Abstract
Vlad tepes (vltm651) is one of only five “bloodless” zebrafish mutants isolated through large-scale chemical mutagenesis screening. It is characterized by a severe reduction in blood cell progenitors and few or no blood cells at the onset of circulation. We now report characterization of the mutant phenotype and the identification of the gene mutated in vltm651. Embryos homozygous for the vltm651 mutation had normal expression of hematopoietic stem cell markers through 24 h postfertilization, as well as normal expression of myeloid and lymphoid markers. Analysis of erythroid development revealed variable expression of erythroid markers. Through positional and candidate gene cloning approaches we identified a nonsense mutation in the gata1 gene, 1015C → T (Arg-339 → Stop), in vltm651. The nonsense mutation was located C-terminal to the two zinc fingers and resulted in a truncated protein that was unable to bind DNA or mediate GATA-specific transactivation. A BAC clone containing the zebrafish gata1 gene was able to rescue the bloodless phenotype in vltm651. These results show that the vltm651 mutation is a previously uncharacterized gata1 allele in the zebrafish. The vltm651 mutation sheds new light on Gata1 structure and function in vivo, demonstrates that Gata1 plays an essential role in zebrafish hematopoiesis with significant conservation of function between mammals and zebrafish, and offers a powerful tool for future studies of the hematopoietic pathway.
The hematopoietic compartment derives from a stem cell that differentiates through successive commitment steps to produce erythroid, myeloid, and lymphoid lineages (1). Studies in the zebrafish have shown many parallels between zebrafish hematopoiesis and that of other vertebrates (2). Primitive zebrafish hematopoiesis takes place ventral to the notochord in a region termed the intermediate cell mass (ICM) that forms from lateral plate mesoderm. Myeloid precursors appear to originate in an independent site from ventral mesoderm near the head (3). After 12 days post-fertilization, the kidney serves as the major site of hematopoiesis in the zebrafish.
The zebrafish system provides a powerful tool for the analysis of hematopoiesis. External fertilization, transparent embryos, and random mutagenesis and screening techniques allow phenotype-driven, “forward” genetic analyses to be performed (4). Two large-scale chemical mutagenesis screens (5, 6) identified 26 hematopoietic mutants. Five independent mutants, including vltm651, had greatly reduced or absent blood cell numbers, suggesting defects in blood cell specification or differentiation; however, none of the genes involved in these mutations have been published to date.
A number of transcription factors in the GATA family have been shown to play important roles in mammalian hematopoietic development (7). The family members are characterized by a highly conserved zinc finger region responsible for binding a consensus (A/T)GATA(A/G) site. GATA1, the first described gene in this family, is expressed in hematopoietic cells of the erythroid lineage, and has been shown to be required for both primitive and definitive erythropoiesis in mouse gene disruption studies (8, 9).
We now report the characterization of the “bloodless” phenotype in vltm651 homozygous mutants. We show that vltm651 has a defect in the erythroid pathway with intact hematopoietic stem cell, myeloid, and lymphoid development. Through positional and candidate gene cloning, we identified a nonsense mutation in gata1, which results in a truncated protein that is unable to bind DNA or mediate transactivation through a GATA site. Therefore, vltm651 defines a previously uncharacterized, null allele for gata1 and demonstrates the essential function of gata1 in zebrafish hematopoiesis.
Materials and Methods
Whole-Mount in Situ Hybridization.
vltm651 fish were bred and maintained as described (10) under an approved National Institutes of Health animal use protocol. Embryos were staged as described (10). Whole-mount RNA in situ hybridization was performed as described (11). RNA antisense probes were generated with UTP-digoxigenin according to the manufacturer's instructions (Roche Diagnostics). scl (12), gata1 (13), band3 (14), alas2 (15), sptb (16) (pBK-CMV BS12), eα1 and eα2 globin plasmids, and pu.1 were kindly provided by Leonard Zon (Harvard Medical School, Boston). ikaros was generously provided by Chris Amemiya (Boston University), rag1 (17) by Catherine Willett (Massachusetts Institute of Technology, Boston), and l-plastin by Bernard Thisse (IGBMC, Strasbourg, France; ref. 3).
Centromeric Linkage Analysis and Diploid Mapping.
Gynogenetic diploid embryos were generated from vltm651 heterozygous females for centromeric linkage as described (10). DNA was prepared from individual embryos at 4 days postfertilization and used in pools of 30 embryos, or as individuals for PCR. Heterozygote incrosses of vltm651 fish were performed to obtain embryos for diploid mapping. DNA from wild-type and mutant embryos was prepared from individual embryos as described above. Microsatellite markers (Invitrogen) were used in PCR with Platinum Genotype Tsp Taq polymerase (Invitrogen) according to the manufacturer's specifications.
Zebrafish RNA and DNA Preparation.
To prepare DNA from single embryos, embryos were placed into 100 μl of DNA lysis buffer (10 mM Tris⋅HCl (pH 8.0)/50 mM EDTA (pH 8.0)/200 mM NaCl/0.5% SDS/0.5 mg/ml proteinase K) in 96-well format and incubated overnight at 50°C. The preparation was purified through Sephacryl S-400 (Amersham Pharmacia) columns, made by placing Sephacryl into each well of a MultiScreen filtration plate (Millipore, MAGVS2210). RNA was prepared using RNA-stat 60 (Tel-Test, Friendswood, TX).
PCR, Sequencing, and gata1 Genotyping.
RNA was reverse transcribed into cDNA by using a cDNA cycle kit (Invitrogen). PCR was performed on cDNA or genomic DNA with multiple primers (described in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org) spanning the gata1 gene. PCR products were purified using a PCR purification kit (Qiagen), followed by sequencing (ACGT, Northbrook, IL).
PCR was performed on DNA with primers Arg-339-S and -AS (see Supporting Text), using an annealing temperature of 60°C to genotype embryos for the gata1 (1015C → T) mutation. Arg-339-S is located in intron 5 so that the PCR reaction amplified only from genomic DNA, eliminating potential PCR contamination from plasmid. The PCR product was digested with TaqI at 65°C for 4 h, followed by gel electrophoresis in a 2% agarose gel.
Microinjection and Rescue Analysis.
gata1 containing BACs were obtained from a zebrafish BAC library (Incyte Genomics) screened by PCR using primers (see Supporting Text) to the most 5′ region of the gata1 promoter and to the 3′ UTR.
BACs positive by PCR screening with both primer sets were isolated. Additional PCR using primers spanning the gata1 gene confirmed the presence of the entire gata1 gene. Embryos from heterozygote incrosses were injected at the one-cell stage, essentially as described (10), with 100 pg of a BAC containing the gata1 gene. BAC DNA for injection was isolated using a Qiagen protocol for large plasmid isolation with the Qiagen maxi-prep kit. Injected embryos were harvested at 21 somites and used for in situ hybridization.
Electrophoretic Mobility Shift Assay.
Zebrafish gata1, gata1 (Arg-339 → STOP), or luciferase (luc) were used for in vitro translation using a transcription-translation-coupled reticulocyte lysates (Promega) according to the manufacturer's instructions. Aliquots of translated protein generated in the presence of 35S-labeled methionine were analyzed on a 4–12% Bis-Tris gel (Invitrogen) and visualized by autoradiography. Proteins translated without radioactive methionine were used for electrophoretic mobility shift assay. Electrophoretic mobility shift assay was performed essentially as described (18), using 2 μl of lysate per reaction with 1 μg poly[dI][dC] and 32P-labeled probe (50,000 cpm) per reaction. 32P-labeled double-stranded probes were generated as described (18) by annealing sense (S) and antisense (AS) primers (described in Supporting Text) based on the sequences from ref. 19.
Transactivation Assay.
NIH 3T3 cells were transfected using Superfect (Qiagen) according to the manufacturer's instructions. Three micrograms of pCS2+-gata1, gata1 (1015C → T), or the positive control, mouse Gata1 (pXmGata1) and 2 μg of reporter constructs M1α or M6α, containing one or six copies of the GATA-binding site of the mouse α1-globin promoter, respectively, and the human GH gene (courtesy of M. Weiss and S. Orkin; ref. 19) were transfected. Supernatants were collected after 48 h and assayed for levels of GH by using an RIA per the manufacturer's protocol (Nichols Institute, San Juan Capistrano, CA). All values were within a standard curve determined using control proteins supplied with the assay kit.
Results
Phenotypic Characterization of vltm651.
vltm651 homozygotes are identified by an almost complete lack of circulating blood cells at the onset of circulation (5). Heterozygote incrosses produce approximately 25% bloodless progeny, consistent with a recessive mode of inheritance. Hematopoietic gene expression in vltm651 was examined by whole-mount RNA in situ hybridization of embryos from vltm651/+ incrosses. A difference in expression of a given marker in mutant embryos is indicated when a unique pattern of expression is observed in approximately 25% of the embryos from these incrosses. Expression of lmo2, scl, and cbfb (20), all markers of hematopoietic stem cells, was normal in all embryos at 21 somites through 24 hours postfertilization (hpf) (data not shown). scl was initiated and expressed normally until approximately 24 hpf in all embryos. However, by 26 hpf scl expression in the ICM was lost from mutants with only a small amount of residual staining in the posterior ICM, where hematopoietic progenitors are thought to reside (Fig. 1b), that disappeared by 30 hpf (data not shown), in contrast to the continued expression of scl in wild-type siblings (Fig. 1a; refs. 12 and 21). Expression of gata2, a marker of hematopoietic progenitors, was normal in the mutants with expression through approximately 24 hpf, followed by a decline to undetectable levels as in wild-type siblings (data not shown).
Figure 1.
Expression of hematopoietic genes in vltm651 by whole-mount RNA in situ hybridization. Embryos from vltm651 heterozygote incrosses are shown in lateral views with the heads to the left. Embryo stages are 26 hpf (a–d) and 21 somites (e–l). In situ analyses were performed on embryos from at least three independent crosses. Wild-type sibling embryos (WT) are shown in a, c, e, g, i, and k, and mutant embryos (vltm651) are shown in b, d, f, h, j, and l with scl (a and b), gata1 (c and d), globin eα2 (e and f), band3 (g and h), sptb (i and j), and alas2 (k and l) RNA probes. An arrow in b shows residual staining in the posterior ICM with scl. Arrows in l demarcate the reduced staining in the ICM with alas2.
Development of myeloid and lymphoid lineages was examined using genes expressed in these lineages. RNA in situ hybridization of myeloid markers [pu.1, l-plastin (3), and c/ebp1 (11)] revealed no differences between wild-type and mutant embryos throughout development (data not shown). Lymphoid cells were examined using ikaros (22) and rag1 (17) at 72 hpf. Both these genes were expressed normally in the thymus, consistent with intact lymphoid cell development (data not shown). We conclude that vltm651 mutant embryos had a specific loss of circulating erythroid cells, apparent by visual inspection of embryos after the onset of circulation, with intact expression of stem cell, myeloid, and lymphoid markers.
Analysis of erythroid gene expression revealed variable effects of the vltm651 mutation on different erythroid genes. gata1 expression was initiated normally and expressed through 24 hpf (data not shown), but was greatly reduced or absent by 26 hpf (Fig. 1d), in contrast to wild-type embryos in which strong ICM staining continued (Fig. 1c). All gata1 staining was absent from mutants by 27–28 hpf. Expression of two embryonic α-globin genes (ea1 and ea2) was retained in the mutants from 14 somites through 24 hpf (shown at 21 somites, Fig. 1 e and f). In addition, one hematopoietic klf gene, biklf (23) or klf4 (24), was expressed normally in the mutants through 24 hpf. There is no known EKLF ortholog in the zebrafish; however, biklf is a klf family member that is expressed in a pattern similar to gata1 (24) and is required for erythroid cell differentiation (25). Expression of the α-globin genes and biklf was lost in mutants, but not wild type siblings by 26 hpf, coincident with the loss of gata1 expression at this stage in the mutants (data not shown). A number of other erythroid markers were greatly reduced or absent throughout development in the mutants. From 21 somites through 24 hpf, band3 (Fig. 1 g and h) and sptb (Fig. 1 i and j) were absent in the mutants. alas2 (Fig. 1 k and l) was severely reduced with a greatly reduced signal in the ICM in the mutants compared with wild-type siblings.
Identification of a Nonsense Mutation in the gata1 Locus in vltm651.
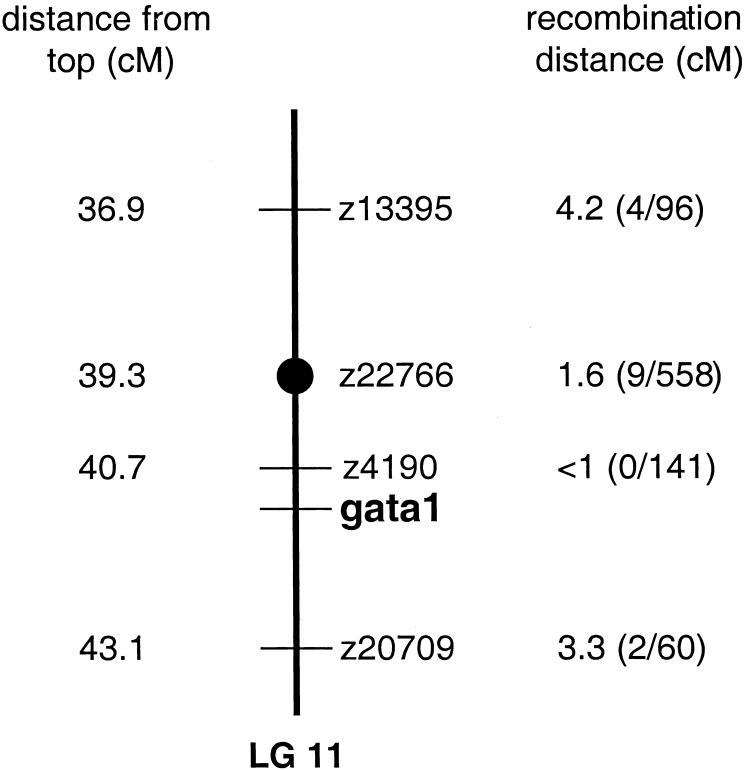
The vltm651 gene was mapped to linkage group 11 (LG11) through half-tetrad analysis (26) by using gynogenetic diploid embryos, which are homozygous for each maternal chromosome without contribution from paternal chromosomes. They were generated from vltm651 heterozygous females by using early pressure to block the second meiotic division, and mutant embryos were identified by visual inspection.
In the absence of crossing-over in first meiosis, 50% of gynogenetic diploid embryos from a heterozygote female will display a mutant phenotype. When crossing-over occurs, only phenotypically wild-type progeny are generated. The probability of crossing-over is proportional to the distance from the centromere. As a result, the distance of the mutant gene from the centromere can be calculated using the following equation: distance in centimorgans (cM) = 50[1–2(M/n)], where M is the number of mutant embryos and n is the total number of embryos (26). Using this equation, a distance of 2.3–3.2 cM from the centromere was calculated based on the findings in gynogenetic diploid embryos from two heterozygous females (#1, M = 236 and n = 496; #2, M = 47 and n = 90). Based on these findings, centromeric linkage analysis was expected to yield a linkage quickly and efficiently.
Pooled DNA from mutant and wild-type embryos was analyzed with centromeric markers to assess linkage. The centromeric markers, z13395, z22766, and z1393, all located on LG11, demonstrated linkage with the vltm651 gene in this analysis. z22766, z13395, and additional markers in the region of these markers on LG11 were used to genotype individual embryos from heterozygote incrosses. Based on the recombination distances calculated (Fig. 2), the vltm651 locus was localized between markers z4190 and z20709. The gene encoding the hematopoietic transcription factor, gata1, mapped to this location (27) and was therefore a candidate gene (Fig. 2).
Figure 2.
Mapping of the vltm651 mutation to LG11. Initial linkage of the vltm651 locus to the centromeric marker z22766 on LG11 was achieved through bulk segregant analysis of gynogenetic diploid embryos from vltm651 heterozygote females. z22766 and additional markers were then mapped in relation to the vltm651 locus by using individual diploid embryos from a vltm651 map cross. The distances of the markers from the top of the map, taken from http://zebrafish.mgh.harvard.edu/cgi-bin/ssr_map/view_lg.cgi (last updated July 2001), are shown in the left column in centimorgans (cM). Recombination distances between a marker and the vltm651 locus are shown on the right of the map in cM. The fractions in parentheses indicate the number of recombinant events per total number of meioses analyzed for each marker.
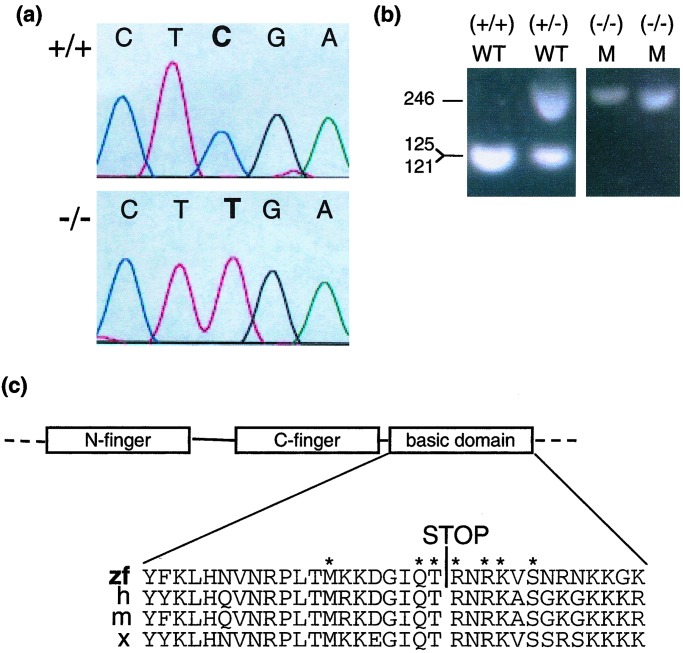
gata1 was amplified using PCR from both cDNA and genomic DNA from vltm651 embryos. The entire ORF of gata1 and the intron–exon junctions from genomic DNA were sequenced and analyzed. A C → T transition at position 1015, resulting in a nonsense mutation at Arg-339, was identified in vltm651 embryos (Fig. 3a). The C → T transition destroyed a TaqI restriction enzyme site at position 1014, allowing genotyping to be performed by PCR and TaqI digestion of the PCR product (Fig. 3b). By this analysis of phenotypically wild-type embryos from heterozygote incrosses, there were no recombinants at the gata1 locus in 774 meioses (387 embryos). However, an analysis of phenotypically mutant embryos revealed 4 heterozygous embryos per 265 embryos assessed. An analysis of embryos generated from two crosses between vltm651/+ fish and wild-type siblings (+/+) revealed 5 bloodless embryos of a total of 871 embryos including 3 heterozygotes and two homozygous wild-type by genotyping. Thus, the discordance between genotype and phenotype in mutant embryos is likely due to a low-frequency defect in the background of vltm651 that results in a bloodless phenotype.
Figure 3.
Detection of a nonsense point mutation in gata1. (a) The chromatograms show sequence of PCR products derived from a homozygous wild-type embryo (+/+) (Upper) and a homozygous mutant embryo (−/−) (Lower) for the gata1 gene nt 1013–1017. (b) Genotyping of vltm651 embryos. PCR products using primers Arg-339-S and Arg-339-AS synthesized from embryo DNA of vltm651 incrosses were digested with TaqI and electrophoresed on a 2% agarose gel. After TaqI digestion, mutant alleles appear as 246-bp and wild-type alleles as 121- and 125-bp products, which migrate together in the gel shown. Phenotypically wild-type embryos (WT) identified by presence of circulating blood and phenotypically mutant (M) embryos identified by absence of circulating blood are shown. (c) A schematic representation of the N-finger, C-finger, and basic domain of Gata1 and a sequence alignment of the basic domain from different species zebrafish (zf), human (h), mouse (m), and Xenopus (x) are shown with the Arg-339 → Stop mutation identified by a STOP. The asterisks mark the amino acids that make direct contact with the minor groove of DNA (29).
The conceptual translation of gata1 (1015C → T) predicts a nonsense mutation at Arg-339, truncating the last 79 aa of Gata1. Based on homology with mammalian Gata1 proteins, the two highly conserved zinc fingers will remain intact in this truncated protein (Fig. 3c). The truncation removes 13 aa of the basic domain that is highly conserved between all species examined to date (Fig. 3c). An additional nonconserved 66 aa downstream are also absent in the truncated Gata1.
A number of other sequence differences were also found in both the mutant and wild-type alleles when compared with the previously published gata1 sequence (GenBank accession no. U18311). Most of the sequence differences occurred at the wobble position of the codon and would not result in a protein coding change. However, two DNA differences from the previously published sequence were noted in both wild-type and mutant alleles that resulted in amino acid differences in the conceptual translation of the published sequence (nucleotide 331C → G), Pro-111 → Ala and (nucleotide 752A → G) Gln-251 → Arg. Analysis of the amino acid sequence in comparison to GATA1 from other species showed that in the Pro-111 → Ala position, there are glycine, alanine, leucine, or serine residues in other species, but no proline residues. In the Gln-251 → Arg position, other species studied to date all encode an arginine. Therefore, the two DNA differences identified here are consistent with sequences in other species, and are unlikely to be responsible for the vltm651 phenotype.
Functional Analysis of Gata1 (Arg-339 → Stop).
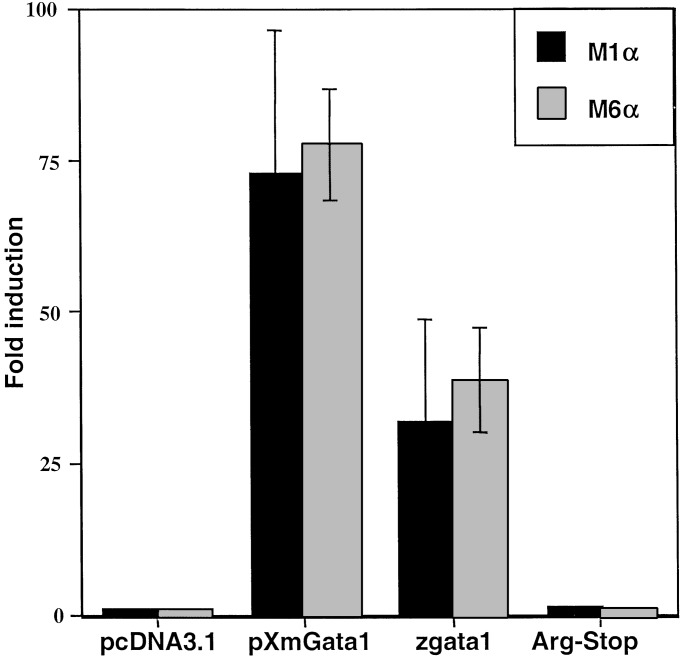
To analyze the functional consequences of the nonsense mutation in Gata1, zebrafish gata1 or gata1 (1015C → T) cDNA constructs were transfected into NIH 3T3 cells with Gata1 reporter plasmids to assay transactivation. Gata1 (Arg-339 → Stop) was unable to activate mammalian promoter constructs containing one (M1α) or six copies (M6α) of a Gata1 binding site from mouse α1-globin gene (19). Wild-type Gata1, however, was able to transactivate the mammalian promoter over 30-fold, comparable to mouse Gata1 (Fig. 4).
Figure 4.
Transactivation analysis of Gata1 and Gata1 (Arg-339 → Stop). The indicated constructs (on the x axis) were transfected into NIH 3T3 cells with either the M1α (black) or the M6α (gray) Gata1 reporter gene, GH levels in the medium were measured and calculated using a standard curve. All values were within the linear range for the assay. The experiments shown are each representative of three independent experiments with samples transfected in triplicate and GH levels measured in duplicate. The y axis represents fold inductions relative to the activation of the reporters by using a pcDNA3 vector control. pcDNA3.1, control empty vector; pXmGata1, mouse Gata1; zgata1, zebrafish gata1; Arg-Stop, gata1 (1015C → T).
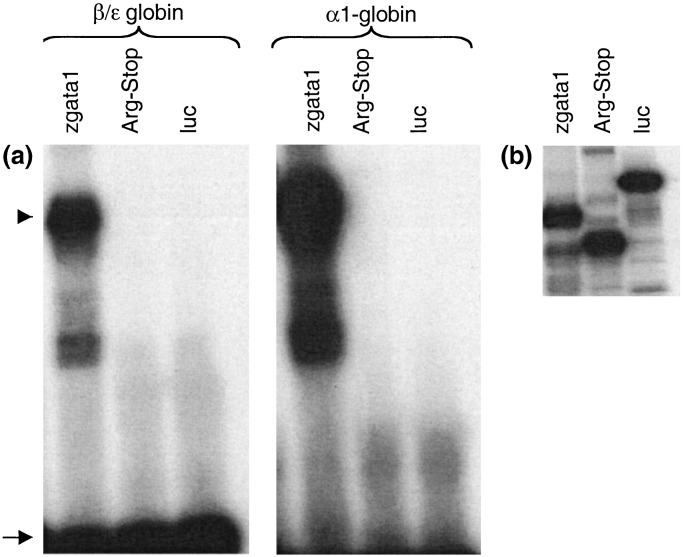
Although the zinc finger domains required for DNA binding were intact in Gata1 (Arg-339 → Stop), a 13-aa downstream basic region that has been demonstrated in vitro to participate in DNA binding was absent. DNA binding ability of Gata1 (Arg-339 → Stop) was tested in an electrophoretic mobility shift assay. In vitro-translated wild-type zebrafish Gata1 bound mouse β/ɛ globin and α1-globin promoter sites, whereas Gata1 (Arg-339 → Stop) demonstrated no DNA binding (Fig. 5a).
Figure 5.
DNA-binding activity of Gata1 and Gata1 (Arg-339 → STOP). (a) Unlabeled in vitro translated proteins, wild-type zebrafish Gata1 (zgata1), zebrafish Gata1 (Arg-339 → Stop) (Arg-Stop), and negative control protein, luciferase (luc), were used in an electrophoretic mobility shift assay with the indicated 32P-labeled probes, β/ɛ globin or α1-globin. The DNA–protein complexes are seen as slower migrating bands, marked by an arrowhead. The arrow marks free probe, demonstrating excess of DNA in the mixture. (b) Polyacrylamide gel electrophoresis of in vitro translated proteins generated in the presence of [35S]methionine and visualized by autoradiography.
Rescue of Mutant Phenotype by Injection of a gata1 BAC in vltm651.
To assess the ability of the gata1 gene to rescue the vltm651 phenotype, either mRNA or cDNA encoding Gata1 or Gata1 (Arg-339 → Stop) was injected into one-cell embryos generated through vltm651/+ incrosses. Embryos injected with gata1 mRNA, or cDNA under a cytomegalovirus promoter, had severe morphologic abnormalities before the onset of circulation. Morphologic abnormalities were apparent with as little as 5 pg of injected gata1 mRNA or 25 pg of cDNA. This phenotype may have resulted from widespread activation of GATA-responsive promoters. In contrast, injection of 25 pg of gata1 (1015C → T) mRNA or 75 pg of cDNA did not produce any morphologic abnormalities in injected embryos. The absence of defects in the gata1 (1015C → T) injected embryos is consistent with production of a nonfunctional protein.
Given the defects caused by misexpression of gata1 in early embryos, a gata1-containing BAC identified through screening a zebrafish BAC library by PCR was used for injections to assess rescue. In this approach, gata1 is expressed under its own promoter, so that hematopoietic-specific expression is expected. The phenotype analyzed for rescue was band3 positivity by in situ hybridization at 21 somites through 24 hpf because vltm651 mutant embryos are easily identified by absolute absence of band3 expression. Genotyping could not be performed because of the injection of a gata1-containing BAC, so the number of band3-negative and band3-positive embryos were counted. The control samples (uninjected or injected with an irrelevant PAC) had 23.4% band3 negative embryos, which was consistent with a recessive mode of inheritance by χ2 analysis (Fig. 6d). In contrast, samples injected with the gata1 BAC had only 14.9% band3-negative embryos, which deviated significantly from recessive inheritance (P < 0.005). Therefore, the gata1 containing BAC was able to rescue some of the mutant embryos. In addition, injection of DNA usually results in mosaic expression of the injected DNA. Whereas control embryos were either band3 positive throughout the ICM (Fig. 6a) or completely negative (Fig. 6b), mosaic expression of band3 was apparent in gata1-BAC-injected band3-positive embryos (Fig. 6c), possibly resulting from mosaic rescue of mutant embryos by the injected gata1-BAC.
Figure 6.

Phenotypic rescue of vltm651 embryos by injection of gata1-BAC DNA. (a–c) Lateral views of the ICM regions of embryos stained by in situ hybridization with band3 (×400 magnification). (a) Uninjected phenotypically wild-type embryo (band3-positive), (b) uninjected phenotypically mutant embryo (band3-negative), (c) gata1-BAC injected embryo showing mosaic expression of band3. (d) χ2 analysis of injection data. The null hypothesis tested was that injections did not change the inheritance of the mutant phenotype (i.e., recessive or 1:3 mutant to wild-type ratio). The data shown indicate the number of embryos obtained for each phenotype, and the numbers shown in parentheses are the number of embryos expected for each phenotype assuming recessive inheritance.
Discussion
In this report, we have identified a zebrafish gata1 mutation in a “bloodless” zebrafish mutant, vltm651. The nonsense mutation in vltm651 is a previously uncharacterized allele of gata1. While the truncated protein retains both intact zinc fingers, in vitro DNA binding and transactivation are completely abolished. This result confirms a role of the basic domain carboxy-terminal to the zinc finger domain for DNA binding. Previous studies have demonstrated that the basic region of chicken Gata1 is essential for DNA binding in vitro (28). Furthermore, the basic domain of chicken Gata1 was shown to wrap around and bind the minor groove of DNA by NMR spectroscopy (29). In that study, seven amino acids from the basic region made direct contact with residues in the minor groove of DNA. These 7 aa are conserved in Gata-1, -2, and -3 in species ranging from Xenopus and zebrafish through humans, consistent with a crucial role for these amino acids. In vltm651, 4 of the 7 aa (marked by asterisks in Fig. 3c) are absent in the truncated protein (Arg-339 → Stop). Further, a truncation deletion starting 3 aa C-terminal to the vltm651 mutation in the murine Gata-1 protein (and therefore missing only two of the amino acids that contact DNA) retained in vitro DNA binding ability, but had markedly reduced transactivation and differentiation capability in a megakaryocytic cell line (30). Thus, vltm651 adds in vivo support for the importance of this domain to DNA binding and transactivation.
In vltm651, the presence of gata1 mRNA seen by in situ hybridization demonstrates that transcription of gata1 occurs at relatively normal levels in the absence of Gata1 function and shows that nonsense mediated decay does not play a significant role in vltm651. In mouse and chicken, multiple GATA sites in the GATA1 promoter have been shown to be required for erythroid-specific expression (31–33), suggesting possible autoregulation. However, analysis of a mouse Gata1 promoter/lacZ transgene in a Gata1 null background showed the ability of Gata1 to be expressed in primitive red blood cells in the absence of Gata1 (33). More recently, positive autoregulation by ectopic expression of gata1 was demonstrated in zebrafish (34). Further studies using vltm651 could help to elucidate the regulation of zebrafish gata1.
Analysis of stem cell and erythroid gene expression in vltm651 showed relatively normal RNA expression of a number of genes believed to be regulated by Gata1 in mammals. Mammalian Scl (35), multiple globin genes (36), and Gata1 itself all contain GATA sites in their promoter and/or enhancer regions. In vltm651, scl, eα1, and eα2 globin, and gata1 were expressed at normal levels until approximately 24 hpf. These results are similar to findings of normal globin expression both in Gata1 null mice at E9.5 (37) and in Gata1 null definitive proerythroblasts (38). Proerythroblasts derived from in vitro differentiation of Gata1 null ES cells have also demonstrated relatively normal levels of scl transcript (38).
These results could be explained through compensation by Gata2, as has been hypothesized in the mouse (38). Alternatively, the above genes may not require Gata1 for expression. In mouse Gata1 null definitive proerythroblasts, the levels of Gata2 were markedly elevated (20-fold) in a quantitative PCR analysis (38), leading to the hypothesis that Gata2 may compensate for loss of Gata1. In contrast, RNA in situ hybridization of gata2 in vltm651 appeared normal in our studies, without any significant elevation. However, up-regulation of gata2 within a subset of cells such as the proerythroblasts would be difficult to detect by whole-mount in situ hybridization or RNA analysis using RNA derived from whole embryos. Unfortunately, it is currently not feasible to culture hematopoietic cells from zebrafish to analyze isolated cell populations, as was done in mice.
In contrast to the normal expression of the erythroid genes described above, band3, sptb, and alas2 expression were absent or severely reduced in vltm651, at all stages examined. In mammals, sptb (39) and alas2 (40–42) genes have been shown to have GATA binding sites within their promoters that are required for transactivation in tissue culture. In the zebrafish, embryonic globin gene expression was observed earlier (at 3–6 somites) than sptb, alas2, and band3 (at 16 somites) (S.E.L., unpublished results). Therefore, the results may indicate erythroid precursor differentiation arrest in vltm651 before expression of the more terminal erythroid markers (43), sptb, alas2, and band3. Alternatively, the absence of band3, sptb, and alas2 expression may reveal an absolute requirement for Gata1 for their transcriptional regulation.
The mutation in vltm651 represents a novel gata1 allele and is the first gata1 mutation identified in the zebrafish. The characterization of vltm651 demonstrates significant functional conservation between mammalian and zebrafish hematopoiesis. Further, the finding of a gata1 nonsense allele in the zebrafish will allow future studies of the hematopoietic pathway, using the strengths of the zebrafish model.
Supplementary Material
Acknowledgments
We thank Mitchell Weiss, Stuart Orkin, Chris Amemiya, Catherine Willett, Bernard Thisse, and Leonard Zon for plasmids. We appreciate insightful discussions with Mitchell Weiss and David Bodine and insights on Gata1 structure from John Bushweller.
Abbreviations
- hpf
hours postfertilization
- ICM
intermediate cell mass
- LG
linkage group
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dzierzak E. Ann NY Acad Sci. 1999;872:256–264. doi: 10.1111/j.1749-6632.1999.tb08470.x. [DOI] [PubMed] [Google Scholar]
- 2.Bahary N, Zon L I. Stem Cells. 1998;16:89–98. doi: 10.1002/stem.160089. [DOI] [PubMed] [Google Scholar]
- 3.Herbomel P, Thisse B, Thisse C. Development (Cambridge, UK) 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 4.Talbot W S, Hopkins N. Genes Dev. 2000;14:755–762. [PubMed] [Google Scholar]
- 5.Weinstein B M, Schier A F, Abdelilah S, Malicki J, Solnica-Krezel L, Stemple D L, Stainier D Y, Zwartkruis F, Driever W, Fishman M C. Development (Cambridge, UK) 1996;123:303–309. doi: 10.1242/dev.123.1.303. [DOI] [PubMed] [Google Scholar]
- 6.Ransom D G, Haffter P, Odenthal J, Brownlie A, Vogelsang E, Kelsh R N, Brand M, van Eeden F J, Furutani-Seiki M, Granato M, et al. Development (Cambridge, UK) 1996;123:311–319. doi: 10.1242/dev.123.1.311. [DOI] [PubMed] [Google Scholar]
- 7.Weiss M J, Orkin S H. Exp Hematol. 1995;23:99–107. [PubMed] [Google Scholar]
- 8.Pevny L, Lin C S, D'Agati V, Simon M C, Orkin S H, Costantini F. Development (Cambridge, UK) 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D'Agati V, Orkin S H, Costantini F. Nature (London) 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 10.Westerfield M. The Zebrafish Book. 3rd Ed. Eugene, OR: Univ. of Oregon Press; 1995. [Google Scholar]
- 11.Lyons S E, Shue B C, Oates A C, Zon L I, Liu P P. Blood. 2001;97:2611–2617. doi: 10.1182/blood.v97.9.2611. [DOI] [PubMed] [Google Scholar]
- 12.Liao E C, Paw B H, Oates A C, Pratt S J, Postlethwait J H, Zon L I. Genes Dev. 1998;12:621–626. doi: 10.1101/gad.12.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detrich H W r, Kieran M W, Chan F Y, Barone L M, Yee K, Rundstadler J A, Pratt S, Ransom D, Zon L I. Proc Natl Acad Sci USA. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paw B H. Blood Cells Mol Dis. 2001;27:62–64. doi: 10.1006/bcmd.2000.0354. [DOI] [PubMed] [Google Scholar]
- 15.Brownlie A, Donovan A, Pratt S J, Paw B H, Oates A C, Brugnara C, Witkowska H E, Sassa S, Zon L I. Nat Genet. 1998;20:244–250. doi: 10.1038/3049. [DOI] [PubMed] [Google Scholar]
- 16.Liao E C, Paw B H, Peters L L, Zapata A, Pratt S J, Do C P, Lieschke G, Zon L I. Development (Cambridge, UK) 2000;127:5123–5132. doi: 10.1242/dev.127.23.5123. [DOI] [PubMed] [Google Scholar]
- 17.Willett C E, Zapata A G, Hopkins N, Steiner L A. Dev Biol. 1997;182:331–341. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- 18.Duckett C S, Gedrich R W, Gilfillan M C, Thompson C B. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin D I, Orkin S H. Genes Dev. 1990;4:1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 20.Blake T, Adya N, Kim C, Oates A C, Zon L, Chitnis A, Weinstein B M, Liu P P. Blood. 2000;96:4178–4184. [PubMed] [Google Scholar]
- 21.Gering M, Rodaway A R, Gottgens B, Patient R K, Green A R. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen J D, Zapata A G. Immunol Rev. 1998;166:199–220. doi: 10.1111/j.1600-065x.1998.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara A, Dawid I B. Mech Dev. 2000;97:173–176. doi: 10.1016/s0925-4773(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 24.Oates A C, Pratt S J, Vail B, Yan Y, Ho R K, Johnson S L, Postlethwait J H, Zon L I. Blood. 2001;98:1792–1801. doi: 10.1182/blood.v98.6.1792. [DOI] [PubMed] [Google Scholar]
- 25.Kawahara A, Dawid I B. Curr Biol. 2001;11:1353–1357. doi: 10.1016/s0960-9822(01)00398-0. [DOI] [PubMed] [Google Scholar]
- 26.Johnson S L, Africa D, Horne S, Postlethwait J H. Genetics. 1995;139:1727–1735. doi: 10.1093/genetics/139.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly P D, Chu F, Woods I G, Ngo-Hazelett P, Cardozo T, Huang H, Kimm F, Liao L, Yan Y L, Zhou Y, et al. Genome Res. 2000;10:558–567. doi: 10.1101/gr.10.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omichinski J G, Trainor C, Evans T, Gronenborn A M, Clore G M, Felsenfeld G. Proc Natl Acad Sci USA. 1993;90:1676–1680. doi: 10.1073/pnas.90.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omichinski J G, Clore G M, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl S J, Gronenborn A M. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 30.Visvader J E, Crossley M, Hill J, Orkin S H, Adams J M. Mol Cell Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartzbauer G, Schlesinger K, Evans T. Nucleic Acids Res. 1992;20:4429–4436. doi: 10.1093/nar/20.17.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onodera K, Takahashi S, Nishimura S, Ohta J, Motohashi H, Yomogida K, Hayashi N, Engel J D, Yamamoto M. Proc Natl Acad Sci USA. 1997;94:4487–4492. doi: 10.1073/pnas.94.9.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDevitt M A, Fujiwara Y, Shivdasani R A, Orkin S H. Proc Natl Acad Sci USA. 1997;94:7976–7981. doi: 10.1073/pnas.94.15.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi M, Nishikawa K, Yamamoto M. Development (Cambridge, UK) 2001;128:2341–2350. doi: 10.1242/dev.128.12.2341. [DOI] [PubMed] [Google Scholar]
- 35.Bockamp E O, McLaughlin F, Murrell A M, Gottgens B, Robb L, Begley C G, Green A R. Blood. 1995;86:1502–1514. [PubMed] [Google Scholar]
- 36.Orkin S H. Cell. 1990;63:665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara Y, Browne C P, Cunniff K, Goff S C, Orkin S H. Proc Natl Acad Sci USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss M J, Keller G, Orkin S H. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher P G, Sabatino D E, Romana M, Cline A P, Garrett L J, Bodine D M, Forget B G. J Biol Chem. 1999;274:6062–6073. doi: 10.1074/jbc.274.10.6062. [DOI] [PubMed] [Google Scholar]
- 40.Kramer M F, Gunaratne P, Ferreira G C. Gene. 2000;247:153–166. doi: 10.1016/s0378-1119(00)00103-7. [DOI] [PubMed] [Google Scholar]
- 41.Surinya K H, Cox T C, May B K. J Biol Chem. 1997;272:26585–26594. doi: 10.1074/jbc.272.42.26585. [DOI] [PubMed] [Google Scholar]
- 42.Surinya K H, Cox T C, May B K. J Biol Chem. 1998;273:16798–16809. doi: 10.1074/jbc.273.27.16798. [DOI] [PubMed] [Google Scholar]
- 43.Wickrema A, Koury S T, Dai C H, Krantz S B. J Cell Physiol. 1994;160:417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.