Abstract
Stem cell treatment may enhance erectile dysfunction (ED) in individuals with cavernous nerve injury (CNI). Nevertheless, no investigations have directly ascertained the implications of varying amounts of human umbilical cord-derived mesenchymal stem cells (HUC-MSCs) on ED. We compare the efficacy of three various doses of HUC-MSCs as a therapeutic strategy for ED. Sprague–Dawley rats (total = 175) were randomly allocated into five groups. A total of 35 rats underwent sham surgery and 140 rats endured bilateral CNI and were treated with vehicles or doses of HUC-MSCs (1 × 106 cells, 5 × 106 cells, and 1 × 107 cells in 0.1 ml, respectively). Penile tissues were harvested for histological analysis on 1 day, 3 days, 7 days, 14 days, 28 days, 60 days, and 90 days postsurgery. It was found that varying dosages of HUC-MSCs enhanced the erectile function of rats with bilateral CNI and ED. Moreover, there was no significant disparity in the effectiveness of various dosages of HUC-MSCs. However, the expression of endothelial markers (rat endothelial cell antigen-1 [RECA-1] and endothelial nitric oxide synthase [eNOS]), smooth muscle markers (alpha smooth muscle actin [α-SMA] and desmin), and neural markers (neurofilament [RECA-1] and neurogenic nitric oxide synthase [nNOS]) increased significantly with prolonged treatment time. Masson’s staining demonstrated an increased in the smooth muscle cell (SMC)/collagen ratio. Significant changes were detected in the microstructures of various types of cells. In vivo imaging system (IVIS) analysis showed that at the 1st day, the HUC-MSCs implanted moved to the site of damage. Additionally, the oxidative stress levels were dramatically reduced in the penises of rats administered with HUC-MSCs.
Keywords: cavernous nerve injury, doses, erectile dysfunction, human umbilical cord-derived mesenchymal stem cells
INTRODUCTION
Erectile dysfunction (ED) is a common clinical condition in males, impacting over 150 million men globally, mainly occurring over the age of 40 years.1 The increasing prevalence of hypertension, diabetes, and cardiovascular disease is directly linked to the rising incidence of ED; and by 2025, around 322 million men globally may have varying levels of ED.2 The etiology of ED is complex, including psychological, vascular, neurological, and endocrine function impairments.3 Pelvic surgery (radical prostate cancer surgery) is a common cause of neurologic ED. Surgery inevitably causes cavernous nerve (CN) dissection, burns, and instrument pulling, resulting in cavernous nerve injury (CNI), greatly impacting the patient’s postoperative quality of life. Radical prostatectomy (RP) with preservation of bilateral sexual nerves has been effectively promoted in recent years; nevertheless, the prevalence of postoperative cavernous nerve injury-related erectile dysfunction (CNI-ED) remains at 20%–90%.4 Current treatments such as oral posphodiesterase-5 (PDE-5) inhibitors, vacuum device, and local injection of vasoactive drugs into the penile corpus cavernosum (CC) are sometimes ineffective; therefore, ED after RP remains a persistent issue for the majority of patients in the long term. In addition, other pelvic surgeries, such as radical cystectomy for bladder cancer, radical rectal surgery for rectal cancer, and pelvic trauma, may lead to CNI-ED.5 Therefore, the search for an effective treatment for CNI-ED has become a crucial clinical concern.
Stem cells have multidirectional differentiation potential and paracrine function. Several preclinical investigations have manifested that stem cells have the ability to enhance erectile function, which is expected to be an ideal treatment for CNI-ED.6,7,8,9,10,11,12 Presently, stem cell therapy has emerged as a prominent area of study for treating ED. Recent clinical investigations have manifested that stem cells may effectively treat ED patients following prostatectomy, leading to a significant improvement in erectile function.13,14 Prior investigations have emerged on the potential therapeutic consequences of different ED models and different sources of cells for ED; however, the optimal dose to benefit patients remains unanswered.
This study used a CNI-ED model to ascertain the therapeutic effectiveness of human umbilical cord-derived mesenchymal stem cells (HUC-MSCs). Additionally, we compared the therapeutic effects of various doses of HUC-MSCs and explored the potential therapeutic mechanisms.
MATERIALS AND METHODS
Study design
A total of 175 male Sprague–Dawley (SD) rats, aged 8 weeks, were procured from the Animal Center of Xuzhou Medical University (Xuzhou, China). The animal operations and care were executed in compliance with the Animal Research: Reporting of In Vivo Experiment (ARRIVE) criteria and were accepted by the Institutional Animal Care and Use Committee of Xuzhou Medical University (Approval No. 202302T006). All methods were performed in accordance with the relevant guidelines and regulations. The animals were housed in a regulated environment maintained at a temperature of 23°C ± 2°C and subjected to a light cycle consisting of 12 h of illumination followed by 12 h of darkness. They were provided with a normal food and access to water.
The rats were administered 3% pentobarbital sodium to induce anesthesia. A surgical cut was performed in the middle of the abdomen to uncover the prostate gland and locate the CN and major pelvic ganglion (MPG) on both sides of the prostate gland’s dorsolateral aspect. A total of 35 SD rats were allocated in a random manner to the sham-operated group (sham control), CNs were exposed and isolated without damaging and then rats received an intracavernous injection (ICI) with 0.1 ml of phosphate-buffered solution (PBS).
In the remaining 140 rats, the isolated CN was clamped with hemostatic forceps at 5 mm distal to MPG for 1 min.15 On the day of operation, rats with bilateral CNI were randomly assigned to four equal groups: ICI with 0.1 ml PBS (crush control), 1 × 106 cells in ICI with 0.1 ml PBS (crush LHUCMSC), 5 × 106 cells in ICI with 0.1 ml PBS (crush MHUCMSC), and 1 × 107 cells in ICI with 0.1 ml PBS (crush HHUCMSC). In each group, five rats were sacrificed on 1 day, 3 days, 7 days, 14 days, 28 days, 60 days, and 90 days postsurgery, respectively. Tissues were harvested to ascertain the HUC-MSCs implications on penile tissues. The erectile function test was performed before the rats were killed. Umbilical cord hematopoietic stem cells were provided by Zhongyuan Xiehe Cellular Gene Engineering Co (Tianjin, China).
A total of 12 male CNI-ED rats were employed for the in vivo imaging investigations. The participants were allocated to the crush LHUCMSC group based on the postoperative day endpoints, which were categorized as 0, 1 day, 3 days, 7 days, 14 days, and 28 days postsurgery. After modeling utilizing labeled HUC-MSCs, two animals were allocated to each time endpoint, resulting in a total of 12 rats. In vivo imaging for cell tracking was performed to observe the survival and distribution of HUC-MSCs. Euthanasia in rats used CO2 inhalation.
Cell tracking
The labeled with HUC-MSCs were tracked in vivo utilizing an in vivo imaging system (IVIS), Lumina II (Revvity, Shanghai, China). Briefly, the substrate (D-luciferin sodium salt, 150 mg kg−1) was administered into the peritoneal cavity of rats. The animals were placed in a camera unit, and local images were obtained. IVIS pictures were acquired 5 min after the injection of the substrate. Furthermore, pictures were obtained promptly after the injury at the time intervals of 5 min, 20 min, and 45 min, as well as on 1 day, 3 days, 7 days, and 14 days postsurgery. Animals in the control group did not receive injections of labeled HUC-MSCs or substrate.
Erectile function evaluation
The rats were anesthetized using a 3% solution of pentobarbital sodium, and a surgical incision was made along the midline of the abdomen to fully expose the bladder and prostate. Bilateral MPG and CN can be found in both dorsolateral regions of the prostate gland. Before placing the CC, the tube was flushed with a sterile heparin saline solution (200 U ml−1). A 25-G needle was inserted in a parallel direction to the path of the penile CC, pushing about 5 mm into the CC. The other side was linked to a bioelectrical signal collection system (Chengdu Taimeng Software Co., Chengdu, China). The CN was connected to a bipolar stimulating electrode positioned 2–4 mm away from the MPG. The stimulation settings were set as follows: the wave amplitude of 5.0 V, a frequency of 20 Hz, a wave width of 5 ms, and a period of 1 min. Three electrical stimulations were performed on both CNs, and the ICPmax amplitude was calculated from the baseline values. During CN electrical stimulation, the mean arterial pressure (MAP) was quantified by inserting a 25 G needle into the left carotid artery. The greatest ratio between ICP and MAP was computed to standardize the fluctuations in systemic blood pressure. Following erectile function testing, the rats were euthanized using a bilateral thoracotomy approach, after which the midportion of the penis was removed for histological analysis.
Immunofluorescence staining
Penile tissues were collected to make 10 μm frozen slices. To detect fluorescence, penis slices were fixed with methanol at 4°C for 15 min and the sections were washed with PBS and closed with a mixture of bovine serum albumin and Triton. Penis samples were covered with antibodies against endothelial nitric oxide synthase (eNOS; 1:200), neuronal NOS (nNOS; 1:200), neurofilament (NF; 1:500), alpha-smooth muscle actin (α–SMA; 1:400), rat endothelium cell antigen-1 (RECA-1; 1:300), and desmin (1:200), and all antibodies were purchased from Servicebio Biotechnology Co. (Wuhan, China). Subsequently, the sections were washed with PBS and incubated with conjugated antibody for Alexa Fluor 555 (Servicebio Biotechnology Co.). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI; Servicebio Biotechnology Co.). The fluorescent microscope (BX51; Olympus, Tokyo, Japan) was employed to capture the digital pictures.
Transmission electron microscopy (TEM)
TEM was deployed to analyze and evaluate the ultrastructural changes in the penile CC. Penile midsection tissues (1 mm3) were collected and fixed using an electron microscope fixative at 4°C for at least 2 h. The tissue samples underwent dehydration employing a sequence of ethanol gradients, infiltrated into a propylene oxide mixture overnight, and embedded in resin. The ultrathin sections, measuring 70 nm in thickness, were treated with a 2% solution of uranyl acetate in alcohol until saturation, followed by staining with lead citrate for 15 min each. The sections were then left to dry overnight at the room temperature. At least 30 micrographs from each rat were randomly selected for the analysis.
Masson’s trichrome staining
Slides were stained with Masson’s trichrome stain for the histological examination of connective tissues and smooth muscles. The ratio between smooth muscle and collagen was employed as a quantitative measure of the extent of cavernous fibrosis.
Oxidative stress (OS) measurement
The concentrations of malondialdehyde (MDA) and superoxide dismutase (SOD) were indicative of the levels of oxidative and antioxidant activity, respectively. MDA in the serum was detected by colorimetric thiobarbituric acid assay using the Lipid Peroxidation MDA Assay Kit (Labgic Technology Co., Hefei, China) based on the manufacturer’s recommendations. Similarly, SOD in the serum was assessed by visible spectrophotometry using the total SOD Assay Kit with 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (Labgic Technology Co.).
Measurement of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) levels
NO levels were measured utilizing a total NO content test kit (Labgic Technology Co.), while cGMP levels were monitored using an enzyme-linked immunosorbent assay (ELISA) kit for cGMP from Enzo Life Sciences (Farmingdale, NY, USA). The procedures were executed in strict adherence to the manufacturer’s prescribed methodology. All specimens were repeated, and the outcomes were presented as percentages obtained as opposed to the crush control.
Statistical analyses
The images were processed employing Image-Pro Plus software (version 6.0; Media Cybernetics, Silver Spring, MD, USA), and the data were analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). All the data collected from the experiment using SPSS 21.0 software (IBM Corporation, Armonk, NY, USA) were processed, and the outcomes were presented as the mean ± standard deviation (s.d.). A two-way analysis of variance (ANOVA) was used to ascertain the disparities across groups, followed by post hoc comparisons employing the Tukey–Kramer test. Statistical significance was established by setting the threshold at P < 0.05.
RESULTS
HUC-MSC survival and distribution after local injections
The animals were imaged using Renilla luciferase bioluminescence at various postoperative time points. Following the injection, the bioluminescence emitted by the tagged HUC-MSCs was promptly detected inside the CC using IVIS. At 1 day postsurgery, several rats substrate injections manifested the presence of HUC-MSCs in the vesicles and periprostatic area. Furthermore, cells were not observed in the penis or pelvis on 3 days postsurgery. The same was observed for 7 days and 14 days postsurgery. In addition, the control animals did not exhibit bioluminescence (Figure 1).
Figure 1.
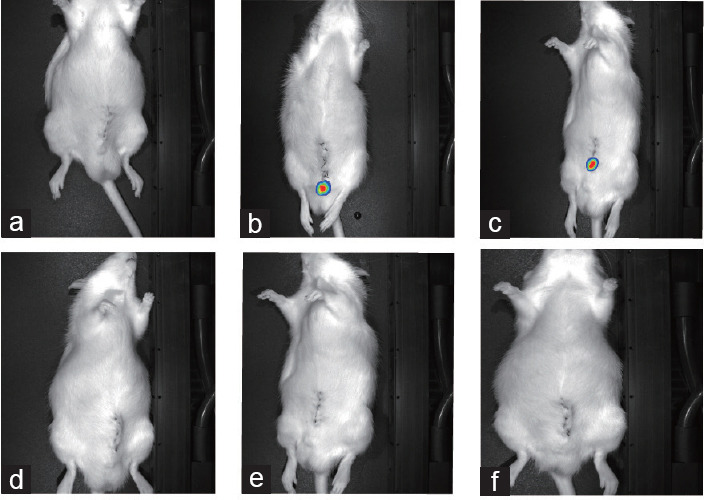
Images from in vivo imaging systems. Images of (a) control animals, (b) immediately after surgery (10 min), (c) 1 day postsurgery, (d) 3 days postsurgery, (e) 7 days postsurgery, and (f) 14 day postsurgery. No bioluminescence was detected in d, e, and f.
HUC-MSCs could improve CNI-induced ED
The ratio of the ICP and MAP is utilized as a measure of erectile function. Electrical stimulation was employed to ascertain erectile function in all groups by targeting the distal CN 2–4 mm distal to MPG (Figure 2 and Supplementary Figure 1 (188.4KB, tif) ). The ICPmax/MAP in the crush control group exhibited a significant hindrance relative to the sham group, indicating a compromised erectile function following bilateral CNI. In CNI-ED rats, the ICP-to-MAP ratio in the crush control group mitigated significantly at 1 day postsurgery, reached a minimum at 14 days postsurgery, progressively recovered over time, and returned to normal at 60 days and 90 days postsurgery. The ICP-to-MAP ratio manifested a significant improvement in the rats of the HUC-MSCs group, indicating that ICI injection of HUC-MSCs could elevate ED. The ICP-to-MAP ratio became increasingly pronounced with the prolongation of the treatment time and basically recovered to normal at 28 days postsurgery. Moreover, no significant difference was found compared to the sham group at 60 days and 90 days postsurgery (P > 0.05). After 4 weeks of HUC-MSC treatment, ICPmax showed the highest penile blood and the best ICPmax/MAP ratio among all treatment groups. A comparison of pre- and posttreatment times for the same treatment groups exhibited an increase in ICPmax in all treatment groups, suggesting that the duration of treatment may be positively related to improvement in penile blood (P < 0.05). There was no significant variation in the therapeutic efficacy of various dosages of HUC-MSCs on CNI-ED rats. Moreover, there was no significant disparity in MAP across the groups (all P > 0.05).
Figure 2.

ICP and MAP were recorded for the 4 groups (crush control, crush LHUCMSC, crush MHUCMSC, and crush HHUCMSC) at 7 different time points (1 day, 3 days, 7 days, 14 days, 28 days, 60 days, and 90 days postsurgery). Crush control: bilateral cavernous nerve crush and intracavernous injection of phosphate-buffered saline; crush LHUCMSC: bilateral cavernous nerve crush and intracavernous injection of 1 × 106 HUC-MSCs; crush MHUCMSC: bilateral cavernous nerve crush and intracavernous injection of 5 × 106 HUC-MSCs; crush HHUCMSC: bilateral cavernous nerve crush and intracavernous injection of 1 × 107 HUC-MSCs. ICP: intracorporeal pressure; MAP: mean arterial pressure.
HUC-MSCs improved histological changes in the CC
Smooth muscle and collagen in the cavernous tissue of the rat penis were identified and quantified by Masson’s staining (Figure 3 and Supplementary Figure 1 (188.4KB, tif) ). In the sham group, the SMC/collagen ratio (mean ± s.d.) was 66.8% ± 0.7%, while in the crush control group, the smooth muscle-to-collagen ratio (mean ± s.d.) was 23.9% ± 12.4%, which was significantly different (P < 0.05). The SMC/collagen ratio (mean ± s.d.) in the penile tissues of rats in the crush LHUCMSC group was 34.4% ± 12.3%, which was significantly greater than the ratio seen in the crush control group (P < 0.05). The SMC/collagen ratio (mean ± s.d.) was 39.4% ± 15.7% in the crush HHUCMSC group; however, no significant difference was found among crush LHUCMSC, crush MHUCMSC, and crush HHUCMSC groups (all P > 0.05).
Figure 3.

Treatment improved the smooth muscle-to-collagen ratio and smooth muscle content in the corpus cavernosum. Masson trichrome staining of smooth muscle (red) and collagen (blue) tissue. Scale bar = 100 µm. The groups’ description is the same as that shown in Figure 2.
HUC-MSCs improved the ultrastructure of penile tissue on TEM
TEM revealed the ultrastructural features of penile tissue cells. In the penile CC of CNI-ED rats, endothelial and smooth muscle cells (Figure 4) and the plasma membrane were damaged; the cytoplasm was condensed; and the nuclei were condensed. There is less damage to various cell types in the penile tissue after HUC-MSC treatment (Figure 5). A certain degree of difference in smooth muscle cell damage in each group, crush control group had relatively obvious smooth muscle cell damage compared with sham control group, with large bulging cell membrane, uneven distribution of intracellular myofilaments, large aggregation to form dense bands, and obvious swelling and vacuolization of organelles (Figure 5a). Some degree of difference in fibroblasts, with the crush LHUCMSC group having relatively the least cellular damage compared to the crush control group, and the cellular results being blurred, organelle-rich, and swollen relative to sham control group (Figure 5b). Some degree of difference is visible among the three groups of vascular endothelial cells, with relatively obvious vascular endothelial damage in the crush control group, with cellular consolidation, intracellular matrix condensation, and high electron density, and most of the organelles are obviously swollen and aggregated, and a significant improvement in the LHUCMS control group (Figure 5c).
Figure 4.

Immunofluorescence showed positive expression of neurogenic nitric oxide synthase. Scale bar = 100 µm. The groups’ description is the same as that shown in Figure 2.
Figure 5.

The ultrastructural changes of HUC-MSCs therapy on (a) smooth muscle cells, (b) fibroblasts, and (c) endothelial cells of rat penile corpus cavernosum were observed by the electron microscopy. Sham control: sham operation and intracavernous injection of phosphate-buffered saline; the other groups’ description is the same as that shown in Figure 2. Scale bar = 1 µm.
Effect of HUC-MSCs on NO and cGMP activities
We deployed ELISA kits to quantify the amounts of NO and cGMP in the penises of rats. The findings demonstrated that NO and cGMP generation was severely inhibited in the penises of CNI-ED rats but enhanced after stem cell injection (Supplementary Figure 2 (180.2KB, tif) ). Next, immunofluorescence was used to ascertain the amounts of eNOS and nNOS. The NO generation in the penile CC is facilitated by these two enzymes, which maintain an erection in the rat penis. Moreover, the eNOS and nNOS levels were mitigated in the crush control group contrasted with the sham group, and greater in each HUC-MSCs group compared to the sham group (Figure 4 and 6, and Supplementary Figure 1 (180.2KB, tif) ). At 1 day, 3 days, 7 days, 14 days, 28 days, 60 days, and 90 days postsurgery, nNOS expression levels in the dorsal nerve of the penis significantly decreased by 74.0%, 75.4%, 84.3%, 88.0%, 80.3%, 70.1%, and 62.3%, and eNOS expression levels significantly decreased by 88.5%, 92.4%, 90.2%, 86.3%, 72.4%, 60.4%, and 46.3%, respectively, in the crush control group (all P < 0.05). In the crush LHUCMSC group, the decline was 73.5%, 67.4%, 64.3%, 46.4%, 35.4%, and 30.2%, as well as 87.0%, 81.0%, 72.4%, 59.4%, 46.3%, 42.3%, and 34.1%, respectively (all P < 0.05). In the crush HHUCMSC group, the reduction was 70.9%, 61.3%, 45.5%, 47.4%, 20.9%, and 17.4% and 84.9%, 75.2%, 62.7%, 55.9%, 39.2%, 35.7%, and 23.4%, respectively. At 1 day, 3 days, 60 days, and 90 days postsurgery, nNOS expression decreased similarly in the crush and crush + HUC-MSCs groups (all P < 0.05); however, the regeneration rates at 7 days, 14 days, and 28 days postsurgery were significantly different (P < 0.05). Specifically, the disparity between the crush and crush + HUC-MSCs groups at 28 days postsurgery was about twice as large. Crush LHUCMSC, crush MHUCMSC, and crush HHUCMSC groups showed different reductions in nNOS and eNOS expression, and the crush HHUCMSC group exhibited a slight decrease; however, there were no significant differences between the three groups: the crush LHUCMSC group, the crush MHUCMSC group, and the crush HHUCMSC group (all P > 0.05).
Figure 6.

Immunofluorescence showed positive expression of endothelial nitric oxide synthase. Scale bar = 100 µm. The groups’ description is the same as that shown in Figure 2.
Effects of HUC-MSCs on endothelial, smooth muscle, and neuronal cells
The regeneration of endothelial, smooth muscle, and neuronal cells by HUC-MSCs was explored by detecting the amount of each marker in the rat’s penis using immunofluorescence. It was found that in the crush control group, the expression levels of endothelium markers (RECA-1), smooth muscle markers (desmin and α-SMA), and neuronal markers (NF) were lower compared to the sham group. In addition, the levels of endothelium markers (RECA-1), smooth muscle indicators (desmin and α-SMA), and neural markers (NF) were greater in the groups of each MSC than those in the crush control group (P < 0.05; Figure 4 and 6, and Supplementary Figure 1 (188.4KB, tif) and 3 (330.7KB, tif) –6 (317.5KB, tif) ). The expression of various markers was significantly hindered in CN-injured rats than those in sham-operated rats (Figure 4 and 6, and Supplementary Figure 1 (188.4KB, tif) and 3 (330.7KB, tif) –6 (317.5KB, tif) ). Crush HUC-MSCs groups were significantly improved following HUC-MSCs injection compared with the crush control group. At 1 day, 3 days, 60 days, and 90 days postsurgery, the crush control and the HUC-MSCs groups displayed similar decreases in the expression of various markers; nonetheless, the regeneration rates at 7 days, 14 days, and 28 days postsurgery were significantly different (P < 0.05). The distinction between the crush control and HUC-MSC groups at 28 days was significant. There was no significant disparity between the sham and the HUC-MSCs groups (P > 0.05). Furthermore, no significant disparities were identified among the crush LHUCMSC groups, the crush MHUCMSC groups, and the crush HHUCMSC groups (all P > 0.05).
HUC-MSCs inhibited OS in vivo in rats
The SOD and MDA levels were assessed in rats. MDA, which is a hallmark of OS, showed a decrease. The existence of HUC-MSCs in CNI-ED rats resulted in a significant hindrance in MDA concentrations (Supplementary Figure 7 (174.3KB, tif) ). Conversely, SOD functioned as an antioxidant in vivo and manifested contrasting alterations compared to MDA (Supplementary Figure 7 (174.3KB, tif) ). The results found that the expression of intracellular reactive oxygen species (ROS) was significantly elevated in the CNI-ED group and significantly mitigated in the crush LHUCMSC, crush MHUCMSC, and crush HHUCMSC groups (P < 0.05).
DISCUSSION
ED is characterized by a man’s constant failure to produce or maintain an adequate penile erection throughout sexual engagement.16 Cavernous fibrosis after nerve injury poses a challenge in clinically treating patients with CNI-ED, and conventional therapies such as PDE-5 inhibitors are ineffective.17 Stem cell therapy gives new hope for treating CNI-ED. Stem cells extracted from various sources encompassing bone marrow, fat, muscle, placenta, urine, and gingiva have been effectively employed for treating CNI-ED.18,19,20,21,22 Recently, stem cell therapy has become an option for treating ED and has demonstrated the ability to regenerate CNs and stimulate smooth muscle cells.23,24,25 Our study found that HUC-MSCs improved the expression of nNOS, eNOS, NF, α-SMA, RECA-1, and desmin in CNI-ED rats, indicating that stem cells could stimulate regeneration of endothelial, smooth muscle, and neuronal cells and protect against degenerative structural changes in cavernous tissues. Ti et al.26 compared the therapeutic implications of HUC-MSCs and adipose-derived stem cells (ADSCs) on ED in rats with bilateral CNI. They found that HUC-MSCs secreted enriched factors and ADSCs were more effective in restoring erectile function in CNI-ED rats. Cord blood mesenchymal stem cells (CBMSCs) isolated from newborn umbilical cord blood are more primitive and less immunogenic than other adult MSCs. With the current widespread establishment of cord blood banks, HUC-MSCs are readily available. Moreover, HUC-MSCs are naïve stem cells existing in the human body and have been extensively employed in regenerative medicine and for treating various diseases because of their noninvasive collection method, high proliferation rate, and low immunogenicity.27 CNI-ED rats displayed significant improvement in erectile function following HUC-MSC injection without noticeable adverse reactions, such as rejection after injection, thus demonstrating the biological safety of HUC-MSCs. Therefore, treating CNI-ED with HUC-MSC could be a promising therapeutic method.
While the efficacy of stem cell treatment for ED has been shown, the precise mechanism of action remains incompletely comprehended. In order to study the process by which stem cell functions, we initially endeavored to monitor the destiny of HUC-MSCs that were injected.28 We used these tagged cells to investigate cell migration by means of in vivo luminescence of luciferase. IVIS exposed that labeled cells appeared in the CC immediately after injection at 1 day postsurgery in the CC but not at 3 days postsurgery. The combination of rapid migration of HUC-MSCs and cellular visualization suggested that injected HUC-MSCs did not merge into natural tissues through transdifferentiation but instead appeared to provide value by recruiting natural stem cells and paracrine release of trophic factors.29 HUC-MSCs function by migrating to the site of injury; however, the process of fast migration does not have the ability to release enough trophic elements to enable a nearly flawless restoration of erectile function. In addition, the injection of human HUC-MSCs into rats was a xenograft, which could have prevented the transdifferentiation of HUC-MSCs.
Erectile function was measured using the ICPmax/MAP ratio. Kim et al.30 discovered a significant and natural improvement in erectile function 6 months following damage to the CN caused by crushing. Our investigation manifested that the regeneration of the damaged CN requires time, the erectile function of MSCs in all groups recovered gradually over time, and the higher the concentration of cells in the early stage, the shorter the recovery time. As the cell concentration increased, ICP recovery was approximately significant. Aligning with the functional outcomes, the number of nNOS-positive fibers progressively decreased after injury until 14 days postsurgery and progressively increased at 28 days postsurgery.
In the present investigation, the nNOS levels were significantly mitigated in crush control group. Additionally, an elevation in nNOS expression was seen at 28 days postsurgery. These outcomes were in line with the reported alterations in the microscopic ultrastructure and functional outcomes. The observed microscopic alterations were in line with the ICP measurements. At 7 days and 14 days postsurgery, the following observations were made: central nervous system (CNS) axonal loss and demyelination, irregular increase in myelin sheaths and tighter myelin sheath formation in the dorsal nerve of the penis, and less mitochondrial damage, suggesting partial spontaneous nerve recovery.
Penile erection is primarily a NO/cGMP-mediated response that includes nNOS produced by CN terminals and eNOS secreted by endothelial cells.31 When sexual stimulation is transmitted to the cavernous body of the penis via the CN, NO is produced by the nitrifying nerve endings and endothelial cells of the cavernous body of the penis under the activation of nNOS and eNOS, respectively. Studies have shown that the penis of CNI rats exhibits mitigated nNOS protein levels production and activity, resulting in a decline in nNOS, which in turn affects penile cavernous smooth muscle relaxation and erectile function.20,32,33 Furthermore, nNOS enhances the functionality of eNOS by promoting increased blood circulation in the penile region and exerting pressure on the blood vessels. Therefore, when the expression and activity of nNOS are reduced, it leads to a decrease in the production of NO by eNOS. This results in a prolonged state of flaccidity in the penis and inadequate oxygen supply. NO is generated and subsequently diffuses into the cavernous smooth muscle of the penis, where it transforms guanosine triphosphate (GTP) into cGMP, which activates cGMP-dependent protein kinase (PKG) that phosphorylates and regulates various ion channels, leading to penile CC erection. Furthermore, we found that HUC-MSCs could promote the production of NO and cGMP to enhance the expression of nNOS and eNOS in CNI-ED. This further validates that HUC-MSCs may improve CNI-ED via the NO/cGMP pathway, thereby affecting erectile function. This investigation also manifested a hindrance in the nNOS and eNOS expression in the penile tissues of CNI rats, resulting in an impact on the relaxation and erectile activities of the smooth muscle in the penile cavernous region. Thus, we postulated that a combination of nNOS inactivation and OS contributes to the development of neurogenic ED. HUC-MSCs can reduce OS and prevent nNOS uncoupling, thereby improving erectile function. Our research showed that the hindrance in nNOS expression and activity following CNI causes a similar hindrance in eNOS-dependent NO production. This leads to long-lasting penile flaccidity and OS.
OS is a state of oxidative and antioxidant imbalance and is an important contributor to various diseases.34 Previous studies have found that OS reduces NO levels, induces penile CC fibrosis, promotes smooth muscle cell apoptosis, and leads to endothelial dysfunction.32,35 OS has been shown to inhibit CN regeneration and stimulate apoptosis in MPG neurons after CNI.36 Nerve injury results in the generation of ROS. Multiple studies have shown that OS, which diminishes blood flow to the cavernous sinus, hinders the function of the endothelium and interferes with the cavernous muscle relaxation.37,38,39 The OS is essential in advancing CNI-ED.40 Hence, decreasing the degree of OS is a viable and efficient approach to enhance erectile function after CNI. OS may be evaluated by quantifying the serum levels of MDA and SOD activity.41 MDA is the final result of the process of lipid oxidation. The MDA level is an indicator of the degree of damage caused by OS. SOD is the primary enzyme responsible for removing excessive ROS and is regarded as an indicator of the body’s ability to counteract OS. To study the impact of HUC-MSCs on OS in rats, we analyzed the levels of MDA content and SOD activity in the serum of three groups of rats. We found that MDA levels were higher and SOD activity was lower in the crush group; nevertheless, following HUC-MSCs intervention, MDA levels were significantly lower, and SOD activity was significantly higher, implying that HUC-MSCs can inhibit OS in CNI-ED rats. Antioxidant capacity of the stem cells can cause changes in SOD and MDA. The neuroinflammatory response after CNI leads to lower SOD levels and higher MDA levels in the OS, which reduces the body’s ability to scavenge oxygen free radicals. OS and ED are closely related. OS can damage the penile endothelium and change the structure and function of the penile CC.42 Therefore, OS plays a vital role in ED. Wu et al.43 reported that oxidative emergency occurs in the CC tissue immediately after CNI. HUC-MSCs promote antioxidant enzyme activity and free radical scavenging by resisting OS-induced neuronal damage. OS in CNI-induced CC in rats was more pronounced in the crush control group. Our study found that HUC-MSCs reduced the level of OS, including MDA and SOD, in the penises of CNI-ED rats. Among them, the best effect was observed at high doses.
Normal penile erection is primarily coordinated through the interplay of properly functioning penile smooth muscles, intact endothelial cells, and characteristic nitrogenous nerves.44 Continued penile denervation can lead to alterations in the CC of the penis, primarily in the form of penile fibrosis.45 Previous studies have stated that fibrosis develops in the penis early after injury and progresses over time.46,47,48,49 The crush control group showed significant fibrosis starting approximately 1 week after CNI and progressed over time. While the fibrotic response might have a protective effect, the advancement of fibrosis can result in permanent cellular malfunction or organ failure.50 Therefore, it is essential to promptly restore the functionality of the penile tissue. In addition, we assessed the erectile function of the penis and the occurrence of fibrosis in the penile CC in CNI-ED rats. The crush control group was characterized by a lower ratio of smooth muscle to collagen, as well as reduced production of junctional proteins, which are the markers of smooth muscle. In contrast, the control HUC-MSCs group exhibited a significant elevation in the smooth muscle/collagen ratio and junctional protein expression in the penile CC compared to the crush group. This suggests that the development of penile fibrosis was effectively hindered. No recovery was noted 28 days after the injury, and these results suggested that CNI is primarily a matter of nerve recovery rather than recovery from the cavernous body. Therefore, for CNI-ED, future treatment should prioritize protecting the cavernous body of the penis than the nerves.
This study has some limitations. First, the alterations observed were quantified using the semiquantitative immunofluorescence microscopy. Second, the experimental mechanisms were incompletely studied and have not been validated by the silencing of key targets. Third, we used the CNI-ED rat model to explore the effects of HUC-MSCs on ED and their underlying mechanisms. Therefore, additional studies are required to gain a comprehensive understanding of the safety, efficacy, and other unknowns of HUC-MSCs in the ED before they can be used in clinical practice. Moreover, we need to establish the animal models, including rabbits, dogs, and monkeys, for further validation of the therapeutic effects of HUC-MSCs on ED. Finally, HUC-MSCs secrete cytokines,51 chemokines,52 growth factors,53 proteases,54 and extracellular vesicles55 to exert paracrine effects; however, we have not yet clarified by which paracrine mode HUC-MSCs function. We will further elucidate the mechanism of HUC-MSCs in treating CNI-ED through appropriate ex vivo experiments.
AUTHOR CONTRIBUTIONS
WW, YL, and ZBZ designed this study. WW, YL, ZHZ, JKW, and TZ performed the experiments and collected and analyzed the data. WW and YL wrote the manuscript. PFH, KP, JRL, and CHH provided technical support for the analysis and critical revision of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
(a) Immunofluorescence showing positive expression of various markers in the sham group. (b) Each column depicts the mean ± standard deviation for per group (n = 5). NF: neurofilament; nNOS: neurogenic nitric oxide synthase; eNOS: endothelial nitric oxide synthase; α-SMA: alpha smooth muscle actin; RECA-1: rat endothelial cell antigen-1. The groups’ description is the same as that shown in Figure 2.
HUC-MSCs treatment restored NO and cGMP components. HUC-MSC: human umbilical cord-derived mesenchymal stem cell; NO: nitric oxide; cGMP: cyclic guanosine monophosphate. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of NF. NF: neurofilament. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of RECA-1. RECA-1: rat endothelial cell antigen-1. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of α-SMA. α-SMA: alpha smooth muscle actin. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of Desmin. The groups’ description is the same as that shown in Figure 2.
HUC-MSC treatment altered SOD and MDA expression. HUC-MSC: human umbilical cord-derived mesenchymal stem cell; MDA: malondialdehyde; SOD: superoxide dismutase. The groups’ description is the same as that shown in Figure 2.
ACKNOWLEDGMENTS
This study was supported by the Xuzhou City 2022 Special Program for Promoting Science and Technology Innovation (grant No. KC22096) and Shandong Provincial Hospital Research Incubation Fund (No. 2022FY063).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, et al. Erectile dysfunction:AUA guideline. J Urol. 2018;200:633–41. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Najari BB, Kashanian JA. Erectile dysfunction. JAMA. 2016;316:1838. doi: 10.1001/jama.2016.12284. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–17. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Fode M, Ohl DA, Ralph D, Sønksen J. Penile rehabilitation after radical prostatectomy:what the evidence really says. BJU Int. 2013;112:998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
- 6.Mangır N, Türkeri L. Stem cell therapies in post-prostatectomy erectile dysfunction:a critical review. Can J Urol. 2017;24:8609–19. [PubMed] [Google Scholar]
- 7.Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7:321–8. doi: 10.1016/j.sxmr.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Fu X, Liu G, Halim A, Ju Y, Luo Q, et al. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8:784. doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naderi N, Combellack EJ, Griffin M, Sedaghati T, Javed M, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14:112–24. doi: 10.1111/iwj.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grochowski C, Radzikowska E, Maciejewski R. Neural stem cell therapy-brief review. Clin Neurol Neurosurg. 2018;173:8–14. doi: 10.1016/j.clineuro.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome:toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bento G, Shafigullina AK, Rizvanov AA, Sardão VA, Macedo MP, et al. Urine-derived stem cells:applications in regenerative and predictive medicine. Cells. 2020;9:573. doi: 10.3390/cells9030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yiou R, Hamidou L, Birebent B, Bitari D, Lecorvoisier P, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction:an open dose-escalation pilot study. Eur Urol. 2016;69:988–91. doi: 10.1016/j.eururo.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy:an open-label phase I clinical trial. EBioMedicine. 2016;5:204–10. doi: 10.1016/j.ebiom.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Yin Y, He K, Ye K, Zhou J, et al. Intracavernous pressure recording in a cavernous nerve injury rat model. J Vis Exp. 2021;175:63024. doi: 10.3791/63024. [DOI] [PubMed] [Google Scholar]
- 16.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–65. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 17.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, et al. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction:a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Chen X, Zheng T, Han D, Zhang H, et al. Transplantation of human urine-derived stem cells transfected with pigment epithelium-derived factor to protect erectile function in a rat model of cavernous nerve injury. Cell Transplant. 2016;25:1987–2001. doi: 10.3727/096368916X691448. [DOI] [PubMed] [Google Scholar]
- 19.Gu X, Thakker PU, Matz EL, Terlecki RP, Marini FC, et al. Dynamic changes in erectile function and histological architecture after intracorporal injection of human placental stem cells in a pelvic neurovascular injury rat model. J Sex Med. 2020;17:400–11. doi: 10.1016/j.jsxm.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Chen Z, Zhong F, Yang W, Ouyang X, et al. Transplantation of human gingiva-derived mesenchymal stem cells ameliorates neurotic erectile dysfunction in a rat model. Front Bioeng Biotechnol. 2021;9:630076. doi: 10.3389/fbioe.2021.630076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Nie P, Yang W, Ma X, Chen Z, et al. Lipopolysaccharide-preconditioned allogeneic adipose-derived stem cells improve erectile function in a rat model of bilateral cavernous nerve injury. Basic Clin Androl. 2022;32:5. doi: 10.1186/s12610-022-00156-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Z, Chai M, Guo F, Fu X, Lan Y, et al. MicroRNA-126 engineered muscle-derived stem cells attenuate cavernosa injury-induced erectile dysfunction in rats. Aging (Albany, NY) 2021;13:14399–415. doi: 10.18632/aging.203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Zhang K, Ruan Z, Sun D, Zhang H, et al. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. Stem Cell Res Ther. 2020;11:302. doi: 10.1186/s13287-020-01788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo W, Li Y, Zhang Y, Li H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. FASEB J. 2020;34:13345–60. doi: 10.1096/fj.202000102RR. [DOI] [PubMed] [Google Scholar]
- 25.Kadihasanoglu M, Ozbek E. Intravenous preload of mesenchymal stem cells rescues erectile function in a rat model of cavernous nerve injury. J Sex Med. 2017;14:1175. doi: 10.1016/j.jsxm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Ti Y, Yang M, Chen X, Zhang M, Xia J, et al. Comparison of the therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells and adipose-derived stem cells on erectile dysfunction in a rat model of bilateral cavernous nerve injury. Front Bioeng Biotechnol. 2022;10:1019063. doi: 10.3389/fbioe.2022.1019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. doi: 10.1016/j.lfs.2019.116733. [DOI] [PubMed] [Google Scholar]
- 28.Pozzi E, Muneer A, Sangster P, Alnajjar HM, Salonia A, et al. Stem-cell regenerative medicine as applied to the penis. Curr Opin Urol. 2019;29:443–9. doi: 10.1097/MOU.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell based therapy:current status and perspectives. Cell Transplant. 2014;23:104. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kim HY, Kim SY, Lee SH, Lee WK, et al. Spontaneous recovery of cavernous nerve crush injury. Korean J Urol. 2011;52:560–5. doi: 10.4111/kju.2011.52.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, et al. An overview of NO signaling pathways in aging. Molecules. 2021;26:4533. doi: 10.3390/molecules26154533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karakus S, Musicki B, La Favor JD, Burnett AL. cAMP-dependent post-translational modification of neuronal nitric oxide synthase neuroprotects penile erection in rats. BJU Int. 2017;120:861–72. doi: 10.1111/bju.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Han X, Ouyang X, Fang J, Huang X, et al. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics. 2019;9:6354–68. doi: 10.7150/thno.34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Ding XG, Li SW, Zheng H, Zheng XM, et al. Role of oxidative stress in surgical cavernous nerve injury in a rat model. J Neurosci Res. 2015;93:922–9. doi: 10.1002/jnr.23545. [DOI] [PubMed] [Google Scholar]
- 35.Kimura M, Rabbani ZN, Zodda AR, Yan H, Jackson IL, et al. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J Sex Med. 2012;9:1535–49. doi: 10.1111/j.1743-6109.2012.02716.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao ZK, Yu HL, Liu B, Wang H, Luo Q, et al. Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regen Res. 2016;11:1312–21. doi: 10.4103/1673-5374.189197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song G, Wang J, Liu J, Ruan Y. Dimethyl fumarate ameliorates erectile dysfunction in bilateral cavernous nerve injury rats by inhibiting oxidative stress and NLRP3 inflammasome-mediated pyroptosis of nerve via activation of Nrf2/HO-1 signaling pathway. Redox Biol. 2023;68:102938. doi: 10.1016/j.redox.2023.102938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao CB, Chen WB, Wang WZ, Gong FX, Fan CQ, et al. Nitro-oleic acid ameliorates erectile dysfunction in a streptozotocin-induced rat model of diabetes by inhibiting oxidative stress and apoptosis and activating the NO/cGMP pathway. Asian J Androl. 2024;26:57–66. doi: 10.4103/aja202331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Z, Wang W, Pan C, Fan C, Li Y, et al. N-acetylcysteine improves diabetic associated erectile dysfunction in streptozotocin-induced diabetic mice by inhibiting oxidative stress. J Cell Mol Med. 2022;26:3527–37. doi: 10.1111/jcmm.17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu H, Bai X, Le L, Tian D, Gao H, et al. Eucommia ulmoides Oliv. leaf extract improves erectile dysfunction in streptozotocin-induced diabetic rats by protecting endothelial function and ameliorating hypothalamic-pituitary-gonadal axis function. Evid Based Complement Alternat Med 2019. 2019:1782953. doi: 10.1155/2019/1782953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases:mechanism and clinical study. Signal Transduct Target Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Wang Y, Cong R, Tian Y, Chen C, et al. Restoration of erectile function by suppression of corporal apoptosis and oxidative stress with losartan in aged rats with erectile dysfunction. Andrology. 2020;8:769–79. doi: 10.1111/andr.12757. [DOI] [PubMed] [Google Scholar]
- 43.Wu YN, Chen KC, Liao CH, Chiang HS. Spontaneous regeneration of nerve fiber and irreversibility of corporal smooth muscle fibrosis after cavernous nerve crush injury:evidence from serial transmission electron microscopy and intracavernous pressure. Urology. 2018;118:98–106. doi: 10.1016/j.urology.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004;83:189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Cho SY, Park K, Chai JS, Son H, et al. Role of inhibiting LIM-kinase2 in improving erectile function through suppression of corporal fibrosis in a rat model of cavernous nerve injury. Asian J Androl. 2018;20:372–8. doi: 10.4103/aja.aja_82_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho MC, Lee J, Son H, Kim SW. Rectification of cavernosal fibrosis and veno-occlusive dysfunction by administration of suberoylanilide hydroxamic acid in a rat model of cavernosal nerve injury:comparison with a PDE5 inhibitor. Andrology. 2021;9:720–7. doi: 10.1111/andr.12922. [DOI] [PubMed] [Google Scholar]
- 47.Ismy J, Khalilullah SA, Maulana R, Hidayatullah F. A potential treatment for erectile dysfunction:effect of platelet-rich plasma administration on axon and collagen regeneration in cavernous nerve injury. Narra J. 2024;4:e880. doi: 10.52225/narra.v4i2.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W, Qiu C, Zhai J, Zhang W, Huang C, et al. Ultrasound-targeted microbubble destruction mediates PDE5i/NO integration for cavernosum remodeling and penile rehabilitation. Bioeng Transl Med. 2023;8:e10568. doi: 10.1002/btm2.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choe S, Veliceasa D, Bond CW, Harrington DA, Stupp SI, et al. Sonic hedgehog delivery from self-assembled nanofiber hydrogels reduces the fibrotic response in models of erectile dysfunction. Acta Biomater. 2016;32:89–99. doi: 10.1016/j.actbio.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockey DC, Bell PD, Hill JA. Fibrosis –a common pathway to organ injury and failure. N Engl J Med. 2015;373:96. doi: 10.1056/NEJMc1504848. [DOI] [PubMed] [Google Scholar]
- 51.Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 2019;10:247. doi: 10.1186/s13287-019-1366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Praveen Kumar L, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K, et al. The mesenchymal stem cell secretome:a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Baer PC, Geiger H. Mesenchymal stem cell interactions with growth factors on kidney repair. Curr Opin Nephrol Hypertens. 2010;19:1–6. doi: 10.1097/MNH.0b013e328333062c. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Rong P, Ma X, Nie W, Chen Y, et al. Mouse umbilical cord mesenchymal stem cell paracrine removes renal fibrosis in diabetic nephropathy by reducing myofibroblast transdifferentiation and cell proliferation and upregulating mmmps in mesangial cells. J Diabetes Res 2020. 2020:3847171. doi: 10.1155/2020/3847171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hur YH, Cerione RA, Antonyak MA. Extracellular vesicles and their roles in stem cell biology. Stem Cells. 2020;38:469–76. doi: 10.1002/stem.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Immunofluorescence showing positive expression of various markers in the sham group. (b) Each column depicts the mean ± standard deviation for per group (n = 5). NF: neurofilament; nNOS: neurogenic nitric oxide synthase; eNOS: endothelial nitric oxide synthase; α-SMA: alpha smooth muscle actin; RECA-1: rat endothelial cell antigen-1. The groups’ description is the same as that shown in Figure 2.
HUC-MSCs treatment restored NO and cGMP components. HUC-MSC: human umbilical cord-derived mesenchymal stem cell; NO: nitric oxide; cGMP: cyclic guanosine monophosphate. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of NF. NF: neurofilament. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of RECA-1. RECA-1: rat endothelial cell antigen-1. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of α-SMA. α-SMA: alpha smooth muscle actin. The groups’ description is the same as that shown in Figure 2.
Immunofluorescence showed positive expression of Desmin. The groups’ description is the same as that shown in Figure 2.
HUC-MSC treatment altered SOD and MDA expression. HUC-MSC: human umbilical cord-derived mesenchymal stem cell; MDA: malondialdehyde; SOD: superoxide dismutase. The groups’ description is the same as that shown in Figure 2.


