescence staining of frozen footpad skin sections from 22-week-old db/m and db/db mice, labeling IENFs. At this time point, db/db mice received 12 doses of PBS, empty LNP, VEGFA mRNA-LNP, NGFR100W mRNA-LNP, or VEGFA+NGFR100W mRNA-LNP injected into the paw pads, while untreated db/m mice served as controls. White arrows indicate IENFs. Scale bar, 50 µm. C: Quantitative analysis was performed on the number of CD31-positive blood vessels in the different groups shown in A. Error bars represent the SD (n = 6). P values were calculated using one-way ANOVA. **P < 0.01, ****P < 0.0001. D: Quantitative analysis of IENFD was performed for the different groups shown in B. Error bars represent the SD (n = 6). P values were calculated using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. E: Representative images of H-E staining in the kidneys (top), livers (middle). and hind paw (bottom) of db/m mice and db/db mice after the administration of PBS, empty LNP, VEGFA mRNA-LNP, NGFR100W mRNA-LNP, and VEGFA+NGFR100W mRNA-LNP (left to right). Scale bar, 200 µm; n = 3.
Immunofluorescence staining with PGP9.5 on frozen sections of the footpad skin revealed significantly higher density of intraepidermal nerve fibers (IENFs) in the group receiving the combined NGFR100W and VEGFA application (Fig. 5B and D), indicating that improved nerve growth or enhanced neuroprotective effects reduced the loss of nerve fibers. While NGFR100W alone also promoted nerve growth, its effect on restoring nerve function was less effective than the combined treatment. To further assess nerve regeneration, we examined the expression of GAP43, a recognized marker of neural repair, via immunofluorescence. The PBS and empty LNP groups showed minimal GAP43-positive nerve fibers, while the treatment groups, particularly those receiving the combination of NGFR100W and VEGF, demonstrated marked nerve regeneration (Supplementary Fig. 5A). Quantitative analysis showed that the combination therapy group had a significantly higher GAP43-positive IENFD (23.4 fibers per mm) compared with monotherapy groups, while the control group had about 11 fibers per mm (Supplementary Fig. 5B), highlighting the enhanced regenerative effect of the combination therapy.
To assess safety, inflammation after LNP injection was monitored in db/db mice treated with empty LNP or NGFR100W+VEGFA mRNA-LNP on days 1 and 14. H-E staining showed localized neutrophil infiltration at the injection site in both groups, decreasing but persisting by day 14 (Supplementary Fig. 5C). Serum IL-6 and TNF-α levels remained undetectable (Supplementary Fig. 5D and E), indicating no systemic inflammation. After the 22-week treatment, histological analysis of major organs showed no structural damage, and serum ALT, AST, blood urea nitrogen, and creatinine levels showed no significant differences between treatment groups (Fig. 5E and Supplementary Fig. 5H), indicating no hepatic or renal damage. These results suggest that mRNA-LNP treatment is relatively safe, with no significant toxic side effects.
Discussion
The nervous and vascular systems are interdependent, sharing growth factors that regulate their development and homeostasis. In DPN, vascular damage reduces blood flow, depriving nerves of oxygen and nutrients, while nerve dysfunction worsens microvascular health. The inability of single-factor supplementation (either NGF or VEGFA alone) to fully reverse microvascular damage in DPN highlights the need for a combined therapeutic approach. VEGFA primarily supports vascular repair by promoting angiogenesis, enhancing local blood flow, and exerting neuroprotective effects through the VEGFR-2 receptor (28,29). These functions are essential for improving the microvascular environment and indirectly aiding nerve repair. In contrast, NGF mainly drives nerve regeneration and protects neurons, with limited effects on vascular repair. The combined use of NGF and VEGFA leverages their complementary roles: VEGFA enhances blood flow to support regenerating nerves, while NGF promotes nerve growth and induces angiogenesis (30), further amplifying VEGFA’s vascular benefits. This synergistic approach addresses the multifaceted pathology of DPN more effectively than either factor alone, with VEGFA contributing more significantly to the vascular repair aspect. Supporting this, previous studies using DNA hydrogels loaded with VEGFA and NGF showed enhanced peripheral nerve repair (31). In our study, the combination of “painless” NGFR100W and VEGFA mRNA significantly promoted microvascular regeneration and nerve fiber growth in diabetic mice, demonstrating dual protective effects.
To investigate the molecular mechanism by which NGFR100W uncouples neurotrophic and nociceptive pathways, we compared the transcriptomes of NGFR100W- and NGFWT-transfected PC12 cells. Pain-related genes, such as Efnb2, Rgs5, and Npy, were downregulated in NGFR100W-transfected cells, suggesting a potential reduction in pain signaling. Meanwhile, nerve growth–related genes like Gap43 were also downregulated compared with NGFWT, indicating a slightly diminished nerve growth–promoting effect. GAP43 is a recognized marker of nerve regeneration associated with neuronal growth, development, and synaptic remodeling, although its detailed mechanisms remain unclear. It is known to be upregulated during small-fiber regeneration in the skin and following NGF treatment. However, GAP43 is also implicated in pain perception (32), as elevated levels have been observed in patients with diabetic neuropathy experiencing chronic pain and in models of postoperative pain under high-fat diets (33). In our study, NGFWT-treated PC12 cells showed a significant increase in GAP43 expression, while NGFR100W-treated cells displayed a lower, yet still elevated, level compared with the untreated group. Despite no significant difference in axon elongation between the two treatments, NGFR100W exhibited a slightly weaker effect. Given that NGFWT strongly induces pain at therapeutic doses while NGFR100W has been shown to be painless, we speculate that the milder upregulation of GAP43 by NGFR100W may help balance nerve regeneration and pain mitigation. This balance potentially allows NGFR100W to support nerve growth while minimizing pain-related effects, making it a promising candidate for therapeutic applications.
In this study, we conducted a three-dimensional structure-based design of NGF mutants for potentially beneficial mutant screen. We found that p75NTR interaction was critical for NGF-guided neurite outgrowth. Notably, NGFR100W showed higher expression and greater axonal growth than other mutants, demonstrating the value of in vitro–transcribed mRNA for rapid design and validation. Additionally, local delivery of NGFR100W and VEGFA mRNA via LNPs protected the peripheral microvasculature and nerve fibers in diabetic mice. However, there are limitations to our approach. The delivery of empty LNPs exacerbated neurovascular dysfunction, likely due to localized inflammation at the injection site. In previous studies, systemic inflammatory markers temporarily increased 6 h post–LNP injection, returning to baseline within 24 h (14), suggesting that LNP-induced inflammation is transient. Despite detectable mRNA-LNP proteins for up to 72 h, extended dosing was insufficient to prevent damage, leading us to use multiple injections. These findings highlight the need to reduce the immunogenicity of mRNA and delivery platforms (e.g., LNPs) and to enhance mRNA stability and half-life for advancing mRNA-based protein replacement therapies.
mRNA-based protein replacement therapies have demonstrated significant potential across various disease areas, from rare genetic disorders like propionic acidemia (34) and hemophilia A (35) to metabolic conditions like Fabry disease (36), highlighting the versatility, efficacy, and safety of this technology. In diabetes, Gan et al. (9) demonstrated that mRNA-based therapies, evidenced by sustained local VEGFA protein expression and improved blood flow without severe adverse events, are feasible and safe in humans. Our findings in a type 2 diabetes animal model showed enhanced local blood flow and improved nerve fiber density with NGFR100W and VEGFA mRNA-LNP, targeting both neurotrophic support and vascularization to address DPN’s dual pathology. For clinical translation, this therapy could use microneedles or subcutaneous injections, with efficacy measured by laser Doppler and von Frey testing. This dual-action approach could be a promising targeted therapy for DPN in type 2 diabetes patients.
In summary, our study demonstrated that a combined treatment using NGFR100W and VEGFA maintained blood flow supply and protected peripheral nerve fiber function in diabetic mice. Advances in mRNA biology and delivery platforms may further enhance IVT mRNA’s potential in protein replacement therapies, while structure-based protein design combined with mRNA technology could open new avenues for disease treatment.
This article contains supplementary material online at https://doi.org/10.2337/figshare.28930736.
Article Information
Acknowledgments. The authors thank Professor He Wang, Yuwen Zhang, and Dr. Tan Li, from Fudan University, as well as Professors Lin Lu, Dr. Yang Dai, and Linshuang Mao, Shiyu Ji, and Yutong Hou from Ruijin Hospital for providing the laser Doppler blood flow meter (MoorLDI2-HIR, Moor Instruments) and related technical support. We also thank Professors Qi Han and Jin Cheng from Shanghai Jiao Tong University School of Medicine for their technical assistance in the extraction and culture of DRG.
Duality of Interest. Y.X. is a cofounder of RNAcure Biopharma. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.X. and W.X. conceived the idea, designed the study, and directed the project. W.W. and Z.Y. prepared the immunofluorescence on frozen sections of mouse plantar skin and conducted the mouse mechanical nociception behavioral experiments. W.W. and Y.Z. prepared the mRNA-LNP and performed its related quality control. X.Y. conducted the protein structure analysis and the construction and expression analysis of mutant mRNA. W.W. completed all other experiments and data analysis. W.Y. provided conceptual advice. W.W. wrote the manuscript and revised according to the comments of X.Y., Y.X., and W.X. All authors were asked to comment on the manuscript. W.Y. and Y.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (82471394) and the Fundamental and Translational Research on mRNA-Based Therapeutics (JZ202409).
Contributor Information
Yingjie Xu, Email: xuyingjie@shsmu.edu.cn.
Wei Xu, Email: xw11246@rjh.com.cn.
Supporting information
References
- 1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013;93:137–188 [DOI] [PubMed] [Google Scholar]
- 3. Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging targets in type 2 diabetes and diabetic complications. Adv Sci (Weinh) 2021;8:e2100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol 2019;7:938–948 [DOI] [PubMed] [Google Scholar]
- 5. Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol 2021;17:400–420 [DOI] [PubMed] [Google Scholar]
- 6. Cernea S, Raz I. Management of diabetic neuropathy. Metabolism 2021;123:154867. [DOI] [PubMed] [Google Scholar]
- 7. Andreassen CS, Jakobsen J, Flyvbjerg A, Andersen H. Expression of neurotrophic factors in diabetic muscle–relation to neuropathy and muscle strength. Brain 2009;132:2724–2733 [DOI] [PubMed] [Google Scholar]
- 8. Pérez-Gutiérrez L, Ferrara N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat Rev Mol Cell Biol 2023;24:816–834 [DOI] [PubMed] [Google Scholar]
- 9. Gan L-M, Lagerström-Fermér M, Carlsson LG, et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun 2019;10:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narumi S, Fujita T. Stimulatory effects of substance P and nerve growth factor (NGF) on neurite outgrowth in embryonic chick dorsal root ganglia. Neuropharmacology 1978;17:73–76 [DOI] [PubMed] [Google Scholar]
- 11. McArthur JC, Yiannoutsos C, Simpson DM, et al. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology 2000;54:1080–1088 [DOI] [PubMed] [Google Scholar]
- 12. Yang W, Sung K, Zhou F, et al. Targeted mutation (R100W) of the gene encoding NGF leads to deficits in the peripheral sensory nervous system. Front Aging Neurosci 2018;10:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung K, Ferrari LF, Yang W, et al. Swedish nerve growth factor mutation (NGFR100W) defines a role for TrkA and p75NTR in nociception. J Neurosci 2018;38:3394–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu X, Yang Z, Zhang Y, et al. Lipid nanoparticle delivery of chemically modified NGFR100W mRNA alleviates peripheral neuropathy. Adv Healthc Mater 2023;12:e2202127. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Xi X, Yu H, et al. Chemically modified in-vitro-transcribed mRNA encoding thrombopoietin stimulates thrombopoiesis in mice. Mol Ther Nucleic Acids 2022;29:657–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan KA, Hayes JM, Wiggin TD, et al. Mouse models of diabetic neuropathy. Neurobiol Dis 2007;28:276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. Ilar J 2014;54:259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–462 [DOI] [PubMed] [Google Scholar]
- 19. Kochi T, Imai Y, Takeda A, et al. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One 2013;8:e84047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellingman AA, Bastiaansen AJNM, de Vries MR, et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg 2010;40:796–803 [DOI] [PubMed] [Google Scholar]
- 21. He X-L, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 2004; 7304:870–875 [DOI] [PubMed] [Google Scholar]
- 22. AlphaFold3 - why did Nature publish it without its code? Nature 2024;629:728. [DOI] [PubMed] [Google Scholar]
- 23. Moser KV, Reindl M, Blasig I, Humpel C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res 2004;1017:53–60 [DOI] [PubMed] [Google Scholar]
- 24. Cattin A-L, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015;162:1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999;19:5731–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol 2003;140:614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Antony V, Wang Y, Wu G, Liang G. Pattern recognition receptor-mediated inflammation in diabetic vascular complications. Med Res Rev 2020; Nov40:2466–2484 [DOI] [PubMed] [Google Scholar]
- 28. Anttila V, Saraste A, Knuuti J, et al. Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol Ther 2023;31:866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calvo PM, Hernández RG, de la Cruz RR, Pastor AM. VEGF is an essential retrograde trophic factor for motoneurons. Proc Natl Acad Sci U S A 2022;119:e2202912119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarnawski AS, Ahluwalia A. The critical role of growth factors in gastric ulcer healing: the cellular and molecular mechanisms and potential clinical implications. Cells 2021;10:1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Liu Y, Zhou L, et al. XT-type DNA hydrogels loaded with VEGF and NGF promote peripheral nerve regeneration via a biphasic release profile. Biomater Sci 2021;9:8221–8234 [DOI] [PubMed] [Google Scholar]
- 32. Galosi E, La Cesa S, Di Stefano G, et al. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain 2018;22:1727–1734 [DOI] [PubMed] [Google Scholar]
- 33. Song Z, Xie W, Strong JA, et al. High-fat diet exacerbates postoperative pain and inflammation in a sex-dependent manner. Pain 2018;159:1731–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koeberl D, Schulze A, Sondheimer N, et al. Interim analyses of a first-in-human phase 1/2 mRNA trial for propionic acidaemia. Nature 2024;628:872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramaswamy S, Tonnu N, Tachikawa K, et al. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc Natl Acad Sci U S A 2017;114:E1941–E1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu X, Yin L, Theisen M, et al. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wild-type mice, Fabry mouse model, and wild-type non-human primates. Am J Hum Genet 2019;104:625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.